Abstract
Background
Ceramides exhibit multiple biological activities that may influence the pathophysiological characteristics of atrial fibrillation (AF). Whether the length of the saturated fatty acid carried by the ceramide or their sphingomyelin precursors are associated with AF risk is not known.
Methods and Results
Among 4206 CHS (Cardiovascular Health Study) participants (mean age, 76 years; 40% men) who were free of prevalent AF at baseline, we identified 1198 incident AF cases over a median 8.7 years of follow‐up. We examined 8 sphingolipid species: ceramide and sphingomyelin species with palmitic acid and species with very‐long‐chain saturated fatty acids: arachidic; behenic; and lignoceric. In adjusted Cox regression analyses, ceramides and sphingomyelins with very‐long‐chain saturated fatty acids were associated with reduced AF risk (ie, per 2‐fold higher ceramide with behenic acid hazard ratio, 0.71; 95% CI, 0.59–0.86; sphingomyelin with behenic acid hazard ratio, 0.60; 95% CI, 0.46–0.77). In contrast, ceramides and sphingomyelins with palmitic acid were associated with increased AF risk (ceramide with palmitic acid hazard ratio, 1.31; 95% CI, 1.03–1.66; sphingomyelin with palmitic acid hazard ratio, 1.73; 95% CI, 1.18–2.55). Associations were attenuated with adjustment for NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), but did not differ significantly by age, sex, race, body mass index, or history of coronary heart disease.
Conclusions
Our findings suggest that several ceramide and sphingomyelin species are associated with incident AF, and that these associations differ on the basis of the fatty acid. Ceramides and sphingomyelins with palmitic acid were associated with increased AF risk, whereas ceramides and sphingomyelins with very‐long‐chain saturated fatty acids were associated with reduced AF risk.
Keywords: atrial fibrillation, biomarker, epidemiology, lipid metabolites, lipids
Subject Categories: Atrial Fibrillation, Epidemiology, Risk Factors, Biomarkers
Clinical Perspective
What Is New?
Several ceramides and sphingomyelins were associated with atrial fibrillation risk among 4206 participants in the CHS (Cardiovascular Health Study).
Ceramide and sphingomyelin species with palmitic acid were associated with increased risk of atrial fibrillation, whereas species with a very‐long‐chain saturated fatty acid were associated with reduced risk.
What Are the Clinical Implications?
Although the study design precludes causality, the study indicates that plasma ceramides and sphingomyelins may influence the risk of atrial fibrillation independent of traditional cardiovascular disease risk factors.
Attenuation of associations after adjustment for NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) suggests that ceramides and sphingomyelins may impact atrial fibrillation risk in part by influencing myocyte pressure load.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with increased risk of stroke and death. More than 3 million people in the United States currently live with AF, a number that is expected to grow to 12 million by 2030, and identification of new risk factors associated with AF later in life is of considerable public health importance.1, 2
Ceramides are lipids made of a sphingoïd backbone to which one fatty acid is N acylated. Ceramides play a role in oxidative stress and inflammation, processes that influence atrial fibrosis and remodeling.3, 4, 5, 6, 7 In addition, ceramides are involved in apoptosis, and animal studies suggest apoptosis in the context of fibrosis may be part of the pathophysiological characteristics of AF.8, 9, 10, 11, 12 More important, ceramide species that have saturated fatty acids of different lengths show different biological activities in experimental studies; in particular, ceramide with palmitic acid (Cer‐16) promotes apoptosis, whereas ceramide with a very‐long‐chain saturated fatty acid (VLSFA) actually prevent apoptosis.13
Sphingomyelins, which may also be related to AF risk, have the same base structure as ceramides but with the addition of a choline head group. Ceramides can be derived from sphingomyelins by sphingomyelinases activated by proinflammatory cytokines, oxidative stress, or ischemia.14, 15 Of note, we have reported that higher levels of several VLSFAs (arachidic [20:0], behenic [22:0], and lignoceric [24:0]) measured in phospholipids, which include phosphoglycerolipid and sphingomyelin fatty acids, were associated with lower risk of AF.16 Whether circulating levels of ceramides and sphingomyelins are associated with incident AF, and whether the associations vary with the length of the fatty acid, is not known.
The goals of this analysis were to assess whether ceramide and sphingomyelin species with a VLSFA are associated with reduced risk of AF and whether species with palmitic acid are associated with increased risk of AF, within the CHS (Cardiovascular Health Study).
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purpose of reproducing the results or replicating the procedure. The authors are not authorized to share CHS data.
Study Design and Setting
The CHS is a prospective cohort study of risk factors for cardiovascular disease in community‐dwelling adults aged ≥65 years. A detailed description of the study design and procedures can be found elsewhere.17, 18 Briefly, in 1989/1990 and 1992/1993, 5888 participants were randomly selected from Medicare beneficiary lists and recruited from 4 field centers located in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Institutional Review Boards at each field center approved the CHS, and written informed consent was obtained from all study participants.
Data Collection
Between 1989 and 1999, participants underwent annual study examinations that included personal interviews, physical examinations, laboratory assessments, and diagnostic tests. This included height, weight, and blood pressure measurement, questions about tobacco and alcohol use, medical history, and an ECG. High‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) cholesterol, CRP (C‐reactive protein), fibrinogen, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and troponin T were measured at the 1992 to 1993 examination. The study examination from which sphingolipids were measured (1992–1993 or 1994–1995) formed the study baseline for this analysis, and participant characteristics were drawn from that examination.
Sphingolipid Measurement
Ceramide and sphingomyelin species were measured using fasting EDTA‐plasma samples collected at the 1994 to 1995 examination (N=4026) and from the 1992 to 1993 examination for participants without a 1994 to 1995 plasma sample (N=586). Plasma samples were stored at −70°C until they were extracted. Sphingolipids were then quantified by liquid chromatography–tandem mass spectrometry in the laboratory of A.N.H. at the University of Washington; a detailed description of the measurement methods has been described previously.19 We examined 8 sphingolipid species: ceramide and sphingomyelin with palmitic acid (Cer‐16 and SM‐16), with arachidic acid (Cer‐20 and SM‐20), with behenic acid (Cer‐22 and SM‐22), and with lignoceric acid (Cer‐24 and SM‐24; Cer‐24 was computed as the sum of 2 ceramide species with distinct “d181” and “d182” sphingoïd backbones. Sphingolipid concentrations were determined using a single point calibrator, made from a pooled EDTA plasma sample that was added to each batch in 5 replicates. A quality control sample from an independent pool of EDTA plasma was added to each batch and run in duplicate; over 52 batches, coefficients of variation for each of the sphingolipid measurements were <20%.
Identification of Incident AF
Incident AF, which we defined as AF and/or flutter, was identified through ECGs performed at annual study examinations (from 1992/1993 through 1999) and through hospital discharge records (through 2012). Study ECGs were read by the CHS Electrocardiography Reading Center, which validated diagnoses of AF. Hospitalization records were reviewed for International Classification of Diseases, Ninth Revision (ICD‐9), codes for AF or atrial flutter (427.3, 427.31, or 427.32). AF that occurred during hospitalizations for open heart surgery were excluded; however, if subsequent records indicated AF unrelated to open heart surgery, the date of the subsequent AF occurrence was identified as the onset date for AF.
Statistical Analysis
Analyses were limited to participants without a history of AF or AF on the study ECG at the time of sphingolipid measurement (baseline). Associations of sphingolipid levels with incident AF were assessed using Cox proportional hazards models. Participants began accruing time at risk at the time of their sphingolipid measurement and were followed up until the earliest of diagnosis of AF, death or dropout, or November 30, 2012. Sphingolipid levels were log (base 2) transformed, and results are presented per 2‐fold higher concentration of each sphingolipid, which is comparable to the difference between the 90th and 10th percentiles of each sphingolipid species (Table S1). We assessed 3 sets of models with a priori selected baseline characteristics as adjustment terms: model 1 (minimally adjusted model) included adjustment for baseline age, sex, race (black versus other), and study site; model 2 (adjusted model) included model 1 with additional adjustment terms for body mass index (BMI), systolic blood pressure, treated hypertension, HDL, LDL, PR interval, smoking, alcohol use, and prevalent diabetes mellitus, heart failure, and myocardial infarction; and model 3 (primary model) included model 2 with additional adjustment for one of the other species: Cer‐16 and SM‐16 models include adjustment for Cer‐22 and SM‐22, respectively; Cer‐20, Cer‐22, and Cer‐24 and SM‐20, SM‐22, and SM‐24 models include adjustment for Cer‐16 and SM‐16, respectively.
Missing values of HDL (n=276), LDL (n=203), and PR interval (n=304) were multiply imputed using information on age, sex, race, BMI, and smoking. Twenty imputed data sets were generated, and model fitting results were pooled using standard methods.20 To correct for multiple comparisons, we assessed statistical significance at a P<0.0063 (0.05/8 sphingolipid species) threshold.
In sensitivity analyses, we repeated our primary analysis with additional adjustment for log‐transformed CRP, NT‐proBNP, troponin T, and fibrinogen, which were measured at the 1992 to 1993 examination. We also evaluated models with and without adjustment for plasma phospholipid saturated fatty acid of the same length as the one in the ceramide or sphingomyelin species among participants with plasma phospholipid fatty acid measures at the 1992 to 1993 examination (n=3230). Only participants with HDL and LDL measurements at the 1992 to 1993 examination were included in the sensitivity analyses.
We also examined whether associations between ceramides and sphingomyelins and AF risk were modified by age, sex, race, BMI, and prevalent coronary heart disease as a sensitivity analysis by adding product interaction terms to the models above. Ten‐year C‐statistics for models with and without each ceramide and sphingomyelin species were based on receiver‐operating characteristic curve estimates that can accommodate censored data; specifically, the nearest neighbor estimation method was used.21 Schoenfeld residuals and models stratified by quartile of survival time were reviewed to assess whether assumptions of proportional hazards were violated, and models of log‐transformed sphingolipids fit with cubic splines were used to check for nonlinearity. Analyses were performed using Stata, version 14, and R, version 3.6.2.
Of the 4612 participants from whom sphingolipids were measured, 406 had a history of AF or AF on the study ECG at the time of blood draw and were excluded, leaving 4206 participants eligible for analyses.
Results
Baseline characteristics of the 4206 included participants, as well as the distributions of these characteristics across quartiles of each of the sphingolipids, are presented in Figure 1. Within this population of older (mean age, 76 years) and predominantly female (60%) adults, 16% of participants self‐reported as black, smoking and alcohol consumption were uncommon, and most were free of underlying prevalent cardiovascular disease at baseline.
Figure 1.
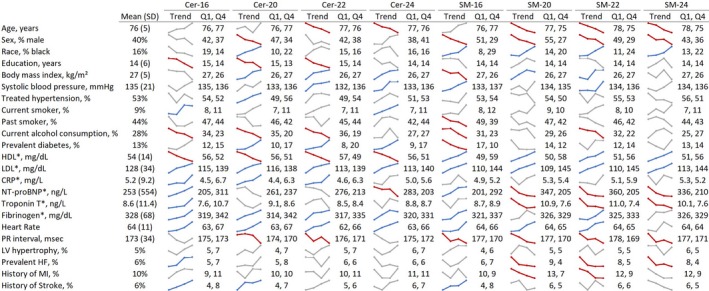
Participant mean baseline characteristics and trends across quartiles of sphingolipids among 4206 CHS (Cardiovascular Health Study) participants. *Measured in 1992 to 1993 for all participants. The colored graphics show means or percentages of each characteristic across quartiles of each of the sphingolipids. Unadjusted linear and logistic regression models were used to assess statistically significant (P<0.0022; 0.05/23 characteristics) associations of log‐transformed sphingolipids with each characteristic; statistically significant positive trends are blue, statistically significant negative trends are red, and gray indicates P>0.0022. Cer‐16 indicates ceramide with palmitic acid; Cer‐20, ceramide with arachidic acid; Cer‐22, ceremide with behenic acid; Cer‐24, ceremide with lignoceric acid; CRP, C‐reactive protein; HDL, high‐density lipoprotein; HF, heart failure; LDL, low‐density lipoprotein; LV, left ventricle; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; Q1, quartile 1 (the mean or percentage of each characteristic among participants with a sphingolipid level in the lowest 25% of the distribution); Q4, quartile 4 (the mean or percentage among participants with a sphingolipid level in the highest 25% of the distribution); SM‐16, sphingomyelin with palmitic acid; SM‐20, sphingomyelin with arachidic acid; SM‐22, sphingomyelin with behenic acid; SM‐24, sphingomyelin with lignoceric acid.
In univariate analyses, presented as trend lines in Figure 1, participants with high levels of circulating ceramides were more likely to have higher BMIs, prevalent diabetes mellitus, higher LDL and CRP levels, lower HDL levels, and higher heart rates than those with lower ceramide levels. Participants with high sphingomyelin levels were more likely to be younger, women, and black, more likely to have higher HDL and LDL levels and shorter PR intervals, and less likely to have prevalent heart failure than those with lower sphingomyelin levels.
Over an average 8.7 years of follow‐up (median, 8.9 years; maximum, 17 years), 1198 cases of incident AF were identified (incidence rate, 33 per 1000 person‐years); hazard ratios (HRs) and 95% CIs of AF risk with sphingolipid levels are presented in Figure 2 and Table S2. A 2‐fold higher level of each of the ceramides with VLSA (Cer‐20, Cer‐22, and Cer‐24) was associated with a 28% to 36% reduced risk of incident AF in models adjusted for age, sex, race, study site, BMI, systolic blood pressure, treated hypertension, smoking, alcohol use, HDL, LDL, PR interval, prevalent diabetes mellitus, heart failure, myocardial infarction, and Cer‐16 (Cer‐20 HR, 0.72; 95% CI, 0.61–0.84; Cer‐22 HR, 0.71; 95% CI, 0.59–0.86; Cer‐24 HR, 0.64; 95% CI, 0.50–0.81). In similar models that included adjustment for SM‐16 instead of Cer‐16, 2‐fold higher levels of SM‐20 and SM‐22 were associated with a 40% to 45% reduced risk of incident AF (SM‐20 HR, 0.55; 95% CI, 0.43–0.71; SM‐22 HR, 0.60; 95% CI, 0.46–0.77).
Figure 2.
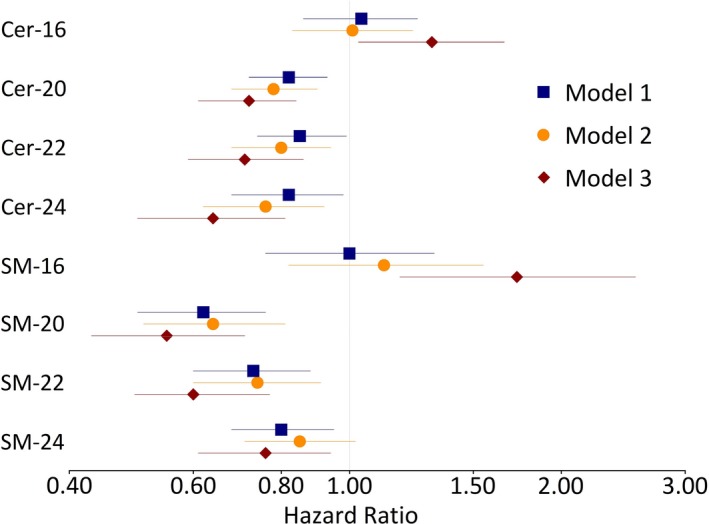
Risk of incident atrial fibrillation per 2‐fold higher sphingolipid level. Hazard ratios and 95% CIs are presented; each line represents a separate model. Model 1 includes adjustment for age, sex, race, and study site; model 2 includes model 1 adjustment terms and additional adjustment for body mass index, systolic blood pressure, treated hypertension, smoking, alcohol use, high‐density lipoprotein, low‐density lipoprotein, PR interval, prevalent diabetes mellitus, heart failure, and history of myocardial infarction; in model 3, in addition to model 2 adjustment terms, ceramide with palmitic acid (Cer‐16) and sphingomyelin with palmitic acid (SM‐16) include adjustment for ceremide with behenic acid (Cer‐22) and sphingomyelin with behenic acid (SM‐22), respectively; ceramide with arachidic acid (Cer‐20), Cer‐22, ceremide with lignoceric acid (Cer‐24) and sphingomyelin with arachidic acid (SM‐20), SM‐22, and sphingomyelin with lignoceric acid (SM‐24) include adjustment for Cer‐16 and SM‐16, respectively.
In similar ceramide and sphingomyelin models adjusted for ceramide and sphingomyelin species with behenic acid (Cer‐22 and SM‐22), SM‐16 was associated with an increased risk of AF (per 2‐fold higher SM‐16 HR, 1.73; 95% CI, 1.18–2.55). There was a suggestion that Cer‐16 was associated with an increased risk of AF, although the association with Cer‐16 did not meet our prespecified threshold of statistical significance (per 2‐fold higher Cer‐16 HR, 1.31; 95% CI, 1.03–1.66).
Models that included adjustment for CRP, fibrinogen, troponin T, and NT‐proBNP are presented in Table S3. Adjustment for CRP and fibrinogen resulted in similar estimates relative to the primary analysis; adjustment for NT‐proBNP and, to a lesser extent, troponin T resulted in attenuated associations.
There was no evidence that associations of sphingolipids with incident AF were modified by age, sex, race, BMI, or prevalent coronary heart disease (Table S4). Adjustment for phospholipid saturated fatty acids of corresponding length did not alter associations of the ceramides and sphingomyelins carrying the same fatty acid with AF risk (Table S5). The addition of each of the ceramide and sphingomyelin species resulted in small improvements to AF classification (Table S6). The assumption of proportional hazards was not violated in any analysis, and there was no evidence of departure from linearity.
Discussion
In this population of older adults in whom incident AF was relatively common, ceramides and sphingomyelins with palmitic acid were associated with increased AF risk, whereas ceramides and sphingomyelins with a VLSFA were associated with reduced AF risk. These associations were independent of other risk factors, and did not differ by subgroups, including age, sex, race, BMI, or prevalent coronary heart disease.
Our underlying hypothesis is that ceramide species with a VLSFA (Cer‐20, Cer‐22, and Cer‐24) may influence AF via an action on apoptosis. Although population‐level studies in this area are limited, experimental studies suggest that apoptosis contributes to AF risk; in particular, expression of apoptotic inducers is elevated in atrial tissue, and inactivation of caspase 3, a key apoptotic enzyme, suppresses apoptosis and prevents intra‐atrial conduction delay and AF.10, 11, 12 Ceramides with a VLSFA have been shown to protect from apoptosis in a variety of cell lines and animal systems, and protect from cardiomyocyte loss in animal studies.4, 13, 22, 23 Our findings, complimented by these cell and animal studies, suggest that reduced AF risk with greater levels of ceramides with VLSFA may be attributable to suppression of apoptosis. Furthermore, associations of ceramides and sphingomyelins with AF were attenuated after adjustment for NT‐proBNP, a marker of myocyte pressure load, indicating a possible impact of ceramides and sphingomyelins on cardiac function.
Mechanisms through which sphingomyelin species contribute to AF risk have not been established. The major biological role of sphingomyelin is as a structural component of membranes, where it contributes to the stability of membrane domains and may influence integral membrane proteins and ion channels.24 In addition, sphingomyelin in plasma membranes and circulating lipoproteins are converted into ceramide by the neutral sphingomyelinase‐2 when activated by cytokines, oxidative stress, or ischemia reperfusion.25 It is possible that the parallel results we observed between sphingomyelin and ceramide species with the risk of AF reflects a single mechanistic pathway resulting from ceramide being produced from sphingomyelin. Adjustment for plasma phospholipid saturated fatty acids did not alter the magnitude of association of sphingolipid species with AF risk, indicating that the sphingolipid associations are independent of associations previously observed with phospholipid fatty acids.16
We observed that the associations of ceramide and sphingomyelin with AF risk are similar after adjustment for CRP and fibrinogen, markers of inflammation. Ceramides play a role in inflammation and cytokines and are among factors known to influence atrial fibrosis, suggesting a possible mechanism for Cer‐16 association with AF.3, 4 In our study, both CRP and fibrinogen were measured 2 years before the sphingolipid measurement, so we cannot assess with certainty whether Cer‐16 is a marker of inflammation or whether the association of Cer‐16 with AF risk is mediated by general inflammation.
Strengths of this study include the prospective study design, the detailed and thorough assessment of ceramide and sphingomyelin species and other covariates, and the large number of identified incident AF cases. The study also has several limitations. By nature, our study was observational and we cannot infer causation. We were not able to differentiate between permanent AF and paroxysmal AF, and because AF is often transient and/or asymptomatic, it is likely that we failed to identify cases who were not in AF at the time of their study ECG or who were not hospitalized for AF during follow‐up. Study participants were predominately white, with a mean baseline age of 76 years, and our findings may not generalize to other ethnicities or younger populations. Finally, HDL, LDL, CRP, fibrinogen, NT‐proBNP, and troponin T were only measured at the 1992 to 1993 study examination, and may not accurately reflect their levels at baseline for participants whose sphingolipids were measured in 1994 to 1995.
Previous research linked plasma phospholipid VLSFA with AF risk16; our findings further advance that research by identifying specific ceramide and sphingomyelin species that are associated with AF risk. Additional studies will be needed to replicate these findings in younger populations, to establish determinants of plasma levels of ceramide and sphingomyelin species, and to evaluate whether ceramide and sphingomyelin levels may have clinical utility as potential components of AF risk scores.
In summary, we report novel associations of ceramide and sphingomyelin species with incident AF. If the associations prove to be causal, increasing levels of ceramide and sphingomyelin species with a VLSFA and lowering levels of species with palmitic acid may be useful therapeutic targets for the prevention of AF.
Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295, U01HL130114, and HL128575 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging and DK103657 and P30 DK035816 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosures
Dr Psaty serves on the Steering Committee of the Yale Open Data Access Project, funded by Johnson & Johnson. Dr Hoofnagle reports grants from the National Institutes of Health during the conduct of the study and grants from Waters, Inc, outside the submitted work. Dr Mozaffarian reports research funding from the National Institutes of Health and the Gates Foundation; personal fees from GOED, Nutrition Impact, Pollock Communications, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, Cleveland Clinic Foundation, America's Test Kitchen, and Danone; scientific advisory board, Elysium Health (with stock options), Omada Health, and DayTwo; and chapter royalties from UpToDate, all outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1. Plasma Concentrations and Correlations of Sphingolipid Species
Table S2. Risk of Incident Atrial Fibrillation Per 2‐Fold Higher Sphingolipid Level
Table S3. Risk of Incident Atrial Fibrillation Per 2‐Fold Higher Sphingolipid, Adjusted for Additional Biomarkers
Table S4. Beta Coefficients and 95% Confidence Intervals for Interactions Terms of Sphingolipids With Age, Sex, BMI, Race, and Prevalent CHD in Cox Regression Models of Incident Atrial Fibrillation
Table S5. Risk of Incident Atrial Fibrillation in Subset of Participants With Plasma Phospholipid Fatty Acid Measures at 1992–1993 (n=3230), With and Without Adjustment for Plasma Phospholipid Fatty Acids
Table S6. C‐Statistics From Models of Incident Atrial Fibrillation at 10 Years
Acknowledgments
We thank the study participants and the CHS (Cardiovascular Health Study) Coordinating Center. A full list of principal CHS investigators and institutions can be found at CHS‐NHLBI.org.
(J Am Heart Assoc. 2020;9:e012853 DOI: 10.1161/JAHA.119.012853.)
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. [DOI] [PubMed] [Google Scholar]
- 4. Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. 2017;3:425–435. [DOI] [PubMed] [Google Scholar]
- 5. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- 6. Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. [DOI] [PubMed] [Google Scholar]
- 7. Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 8. Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(suppl):S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochem Biophys Acta. 2002;1585:114–125. [DOI] [PubMed] [Google Scholar]
- 10. Heinke MY, Yao M, Chang D, Einstein R, dos Remedios CG. Apoptosis of ventricular and atrial myocytes from pacing‐induced canine heart failure. Cardiovasc Res. 2001;49:127–134. [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Gong ZH, Sheng L, Gong YT, Tan XY, Li WM, Dong DL, Yang BF, Fu SB, Xue HJ. Anti‐apoptotic effects of a calpain inhibitor on cardiomyocytes in a canine rapid atrial fibrillation model. Cardiovasc Drugs Ther. 2009;23:361–368. [DOI] [PubMed] [Google Scholar]
- 12. Trappe K, Thomas D, Bikou O, Kelemen K, Lugenbiel P, Voss F, Becker R, Katus HA, Bauer A. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre‐clinical pilot study. Eur Heart J. 2013;34:147–157. [DOI] [PubMed] [Google Scholar]
- 13. Grosch S, Schiffmann S, Geisslinger G. Chain length‐specific properties of ceramides. Prog Lipid Res. 2012;51:50–62. [DOI] [PubMed] [Google Scholar]
- 14. Pavoine C, Pecker F. Sphingomyelinases: their regulation and roles in cardiovascular pathophysiology. Cardiovasc Res. 2009;82:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2010;584:1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB, McKnight B, Sitlani C, Sacks FM, Song X, Sotoodehnia N, Spiegelman D, Wallace ER, Lemaitre RN. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3:e000889 DOI: 10.1161/JAHA.114.000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 18. Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 19. Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM, Umans JG, Howard BV, Sitlani CM, Siscovick DS, King IB, Sotoodehnia N, McKnight B. Circulating sphingolipids, insulin, HOMA‐IR, and HOMA‐B: the strong heart family study. Diabetes. 2018;67:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 21. Heagerty PJ, Lumley T, Pepe MS. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 22. Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, Davidson MM, Choi CS, Shin KO, Lee YM, Park WJ, Park IS, Jiang XC, Goldberg IJ, Park TS. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem. 2012;287:18429–18439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crowder CM. Cell biology: ceramides–friend or foe in hypoxia? Science. 2009;324:343–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slotte JP. Biological functions of sphingomyelins. Prog Lipid Res. 2013;52:424–437. [DOI] [PubMed] [Google Scholar]
- 25. Shamseddine AA, Airola MV, Hannun YA. Roles and regulation of neutral sphingomyelinase‐2 in cellular and pathological processes. Adv Biol Regul. 2015;57:24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Plasma Concentrations and Correlations of Sphingolipid Species
Table S2. Risk of Incident Atrial Fibrillation Per 2‐Fold Higher Sphingolipid Level
Table S3. Risk of Incident Atrial Fibrillation Per 2‐Fold Higher Sphingolipid, Adjusted for Additional Biomarkers
Table S4. Beta Coefficients and 95% Confidence Intervals for Interactions Terms of Sphingolipids With Age, Sex, BMI, Race, and Prevalent CHD in Cox Regression Models of Incident Atrial Fibrillation
Table S5. Risk of Incident Atrial Fibrillation in Subset of Participants With Plasma Phospholipid Fatty Acid Measures at 1992–1993 (n=3230), With and Without Adjustment for Plasma Phospholipid Fatty Acids
Table S6. C‐Statistics From Models of Incident Atrial Fibrillation at 10 Years


