Abstract
Background
Domestic abuse (DA) against women is a global public health problem. Although the possible health burden could be substantial, the associations between DA and subsequent cardiometabolic disease (cardiovascular disease, hypertension, and type 2 diabetes mellitus) and all‐cause mortality are poorly understood.
Methods and Results
This retrospective cohort study consisted of UK‐based primary care patients between January 1, 1995, to December 1, 2017. Overall, 18 547 women exposed to DA were matched to 72 231 unexposed women by age and lifestyle factors. The main outcomes, presented as adjusted incidence rate ratios (IRRs), were the risk of developing cardiovascular disease, hypertension, type 2 diabetes mellitus, and all‐cause mortality. In total, 181 exposed women experienced a cardiovascular disease event compared with 644 of the unexposed control group, relating to an increased adjusted IRR of 1.31 (95% CI, 1.11–1.55; P=0.001). There was also an increased risk of subsequent type 2 diabetes mellitus (adjusted IRR: 1.51; 95% CI, 1.30–1.76; P<0.001) and all‐cause mortality (adjusted IRR: 1.44; 95% CI, 1.24–1.67; P<0.001) following exposure to DA. This observation was not seen with hypertension (adjusted IRR: 0.99; 95% CI, 0.88–1.12; P=0.873).
Conclusions
There is an increased risk of subsequent cardiovascular disease, type 2 diabetes mellitus, and all‐cause mortality in female survivors of DA. However, there is no association with the development of hypertension in this group, in keeping with previous literature. Considering the high prevalence of DA, clinicians should be made aware of the disproportionally increased risk and thus are encouraged to manage modifiable risk factors actively in this group.
Keywords: cardiovascular disease, domestic abuse, epidemiology, hypertension, type 2 diabetes mellitus
Subject Categories: Cardiovascular Disease; Diabetes, Type 2; Risk Factors; Women
Clinical Perspective
What Is New?
The global health burden of domestic abuse (DA) is substantial and a key cause of morbidity and mortality for women; however, few studies have explored the relationship between DA and the subsequent development of cardiometabolic disease or all‐cause mortality.
We demonstrate 31%, 51%, and 44% increased risk of subsequent cardiovascular disease, type 2 diabetes mellitus and all‐cause mortality following consideration of important lifestyle factors.
There is no clear increased risk of hypertension following DA in line with previous literature.
What Are the Clinical Implications?
Considering the high prevalence of DA among women, clinicians should be made aware of the disproportionally increased risk and thus are encouraged to consider the need for intervention in this cohort.
Women exposed to DA also appear to have a higher burden of lifestyle risk factors such as smoking and excessive alcohol use compared with the general population.
This lifestyle risk may play a role in the described association and will require a coordinated public health approach to manage.
Introduction
Domestic abuse (DA) against women is a global public health problem and a violation of human rights.1 It is defined by the UK government as “any incident or patterns of incidents of controlling, coercive, threatening behavior, violence or abuse between those aged 16 or over who are, or have been, intimate partners or family members regardless of gender or sexuality.”2 The UK prevalence of lifetime exposure to DA in the female population is estimated to be 27.1%.3
Population‐based studies have identified an association between being a survivor of DA and a variety of physical and psychological consequences in this group.4, 5, 6, 7 A previous systematic review8 assessed the impact of being a survivor of DA and the development of cardiovascular disease (CVD) and hypertension. This review8 included 13 cross‐sectional studies and 2 prospective studies (both examining the development of hypertension). The included cross‐sectional studies provided conflicting evidence of a potential association between exposure to DA and the development of CVD. The largest cross‐sectional study9 (n=70 156) included both male and female participants and found a higher risk of developing self‐reported coronary heart disease and heart attacks among DA survivors. This result was consistent with findings from a more recent small (n=151) study in a Polish population10 for which self‐reported DA was associated with a 6‐fold increased risk of developing ischemic heart disease (IHD). In contrast, a cross‐sectional study11 conducted in South Africa suggested that survivors of DA were not at increased risk of developing heart disease or heart attacks. More recent systematic reviews examining physical outcomes of DA survivors5, 12 have not identified any global cohort study designed to assess CVD risk following DA exposure.
Interestingly, despite the possible association with CVD development, many studies report no association between DA and the subsequent development of hypertension,11, 13, 14, 15, 16, 17 suggesting that hypertension may not be a mediating factor in the relationship between DA and CVD. The NHSII (Nurses’ Health Study II)18 was a prospective study that demonstrated being a victim of severe emotional abuse is associated with the development of hypertension, but this relationship was not present in survivors of sexual and violent forms of DA. The other relevant prospective study19 was conducted in Norway (N=5593 women) and discerned a positive association between physical and sexual DA with the subsequent use of antihypertensive drugs. However, neither of these prospective studies used clinical CVD end points such as IHD, stroke, transient ischemic attack (TIA), peripheral vascular disease, or heart failure.
Similar to CVD, there is sparse literature assessing the relationship between DA and the development or incidence of type 2 diabetes mellitus (T2DM). The association between childhood abuse and development of T2DM in later life20, 21, 22 has been well documented; however, few studies have explored this association in the DA population. The NHSII was one of the few studies investigating this link and identified a 61% adjusted increased risk in the incidence of T2DM in DA survivors who had undergone severe psychological abuse, but this finding was not statistically significant in survivors of physical and sexual abuse.23
The pathophysiologic link between DA and cardiometabolic disease is complex and unclear. It is thought that the acute and sustained elevated stress response triggered by DA can compromise the neuroendocrine and immune systems and induce changes in brain structure, leading to an increased risk of a variety of physical and psychological disorders.1 Similar to survivors of childhood abuse,24 female survivors of DA25 appear to have elevated levels of CRP (C‐reactive protein) in their plasma and salivary glands that may be associated with the development of cardiometabolic disease26, 27; however, little research on this topic has been conducted in a population exposed to DA. A recent consensus on the association between childhood adversity and subsequent cardiometabolic development considered that the relationship may be tied to 3 pathways that may occur following abuse: adoption of poor lifestyle behaviors (physical inactivity, poor diet, disrupted sleep, substance misuse, and smoking); mental ill health; and alteration of immune, metabolic, neuroendocrine, and autonomic nervous systems.28 It is likely that similar pathways may be mediating the relationship between DA and cardiometabolic disease. For example, previous research has identified that DA survivors experience higher rates of smoking, obesity, and excessive alcohol use compared with the general population.5, 29, 30, 31, 32, 33 However, previous studies have not been able to address the impact of these lifestyle factors.
Globally, cardiometabolic disease still remains an important, albeit preventable, cause of mortality.34 Literature exploring the association between DA and mortality risk is scarce. Of the available evidence, it is clear that being a victim of DA is associated with a disproportionally increased risk of homicide and nonaccidental injuries as causes of death.35, 36 However, there is absence of evidence examining the association between DA and all‐cause mortality risk compared with the general population.
It is evident that no previous cohort study has investigated the relationship between exposure to DA and the development of CVD and all‐cause mortality in the female population, and only a few studies have explored the relationship between DA and the development of hypertension and T2DM in a cohort setting. Consequently, considering the sizeable prevalence of DA and the public health burden posed by cardiometabolic disease, we aimed to explore the relationship between DA and cardiometabolic disease (CVD, hypertension, and T2DM) alongside mortality.
Methods
The anonymized data that support the findings of this study are available from the senior author (k.nirantharan@bham.ac.uk). However, this will be subject to approval from the data providers and data owners (IQVIA and Cegedim). Ethics approval: anonymized data were used throughout the study, provided by the data provider IQVIA to the University of Birmingham. Studies using the Health Improvement Network (THIN) database have had initial ethics approval from the NHS South‐East Multicentre Research Ethics Committee, subject to prior independent scientific review. The Scientific Review Committee (IMS Health) approved the study protocol (SRC Reference Number: SRC18THIN034) before its undertaking. Informed consent was not required in this study as the data were anonymized.
Study Design and Data Source
This population‐based, retrospective, open, cohort study using the Health Improvement Network (THIN) database compared female patients coded with previous exposure of DA with female patients not coded to have experienced DA. The THIN database consists of UK electronically recorded person‐level medical records derived from >750 general practices and is considered to be representative of the UK population.37 Patient information is entered into electronic record software that uses Read codes.38 Diagnosis and clinical presentations are represented in the Read code hierarchy system. Other available data in the database relate to demographic, prescription, biochemistry results, and mortality data. To reduce underrecording of events, individual practices were included 12 months following their electronic practice system was installed or from the practice's acceptable mortality recording date.
Study Population
Women noted by general practitioners to have been exposed to DA (exposed cohort) were identified during the study period (January 1, 1995, to December 1, 2017). The index date in the exposed group was stated to be the first documentation of an DA Read code once a patient was eligible to take part in the study during the study period or the study start date for patients with a previous record of DA. An open cohort study allows for patients to enter and exit the study at different time points, with each individual patient contributing only person‐years of follow‐up from the time of cohort entry (index date) to the time she leaves the cohort (exit date).
Each exposed woman was matched to up to 4 women who had no DA Read code from random general practitioner practices in the data set (unexposed cohort). Controls from the unexposed group were matched individually to cases based on age at index date (±1 year), body mass index (to within 2 kg/m2), smoking status, and Townsend deprivation score39 at baseline.
To mitigate immortality time bias,40 the same index date was assigned to the corresponding unexposed patient. The follow‐up period for each patient was from the index date until the exit date. Exit date is defined as the earliest of the following dates: study end date, last date of data collection from a given general practice, date the patient transferred from general practice, date of death, or date the outcome of interest occurred.
The primary outcome was the development of cardiometabolic disease, exploring the outcomes of CVD (IHD, heart failure, peripheral vascular disease, and stroke or TIA during the observation period), hypertension, and T2DM as identified through Read codes. In the UK primary setting, there is a mandatory requirement to maintain an accurate register of CVD, hypertension, and T2DM patients that is further incentivized through the national Quality and Outcome Framework system recording an individual's identification and management of these conditions.41 All patients with a recorded code of DA who were eligible during the study period with their corresponding controls were included in the risk calculation for all‐cause mortality. The details for these patients are highlighted in Table 1. However, when examining each outcome of interest (CVD, hypertension, or T2DM), patients were excluded from the study if they had a diagnosis relating to the corresponding condition before the index date of the study. For example, patients highlighted in Table 1 with existing diagnoses of hypertension at baseline were excluded from the main cohort when we examined the risk of developing hypertension.
Table 1.
Baseline Characteristics
| Exposed Group | Unexposed Group | |
|---|---|---|
| Patients, n | 18 547 | 73 231 |
| Follow‐up period (person‐years) | 2.2±2.3 | 3.1±2.7 |
| Age, y | 36.9±12.5 | 36.9±12.4 |
| Body mass index | ||
| <25 | 7916 (42.7) | 31 692 (42.3) |
| 25–30 | 3999 (21.6) | 15 940 (21.8) |
| >30 | 3568 (19.2) | 13 680 (18.7) |
| Not available | 3064 (16.5) | 11 919 (16.3) |
| Current smoking | 8096 (44.7) | 32 064 (44.7) |
| Hypertension | 1106 (6.0) | 4200 (5.7) |
| Lipid‐regulating medications | 955 (5.2) | 3418 (4.7) |
| T2DM | 463 (2.5) | 1431 (2.0) |
| Drinking status | ||
| Nondrinker | 5149 (27.8) | 13 709 (18.7) |
| Drinker not excess | 8353 (45.0) | 41 993 (57.3) |
| Excessive drinker | 1870 (10.1) | 2558 (3.5) |
| Not available | 3175 (17.1) | 14 971 (20.4) |
| Townsend index | ||
| (Least deprived) 1 | 1773 (9.6) | 8303 (11.3) |
| 2 | 2104 (11.3) | 9553 (13.1) |
| 3 | 3149 (17.0) | 13 695 (18.7) |
| 4 | 4215 (22.7) | 16 818 (23.0) |
| 5 | 4266 (23.0) | 16 110 (22.0) |
| Not available | 3040 (16.4) | 8752 (12.0) |
| Charlson comorbidity index | ||
| 0 (Least comorbid) | 13 569 (73.2) | 57 030 (77.9) |
| 1 | 4130 (22.3) | 13 352 (18.2) |
| 2 | 571 (3.1) | 1983 (2.7) |
| 3 | 170 (0.9) | 542 (0.7) |
| ≥4 | 108 (0.6) | 324 (0.4) |
| CVD at baseline | ||
| IHD | 199 (1.1) | 539 (0.7) |
| Stroke/TIA | 208 (1.2) | 488 (0.7) |
| Heart failure | 24 (0.1) | 109 (0.2) |
| Peripheral vascular disease | 44 (0.2) | 136 (0.2) |
| All CVD | 413 (2.2) | 1116 (1.5) |
Data are shown as mean±SD or n (%) except as noted. CVD indicates cardiovascular disease; IHD, ischemic heart disease; TIA indicates transient ischemic attack; T2DM, type 2 diabetes mellitus.
Covariates known to affect the development of CVD, hypertension, or T2DM were included in the study population baseline data. These included body mass index, Townsend deprivation score, the use of lipid‐lowering medications, smoking status, hypertension and diabetes mellitus, alcohol excess, and comorbidity identified through the Charlson comorbidity index.42
Read code lists for exposure, outcomes, and covariates are provided in Table S1. Table S2 contains the RECORD (Reporting of studies conducted using observational routinely‐collected data) reporting checklist for this study.43
Statistical Analysis
Categorical baseline data were described using proportions, and parametric continuous variables were described using mean±SD. Missing data are described in the baseline characteristics (Table 1).
The number of outcomes in each group is described and then an incidence rate (IR) per 1000 person‐years was calculated. Poisson regression was then used to calculate an IR ratio (IRR) for each outcome of interest, offsetting for person‐years of follow‐up. The IRR was adjusted for known covariates independently affecting the outcome of interest (eg, age, deprivation, body mass index, use of lipid‐lowering medications, smoking status, hypertension, and T2DM [for individual components of CVD] and alcohol excess and Charlson comorbidity score [for mortality]), and an adjusted IRR for each outcome of interest was calculated. IRRs were calculated with 95% CIs, and statistical significance was set at P<0.05. In addition, for the calculation of the risk of individual components of CVD, a composite CVD risk was calculated, composed of the risk of developing IHD, stroke or TIA, heart failure, and peripheral vascular disease.
Stata version 14.2 software (StataCorp) was used to conduct all analyses throughout the study. This particular study obtained study specific approval from the scientific review committee in May 2018 (SRC18THIN034).
Results
Baseline Characteristics
A total of 18 547 women who had experienced DA were matched to 73 231 unexposed women as controls. The mean length of follow‐up was shorter at 2.2±2.3 years in the exposed group compared with 3.1±2.7 years in the unexposed group. This result was due to women in the exposed group transferring practice more frequently than those in the unexposed group. The mean age (37 years) was similar in both exposed and unexposed groups. Given the matching process, body mass index, smoking, and deprivation levels were similar between groups. Of particular note, both groups had high prevalence of smoking (44.7%) and high levels of deprivation compared with the national UK average. Excessive drinking at baseline was more prevalent at 10.1% in the exposed group compared with 3.5% in the unexposed group. The exposed group at baseline had higher proportion of patients with T2DM, greater comorbidity, higher prevalence of hypertension, and more use of lipid‐lowering medications. Baseline characteristics are presented in Table 1.
Association Between DA and Subsequent Development of Cardiometabolic Disease
During the study period, 181 women (IR: 3.1 per 1000 person‐years) in the exposed group compared with 644 women (IR: 2.3 per 1000 person‐years) in the unexposed group developed a CVD outcome. This translated into an increased adjusted IRR of composite CVD (1.31; 95% CI, 1.11–1.55; P=0.001). Specifically, the DA group had a significantly higher risk of developing IHD (1.40; 95% CI, 1.09–1.79; P=0.007) and stroke or TIA (1.29; 95% CI, 1.02–1.63; P=0.035). There were not statistically significant differences between the likelihood of developing heart failure or peripheral vascular disease.
Overall, 316 women in the exposed group developed hypertension (IR: 5.7 per 1000 person‐years) compared with 1496 women in the unexposed group (IR: 5.6 per 1000 person‐years). There was no association observed between DA and hypertension (IRR: 0.99; 95% CI, 0.88–1.12; P=0.873). In total, 222 women (IR: 3.8 per 1000 person‐years) in the exposed group compared with 678 women (IR: 2.4 per 1000 person‐years) in the unexposed group developed T2DM. The risk of developing T2DM was found to be higher in the exposed cohort than in the unexposed cohort (IRR: 1.51; 95% CI, 1.30–1.76; P<0.001). These results are presented in Figure and Table 2.
Figure 1.
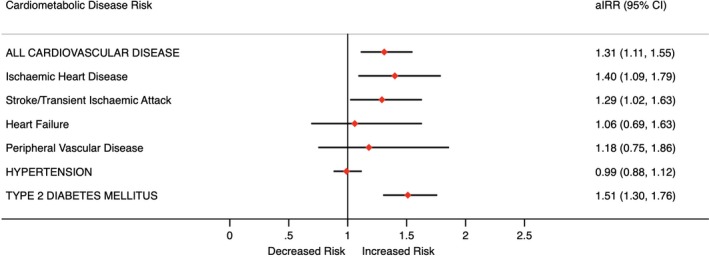
Cardiometabolic disease risk. aIRR indicates adjusted incidence rate ratio.
Table 2.
Risk of Subsequent Development of Cardiometabolic Disease in Exposed and Unexposed Groups
| All CVD | IHD | Stroke/TIA | Heart Failure | Peripheral Vascular Disease | Hypertension | T2DM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | |
| Patients, n | 18 134 | 72 115 | 18 348 | 72 692 | 18 339 | 72 743 | 18 523 | 73 122 | 18 503 | 73 095 | 17 441 | 69 031 | 18 084 | 71 800 |
| No. of outcomes | 181 | 644 | 83 | 270 | 89 | 316 | 26 | 106 | 24 | 89 | 316 | 1469 | 222 | 678 |
| Person‐years | 58 732 | 278 372 | 59 635 | 281 484 | 59 686 | 281 778 | 60 507 | 283 995 | 60 421 | 283 761 | 55 373 | 261 695 | 58 462 | 277 134 |
| IR (per 1000 person years) | 3.1 | 2.3 | 1.4 | 1.0 | 1.5 | 1.1 | 0.4 | 0.4 | 0.4 | 0.3 | 5.7 | 5.6 | 3.8 | 2.4 |
| IRR (95% CI)* | 1.33 (1.13–1.57) | 1.45 (1.130–1.85) | 1.33 (1.05–1.68) | 1.15 (0.75–1.77) | 1.27 (0.81–1.99) | 1.02 (0.90–1.15) | 1.55 (1.33–1.81) | |||||||
| P value | 0.001 | 0.003 | 0.018 | 0.520 | 0.304 | 0.790 | <0.001 | |||||||
| aIRR (95% CI)† | 1.31 (1.11–1.55) | 1.40 (1.09–1.79) | 1.29 (1.02–1.63) | 1.06 (0.69–1.63) | 1.18 (0.75–1.86) | 0.99 (0.88–1.12) | 1.51 (1.30–1.76) | |||||||
| P value | 0.001 | 0.007 | 0.035 | 0.796 | 0.465 | 0.873 | <0.001 | |||||||
aIRR indicates adjusted incidence rate ratio; CVD, cardiovascular disease; IHD, ischemic heart disease; IR, incidence rate; IRR, incidence rate ratio; TIA indicates transient ischemic attack; T2DM, type 2 diabetes mellitus.
*Unadjusted incidence rate ratio.
All CVD, IHD, stroke/TIA, heart failure, and peripheral vascular disease outcomes were adjusted for body mass index, age, sex, smoking, diabetes mellitus status, lipid‐lowering drug use, hypertension, and Townsend deprivation score at baseline. The hypertension outcome was adjusted for these factors excluding hypertension. The T2DM outcome was adjusted for these factors excluding hypertension and diabetes mellitus status.
Mortality Analysis
Table 3 demonstrates the variation in risk of all‐cause mortality between the exposed and unexposed groups. A total of 248 patients in the DA group died during the study period compared with 700 in the unexposed group. This relates to an IR per 1000 person‐years of 6.0 and 3.1 between the DA and unexposed groups, respectively. Following adjustment, this translated into an IRR of 1.44 (95% CI, 1.24–1.67; P<0.001).
Table 3.
Risk of Mortality in the Exposed and Unexposed Groups
| Exposed | Unexposed | |
|---|---|---|
| Patients, n | 18 547 | 73 231 |
| No. of outcomes | 248 | 700 |
| Person‐years | 41 213 | 225 438 |
| IR (per 1000 person‐years) | 6.0 | 3.1 |
| IRR (95% CI)* | 1.94 (1.68–2.24) | |
| P value | <0.001 | |
| aIRR (95% CI)† | 1.44 (1.24–1.67) | |
| P value | <0.001 | |
aIRR indicates adjusted incidence rate ratio; IR, incidence rate; IRR, incidence rate ratio.
*Unadjusted incidence rate ratio.
Adjusted for body mass index, age, sex, smoking status, diabetes mellitus status, lipid‐lowering drug use, hypertension, Charlson comorbidity score, alcohol status, and Townsend deprivation score.
Discussion
Summary of Key Results
After matching and adjusting for key lifestyle factors, this study found that women who were coded with exposure to DA were at a higher risk of developing CVD (particularly IHD and stroke or TIA) and T2DM than those not coded for exposure to DA. In addition, the risk of all‐cause mortality was higher in the group of women who had experienced DA. However, there was no association between exposure to DA and the development of hypertension.
Relationship to Current Literature
To the authors knowledge, this cohort study is the first to assess the CVD outcomes and all‐cause mortality risk associated with DA and has added to the literature exploring the association between DA and the development of hypertension and T2DM.
Because no cohort study has previously been designed to compare the IR of CVD in the cohort of women who have experienced DA, it is difficult to directly compare this study with studies that demonstrate an increased risk of developing of CVD outcomes.19, 44 However, this study supports the suggestion that exposure to DA increases the risk of developing CVD suggested by previously cross‐sectional studies.8 In particular, our study is consistent with the assertion that DA may particularly correlate with the development of IHD.8, 10 Interestingly, in agreement with previous work (cross‐sectional studies13, 14, 15, 16, 17 and 1 prospective study18), our study supports no statistical association between being a survivor of DA and hypertension. Our study also demonstrates an association between DA and T2DM. This relationship mirrors the positive association between DA and T2DM previously reported in the NHSII23 in survivors of severe psychological forms of DA.
This study made efforts to account for the impact of lifestyle choices on the development of cardiometabolic disease by matching similar patients. Previous studies suggested that women exposed to DA had high rates of smoking, alcohol use, and obesity,5, 29, 30, 31, 32, 33 which was clearly demonstrated in our study. Both cohorts had high baseline rates of smoking (44.7%), and more patients in the DA cohort were excessive drinkers. Interestingly, however, following matching and adjustment for these factors, there still appeared to be a relationship between DA and cardiometabolic disease. This finding suggests that lifestyle risk factors may not be the sole explanation for the observed increased risk of cardiometabolic disease in this population. Further investigation must be conducted into other possible mediating pathways that are responsible for this relationship.
Similarly, because there have been no cohorts examining the association between all‐cause mortality and DA exposure, it is not possible to compare the IR in our study with others. We show an increased mortality risk associated with DA, and this may correlate with literature identifying that victims of DA are at an increased risk of violent injury that may lead to death.35, 36 However, it may also raise the possibility that there is an increased burden of CVD and other morbidities that may increase mortality.
Study Limitations
The use of a primary care database to undertake retrospective analysis relies on the correct coding of Read codes by general practitioners. Therefore, the validity of coding will be affected by differing coding practices. An important limitation to note is that the overall number of women experiencing DA appears to be extremely low in this study population compared with the estimates provided previously in the United Kingdom.3 Consequently, it is possible that women of the unexposed group may actually have experienced DA but were misclassified as unexposed. This possible misclassification bias may lead to underestimation of the true effect size. Similarly, we may have identified only women with severe DA, which may result in an overestimate of the effect size. To minimize this risk, we attempted to use multiple codes relating to DA to capture as many patients as possible. It is worth highlighting that the underrecording of DA in a primary care setting suggests the importance of ensuring that clinicians make a concerted effort to ask at‐risk patients about a history of abuse.
Given the nature of the data collected, we were unable to assess for the dose relationship between the severity of DA and CVD, hypertension, and T2DM, which could have provided further useful information. It is possible, for example, that more severe abuse may be correlated with worse cardiometabolic outcomes. It is also important to identify that although we matched women's lifestyle choices at baseline, it would be interesting to identify whether exposure to DA leads to poorer lifestyle choices in the future, which also may mediate the described effect size.
Conclusions
This is the first cohort study to demonstrate an association between DA and the development of CVD. This study has reaffirmed the relationship between exposure to DA and the development of T2DM, even after consideration of the impact of contributing lifestyle risk factors, and identifies this population as having higher risk of all‐cause mortality. This cohort study also supports previous research demonstrating no association between the development of hypertension and being a female survivor of DA. However, this result needs to be viewed in light of the limitations of the study design. Regardless, given the sizeable population that these results may affect, physicians should pay particular notice to managing risk factors for CVD and T2DM in this group. Further studies in other cohorts are needed to confirm this relationship, and basic scientific research is required to understand the biological plausibility of the associations between DA exposure and the subsequent development of cardiometabolic disease.
Disclosures
None.
Supporting information
Table S1. Read Codes for Domestic Abuse, Outcomes, Baseline Data, and Covariates
Table S2. RECORD (Reporting of studies conducted using observational routinely‐collected data) Statement—Checklist of Items, Extended From the STROBE (Strengthening the reporting of observational studies in epidemiology) Statement That Should Be Reported in Observational Studies Using Routinely Collected Health Data
Acknowledgments
Author Contributions: This study contributed to the PhD thesis for the main author (J.S.C.). J.S.C., T.T., C.B.J., J.T., S.B., and K.N. were responsible for initial conception of the study. J.S.C. and T.T. were responsible for data extraction, analysis, and the first draft of the manuscript. The final manuscript was authorized by all the authors, with C.B.J., J.T., and S.B. providing expert opinions on domestic abuse and K.N. providing expert opinion on cardiovascular risk in young women and methodological expertise.
(J Am Heart Assoc. 2020;9:e014580 DOI: 10.1161/JAHA.119.014580.)
References
- 1. World Health Organization . WHO | Violence Against Women. WHO; 2018. Available at: http://www.who.int/mediacentre/factsheets/fs239/en/. [Google Scholar]
- 2. UK Government . Guidance: Domestic Violence and Abuse. UK Gov; 2016. Available at: https://www.gov.uk/government/news/new-definition-of-domestic-violence. [Google Scholar]
- 3. Flatley J. Intimate Personal Violence and Partner Abuse. UK: Office for National Statistics; (2016). Available at: https://www.istat.it/it/files/2017/11/Intimate-personal-violence-and-partner-abuse.pdf. [Google Scholar]
- 4. Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R. World Report on Violence and Health. WHO: 2002. Available at: http://handle/10665/42495/9241545615_eng.pdf?sequence=1. [Google Scholar]
- 5. Bacchus LJ, Ranganathan M, Watts C, Devries K. Recent intimate partner violence against women and health: a systematic review and meta‐analysis of cohort studies. BMJ Open. 2018;8:e019995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandan JS, Thomas T, Bradbury‐Jones C, Russell R, Bandyopadhyay S, Nirantharakumar K, Taylor J. Female survivors of intimate partner violence and risk of depression, anxiety and serious mental illness. Br J Psychiatry. 2019;1–6. [DOI] [PubMed] [Google Scholar]
- 7. Chandan JS, Thomas T, Bradbury‐Jones C, Taylor J, Bandyopadhyay S, Nirantharakumar K. Intimate partner violence and temporomandibular joint disorder. J Dent. 2019;82:98–100. [DOI] [PubMed] [Google Scholar]
- 8. Suglia SF, Sapra KJ, Koenen KC. Violence and cardiovascular health: a systematic review. Am J Prev Med. 2015;48:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The King's Fund . Briefing: General practice in England | The King's Fund. 2009. Available at: https://www.kingsfund.org.uk/publications/briefing-general-practice-england.
- 10. Łukasik P, Karakuła‐Juchnowicz H, Morylowska‐Topolska J, Flis M, Krukow P. [Long‐term somatic consequences of intimate partner violence in primary care female patients]. Pol Merkur Lekarski. 2015;39:372–376. [PubMed] [Google Scholar]
- 11. Gass JD, Stein DJ, Williams DR, Seedat S. Intimate partner violence, health behaviours, and chronic physical illness among South African women. S Afr Med J. 2010;100:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Neil A, Scovelle AJ. Intimate partner violence perpetration and cardiovascular risk: a systematic review. Prev Med Rep. 2018;10:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coker AL, Smith PH, Bethea L, King MR, McKeown RE. Physical health consequences of physical and psychological intimate partner violence. Arch Fam Med. 2000;9:451–457. [DOI] [PubMed] [Google Scholar]
- 14. Bonomi AE, Anderson ML, Reid RJ, Rivara FP, Carrell D, Thompson RS. Medical and psychosocial diagnoses in women with a history of intimate partner violence. Arch Intern Med. 2009;169:1692. [DOI] [PubMed] [Google Scholar]
- 15. Sparrenberger F, Fuchs SC, Moreira LB, Fuchs FD. Stressful life events and current psychological distress are associated with self‐reported hypertension but not with true hypertension: results from a cross‐sectional population‐based study. BMC Public Health. 2008;8:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golding JM. Intimate partner violence as a risk factor for mental disorders: a meta‐analysis. J Fam Violence. 1999;14:99–132. [Google Scholar]
- 17. Ruiz‐Perez I, Plazaola‐Castano J, del Rio‐Lozano M. Physical health consequences of intimate partner violence in Spanish women. Eur J Public Health. 2007;17:437–443. [DOI] [PubMed] [Google Scholar]
- 18. Mason SM, Wright RJ, Hibert EN, Spiegelman D, Forman JP, Rich‐Edwards JW. Intimate partner violence and incidence of hypertension in women. Ann Epidemiol. 2012;22:562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stene LE, Jacobsen GW, Dyb G, Tverdal A, Schei B. Intimate partner violence and cardiovascular risk in women: a population‐based cohort study. J Women's Health. 2013;22:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shields ME, Hovdestad WE, Pelletier C, Dykxhoorn JL, O'Donnell SC, Tonmyr L. Childhood maltreatment as a risk factor for diabetes: findings from a population‐based survey of Canadian adults. BMC Public Health. 2016;16:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rich‐Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun H‐J, Todd TJ, Kawachi I, Wright RJ. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP. The effect of multiple adverse childhood experiences on health: a systematic review and meta‐analysis. Lancet Public Health. 2017;2:e356–e366. [DOI] [PubMed] [Google Scholar]
- 23. Mason SM, Wright RJ, Hibert EN, Spiegelman D, Jun H‐J, Hu FB, Rich‐Edwards JW. Intimate partner violence and incidence of type 2 diabetes in women. Diabetes Care. 2013;36:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasmussen LJH, Moffitt TE, Eugen‐Olsen J, Belsky DW, Danese A, Harrington H, Houts RM, Poulton R, Sugden K, Williams B, Caspi A. Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J Child Psychol Psychiatry. 2018;60:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Out D, Hall RJ, Granger DA, Page GG, Woods SJ. Assessing salivary C‐reactive protein: longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav Immun. 2012;26:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 27. Esser N, Legrand‐Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. [DOI] [PubMed] [Google Scholar]
- 28. Suglia SF, Koenen KC, Boynton‐Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, Pratt CA. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. 2018;137:e15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott‐Storey K, Wuest J, Ford‐Gilboe M. Intimate partner violence and cardiovascular risk: is there a link? J Adv Nurs. 2009;65:2186–2197. [DOI] [PubMed] [Google Scholar]
- 30. Crane CA, Hawes SW, Weinberger AH. Intimate partner violence victimization and cigarette smoking. Trauma, Violence, Abuse. 2013;14:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yount KM, Li L. Domestic violence and obesity in Egyptian women. J Biosoc Sci. 2011;43:85–99. [DOI] [PubMed] [Google Scholar]
- 32. Afifi TO, Henriksen CA, Asmundson GJG, Sareen J. Victimization and perpetration of intimate partner violence and substance use disorders in a nationally representative sample. J Nerv Ment Dis. 2012;200:684–691. [DOI] [PubMed] [Google Scholar]
- 33. Davies R, Lehman E, Perry A, McCall‐Hosenfeld JS. Association of intimate partner violence and health‐care provider‐identified obesity. Women Health. 2016;56:561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. GBD 2017 Causes of Death Collaborators GA , Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe HT, Abebe M, Abebe Z, Abejie AN, Abera SF, Abil OZ, Abraha HN, Abrham AR, Abu‐Raddad LJ, Accrombessi MMK, Acharya D, Adamu AA, Adebayo OM, Adedoyin RA, Adekanmbi V, Adetokunboh OO, Adhena BM, Adib MG, Admasie A, Afshin A, Agarwal G, Agesa KM, Agrawal A, Agrawal S, Ahmadi A, Ahmadi M, Ahmed MB, Ahmed S, Aichour AN, Aichour I, Aichour MTE, Akbari ME, Akinyemi RO, Akseer N, Al‐Aly Z, Al‐Eyadhy A, Al‐Raddadi RM, Alahdab F, Alam K, Alam T, Alebel A, Alene KA, Alijanzadeh M, Alizadeh‐Navaei R, Aljunid SM, Alkerwi A, Alla F, Allebeck P, Alonso J, Altirkawi K, Alvis‐Guzman N, Amare AT, Aminde LN, Amini E, Ammar W, Amoako YA, Anber NH, Andrei CL, Androudi S, Animut MD, Anjomshoa M, Ansari H, Ansha MG, Antonio CAT, Anwari P, Aremu O, Ärnlöv J, Arora A, Arora M, Artaman A, Aryal KK, Asayesh H, Asfaw ET, Ataro Z, Atique S, Atre SR, Ausloos M, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Ayele Y, Ayer R, Azzopardi PS, Babazadeh A, Bacha U, Badali H, et al Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melville JD, McDowell JD. Domestic Violence. Forensic Odontol. 2018;121–144. [Google Scholar]
- 36. Stöckl H, Devries K, Rotstein A, Abrahams N, Campbell J, Watts C, Moreno CG. The global prevalence of intimate partner homicide: a systematic review. Lancet. 2013;382:859–865. [DOI] [PubMed] [Google Scholar]
- 37. Cegedim . The health improvement network. 2019. Available at: https://www.cegedim-health-data.com/cegedim-health-data/thin-the-health-improvement-network/. [Google Scholar]
- 38. NHS Digital . Read Codes – NHS Digital. 2017. Available at: https://digital.nhs.uk/services/terminology-and-classifications/read-codes.
- 39. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North, London: Croom Helm, 1988. [Google Scholar]
- 40. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 41. NHS Digital . Quality and Outcomes Framework. 2017. Available at: https://qof.digital.nhs.uk/.
- 42. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 43. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clark CJ, Alonso A, Everson‐Rose SA, Spencer RA, Brady SS, Resnick MD, Borowsky IW, Connett JE, Krueger RF, Nguyen‐Feng VN, Feng SL, Suglia SF. Intimate partner violence in late adolescence and young adulthood and subsequent cardiovascular risk in adulthood. Prev Med. 2016;87:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Read Codes for Domestic Abuse, Outcomes, Baseline Data, and Covariates
Table S2. RECORD (Reporting of studies conducted using observational routinely‐collected data) Statement—Checklist of Items, Extended From the STROBE (Strengthening the reporting of observational studies in epidemiology) Statement That Should Be Reported in Observational Studies Using Routinely Collected Health Data


