Abstract
Background
Arterial closure devices reduce the length of bedrest after invasive cardiac procedures via the femoral approach, but there are conflicting data on their association with major bleeding and vascular complications. We thus sought to evaluate the contemporary use of femoral arterial closure devices and their association with major bleeding among patients undergoing percutaneous coronary intervention.
Methods and Results
We identified patients undergoing percutaneous intervention via the femoral approach within the Veterans Affairs Healthcare System from December 2004 through September 2018. The association between arterial closure device use and major bleeding was evaluated using both propensity matching and instrumental variable analyses, incorporating contrast‐induced nephropathy as a falsification end point. We identified 132 373 percutaneous coronary interventions performed by 681 operators, with closure device use increasing 1.2% each year (linear trend P<0.001). In a propensity‐matched cohort, closure devices were associated with a 1.1% reduction in periprocedural bleeding (95% CI, −1.5% to −0.6%). Closure devices were also associated with a numerical decrease in contrast‐inducted nephropathy that did not reach statistical significance (−0.6%; 95% CI, −1.3% to 0.1%). In an instrumental variable analysis of closure device use, there was no difference in the bleeding rate between those who received a closure device and those who did not (0.2%; 95% CI, −0.9% to 1.2%).
Conclusions
Arterial closure devices are associated with a reduction in major bleeding within a propensity‐matched cohort. This association dissipates in an instrumental variable analysis, highlighting some of the methodologic limitations of comparative effectiveness research in observational analyses.
Keywords: arterial closure devices, falsification end point, percutaneous coronary intervention, residual confounding
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Health Services, Quality and Outcomes
Clinical Perspective
What Is New?
Arterial closure devices reduce bedrest after cardiac procedures, but there are conflicting data on their association with major bleeding.
A propensity‐matched cohort demonstrated a reduction in major bleeding among patients who received a closure device, but also a numerical decrease in the falsification end point of contrast‐induced nephropathy.
An instrumental variable analysis demonstrated no difference in major bleeding.
What Are the Clinical Implications?
These findings have implications for the use of closure devices, as well as the performance and interpretation of comparative effectiveness research in observational data sets.
Introduction
Vascular complications associated with catheterization have been shown to lead to significant morbidity and mortality.1, 2, 3, 4 Compared with the femoral approach, radial arterial access has been suggested as a safer alternative to reduce the risk of adverse events with percutaneous procedures.5, 6 However, radial access is not consistently feasible for several peripheral and structural interventions. Furthermore, many operators continue to use the femoral approach because of increased familiarity with the procedure. Prior research has demonstrated that these considerations contribute to the persistent overwhelming use of the femoral approach for invasive coronary procedures in the United States.7
Arterial closure devices (ACDs) are one strategy that has been proposed to decrease vascular complications after femoral catheterization.8 Prior research has suggested that the use of these devices is associated with a reduction in major bleeding, although the data on clinical outcomes have been inconsistent because of methodological limitations.9, 10, 11 More specifically, comparative effectiveness analyses of closure devices compared with manual compression for arterial hemostasis are often plagued by residual confounding.8 The specifics of vascular anatomical characteristics at the puncture site (eg, the preponderance of atherosclerotic plaque) are rarely available, making it challenging to incorporate characteristics associated with bleeding complications into risk adjustment models. Because of this, there are no professional society guidelines available for the use of closure devices, and conventional practices have been drawn from consensus opinion. Furthermore, there has not been a contemporary analysis of closure device use in a large cohort of patients to assess the present state of use and efficacy with the advent and increased use of transradial techniques.
Accordingly, we leveraged clinical and procedural data from the Veterans Affairs (VA) Clinical Assessment, Reporting, and Tracking Program to define the temporal trends and clinical outcomes associated with closure device use among patients undergoing percutaneous coronary intervention within the largest integrated healthcare system in the United States, the VA Healthcare System.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request, although will be subject to the stringent data privacy rules of the VA Healthcare System and the US government.
Population
The VA Clinical Assessment, Reporting, and Tracking Program is a national quality and safety oversight organization for invasive cardiac procedures performed by cardiologists throughout the VA Healthcare System. As described previously, this program captures and compiles standardized patient and procedural data elements for all coronary procedures performed in VA cardiac catheterization laboratories.12 The data elements surveyed are derived from previously established data definitions from the National Cardiovascular Data Registry.13 The present analysis includes patients who underwent percutaneous coronary intervention through a single femoral access site within this healthcare system between December 1, 2004, and September 30, 2018. Patients who underwent emergent procedures or those who required mechanical support (intra‐aortic balloon pump/Impella) and thus larger‐bore access were excluded from the analysis. In addition, interventions performed by an operator who had performed <20 cases were excluded. This cohort was used to define temporal trends and variability in closure device use. The analytic cohort was further refined for the assessment of clinical outcomes. For patients who underwent multiple interventions during the study period, one procedure for each patient was randomly chosen for inclusion in the analysis. Patients were excluded for multiple access points during a single procedure, and for missing key data points, such as complete periprocedural (before and after) hemoglobin levels. This study was approved by the Colorado Multiple Institutional Review Board, which includes the Rocky Mountain Regional VA Medical Center, with a waiver of informed consent.
Definitions
The mechanism of femoral artery hemostasis was derived from clinical documentation from the operator performing the procedure. Documentation of a suture, collagen plug, or clip to close the femoral artery was defined as cases that used a closure device. Alternatively, documentation of manual hemostasis to close the femoral artery was defined as cases that did not use a closure device. Cases using other closure methods or those that did not have a closure method documented were necessarily excluded.
Measurements and Outcomes
Patient and procedural information was derived from the electronic medical record and cardiac catheterization report documentation. The primary outcome was the occurrence of bleeding, defined as a decline in postprocedure hemoglobin by at least 3 g/dL compared with preprocedure values or the administration of a blood transfusion within 2 days of the coronary intervention. These values approximate Bleeding Academic Research Consortium 3a criteria.14 The occurrence of contrast‐induced nephropathy was also assessed as a secondary falsification end point. This was defined as an absolute increase of serum creatinine of ≥0.3 mg/dL or a relative increase of 50% in serum creatinine within 72 hours of coronary intervention.
Statistical Analysis
The temporal trends and variation in the use of closure devices across facilities and operators were performed using standard descriptive statistics. Baseline patient and procedural characteristics were compared among patients who received a closure device and those who received manual hemostasis. Continuous variables were presented as means and SDs, whereas categorical variables are reported as counts and percentages. Standardized differences were provided for comparisons independent of sample size.
Propensity score
A propensity score was estimated on the basis of the conditional probability that a closure device would be used, including covariates accounting for patient characteristics (age/sex/body mass index), medical comorbidities (cerebrovascular disease/chronic kidney disease/chronic obstructive lung disease/hypertension/hyperlipidemia/prior percutaneous intervention), medical presentation (acute coronary syndromes), and facility characteristics (training facility) as well as the time of the procedure. Additional covariates for concomitant medication use (IIb/IIIa) were also included. Patients were matched using a caliper width of 0.05 SDs of the logit of the propensity score, and preferentially matched to others within their same facility. Standardized differences <10% were considered to indicate adequate balance across groups.15 Finally, a model was constructed using our matched cohort to estimate the adjusted risk difference for the effect of a closure device on bleeding. All analyses were performed using R, version 3.4.1, with matching preformed with the Matching package.
Instrumental variable
As the decision to use a closure device has the potential to be influenced by unmeasured confounding, an instrumental variable approach was chosen as an alternative method to estimate the effectiveness of closure device use. The instrumental variable used in this analysis was the variation in closure device use by operator, quantified as the proportion of cases in which a closure device was used in the prior 20 interventions. This preference‐based instrumental variable measures the operator proclivity for using a closure device.16 To further strengthen the instrumental variable, a binary instrument was defined as being performed by a high‐ versus low‐ACD use preferring operator. Specifically, the intervention was identified as being done by a high‐ACD use operator if the ratio of percutaneous coronary interventions with ACD use in the prior 20 percutaneous coronary interventions for the operator was in the top quartile of proportion of ACD use and by a low‐ACD use operator if the ratio was in the bottom quartile.
The 2‐stage least squares linear regression method was used to perform the instrumental variable analysis to estimate the effect of closure devices on our primary outcome and falsification end point. This method includes 2 sequential linear regression models where in the first‐stage model we regressed ACD status on our instrument while also adjusting for all patient, procedural, and facility characteristics as well as fiscal year quarter. The second‐stage model included the regression of our binary outcome on the predicted value of receiving a closure device as estimated from the first‐stage model with adjustment for listed covariates. Huber‐White cluster robust SEs were estimated for the second‐stage model to account for heteroscedasticity, for sampling error in the first‐stage estimate, and for clustering of patients within site. The coefficient of the predicted value of receiving a closure device in the second‐stage model gave us our estimated effect of interest of the adjusted risk difference in site bleeding with closure device use in the subpopulation of compliers, those who would receive a closure device only if they went to high‐use operators. All analyses were performed using R, version 3.4.1, with the 2‐stage least squares linear regression method executed with the package AER and cluster robust SEs calculated with the package ivpack. P<0.05 was considered statistically significant.
Results
During the time period under investigation, 132 373 coronary interventions were performed by 681 operators. Emergent cases requiring mechanical support (n=1090) or a salvage intervention (n=79) were excluded. Similarly, cases that were not performed via the femoral approach (n=30 892) or used other or unidentified closure methods (n=13 196) were also removed from the base cohort. Finally, cases where an operator was not listed (n=1385) were removed, leaving 85 731 interventions available to analyze temporal trends and site variation. The cohort was further refined for the analysis of clinical outcomes, with 3519 cases excluded for multiple access sites and 24 936 excluded for missing data necessary to assess risk of bleeding or bleeding outcomes, such as body mass index or periprocedural hemoglobin. The final analytic cohort subsequently consisted of 40 718 interventions performed on unique patients by 323 different operators (Figure 1).
Figure 1.
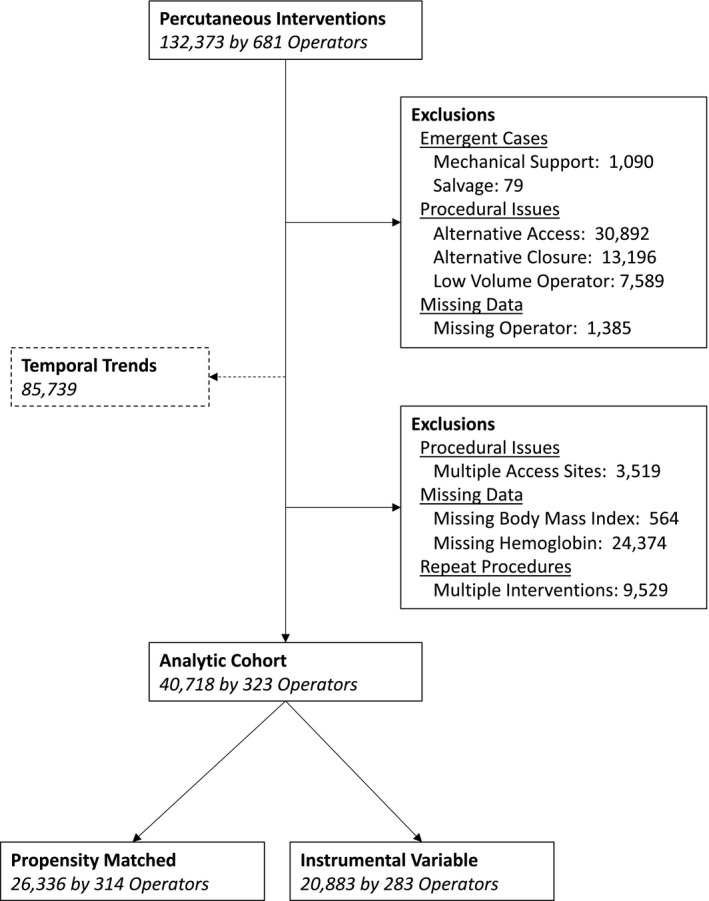
Diagram of cohort construction.
Temporal Trends
The temporal trends in closure device use among patients undergoing coronary intervention via the femoral approach are demonstrated in Figure 2. As shown, the proportion of cases that used a mechanical closure device increased from 60.4% in fiscal year 2006 to 75.3% in fiscal year 2018, consistent with an average increase of 1.2% each year (linear trend P<0.0001).
Figure 2.
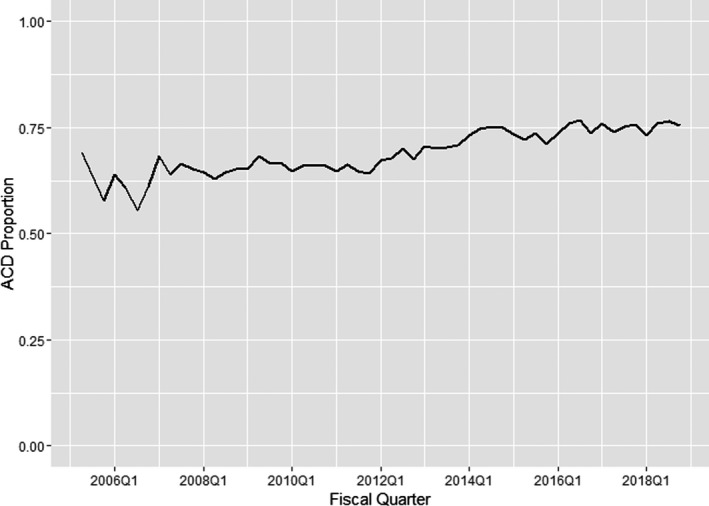
Temporal trends in the use of arterial closure devices (ACDs). The proportion of cases that used a mechanical closure device increased from 60.4% in fiscal year 2006 to 75.3% in fiscal year 2018, consistent with an average increase of 1.2% each year (linear trend P<0.0001). Q indicates quarter.
Operator and Site Variation
There was significant variation in the use of closure devices dependent on both operator and site. The proportion of closure device use for operators ranged from 0% to 100%, with a median of 72.1% (interquartile range, 44.9%–88.8%). Similarly, sites varied in the proportion of closure device use, with a range of 0% to 100% and a median of 73.1% (interquartile range, 52.9%–87.1%).
Clinical Outcomes
The characteristics of the patients in analytic cohort for clinical outcomes are presented in Table 1, stratified by the use of a closure device. Most notably, patients who received a closure device had significantly lower rates of peripheral artery disease (19.8% versus 26.1%; Absolute Standardized Difference [ASD], 19.8) and prior bypass surgery (29.3% versus 34.4%; ASD, 10.8) compared with those who did not. Fewer patients treated with a closure device were also treated with concomitant IIb/IIIa inhibitors (13.1% versus 17.4%; ASD, 12.0).
Table 1.
Patient Characteristics
| Characteristic | Overall (n=40 718) | ACD (n=27 505) | No ACD (n=13 213) | Standardized Difference, % |
|---|---|---|---|---|
| Closure device type | ||||
| Manual | 13 213 (32.5) | … | 13 213 (100.0) | … |
| Collagen plug | 504 (1.2) | 504 (1.8) | … | … |
| Extravascular plug | 2 (0.0) | 2 (0.0) | … | … |
| Seal | 18 489 (45.4) | 18 489 (67.2) | … | … |
| StarClose/clip | 1072 (2.6) | 1072 (3.9) | … | … |
| Suture | 7438 (18.3) | 7438 (27.0) | … | … |
| Demographics | ||||
| Age, mean (SD), y | 66.4 (9.4) | 66.3 (9.4) | 66.7 (9.3) | 5.1 |
| Men | 39 975 (98.2) | 27 025 (98.3) | 12 950 (98.0) | 1.8 |
| White | 34 116 (83.8) | 22 967 (83.5) | 11 149 (84.4) | 2.4 |
| BMI, mean (SD), kg/m2 | 30.2 (5.7) | 30.3 (5.6) | 29.9 (5.8) | 7.2 |
| Tobacco use | 25 694 (63.1) | 17 124 (62.3) | 8570 (64.9) | 5.4 |
| Hypertension | 37 026 (90.9) | 24 868 (90.4) | 12 158 (92.0) | 5.7 |
| Hyperlipidemia | 36 805 (90.4) | 24 871 (90.4) | 11 934 (90.3) | 0.4 |
| Renal failure (GFR <30 ml/min/1.73 m2) | 2066 (5.1) | 1265 (4.6) | 801 (6.1) | 6.5 |
| Chronic kidney disease | 9831 (24.1) | 6409 (23.3) | 3422 (25.9) | 6.0 |
| Cerebrovascular disease | 7811 (19.2) | 5035 (18.3) | 2776 (21.0) | 6.8 |
| Peripheral arterial disease | 9166 (22.5) | 5439 (19.8) | 3727 (28.2) | 19.8 |
| Obstructive lung disease | 9648 (23.7) | 6201 (22.5) | 3447 (26.1) | 8.3 |
| Diabetes mellitus | 20 486 (50.3) | 13 700 (49.8) | 6786 (51.4) | 3.1 |
| Congestive heart failure | 11 755 (28.9) | 7594 (27.6) | 4161 (31.5) | 8.5 |
| Chronic depression | 12 439 (30.5) | 8378 (30.5) | 4061 (30.7) | 0.6 |
| Posttraumatic stress | 6690 (16.4) | 4564 (16.6) | 2126 (16.1) | 1.4 |
| Prior myocardial infarction | 17 496 (43.0) | 11 586 (42.1) | 5910 (44.7) | 5.3 |
| Prior PCI | 18 326 (45.0) | 12 301 (44.7) | 6025 (45.6) | 1.8 |
| Prior CABG | 12 613 (31.0) | 8072 (29.3) | 4541 (34.4) | 10.8 |
| Presentation characteristics | ||||
| Medication IIb/IIIa | 5891 (14.5) | 3596 (13.1) | 2295 (17.4) | 12.0 |
| Outpatient PCI | 217 (0.5) | 149 (0.5) | 68 (0.5) | 0.4 |
| Presentation type | ||||
| STEMI | 2855 (7.0) | 1909 (6.9) | 946 (7.2) | 3.4 |
| NSTEMI | 9786 (24.0) | 6647 (24.2) | 3139 (23.8) | |
| Unstable angina | 9995 (24.5) | 6778 (24.6) | 3217 (24.3) | |
| Stable angina | 11 681 (28.7) | 7927 (28.8) | 3754 (28.4) | |
| Chest pain | 1705 (4.2) | 1135 (4.1) | 570 (4.3) | |
| Othera | 778 (1.9) | 530 (1.9) | 248 (1.9) | |
| Unknown | 3527 (8.7) | 2335 (8.5) | 1192 (9.0) | |
Data are presented as counts (proportions) unless otherwise noted.
ACD indicates arterial closure device; BMI, body mass index; CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Other: presentations for valve diseases, arrhythmia, asymptomatic, cardiomyopathy, heart failure, positive functional study, noncardiac preoperation, pulmonary hypertension, syncope, and transplant evaluation.
Propensity matching
The association of closure device use and bleeding was first assessed using propensity matching. Using this approach, 26 336 (65%) patients were included in the matched analysis, with inclusion of 13 168 patients who did not receive a closure device (99.6% of the manual device cohort). A summary of the unmatched and matched cohorts, stratified by propensity score, is depicted in Figure S1, demonstrating excellent overlap in the matched population. This is consistent with the clinical and procedural characteristics of in the matched cohort, with evidence of excellent balance between those who did or did not receive a closure device (Table S1). The facility characteristics in the propensity‐matched cohort were also similar (Table S2). After binomial regression, the estimated average treatment effect for those who received a closure device suggested a 1.1% reduction in risk (95% CI, −1.5% to −0.6%) in periprocedural bleeding. Closure devices were also associated with a reduction in the point estimate of contrast‐induced nephropathy (−0.6%; 95% CI, −1.3% to 0.1%) that did not reach statistical significance (P=0.10).
Instrumental variable
The instrumental variable used for this analysis was the proportion of 20 prior interventions that an operator performed using a closure device, divided into the upper (high‐use) and lower (low‐use) quartiles, at the time of the intervention of interest (Figure S2). Using this as the instrument, Table S3 summarizes the difference in patient characteristics treated by high‐use and low‐use operators. This constituted a total of 20 883 (51%) patients, removing the patients in the middle quartiles to strengthen the instrumental variable. Once again, the use of a concomitant IIb/IIIa inhibitor was lower among those with a closure device compared with those without (12.9% versus 18.6%; ASD, 15.9). The facility characteristics stratified by the instrument are reproduced in Table S4, demonstrating a lower proportion of academic affiliations (92.9% versus 100.0%; ASD, 39.0) and trainees (82.3% versus 98.1%; ASD, 54.9) at sites with high closure device use operators. The crude bleeding rates of patients treated by these operators are summarized in Table S5, with the rates similar among high‐use operators (3.54 per 100 cases) and low‐use operators (3.58 per 100 cases). After adjustment for patient, procedural, and facility characteristics, there was no difference in bleeding rate using the instrumental variable approach, with an average treatment effect of 0.2% (95% CI, −0.9% to 1.2%). There was also no difference in the falsification end point, contrast‐induced nephropathy, between those who received a closure device and those who did not (0.5%; 95% CI, −1.4% to 2.4%).
The clinical outcomes stratified by analytic method are reproduced in Table 2. As shown, unadjusted regression (P=0.004) and propensity‐matched analyses (P<0.001) suggest that closure devices are associated with a reduction in periprocedural bleeding. However, adjusted regression (P=0.08) and an instrumental variable analysis (P=0.74) designed to address residual confounding did not demonstrate the same relationship.
Table 2.
Risk Difference Estimates for the Association Between Closure Device and Bleeding Using Different Methods
| Model | Adjusted Risk Difference (95% CI) | P Value |
|---|---|---|
| Unadjusted regression | −0.00754 (−0.0127 to −0.00241) | 0.004 |
| Adjusted regression | −0.00473 (−0.0100 to 0.000574) | 0.08 |
| Instrumental variable | 0.00178 (−0.00887 to 0.0124) | 0.74 |
| Propensity score | −0.0106 (−0.0152 to −0.00611) | <0.0001 |
Discussion
The present study evaluated the temporal trends and clinical outcomes associated with the use of ACDs after coronary revascularization. As the data demonstrate, the use of closure devices has significantly increased over the past decade, albeit with significant variation across different medical centers. A propensity‐matched analysis demonstrates that use of these devices is associated with a reduction in major bleeding. However, these devices were also associated with a trend toward a reduction in contrast‐induced nephropathy in the same matched cohort, suggesting the possibility of residual confounding. With this in mind, an instrumental variable analysis was performed that demonstrated similar major bleeding risk between patients who did and did not receive closure devices. These data have implications both for the use of closure devices during invasive cardiac procedures, but also for the performance and interpretation of comparative effectiveness research using observational data sets.
The use of femoral ACDs has increased over time with site‐level variation. A survey of international interventional cardiologists has suggested that vascular closure devices are used in >40% of coronary angiograms and interventions performed via the femoral approach.17 This is consistent with data derived from large registries in the United States, where >50% of patients undergoing coronary intervention via the femoral approach received an ACD.8 In the present analysis, we demonstrate that closure device use in the Veterans Health Administration is increasing by >1% each year, such that 75% of coronary interventions performed via femoral approach were closed with a device in the most recent year analyzed. Increased use of closure devices likely reflects the decreased bedrest required once these devices are used, significantly increasing patient comfort. Multiple prior analyses have also demonstrated that these devices are cost‐effective as they facilitate earlier patient ambulation and potential discharge.18, 19 Our data do demonstrate significant operator and site‐level variation in closure device use, perhaps related to the experience each site has had with these products. A further evaluation of the clinical risks associated with closure device use is thus warranted.
The association between ACDs and clinical outcomes has been inconsistent, largely because of methodologic limitations in observational analyses. Previous research demonstrated that the use of closure devices may be associated with a reduction in vascular complications when compared with manual compression.20, 21 Further studies suggested that ACDs may be associated with a reduction in short‐term mortality among propensity‐matched patients.10 The positive association between closure devices and clinical outcomes was also demonstrated in the present propensity‐matched analysis, which suggested a modest 1.1% reduction in the risk of major bleeding with the use of a closure device. However, our propensity analysis also demonstrated that closure devices may be associated with a reduction in the point estimate for contrast‐induced nephropathy. The concomitant reduction in this falsification end point suggests potential differences in the underlying patient population that could not be accounted for during propensity matching, and it is consistent with the known variation in the use of closure devices on the basis of poorly captured patient characteristics. Conversely, when analyzed with an instrumental variable approach, similar bleeding rates were demonstrated between those who received a closure device and those who did not. Instrumental variable analyses are specifically designed with the intent of isolating a treatment effect, independent of unmeasured variation. An instrumental variable (operator preference for use of closure devices in the present analysis) is associated with the exposure (closure device use) but not with the outcome (bleeding), except through its association with the exposure. Thus, by using a method to compare cases where an operator chooses whether to use a closure device independent of patient factors, one can minimize the impact of unmeasured patient factors that may influence the outcome. Interestingly, this is consistent with the results from a large randomized clinical trial demonstrating that vascular closure devices were noninferior to manual compression among patients undergoing coronary angiography.22
The present analyses demonstrate the potential limitations of comparative effectiveness analyses using observational data sets. A strong association between closure devices and major bleeding was established using unadjusted regression and propensity‐matched methods, with a nonsignificant trend also demonstrated with adjusted regression. Each of these methods has been used to evaluate this association previously, often with positive findings.10, 20, 21 Some have questioned the use of these methods because of the significant risk of residual confounding by factors that could not be identified or incorporated into the regression or propensity models.23 These unknown unknowns can lead to erroneous conclusions about the association between a predictor and an outcome, potentially influencing clinical practice in a wide range of disciplines. A large observational study comparing percutaneous and surgical revascularization using a propensity‐matched cohort, for example, demonstrated superior outcomes with surgery.24 The authors recognized the potential role of unknown confounders, however, and constructed a sensitivity analysis demonstrating that the results would be questioned if 20% of the cohort had an unmeasured characteristic that increased the risk of percutaneous revascularization by >3‐fold. A confounder with these characteristics, surgical ineligibility, was later identified in these proportions with this effect size in another data set.25 In the present analysis, propensity methods suggested an association between closure device use and reductions in bleeding. However, a similar association between closure device use and the falsification end point of contrast nephropathy raises concern that residual confounding is present, wherein patients not receiving closure devices may be selected against because of factors that may associate with increased bleeding, like peripheral vascular disease. Alternative analytic methods can be used to address the residual confounding that plagues observational analyses, including falsification end points and instrumental variable analyses. These analytic tools were valuable in identifying potential residual confounding among patients receiving closure devices and should be considered when performing other comparative effectiveness analyses using observational data sets in the future.
Limitations
The present project should be interpreted in the context of several limitations. Similar to prior studies, the current analysis is derived from data reported by the clinicians providing clinical care. Incomplete or incorrect data entry is possible, although steps have been taken to validate and adjudicate the recorded information. As referenced, unmeasured confounding can be present in observational analyses. We have used multiple different analytic methods to assess for residual confounding when formulating our conclusions. Heterogeneous results produced by these methods could reflect residual confounding, but may also be valid representations of the effect of a therapy in different subpopulations as the instrumental variable cohort was distinct from the cohort used for propensity matching. In addition, clinical outcomes were derived from laboratory data or the administration of blood products. The data set does not allow us to easily discriminate between access or nonaccess site bleeding, such that it is possible that some episodes of major bleeding were unrelated to the use of closure devices. Alternative end points, such as limb ischemia or arterial dissection, were not frequently reported and thus could not be included in the analysis. Use of a closure device was assumed to be successful, and it is possible that some bleeding events were related to unsuccessful deployment or device failure in the hands of inexperienced operators and not attributable to appropriate device use. Furthermore, we analyzed all closure devices as a group, and it is likely that some closure devices have greater efficacy than others.26 An analysis of the differences in efficacy was not entertained because of the concerns about residual confounding that were highlighted in the primary analysis. Finally, the VA patient population is unique and may not be representative of a broader national population that includes a higher proportion of women and minorities. A contemporary randomized trial could obviate some of these limitations.
Conclusions
In conclusion, the use of ACDs has increased over time, with significant site variation. ACDs are associated with a reduction in major bleeding within a propensity‐matched cohort, which dissipates in an instrumental variable analysis. These data highlight some of the methodologic limitations of comparative effectiveness research in observational analyses.
Disclosures
E.J. Armstrong is a consultant to Abbott Vascular, Boston Scientific, Cardiovascular Systems, Janssen, Medtronic, and Philips. Dr Waldo receives unrelated investigator‐initiated research support to the Denver Research Institute from Abiomed, Cardiovascular Systems Incorporated, and Merck Pharmaceuticals. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline clinical and procedural characteristics stratified by closure device in a propensity matched cohort.
Table S2. Facility characteristics by closure device use in the propensity matched cohort.
Table S3. Baseline clinical and procedural characteristics by high and low use of closure devices.
Table S4. Facility characteristics by high and low closure device utilization.
Table S5. Crude bleeding events stratified by instrumental variable.
Figure S1. Histogram of unmatched (A) and matched (B) propensity scores.
Figure S2. Histogram of proportion of providers previous twenty cases that utilized a closure device.
Acknowledgments
This material is the result of work supported with resources and use of facilities at the Rocky Mountain Regional VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
(J Am Heart Assoc. 2020;9:e015223 DOI: 10.1161/JAHA.119.015223.)
References
- 1. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, Ohman EM, Stone GW. Impact of major bleeding on 30‐day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. [DOI] [PubMed] [Google Scholar]
- 2. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 3. Doyle BJ, Rihal CS, Gastineau DA, Holmes DR. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. 2009;53:2019–2027. [DOI] [PubMed] [Google Scholar]
- 4. Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67:541–545. [DOI] [PubMed] [Google Scholar]
- 5. Bertrand OF, Bélisle P, Joyal D, Costerousse O, Rao SV, Jolly SS, Meerkin D, Joseph L. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta‐analysis. Am Heart J. 2012;163:632–648. [DOI] [PubMed] [Google Scholar]
- 6. Agostoni P, Biondi‐Zoccai GGL, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta‐analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. [DOI] [PubMed] [Google Scholar]
- 7. Waldo SW, Gokhale M, O'Donnell CI, Plomondon ME, Valle JA, Armstrong EJ, Schofield R, Fihn SD, Maddox TM. Temporal trends in coronary angiography and percutaneous coronary intervention: insights from the VA clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. 2018;11:879–888. [DOI] [PubMed] [Google Scholar]
- 8. Wimmer NJ, Secemsky EA, Mauri L, Roe MT, Saha‐Chaudhuri P, Dai D, McCabe JM, Resnic FS, Gurm HS, Yeh RW. Effectiveness of arterial closure devices for preventing complications with percutaneous coronary intervention: an instrumental variable analysis. Circ Cardiovasc Interv. 2016;9:e003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Applegate RJ, Sacrinty MT, Kutcher MA, Kahl FR, Gandhi SK, Santos RM, Little WC. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1:317–326. [DOI] [PubMed] [Google Scholar]
- 10. Farooq V, Goedhart D, Ludman P, de Belder MA, Harcombe A, El‐Omar M; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Relationship between femoral vascular closure devices and short‐term mortality from 271 845 percutaneous coronary intervention procedures performed in the United Kingdom between 2006 and 2011: a propensity score‐corrected analysis from the British cardiovascular intervention society. Circ Cardiovasc Interv 2016;9:e003560. [DOI] [PubMed] [Google Scholar]
- 11. Koreny M, Riedmüller E, Nikfardjam M, Siostrzonek P, Müllner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta‐analysis. JAMA. 2004;291:350–357. [DOI] [PubMed] [Google Scholar]
- 12. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, Rumsfeld JS. A national clinical quality program for veterans affairs catheterization laboratories (from the veterans affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. [DOI] [PubMed] [Google Scholar]
- 13. Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. [DOI] [PubMed] [Google Scholar]
- 14. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 15. Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat. 2009;38:1228–1234. [Google Scholar]
- 16. Brookhart MA, Schneeweiss S. Preference‐based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat. 2007;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damluji AA, Nelson DW, Valgimigli M, Windecker S, Byrne RA, Cohen F, Patel T, Brilakis ES, Banerjee S, Mayol J, Cantor WJ, Alfonso CE, Rao SV, Moscucci M, Cohen MG. Transfemoral approach for coronary angiography and intervention: a collaboration of international cardiovascular societies. JACC Cardiovasc Interv. 2017;10:2269–2279. [DOI] [PubMed] [Google Scholar]
- 18. Resnic FS, Arora N, Matheny M, Reynolds MR. A cost‐minimization analysis of the angio‐seal vascular closure device following percutaneous coronary intervention. Am J Cardiol. 2007;99:766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerré S, Kustermans L, Vandendriessche T, Bosmans J, Haine SE, Miljoen H, Vrints CJ, Beutels P, Wouters K, Claeys MJ. Cost‐effectiveness of contemporary vascular closure devices for the prevention of vascular complications after percutaneous coronary interventions in an all‐comers PCI population. EuroIntervention. 2014;10:191–197. [DOI] [PubMed] [Google Scholar]
- 20. Resnic FS, Blake GJ, Ohno‐Machado L, Selwyn AP, Popma JJ, Rogers C. Vascular closure devices and the risk of vascular complications after percutaneous coronary intervention in patients receiving glycoprotein IIb‐IIIa inhibitors. Am J Cardiol. 2001;88:493–496. [DOI] [PubMed] [Google Scholar]
- 21. Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153:606–611. [DOI] [PubMed] [Google Scholar]
- 22. Schulz‐Schüpke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, Hoppe K, Böttiger C, Groha P, Bradaric C, Schmidt R, Bott‐Flügel L, Ott I, Goedel J, Byrne RA, Schneider S, Burgdorf C, Morath T, Kufner S, Joner M, Cassese S, Hoppmann P, Hengstenberg C, Pache J, Fusaro M, Massberg S, Mehilli J, Schunkert H, Laugwitz K‐L, Kastrati A; Instrumental Sealing of Arterial Puncture Site—CLOSURE Device vs Manual Compression (ISAR‐CLOSURE) Trial Investigators. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR‐CLOSURE randomized clinical trial. JAMA. 2014;312:1981–1987. [DOI] [PubMed] [Google Scholar]
- 23. Secemsky EA, Wimmer NJ, Yeh RW. Letter by Secemsky et al. regarding article, “relationship between femoral vascular closure devices and short‐term mortality from 271 845 percutaneous coronary intervention procedures performed in the United Kingdom between 2006 and 2011: a propensity score‐corrected analysis from the British cardiovascular intervention society.” Circ Cardiovasc Interv 2016;9. [DOI] [PubMed] [Google Scholar]
- 24. Weintraub WS, Grau‐Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waldo SW, Secemsky EA, O'Brien C, Kennedy KF, Pomerantsev E, Sundt TM, McNulty EJ, Scirica BM, Yeh RW. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014;130:2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Resnic FS, Majithia A, Marinac‐Dabic D, Robbins S, Ssemaganda H, Hewitt K, Ponirakis A, Loyo‐Berrios N, Moussa I, Drozda J, Normand S‐L, Matheny ME. Registry‐based prospective, active surveillance of medical‐device safety. N Engl J Med. 2017;376:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical and procedural characteristics stratified by closure device in a propensity matched cohort.
Table S2. Facility characteristics by closure device use in the propensity matched cohort.
Table S3. Baseline clinical and procedural characteristics by high and low use of closure devices.
Table S4. Facility characteristics by high and low closure device utilization.
Table S5. Crude bleeding events stratified by instrumental variable.
Figure S1. Histogram of unmatched (A) and matched (B) propensity scores.
Figure S2. Histogram of proportion of providers previous twenty cases that utilized a closure device.


