Abstract
Background
People living with HIV (PLWH) are at increased risk of cardiovascular disease, including hypertension, which persists despite effective plasma viral suppression on antiretroviral therapy. HIV infection is characterized by long‐term alterations in immune function, but the contribution of immune factors to hypertension in PLWH is not fully understood. Prior studies have found that both innate and adaptive immune cell activation contributes to hypertension.
Methods and Results
We hypothesized that chronic inflammation may contribute to hypertension in PLWH. To test this hypothesis, we enrolled a cohort of 70 PLWH (44% hypertensive) on a long‐term single antiretroviral therapy regimen for broad phenotyping of inflammation biomarkers. We found that hypertensive PLWH had higher levels of inflammatory cytokines, including tumor necrosis factor‐α receptor 1, interleukin‐6, interleukin‐17, interleukin‐5, intercellular adhesion molecule 1 and macrophage inflammatory protein‐1α. After adjustment for age, sex, and fat mass index, the circulating eosinophils remained significantly associated with hypertension. On the basis of these results, we assessed the relationship of eosinophils and hypertension in 2 cohorts of 50 and 81 039 similar HIV‐negative people; although eosinophil count was associated with prevalent hypertension, this relationship was abrogated by body mass index.
Conclusions
These findings may represent a unique linkage between immune status and cardiovascular physiological characteristics in HIV infection, which should be evaluated further.
Keywords: eosinophilia, HIV, hypertension, inflammation, interleukin‐5
Subject Categories: Inflammation
Clinical Perspective
What Is New?
Hypertensive people living with HIV (PLWH) on antiretroviral therapy with long‐term viral suppression exhibit higher levels of circulating eosinophils and the eosinophil maturation marker interleukin‐5 compared with normotensive PLWH.
There may be a novel pathway linking eosinophils and hypertension in PLWH, and further studies are warranted to validate and explore this finding.
What Are the Clinical Implications?
PLWH are at increased risk of cardiovascular disease compared with HIV‐negative people, and the management of hypertension is essential to reducing disease burden in this population.
Targeting inflammation, especially eosinophils and their maturation, may provide therapeutic benefit in treating and/or preventing hypertension in PLWH.
Introduction
With the introduction of effective antiretroviral therapy (ART), people living with HIV (PLWH) can now survive decades, but this success is tempered by an increasing burden of cardiovascular disease in this population.1, 2, 3, 4 One contributor to the excess cardiovascular mortality in PLWH may be the increased prevalence of hypertension compared with the general population, which persists despite suppression of plasma viremia with ART.5 Recent evidence suggests that HIV may contribute to hypertension through direct viral effects on the lining of vessels, and indirectly through eliciting an inflammatory cascade that contributes to the genesis and propagation of hypertension and atherosclerosis.6
Over the past 50 years, research in several laboratories, including our own, has demonstrated that both the innate and adaptive immune systems contribute to hypertension. T cells infiltrate the kidneys and perivascular space in response to hypertensive stimuli and release inflammatory cytokines that promote renal and vascular dysfunction, leading to elevated blood pressure and end organ damage.7 However, the mechanisms of inflammation contributing to hypertension are not well explored in PLWH. In this study, we used a well‐characterized cohort of PLWH on long‐term ART to investigate the relationship between immune cell subtypes, soluble biomarkers of inflammation, and prevalent hypertension. We also compared our findings in hypertension in HIV‐negative participants with or without hypertension.
Methods
Availability of Data and Material
The data sets used and/or analyzed during the current study will be made available on request from the corresponding author.
Human Study Population
We used the Adiposity and Immune Activation Cohort of 70 PLWH recruited at the Vanderbilt Comprehensive Care Clinic from 2013 to 2014, who have been previously described.8, 9, 10, 11, 12 All subjects were on efavirenz, tenofovir, and emtricitabine (ie, the combination pill Atripla) for at least the 6 months before enrollment, and had been on ART with persistent HIV‐1 RNA measurements <50 copies/mL for at least the previous 2 years. All participants were nondiabetic, with no history of acute coronary events, stroke, known rheumatologic or inflammatory conditions (aside from HIV), or concomitant comorbidities that might modify eosinophil counts (allergic rhinitis, asthma, dermatoses, and parasitic disease). Use of antihypertensive medication at the time of the study visit and/or >2 sequential outpatient systolic blood pressure measurements >140 mm Hg preceding the visit were used to classify hypertension status. A cohort of 50 HIV‐negative people, also from Vanderbilt University Medical Center, served as controls. Data on this cohort were obtained by review of medical records of volunteers who provided consent for participation in research conducted at the Division of Clinical Pharmacology. Data in normotensive and hypertensive HIV‐negative subjects with available differential counts of white blood cells were collected, eliminating participants with comorbidities known to modify eosinophil counts, such as allergic rhinitis, asthma, dermatoses, and parasitic disease. Each subject could have ≥1 eosinophil count over time (mean, 3.9±0.4; median, 3). If counts were >1, values were averaged. As an additional HIV‐negative control cohort, individuals of interest were sought in the Synthetic Derivative, a Vanderbilt University Medical Center database of ~2.5×106 deidentified electronic medical records. The search strategy consisted of identifying subjects without HIV/AIDS and dividing them by the presence versus absence of essential hypertension diagnostic codes. Only subjects containing ≥1 data point on eosinophil counts and body mass indexes (BMIs) were used. Exclusion criteria were all diseases known to produce eosinophilia, including but not limited to respiratory and cutaneous allergic disorders, hematologic malignancies, some infections, collagen vascular disorders, and ages <30 years and >60 years. For subjects with >1 measurement of eosinophils or BMI, all values were averaged. All participants provided written informed consent, and the study was approved by the Vanderbilt Institutional Review Board.
Inflammatory Biomarker Assessment
Venous blood was drawn in the morning between 8 and 11 am and after a minimum 8‐hour fast. Samples were collected in an EDTA‐containing vacutainer and centrifuged for 10 minutes at 4°C, and the plasma was removed and immediately frozen at −80°C. Plasma levels of soluble cluster of differentiation (sCD) 14 and sCD163, 2 surface markers released into circulation by activated macrophages, were measured using ELISA (R&D Systems, Minneapolis, MN). Other plasma cytokines and immune biomarkers, including interleukins, MCP1 (monocyte chemoattractant protein‐1), MIP‐1α/β (macrophage inflammatory protein‐1 α and β), tumor necrosis factor‐α (TNF‐α), and soluble TNF‐α receptors 1 and 2 (sTNFR1 and sTNFR2, respectively), as well as vascular cell adhesion molecule‐1 and intercellular adhesion molecule‐1 (ICAM‐1), were measured in duplicate using a standard multiple immunoassay panel (MesoScale, Rockville, MD).
Flow Cytometry
Peripheral blood mononuclear cells were obtained from fasting whole blood samples, as previously described.10 Flow cytometry was performed using the Fortessa (Becton Dickson Biosciences) flow cytometer to measure activated, senescent, exhausted, and memory T‐cell subsets using previously described fluorochrome panels.8
Eosinophil Counts
Eosinophil counts and percentages were obtained in normotensive and hypertensive participants with and without HIV from automated differential cell counts performed in the Vanderbilt University Medical Center Clinical Laboratory.
Assessment of Body Composition
A full‐body Dual‐energy X‐ray absorptiometry (GE Lunar Prodigy; GE Healthcare, Little Chalfont, UK) measured total body fat mass to calculate fat mass index (FMI; total fat in kilograms divided by height in meters, squared). FMI is a variant of BMI that accounts for individual variability in the ratio of fat/lean mass.13 The assessment of FMI is shown in Table S1.
Statistical Analyses
We assessed the normality of data using kurtosis and skewness values as well as graphing using Q‐Q plots. Medians and interquartile ranges were calculated for continuous variables, and percentages were calculated for categorical variables. Demographics, clinical characteristics, and the outcome variables were compared between hypertensive and normotensive people using Mann‐Whitney U test or χ2 test, as appropriate. We conducted statistical analysis in R software (http://www.r-project.com). Logistic regression was performed to analyze the association between hypertension and inflammation markers, with adjustment of age, sex, and FMI/BMI for the HIV‐positive participants and age, sex, and BMI for the HIV‐negative participants. Log2 transformation was performed on variables with highly skewed data distribution. Multiple imputation was performed to impute missing data with Hmisc package in R (cran.r‐project.org/package=Hmisc). No adjustments were made for multiple comparisons for this exploratory study, although results and the potential for false discovery were interpreted in the context of known biological pathways.14 P=0.05 was used to infer statistical significance.
Results
Clinical characteristics of the HIV‐positive study cohort are shown in Table 1. In unadjusted comparisons, we observed no significant differences among CD4+ and CD8+ naïve and memory T‐cell subtypes between hypertensive and normotensive individuals. We also found no significant differences among CD4+ and CD8+ T cells expressing CD57 and Programmed cell death protein 1. However, we found that hypertension was associated with lower CD4+ T cells expressing the CD38 activation marker, but not CD8+ CD38+ T cells, and there were no significant differences in dual‐expressing CD38+ and Human Leukocyte Antigen – DR isotype+ cells (Figure S1). The gating strategy to identify T‐cell subpopulations is shown in Figure S2.
Table 1.
HIV‐Positive Participant Characteristics
| Variables | Total Participants (n=70) | |||
|---|---|---|---|---|
| Normotensive (n=39) | Hypertensive (n=31) | P Value | ||
| Age, median (IQR), y | 42 (35–47) | 43 (35–47) | 49 (43–52) | 0.002a |
| Women, n (%) | 30 (43) | 16 (41) | 14 (45) | 0.810 |
| Duration on treatment, median (IQR), y | 6.2 (4.3–10.1) | 6.1 (4.3–11.1) | 6.4 (4.3–8.2) | 0.804 |
| BMI, median (IQR), kg/m2 | 32.3 (26.3–37.1) | 26.5 (22.8–32.8) | 33.9 (28.2–40.0) | 0.001a |
| FMI×103, median (IQR) | 12.8 (8.8–16.3) | 8.8 (5.7–13.2) | 13.3 (9.9–17.5) | 0.003a |
| CD4+ count, median (IQR), cells/μL | 701 (540–953) | 700 (523–924) | 690 (581–969) | 0.554 |
| Nadir CD4+ count, median (IQR), cells/μL | 257 (140–378) | 276 (183–393) | 240 (100–371) | 0.301 |
| CD8+ count, median (IQR), cells/μL | 752 (600–1004) | 774 (630–949) | 675 (585–1062) | 0.582 |
| Smokers, n (%) | 25 (36) | 14 (36) | 11 (35) | 1.000 |
| Cigarettes per day (N=69), median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.787 |
| Nonwhite, n (%) | 38 (54) | 23 (59) | 15 (48) | 1.000 |
| Hepatitis C infection, n (%) | 8 (11) | 2 (5) | 6 (19) | 0.127 |
| Fasting total cholesterol, median (IQR), mg/dL | 176 (154–202) | 176 (160–203) | 171 (142–200) | 0.460 |
| Fasting LDL, median (IQR), mg/dL | 105 (88–123) | 111 (89–123) | 101 (85–124) | 0.435 |
| Fasting HDL, median (IQR), mg/dL | 45 (36–52) | 47 (35–55) | 43 (36–50) | 0.619 |
| Fasting triglycerides, median (IQR), mg/dL | 101 (73–139) | 98 (80–147) | 102 (73–138) | 0.953 |
| Obese, n (%) | 35 (50) | 14 (35.9) | 21 (67.7) | 0.008a |
| Average waist circumference, median (IQR), cm | 104 (88–122) | 96 (82–110) | 114 (101–128) | 0.001a |
| Average mid‐upper arm circumference, median (IQR), cm | 33 (30–36) | 31 (28–35) | 35 (31–38) | 0.006a |
| Visceral adipose tissue, median (IQR), cm | 1554 (630–2219) | 906 (381–2061) | 1950 (1316–3014) | 0.002a |
| Taking NSAIDS, n (%) | 10 (14.3) | 5 (12.8) | 5 (16.1) | 0.694 |
| Taking daily aspirin, n (%) | 6 (8.6) | 0 (0.0) | 6 (19.4) | 0.006 |
| Highly sensitive CRP, median (IQR), mg/L | 2.4 (1.1–6.2) | 2.1 (0.8–3.4) | 3.2 (1.5–6.6) | 0.05 |
| Amyloid A, median (IQR), pg/mL ×106 | 1.8 (0.9–5.0) | 1.4 (0.7–2.2) | 4.2 (1.8–9.7) | <0.001a |
| Leptin, median (IQR), ng/mL | 17.3 (8.5–32.1) | 11.5 (5.9–30.2) | 23.6 (12.4–37.2) | 0.02a |
| Adiponectin, median (IQR), pg/mL ×106 | 9.9 (5.9–14.6) | 10.6 (6.2–18.5) | 9.5 (5.4–13.8) | 0.200 |
For quantitative variables age, duration on treatment, BMI, FMI, and CD4 and CD8 counts, we used the Wilcoxon rank sum test; and we used the χ2 test for the remaining binary categorical variables. BMI indicates body mass index; CD, cluster of differentiation; CRP, C‐reactive protein; FMI, fat mass index; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein.
P<0.05.
Increased Macrophage Activation in PLWH Is Associated With Prevalent Hypertension
We have previously shown that cells of the innate immune system, including monocyte‐derived dendritic cells, are activated during experimental and human hypertension.15, 16 We observed no significant difference in sCD14 in normotensive compared with hypertensive participants with HIV (Figure 1A). However, we found that hypertension was associated with a marked increase in macrophage activation markers and chemokines, including sCD163 (Figure 1B) and MIP‐1α (Figure 1C). In addition, we found that hypertension was associated with a significant increase in MCP1 (Figure 1D) and both vascular cell adhesion molecule‐1 (Figure 1E) and ICAM‐1 (Figure 1F). These results suggest that increased macrophage activation and expression of vascular adhesion molecules are a feature of inflammation and hypertension among PLWH on ART.
Figure 1.
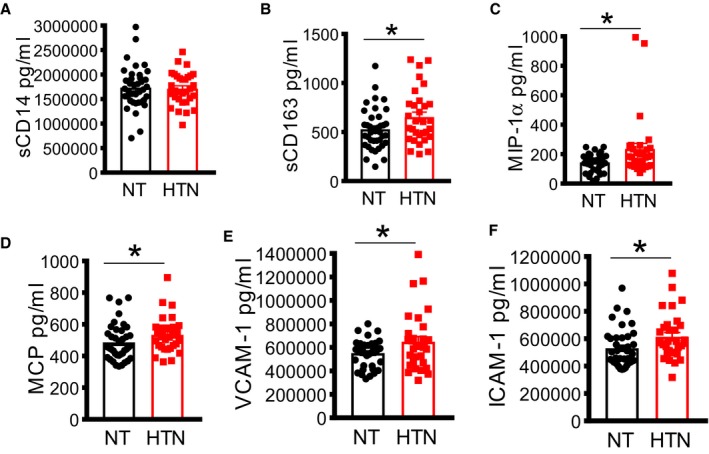
Increased macrophage activation in virally suppressed HIV + participants on antiretroviral therapy is associated with blood pressure elevation. Analysis of monocyte/macrophage activation in plasma using ELISA assay in people living with HIV showing soluble cluster of differentiation (sCD) 14 (A), sCD163 (B), MIP (macrophage inflammatory protein) (C), MCP (monocyte chemoattractant protein) (D) and adhesion molecules, including vascular cell adhesion molecule 1 (VCAM‐1) (E) and intercellular adhesion molecule 1 (ICAM‐1) (F). HTN indicates hypertensive; NT, normotensive. *P<0.05 using the Mann‐Whitney U test.
Cytokines Associated With Hypertension in PLWH
Previous studies have found that inflammatory cytokines, including interleukin‐17, contribute to the development of hypertension.17, 18, 19 We sought to determine if hypertension in PLWH is associated with increased cytokine production. We found that hypertensive PLWH have significantly higher levels of interleukin‐17 (Figure 2A). We also found that hypertension was associated with increased circulating interleukin‐6 (Figure 2B). In addition, we observed lower levels of the anti‐inflammatory cytokine interleukin‐10 in hypertensive participants, but this did not reach statistical significance (Figure 2C). There was no significant difference in plasma TNF‐α or sTNFR2, but we found a significant elevation of sTNFR1 (Figure 2D through 2F). Thus, hypertension in PLWH is associated with higher circulating levels of interleukin‐17, interleukin‐6, and sTNFR1.
Figure 2.
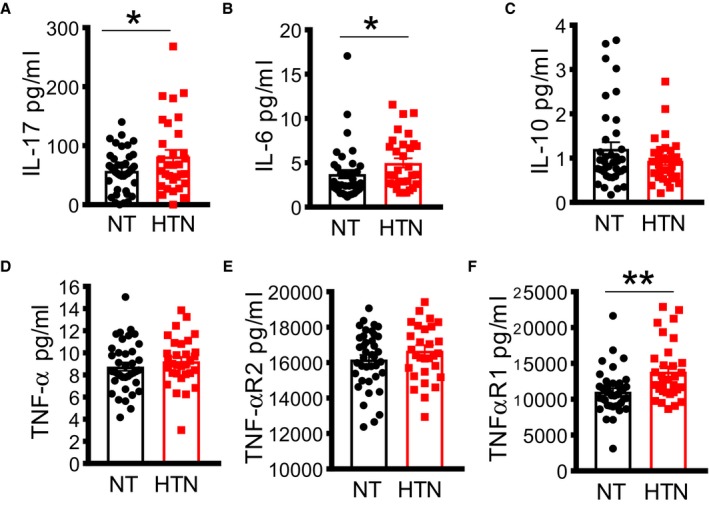
Increased cytokine production in virally suppressed HIV + participants on antiretroviral therapy is associated with hypertension. Cytokine production was analyzed in plasma using ELISA, including interleukin (IL)‐17 (A), IL‐6 (B), IL‐10 (C), tumor necrosis factor (TNF)‐α (D), TNF‐α receptor 2 (TNF‐αR2) (E), and TNF‐α receptor 1 (TNF‐αR1) (F). HTN indicates hypertensive; NT, normotensive. *P<0.05, ** P<0.01 using the Mann‐Whitney U test.
Elevated Eosinophils Were Associated With Increased Hypertension in Virally Suppressed PLWH
Prior studies have indicated that eosinophils play a role in several immune‐mediated diseases.20 As shown in Figure 3A, we found that among PLWH, participants with hypertension had a significantly higher percentage of eosinophils when compared with the normotensive participants. The absolute numbers of eosinophils were also similarly elevated in hypertensive PLWH when compared with normotensive participants (Figure 3B). The positive correlation between eosinophils and hypertension in virally suppressed PLWH remained significant after adjusting for age, sex, and FMI in a multivariate analysis (Table 2). We repeated a similar analysis using BMI instead of FMI, and eosinophils remained significantly associated with hypertension in PLWH (Table S2). Notably, we found that the eosinophil maturation and differentiation factor interleukin‐5 was also associated with hypertension in virally suppressed HIV+ people in a univariate analysis (Figure 3C). This association was robust but did not reach statistical significance in the adjusted model (Table 2). The finding of both elevated circulating eosinophils and increased plasma levels of a key maturation factor strongly suggests that expansion of the eosinophil population may be a feature of hypertension in HIV. Given the small sample size, we performed goodness‐of‐fit analysis using the P values and the Nagelkerke R 2 as well as the C‐index analysis. Both these tests indicated goodness of fit (Tables S3 and S4).
Figure 3.

Elevated eosinophils in blood are associated with increased hypertension. Analysis of eosinophils was performed using automated differential count of white blood cells and expressed as percentage (A) and absolute counts (B) in people living with HIV. Interleukin (IL)‐5 was analyzed using ELISA in plasma of people living with HIV (C). Eosinophil counts in HIV‐negative normotensive (NT) and hypertensive (HTN) participants in two different cohorts (D). *P<0.01 using the Mann‐Whitney U test.
Table 2.
Association Between Hypertension and Inflammatory Cell Subset/Biomarkers in HIV Using Logistic Regression Adjusted for FMI, Age, and Sex
| Variable | 25% Quantile | 75% Quantile | Value Difference (75%–25%) | P Value | Adjusted OR (75%–25%) | Adjusted OR (75%–25%, 95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|
| OR (Difference: 75%–25%) | OR (Difference, Lower 95%) | OR (Difference, Upper 95%) | ||||||
| Interleukin‐17 | 36.25 | 87 | 50.75 | 0.056 | 1.4 | 0.708 | 2.766 | 0.333 |
| Interleukin‐6 | 2.282 | 5.743 | 3.46 | 0.09 | 1.064 | 0.492 | 2.301 | 0.874 |
| TNFαR1 | 9927.125 | 13 650.625 | 3723.5 | 0.005 | 1.616 | 0.817 | 3.196 | 0.168 |
| MIP‐1α | 121.75 | 199.75 | 78 | 0.025 | 1.905 | 0.961 | 3.775 | 0.065 |
| MCP1 | 421.75 | 574.5 | 152.75 | 0.094 | 1.458 | 0.7 | 3.036 | 0.313 |
| ICAM‐1 | 445 708 | 646 911.125 | 201 203.125 | 0.033 | 1.814 | 0.821 | 4.008 | 0.141 |
| VCAM1 | 471 585.5 | 729.025 | 312.05 | 0.04 | 1.733 | 0.787 | 3.815 | 0.172 |
| sCD163 | 416.975 | 5.743 | 3.46 | 0.09 | 1.064 | 0.492 | 2.301 | 0.874 |
| CD4+CD3+ T cells | 5.3 | 10.3 | 5.6 | 0.006 | 0.394 | 0.158 | 0.985 | 0.063 |
| Interleukin‐5 | 4.645 | 9.448 | 4.803 | 0.013 | 1.988 | 0.975 | 4.055 | 0.059 |
| Eosinophils | 1.1 | 2.7 | 1.6 | 0.007 | 2.797 | 1.106 | 7.078 | 0.03 |
| Eosinophil % | 1.787 | 2.659 | 0.872 | 0.009 | 1.735 | 0.804 | 3.746 | 0.043 |
CD indicates cluster of differentiation; FMI, fat mass index; ICAM‐1, intercellular adhesion molecule 1; MCP1, monocyte chemoattractant protein 1; MIP‐1α, macrophage inflammatory protein‐1α; OR, odds ratio; sCD, soluble CD; TNFαR1, tumor necrosis factor‐α receptor 1; VCAM1, vascular cell adhesion molecule‐1.
To determine whether hypertension is also associated with elevated eosinophil counts in HIV‐negative individuals, we recruited an additional cohort of 50 HIV‐negative participants, including 25 normotensive and 25 hypertensive participants. Characteristics of these participants are shown in Table S5. The eosinophil counts were obtained by retrospective review of the medical records for differential counts of white blood cells. We found that eosinophil counts were elevated in hypertensive when compared with normotensive people without HIV (Figure 3D). However, in a multivariate analysis after adjusting for sex, age, and BMI, eosinophil counts were not associated with hypertension, but they were significantly associated with increased BMI (Table S6). In addition, we performed a regression analysis on the combined cohort comprising both the 70 HIV‐positive and the 50 HIV‐negative individuals. The clinical characteristics for the combined group are shown in Table S7. After multivariate analysis of this combined cohort, controlling for age, sex, and BMI, the association between eosinophils and hypertension was robust but did not reach statistical significance (Table S8).
In additional studies, we used the Synthetic Derivative, a Vanderbilt University Medical Center database of ~2.5×106 deidentified electronic medical records, to identify additional HIV‐negative normotensive and hypertensive participants. We excluded participants who did not have information on eosinophil counts, those who were diabetic, and those with a history of acute coronary events, stroke, known rheumatologic or inflammatory conditions, or concomitant comorbidities that might modify eosinophil counts (allergic rhinitis, asthma, dermatoses, and parasitic disease). Pediatric subjects, who constitute a large number of the Vanderbilt Synthetic Derivative, were also excluded. The clinical characteristics for this cohort are shown in Table S9. We found that hypertensive HIV‐negative subjects had a significantly higher eosinophil count when compared with the normotensive subjects (Figure 3D 165.81±1.29 [n=9725 hypertensive subjects] versus 151.97±0.49 [n=71 314 normotensive subjects]; P<0.0001). However, like our smaller cohort of 50 HIV‐negative participants, higher eosinophil count was significantly corelated with the higher BMI in the hypertensive participants (P<0.000001). The eosinophil count remained significantly associated with hypertension after a multivariate analysis (Table S10).
We performed an additional analysis combining the HIV‐positive and HIV‐negative cohorts to determine the interaction between elevated eosinophils and HIV. This analysis confirmed that elevated eosinophils were significantly associated with hypertension (P=0.045) in the HIV‐positive cohort, but not significant in HIV‐negative cohort (P=0.41) (Table S11 and Figure S3). The prediction results with one unit increase of eosinophilia in HIV‐positive and HIV‐negative cohorts are shown in Table S12. These results suggest that unlike in HIV‐negative participants, in whom higher BMI explains the elevated eosinophil counts, HIV infection is accompanied by adipose tissue dysfunction that could mimic obesity, and this may increase eosinophil counts in HIV even without increase in BMI.
Discussion
In the current studies, we found that virally suppressed hypertensive PLWH and on ART exhibited higher levels of circulating eosinophils and their maturation marker interleukin‐5. They also had higher levels of inflammatory cytokines, including TNF‐α1, interleukin‐6, and interleukin‐17, when compared with normotensive controls. In addition, ICAM‐1 and MIP‐1α were elevated in hypertensive PLWH. After adjustment for multiple clinical and demographic factors, the circulating eosinophil counts remained significantly associated with hypertension in this population. However, elevated eosinophil counts were not significantly associated with hypertension in HIV‐negative participants after adjusting for multiple clinical and demographic factors.
Our results are summarized in Figure 4, in which we propose that HIV infection and/or ART is/are associated with increased endothelial dysfunction, with increased expression of vascular cell adhesion molecule‐1 and ICAM‐1. This increases the propensity of activated monocytes, with increased production of sCD163, to diapedese into tissues where they encounter dysfunctional adipose tissue and convert into activated macrophages and dendritic cells. The activated antigen‐presenting cells, including macrophages and dendritic cells, produce MCP1, which further increases migration and infiltration of monocyte/macrophages into tissues. They also produce chemotactic cytokine MIP‐1a, which activates eosinophils and induces release of interleukin‐6 and TNF‐α from macrophages and dendritic cells. These antigen‐presenting cells activate T cells to produce interleukin‐17, which contributes to hypertension. They also produce interleukin‐5, which induces differentiation of eosinophils. Thus, these findings suggest an association between higher eosinophil levels, potentially driven by elevated plasma interleukin‐5, and hypertension in HIV‐positive but not HIV‐negative people. The causal relationship between increased interleukin‐5 and eosinophils in inducing hypertension is not known.
Figure 4.
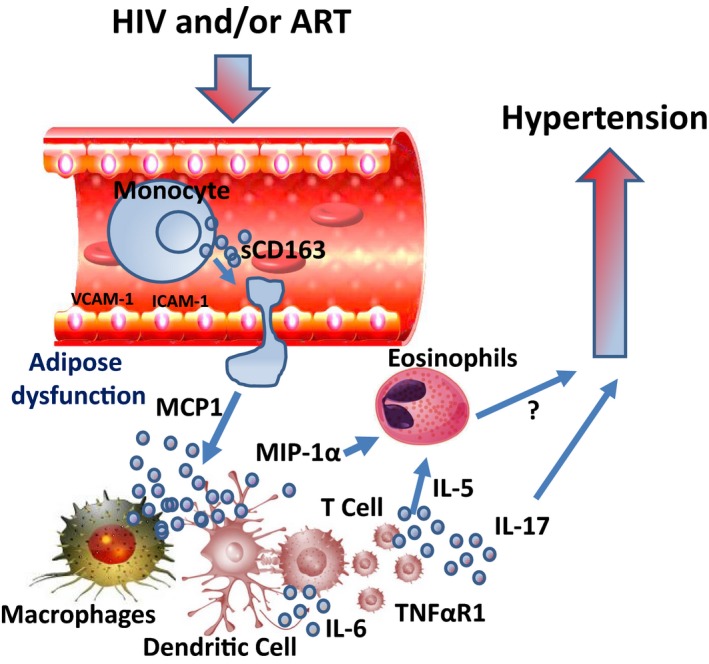
Hypothesized model summarizing all the inflammatory components associated with hypertension in people living with HIV. HIV infection and/or antiretroviral therapy (ART) is/are associated with increased endothelial dysfunction with increased expression of vascular cell adhesion molecule‐1 (VCAM‐1) and intracellular adhesion molecule 1 (ICAM‐1). This increases the propensity of activated monocytes, with increased production of soluble cluster of differentiation (sCD) 163, to diapedese into tissues where they encounter dysfunctional adipose tissue and convert into activated macrophages and dendritic cells. The activated antigen‐presenting cells, including macrophages and dendritic cells, produce MCP1 (monocyte chemoattractant protein 1), which further increases migration and infiltration of monocyte/macrophages into tissues. They also produce chemotactic cytokine MIP‐1α (macrophage inflammatory protein‐1α), which activates eosinophils and induces release of interleukin (IL)‐6 and tumor necrosis factor (TNF)‐α from macrophages and dendritic cells. These antigen‐presenting cells activate T cells to produce IL‐17, which contributes to hypertension. They also produce IL‐5, which induces differentiation of eosinophils. TNF‐αR1 indicates TNF‐α receptor 1.
Although ART has significantly increased life expectancy among PLWH, there is now increased risk for hypertension and cardiovascular disease.5, 21 In humans, observational studies have reported a higher prevalence of hypertension in HIV‐positive people compared with the general population.5, 22 Recent studies suggest that HIV infection and its treatment with ART may play a role in the development of hypertension.22 One contributor may be the accumulation of central adiposity because of effects of both primary infection and antiretroviral agents on fat distribution. Notably, hypertensive people had similar quantities of fat mass in the lower extremities compared with normotensive people, but significantly higher quantities of android and truncal fat. Together, these findings likely represent a lipodystrophy phenotype characterized by central lipohypertrophy without a pronounced limb lipoatrophy, potentially reflecting the limitation of enrollment to people on efavirenz/tenofovir disoproxil fumarate/emtricitabin as opposed to older thymidine analogue medications (eg, zidovudine or stavudine). Although the accumulation of central adiposity may contribute to inflammation, the HIV‐specific inflammatory factors predisposing to hypertension are poorly understood. Herein, we found some similarity as well as unique immune components associated with hypertension in PLWH when compared with the general population.
An interesting finding of the present study is that elevated blood pressure in PLWH was associated with low levels of CD38 expression on CD4+ T cells, which is a transmembrane glycoprotein indicating T‐cell activation. Prior studies have found that lower percentages and absolute numbers of CD4+CD38+ T cells were associated with severe HIV disease pathogenesis and immunologic deterioration in children perinatally infected with HIV and surviving for >5 years.23 We found no significant difference in expression of CD38 on CD8+ T cells in hypertensive versus normotensive subjects, which has been found to be a marker of residual virus replication in chronically HIV‐infected patients receiving Combination Antiretroviral Therapy.24 Indeed, all participant in the current studies had been virally suppressed (viral load<50 copies/mL limit of detection) for a minimum 12 months before the study. Further research is needed to determine the role of CD4+ T cells expressing CD38 and how they may interact with other immune cells in the setting of hypertension in HIV‐positive individuals.
We found that monocyte/macrophage activation was associated with hypertension in PLWH. This was indicated by a marked increase in the shedding of the hemoglobin‐haptoglobin scavenger receptor CD163 into the circulation as sCD163 by the activated macrophages and monocytes. In addition, we found increased MIP‐1α and MCP in hypertensive when compared with normotensive PLWH. Our current findings are in keeping with increasing evidence indicating that cells of the innate immune system, including monocytes and macrophages, play a role in the pathogenesis of hypertension. Previous studies have found that activated macrophages contribute to hypertension and deletion of monocytes markedly reduces experimental hypertension.25, 26, 27 Macrophages accumulate in the kidney and the vasculature in experimental models of hypertension and promote hypertension.28, 29 Moreover, we found that hypertension in PLWH was associated with increased expression of adhesion molecules, including vascular cell adhesion molecule‐1 and ICAM‐1, which govern migration and infiltration of immune cells into tissues and have been implicated in the pathogenesis of hypertension in experimental animal models.30
A notable finding of our current study is that hypertension in PLWH was associated with increased cytokine production and signaling, including interleukin‐6, TNF‐α, and interleukin‐17. Previous studies have found that T cells and their cytokines contribute to hypertension.31, 32 Mice lacking T cells (recombination‐activating gene‐1−/− mice) and mice lacking the T‐cell cytokine interleukin‐17A develop blunted hypertension.17, 33 We previously found that hypertension augments the capacity of dendritic cells to produce interleukin‐6 and these polarized T cells to produce interleukin‐17 and TNF‐α.16, 34 Thus, hypertension in PLWH is associated with some inflammatory components similar to those that have been implicated in hypertension in the general population.
In this cohort of virally suppressed PLWH, eosinophils were significantly associated with hypertension, independent of age, sex, and adiposity. Interleukin‐5 and eosinophils have been implicated in several inflammatory conditions, including asthma, drug hypersensitivity, neoplastic disorders, and helminths infections.35 Interleukin‐5 is produced by T helper 2 cells, mast cells, γδT cells, natural killer, and natural killer T cells and other nonhematopoietic cells.36, 37, 38, 39, 40 It was originally identified as a T‐cell–derived cytokine involved in antibody production from B cells.38 Recently, interleukin‐5 has been implicated in mediating maturation and differentiation of eosinophils in mice and humans.38 In addition, eosinophils play a controversial role in obesity‐mediated inflammation.41, 42 In the LifeLines Cohort Study of >13 000 members of the general population in Europe, higher eosinophil counts were associated with higher triglycerides, total cholesterol, and hemoglobin A1c, but lower high‐density lipoprotein, in addition to a higher odds of obesity and metabolic syndrome.43 Despite this association between eosinophils and cardiometabolic disease, no prior studies have found any association between eosinophils and hypertension.
Our results indicate that although eosinophil counts are elevated in hypertensive PLWH independent of BMI, they are dependent on BMI in HIV‐negative people. Previously, eosinophils were thought to be only associated with Th2 inflammatory disorders, including parasitic infections and allergic reactions.44 However, recent studies have found that eosinophils can infiltrate adipose tissue and regulate its function.41 Wither et al found that eosinophils play a role in obesity‐related hypertension, where they regulate perivascular adipose tissue and vascular function.45 Mice lacking eosinophils do not elicit an anticontractile effect resulting from adiponectin and adipocyte‐derived NO.46 This effect mimics the obese phenotype and is restored by reconstitution of eosinophils. The role of eosinophils in hypertension in the setting of HIV infection is not known. Acquired lipodystrophy occurs in 60% to 80% of patients with HIV on cART,47 and is associated with endothelial dysfunction leading to cardiovascular disease.48, 49, 50, 51 Thus, it is likely that although elevated eosinophil counts were independent of increased BMI in the hypertensive PLWH, they may be associated with acquired lipodystrophy, which creates a metabolic milieu similar to increased BMI. Understanding how eosinophils impact cardiovascular function and hypertension may have important implications for the treatment of metabolic disorders associated with obesity and HIV.
Eosinophils have been found to regulate macrophage activation in adipose tissue. Wu et al41 found that in mice, eosinophils are major producers of interleukin‐4 in white adipose tissues and increase infiltration of alternatively activated macrophages. Mice fed a high‐fat diet develop increased body fat, impaired glucose tolerance, and insulin resistance in the absence of eosinophils. These studies suggest that eosinophils play a role in metabolic homeostasis through maintenance of adipose alternatively activated macrophages. Indeed, we found that macrophage activation factors, including MIP‐1α, were elevated in hypertensive PLWH. However, unlike in the HIV‐negative participants, we found that eosinophil counts are increased in HIV independent of BMI. It is not clear what role these cells play in hypertension associated with HIV and whether they contribute to macrophage activation. Moreover, peripheral blood eosinophilia is often observed in patients with chronic kidney disease, those with acute kidney injury, or patients on renal replacement therapy, and this may be associated with bioincompatibility of the dialysis material, acute allograft rejection, or Strongyloides hyperinfection. Further scientific effort is required to determine if eosinophilia is associated with kidney disease in hypertensive PLWH.52
Our results suggest an association between higher eosinophil levels, potentially driven by elevated plasma interleukin‐5, and hypertension in PLWH. This association is present but abrogated by BMI in HIV‐negative hypertensive people. The findings that eosinophils and their maturation cytokine interleukin‐5 are elevated in hypertensive PLWH are interesting but only hypothesis generating and do not confirm any new pathogenesis of hypertension. However, these 2 related findings provide a pragmatic and strong rationale to study this pathway further in either hypertension or hypertension in HIV.
There is evidence that CD8+ T cells activated in presence of interleukin‐4 can exhibit a Th2‐like phenotype and produce cytokines interleukin‐4, interleukin‐5, interleukin‐6, and interleukin‐10. The role of CD8+ T cells in the context of the HIV‐1 infection is not fully understood; however, it is possible that they are responsible for production of high levels of interleukin‐5,53, 54 which may drive the eosinophilia. Although we did not observe any significant differences in CD8+ T cells between normotensive and hypertensive PLWH, previous studies have found that adipose tissue from PLWH is enriched for CD8+ T cells compared with HIV‐negative controls; and similar changes seen have been observed in obesity.55, 56 This role for CD8+ T cells in interleukin‐5 production does raise the possibility that the elevated eosinophils we observed in hypertensive PLWH may not represent a causal mechanism, but rather be a reflection of a link between the CD8+ T‐cell antiviral immune response and cardiovascular function. In keeping with this concept, absence of eosinophils has been associated with worsening of cardiometabolic health and restoration of eosinophils leads to increased vascular relaxation and improved glucose homeostasis.41 However, other studies have failed to demonstrate an effect of these cells in rescuing metabolic impairment,42 and Amini et al found that higher eosinophil counts were associated with risk factors of metabolic syndrome, including higher triglycerides, total cholesterol, and hemoglobin A1c.43 Studies have found increased levels of other cells known to be anti‐inflammatory, such as T‐regulatory cells in disease. For example, T‐regulatory cells are increased in the circulation of patients with idiopathic pulmonary arterial hypertension.57, 58 Thus, anti‐inflammatory cells may become dysfunctional during disease pathogenesis. Further research is needed to determine how the function of eosinophils may be affected during hypertension in HIV.
In summary, we discovered a significant association between elevated eosinophils and hypertension that might represent a novel pathway leading to hypertension in HIV‐infected adults. Further research is needed to determine the specific contribution of the HIV‐positive status versus ART on inflammation and how they may contribute to hypertension and cardiovascular disease. We acknowledge that this is a cross‐sectional study in a limited cohort of 70 HIV‐positive participants and therefore cannot address causality. Although our findings obviously warrant further validation, we believe there is clinical utility for treatment of hypertension in or outside the context of HIV.
Sources of Funding
This work was supported by the Fogarty International Center of the National Institutes of Health grant D43 TW009744, National Institute of Allergy and Infectious Diseases grant K23 AI100700, National Center for Advancing Translational Sciences grant UL1 TR002243, the Tennessee Center for AIDS Research grant P30 AI110527, and National Heart, Lung, and Blood Institute grant K01HL130497. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures
None.
Supporting information
Figure S1. Increased CD4 T cell activation in people living with HIV is negatively associated with hypertension. Flow cytometry analysis of T cell subsets in people living with HIV showing (A) naïve and (B) memory CD4+ and CD8+T cell subtypes, (C) T cells expressing CD57, (D) PD1, (E) CD38and (F) the CD38 receptor in normotensives compared to hypertensives. *** P<0.001.
Figure S2. Gating strategy to identify T cell subtypes.
Figure S3. Analysis combining the HIV positive and negative cohorts to determine the Interaction between elevated eosinophils and HIV. Log odds of elevated eosinophils in HIV positive and negative cohort (P=0.045 in the HIV positive cohort and P=0.41 in the HIV negative cohort).
Table S1. Fat Mass Measurements Between Hypertensives and Normotensive
Table S2. Association Between Hypertension and Inflammatory Cell Subset/Biomarkers in HIV Using Logistic Regression Adjusted for Body Mass Index, Age, and Sex
Table S3. Analysis of P Values of Model Likelihood Ratio chi‐square test and Nagelkerke R 2 Index. Most of the Interesting Variables Have P<0.01 and R 2>0.3, Which Indicated the Goodness of Fit for the HIV‐Positive Cohort
Table S4. Analysis of C‐Index to Measure Goodness of Fit. All variables of interest have C‐index >0.75 indicating goodness of fit. See last column (C) of each Table
Table S5. Demographics and Clinical Data for HIV‐Negative Subjects
Table S6. Association Between Hypertension and Demographic and Clinical Characteristics in the HIV‐Negative Cohort Using Logistic Regression Adjusted for Body Mass Index, Age, and Sex
Table S7. Demographics and Clinical Data for Both the 50 HIV‐Negative and 70 HIV‐Positive Cohorts Combined
Table S8. Association Between Hypertension and Demographic/Clinical Characteristics in the Both the 50 HIV‐Negative and 70 HIV‐Positive Cohorts Logistic Regression
Table S9. Demographics and Clinical Data for the Confirmatory HIV‐Negative Cohort From the Synthetic Derivative
Table S10. Association Between Hypertension and Demographic and Clinical Characteristics in the HIV‐Negative Cohort From the Synthetic Derivative Using Logistic Regression
Table S11. Model Result Analysis Combining the HIV Positive and Negative Cohorts to Determine the Interaction Between Elevated Eosinophils and HIV
Table S12. Prediction Results With One Unit Increase of Eosinophilia in HIV Positive and Negative Cohorts
Acknowledgments
Graphics were produced using the online ePath3D toll (Protein Lounge, Inc).
(J Am Heart Assoc. 2020;9:e011450 DOI: 10.1161/JAHA.118.011450.)
References
- 1. Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV‐infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. [DOI] [PubMed] [Google Scholar]
- 2. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV‐infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18:264–276. [DOI] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, Fitzgerald DW, Kataraihya JB. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross‐sectional study. BMC Med. 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bigna JJR, Nansseu JRN, Um LN, Noumegni SRN, Sime PSD, Aminde LN, Koulla‐Shiro S, Noubiap JJN. Prevalence and incidence of pulmonary hypertension among HIV‐infected people in Africa: a systematic review and meta‐analysis. BMJ Open. 2016;6:e011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end‐organ damage. Circ Res. 2015;116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koethe JR, Jenkins CA, Furch BD, Lake JE, Barnett L, Hager CC, Smith R, Hulgan T, Shepherd BE, Kalams SA. Brief report: circulating markers of immunologic activity reflect adiposity in persons with HIV on antiretroviral therapy. J Acquir Immune Defic Syndr. 2018;79:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailin SS, Jenkins CA, Petucci C, Culver JA, Shepherd BE, Fessel JP, Hulgan T, Koethe JR. Lower concentrations of circulating medium and long‐chain acylcarnitines characterize insulin resistance in persons with HIV. AIDS Res Hum Retroviruses. 2018;34:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long‐term antiretroviral therapy. AIDS. 2016;30:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T cell and macrophage activation with arterial vascular health in HIV. AIDS Res Hum Retroviruses. 2017;33:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koethe JR, Jenkins CA, Petucci C, Culver J, Shepherd BE, Sterling TR. Superior glucose tolerance and metabolomic profiles, independent of adiposity, in HIV‐infected women compared with men on antiretroviral therapy. Medicine. 2016;95:e3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height‐normalized indices of the body's fat‐free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. [DOI] [PubMed] [Google Scholar]
- 14. Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142:904–908. [DOI] [PubMed] [Google Scholar]
- 15. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T‐cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J II, Harrison DG. DC isoketal‐modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II‐induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin‐17 causes rho‐kinase‐mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes‐Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension. 2011;57:1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martinez‐Maza O, Bream JH. The effect of haart‐induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV‐infected adults: novel pathophysiologic mechanisms. Hypertension. 2018;72:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–758. [DOI] [PubMed] [Google Scholar]
- 24. Benito JM, Lopez M, Lozano S, Martinez P, Gonzalez‐Lahoz J, Soriano V. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV‐infected patients receiving antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20:227–233. [DOI] [PubMed] [Google Scholar]
- 25. Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate‐like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21:4. [DOI] [PubMed] [Google Scholar]
- 26. Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ. High salt primes a specific activation state of macrophages, M(NA). Cell Res. 2015;25:893–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M‐positive monocytes mediate angiotensin II‐induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. [DOI] [PubMed] [Google Scholar]
- 28. Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL. Resistance artery remodeling in deoxycorticosterone acetate‐salt hypertension is dependent on vascular inflammation: evidence from M‐CSF‐deficient mice. Am J Physiol Heart Circ Physiol. 2007;292:H1789–H1795. [DOI] [PubMed] [Google Scholar]
- 29. Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt‐sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–H1025. [DOI] [PubMed] [Google Scholar]
- 30. Rudemiller NP, Crowley SD. The role of chemokines in hypertension and consequent target organ damage. Pharmacol Res. 2017;119:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II‐dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–R1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride‐sensitive channels mediate sodium‐induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kouro T, Takatsu K. IL‐5‐ and eosinophil‐mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. [DOI] [PubMed] [Google Scholar]
- 36. Takatsu K. Interleukin‐5 and IL‐5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Desreumaux P, Janin A, Colombel JF, Prin L, Plumas J, Emilie D, Torpier G, Capron A, Capron M. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992;175:293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL‐5 for mature B‐1 cells in homeostatic proliferation, cell survival, and IG production. J Immunol. 2004;172:6020–6029. [DOI] [PubMed] [Google Scholar]
- 39. Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL‐5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–3462. [DOI] [PubMed] [Google Scholar]
- 40. Takatsu K, Takaki S, Hitoshi Y. Interleukin‐5 and its receptor system: implications in the immune system and inflammation. Adv Immunol. 1994;57:145–190. [DOI] [PubMed] [Google Scholar]
- 41. Wu D, Molofsky AB, Liang HE, Ricardo‐Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amini M, Bashirova D, Prins BP, Corpeleijn E, LifeLines Cohort Study, Bruinenberg M, Franke L, van der Harst P, Navis G, Wolffenbuttel BHR, Stolk RP, Wijmenga C, Postma DS, Koppelman GH, Boezen HM, Vonk J, Snieder H, Alizadeh BZ. Eosinophil count is a common factor for complex metabolic and pulmonary traits and diseases: the lifelines cohort study. PLoS One. 2016;11:e0168480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Withers SB, Forman R, Meza‐Perez S, Sorobetea D, Sitnik K, Hopwood T, Lawrence CB, Agace WW, Else KJ, Heagerty AM, Svensson‐Frej M, Cruickshank SM. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Withers SB, Agabiti‐Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol. 2011;31:908–913. [DOI] [PubMed] [Google Scholar]
- 47. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. [DOI] [PubMed] [Google Scholar]
- 48. Masia M, Padilla S, Garcia N, Jarrin I, Bernal E, Lopez N, Hernandez I, Gutierrez F. Endothelial function is impaired in HIV‐infected patients with lipodystrophy. Antivir Ther. 2010;15:101–110. [DOI] [PubMed] [Google Scholar]
- 49. Behrens GM, Meyer‐Olson D, Stoll M, Schmidt RE. Clinical impact of HIV‐related lipodystrophy and metabolic abnormalities on cardiovascular disease. AIDS. 2003;17(suppl 1):S149–S154. [DOI] [PubMed] [Google Scholar]
- 50. Bidault G, Garcia M, Vantyghem MC, Ducluzeau PH, Morichon R, Thiyagarajah K, Moritz S, Capeau J, Vigouroux C, Bereziat V. Lipodystrophy‐linked LMNA p. R482W mutation induces clinical early atherosclerosis and in vitro endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2013;33:2162–2171. [DOI] [PubMed] [Google Scholar]
- 51. Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus‐1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. [DOI] [PubMed] [Google Scholar]
- 52. Gauckler P, Shin JI, Mayer G, Kronbichler A. Eosinophilia and kidney disease: more than just an incidental finding? J Clin Med. 2018;7:E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Gros G, Erard F. Non‐cytotoxic, IL‐4, IL‐5, IL‐10 producing CD8+ t cells: their activation and effector functions. Curr Opin Immunol. 1994;6:453–457. [DOI] [PubMed] [Google Scholar]
- 54. Gonzalez SM, Taborda NA, Rugeles MT. Role of different subpopulations of CD8(+) T cells during HIV exposure and infection. Front Immunol. 2017;8:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, Nguyen C, Iyer D, Kozinetz CA, Overbeek PA, Metzker ML, Balasubramanyam A, Lewis DE. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koethe JR, McDonnell W, Kennedy A, Abana CO, Pilkinton M, Setliff I, Georgiev I, Barnett L, Hager CC, Smith R, Kalams SA, Hasty A, Mallal S. Adipose tissue is enriched for activated and late‐differentiated CD8+ T cells and shows distinct CD8+ receptor usage, compared with blood in HIV‐infected persons. J Acquir Immune Defic Syndr. 2018;77:e14–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration. 2008;75:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Austin ED, Rock MT, Mosse CA, Vnencak‐Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med. 2010;104:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Increased CD4 T cell activation in people living with HIV is negatively associated with hypertension. Flow cytometry analysis of T cell subsets in people living with HIV showing (A) naïve and (B) memory CD4+ and CD8+T cell subtypes, (C) T cells expressing CD57, (D) PD1, (E) CD38and (F) the CD38 receptor in normotensives compared to hypertensives. *** P<0.001.
Figure S2. Gating strategy to identify T cell subtypes.
Figure S3. Analysis combining the HIV positive and negative cohorts to determine the Interaction between elevated eosinophils and HIV. Log odds of elevated eosinophils in HIV positive and negative cohort (P=0.045 in the HIV positive cohort and P=0.41 in the HIV negative cohort).
Table S1. Fat Mass Measurements Between Hypertensives and Normotensive
Table S2. Association Between Hypertension and Inflammatory Cell Subset/Biomarkers in HIV Using Logistic Regression Adjusted for Body Mass Index, Age, and Sex
Table S3. Analysis of P Values of Model Likelihood Ratio chi‐square test and Nagelkerke R 2 Index. Most of the Interesting Variables Have P<0.01 and R 2>0.3, Which Indicated the Goodness of Fit for the HIV‐Positive Cohort
Table S4. Analysis of C‐Index to Measure Goodness of Fit. All variables of interest have C‐index >0.75 indicating goodness of fit. See last column (C) of each Table
Table S5. Demographics and Clinical Data for HIV‐Negative Subjects
Table S6. Association Between Hypertension and Demographic and Clinical Characteristics in the HIV‐Negative Cohort Using Logistic Regression Adjusted for Body Mass Index, Age, and Sex
Table S7. Demographics and Clinical Data for Both the 50 HIV‐Negative and 70 HIV‐Positive Cohorts Combined
Table S8. Association Between Hypertension and Demographic/Clinical Characteristics in the Both the 50 HIV‐Negative and 70 HIV‐Positive Cohorts Logistic Regression
Table S9. Demographics and Clinical Data for the Confirmatory HIV‐Negative Cohort From the Synthetic Derivative
Table S10. Association Between Hypertension and Demographic and Clinical Characteristics in the HIV‐Negative Cohort From the Synthetic Derivative Using Logistic Regression
Table S11. Model Result Analysis Combining the HIV Positive and Negative Cohorts to Determine the Interaction Between Elevated Eosinophils and HIV
Table S12. Prediction Results With One Unit Increase of Eosinophilia in HIV Positive and Negative Cohorts
Data Availability Statement
The data sets used and/or analyzed during the current study will be made available on request from the corresponding author.
Human Study Population
We used the Adiposity and Immune Activation Cohort of 70 PLWH recruited at the Vanderbilt Comprehensive Care Clinic from 2013 to 2014, who have been previously described.8, 9, 10, 11, 12 All subjects were on efavirenz, tenofovir, and emtricitabine (ie, the combination pill Atripla) for at least the 6 months before enrollment, and had been on ART with persistent HIV‐1 RNA measurements <50 copies/mL for at least the previous 2 years. All participants were nondiabetic, with no history of acute coronary events, stroke, known rheumatologic or inflammatory conditions (aside from HIV), or concomitant comorbidities that might modify eosinophil counts (allergic rhinitis, asthma, dermatoses, and parasitic disease). Use of antihypertensive medication at the time of the study visit and/or >2 sequential outpatient systolic blood pressure measurements >140 mm Hg preceding the visit were used to classify hypertension status. A cohort of 50 HIV‐negative people, also from Vanderbilt University Medical Center, served as controls. Data on this cohort were obtained by review of medical records of volunteers who provided consent for participation in research conducted at the Division of Clinical Pharmacology. Data in normotensive and hypertensive HIV‐negative subjects with available differential counts of white blood cells were collected, eliminating participants with comorbidities known to modify eosinophil counts, such as allergic rhinitis, asthma, dermatoses, and parasitic disease. Each subject could have ≥1 eosinophil count over time (mean, 3.9±0.4; median, 3). If counts were >1, values were averaged. As an additional HIV‐negative control cohort, individuals of interest were sought in the Synthetic Derivative, a Vanderbilt University Medical Center database of ~2.5×106 deidentified electronic medical records. The search strategy consisted of identifying subjects without HIV/AIDS and dividing them by the presence versus absence of essential hypertension diagnostic codes. Only subjects containing ≥1 data point on eosinophil counts and body mass indexes (BMIs) were used. Exclusion criteria were all diseases known to produce eosinophilia, including but not limited to respiratory and cutaneous allergic disorders, hematologic malignancies, some infections, collagen vascular disorders, and ages <30 years and >60 years. For subjects with >1 measurement of eosinophils or BMI, all values were averaged. All participants provided written informed consent, and the study was approved by the Vanderbilt Institutional Review Board.
Inflammatory Biomarker Assessment
Venous blood was drawn in the morning between 8 and 11 am and after a minimum 8‐hour fast. Samples were collected in an EDTA‐containing vacutainer and centrifuged for 10 minutes at 4°C, and the plasma was removed and immediately frozen at −80°C. Plasma levels of soluble cluster of differentiation (sCD) 14 and sCD163, 2 surface markers released into circulation by activated macrophages, were measured using ELISA (R&D Systems, Minneapolis, MN). Other plasma cytokines and immune biomarkers, including interleukins, MCP1 (monocyte chemoattractant protein‐1), MIP‐1α/β (macrophage inflammatory protein‐1 α and β), tumor necrosis factor‐α (TNF‐α), and soluble TNF‐α receptors 1 and 2 (sTNFR1 and sTNFR2, respectively), as well as vascular cell adhesion molecule‐1 and intercellular adhesion molecule‐1 (ICAM‐1), were measured in duplicate using a standard multiple immunoassay panel (MesoScale, Rockville, MD).
Flow Cytometry
Peripheral blood mononuclear cells were obtained from fasting whole blood samples, as previously described.10 Flow cytometry was performed using the Fortessa (Becton Dickson Biosciences) flow cytometer to measure activated, senescent, exhausted, and memory T‐cell subsets using previously described fluorochrome panels.8
Eosinophil Counts
Eosinophil counts and percentages were obtained in normotensive and hypertensive participants with and without HIV from automated differential cell counts performed in the Vanderbilt University Medical Center Clinical Laboratory.
Assessment of Body Composition
A full‐body Dual‐energy X‐ray absorptiometry (GE Lunar Prodigy; GE Healthcare, Little Chalfont, UK) measured total body fat mass to calculate fat mass index (FMI; total fat in kilograms divided by height in meters, squared). FMI is a variant of BMI that accounts for individual variability in the ratio of fat/lean mass.13 The assessment of FMI is shown in Table S1.
Statistical Analyses
We assessed the normality of data using kurtosis and skewness values as well as graphing using Q‐Q plots. Medians and interquartile ranges were calculated for continuous variables, and percentages were calculated for categorical variables. Demographics, clinical characteristics, and the outcome variables were compared between hypertensive and normotensive people using Mann‐Whitney U test or χ2 test, as appropriate. We conducted statistical analysis in R software (http://www.r-project.com). Logistic regression was performed to analyze the association between hypertension and inflammation markers, with adjustment of age, sex, and FMI/BMI for the HIV‐positive participants and age, sex, and BMI for the HIV‐negative participants. Log2 transformation was performed on variables with highly skewed data distribution. Multiple imputation was performed to impute missing data with Hmisc package in R (cran.r‐project.org/package=Hmisc). No adjustments were made for multiple comparisons for this exploratory study, although results and the potential for false discovery were interpreted in the context of known biological pathways.14 P=0.05 was used to infer statistical significance.


