Abstract
Background
People living with HIV have an increased risk of left ventricular diastolic dysfunction (LVDD) and heart failure. HIV‐associated LVDD may reflect both cardiomyocyte and systemic metabolic derangements, but the underlying pathways remain unclear.
Methods and Results
To explore such pathways, we conducted a pilot study in the Bronx and Brooklyn sites of the WIHS (Women's Interagency HIV Study) who participated in concurrent, but separate, metabolomics and echocardiographic ancillary studies. Liquid chromatography tandem mass spectrometry–based metabolomic profiling was performed on plasma samples from 125 HIV‐infected (43 with LVDD) and 35 HIV‐uninfected women (9 with LVDD). Partial least squares discriminant analysis identified polar metabolites and lipids in the glycerophospholipid‐metabolism and fatty‐acid‐oxidation pathways associated with LVDD. After multivariable adjustment, LVDD was significantly associated with higher concentrations of diacylglycerol 30:0 (odds ratio [OR], 1.60, 95% CI [1.01–2.55]); triacylglycerols 46:0 (OR 1.60 [1.04–2.48]), 48:0 (OR 1.63 [1.04–2.54]), 48:1 (OR 1.62 [1.01–2.60]), and 50:0 (OR 1.61 [1.02–2.53]); acylcarnitine C7 (OR 1.88 [1.21–2.92]), C9 (OR 1.99 [1.27–3.13]), and C16 (OR 1.80 [1.13–2.87]); as well as lower concentrations of phosphocholine (OR 0.59 [0.38–0.91]). There was no evidence of effect modification of these relationships by HIV status.
Conclusions
In this pilot study, women with or at risk of HIV with LVDD showed alterations in plasma metabolites in the glycerophospholipid‐metabolism and fatty‐acid‐oxidation pathways. Although these findings require replication, they suggest that improved understanding of metabolic perturbations and their potential modification could offer new approaches to prevent cardiac dysfunction in this high‐risk group.
Keywords: heart failure, HIV, left ventricular diastolic dysfunction, metabolomics
Subject Categories: Heart Failure, Metabolism
Clinical Perspective
What Is New?
This is the first investigation of metabolomic perturbations associated with left ventricular diastolic dysfunction in the context of HIV infection.
What Are the Clinical Implications?
These findings highlight the potential role of impaired fatty acid metabolism, mitochondrial dysfunction, and increased oxidative stress in early‐stage cardiac dysfunction.
Pending replication, these findings suggest that further work to understand the pathways involved and testing pharmacological interventions could lead to new strategies to prevent heart failure, both among women with or at risk for HIV and more broadly.
Introduction
Progress in delineating risk factors for HIV infection, along with advances in treatment, have redefined the contemporary epidemiology of this condition.1, 2, 3, 4 Incidence of HIV infection has been declining over time, yet the number of people living with HIV continues to rise, driven by the markedly improved survival conferred by antiretroviral therapy (ART).1, 2, 3, 4 With ART's transformation of HIV infection into a chronic condition has come the recognition that people living with HIV appear to be susceptible to a range of common aging‐related disorders.4, 5, 6
People living with HIV have been reported to have a higher risk of cardiovascular disease than uninfected individuals.7 Apart from a well‐documented increased risk of myocardial infarction in persons with HIV,8, 9 available evidence also points to a higher risk of heart failure in the absence of previous coronary artery disease,10 including heart failure with preserved ejection fraction.11 Consistent with this finding, a meta‐analysis of echocardiographic studies of HIV‐positive individuals receiving ART (mean age, 41 years) showed a prevalence of left ventricular (LV) diastolic dysfunction (LVDD) of 43%,12 far exceeding the prevalence of 8% reported in an older (mean age, 63 years) HIV‐negative population.13 More‐recent classification schemes used more‐stringent criteria for LVDD, leading to lower prevalences,14 but among mostly middle‐aged participants with or at risk for HIV in the WIHS (Women's Interagency HIV Study), frequencies of LVDD based on the latest guidelines15, 16 were still substantial at 19% to 29%.17
The high prevalence of HIV‐related LVDD reflects an increased burden of subclinical myocardial disease, with increased myocardial fibrosis and cardiomyocyte steatosis documented by magnetic resonance imaging in persons with HIV.18 The precise basis for such myocardial disease/dysfunction has not been determined, but various factors are likely implicated, including chronic inflammation and immune activation, adverse effects of ART, or increased behavioral and clinical risk factors for cardiovascular diseases.7
Women with or at risk for HIV have a high burden of obesity and metabolic dysregulation,19 which along with inflammation and oxidative stress can alter systemic and myocardial energetics.20 In this context, the advent of metabolomics allowing untargeted assessment of a wide array of metabolic intermediates offers the opportunity to probe biochemical pathways involved in cardiac dysfunction.21 Given that LVDD reflects early myocardial functional derangements that can eventuate in clinical heart failure,22 and is of particular relevance to women and race/ethnic minorities,23, 24 evaluating the metabolomic signatures of LVDD could lead to improved risk stratification, novel mechanistic insights, and potential new prevention approaches for this important condition.21 In this pilot study, we leveraged availability of plasma metabolomics and lipidomics measured among women who completed echocardiograms in a substudy of the WIHS25, 26 to investigate the plasma metabolite profiles of LVDD in this high‐risk cohort.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
The study was conducted in a sample of participants enrolled in the WIHS, an ongoing multicenter study of the natural history of HIV disease in women.25, 26 Women in the present analysis were drawn from those participating in 2 WIHS ancillary studies, an echocardiographic study performed in the Bronx and Brooklyn sites in 2004–2005,27 and a metabolomic study performed in nondiabetic participants with available carotid sonograms.28 Relevant demographic, behavioral, anthropometric, and clinical information was obtained from the included participants, as previously reported.28
The study was approved by the institutional review boards of both study sites, and all subjects provided written informed consent.
Echocardiography Procedures
Echocardiogram acquisition followed a standardized protocol involving 2‐dimensional, M‐mode, spectral, tissue‐Doppler, and color‐Doppler imaging, as previously reported.27 Transmitral diastolic velocities were obtained by placing the sample volume at the leaflet tips. Tissue Doppler imaging was performed at the medial mitral annulus. Data were recorded on VHS tapes and analyzed by a single experienced echocardiographer (J.L.). LV wall thicknesses and internal diameters were determined in accordance with the American Society of Echocardiography (ASE) guidelines.29 LV ejection fraction was calculated using the biplane method of discs from the apical‐4‐chamber and apical‐2‐chamber views and confirmed by visual estimation. Left atrial volumes were determined by the biplane method of discs.
For our primary evaluation of LV diastolic function, we used the 2009 ASE/European Association of Cardiovascular Imaging (EACVI) guidelines,16 applying the 2016 ASE/EACVI guidelines in a sensitivity analysis.15 This approach was chosen to enhance identification of metabolomic profiles associated with earlier and milder stages of LVDD occurring in our younger population, detectable with the less‐restrictive criteria formulated by the 2009 ASE/EACVI classification.
The 2009 ASE/EACVI guidelines define LVDD based on septal and lateral mitral annular early diastolic (e’) velocities and left atrial volume. We applied the 2009 ASE/EACVI criteria incorporating the presence of left atrial enlargement (≥34 mL/m2) and either septal e’ velocity <8 cm/s or lateral e’ velocity <10 cm/s to define LVDD, but modified these to include only septal e’ because lateral e’ was not obtained.17 Similarly, we adapted the 2016 ASE/EACVI guidelines15 to define LVDD as the presence of >2 of the following echocardiographic findings: (1) early mitral inflow (E)/septal e’ ratio >15; (2) septal e’ velocity <7 cm/s; (3) tricuspid regurgitation velocity >2.8 m/s; and (4) left atrial volume >34 mL/m2. For the 2016 ASE/EACVI guidelines, cases with indeterminate diastolic function were reclassified as LVDD if LV end‐diastolic volume index was >61 mL/m2 or LV mass index was >95 g/m2. Grading of LVDD was done in accordance with each set of guidelines.15, 17 For both LVDD definitions, we included all grades of LVDD.
Metabolomics
Fasting plasma samples were sent to the Broad Institute Metabolomics Platform (Cambridge, MA), where metabolomic technology was used to quantitatively profile metabolites. Metabolomic analysis was performed utilizing tandem liquid chromatography/mass spectrometry. Liquid chromatography affords reproducible separation of metabolites on the basis of their physical properties, and mass spectrometry enables further resolution of metabolites on the basis of mass‐to‐charge ratio (m/z). Two separate liquid chromatography/mass spectrometry methods were performed to measure polar metabolites and lipids in each sample, as previously described (Data S1).28, 30, 31 Raw data from Orbitrap mass spectrometers were processed using Progenesis QI software (NonLinear Dynamics, Durham, NC) for feature alignment, untargeted signal detection, and signal integration. Metabolites were quantified using area under the curve of the peaks. The targeted processing of a subset of known metabolites was conducted using TraceFinder software (version 3.1; Thermo Fisher Scientific; Waltham, MA). In the current analysis, a total of 325 metabolites (114 polar metabolites and 211 lipids) were included after excluding metabolites with coefficient of variation of ≥20% or percentage of missing values ≥20%.
Statistical Analysis
Continuous variables were summarized with the mean and SD, if the data were normally distributed, and the median and interquartile range otherwise. Means were compared utilizing the parametric 2‐tailed Student t test, and comparisons between medians were performed using the nonparametric McNemar test. The chi‐squared test was performed to compare proportions, and Fisher's exact test was utilized when the number in a cell was <5 in the 2×2 table. Given that the data obtained from metabolomics are semiquantitative, that is, the values are not the actual levels with a unit, but are instead relative levels, we applied rank‐based inverse normal transformation to all plasma polar metabolites and lipids to transform the measurements to a normal distribution. Given that the number of polar metabolites and lipids was greater than the number of participants in this study, we used the dimension‐reduction technique, partial least squares discriminant analysis (PLS‐DA), to identify polar metabolites and lipids with high contributions to group separation. Because the polar metabolites and lipids were analyzed in different platforms, we performed PLS‐DA separately for each group of metabolites. Thereafter, we selected the top 10 polar metabolites and top 10 lipids with the highest loading coefficients and variable importance projection scores of the first principal component, using the mixOmics package (mixomics.org) for further analyses. We assessed the associations between these polar metabolites and lipids with the presence of LVDD using logistic regression, adjusting for age, race/ethnicity, level of education, smoking or drinking history, hepatitis C virus infection, and history of intravenous drug use (model 1); HIV status, baseline viral load, and ART status (included in model 2, in addition to variables in model 1); and total cholesterol, high‐density lipoprotein cholesterol, systolic blood pressure, homeostasis model assessment of insulin resistance, and current use of lipid‐lowering or antihypertensive medication (included in model 3, in addition to variables in model 2). The interpretation was the odds ratio of LVDD per SD of plasma polar metabolite and lipid levels. We tested the difference by HIV status in associations between polar metabolites/lipids and LVDD by adding an interaction term of polar metabolites/lipids and HIV status in the logistic models and reported P for interaction.
In order to evaluate the joint associations of the top 10 polar metabolites in addition to the top 10 lipids, we examined the score of each metabolite in association with LVDD using 3 models. We generated a weighted score of the 20 selected metabolites by summing the weighted rank‐based inverse normal transformed levels of these metabolites. The weight for each metabolite is the coefficient of the metabolite generated from logistic regression, with LVDD as the dependent variable and each metabolite and covariates in model 1 as independent variables.
All analyses were conducted using SPSS (version 15; SPSS, Inc, Chicago, IL) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). Visualization of pathway mapping was performed in Cytoscape (version 3.7.1; MetScape application version 3.1.3).32 Because of the pilot nature of the study, statistical significance was defined as P<0.05 without correction for multiple testing. The Benjamini–Yekutieli procedure was applied to explore whether any associations met significance by controlling the study‐wide false discovery rate at q≤0.05.
Results
Baseline Characteristics
Among 160 participants included in this study, 52 (32.5%) women had echocardiographic evidence of LVDD. Of these, 42 women had grade I and 10 had grade II LVDD. A total of 125 women were HIV positive, of whom 43 (34.4%) had evidence of LVDD; and among the 35 HIV‐negative women, 9 (25.7%) had evidence of LVDD. A small minority of participants overall had LV systolic dysfunction, 4 (2.5%), without significant difference by HIV status. This and other pertinent information are listed in Table 1.
Table 1.
Characteristics of Participants
| All | LVDD | P Values | ||
|---|---|---|---|---|
| No | Yes | |||
| No. of participants | 160 | 108 | 52 | |
| Demographic and behavioral | ||||
| Age, y | 41.5 [39.0–46.0] | 41.0 [39.0–45.0] | 44.5 [39.0–51.0] | 0.02 |
| Race/ethnicity | 0.53 | |||
| Non‐Hispanic black | 108 (68%) | 75 (69%) | 33 (63%) | |
| Hispanic | 47 (29%) | 29 (27%) | 18 (35%) | |
| Non‐Hispanic white/other | 5 (3%) | 4 (4%) | 1 (2%) | |
| Body mass index, kg/m2 | 27.9 [24.6–31.5] | 27.8 [24.6–31.3] | 28.4 [24.6–31.8] | 0.79 |
| Waist circumference, cm | 88.7 [81.3–97.2] | 88.0 [80.7–95.5] | 91.6 [82.6–100.1] | 0.31 |
| Hip circumference, cm | 99.1 [93.4–108.0] | 101.2 [93.4–108.0] | 96.4 [92.9–109.3] | 0.13 |
| Current crack/cocaine use | 9 (6%) | 7 (6%) | 2 (4%) | 0.72 |
| History of intravenous drug use | 38 (24%) | 20 (19%) | 18 (35%) | 0.03 |
| Current smoker | 87 (54%) | 61 (56%) | 26 (50%) | 0.44 |
| Alcohol abuse (>7 drinks per wk) | 18 (11%) | 10 (9%) | 8 (15%) | 0.25 |
| Laboratory parameters | ||||
| Total cholesterol, mg/dL | 180.6 (37.7) | 182.5 (39.0) | 176.6 (34.7) | 0.35 |
| LDL cholesterol, mg/dL | 105.0 (31.0) | 106.6 (33.5) | 102.0 (25.1) | 0.37 |
| HDL cholesterol, mg/dL | 48.7 (17.2) | 50.1 (17.8) | 45.6 (15.5) | 0.12 |
| Triglycerides, mg/dL | 108.0 [74.0–144.0] | 110.5 [74.0–140.0] | 105.0 [75.0–152.0] | 0.63 |
| HOMA‐IR | 2.4 [1.6–3.6] | 2.4 [1.7–3.6] | 2.8 [1.5–3.8] | 0.44 |
| eGFR, mL/min/1.73 m2 | 93.4 (21.6) | 95.7 (20.6) | 88.5 (23.0) | 0.05 |
| hs‐CRP, μg/mL | 2.0 [0.8–4.9] | 2.2 [0.8–4.9] | 1.8 [0.8–5.8] | 0.96 |
| Comorbidities | ||||
| Systolic blood pressure, mm Hg | 119.3 (19.0) | 117.3 (16.3) | 123.2 (23.2) | 0.1 |
| Diastolic blood pressure, mm Hg | 74.5 (11.4) | 73.6 (11.1) | 76.3 (11.9) | 0.17 |
| Current antihypertensive medication use | 31 (19%) | 19 (18%) | 12 (23%) | 0.41 |
| Current lipid‐lowering medication use | 4 (3%) | 2 (2%) | 2 (4%) | 0.6 |
| Hepatitis C virus infection | 48 (30%) | 25 (23%) | 23 (44%) | 0.006 |
| HIV and related factors | ||||
| HIV infection | 125 (78%) | 82 (76%) | 43 (83%) | 0.33 |
| CD4+ count, cell/mm3 | 429 [282–602] | 469 [299–636] | 367 [267–564] | 0.23 |
| HIV+ viral load, copies/mL | 260 [80–5000] | 195 [80–4000] | 540 [80–7700] | 0.57 |
| Current antiretroviral therapy use | 100 (80%) | 64 (78%) | 36 (84%) | 0.45 |
| Echocardiographic parameters | ||||
| LV ejection fraction (%) | 59.8 (6.2) | 60.1 (5.8) | 59.2 (7.1) | 0.4 |
| LV ejection fraction <50% | 4 (2.5) | 2 (1.9) | 2 (3.9) | 0.60 |
| LV mass index, g/m2 | 82.1 (17.7) | 80.2 (17.4) | 86.1 (17.7) | 0.05 |
| Left atrial volume index, mL/m2 | 27.5 (6.5) | 26.4 (4.7) | 29.8 (8.8) | 0.01 |
| LV end‐diastolic volume index, mL/m2 | 45.8 (10.6) | 45.6 (10.6) | 46.0 (10.6) | 0.85 |
| LV early filling velocity, E (m/s) | 0.8 [0.7–0.9] | 0.8 [0.7–0.9] | 0.7 [0.6–0.9] | 0.04 |
| LV late filling velocity, A (m/s) | 0.7 [0.6–0.8] | 0.6 [0.5–0.8] | 0.7 [0.6–0.8] | 0.01 |
| E/A ratio | 1.2 [0.9–1.4] | 1.3 [1.1–1.5] | 1.0 [0.8–1.2] | 0.0007 |
| Septal e’ at mitral annulus, ms | 0.10 [0.08–0.13] | 0.11 [0.10–0.14] | 0.07 [0.06–0.10] | <0.0001 |
| E/e’ ratio | 8.0 [6.2–10.0] | 7.1 [5.5–8.8] | 10.0 [7.5–12.0] | <0.0001 |
eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; LV, left ventricular; LVDD, left ventricular diastolic dysfunction.
Metabolomic and Lipidomic Profiles With LVDD
PLS‐DA showed that using the first and second principal components of metabolomic and lipidomic profiles, respectively, there were some distinctions between participants with and without LVDD (Figure 1) and also in the presence or absence of HIV (Figure S1), but these distinctions were at a relatively low level. We then focused on the top 10 polar metabolites and top 10 lipids in the first principal components selected by the PLS‐DA (Table 2 and Table S1). None of these top polar metabolites or lipids showed significant associations with LVDD by the Benjamini–Yekutieli false discovery rate threshold.
Figure 1.
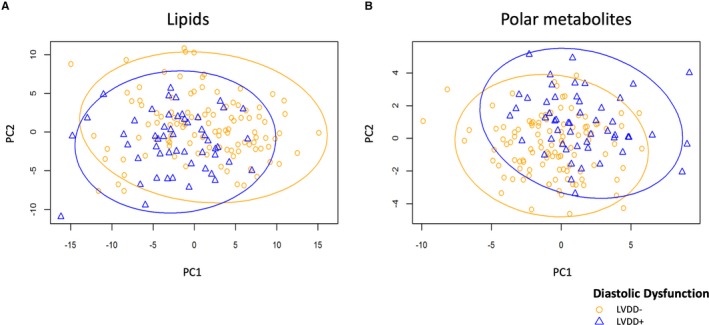
Scores plot of metabolites and lipids according to presence of LVDD. Scores plot showing metabolites from principal component 1 and 2 obtained with PLS‐DA analysis of participants with LVDD (blue triangles and line) vs without LVDD (orange circles and line) for (A) lipids and (B) polar metabolites. LVDD indicates left ventricular diastolic dysfunction; PC, principal component; PLS‐DA, partial least squares discriminant analysis.
Table 2.
Relationship Between Top Polar Metabolites/Lipids Concentration and LVDD
| All | HIV+ | HIV− | P‐inta | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | OR (95% CI) | ||
| Polar metabolites | |||||
| Phosphocholine | |||||
| Model 1 | 0.59 (0.39–0.87) | 0.008 | 0.56 (0.37–0.87) | 0.65 (0.26–1.62) | 0.26 |
| Model 2 | 0.56 (0.37–0.85) | 0.006 | 0.55 (0.35–0.86) | 0.63 (0.25–1.59) | 0.64 |
| Model 3 | 0.60 (0.39–0.93) | 0.022 | 0.52 (0.32–0.86) | 1.06 (0.41–2.71) | 0.92 |
| C7‐Carnitine | |||||
| Model 1 | 1.60 (1.09–2.36) | 0.017 | 1.61 (1.04–2.50) | 1.55 (0.68–3.54) | 0.25 |
| Model 2 | 1.62 (1.09–2.41) | 0.018 | 1.63 (1.04–2.56) | 1.58 (0.69–3.63) | 0.38 |
| Model 3 | 1.88 (1.18–2.98) | 0.008 | 1.94 (1.16–3.25) | 1.67 (0.68–4.06) | 0.27 |
| C9‐Carnitine | |||||
| Model 1 | 1.73 (1.18–2.55) | 0.006 | 1.96 (1.24–3.12) | 1.23 (0.56–2.71) | 0.35 |
| Model 2 | 1.82 (1.21–2.72) | 0.004 | 2.07 (1.28–3.35) | 1.23 (0.56–2.72) | 0.35 |
| Model 3 | 1.96 (1.25–3.08) | 0.004 | 2.22 (1.32–3.75) | 1.27 (0.51–3.17) | 0.62 |
| C16‐Carnitine | |||||
| Model 1 | 1.67 (1.13–2.48) | 0.011 | 1.72 (1.10–2.70) | 1.44 (0.58–3.60) | 0.30 |
| Model 2 | 1.73 (1.14–2.63) | 0.010 | 1.81 (1.12–2.91) | 1.47 (0.59–3.67) | 0.37 |
| Model 3 | 1.94 (1.18–3.21) | 0.010 | 2.14 (1.22–3.75) | 1.30 (0.47–3.63) | 0.28 |
| Lipids | |||||
| DAG 30:0 | |||||
| Model 1 | 1.65 (1.10–2.49) | 0.016 | 1.46 (0.93–2.30) | 2.65 (1.05–6.73) | 0.32 |
| Model 2 | 1.74 (1.14–2.66) | 0.011 | 1.56 (0.98–2.49) | 2.64 (1.04–6.68) | 0.28 |
| Model 3 | 1.70 (1.05–2.76) | 0.032 | 1.48 (0.87–2.53) | 2.76 (1.03–7.37) | 0.60 |
| TAG 46:0 | |||||
| Model 1 | 1.62 (1.08–2.41) | 0.018 | 1.45 (0.94–2.23) | 2.89 (1.06–7.90) | 0.36 |
| Model 2 | 1.69 (1.12–2.54) | 0.012 | 1.51 (0.97–2.35) | 2.89 (1.06–7.92) | 0.34 |
| Model 3 | 1.78 (1.10–2.87) | 0.018 | 1.56 (0.93–2.62) | 3.17 (1.09–9.23) | 0.52 |
| TAG 48:0 | |||||
| Model 1 | 1.61 (1.08–2.40) | 0.019 | 1.40 (0.91–2.14) | 3.75 (1.28–11.00) | 0.32 |
| Model 2 | 1.69 (1.12–2.54) | 0.012 | 1.46 (0.94–2.26) | 3.76 (1.28–11.08) | 0.37 |
| Model 3 | 1.77 (1.08–2.88) | 0.023 | 1.49 (0.89–2.51) | 4.39 (1.32–14.63) | 0.50 |
| TAG 48:1 | |||||
| Model 1 | 1.65 (1.10–2.49) | 0.016 | 1.48 (0.96–2.27) | 3.40 (1.09–10.59) | 0.35 |
| Model 2 | 1.70 (1.12–2.57) | 0.012 | 1.53 (0.99–2.38) | 3.39 (1.09–10.56) | 0.32 |
| Model 3 | 1.79 (1.06–3.03) | 0.029 | 1.60 (0.93–2.77) | 3.61 (1.06–12.32) | 0.41 |
| TAG 50:0 | |||||
| Model 1 | 1.59 (1.08–2.35) | 0.019 | 1.39 (0.91–2.12) | 3.22 (1.16–8.96) | 0.30 |
| Model 2 | 1.65 (1.10–2.47) | 0.015 | 1.44 (0.93–2.23) | 3.25 (1.16–9.06) | 0.33 |
| Model 3 | 1.73 (1.05–2.83) | 0.031 | 1.47 (0.86–2.50) | 3.75 (1.19–11.74) | 0.41 |
Regression was performed adjusted for age, race, education level, smoking, drinking habits, HCV infection, and intravenous drug use (model 1). Further adjustment was performed for HIV status, baseline viral load, and ART treatment status (model 2); and, additionally, for total cholesterol, HDL cholesterol, systolic blood pressure, HOMA‐IR, lipid‐lowering medication, and antihypertensive medication use (model 3). Metabolites underwent rank‐based inverse normal transformation. The OR for LVDD for each 1‐unit increase in the standardized plasma metabolite level (ie, per SD increase in the rank‐based inverse‐normal transformed level). ART indicates antiretroviral therapy; DAG indicates diacylglycerol; HCV, hepatitis C virus; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LVDD, left ventricular diastolic dysfunction; OR, odds ratio; TAG, triacylglycerol.
P for interaction test.
Polar Metabolites
Of the top 10 polar metabolites that PLS‐DA identified (Table S1), after adjustment for age, race/ethnicity, level of education, smoking or drinking history, hepatitis C virus infection, and history of intravenous drug use, 4 polar metabolites that belong to the glycerophospholipid‐metabolism pathway (ie, phosphocholine) and the fatty‐acid‐oxidation pathway (ie, acylcarnitines [C7, C9, and C16‐carnitine]) were significantly associated with LVDD. The same polar metabolites were significantly different in participants with and without LVDD after additional adjustment for HIV‐specific variables: HIV status, baseline viral load, and ART treatment status; and after further adjustment was performed using conventional cardiovascular risk factors: total cholesterol and high‐density lipoprotein‐cholesterol concentration, systolic blood pressure, and lipid‐lowering and antihypertensive medication use (Table 2; Figure 2). According to a test for HIV‐polar metabolite interaction, there was no significant difference in the associations of these 4 polar metabolites with LVDD between HIV‐positive and ‐negative participants (Table 2).
Figure 2.
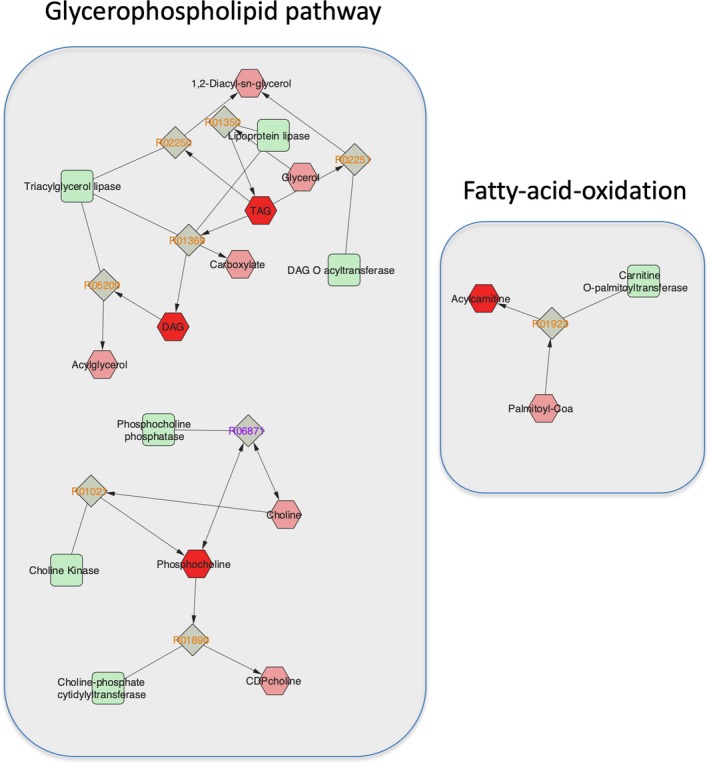
Glycerophospholipid and fatty‐acid‐oxidation pathways. Metabolic pathways constructed utilizing Cytoscape software (version 3.7.1; MetScape application version 3.1.3). The red‐colored hexagons represent the individual metabolites and lipids that were associated with the presence of LVDD within the first principal component, and the light red hexagons are other relevant compounds of those pathways. Light green squares represent the enzymes, and gray rotated squares are the reactions (KEGG ID number) of key metabolic steps of those pathways. DAG indicates diacylglycerol; KEGG, Kyoto Encyclopedia of Genes and Genomes; LVDD, left ventricular diastolic dysfunction.
Lipids
Of the top 10 lipids that PLS‐DA identified (Table S1), after adjustment for age, race/ethnicity, level of education, smoking or drinking history, hepatitis C virus infection, and history of intravenous drug use, 5 lipids that belong to the glycerophospholipid‐metabolism pathway, that is, diacylglycerol (DAG; 30:0) and triacylglycerol (TAG; 46:0, 48:0, 48:1, and 50:0), were associated with LVDD. The same lipids were significantly different between participants with and without LVDD after additional adjustment for HIV‐specific variables; and after further adjustment was performed using conventional cardiovascular risk factors (Table 2; Figure 2). No significant effect modification by HIV status was observed (Table 2).
Joint Associations of Polar Metabolites and Lipids
In an additional exploratory analysis of the joint associations involving the top 10 polar metabolites along with the top 10 lipids, we found that the score of the 20 metabolites was significantly related to an increased risk of LVDD (odds ratio, 1.25; 95% CI, 1.11–1.40; P<0.0001). We did not find evidence of interaction for this association by HIV status (Table S2).
Sensitivity Analyses
In sensitivity analyses, we explored the associations of PLS‐DA–identified polar metabolites and lipids above with LVDD defined using the 2016 ASE/EACVI guidelines,15 which identified 26 women with LVDD. After adjustment for age, race/ethnicity, level of education, smoking or drinking history, hepatitis C virus infection, and history of intravenous drug use, none of the polar metabolites were significantly associated with newly defined LVDD. After multivariable adjustment, 2 lipids that belong to the glycerophospholipid‐metabolism pathway (ie, DAG 30:0 and TAG 50:0) were associated with the newly defined LVDD. However, the associations between lipids and LVDD did not remain statistically significant after additional adjustment for HIV‐specific variables and conventional cardiovascular risk factors (Table S3).
Discussion
In this sample of early‐middle‐aged women with or at risk for HIV, mostly of black and Hispanic race/ethnicity and free of diabetes mellitus, there were no significant differences in metabolite principal components between those with and without echocardiographic LVDD. Evaluation of the top 10 polar metabolites and top 10 lipids within the first principal component, however, showed that LVDD was associated with a lower concentration of phosphocholine and higher concentration of acylcarnitine, as well as higher concentrations of TAGs and DAG. Further exploration of the joint relationship of the top 10 polar metabolites and top 10 lipids with LVDD also showed that the corresponding metabolite score was significantly associated with this outcome. There was no evidence in our sample of effect modification of these associations by HIV status. Additional analysis using the least absolute shrinkage and selection operator method confirmed the findings obtained using PLS‐DA.
To our knowledge, the present exploratory study is the first to evaluate the metabolomic signatures of LVDD in a sample of women with or at risk of HIV. Our investigation relied on the WIHS, a long‐running study originally designed to characterize the natural history of HIV infection in women.25, 26 The participants enrolled are at increased risk of cardiovascular diseases, exhibiting high frequencies of smoking, substance abuse, and other risk factors. These characteristics are commonly noted in people living with HIV, but as our sample shows, the WIHS counts as a strength that the population at risk for HIV included as a comparator has a similar frequency of risk behaviors and risk factors.33
Previously, a metabolomic analysis of people with symptomatic heart failure with preserved ejection fraction of the HEART (Alberta Heart Failure Etiology and Analysis Research Team) project showed some similar metabolic features to those found in our study. Among the metabolomic differences uncovered, the study documented that patients with heart failure with preserved ejection fraction and heart failure with reduced ejection fraction had higher concentrations of acylcarnitines, but lower concentrations of phosphatidylcholines, as compared with controls.34 In turn, metabolomic analysis of blood samples from the CATHGEN (Catheterization Genetics) study showed that patients with heart failure with preserved ejection fraction and, even more so, heart failure with reduced ejection fraction had higher concentrations of acylcarnitines compared with a control group without evidence of heart failure.35
Like previous studies of patients with heart failure, the present study found that higher acylcarnitine levels were associated with LVDD. Through the carnitine shuttle, acylcarnitines are carriers of fatty acids from the cytoplasm into the mitochondria, where the fatty acids undergo beta‐oxidation and ATP production (Figure S2). High plasma concentrations of acylcarnitines might reflect mitochondrial dysfunction, either in the myocardium, skeletal muscle, or both. Insulin resistance can also affect the fatty acid oxidation and plasma concentrations of acylcarnitine.36 In our study, however, there was no difference in homeostasis model assessment of insulin resistance between participants with LVDD versus those with normal LV diastolic function, as noted above.
Under physiological conditions, fatty‐acid‐beta oxidation is the source of 50% to 70% of cardiomyocyte energy production, and glucose is only a minor contributor.37 Evidence from animal and human studies shows that heart failure, depending on the etiology, is associated with reprogramming of energy metabolism and change in fuel preference.38, 39 In our cohort, we found that participants with LVDD had a higher plasma concentration of DAG and TAGs, which, in turn, are sources of fatty acids (Figure S2). Furthermore, TAG, acting through PPARα (peroxisome proliferator–activated receptor α), can activate the transcription of genes that encode important regulatory fatty‐acid‐oxidation proteins, resulting in enhanced lipid metabolism.40 Diabetes mellitus and obesity can be associated with higher concentrations of DAGs and TAGs, but in our study diabetic women were excluded. Furthermore, insulin resistance (homeostasis model assessment of insulin resistance), body mass index, and lipid profiles were similar between participants with LVDD versus those with normal LV diastolic function. It is possible, however, that the associations observed could reflect residual confounding, wherein greater lipolysis or hepatic DAG or TAG production may signal incompletely measured insulin resistance or related metabolic perturbations.
Another notable association identified by our study is that of lower phosphocholine with presence of LVDD. Despite the documented association of lower phosphocholine with clinical heart failure,34 available findings on the relationship of this metabolite with different measures of LVDD are contradictory.41 Our finding lends support to deficiency of this metabolite, and not excess, as implicated in subclinical cardiac dysfunction or associated disorders. Phosphocholine is a metabolic intermediate in the synthesis of phosphatidylcholine from choline, the latter primarily derived from dietary sources. Choline levels are reduced by oxidative stress, which could account for the observed association of low phosphocholine with LVDD. Moreover, phosphatidylcholine is a key component of biological membranes, suggesting that reduced levels could alter membrane function in ways that are unfavorable to cardiac function or its determinants.
The overall higher availability of fatty acids and acylcarnitines could potentially lead to increased cardiomyocyte reliance on fatty acid oxidation, heightening myocardial susceptibility to ischemia, because ATP production by beta‐oxidation requires greater oxygen consumption than that required by glycolysis.37 Our participants were free of self‐reported coronary heart disease, however, diminishing this as a contributing factor to LVDD in the present study. Higher fatty acid delivery to cardiomyocytes may lead to mismatch between supply and mitochondrial oxidative capacity, resulting in cardiac steatosis and lipotoxicity as observed in diabetics and obese patients.42 In addition, higher concentration of acylcarnitines, apart from reflecting impaired fatty acid oxidation, could itself contribute to cardiac dysfunction by fostering inflammation and oxidative stress.43
Echocardiographic LVDD is a measure of subclinical cardiac dysfunction that portends an increased risk of progression to overt heart failure. The echocardiographic diagnosis of LVDD has been a matter of debate for the past several years, as reflected by the different diagnostic criteria used in the 1998 European Society of Cardiology guidelines,44 and subsequently in newer versions published on 2009 and 2016 by the ASE/EACVI.15, 16 Observational studies have shown that compared with the 1998 and 2009 guidelines, prevalence of LVDD by the 2016 classification is lower, but appears to correlate better with clinical outcomes and N‐terminal pro b‐type natriuretic peptide plasma level.17 We applied the 2009 classification16 for this study, leveraging its higher sensitivity to improve our ability to detect early abnormalities in this modest sample that likely also portend progression to full‐fledged LVDD and, ultimately, LVDD, albeit with lower specificity than the 2016 classification. In support of this approach, sensitivity analysis using the 2016 ASE/EACVI guidelines,15 which identified 28 women with LVDD (as compared with 52 women using the 2009 guidelines),16 also showed an association between LVDD and high concentrations of TAGs and DAG, though not acylcarnitines. The extent to which these differences might reflect differences in echocardiographic features captured by 2009 and 2016 guideline definitions will require further study.
The strengths of this exploratory study are its inclusion of participants from a well‐characterized cohort of women with or at risk for HIV and predominantly of black or Hispanic race/ethnicity and leveraging of state‐of‐the‐art metabolomic profiling and standardized echocardiographic assessments. Owing to the modest sample size, no individual metabolites remained significant after correction for multiple testing in this exploratory study. Accordingly, the findings of this study can only be considered as hypothesis generating, although, interestingly, the association of the metabolite score with LVDD proved to be highly statistically significant. As such, the results of this pilot study do suggest that perturbations in distinct pathways are related to early‐stage myocardial abnormalities in high‐risk middle‐aged women. The study's modest size also prevented well‐powered assessment of differences in metabolomic associations between HIV‐positive and ‐negative groups, a question that requires investigations of substantially larger scale. Another important limitation is the study's cross‐sectional observational design, which prevents determinations of direction of associations or their casual basis. Although we undertook extensive adjustment for covariates, residual confounding by partly measured or unmeasured factors cannot be excluded. In addition, blood metabolites provide an integrative picture of metabolism, but cannot distinguish the tissue sources of the metabolites in question. Such determinations can only be accomplished through experimental studies focused on specific organs. Last, these exploratory results are not necessarily generalizable to men or other race/ethnic groups.
In conclusion, the present exploratory study shows that metabolite profiles reflecting glycerophospholipid metabolism and fatty acid oxidation are associated with subclinical LVDD in women with or at risk for HIV. These results suggest that better understanding of these metabolic abnormalities and their determinants could offer new therapeutic strategies for prevention of cardiac dysfunction and heart failure in high‐risk individuals with or at risk for HIV.
Sources of Funding
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) K01HL129892 and R01HL140976 to Qi, and other funding sources for this study include R01 HL126543, R01 HL132794, R01HL083760, and R01HL095140 to Kaplan, K01HL137557 to Hanna, and R01 HL132794 and K24 HL135413 to Kizer. WIHS (Principal Investigators): UAB‐MS WIHS (Mirjam‐Colette Kempf and Deborah Konkle‐Parker), U01‐AI‐103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01‐AI‐103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01‐AI‐035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01‐AI‐031834; Chicago WIHS (Mardge Cohen and Audrey French), U01‐AI‐034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01‐AI‐034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01‐AI‐103397; UNC WIHS (Adaora Adimora), U01‐AI‐103390; Connie Wofsy Women's HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01‐AI‐034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01‐AI‐042590; Southern California WIHS (Joel Milam), U01‐HD‐032632 (WIHS I—WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1‐TR000004 (UCSF CTSA), UL1‐TR000454 (Atlanta CTSA), P30‐AI‐050410 (UNC CFAR), and P30‐AI‐027767 (UAB CFAR).
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Relationship Between Top 10 Polar Metabolites/Lipids Concentration and LVDD
Table S2. Joint Effect of the First 20 Metabolites of the First Principal Component
Table S3. Sensitivity Analysis of Relationship Between Top Polar Metabolites/Lipids Concentration and LVDD
Figure S1. Scores plot of polar metabolites and lipids according to presence of LVDD and HIV status.
Figure S2. Diagram of glycerophospholipid and fatty acid oxidation in the cardiomyocyte.
Acknowledgments
Data in this article were collected by the WIHS (Women's Interagency HIV Study). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
(J Am Heart Assoc. 2020;9:e013522 DOI: 10.1161/JAHA.119.013522.)
References
- 1. CDC . Estimated HIV incidence and prevalence in the United States, 2010–2015. HIV Surveillance Supplemental Report, Vol 23. Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 2. UNAIDS . Fact sheet—latest statistics on the status of the AIDS epidemic. Geneva, Switzerland: UNAIDS Secretariat; 2017.
- 3. Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 4. Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS‐defining opportunistic illnesses: results from 16 years of population‐based AIDS surveillance. AIDS. 2013;27:597–605. [DOI] [PubMed] [Google Scholar]
- 5. Shah SS, McGowan JP, Smith C, Blum S, Klein RS. Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clin Infect Dis. 2002;35:1238–1243. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV‐infected adults. Curr HIV/AIDS Rep. 2016;13:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friis‐Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El‐Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD; The Data Collection on Adverse Events of Anti‐HIV Drugs (DAD) Study Group . Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 9. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez‐Barradas MC, Lim J, Kazis LE, Gottlieb S, Justice AC, Freiberg MS. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So‐Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez‐Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerrato E, D'Ascenzo F, Biondi‐Zoccai G, Calcagno A, Frea S, Grosso Marra W, Castagno D, Omede P, Quadri G, Sciuto F, Presutti D, Frati G, Bonora S, Moretti C, Gaita F. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta‐analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–1436. [DOI] [PubMed] [Google Scholar]
- 13. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 14. Huttin O, Fraser AG, Coiro S, Bozec E, Selton‐Suty C, Lamiral Z, Frikha Z, Rossignol P, Zannad F, Girerd N. Impact of changes in consensus diagnostic recommendations on the echocardiographic prevalence of diastolic dysfunction. J Am Coll Cardiol. 2017;69:3119–3121. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 16. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 17. Hanna DB, Lazar JM, Avadhani S, Kaplan RC, Anastos K, Gange SJ, Holman S, Minkoff HL, Kizer JR. Assessment of different classification schemes for identification of diastolic dysfunction in the Women's Interagency HIV Study. J Am Soc Echocardiogr. 2019;32:547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. [DOI] [PubMed] [Google Scholar]
- 19. Tien PC, Schneider MF, Cox C, Karim R, Cohen M, Sharma A, Young M, Glesby MJ. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol. 2014;64:1388–1400. [DOI] [PubMed] [Google Scholar]
- 21. Hunter WG, Kelly JP, McGarrah RW III, Kraus WE, Shah SH. Metabolic dysfunction in heart failure: diagnostic, prognostic, and pathophysiologic insights from metabolomic profiling. Curr Heart Fail Rep. 2016;13:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Echouffo‐Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta‐analysis. JACC Heart Fail. 2016;4:237–248. [DOI] [PubMed] [Google Scholar]
- 23. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez CJ, Diez‐Roux AV, Moran A, Jin Z, Kronmal RA, Lima J, Homma S, Bluemke DA, Barr RG. Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non‐Hispanic blacks and whites: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;55:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barkan SE, Melnick SL, Preston‐Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 26. Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansoor A, Golub ET, Dehovitz J, Anastos K, Kaplan RC, Lazar JM. The association of HIV infection with left ventricular mass/hypertrophy. AIDS Res Hum Retroviruses. 2009;25:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, Haberlen SA, Shah SJ, Glesby MJ, Lazar JM, Burk RD, Hodis HN, Landay AL, Post WS, Anastos K, Kaplan RC. Plasma tryptophan‐kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. 2018;67:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 30. Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martinez‐Gonzalez MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB, Rexrode KM. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao W, Wang X, Deik AA, Hanna DB, Wang T, Haberlen SA, Shah SJ, Lazar JM, Hodis HN, Landay AL, Yu B, Gustafson D, Anastos K, Post WS, Clish CB, Kaplan RC, Qi Q. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection. Circulation. 2019;139:2003–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karnovsky A, Weymouth T, Hull T, Tarcea VG, Scardoni G, Laudanna C, Sartor MA, Stringer KA, Jagadish HV, Burant C, Athey B, Omenn GS. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics. 2012;28:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, Mack WJ, Cohen MH, Jacobson L, Gange SJ. Ten‐year predicted coronary heart disease risk in HIV‐infected men and women. Clin Infect Dis. 2007;45:1074–1081. [DOI] [PubMed] [Google Scholar]
- 34. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JR, Alberta H. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One. 2015;10:e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunter WG, Kelly JP, McGarrah RW III, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, Newgard CB, Felker GM, Hernandez AF, Velazquez EJ, Kraus WE, Shah SH. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5:e003190 DOI: 10.1161/JAHA.115.003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 38. Massie BM, Schaefer S, Garcia J, McKirnan MD, Schwartz GG, Wisneski JA, Weiner MW, White FC. Myocardial high‐energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91:1814–1823. [DOI] [PubMed] [Google Scholar]
- 39. Paolisso G, Gambardella A, Galzerano D, D'Amore A, Rubino P, Verza M, Teasuro P, Varricchio M, D'Onofrio F. Total‐body and myocardial substrate oxidation in congestive heart failure. Metabolism. 1994;43:174–179. [DOI] [PubMed] [Google Scholar]
- 40. Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator‐activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92:518–524. [DOI] [PubMed] [Google Scholar]
- 41. Zhang ZY, Marrachelli VG, Thijs L, Yang WY, Wei FF, Monleon D, Jacobs L, Nawrot T, Verhamme P, Voigt JU, Kuznetsova T, Redon J, Staessen JA. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a general population. J Am Heart Assoc. 2016;5:e002681 DOI: 10.1161/JAHA.115.002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H‐magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. [DOI] [PubMed] [Google Scholar]
- 43. Rutkowsky JM, Knotts TA, Ono‐Moore KD, McCoin CS, Huang S, Schneider D, Singh S, Adams SH, Hwang DH. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab. 2014;306:E1378–E1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J. 1998;19:990–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Relationship Between Top 10 Polar Metabolites/Lipids Concentration and LVDD
Table S2. Joint Effect of the First 20 Metabolites of the First Principal Component
Table S3. Sensitivity Analysis of Relationship Between Top Polar Metabolites/Lipids Concentration and LVDD
Figure S1. Scores plot of polar metabolites and lipids according to presence of LVDD and HIV status.
Figure S2. Diagram of glycerophospholipid and fatty acid oxidation in the cardiomyocyte.


