Abstract
Background
Coexistence of cancer and cardiovascular disease is increasingly frequent, but nationwide data covering cancer patients with myocardial infarction (MI) are scarce. We sought to investigate the prevalence of cancer in patients with first MI, and its impact on cardiovascular and bleeding outcome.
Methods and Results
Using nationwide Swedish quality registries, all patients admitted for first MI between 2001 and 2014 were identified. Data on comorbidity, cancer, and outcome were obtained from the national cancer and patient registries. Stratification was performed according to cancer during the 5 years before MI. Multivariable Cox proportional hazards analyses adjusting for cardiovascular risk factors and invasive treatment assessed the association of cancer with outcome. In total, 175 146 patients with first MI were registered, of whom 9.3% (16 237) had received care for cancer in the 5 years before admission. The cancer rate increased from 6.7% in the years 2001–2002 to 10.7% in 2013–2014, independent of sex and cancer type. The presence of a new cancer diagnosis within 5 years increased from 4.9% to 6.2%. During a median follow‐up of 4.3 years, cancer was associated with all‐cause mortality (hazard ratio, 1.44; 95% CI, 1.40–1.47), recurrent MI (hazard ratio, 1.08; 95% CI, 1.04–1.12), heart failure (hazard ratio, 1.10; 95% CI, 1.06–1.13), and major bleeding (hazard ratio, 1.45; 95% CI, 1.34–1.57). Risk for adverse events varied strongly according to cancer extent, timing, and type.
Conclusions
Cancer as a comorbid disorder is increasing and is strongly associated with mortality, severe bleeding, and adverse cardiovascular outcome after first MI.
Keywords: cancer, cardio‐oncology, myocardial infarction
Subject Categories: Myocardial Infarction
Clinical Perspective
What Is New?
Our nationwide study shows that the prevalence of cancer in patients with first myocardial infarction is increasing.
Furthermore, cancer is associated with long‐term adverse outcome, including mortality, severe bleeding, recurrent myocardial infarction, and heart failure.
What Are the Clinical Implications?
Risk for adverse events varied strongly according to cancer extent, timing, and type and need to be taken into consideration when treating these high‐risk patients.
Due to the enormous heterogeneity of the cancer population, multidisciplinary evaluation with care tailored to the individual patient by both cardiologists and oncologists is needed to reduce risk for cardiovascular and bleeding complications.
Introduction
The increasing cancer incidence and improving treatment regimens for malignancy are resulting in a higher prevalence of cancer as well as more individuals considered cancer survivors after successful treatment.1, 2 As such, the occurrence of cardiovascular disease in patients with current or previous cancer is expected to increase, since more patients with cancer live to develop cardiovascular disease. Additional co‐occurrence of cancer and cardiovascular disease may be explained by shared risk factors and biological mechanisms such as inflammation and oxidative stress.3 Cancer treatment may also directly cause cardiovascular complications such as heart failure, ischemia, and thromboembolism.4 Because of the exclusion of patients with cancer from most cardiovascular trials and a paucity of large unselected observational studies, there are limited data on the prognosis and treatment of these complex patients, with risk of both undertreatment and treatment complications.
The current nationwide registry study sought to investigate temporal trends in the prevalence of cancer in patients admitted with first myocardial infarction (MI) and its impact on outcome.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Population
Patients with acute MI admitted to a cardiac care unit in Sweden between January 1, 2001, and December 31, 2014, were identified using the national quality registry for MI (SWEDEHEART [Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies]). SWEDEHEART includes all consecutive patients and contains data on general demographics, cardiovascular risk factors, pharmacological treatment during hospitalization, in‐hospital complications, and invasive management. The database was linked to the patient registry (containing International Classification of Diseases, Ninth Revision and 10 [ICD‐9 and ‐10] codes for inpatient and outpatient care), providing data on comorbidity before index hospitalization, as well as events after discharge. ICD codes concerning cancer care were also obtained from the patient registry. Time of diagnosis as well as cancer stage were obtained from the national cancer registry. The most recent registration was chosen from the national cancer registry in the case of multiple registrations. Mortality statistics, including cause of death, were obtained from the national cause‐of‐death registry. The information in these registries is registered by the treating physicians. Patients were followed from admission date to either the occurrence of an outcome or until December 31, 2014, using the personal identification number that all Swedish citizens have. Therefore, no patients are lost to follow‐up, except in the case of emigration. In accordance with Swedish law, all patients are informed about their participation in the SWEDEHEART registry and the right to get their data erased from the registry on request. The study complied with the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Uppsala University, Sweden (registration number, dnr 2013/525).
Definitions
Patients were designated into the cancer subpopulation if 1 of 2 criteria were met:
A cancer ICD code was recorded in the patient registry during the 5 years before MI, indicating hospital admission for cancer or outpatient care for cancer.
A diagnosis of cancer was registered in the national cancer registry during the 5 years before MI, indicating the time of cancer diagnosis.
Either one of these criteria sufficed, and a majority of patients met both criteria. The 5‐year time period was decided through clinical judgement.
Cancer was defined according to ICD coding categories (specified in Data S1). Nonmelanoma skin cancer, benign neoplasms, and neoplasms of uncertain or unknown behavior were not included in the cancer group but in the group of patients without cancer.
Baseline characteristics as well as outcome definitions are specified in Data S1. Mortality was considered to be attributable to cardiovascular causes if the main cause of death was registered as a cardiovascular ICD code (category I), and cancer related with the use of a cancer ICD code (category C). MI and hospitalization for heart failure were defined by ICD coding (Data S1). Classification of index MI into subtypes was available from 2010 and forward.5, 6 This classification was also used to evaluate the rate of type 2 MI after discharge for patients admitted from 2010 and forward.
Bleeding was defined according to categories as validated by Friberg and Skeppholm.7 Bleeding was divided into fatal bleeding, nonfatal major bleeding, bleeding requiring hospitalization, and gastrointestinal bleeding (Data S1). Furthermore, a composite end point of fatal bleeding and nonfatal major bleeding was created. Stroke was categorized as ischemic or hemorrhagic, and venous thromboembolism was reported including pulmonary embolism.
Statistical Analysis
For the current analysis, only patients with no prior MI, as evaluated using the SWEDEHEART and patient registry, were selected. This was done to reduce selection bias. Patients with unstable angina were excluded. To identify trends over time, population characteristics were evaluated according to year of admission grouped into 2‐year blocks, with subsequent stratification according to sex. Furthermore, the population was stratified according to cancer in the 5 years before hospitalization. Categorical variables are presented as percentages and compared by chi‐squared test. Continuous variables are presented as median and interquartile range and compared using Kruskall–Wallis test. A P value <0.05 was considered statistically significant. For the trend analyses of cancer types, correction for multiple comparisons was performed according to Bonferroni. Unadjusted survival rates were shown using Kaplan–Meier curves. Cox proportional hazard regression analyses were performed to investigate the association between cancer and outcome, adjusting for age, sex, diabetes mellitus, hypertension, smoking status, previous heart failure, previous percutaneous coronary intervention, previous coronary artery bypass grafting, chronic kidney disease, peripheral arterial disease, indication for admission, previous stroke, year of admission (as a categorical variable), and percutaneous coronary intervention during hospitalization. These variables were selected according to clinical relevance, and all variables were included in the models simultaneously. Cases with missing covariate values (N=3565; 2.0%) were dropped from the multivariable analyses. The proportional hazards assumption was assessed visually for the covariates of the Cox proportional hazards models using Kaplan–Meier curves, with subsequent plotting of the Schoenfeld residuals in those variables possibly violating the proportional hazards assumption. These analyses showed that 1 confounder, type of myocardial infarction, violated the proportional hazard assumption because of a higher initial mortality in patients with ST‐elevation MI compared with patients with non–ST‐elevation MI, but a lower late mortality in patients with ST‐elevation MI. Because the differences between patients with and without cancer were limited with regard to the distribution of the type of MI, no further measures were taken. Echocardiographic left ventricular ejection fraction was considered as a covariate but was not included to the large number of missing values (33.9%), which were likely to be nonrandomly missing in the sickest patients.
Using the same models, multivariable Cox proportional hazards analyses were performed to evaluate the association of prespecified cancer subgroups with outcome, including extent of cancer (according to tumor classification8) and the 5 most common types of cancer. Furthermore, stratification was performed according to timing of cancer diagnosis. The following categories were used: cancer diagnosis in the year before MI, and cancer diagnosis between 1 and 5 years before MI. Finally, the cancer population was stratified according to care for cancer according to the following categories: care for cancer in the year before MI, and care for cancer between 1 and 5 years before MI. Results from the Cox proportional hazards analyses were displayed as hazard ratios with 95% CI. Analyses were performed using IBM SPSS version 24 (SPSS, IBM Corporation, Armonk, NY). The forest plots were created using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results
Population Selection
In total, 805 717 hospitalizations were registered in SWEDEHEART between January 1, 2001, and December 31, 2014. After selection of patients admitted for first MI, 178 621 hospital admissions remained. The first hospital admission was chosen in patients with multiple admissions (which may occur with transfer between hospitals), excluding an additional 3020 admissions. Finally, 445 patients with unstable angina were excluded. Therefore, the final population consisted of 175 146 unique patients with first MI, of whom 9.3% (16 237) had cancer in the 5 years before admission.
Patient Characteristics
Patient characteristics stratified according to year of admission are shown in Table 1. Cancer characteristics are shown in Table 2. Median duration between time of cancer diagnosis and index MI was 1.97 years (interquartile range, 0.79–3.38 years). Median duration between the most recent care for cancer (including diagnosis) and MI was 0.67 years (interquartile range, 0.16–2.11 years). Temporal trends for the most common cancer types showed a gradual increase in cancer care for most types of cancer during the inclusion period (Table S1). For new cancer diagnoses, only melanoma and breast cancer showed a significantly increasing trend. Cancer extent is shown in Table S2.
Table 1.
Patient Characteristics Stratified According to Year of Admission
| 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | P trend | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 23 692 | 24 465 | 24 952 | 26 206 | 24 918 | 25 853 | 25 060 | ··· |
| Age, median (interquartile range) | 72 (19) | 71 (19) | 71 (19) | 70 (19) | 70 (18) | 70 (19) | 70 (18) | <0.001 |
| Male, % (n) | 61.4 (14 545) | 62.0 (15 157) | 62.9 (15 700) | 62.4 (16 364) | 63.6 (15 849) | 63.9 (16 513) | 64.4 (16 128) | <0.001 |
| Cancer care/diagnosis <5 ya | ||||||||
| All patients, % (n) | 6.7 (1598) | 8.1 (1977) | 9.3 (2316) | 9.7 (2552) | 9.9 (2477) | 10.2 (2640) | 10.7 (2677) | <0.001 |
| Men, % (n) | 7.3 (1064) | 8.4 (1278) | 9.9 (1561) | 10.1 (1658) | 10.5 (1658) | 10.9 (1792) | 11.2 (1808) | <0.001 |
| Women, % (n) | 5.8 (534) | 7.5 (699) | 8.2 (755) | 9.1 (894) | 9.0 (819) | 9.1 (848) | 9.7 (869) | <0.001 |
| Cancer diagnosis <5 yb | ||||||||
| All patients, % (n) | 4.9 (1153) | 5.1 (1241) | 5.6 (1405) | 5.6 (1459) | 5.8 (1447) | 5.8 (1493) | 6.2 (1557) | <0.001 |
| Men, % (n) | 5.3 (774) | 5.4 (817) | 6.3 (993) | 6.0 (984) | 6.1 (971) | 6.2 (1016) | 6.5 (1044) | <0.001 |
| Women, % (n) | 4.1 (379) | 4.6 (424) | 4.5 (412) | 4.8 (475) | 5.2 (476) | 5.1 (477) | 5.7 (513) | <0.001 |
The statistical trend was calculated using the chi‐squared test.
Time between most recent cancer care or cancer diagnosis and myocardial infarction.
Time between cancer diagnosis and myocardial infarction.
Table 2.
Characteristics of Patients With Cancer
| Previous Cancer (N=16 237), % (n) | |
|---|---|
| Type of cancer | |
| Prostate | 33.0 (5354) |
| Bladder | 9.6 (1562) |
| Hematological | 9.5 (1543) |
| Breast | 9.4 (1534) |
| Colon | 7.4 (1203) |
| Melanoma | 5.3 (853) |
| Rectum | 4.8 (783) |
| Lung and airway | 3.7 (600) |
| Kidney including renal pelvis | 2.9 (471) |
| Uterus | 2.7 (436) |
| Ovaries including fallopian tube and broad ligament of the uterus | 1.4 (226) |
| Pancreas | 0.8 (130) |
| Primary brain cancer | 0.4 (63) |
| Diagnosis of cancer | |
| Within 1 y before admission | 17.9 (2908) |
| Between 1 and 5 y before admission | 42.2 (6847) |
| Diagnosis of cancer or care for cancer | |
| Within 1 y before admission | 58.3 (9464) |
| Between 1 and 5 y before admission | 41.7 (6773) |
Baseline characteristics stratified by previous cancer showed that patients with previous cancer were older and more burdened with comorbidities compared with patients without cancer (Table 3). Invasive management was less common in patients with cancer. Use of angiography increased from 32.6% in the years 2001 and 2002, to 84% during 2013 and 2014. In case of percutaneous coronary intervention, use of stents, including drug‐eluting stents, was slightly less common in patients with cancer.
Table 3.
Patient Characteristics Stratified According to Presence of Cancer
| No Previous Cancer (N=158 909), % (n) | Previous Cancer (N=16 237), % (n) | P Value | |
|---|---|---|---|
| Age, y, median (interquartile range) | 70 (19) | 76 (13) | <0.001 |
| Male sex | 62.6 (99 437) | 66.6 (10 819) | <0.001 |
| Indication for hospitalization | <0.001 | ||
| Non–ST‐elevation myocardial infarction | 51.9 (82 459) | 56.0 (9092) | |
| ST‐elevation myocardial infarction | 35.9 (57 108) | 32.0 (5198) | |
| Unspecified myocardial infarction | 12.2 (19 342) | 12.0 (1947) | |
| Previous percutaneous coronary intervention | 2.2 (3437) | 2.9 (470) | <0.001 |
| Previous coronary artery bypass grafting | 2.2 (3544) | 2.9 (468) | <0.001 |
| History of diabetes mellitus | 18.5 (29 464) | 20.6 (3337) | <0.001 |
| Current smoker | 24.0 (37 479) | 14.7 (2338) | <0.001 |
| History of hypertension | 42.5 (67 366) | 46.6 (7545) | <0.001 |
| History of heart failure | 5.3 (8406) | 8.8 (1428) | <0.001 |
| History of chronic kidney disease | 2.0 (3131) | 4.5 (726) | <0.001 |
| Any previous stroke (including hemorrhagic) | 7.7 (12 274) | 10.4 (1682) | <0.001 |
| History of peripheral vascular disease | 4.8 (7675) | 7.4 (1208) | <0.001 |
| Previous thromboembolism | 2.4 (3842) | 4.8 (780) | <0.001 |
| Angiography with/without PCI during admission | 69.0 (109 645) | 59.8 (9709) | <0.001 |
| Stents implanted in patients undergoing PCI | 92.0 (76 976) | 90.6 (6541) | <0.001 |
| Drug‐eluting stents implanted | 44.0 (33 626) | 42.6 (2529) | 0.037 |
| Multivessel disease in patients undergoing PCI | 47.2 (35 780) | 50.8 (3387) | <0.001 |
| Successful PCI | 94.1 (78 002) | 93.7 (6724) | 0.329 |
The variables age, sex, indication for hospitalization, prior coronary interventions, history of hypertension, and smoking status were obtained from SWEDEHEART. History of diabetes mellitus, heart failure, chronic kidney insufficiency, stroke, peripheral vascular disease, and thromboembolism were obtained from the patient registry. PCI indicates percutaneous coronary intervention; SWEDEHEART, Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies.
From 2010 and onward, MI was classified according to type in SWEDEHEART. Overall, MI was classified as type one in 87.9% of patients with cancer compared with 91.3% in patients without cancer. Rates of type 2 MI were 9.0% for patients with cancer versus 5.9% for patients without cancer (P<0.001). The rate of type 1 MI was lowest in 2010 (84.6%) but also with the highest rate of unknown MI, at 7.7%. The rates of type 1 MI were between 89.9% and 92.1% during the later years. Type 2 MI varied between 5.8% and 7.1%. Other types were uncommon.
Periprocedural and discharge medication is shown in Table S3. Patients with cancer generally received slightly less extensive periprocedural antithrombotic treatment. Use of aspirin and other antiplatelet agents with discharge was significantly lower in patients with cancer (aspirin, 88.9% versus 91.7%; other antiplatelet agents, 64.7% versus 69.3%). Use of anticoagulants was more common in patients with cancer (8.0% versus 6.7%). Other preventive medications were less commonly used in cancer patients (β‐blocking agents, 86.5% versus 88.2%; statins, 73.6% versus 80.3%; angiotensin‐converting enzyme inhibitors or angiotensin II antagonist, 64.6% versus 65.9%; all differences significant).
Outcome
The association of cancer with mortality, cardiovascular, and bleeding outcome is shown in Table 4. Median follow‐up duration was 4.3 years (interquartile range, 1.5–7.9 years). Figures S1 and S2 show unadjusted Kaplan–Meier curves for all‐cause mortality, fatal or nonfatal major bleeding, MI, and hospitalization for heart failure. Furthermore, the association between extent of cancer, timing of last cancer care, and outcome is shown in Figures 1 and 2. Finally, outcome for the 5 most common types of cancer in the current population is shown in Figures 3 and 4. Table S4 shows a sensitivity analysis of the association of cancer with outcome, after exclusion of patients with prostate cancer. The exclusion of these patients did not alter the observed associations between cancer and outcome.
Table 4.
Association of Cancer With Outcome After Discharge
| Unadjusted Models, Cancer vs. No Cancer (Reference) | Multivariable Adjusted Models, Cancer vs. No Cancer (Reference) | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| All‐cause mortality | 2.01 (1.96–2.06) | <0.001 | 1.44 (1.40–1.47) | <0.001 |
| Cancer mortality | 6.78 (6.48–7.10) | <0.001 | 5.33 (5.09–5.59) | <0.001 |
| Cardiovascular events | ||||
| Cardiovascular mortality | 1.43 (1.39–1.48) | <0.001 | 1.03 (0.996–1.07) | 0.085 |
| Recurrent myocardial infarction | 1.24 (1.20–1.28) | <0.001 | 1.08 (1.04–1.12) | <0.001 |
| Admission for heart failure | 1.51 (1.46–1.56) | <0.001 | 1.10 (1.06–1.13) | <0.001 |
| Ischemic stroke | 1.31 (1.23–1.40) | <0.001 | 1.01 (0.95–1.08) | 0.708 |
| Venous thromboembolism | 1.90 (1.75–2.06) | <0.001 | 1.58 (1.46–1.72) | <0.001 |
| Bleeding events | ||||
| Fatal bleeding | 1.85 (1.60–2.15) | <0.001 | 1.33 (1.15–1.55) | <0.001 |
| Nonfatal major bleeding | 2.02 (1.84–2.22) | <0.001 | 1.49 (1.35–1.64) | <0.001 |
| Fatal bleeding or nonfatal major bleeding | 1.98 (1.83–2.15) | <0.001 | 1.45 (1.34–1.57) | <0.001 |
| Hospitalization for bleeding | 1.80 (1.70–1.90) | <0.001 | 1.42 (1.34–1.51) | <0.001 |
| Gastrointestinal bleeding | 1.68 (1.58–1.79) | <0.001 | 1.35 (1.27–1.44) | <0.001 |
| Hemorrhagic stroke | 1.50 (1.31–1.71) | <0.001 | 1.16 (1.02–1.33) | 0.029 |
Hazard ratios calculated using Cox proportional hazards models adjusted for age, sex, diabetes mellitus, hypertension, smoking status, previous heart failure, previous percutaneous coronary intervention, previous coronary artery bypass grafting, chronic kidney disease, peripheral arterial disease, indication for admission, previous stroke, year of admission, and percutaneous coronary intervention during hospitalization.
Figure 1.
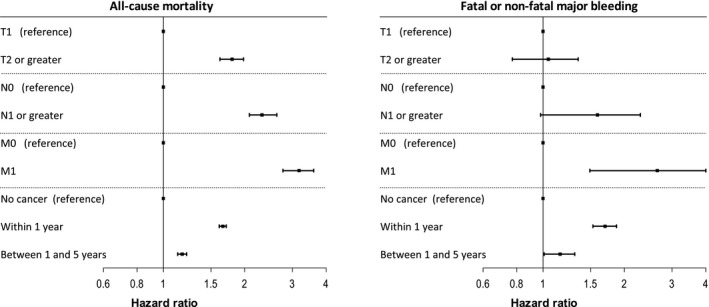
Association of extent and timing of cancer with all‐cause mortality and fatal or nonfatal major bleeding. T provides information about the primary tumor, for which a higher category generally means an increasing size, an increasing local extension, or both. T0, Ta, Tis, and Tx not shown. N0 denotes no regional lymph node involvement. N1 and higher denotes evidence of regional node(s) containing cancer. Nx not shown. M0 means no evidence of distant metastasis, and M1 means distant metastasis is present. Mx not shown. Hazard ratios calculated using Cox proportional hazards models adjusted for age, sex, diabetes mellitus, hypertension, smoking status, previous heart failure, previous percutaneous coronary intervention, previous coronary artery bypass surgery, chronic kidney disease, peripheral arterial disease, indication for admission, previous stroke, year of admission, and percutaneous coronary intervention during hospitalization.
Figure 2.

Association of extent and timing of cancer with myocardial infarction and hospitalization for heart failure. See Figure 1 for multivariable model explanations and abbreviations.
Figure 3.
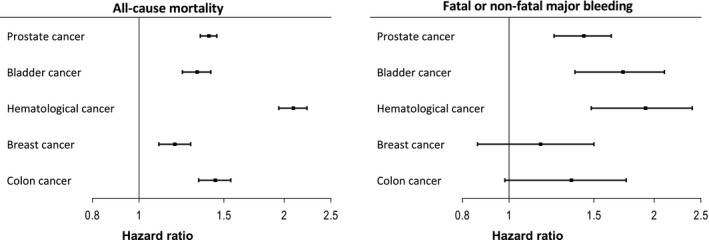
Association of type of cancer with all‐cause mortality and the composite of fatal or nonfatal major bleeding. See Figure 1 for multivariable model explanations. The reference category in the current models was patients without cancer.
Figure 4.
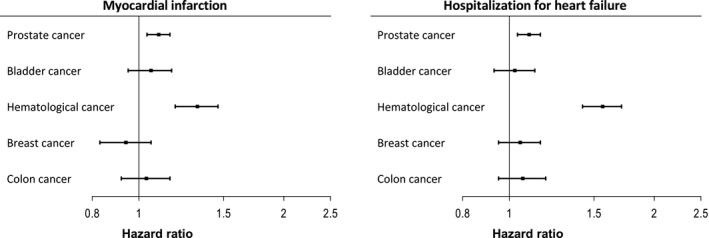
Association of type of cancer with myocardial infarction and hospitalization for heart failure. See Figure 1 for multivariable model explanation. The reference category in the current models was patients without cancer.
Additional sensitivity analyses were performed to evaluate type 2 MI after discharge. For patients admitted during or after 2010, the unadjusted rate of type 2 MI for the remaining follow‐up time was 0.7% for patients with previous cancer, and 0.6% for patients without previous cancer. No further analyses were performed because of low event rates.
Discussion
Cancer as a comorbid disease in the 5 years before first MI was increasing during the 14‐year inclusion period of this nationwide study. The positive trend in recent care for cancer was more pronounced than the increase in the number of new cancer diagnoses by itself, suggesting that improved survival of malignancy is the main driving factor behind this development. International trends showing improving cancer survival rates support this hypothesis.1, 2 The increase in recent cancer care was consistent among most types of cancer, but was not significant for bladder and colon cancer. With regard to new diagnoses, an overall gradual increase was observed, but only breast cancer and melanoma reached significance when tested individually. The overall pattern of the most common types of cancer reflected rates in the general Swedish population, with the exception of hematological malignancies, which were relatively overrepresented.9
Most cancer patients had received some form of care for cancer during the year before MI, but the diagnosis had more commonly been made in earlier years. Importantly, cancer in the 5 years before admission for first MI was associated with higher subsequent risk of mortality and severe bleeding, including fatal bleeding and intracerebral bleeding. Risk for cardiovascular complications, specifically hospitalization for heart failure and recurrent MI, was also modestly increased in these patients. Despite that, an increased risk of cancer mortality but not cardiovascular mortality was observed in patients with cancer.
Patients with cancer tended to be older and more burdened by comorbid diseases and received less extensive treatment. Statistical correction for comorbidity and performance of percutaneous coronary intervention showed that only part of the increased risk for adverse events could be explained by these factors. Correction for in‐hospital antithrombotic treatment and secondary preventive medication on discharge was not performed, but this was unlikely to explain the large gaps in outcome attributable to small differences in medication between groups.
In those years that classification of MI was available, index MI was more often attributable to type 2 infarction (ie, a mismatch between myocardial oxygen demand and supply) in patients with cancer, resulting in a slightly higher rate compared with the overall SWEDEHEART population.6 As such, part of the higher reinfarction rate after discharge may be attributable to higher rates of type 2 MI. A sensitivity analysis could not confirm this because of the low number of type 2 infarctions registered in SWEDEHEART during the inclusion period. Other explanations include withdrawal of antithrombotic treatment because of bleeding or need for surgery, stent thrombosis attributable to a hypercoagulable state, or other still unknown mechanisms.4, 10, 11 Navi et al12 previously observed an increased risk of arterial thromboembolism in newly diagnosed patients with cancer, with risk directly related to overall tumor burden and extent of disease. In the current study, more extensive malignant disease was not associated with higher risk for recurrent MI, but was associated with higher rates of hospitalization for heart failure, as well as bleeding and all‐cause mortality.
Patients with cancer showed an increased risk of hospitalizations for heart failure, with additional risk in those patients with spread disease. Possibly, these patients may have had preexisting myocardial dysfunction attributable to treatment with cardiotoxic drugs such as anthracyclines, trastuzumab, and tyrosine kinase inhibitors, rendering them more vulnerable to heart failure as a complication of the index ischemic insult.4 Supporting this was the high rate of heart failure in patients with hematological malignancy, a group commonly treated with cardiotoxic drugs.13 Patients with hematological malignancy also had high rates of all‐cause mortality, MI, and bleeding, reflecting the risk of bone marrow invasion or suppression with resulting anemia and thrombocytopenia, platelet dysfunction, and higher risk for thrombotic complications.14 Further supporting this was the relatively high rate of hematological malignancies in the current population compared with the Swedish general population.9 Prostate cancer, which was the most common type of cancer, was also associated with an increased risk of MI and heart failure, but likely through other mechanisms. Androgen therapy for prostate cancer has previously been associated with MI and heart failure, possibly by inducing the metabolic syndrome secondary to testosterone deficiency.15, 16 In contrast, bladder, breast, and colon cancer were not significantly associated with adverse cardiovascular outcome.
Stratification of the cancer population according to timing of care for cancer showed a strong association between recent cancer care (within 1 year) and increased all‐cause mortality, recurrent MI, heart failure, and bleeding. Interestingly, cardiovascular risk was not elevated in patients with no care for cancer during the past year. Recent cancer care may reflect more recent cancer treatment, which can provoke cardiac ischemia.4 It may also reflect more extensive or more aggressive disease.
Besides higher rates of cardiovascular complications, patients with cancer had higher risk for all bleeding outcomes, including fatal bleeding, major bleeding, and hospitalizations for bleeding, as well as intracranial bleeding. Of note, the degree of significance for intracranial bleeding was more uncertain because of the low number of events. Bleeding risk increased with more extensive disease and with more recent cancer care. Risk of bleeding was only slightly elevated in patients without cancer care in the year before MI. Of the most common cancer types, risk for bleeding was highest in patients with hematological malignancies, followed by bladder cancer and prostate cancer. Risk of serious bleeding did not reach significance in patients with colon cancer and breast cancer, but CIs were wide.
Our findings support the recent suggestion of cancer as a major risk factor for bleeding in patients undergoing percutaneous coronary intervention, and suggest that these recommendations should be extended to all patients with MI and cancer regardless of invasive management.17 Bleeding in cancer is often multifactorial and may be attributable to malignancy‐related factors (eg, immune‐mediated thrombocytopenia, bone marrow involvement, bleeding from tumor tissue or eroded blood vessel, and disseminated intravascular coagulation) or treatment related (myelotoxicity, anticoagulation).18 Venous thromboembolism was increased in the cancer population, contributing to bleeding complications attributable to the need for anticoagulant therapy. Patients with cancer with venous thromboembolism are known to be extra vulnerable for bleeding.19 For cardiologists, strategies to reduce risk for bleeding include use of less potent antiplatelet therapy, low‐dose aspirin, use of proton pump inhibitors, and shorter‐duration dual antiplatelet therapy. In case of need for anticoagulant therapy, shortened triple‐antithrombotic therapy or only dual‐antithrombotic therapy can be considered.20 For oncologists, strategies to reduce cardiovascular complications include consideration of cardiac disease before initiation of cardiotoxic therapy, optimal treatment of cardiovascular risk factors in cooperation with cardiologists, and use of treatment regimens with limited cardiotoxicity.21
Other studies of varying size have investigated cancer in patients with ischemic heart disease. A large study by Potts et al22 recently reported an increasing prevalence of cancer in patients undergoing percutaneous coronary intervention. They observed an increased risk of in‐hospital mortality and percutaneous coronary intervention complications in patients with cancer, including higher bleeding rates. The importance of timing of cancer as well as extent of cancer was observed, and patients with breast cancer showed the best outcome, similar to our findings.22 However, outcome was limited to in‐hospital events. A Canadian study also observed higher rates of mortality and heart failure but not reinfarction and stroke in patients with cancer after admission for MI.23
Besides these large studies, a number of other studies have been published suggesting elevated rates of mortality,24, 25, 26, 27, 28, 29, 30, 31 recurrent infarction,24 bleeding,24, 31 heart failure,24, 30, 31 and target lesion revascularization.32 However, the current cohort is the first large unselected nationwide study with the whole spectrum of patients with MI, including long‐term follow‐up for clearly defined ischemic and bleeding events.
Some limitations deserve mention. The SWEDEHEART registry includes patients admitted to cardiac care units, and patients diagnosed with MI at oncology units and not transferred to a cardiology department were not included. Likely, these are patients with type 2 MI or palliative patients with type 1 MI deemed unsuitable for transfer to a coronary care unit, as the standard of care for MI in Sweden includes transfer to a coronary care unit. Furthermore, classification of infarction into subtypes was available only in later years. Data on oncological treatment would have helped elucidate additional associations of cancer treatment with outcome but were not available. Although the Swedish national cancer registry has a high degree of completeness, tumor classification was not available for all patients.33 The tumor classification was based on data from the time of cancer diagnosis and may have changed over time.
Conclusions
Cancer as a comorbid disorder is increasing and is strongly associated with mortality, severe bleeding, and adverse cardiovascular outcome after first MI. Cancer timing, extent, and type influence these risks and need to be taken into consideration when treating these high‐risk patients. Because of the enormous heterogeneity of the cancer population, multidisciplinary evaluation with care tailored to the individual patient by both cardiologists and oncologists is needed to reduce risk for cardiovascular and bleeding complications.
Sources of Funding
This work was supported by ALF (“Avtal mellan svenska staten och vissa landsting om samarbete om grundutbildning av läkare, medicinsk forskning och utveckling av hälso‐ och sjukvården”) Uppsala, the Johan Bergström Foundation, the Västmanland Research Foundation (LTV‐646091), and the Swedish Heart‐ and Lung Foundation (20150548). The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication. Dr Velders had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosures
Dr Hagström reports institutional grants from Amgen and Sanofi; and personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, NovoNordisk, and Sanofi. Dr James reports grants and personal fees from AstraZeneca, Bayer, Boston Scientific, and Abbott; and grants from Jansen and The Med Co. Dr Velders has no disclosures to report.
Supporting information
Data S1. Supplemental methods.
Table S1. Temporal Trends for Cancer Types
Table S2. Cancer Extent
Table S3. Periprocedural and Discharge Medication According to Presence of Cancer
Table S4. Association of Cancer With Outcome After Discharge, Excluding Patients With Prostate Cancer
Figure S1. Unadjusted Kaplan–Meier curves for all‐cause mortality and fatal or nonfatal major bleeding.
Figure S2. Unadjusted Kaplan–Meier curves for myocardial infarction and hospitalization for heart failure.
Acknowledgments
The authors thank Elisabet Ärnström for her assistance with database management and Lars Lindhagen for statistical support.
(J Am Heart Assoc. 2020;9:e014383 DOI: 10.1161/JAHA.119.014383.)
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group . Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66:309–325. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction , Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 6. Baron T, Hambraeus K, Sundström J, Erlinge D, Jernberg T, Lindahl B; TOTAL‐AMI Study Group . Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106. [DOI] [PubMed] [Google Scholar]
- 7. Friberg L, Skeppholm M. Usefulness of Health Registers for detection of bleeding events in outcome studies. Thromb Haemost. 2016;116:1131–1139. [DOI] [PubMed] [Google Scholar]
- 8. Gress D, Edge S, Greene F, Washington M, Asare E, Brierley J. Principles of cancer staging In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed New York: Springer; 2017:3–30. [Google Scholar]
- 9. The National Board of Health and Welfare (Socialstyrelsen) . Statistics on Cancer Incidence 2017. Sweden: The National Board of Health and Welfare; 2018. ISSN 1401‐0216. Online appendix. Available at: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-12-50-tabeller.xls. Accessed August 1, 2019. [Google Scholar]
- 10. Rossini R, Capodanno D, Lettieri C, Musumeci G, Nijaradze T, Romano M, Lortkipanidze N, Cicorella N, Biondi Zoccai G, Sirbu V, Izzo A, Guagliumi G, Valsecchi O, Gavazzi A, Angiolillo DJ. Prevalence, predictors, and long‐term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011;107:186–194. [DOI] [PubMed] [Google Scholar]
- 11. van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399–1409. [DOI] [PubMed] [Google Scholar]
- 12. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson SA. Anthracycline‐induced cardiotoxicity in adult hematologic malignancies. Semin Oncol. 2006;33:S22–S27. [DOI] [PubMed] [Google Scholar]
- 14. Franchini M, Frattini F, Crestani S, Bonfanti C. Bleeding complications in patients with hematologic malignancies. Semin Thromb Hemost. 2013;39:94–100. [DOI] [PubMed] [Google Scholar]
- 15. Jespersen CG, Nørgaard M, Borre M. Androgen‐deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population‐based cohort study. Eur Urol. 2014;65:704–709. [DOI] [PubMed] [Google Scholar]
- 16. Van Hemelrijck M, Garmo H, Holmberg L, Ingelsson E, Bratt O, Bill‐Axelson A, Lambe M, Stattin P, Adolfsson J. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population‐Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–3456. [DOI] [PubMed] [Google Scholar]
- 17. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Circulation. 2019;40:2632–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escobar MA. Bleeding in the patient with a malignancy: is it an acquired factor VIII inhibitor? Cancer. 2012;118:312–320. [DOI] [PubMed] [Google Scholar]
- 19. Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. [DOI] [PubMed] [Google Scholar]
- 20. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 21. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. 2017;70:2536–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, De Belder MA, Kwok CS, Rashid M, Fischman DL, Mamas MA. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. 2019;40:1790–1800. DOI: 10.1093/eurheartj/ehy769. [DOI] [PubMed] [Google Scholar]
- 23. Gong IY, Yan AT, Ko DT, Earle CC, Cheung WY, Peacock S, Hall M, Gale CP, Chan KKW. Temporal changes in treatments and outcomes after acute myocardial infarction among cancer survivors and patients without cancer, 1995 to 2013. Cancer. 2018;124:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iannaccone M, D'Ascenzo F, Vadalà P, Wilton SB, Noussan P, Colombo F, Raposeiras Roubín S, Abu Assi E, González‐Juanatey JR, Simao Henriques JP, Saucedo J, Kikkert WJ, Nuñez‐Gil I, Ariza‐Sole A, Song XT, Alexopoulos D, Liebetrau C, Kawaji T, Moretti C, Garbo R, Huczek Z, Nie SP, Fujii T, Correia LC, Kawashiri MA, García Acuña JM, Southern D, Alfonso E, Terol B, Garay A, Zhang D, Chen Y, Xanthopoulou I, Osman N, Möllmann H, Shiomi H, Giordana F, Kowara M, Filipiak K, Wang X, Yan Y, Fan JY, Ikari Y, Nakahashi T, Sakata K, Gaita F, Yamagishi M, Kalpak O, Kedev S. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7:631–638. [DOI] [PubMed] [Google Scholar]
- 25. Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, Cannegieter SC, Jukema JW, Umans VA, Schalij MJ, van Boven AJ. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–1872. [DOI] [PubMed] [Google Scholar]
- 26. Pothineni NV, Shah NN, Rochlani Y, Saad M, Kovelamudi S, Marmagkiolis K, Bhatti S, Cilingiroglu M, Aronow WS, Hakeem A. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann Transl Med. 2017;5:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, Wright RS, Lerman A, Herrmann J. Cancer history portends worse acute and long‐term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST‐segment elevation myocardial infarction. Mayo Clin Proc. 2016;91:1680–1692. [DOI] [PubMed] [Google Scholar]
- 28. Landes U, Kornowski R, Bental T, Assali A, Vaknin‐Assa H, Lev E, Iakobishvili Z. Long‐term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. 2017;28:5–10. [DOI] [PubMed] [Google Scholar]
- 29. Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, Hagstrom E, James SK, Khouri MG, Hirsch BR, Kong DF, Abernethy AP, Krucoff MW. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015;4:e001779 DOI: 10.1161/JAHA.115.001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. 2018;7:639–645. [DOI] [PubMed] [Google Scholar]
- 31. Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Ono K, Shizuta S, Kimura T; CREDO‐KyotoPCI/CABG Registry Cohort‐2 Investigators . Influence of a history of cancer on long‐term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study‐Kyoto Registry Cohort‐2). Eur Heart J Qual Care Clin Outcomes. 2018;4:200–207. [DOI] [PubMed] [Google Scholar]
- 32. Tabata N, Sueta D, Yamamoto E, Takashio S, Arima Y, Araki S, Yamanaga K, Ishii M, Sakamoto K, Kanazawa H, Fujisue K, Hanatani S, Soejima H, Hokimoto S, Izumiya Y, Kojima S, Yamabe H, Kaikita K, Tsujita K; KUMA Study Investigators . Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur Heart J Qual Care Clin Outcomes. 2018;4:290–300. [DOI] [PubMed] [Google Scholar]
- 33. Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Temporal Trends for Cancer Types
Table S2. Cancer Extent
Table S3. Periprocedural and Discharge Medication According to Presence of Cancer
Table S4. Association of Cancer With Outcome After Discharge, Excluding Patients With Prostate Cancer
Figure S1. Unadjusted Kaplan–Meier curves for all‐cause mortality and fatal or nonfatal major bleeding.
Figure S2. Unadjusted Kaplan–Meier curves for myocardial infarction and hospitalization for heart failure.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


