Abstract
Background
Management of patients with hypoplastic left heart syndrome has benefited from advancements in medical and surgical care. Outcomes have improved, although survival and long‐term functional and cognitive deficits remain a concern.
Methods and Results
This is a cohort study of all consecutive patients with hypoplastic left heart syndrome undergoing surgical palliation at a single center. We aimed to examine demographic and perioperative factors from each surgical stage for their association with survival and neurocognitive outcomes. A total of 117 consecutive patients from 1996 to 2010 underwent surgical palliation. Seventy patients (60%) survived to the Fontan stage and 68 patients (58%) survived to undergo neurocognitive assessment at a mean (SD) age of 56.6 months (6.4 months). Full‐scale, performance, and verbal intelligence quotient, as well as visual‐motor integration mean (SD) scores were 86.7 (16.1), 86.3 (15.8), 88.8 (17.2), and 83.2 (14.8), respectively. On multivariable analysis, older age at Fontan, sepsis peri‐Norwood, lowest arterial partial pressure of oxygen postbidirectional cavopulmonary anastomosis, and presence of neuromotor disability pre‐Fontan were strongly associated with lower scores for all intelligence quotient domains. Older age at Fontan and sepsis peri‐Norwood remained associated with lower scores for all intelligence quotient domains in a subgroup analysis excluding patients with disability pre‐Fontan or with chromosomal abnormalities.
Conclusions
Older age at Fontan and sepsis are among independent predictors of poor neurocognitive outcomes for patients with hypoplastic left heart syndrome. Further studies are required to identify the appropriate age range for Fontan completion, balancing a lower risk of acute and long‐term hemodynamic complications while optimizing long‐term neurocognitive outcomes.
Keywords: cognitive, Fontan procedure, Glenn procedure, mortality, neurodevelopment, Norwood procedure, single ventricle
Subject Categories: Cardiovascular Surgery, Mortality/Survival, Quality and Outcomes
Clinical Perspective
What Is New?
Patients with hypoplastic left heart syndrome undergoing 3‐stage single ventricle palliation are at risk for significant neurocognitive deficits and risk factors include older age at Fontan, sepsis, and hypoxemia.
What Are the Clinical Implications?
Older age at Fontan may offer hemodynamic benefits but its possible association with worse neurocognitive outcomes is a novel finding that should be further investigated.
The evolving care of patients with single ventricle undergoing staged surgical palliation, as well as ongoing clinical research in this patient population, should cogitate on the effect of age at surgery on both hemodynamic and neurocognitive outcomes, the importance of further decreasing the risk of infection and sepsis, and the impact of perioperative and more importantly interoperative hypoxemia.
Introduction
The care of patients with hypoplastic left heart syndrome (HLHS) has experienced further improvements in medical and surgical care over the past decade. However, refining the management of such complex patients is an ongoing challenge, aimed at further improving survival and functional long‐term outcomes. There have been several single and multicenter investigations on the outcome of patients with a single ventricle. Recent publications reporting predominantly on patients with HLHS have helped identify many of the outcomes and associated risk factors across the different stages of surgical palliation.1, 2, 3, 4, 5, 6
Neurodevelopmental and neurocognitive outcomes for patients with HLHS after the Fontan palliation have also been reported in several studies.7, 8, 9, 10, 11, 12, 13, 14 Some of these studies were limited by a small study cohort9, 10, 11 while others included only a limited number of clinical variables.7, 8 Of the studies that have reported on predictors of cognitive outcomes, the variables analyzed included mainly Norwood perioperative variables and a limited number of variables from the bidirectional cavopulmonary anastomosis (BCPA) and Fontan procedures. We previously reported on the survival and 2‐year neurodevelopmental outcomes of a large Western Canadian cohort.15 In this study, we report on the 4‐ to 6‐year outcomes of a large consecutive cohort of patients with classic HLHS having completed the 3‐stage surgical palliation. We aimed to examine the impact of surgical timing and detailed perioperative factors, for all 3 palliative procedures, on long‐term survival, neurocognitive, and functional outcomes.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This cohort study included all consecutive patients with HLHS who underwent surgical palliation between September 1996 and March 2010 at the Stollery Children's Hospital in Edmonton, Alberta, Canada. Patients with other forms of congenital heart disease such as an atrioventricular septal defect or isomerism and a hypoplastic left heart were excluded. The Stollery is the main Western Canadian surgical referral program. All patients were followed by the Western Canadian Complex Pediatric Therapies Follow‐up Program (CPTFP) and none were lost to follow‐up. Details of the program's methodology and database created from prospective data collection have been previously published.16, 17 Consents were attained from the patient's parents or legal guardian. Approval for this study was obtained from each institution's ethics board.
All patients were intended to undergo 3‐stage surgical palliation with a Norwood procedure, a BCPA, and a Fontan procedure, performed at the Stollery Children's Hospital. For the Norwood procedure, a modified Blalock‐Taussig shunt was used before September 2002, subsequently predominantly replaced by the right ventricle to pulmonary artery shunt, as previously reported.15 The Fontan operation included the lateral tunnel, intra‐extra cardiac, and preferentially extra‐cardiac conduit technique. All cases were discussed at a combined cardiosurgical conference before surgical planning, as per the institution and program standards. Timing for each surgery was guided by published clinical standards and the patient's clinical status. For the Fontan, the preference was to perform the procedure in patients older than 18 months of age and preferably >12 kg in weight. Patients who underwent cardiac transplantation were excluded from this study. Patients with a chromosomal abnormality or chronic neuromotor or neurosensory disability were not excluded from this study. Chromosomal abnormalities were identified with laboratory testing using G‐banding karyotype and molecular analysis for 22q deletion.
Early Childhood Assessments
Assessments were performed by a multidisciplinary team in patients between 48 and 72 months of age, at least 6 to 8 months post‐Fontan, in the dedicated Western Canadian CPTFP clinics. The Blishen index of occupational status is based on employment of the main wage earner of each family and was used to reflect socioeconomic status (population: mean, 43; SD, 13).18 Maternal education was indicated by years of schooling. Outcome measures were assessed in patients between 48 and 72 months of age and included physical growth and testing of neurocognition and adaptive functioning.19 Experienced pediatric psychologists assessed cognitive ability and visual motor skills using current age‐appropriate standardized measures, including the Wechsler Preschool and Primary Scales of Intelligence (third edition) and the Beery‐Buktenica Developmental Test of Visual‐Motor Integration (fifth edition).20, 21 Collected data included the US‐normed full‐scale intelligence quotient (FSIQ), performance intelligence quotient (PIQ), verbal intelligence quotient (VIQ), and visual‐motor integration (VMI). The population norm score (SD) was 100 (15) for each scale. Deidentified data were sent to the central site and all assessment scores and calculations from raw data were verified for accuracy by one of the investigators. Pediatricians experienced in neurodevelopmental follow‐up examined each child for evidence of chronic neuromotor disability22 or visual impairment, defined as corrected visual acuity in the better eye of <20/60. Sensorineural hearing impairment was defined as responses in the better ear of >25 dB at any frequency from 250 to 4000 Hz.17
To measure the patients’ functional and adaptive abilities, parents or legal guardians were asked to complete the Adaptive Behavior Assessment System—second edition (ABAS) (ages 0–5 years) parent report form at the time of routine assessment.23 This comprehensive normative assessment evaluates realistic, independent behaviors of patients and the effectiveness of interaction with others, while including consideration of community contexts. The measure includes 4 domains: conceptual (communication, functional pre‐academics, and self‐direction), practical (home living, health and safety, community use, and self‐care), social (leisure and social), and overall general adaptive composite (GAC), which includes the aforementioned 3 domains as well as motor skills. The ABAS‐GAC age‐based population score has a mean (SD) of 100 (15).
Variables and Outcomes
Collected data included baseline demographics, perinatal, perioperative (Norwood, BCPA, and Fontan), and cumulative variables (eg, overall hospitalization days, overall ventilations days, sepsis). As previously reported, sepsis was defined as a positive blood culture that was treated for at least 5 days with intravenous antibiotics.24 Potential skin contaminants in the blood culture (coagulase‐negative staphylococci, Aerococcus spp, Micrococcus spp, Corynebacterium spp, Propionibacterium spp, viridians group streptococci, or Bacillus spp [not Bacillus anthracis])25 required 2 positive blood cultures or 1 positive blood culture associated with fever or hypothermia, abnormal white blood cell count, and increase in inotrope score on the day of the culture. The patients with potential skin contaminants in the blood culture were identified as those with a Gram‐positive organism grown from the culture. All of these patients had their charts reviewed to confirm the above criteria. All variables examined in this study are included in Table 1. Inotrope scores were calculated using the modified inotrope score formula.26 Cardiac and noncardiac hospitalization, the use of cardiac and pulmonary medications, and the number of noncardiac medical specialists being seen were ascertained at the time of the follow‐up. The cardiac and non‐cardiac hospitalizations included all admissions to any hospital between the time of the neurodevelopmental assessment at 20 to 24 months and the neurocognitive assessment at 48 to 72 months of age. Medications included those being regularly taken at the time of the follow‐up assessment, and the specialists included those seen since the Fontan or anticipated to be seen in the near future. Outcome variables included postoperative and overall survival, neurocognitive outcomes (FSIQ, PIQ, VIQ), and VMI and GAC scores. Mortality was ascertained by direct contact with the families and primary physician. Postoperative deaths were defined as within 30 days after surgery or before hospital discharge.
Table 1.
Demographic and Perioperative Variables for all Patients With Classic HLHS Undergoing the Norwood Procedure, Postoperative Survivors, Postoperative Deaths, and Survivors to Neurocognitive Assessment
| Variables | Total Cohort (N=117) | Norwood Survivors (N=92) | Norwood Deaths (N=25) | Overall Survivors (N=68)a |
|---|---|---|---|---|
| Demographic | ||||
| Gestational age, wk | 38.7 (1.8) | 39.1 (1.6) | 38.0 (2.1) | 39.0 (1.5) |
| Birth weight, kg | 3.2 (0.6) | 3.3 (0.5) | 2.9 (0.5) | 3.3 (0.5) |
| Male sex | 77 (65.8%) | 63 (68.5%) | 14 (56.0%) | 48 (70.9%) |
| Chromosomal abnormality | 4 (3.4%) | 3 (3.3%) | 1 (4.0%) | 2 (2.9%) |
| Prenatal diagnosis | 63 (53.8%) | 53 (57.6%) | 10 (40.0%) | 38 (55.9%) |
| Preoperative | ||||
| Highest serum lactate, mmol/L | 3.9 (3.3) | 3.6 (2.7) | 5.0 (4.6) | 3.8 (3.0) |
| Lowest arterial pH | 7.21 (0.13) | 7.22 (0.12) | 7.18 (0.14) | 7.20 (0.12) |
| Lowest PaO2 | 31.5 (6.7) | 32.0 (6.5) | 30.1 (5.6) | 32.0 (6.8) |
| Lowest base deficit, mmol/L | −6.4 (5.6) | −6.1 (5.2) | −8.0 (6.7) | −6.2 (5.0) |
| Highest inotrope score | 11.4 (27.9) | 11.4 (30.0) | 11.5 (18.0) | 7.4 (13.1) |
| Ventilation time, d | 8.4 (7.4) | 7.2 (6.1) | 12.0 (11.1) | 7.2 (6.0) |
| CPR | 4 (3.4%) | 2 (2.2%) | 2 (8.0%) | 2 (2.9%) |
| ECMO | 3 (2.6%) | 1 (1.1%) | 2 (8.0%) | 1 (1.5%) |
| Operative | ||||
| Age at surgery, d | 8 (7–11.5) | 8 (7–11) | 8 (5.8–13.3) | 8 (7–12.5) |
| Age at surgery >14 d | 18 (15.4%) | 13 (14.1%) | 5 (20.0%) | 10 (14.7%) |
| Weight, kg | 3.3 (0.6) | 3.4 (0.5) | 3.0 (0.5) | 3.4 (0.6) |
| Year of surgery | 2002.9 (3.9) | 2003.1 (4.0) | 2001.4 (3.5) | 2003.5 (4.1) |
| MBTS | 56 (47.9%) | 40 (43.4%) | 16 (64.0%) | 24 (35.3%) |
| CPB, min | 124.4 (60.0) | 117.5 (52.0) | 150.0 (79.0) | 113.1 (46.0) |
| ACC, min | 45.8 (16.3) | 45.0 (15.1) | 47.3 (20.2) | 44.1 (15.0) |
| DHCA, min | 32.5 (18.8) | 31.2 (18.1) | 37.3 (22.0) | 30.2 (18.2) |
| Postoperative, day 1 | ||||
| Highest serum lactate, mmol/L | 8.4 (4.6) | 8.3 (4.5) | 10.1 (5.0) | 7.7 (3.6) |
| Lactate time to <2 mmol/L, h | 20.1 (17.8) | 18.2 (13.0) | 29.3 (28.1) | 16.1 (12.2) |
| Lowest arterial pH | 7.28 (0.09) | 7.29 (0.07) | 7.25 (0.13) | 7.28 (0.07) |
| Lowest PaO2 | 31.6 (7.0) | 31.5 (3.7) | 32.4 (4.1) | 31.2 (3.7) |
| Lowest base deficit, mmol/L | −1.7 (4.4) | −1.4 (3.7) | −2.9 (6.5) | −1.6 (3.8) |
| Highest inotrope score | 18.0 (19.9) | 15.2 (14.0) | 27.1 (33.1) | 15.3 (12.2) |
| Postoperative, day 2 to 5 | ||||
| Highest serum lactate, mmol/L | 4.5 (5.0) | 3.3 (3.8) | 9.1 (6.7) | 2.9 (2.0) |
| Lowest arterial pH | 7.32 (0.09) | 7.34 (0.05) | 7.25 (0.16) | 7.33 (0.05) |
| Lowest PaO2 | 33.7 (4.8) | 34 (4.1) | 34 (6.9) | 33 (3.9) |
| Lowest base deficit, mmol/L | −1.5 (4.2) | −0.67 (2.7) | −5.0 (6.6) | −0.6 (2.6) |
| Highest inotrope score | 22.8 (50.7) | 14 (10) | 58 (106) | 13 (8.2) |
| Postoperative, day 1 to 30 | ||||
| Ventilation time, d | 15.1 (11.9) | 14.2 (8.0) | 20.3 (20.1) | 14.3 (8.8) |
| ICU stay, d | 25.7 (18.9) | 26.2 (19.0) | 26.1 (20.4) | 25.3 (19.2) |
| Open sternum, d | 6.5 (7.6) | 5.0 (4.2) | 12.1 (4.4) | 5.2 (3.3) |
| Convulsion (patients) | 9 (7.7%) | 8 (8.7%) | 1 (4.0%) | 8 (11.8%) |
| CPR (patients) | 14 (12.0%) | 4 (4.3%) | 10 (40.0%) | 3 (4.4%) |
| ECMO (patients) | 14 (12.0%) | 1 (1.1%) | 13 (52.0%) | 1 (1.5%) |
| Overall hospitalization | ||||
| Ventilation time, d | 23.3 (15.1) | 21.1 (11.2) | 31.2 (24.3) | 21.4 (11.2) |
| Hospitalization, d | 37.6 (24.7) | 38.9 (25.2) | 32.7 (22.6) | 39.2 (26.5) |
| Convulsion (patients) | 12 (10.3%) | 10 (10.9%) | 2 (8.0%) | 9 (13.2%) |
| CPR (patients) | 17 (14.5%) | 5 (5.4%) | 12 (48.0%) | 4 (5.9%) |
| Dialysis (patients) | 33 (28.2%) | 19 (20.1%) | 14 (56.0%) | 11 (16.2%) |
| Sepsis (patients) | 27 (23.1%) | 22 (23.9%) | 5 (20.0%) | 17 (25.0%) |
Data are presented as mean (SD), median (interquartile range), or number (percentage). ACC indicates aortic cross clamp; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; ICU, intensive care unit; MBTS, modified Blalock‐Tausig shunt; PaO2, arterial partial pressure of oxygen.
Survivors after the Fontan procedure, evaluated at 48 to 72 months of age.
Statistical Analysis
Continuous variables were analyzed for the normality of their distribution and are presented as means with SDs or medians with interquartile range (IQR) if non‐normally distributed. The Wilcoxon rank sum test was used for non‐normally distributed data. Categorical variables are presented as numbers with percentages.
Univariate logistic regression was used to screen for independent variables associated with survival post‐Norwood. Multivariable linear regression was used to evaluate for independent variables associated with FSIQ, PIQ, VIQ, VMI, and GAC. The presence of a Fontan fenestration, surgical era, and the Blishen score were forced into all models as determined a priori. A multivariable linear regression model was also used to evaluate for independent variables associated with age at Fontan. The FSIQ, PIQ, and VIQ models included noncorrelated variables that met the P<0.10 criteria for all 3 domains of FSIQ, PIQ, and VIQ, on the corresponding univariate analysis. Models for VMI, GAC, and Fontan age were assessed separately but also included noncorrelated variables with a P<0.10 on the corresponding univariate analysis. When collinearity was identified between 2 variables, the more clinically applicable variable was chosen (eg, lactate instead of base excess). For variables that were measured at more than one point in time, eg, lactate on postoperative day 1 and postoperative days 2 to 5, chronological priority was applied, and the day 1 variable was used if both were statistically significant on univariate analysis. For variables represented as a component as well as an overall variable (eg, “pre‐Norwood ventilation” and “Norwood overall ventilation,” which included preoperative and postoperative ventilation, or “intensive care unit length of stay” and “overall hospitalization”), the component variable was included in the model instead of the overall variable, whenever the component variable was statistically significant.
For each model, the variance inflation factor was used to assess for any evidence of collinearity, defined as a variance inflation factor >5. The lowest Akaike Information Criterion was consistently used for model selection. Statistically significant data were defined as a P<0.05. Data analysis was performed using SAS version 9.4 (SAS Institute Inc).
Results
A total of 117 consecutive patients with classic HLHS were included in this cohort study. The detailed characteristics of the study population including demographic, perioperative, and cumulative variables are presented in Table 1 for the Norwood, Table 2 for the BCPA, and Table 3 for the Fontan procedures.
Table 2.
Demographic and Perioperative Variables for all Patients With Classic HLHS Undergoing the BCPA Procedure (N=76)
| Variables | BCPA Cohort (N=76) | BCPA Survivors (N=73) | BCPA Deaths (N=3) | Overall Survivors (N=68)a |
|---|---|---|---|---|
| Operative | ||||
| Age at surgery, mo | 6.6 (2.2) | 6.7 (2.2) | 5.4 (3.6) | 6.5 (2.2) |
| Weight at surgery, kg | 6.5 (1.1) | 6.5 (1.2) | 6.3 (0.45) | 6.4 (1.0) |
| CPB, min | 62.0 (27.5) | 59.1 (22.2) | 131.2 (55.0) | 60.3 (23.1) |
| DHCA | 6 (7.9%) | 5 (6.8%) | 1 (33.3%) | 5 (7.4%) |
| RVPA shunt occlusion | ||||
| Partial | 20 (26.3%) | 20 (27.4%) | 0 | 20 (29.4%) |
| Complete | 54 (71.1%) | 51 (69.9%) | 3 (100%) | 46 (67.6%) |
| None | 2 (2.6%) | 2 (2.7%) | 0 | 2 (2.9%) |
| Concomitant surgeries | ||||
| Arch repair | 5 (6.6%) | 5 (6.8%) | 0 | 5 (7.4%) |
| Systemic atrioventricular valve repair | 2 (2.6%) | 2 (2.7%) | 0 | 2 (2.9%) |
| Pulmonary artery plasty | 3 (3.9%) | 2 (2.7%) | 1 (33.3%) | 2 (2.9%) |
| Atrial septostomy | 3 (3.9%) | 3 (4.1%) | 0 | 3 (4.4%) |
| Transferred to pediatric ICU, intubated | 70 (92.1%) | 67 (91.8%) | 3 (100%) | 62 (91.2%) |
| Reintubated within 24 h | 3 (3.9%) | 3 (4.1%) | 0 | 3 (4.4%) |
| Postoperative, day 1 | ||||
| Highest serum lactate, mmol/L | 2.4 (1.7) | 2.3 (1.7) | 4.3 (0.66) | 2.4 (1.7) |
| Lactate time to <2 mmol/L, h | 2.0 (4.9) | 1.6 (3.3) | 13.8 (16.7) | 1.8 (3.4) |
| Lowest arterial pH | 7.32 (0.05) | 7.32 (0.05) | 7.30 (0.08) | 7.32 (0.05) |
| Lowest PaO2 | 38.7 (6.2) | 39.2 (6.0) | 30.3 (3.3) | 39.1 (6.2) |
| Lowest hemoglobin, g/L | 112.1 (20.0) | 112.0 (20.3) | 112.3 (7.5) | 111.4 (20.1) |
| Highest serum creatinine, μmol/L | 43.5 (12.6) | 43.3 (12.5) | 51.4 (10.1) | 43.6 (12.5) |
| Highest glucose, mmol/L | 10.5 (4.5) | 10.4 (4.4) | 13.4 (2.3) | 10.5 (4.5) |
| Highest inotrope score | 3.2 (5.9) | 2.9 (5.1) | 10.1 (17.1) | 3.0 (5.2) |
| Postoperative, day 2 to 5 | ||||
| Highest serum lactate, mmol/L | 1.5 (0.9) | 1.4 (0.9) | 3.4 (2.5) | 1.5 (0.9) |
| Lowest arterial pH | 7.34 (0.05) | 7.35 (0.04) | 7.31 (0.07) | 7.35 (0.04) |
| Lowest PaO2 | 38.8 (6.1) | 38.7 (6.0) | 32.3 (6.3) | 38.7 (6.1) |
| Lowest hemoglobin, g/L | 107.2 (17.2) | 107.4 (17.1) | 117.0 (18.0) | 106.1 (16.2) |
| Highest serum creatinine, μmol/L | 39.7 (13.9) | 39.5 (13.7) | 45.7 (22.9) | 39.2 (14.1) |
| Highest glucose, mmol/L | 8.2 (2.3) | 8.0 (1.9) | 10.9 (6.9) | 8.1 (1.8) |
| Highest inotrope score | 1.7 (5.3) | 1.2 (3.7) | 13.3 (19.0) | 1.3 (3.8) |
| Highest BCPA CVP (days 1–5) | 19.6 (4.3) | 19.5 (4.2) | 18 (7.6) | 20 (4.0) |
| Postoperative, day 1 to 30 | ||||
| Ventilation time, d | 2.8 (6.1) | 1.9 (3.1) | 22.7 (20.6) | 2.0 (3.2) |
| ICU LOS, d | 7.6 (3.9) | 5.2 (4.7) | 66.3 (32.0) | 5.3 (4.8) |
| Hospitalization, d | 17.9 (19.5) | 13.4 (18.8) | 128.1 (73.3) | 13.5 (19.4) |
| Cardiac catheterization | 4 (5.3%) | 2 (2.7%) | 2 (66.7%) | 2 (2.9%) |
| Convulsion (patients) | 1 (1.3%) | 1 (1.4%) | 0 | 1 (1.5%) |
| CPR (patients) | 0 | 0 | 0 | 0 |
| ECMO | 2 (2.6%) | 1 (1.4%) | 1 (33.3%) | 1 (1.5%) |
| Inhaled nitric oxide | 9 (11.8%) | 7 (9.6%) | 2 (66.7%) | 7 (10.3%) |
| Dialysis (patients) | 1 (1.3%) | 1 (1.4%) | 0 | 1 (1.5%) |
| Sepsis (patients) | 0 | 0 | 0 | 0 |
Data are presented as mean (SD) or number (percentage). BCPA indicates bidirectional cavopulmonary anastomosis; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; CVP, central venous pressure; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; ICU, intensive care unit; LOS, length of stay; PaO2, arterial partial pressure of oxygen; RVPA, right ventricle to pulmonary artery.
Survivors after the Fontan procedure, evaluated at 48 to 72 months of age.
Table 3.
Demographic and Perioperative Variables for all Patients With Classic HLHS Undergoing the Fontan Procedure (N=70)
| Variables | Fontan Cohort (N=70) | Fontan Deaths (N=2) | Overall Survivors (N=68)a |
|---|---|---|---|
| Operative | |||
| Age at surgery, mo | 41.7 (9.7) | 40.6 (1.6) | 41.7 (9.9) |
| Weight at surgery, kg | 14.0 (1.7) | 15.5 (2.5) | 14 (1.7) |
| CPB, min | 77.5 (23.1) | 79.5 (2.1) | 77.4 (23.5) |
| DHCA | 4 (5.7%) | 0 | 4 (5.9%) |
| Type of Fontan | |||
| Extracardiac | 52 (74.3%) | 2 (100%) | 50 (73.5%) |
| Intra‐extracardiac | 11 (15.7%) | 0 | 11 (16.2%) |
| Lateral tunnel | 7 (10.0%) | 0 | 7 (10.3%) |
| Fenestration | 59 (84.3%) | 2 (100%) | 57 (83.8%) |
| Concomitant surgeries | |||
| Arch repair | 4 (5.7%) | 0 | 4 (5.9%) |
| Systemic atrioventricular valve repair | 16 (23%) | 0 | 16 (23%) |
| Systemic ventriculoarterial valve repair | 2 (2.9%) | 0 | 2 (2.9%) |
| Pulmonary artery plasty | 2 (2.9%) | 0 | 2 (2.9%) |
| Atrial septostomy | 1 (1.4%) | 0 | 1 (1.5%) |
| Transferred to ICU, intubated | 39 (55.7%) | 2 (100%) | 37 (54.4%) |
| Reintubated within 24 h | 5 (7.1%) | 1 (50%) | 4 (5.9%) |
| Postoperative, day 1 | |||
| Highest plasma lactate, mmol/L | 4.5 (1.7) | 4.6 (2.1) | 4.5 (1.7) |
| Lactate time to <2 mmol/L, h | 10.7 (7.3) | 13 (4.0) | 10.7 (7.3) |
| Lowest arterial pH | 7.28 (0.04) | 7.26 (0.01) | 7.28 (0.04) |
| Lowest PaO2 | 55.7 (13.9) | 55.1 (24.1) | 56.3 (14.2) |
| Lowest hemoglobin, g/L | 121.4 (20.3) | 136.5 (37.5) | 121.1 (20.3) |
| Highest serum creatinine, μmol/L | 64.6 (20.3) | 83.5 (13.4) | 64.2 (20.1) |
| Highest glucose, mmol/L | 13.0 (4.6) | 7.5 (1.6) | 14.0 (4.6) |
| Highest inotrope score | 9.5 (9.0) | 19.1 (1.4) | 9.4 (9.0) |
| Postoperative, day 2 to 5 | |||
| Highest plasma lactate, mmol/L | 2.1 (1.2) | 2.8 (0.3) | 2.1 (1.2) |
| Lowest arterial pH | 7.33 (0.06) | 7.21 (0.06) | 7.33 (0.05) |
| Lowest PaO2 | 53.1 (10.9) | 50.2 (13.1) | 53.1 (11.2) |
| Lowest hemoglobin, g/L | 118.7 (17.8) | 113.0 (4.2) | 119.0 (18.0) |
| Highest serum creatinine, μmol/L | 68.5 (54.6) | 153.0 (123) | 66.2 (51.1) |
| Highest glucose, mmol/L | 9.7 (2.8) | 12.1 (0.7) | 9.6 (2.8) |
| Highest inotrope score | 5.6 (10.1) | 19.8 (11.0) | 5.2 (9.8) |
| Highest CVP (days 1–5) | 19.1 (3.5) | 21.5 (4.0) | 9.6 (2.8) |
| Postoperative, day 1 to 30 | |||
| Ventilation, d | 1.8 (2.6) | 8.0 (3.0) | 2.1 (2.5) |
| ICU LOS, d | 4.7 (3.4) | 81.5 (104) | 4.7 (3.4) |
| Hospitalization, d | 14.4 (7.2) | 85.0 (99.0) | 14.1 (7.1) |
| Cardiac catheterization | 2 (2.9%) | 0 | 2 (2.9%) |
| Convulsion (patients) | 4 (5.7%) | 0 | 4 (5.9%) |
| CPR (patients) | 1 (1.4%) | 0 | 1 (1.5%) |
| ECMO (patients) | 0 | 0 | 0 |
| Inhaled nitric oxide | 5 (7.1%) | 1 (50%) | 4 (5.9%) |
| Dialysis (patients) | 2 (2.9%) | 1 (50%) | 1 (1.5%) |
| Sepsis (patients) | 2 (2.9%) | 0 | 2 (2.9%) |
| Overall for all 3 surgical stagesb | |||
| Ventilation, d | 27.2 (21.5) | 100.5 (105) | 25.1 (12.2) |
| Hospitalization, d | 69.3 (45.9) | 145.0 (116) | 67.3 (42.1) |
| Convulsion (patients) | 13 (18.6%) | 0 | 13 (19.1%) |
| CPR (patients) | 5 (7.1%) | 0 | 5 (7.4%) |
| ECMO (patients) | 2 (2.9%) | 0 | 2 (2.9%) |
| Dialysis (patients) | 13 (18.6%) | 1 (50%) | 12 (17.6%) |
| Sepsis (patients) | 21 (30.0%) | 1 (50%) | 20 (29.4%) |
Data are presented as mean (SD) or number (percentage).CPB indicates cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; CVP, central venous pressure; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; ICU, intensive care unit; LOS, length of stay; PaO2, arterial partial pressure of oxygen.
Survivors after the Fontan procedure, evaluated at 48 to 72 months of age
Includes Norwood preoperative data, as well as Norwood, bidirectional cavopulmonary anastomosis, and Fontan postoperative (up to 30 days postoperative) data.
Survival
The early era (1996–2002) included 56 patients having undergone the Norwood modified Blalock‐Taussig shunt procedure. Eighteen patients (32.1%) died postoperatively and 8 additional patients (14.3%) died in the interstage period, before the BCPA procedure. Of the 30 patients (53.6%) undergoing the BCPA, 2 died postoperatively and 3 died in the interim period before the Fontan procedure (Figure 1). There was only 1 death post‐Fontan and all 24 survivors (42.9%) underwent neurocognitive assessment at a mean age of 57.7 months (SD, 7.6 months). The recent era (2002–2010) included 61 patients having undergone the Norwood right ventricle to pulmonary artery procedure. Seven patients (11.5%) died postoperatively and 8 additional patients (13.1%) died in the interstage period, before the BCPA procedure. Of the 46 patients (75.4%) undergoing the BCPA, 1 died postoperatively. There was only 1 death post‐Fontan and all 44 survivors (72.1%) underwent neurocognitive assessment at a mean age of 56.1 months (SD, 5.7 months) (Figure 1).
Figure 1.
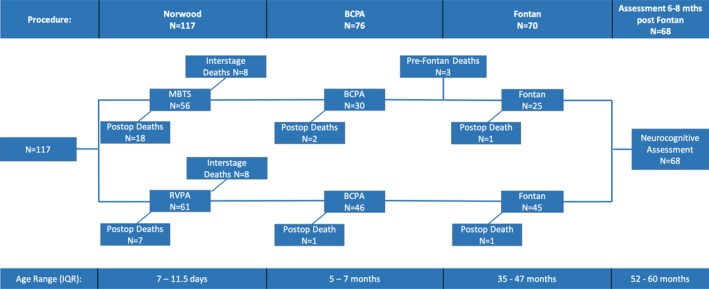
Schematic of the overall cohort 3‐stage palliative course and interval mortality, showing the early era (1996–2002, modified Blalock‐Taussig shunt [MBTS]) and the recent era (2002–2010, right ventricle to pulmonary artery [RVPA]), and interquartile age range for each stage. BCPA indicates bidirectional cavopulmonary anastomosis; IQR, interquartile range.
The overall survival to the Fontan procedure was 59.8% (44.6% and 73.8% for the consecutive eras) and 58% survived to undergo neurocognitive assessment. No patient was lost to follow‐up. Of the 25 total deaths post‐Norwood, 17 patients required cardiopulmonary resuscitation (CPR), of whom 12 died and 14 patients required extracorporeal membrane oxygenation (ECMO), of whom 13 died. On univariate analysis, variables associated with mortality post‐Norwood included birth weight, gestational age, preoperative ventilation and lactate, modified Blalock‐Taussig–type Norwood shunt, cardiopulmonary bypass time, postoperative lactate and pH, delayed sternal closure and the need for CPR, ECMO, or dialysis (Table S1). A strong correlation was identified between preoperative ventilation and birth weight or gestational age, between dialysis and open sternum days, and between pH and lactate. In the multivariable model for mortality post‐Norwood, when CPR and ECMO were included in the model, only those 2 variables were found to be significant: ECMO (odds ratio [OR], 116.85; 95% CI, 11.56–990.09 [P<0.001]) and CPR (OR, 14.31; 95% CI, 3.27–62.67 [P=0.004]). When ECMO and CPR were excluded, the factors associated with increased mortality were lower birth weight, modified Blalock‐Taussig–type shunt, and prolonged open sternum duration (Table 4). Age at Norwood was not statistically significantly associated with survival when analyzed as a continuous variable or as a categorical variable with cutoffs of 5, 7, 10, or 14 days.
Table 4.
Multivariable Logistic Regression Model for Risk Factors Associated With Postoperative Mortality After the Norwood Procedure
| Variables | OR (95% CI) | P Value |
|---|---|---|
| Birth weight, kg | 0.39 (0.15–0.97) | 0.042 |
| Use of RVPA shunt | 0.23 (0.07–0.74) | 0.013 |
| Open sternum, d | 1.14 (1.04–1.25) | 0.005 |
Analysis excludes cardiopulmonary resuscitation (CPR) and extracorporeal membrane oxygenation (ECMO) as these variables are likely to cancel out more informative proximal predictors of outcome. Odds ratio (OR) results for CPR and ECMO, when included in the model, are presented under Results. RVPA indicates right ventricle to pulmonary artery.
Overall interstage mortality (between Norwood and BCPA) was 13.7% (16/117). On univariate analysis, only 3 variables showed a borderline association but did not reach statistical significance: birth weight (OR, 0.38; 95% CI, 0.13–1.06 [P=0.064]), elevated pre‐Norwood modified inotropic score (OR, 1.02; 95% CI, 1.00–1.05 [P=0.055]), and age at Norwood operation >7 days (OR, 3.59; 95% CI, 0.95–13.7 [P=0.060]). Multivariable analysis was not performed.
Clinical Outcomes
On the last follow‐up at a mean age of 56.6 months (SD, 6.4 months), the mean weight and height z scores were −0.96 (1.06) and −0.68 (0.95), respectively. The mean number of total cardiac hospitalizations was 2.5 (SD, 2.1) and noncardiac hospitalizations was 1.8 (SD, 2.1). The majority of patients were taking cardiac medications (92.5%), 11.9% were taking pulmonary medications, and a total of 26 (38.8%) had been fed by a gastrostomy or NG tube as an outpatient at some point in time. At the mean age of assessment of 56.6 months, a total of 18 patients had neuromotor and/or sensory disability: 8 patients (12%) had a chronic neuromotor disability, 1 (1.5%) had visual impairment, 8 (12%) had sensorineural hearing loss, and 2 (3%) had epilepsy.
Neurocognitive and Functional Outcomes
The mean FSIQ score was 86.7 (SD, 16.1), PIQ was 86.3 (SD, 15.8), VIQ was 88.8 (SD, 17.2), and VMI was 83.2 (SD, 14.8). The mean GAC score was 88.2 (SD, 19.9). The results are shown in Table 5, along with the proportion of patients with scores 1 (<85) and 2 (<70) SDs below the population norms. Using FSIQ, severe cognitive impairment (score <70) was identified in 13% of patients. Using the GAC, severe impairment in functional ability (score <70) was reported by 25% of parents/guardians.
Table 5.
Demographic, Clinical, and Neurocognitive Outcomes for 68 Survivors, Assessed Between 6 and 8 Months After the Fontan Procedure
| Variables | N=68 |
|---|---|
| Demographic | |
| Age at assessment, mo |
56.6 (6.4) Range (48–79) |
| Socioeconomic statusa | 45.0 (12) |
| Guardianship, both parents | 61 (90%) |
| Primary language, English | 61 (91%) |
| Mother's schooling, y | 13.9 (2.1) |
| Race | |
| European | 57 (84%) |
| Other | 11 (16%) |
| Clinical parameters | |
| Cardiac hospitalizations | 1.7 (1.0) |
| Noncardiac hospitalizations | 0.6 (0.1) |
| Noncardiac medical specialist seen | 2.5 (1.6) |
| Chronic cardiac medications | 2.0 (1.1) |
| Chronic pulmonary medications | 0.2 (0.5) |
| Outcomes | |
| Chronic neuromotor disability | 8 (12%) |
| Visual impairment | 1 (1.5%) |
| Sensorineural hearing loss | 8 (12%) |
| Epilepsy | 2 (3%) |
| FSIQ |
86.7 (16.1) Range (54–115) |
| FSIQ <85 | 28 (41%) |
| FSIQ <70 | 9 (13%) |
| PIQ |
86.3 (15.8) Range (54–118) |
| PIQ <85 | 30 (44%) |
| PIQ <70 | 10 (15%) |
| VIQ |
88.8 (17.2) Range (54–120) |
| VIQ <85 | 21 (31%) |
| VIQ <70 | 8 (12%) |
| VMI |
83.2 (14.8) Range (47–107) |
| Adaptive behavioral assessment system |
88.2 (19.9) Range (52–117) |
| GAC <85 | 25 (37%) |
| GAC <70 | 17 (25%) |
Data are presented as mean (SD) or number (percentage). FSIQ indicates full‐scale intelligence quotient; GAC, general adaptive composite; PIQ, performance intelligence quotient; VIQ, verbal intelligence quotient; VMI, visual motor integration.
Socioeconomic status as measured by the Blishen index.
Table 6 shows the results for the multivariable linear regression analysis of factors associated with lower scores for each of the intelligence quotient (IQ) outcomes (Table S2 shows the univariate analysis). Sepsis at the time of the Norwood operation, lowest arterial partial pressure of oxygen (PaO2) post‐BCPA operation, older age at Fontan (Figure 2), and the presence of a chronic neuromotor disability pre‐Fontan were associated with lower scores for all 3 outcomes of FSIQ, PIQ, and VIQ. Older age at Norwood was associated with lower VIQ scores, while non‐European race and lower socioeconomic status were associated with lower PIQ scores. The respective multivariable linear regression models for FSIQ, PIQ, and VIQ accounted for 45%, 42%, and 45% of the outcome variability around the mean (adjusted R 2). For sensitivity analysis, the multivariable linear regression analysis for factors associated with FSIQ, PIQ, and VIQ was repeated after excluding patients with chronic neuromotor disability and chromosomal abnormality (n=11). Age at Fontan remained a statistically significant predictor of lower FSIQ and PIQ scores, as did sepsis peri‐Norwood. PaO2 post‐BCPA, non‐European race, and lower socioeconomic status remained associated with PIQ scores (Table 7). Age at Fontan was compared across the study era, with no significant change identified over the years (P=0.790). Noncardiac hospitalizations were included in the models and were not found to be statistically significant independent predictors.
Table 6.
Multivariable Linear Regression Models for Factors Associated With FSIQ, PIQ, and VIQ at 48 to 72 Months of Age After Surgical Palliation for Patients With Classic HLHS (N=68): Linear Regression Coefficient (β) and 95% CI
| Variable | FSIQ | P Value | PIQ | P Value | VIQ | P Value |
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | ||||
| Norwood | ||||||
| Age at Norwood, d | −0.70 (−1.40, −0.03) | 0.041 | ||||
| Sepsis | −9.90 (−17.00, −2.90) | 0.007 | −9.20 (−16.77, −2.10) | 0.012 | −9.20 (−17.02, −1.90) | 0.015 |
| BCPA | ||||||
| Lowest PaO2 (POD 2–5) | 0.58 (0.07–1.09) | 0.027 | 0.71 (0.20–1.23) | 0.008 | 0.51 (−0.04, 1.06) | 0.066 |
| Fontan | ||||||
| Age at Fontan, y | −6.50 (−10.42, −2.80) | <0.001 | −6.40 (−10.51, −2.70) | <0.001 | −5.10 (−9.00, −1.10) | 0.013 |
| Demographics | ||||||
| Race (European) | 8.92 (0.76–17.10) | 0.033 | ||||
| Socioeconomic statusa | 0.21 (−0.01, 0.42) | 0.059 | ||||
| Pre‐Fontan NMD | −9.80 (−19.06, −0.49) | 0.039 | −10.04 (−19.72, −1.20) | 0.026 | −13.74 (−23.45, −3.40) | 0.009 |
The adjusted R 2 values for each model were 0.45 for full‐scale intelligence quotient (FSIQ), 0.42 for performance intelligence quotient (PIQ), and 0.45 for verbal intelligence quotient (VIQ).
BCPA indicates bidirectional cavopulmonary anastomosis; HLHS, hypoplastic left heart syndrome; NMD, neuromotor disability; POD, postoperative day; PaO2, arterial partial pressure of oxygen.
Socioeconomic status as measured by the Blishen index.
Figure 2.
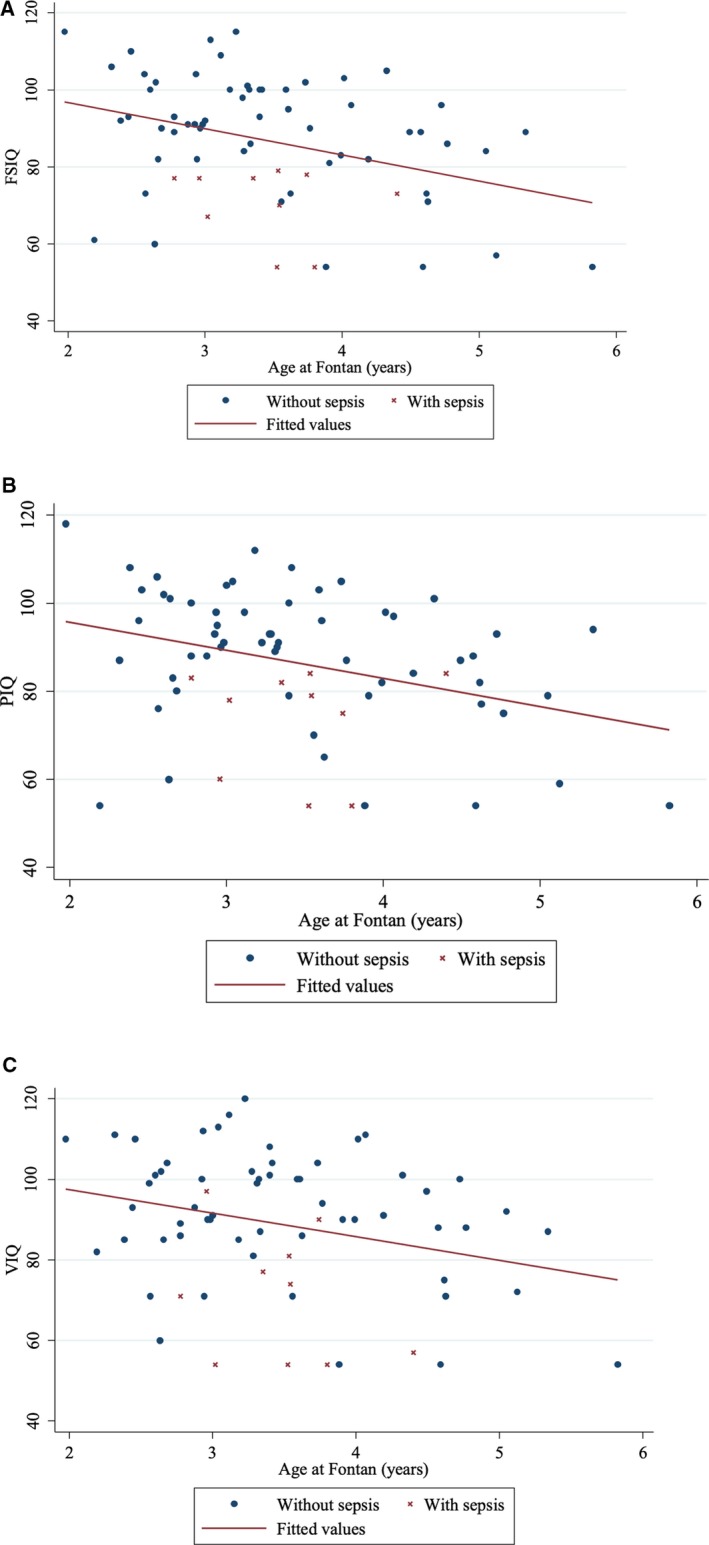
Scatter plot and unadjusted regression line of Fontan age with or without sepsis vs each of full‐scale intelligence quotient (FSIQ) (A), performance intelligence quotient (PIQ) ( B), and verbal intelligence quotient (VIQ) (C).
Table 7.
Multivariable Linear Regression Models for Factors Associated With FSIQ, PIQ, and VIQ at 48 to 72 Months of Age After Surgical Palliation for Classic HLHS, Excluding Patients With Pre‐Fontan Disability and Chromosomal Abnormality (N=57): Linear Regression Coefficient (β) and 95% CI
| Variable | FSIQ | P Value | PIQ | P Value | VIQ | P Value |
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | ||||
| Norwood | ||||||
| Age at Norwood, d | −0.71 (−1.50 to −0.06) | 0.069 | ||||
| Sepsis | −11.09 (−19.41, −2.30) | 0.014 | −11.23 (−19.44, −2.80) | 0.010 | −9.30 (−18.49 to −0.57) | 0.037 |
| BCPA | ||||||
| Lowest PaO2 (POD 2–5) | 0.55 (−0.03, 1.14) | 0.065 | 0.68 (0.13–1.23) | 0.016 | ||
| Fontan | ||||||
| Age at Fontan, y | −6.20 (−10.71, −2.00) | 0.005 | −6.50 (−10.89, −2.60) | <0.001 | −4.05 (−8.30 to −0.31) | 0.068 |
| Demographics | ||||||
| Race (European) | 8.70 (0.49–16.90) | 0.038 | ||||
| Socioeconomic statusa | 0.24 (0.02–0.46) | 0.032 | ||||
The adjusted R 2 values for each model were 0.29 for full‐scale intelligence quotient (FSIQ), 0.39 for performance intelligence quotient (PIQ), and 0.23 for verbal intelligence quotient (VIQ).
BCPA indicates bidirectional cavopulmonary anastomosis; HLHS, hypoplastic left heart syndrome; PaO2, arterial partial pressure of oxygen; POD, postoperative day.
Socioeconomic status as measured by the Blishen index.
For VMI, variables associated with lower scores on multivariable analysis included lowest PaO2 post‐BCPA (effect size, 9.15; 95% CI, 1.36–12.88 [P=0.045]) and noncardiac hospitalizations (effect size, −28.25; 95% CI, −36.75 to −15.66 [P=0.005]) (adjusted R 2=24%). Overall convulsions showed a borderline result (effect size, −35.25; 95% CI, −50.69 to 9.15 [P=0.070]). Age at Fontan was not statistically significant.
For the GAC instrument results, variables associated with a lower score on multivariable analysis included prolonged ventilation pre‐Norwood (effect size, −1.18; 95% CI, −1.91 to −0.45 [P=0.002]), lower weight at the time of the BCPA operation (effect size, 4.11; 95% CI, 0.20–8.03 [P=0.040]), prolonged postoperative ventilation at the time of the BCPA operation (effect size, −1.36; 95% CI, −2.68 to −0.04 [P=0.044]), and overall convulsion at any time during all 3 surgical stages (effect size, −13.27; 95% CI, −23.76 to −2.79 [P=0.014]). The regression model adjusted R 2 was 31%.
Using the median age at Fontan of 40 months (IQR, 35–47 months), baseline and perioperative variables were compared between patients with age at Fontan completion <40 months (n=33) versus ≥40 months (n=35). The median weight at Fontan was 13.7 kg (IQR, 12.8–15.4 kg) versus 14.4 kg (IQR, 13.0–16.1 kg) (P=0.002), and the mean age was 33.6 months (SD, 3.6 months) versus 49.2 months (SD, 7.2 months) (Table S3). On univariate analysis, the only variables associated with age at Fontan as a continuous variable included the use of deep hypothermic circulatory arrest, overall ventilation and convulsion peri‐Norwood, and age at BCPA. On multivariable linear regression analysis, older age at BCPA (months) (effect size, 1.18; 95% CI, 0.14–2.23 [P=0.027]) and convulsions at the time of Norwood (effect size, 6.38; 95% CI, −0.16 to 12.91 [P=0.056]) (adjusted R 2 15%) were associated with an older age at Fontan (months).
Discussion
This study evaluated the survival, neurocognitive, and functional outcomes of a large prospective cohort of 117 consecutive patients with classic HLHS who underwent 3‐stage surgical palliation in a single referral surgical center. Detailed demographic, medical, and perioperative data for all 3 surgical stages were available for all patients and no patients were lost to follow‐up. Mortality post‐Norwood in the recent era was 11.5%, being similar to the 12% reported from the Single Ventricle Reconstruction trial during a similar period.2 Predictors of 30‐day mortality after Norwood were also similar, including low birth weight, prolonged open sternum, and the need for ECMO post‐Norwood.2
Sixty‐eight survivors were assessed at a mean age of 56.6 months. Their average neurocognitive outcome scores, including FSIQ, PIQ, and VIQ, were all within 1 SD below the population mean, similar to previous reports.7, 8, 10, 11 A significantly low FSIQ, defined as <2 SD below the mean (score <70), was present in 13% of survivors. The novel finding in this study is the association between older age at Fontan and lower IQ scores across all 3 parameters, with a 6‐ to 7‐point decline in mean IQ score per additional year of delayed Fontan timing. Other relevant predictors of lower neurocognitive scores included sepsis peri‐Norwood, lowest PaO2 post‐BCPA, and pre‐Fontan disability. When excluding patients with pre‐Fontan disability or chromosomal abnormality, peri‐Norwood sepsis and age at Fontan remained as independent predictors. Additional analysis looking for variables associated with older age at Fontan failed to identify obvious confounders such as a more complicated medical or perioperative course, clustering of patients from the older era, or patients with more comorbidities. The only independently associated factor was age at BCPA, with an effect factor of only 1.18, meaning that for every 1‐month delay in completing the BCPA, the Fontan procedure was delayed by 1.18 months.
Other studies have evaluated predictors of neurocognitive outcomes in 48‐ to 72‐month‐old patients with HLHS post‐Fontan.7, 8, 9, 11 Rotermann and colleagues8 evaluated patients with a single ventricle, including 59 with HLHS, comparing neurodevelopmental outcomes and their association with initial surgical palliation involving the Norwood procedure.8 Outcomes, however, were dichotomized as “below average” versus “average or above.” They identified head circumference, preoperative adverse events, and postoperative stay as independent predictors. Gaynor and colleagues7 evaluated preschool neurodevelopmental outcomes in 112 patients with Fontan, including 91 with HLHS, comparing them with patients who had biventricular repair. Patients with Fontan were found to have worse processing speed, inattention, and impulsivity. The analysis of variables associated with lower IQ scores included predominantly peri‐Norwood variables. Longer cardiopulmonary bypass and Norwood length of stay were consistent predictors of lower FSIQ, PIQ, and VIQ. In the latter study, age at Fontan was not analyzed as a possible predictor. In the study by Rotermann et al8, age at Fontan was higher in the group of patients with below average neurodevelopmental outcomes (2.8 versus 2.6 years, P=0.074), but was not an independent predictor. Of note, the average age at Fontan in that study was lower than our overall cohort but was similar to the subgroup of patients in our cohort with Fontan completion younger than the median age of 40 months (mean age, 2.8 years). The overall average age at Fontan in our study was a mean of 3.5 years (median, 3.3; IQR, 2.9–3.9), in keeping with several larger published series.27, 28, 29
The pathogenesis of the association between older age at Fontan and worse neurocognitive outcomes is unclear. It may correlate with longer cerebral exposure to a hypoxic state, during a period of rapid cognitive development.30 Several investigators have used near‐infrared spectroscopy to assess cerebral oxygenation and identified a correlation between the severity and duration of pre‐Norwood and post‐Norwood hypoxia and worse neurocognitive outcomes (IQ and VMI).31, 32 It is conceivable that a more chronic exposure to less severe hypoxia may have similar consequences. Our data do not allow us to ascertain the relationship between chronic hypoxia and neurocognitive outcomes as oxygen saturation was recorded only in the acute postoperative state. Although post‐Norwood hypoxia was not a predictor in our study, lowest PaO2 post‐BCPA was an independent variable associated with lower IQ scores post‐Fontan. The phenomenon of hypoxia may be compounded by the reported findings of ischemic brain insults peri‐Fontan procedure and associated worse neurocognitive outcomes and parental reports of adaptive behavior.11, 22, 33 However, it is unclear whether the age at Fontan influences the risk of perioperative ischemic injury.
Another possible pathological factor is the change in cardiac output and consequently cerebral perfusion upon Fontan completion. Shiraishi et al34 showed an inverse relationship between age at Fontan and cardiac index at 5 and 10 years post‐Fontan, arguing for Fontan completion at an earlier age. Their aim was to contrast the outcomes of patients undergoing the Fontan procedure before or after 3 years of age. They showed a benefit to younger age at Fontan with better hemodynamics and exercise capacity. Our study suggests that their findings may also be associated with better neurocognitive outcomes. The optimal age at Fontan completion, however, remains an unresolved clinical dilemma. In a single‐center study, Napoleone et al35 reported similar midterm morbidity and mortality outcomes for the Fontan procedure performed before and after 7 years of age. Wallace et al27 reviewed the Society of Thoracic Surgeon database (2747 patients) and concluded that although age at Fontan was not, a weight for age z score <−2 was associated with higher in‐hospital mortality, Fontan failure, and longer length of stay.
Of the other factors associated with worse neurocognitive outcomes, sepsis has been previously reported as an independent predictor of worse neurocognitive outcomes in 502 patients undergoing neonatal open heart surgery and followed by the Western Canadian Complex Pediatric Therapies Follow‐up Program.24 In our center, ongoing quality improvement measures are aimed at decreasing the risk of central line and sternal infection and prolonged open sternum periods, as well as promoting early postoperative extubation and earlier discharge from the intensive care unit.36
In our study, the average results for VMI and adaptive functional ability as reported by the patients’ parents (GAC) were also within 1 SD below the mean. This is again in keeping with other series.7, 10, 11 However, 25% of parents reported significant adaptive deficits on the GAC (score <70), placing these children at risk for difficulties within early childhood educational settings. Such a high risk of delayed functional abilities has been previously identified by our group.22, 37, 38 The extent of these overall cognitive and functional deficits has also been identified in school‐aged patients with HLHS.12, 13 Bergemann and colleagues12 reported on the neuropsychological profile of 40 patients with HLHS. They identified deficits in specific cognitive domains such as memory, executive functions, and processing speed. Overall, such deficits in functional abilities and the children's inability to independently complete different tasks of daily living can represent a significant impediment to the development of essential attributes such as autonomy, self‐care, and social integration.
Limitations
The results of this study must be interpreted in the context of the nonrandomized study design and the single surgical center origin of the data. Although there was no loss to follow‐up, the inception cohort size limited the statistical power of the study. The study included a large number of variables; however, in this observational study, we cannot rule out other unmeasured confounders that account for the findings. Specifically, certain characteristics such as right ventricular function, tricuspid valve regurgitation, pulmonary artery growth and vascular resistance, and cardiac symptoms and oxygen saturation during follow‐up are some of the potentially confounding variables that may have played a role in the timing of the Fontan procedure and that are not directly measured in our data set. However, the overall number of hospitalizations (cardiac and non‐cardiac) and medications (cardiac and pulmonary), as well as the number of patients undergoing concomitant surgery such as atrioventricular valve repair and pulmonary artery plasty at the time of Fontan, were not independently associated with the cognitive outcomes post‐Fontan. It is also possible that certain changes in surgical and medical practice over the years may not have been accounted for. Nevertheless, age at Fontan, the finding of most interest, did not significantly vary over the years. Finally, neuroimaging was not routinely performed in all patients in our study; hence, its correlation with the outcomes could not be systematically evaluated.
Conclusions
Patients with HLHS are at risk for significant neurocognitive and adaptive functional deficits. Measures to avoid risk of sepsis and hypoxia may have a favorable impact on their outcomes. The association between the age at Fontan and neurocognitive outcomes is a new finding. Published studies have provided contrasting evidence on the optimal age for Fontan completion based on hemodynamic outcomes. The findings in this study suggest that younger age at Fontan results in better neurocognitive outcomes when assessed at 48 to 72 months of age. These results need to be confirmed in larger patient populations, as the impact on clinical practice is considerable. A larger series may also help identify the optimal age range for Fontan completion. Additional studies will also need to explore the pathophysiology of such an association, presumed multifactorial, and its application to other single ventricle patients undergoing the Fontan procedure.
Sources of Funding
Financial support was initially provided by the Glenrose Rehabilitation Hospital Research Trust Fund, with operational funding from The Registry and Follow‐up of Complex Pediatric Therapies Project, Alberta Health and Wellness.
Disclosures
None.
Supporting information
Appendix S1. Western Canadian Complex Pediatric Therapies Follow‐up Program participating members.
Table S1. Univariate Logistic Regression Analysis for Risk Factors Associated With Postoperative Mortality After the Norwood Procedure (N=117): OR and 95% CI
Table S2. Univariate Linear Regression Analysis for Factors Associated With FSIQ, PIQ, VIQ, and VMI at 48 to 72 months of Age After Surgical Palliation for Classic HLHS (N=68): Linear Regression Coefficient (β) and 95% CI
Table S3. Demographic, All 3 Perioperative and Overall Variables for All Patients With Classic HLHS Who Underwent Neurocognitive Assessment Post‐Fontan Procedure (N=68) (Data are Presented as Median [Interquartile Range] and Number [Percentage])
Acknowledgments
We would like to thank the families of these children for their active participation in the developmental sites across Western Canada and their commitment to this project. We sincerely thank the research coordinators and psychologists who made this research possible.
(J Am Heart Assoc. 2020;9:e013632 DOI: 10.1161/JAHA.119.013632.)
References
- 1. Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar‐Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW; Pediatric Heart Network I . Comparison of shunt types in the Norwood procedure for single‐ventricle lesions. N Engl J Med. 2010;362:1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen P; Pediatric Heart Network I . Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meza JM, Hickey EJ, Blackstone EH, Jaquiss RD, Anderson BR, Williams WG, Cai S, Van Arsdell GS, Karamlou T, McCrindle BW. The optimal timing of stage 2 palliation for hypoplastic left heart syndrome: an analysis of the pediatric heart network single ventricle reconstruction trial public data set. Circulation. 2017;136:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck‐Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, Chen S, Cnota J, Dunbar‐Masterson C, Ghanayem NS, Goldberg CS, Jacobs JP, Lewis AB, Mital S, Pizarro C, Eckhauser A, Stark P Ohye RG; Pediatric Heart Network I . Transplant‐free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryerson LM, Mackie AS, Atallah J, Joffe AR, Rebeyka IM, Ross DB, Adatia I. Prophylactic peritoneal dialysis catheter does not decrease time to achieve a negative fluid balance after the Norwood procedure: a randomized controlled trial. J Thorac Cardiovasc Surg. 2015;149:222–228. [DOI] [PubMed] [Google Scholar]
- 6. Martin BJ, Ross DB, Aklabi MA, Harder J, Dyck JD, Rebeyka IM. Post‐operative outcomes in children undergoing fontan palliation in a regionalized surgical system. Pediatr Cardiol. 2017;38:1654–1662. [DOI] [PubMed] [Google Scholar]
- 7. Gaynor JW, Ittenbach RF, Gerdes M, Bernbaum J, Clancy RR, McDonald‐McGinn DM, Zackai EH, Wernovsky G, Nicolson SC, Spray TL. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1276–1282; discussion 1282–1283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rotermann I, Logoteta J, Falta J, Wegner P, Jung O, Dutschke P, Scheewe J, Kramer HH, Hansen JH. Neuro‐developmental outcome in single‐ventricle patients: is the Norwood procedure a risk factor? Eur J Cardiothorac Surg. 2017;52:558–564. [DOI] [PubMed] [Google Scholar]
- 9. Knirsch W, Liamlahi R, Dave H, Kretschmar O, Latal B. Neurodevelopmental outcome of children with hypoplastic left heart syndrome at one and four years of age comparing hybrid and norwood procedure. Ann Thorac Cardiovasc Surg. 2016;22:375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brosig C, Mussatto K, Hoffman G, Hoffmann RG, Dasgupta M, Tweddell J, Ghanayem N. Neurodevelopmental outcomes for children with hypoplastic left heart syndrome at the age of 5 years. Pediatr Cardiol. 2013;34:1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarajuuri A, Jokinen E, Mildh L, Tujulin AM, Mattila I, Valanne L, Lonnqvist T. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics. 2012;130:e1636–e1646. [DOI] [PubMed] [Google Scholar]
- 12. Bergemann A, Hansen JH, Rotermann I, Voges I, Scheewe J, Otto‐Morris C, Geiger F, Kramer HH. Neuropsychological performance of school‐aged children after staged surgical palliation of hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2015;47:803–811. [DOI] [PubMed] [Google Scholar]
- 13. Oberhuber RD, Huemer S, Mair R, Sames‐Dolzer E, Kreuzer M, Tulzer G. Cognitive development of school‐age hypoplastic left heart syndrome survivors: a single center study. Pediatr Cardiol. 2017;38:1089–1096. [DOI] [PubMed] [Google Scholar]
- 14. Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, Dunbar‐Masterson C, Bernson D, DeMaso DR, Wypij D, Newburger JW. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the fontan procedure. J Am Heart Assoc. 2015;4:e002302 DOI: 10.1161/JAHA.115.002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Atallah J, Dinu IA, Joffe AR, Robertson CM, Sauve RS, Dyck JD, Ross DB, Rebeyka IM; tWestern Canadian Complex Pediatric Therapies Follow‐Up G . Two‐year survival and mental and psychomotor outcomes after the Norwood procedure: an analysis of the modified Blalock‐Taussig shunt and right ventricle‐to‐pulmonary artery shunt surgical eras. Circulation. 2008;118:1410–1418. [DOI] [PubMed] [Google Scholar]
- 16. Robertson CM, Sauve RS, Joffe AR, Alton GY, Moddemann DM, Blakley PM, Synnes AR, Dinu IA, Harder JR, Soni R, Bodani JP, Kakadekar AP, Dyck JD, Human DG, Ross DB, Rebeyka IM. The registry and follow‐up of complex pediatric therapies program of Western Canada: a mechanism for service, audit, and research after life‐saving therapies for young children. Cardiol Res Pract. 2011;2011:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robertson CM, Joffe AR, Sauve RS, Rebeyka IM, Phillipos EZ, Dyck JD, Harder JR; Western Canadian Complex Pediatric Therapies Project Follow‐Up G . Outcomes from an interprovincial program of newborn open heart surgery. J Pediatr. 2004;144:86–92. [DOI] [PubMed] [Google Scholar]
- 18. Blishen BR, Carroll WK, Moore C. The 1981 socioeconomic index for occupations in Canada. Can Rev Soc Anthropl. 1987;24:465–488. [Google Scholar]
- 19. Creighton DE, Robertson CM, Sauve RS, Moddemann DM, Alton GY, Nettel‐Aguirre A, Ross DB, Rebeyka IM; Western Canadian Complex Pediatric Therapies Follow‐up G . Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120:e478–e486. [DOI] [PubMed] [Google Scholar]
- 20. Beery K, Buktenica N, Beery N. Beery‐Buktenica Developmental Test of Visual‐Motor Integration. 5th ed Minneapolis, MN: NCS Pearson Inc; 2004. [Google Scholar]
- 21. Wechsler D. Manual for the Preschool and Primary Scale of Intelligence. 3rd ed San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- 22. Ricci MF, Martin BJ, Joffe AR, Dinu IA, Alton GY, Guerra GG, Robertson CM; Western Canadian Complex Pediatric Therapies Follow‐Up P . Deterioration of functional abilities in children surviving the Fontan operation. Cardiol Young. 2018;28:868–875. [DOI] [PubMed] [Google Scholar]
- 23. Harrison P, Oakland T. Manual of the Adaptive Behaviour Assessment System II. San Antonio, Texas: Harcourt Assessment Inc; 2003. [Google Scholar]
- 24. Sidhu N, Joffe AR, Doughty P, Vatanpour S, Dinu I, Alton G, Acton B, Robertson CM; Western Canadian Complex Pediatric Therapies Follow‐up P . Sepsis after cardiac surgery early in infancy and adverse 4.5‐year neurocognitive outcomes. J Am Heart Assoc. 2015;4:e001954 DOI: 10.1161/JAHA.115.001954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 26. Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW, Wessel DL. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low‐flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. [DOI] [PubMed] [Google Scholar]
- 27. Wallace MC, Jaggers J, Li JS, Jacobs ML, Jacobs JP, Benjamin DK, O'Brien SM, Peterson ED, Smith PB, Pasquali SK. Center variation in patient age and weight at Fontan operation and impact on postoperative outcomes. Ann Thorac Surg. 2011;91:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. d'Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, Bullock A, Justo RN, Grigg LE, Sholler GF, Hope S, Radford DJ, Gentles TL, Celermajer DS, Winlaw DS. Redefining expectations of long‐term survival after the Fontan procedure: twenty‐five years of follow‐up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–S38. [DOI] [PubMed] [Google Scholar]
- 29. Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, Blaufox AD; Pediatric Heart Network I . Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross‐sectional study. J Am Coll Cardiol. 2010;56:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman GM, Brosig CL, Mussatto KA, Tweddell JS, Ghanayem NS. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2013;146:1153–1164. [DOI] [PubMed] [Google Scholar]
- 32. Hansen JH, Rotermann I, Logoteta J, Jung O, Dutschke P, Scheewe J, Kramer HH. Neurodevelopmental outcome in hypoplastic left heart syndrome: impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J Thorac Cardiovasc Surg. 2016;151:1358–1366. [DOI] [PubMed] [Google Scholar]
- 33. Knirsch W, Mayer KN, Scheer I, Tuura R, Schranz D, Hahn A, Wetterling K, Beck I, Latal B, Reich B. Structural cerebral abnormalities and neurodevelopmental status in single ventricle congenital heart disease before Fontan procedure. Eur J Cardiothorac Surg. 2017;51:740–746. [DOI] [PubMed] [Google Scholar]
- 34. Shiraishi S, Yagihara T, Kagisaki K, Hagino I, Ohuchi H, Kobayashi J, Kitamura S. Impact of age at Fontan completion on postoperative hemodynamics and long‐term aerobic exercise capacity in patients with dominant left ventricle. Ann Thorac Surg. 2009;87:555–560; discussion 560–1. [DOI] [PubMed] [Google Scholar]
- 35. Pace Napoleone C, Oppido G, Angeli E, Giardini A, Resciniti E, Gargiulo G. Results of the modified Fontan procedure are not related to age at operation. Eur J Cardiothorac Surg. 2010;37:645–650. [DOI] [PubMed] [Google Scholar]
- 36. Mutsuga M, Quinonez LG, Mackie AS, Norris CM, Marchak BE, Rutledge JM, Rebeyka IM, Ross DB. Fast‐track extubation after modified Fontan procedure. J Thorac Cardiovasc Surg. 2012;144:547–552. [DOI] [PubMed] [Google Scholar]
- 37. Alton GY, Rempel GR, Robertson CM, Newburn‐Cook CV, Norris CM. Functional outcomes after neonatal open cardiac surgery: comparison of survivors of the Norwood staged procedure and the arterial switch operation. Cardiol Young. 2010;20:668–675. [DOI] [PubMed] [Google Scholar]
- 38. Alton GY, Taghados S, Joffe AR, Robertson CM, Dinu I; Western Canadian Pediatric Therapies Follow‐Up G . Prediction of preschool functional abilities after early complex cardiac surgery. Cardiol Young. 2015;25:655–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Western Canadian Complex Pediatric Therapies Follow‐up Program participating members.
Table S1. Univariate Logistic Regression Analysis for Risk Factors Associated With Postoperative Mortality After the Norwood Procedure (N=117): OR and 95% CI
Table S2. Univariate Linear Regression Analysis for Factors Associated With FSIQ, PIQ, VIQ, and VMI at 48 to 72 months of Age After Surgical Palliation for Classic HLHS (N=68): Linear Regression Coefficient (β) and 95% CI
Table S3. Demographic, All 3 Perioperative and Overall Variables for All Patients With Classic HLHS Who Underwent Neurocognitive Assessment Post‐Fontan Procedure (N=68) (Data are Presented as Median [Interquartile Range] and Number [Percentage])


