Abstract
Background
Sex‐specific differences may influence prognosis after deferred revascularization following fractional flow reserve (FFR) measurement. This study sought to investigate the sex differences in long‐term prognosis of patients with deferred revascularization following FFR assessment.
Methods and Results
A total of 879 patients (879 vessels) with deferred revascularization with FFR >0.75 who underwent FFR and coronary flow reserve measurements were enrolled from 3 countries (Korea, Japan, and Spain). Long‐term outcomes were assessed in 649 men and 230 women by the patient‐oriented composite outcome (POCO, a composite of any death, any myocardial infarction, and any revascularization). We applied inverse‐probability weighting based on propensity scores to account for differences at baseline between women and men (age, hyperlipidemia, diabetes mellitus, diameter stenosis, lesion length, multivessel disease, FFR, coronary flow reserve. The median follow‐up duration was 1855 days (745–1855 days). Median FFR values were 0.88 (0.83–0.93) in men and 0.89 (0.85–0.94) in women, respectively. The occurrences of POCO were significantly high in men compared with that in women (10.5% versus 4.2%, P=0.007). Kaplan–Meier analysis revealed that women had a significantly lower risk of POCO (χ2=7.2, P=0.007). Multivariate COX proportional hazards regression analysis revealed that age, male, diabetes mellitus, diameter stenosis, lesion length, and coronary flow reserve were independent predictors of POCO. After applying IPW, the hazard ratio of males for POCO was 2.07 (95% CI, 1.07–4.04, P=0.032).
Conclusions
This large multinational study reveals that long‐term outcome differs between women and men in favor of women after FFR‐guided revascularization deferral.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT02186093.
Keywords: coronary flow reserve, fractional flow reserve, microvascular dysfunction, sex differences
Subject Categories: Prognosis
Clinical Perspective
What Is New?
In patients from the multinational registry with revascularization deferral after fractional flow reserve (FFR) assessment, long‐term outcomes during 5‐year follow‐up differed significantly between women and men in favor of women, which was confirmed by the propensity score–adjusted inverse‐probability of weighing Cox proportional hazards analysis.
CFR was significantly lower in females in nonobstructive coronary disease, and CFR, but not FFR was an independent predictor of patient‐oriented composite outcomes (all‐cause mortality, any myocardial infarction, and any revascularization) in deferred patients.
What Are the Clinical Implications?
The present hypothesis‐generating study may support the importance of functional assessment of coronary artery disease, particularly in females and may also suggest the potential of a more optimized approach for stable coronary heart disease in females beyond the current equal cut‐off point of FFR value for revascularization decision making.
Introduction
Physiological assessment of epicardial coronary stenosis by fractional flow reserve (FFR) has been used to guide decision making for revascularization in both sexes. Women and men have a different prevalence of ischemic heart disease.1 Previous studies investigating the sex differences in FFR values showed that angiographic lesions of similar stenosis are less likely to cause ischemic FFR in women.2, 3 Sex‐related differences can influence not only FFR assessment but treatment decisions and prognosis. Sex differences in percutaneous coronary intervention (PCI) benefits have been extensively studied but still remains controversial with inconsistent results.4, 5, 6, 7 When considering higher FFR values for given stenosis in women,2 functional guidance by using FFR is more likely to facilitate an appropriate revascularization decision in women than in men. A recent secondary analysis from the FAME (Fractional Flow Reserve Versus Angiography for Mutivessel Evaluation) trial demonstrated that a functionally significant stenosis was less common in women and that FFR‐guided decision making demonstrated an equal benefit in both sexes during 2‐year follow‐up.8 However, it remains undetermined whether long‐term outcome in patients with FFR‐guided revascularization deferral is comparable between women and men. This multinational and multicenter study sought to investigate the difference in long‐term prognosis between women and men in patients with deferred revascularization after FFR assessment.
Methods
Patient Population
The present study was a patient‐level pooled analysis of 3 prospective registries whose results have been previously published.9, 10, 11, 12, 13 The first study prospectively enrolled consecutive patients from 5 university hospitals in Korea (519 patients, 737 vessels), each undergoing clinically indicated invasive coronary angiography, and FFR, coronary flow reserve (CFR), and index of microcirculatory resistance (IMR) measurement for at least 1 coronary artery.10 The second study was an institutional registry of Tsuchiura Kyodo General Hospital, Ibaraki, Japan that included 643 patients (643 vessels) submitting to invasive angiography and physiologic assessment, including FFR, CFR, and IMR.13 The third study prospectively enrolled patients with FFR, CFR, and IMR data on at least 1 intermediate‐grade stenosis from Hospital Clinico San Carlos, Madrid, Spain.11 In all these studies, patients with hemodynamic instability, left ventricular dysfunction, or a culprit vessel of acute coronary syndrome were excluded. Individual patient data for pooled analysis were collected using standardized spreadsheets. For all variables included, standardized definitions were used. Invasive physiologic indices were also cross‐checked and confirmed by each study's principal investigators (T.K., J.E., B.K.).
Among the 1397 patients (1694 vessels) enrolled overall, those undergoing PCI were excluded. In the presence of multiple coronary stenoses, a single vessel with the most severely decreased FFR value was used for the present analysis. Of the remaining 914 patients with deferred revascularization, this study enrolled 879 deferred patients with lesions showing FFR values >0.75 (Figure 1). Only 1 patient of all the study cohort was lost to follow‐up. Study protocols were designed in accordance with the Declaration of Helsinki and were authorized by institutional review boards or ethics committees at corresponding centers. All patients granted written informed consent. The study protocol of the International Collaboration of Comprehensive Physiologic Assessment was registered at http://clinicaltrials.gov (NCT03690713).
Figure 1.
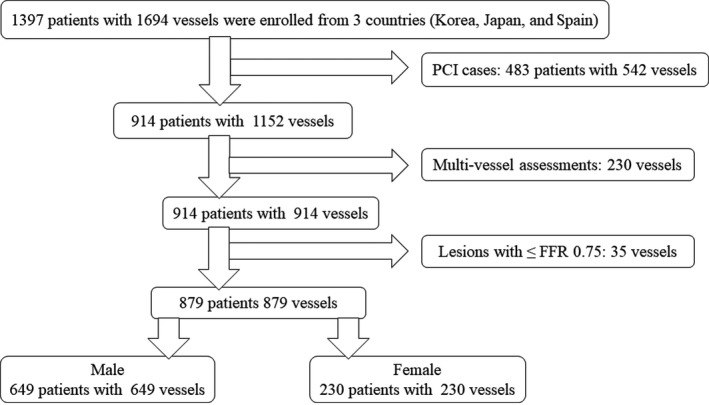
The Consolidated Standards of Reporting Trials flow diagram. FFR indicates fractional flow reserve, PCI, percutaneous coronary intervention.
Coronary Angiographic Analysis
Coronary angiography was performed using standard techniques. All angiograms were analyzed at local core laboratories in blinded fashion. Percent diameter stenosis, minimum luminal diameter, reference‐vessel size, and lesion length were measured. All coronary physiological parameters were measured following diagnostic angiography. A guiding catheter (5–7Fr) without side holes was used to engage coronary arteries, and a pressure/temperature‐sensor guide wire (Abbott Vascular, St. Paul, MN,) was used to measure FFR and CFR.
Coronary Physiological Assessment
FFR, mean transit time (Tmn), and IMR were determined using a RadiAnalyzer Xpress instrument with a Pressure Wire Certus (St. Jude Medical, St. Paul, MN). FFR and IMR were measured in vessels determined to be clinically indicated for evaluation. After nitroglycerine was administered, a pressure‐monitoring guidewire was advanced distal to the stenosis. Hyperemia was induced by an intravenous infusion of adenosine (140 mg/kg per minute). FFR was calculated by dividing the mean distal pressure by the mean aortic pressure during stable hyperemia. For IMR measurements, hyperemic thermodilution curves (measured 3 times each using a 3‐mL saline bolus injection) and hyperemic Tmn were obtained. The IMR was calculated as the product of the mean distal coronary pressure during stable hyperemia and mean hyperemic Tmn.9, 14 The CFR was measured simultaneously with FFR and IMR using the thermodilution method and expressed as the ratio of basal Tmn divided by hyperemic Tmn.15 After physiological measurements, the pressure wire was retracted into the guiding catheter to evaluate pressure drift. For lesions with low FFR (≤0.80), PCI was recommended, as stipulated by current guidelines. However, decisions regarding PCI were at the discretion of operators. Patients who underwent PCI were excluded from the analysis. Of note, receiver operating curves analyses demonstrated that the best cut‐off values of FFR values to predict PCI in this registry were 0.80 for both sexes (Figures S1 through S3).
Clinical Follow‐Up, Outcome Measures, and Adjudication of Clinical Events
Clinical data were obtained at outpatient clinic visits or by telephone contact if needed. The primary study end point was patient‐oriented composite outcomes (POCO) including all‐cause mortality, any myocardial infarction, and any revascularization. All clinical outcomes were defined as stipulated by the Academic Research Consortium, including the addendum to the definition of myocardial infarction.16 In the absence of a clear noncardiac cause, all deaths were considered cardiac related.
Statistical Analysis
Categorical variables were expressed as numbers and relative frequencies (percentages), and continuous variables as means and standard deviations or medians with interquartile ranges (Q1–Q3) according to related distributions. Shapiro–Wilk test was used to assess for departures from normality and the distributions were further confirmed visually. Data were analyzed on a per‐patient basis for clinical characteristics and outcomes for comparison between women and men, and between the patients with or without POCO. Although a single vessel with the most severely decreased FFR value was used for the present analysis in the presence of multiple coronary stenoses, we included any nontarget vessel events that did not undergo physiological evaluation. Since several subjects experienced not only target‐vessel–oriented cardiac events but non–target vessel revascularization, the first event that occurred was censored and counted in the survival analysis using Kaplan–Meier estimates for POCO. Receiver operating curves analysis was applied to assess the best FFR cutoff values for performing or deferring PCI. The optimal cutoff was calculated using Youden's index. Survival curves were estimated using Kaplan–Meier estimates and were compared using log‐rank tests. A Cox proportional hazards regression model was used to identify independent predictors of POCO. The covariates with P<0.10 in the univariate analysis were included in the multivariate analysis. A collinearity index was used for checking linear combinations among covariates, and the Akaike information criterion for avoiding overfitting. The assumption of proportionality was assessed graphically by log‐minus‐log plot, and Cox proportional hazard models for all clinical outcomes satisfied the proportional hazards assumption. Outcomes were evaluated both before and after risk adjustment by using propensity score inverse‐probability of weighing.17 The following variables were used to calculate propensity scores: age, hypertension, diabetes mellitus, dyslipidemia, FFR, CFR, IMR, diameter stenosis, lesion length, lesion location, acute coronary syndrome, and multivessel disease. Using the propensity scores for each group comparison, IPW was used to adjust covariates. A 2‐sided P<0.05 was considered statistically significant. Standard software application (SPSS v23, SPSS Inc, Chicago, IL) and R version 3.0.2 (The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
Results
Baseline Patient Characteristics
Table 1 shows clinical, angiographic, and physiological characteristics of the 879 patients included in this study. Most patients presented with stable coronary artery disease, and in those with acute coronary syndrome nonculprit vessels were physiologically investigated. Women were significantly older than men. Angiographic severity of investigated coronary stenosis was mostly intermediate, median diameter stenosis, 43.2% (31.3–52.8), and median FFR value was 0.87 (0.83–0.91). Figure 2 presents the distribution of FFR values in a total cohort, women, and men, respectively. In women, FFR values tended to be higher, although angiographic stenosis severity was similar between both sexes. For a nonobstructive coronary artery disease (CAD) (diameter stenosis <50%), female patients showed higher FFR values than male patients (Figure 3). CFR values were significantly lower in women (3.0 versus 2.5, P<0.001). No significant difference in IMR was observed between female and male (female versus male; 17.0 versus 17.3, P=0.667).
Table 1.
Patient Characteristics
| Overall (N=879) | Male (N=649) | Female (N=230) | P Value | |
|---|---|---|---|---|
| Age, y | 65.0 (57.0–72.0) | 64.0 (56.0–71.0) | 67.0 (60.0–74.0) | <0.001 |
| Hypertension | 556 (63.3) | 411 (63.3) | 145 (63.0) | 1.000 |
| Dyslipidemia | 556 (63.3) | 418 (64.4) | 138 (60.0) | 0.266 |
| Diabetes mellitus | 295 (33.6) | 226 (34.8) | 69 (30.0) | 0.211 |
| Current smoker | 187 (21.3) | 177 (27.3) | 10 (4.3) | <0.001 |
| ACS nonculprit lesion | 128 (14.6) | 94 (14.5) | 34 (14.8) | 0.999 |
| Physiological characteristics | ||||
| FFR | 0.87 (0.83–0.91) | 0.87 (0.82–0.91) | 0.88 (0.84–0.91) | 0.053 |
| CFR | 2.9 (2.0–4.0) | 3.0 (2.1–4.1) | 2.5 (2.0–3.6) | <0.001 |
| IMR | 17.2 (12.7–24.5) | 17.3 (12.7–24.8) | 17.0 (12.6–23.2) | 0.667 |
| Tmn at rest | 0.73 (0.48–1.04) | 0.76 (0.51–1.08) | 0.59 (0.42–0.87) | <0.001 |
| Tmn at hyperemic | 0.24 (0.17–0.34) | 0.25 (0.18–0.35) | 0.23 (0.17–0.33) | 0.064 |
| Angiographic characteristics | 0.15 (0.05–0.50) | 0.16 (0.04–0.52) | 0.12 (0.06–0.44) | 0.973 |
| Lesion location (LAD/LCX/RCA) | 610/108/161 | 422/90/135 | 188/18/26 | 0.001 |
| Reference diameter | 2.91 (2.48–3.29) | 2.99 (2.52–3.34) | 2.77 (2.43–3.09) | <0.001 |
| Minimum lumen diameter | 1.64 (1.33–2.06) | 1.67 (1.36–2.08) | 1.58 (1.29–2.00) | 0.078 |
| Diameter stenosis | 43.2 (31.3–52.8) | 42.4 (31.5–53.1) | 43.2 (30.1–51.8) | 0.732 |
| Lesion length | 10.1 (6.6–15.0) | 10.2 (6.7–15.1) | 10.0 (6.3–14.4) | 0.548 |
| Multivessel disease | 382 (43.5) | 303 (46.7) | 79 (34.3) | 0.002 |
Data are presented as n (%) or median (Q1–Q3). ACS indicates acute coronary syndrome; CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; Tmn, mean transit time.
Figure 2.
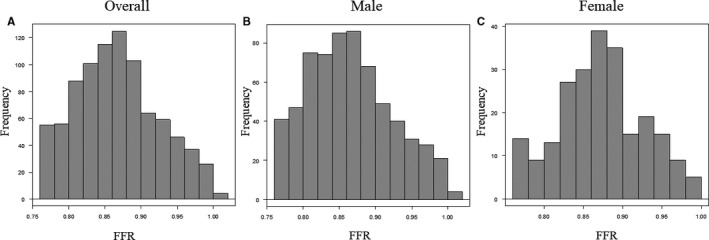
Distribution of the FFR. A, Patient‐level histogram of FFR values in the total cohort; (B) in males; (C) in females. FFR indicates fractional flow reserve.
Figure 3.
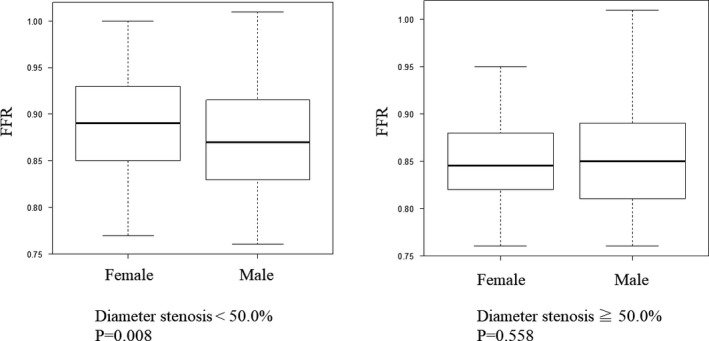
FFR values according to angiographic stenosis severity; left, angiographic stenosis <50%, right, angiographic stenosis ≥50%. FFR indicates fractional flow reserve.
Clinical Outcomes of Deferred Patients After FFR Assessment
During 5‐year follow‐up, POCO occurred in 83 patients (16 deaths, 14 myocardial infarctions, and 53 any revascularization, women versus men; [4.8% versus 11.1%, P=0.007]) (Table 2). The median follow‐up duration was 5 years (2.0–5.0 years). There was no significant difference in time to POCO between the sexes (male versus female; 1.8 years versus 1.0 years, P=0.110). In the subgroup analysis of deferred patients with FFR >0.8, male patients were also significantly associated with poor prognosis (POCO: χ2=10.0, P=0.002) (Figure S4). Figure 4 shows that there were no differences in cumulative rates of POCO at 1‐, 2‐, or 3‐year follow‐up between the groups. The cumulative rates of POCO were higher in male than in female both at the 4‐year (hazard ratio: 2.22, P=0.012) and 5‐year (hazard ratio: 2.33, P=0.007) follow‐up examination. Multivariate Cox proportional hazard models revealed that age, sex, diabetes mellitus, diameter stenosis, lesion length, and CFR were independently significant predictors of POCO in a total cohort (Table 3). Of interest, age, diabetes mellitus, and CFR were significant predictors of POCO in men, while multivessel disease was a significant predictor in women (Tables 4 and 5). Propensity score–adjusted inverse‐probability of weighing Cox proportional hazards analysis showed that male patients showed significantly higher risk of POCO than female patients (adjusted hazard ratio 2.14, 95% CI 1.05–4.34, P=0.036).
Table 2.
Clinical Events During Follow‐Up Period
| Male (n=649) | Female (n=230) | P Value | |
|---|---|---|---|
| POCO | 72 (11.1%) | 11 (4.8%) | 0.007 |
| Death | 20 (3.1%) | 1 (0.4%) | 0.022 |
| Cardiac death | 15 (2.3%) | 1 (0.4%) | |
| Noncardiac death | 5 (0.8%) | 0 | |
| Nonfatal myocardial infarction | 11 (1.7%) | 3 (1.3%) | 1.000 |
| Any revascularization | 41 (6.3%) | 7 (3.0%) | 0.087 |
| TVR | 25 (3.9%) | 6 (2.6%) | |
| Non‐TVR | 16 (2.5%) | 1 (0.4%) |
Data are presented as n (%). POCO indicates patient‐oriented cardiovascular events; TVR, target vessel revascularization.
Figure 4.
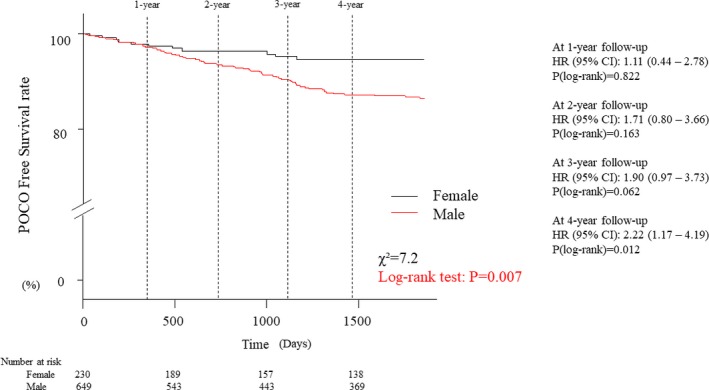
Kaplan–Meier curves of freedom from POCO. The incidence of POCO was significantly higher in males at 5‐year follow‐up. On the other hand, there were no differences in cumulative rates of POCO at 1‐, 2‐, or 3‐year follow‐up between the groups. HR indicates hazard ratio; POCO, patient‐oriented composite outcome.
Table 3.
Univariate and Multivariate Cox Proportional Hazards Analysis for POCO
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | 1.05 | 1.02–1.07 | <0.001 | 1.04 | 1.01–1.07 | 0.002 |
| Male | 2.33 | 1.23–4.38 | 0.009 | 2.93 | 1.54–5.58 | 0.001 |
| Hyperlipidemia | 1.30 | 0.82–2.07 | 0.264 | |||
| Diabetes mellitus | 2.12 | 1.38–3.25 | <0.001 | 1.81 | 1.17–2.80 | 0.007 |
| Smoker | 1.16 | 0.69–1.93 | 0.574 | |||
| Diameter stenosis | 1.03 | 1.01–1.04 | <0.001 | 1.02 | 1.00–1.03 | 0.026 |
| Lesion length | 1.04 | 1.02–1.06 | 0.001 | 1.03 | 1.00–1.06 | 0.021 |
| Multivessel disease | 1.72 | 1.11–2.65 | 0.015 | |||
| FFR | 0.02 | 0.04×10−2 to 0.89 | 0.044 | |||
| CFR | 0.69 | 0.57–0.84 | <0.001 | 0.75 | 0.61–0.91 | 0.004 |
| IMR | 1.01 | 1.00–1.02 | 0.220 | |||
| Tmn (at hyperemic) | 2.75 | 1.11–6.85 | 0.030 | |||
CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; IMR, index of microcirculatory resistance; POCO, patient‐oriented cardiovascular events; Tmn, mean transit time.
Table 4.
Univariate and Multivariate Cox Proportional Hazards Analysis for POCO (Male)
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | 1.05 | 1.03–1.08 | <0.001 | 1.04 | 1.02–1.07 | 0.001 |
| Diabetes mellitus | 1.96 | 1.23–3.11 | 0.004 | 1.72 | 1.08–2.75 | 0.023 |
| Diameter stenosis | 1.02 | 1.01–1.04 | 0.002 | 1.02 | 1.00–1.03 | 0.058 |
| Lesion length | 1.04 | 1.01–1.06 | 0.010 | 1.03 | 1.00–1.06 | 0.051 |
| Multivessel disease | 1.37 | 0.86–2.17 | 0.186 | |||
| FFR | 0.01 | 0.02×10−1 to 6.74 | 0.307 | |||
| CFR | 0.64 | 0.52–0.79 | <0.001 | 0.71 | 0.58–0.88 | 0.002 |
| Tmn (at hyperemic) | 2.85 | 1.17–6.94 | 0.021 | |||
CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; POCO, patient‐oriented cardiovascular events; Tmn, mean transit time.
Table 5.
Univariate and Multivariate Cox Proportional Hazards Analysis for POCO (Female)
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | 1.03 | 0.96–1.10 | 0.379 | |||
| Diabetes mellitus | 2.95 | 0.90–9.67 | 0.074 | |||
| Diameter stenosis | 1.05 | 1.01–1.10 | 0.016 | |||
| Lesion length | 1.06 | 1.01–1.11 | 0.030 | |||
| Multivessel disease | 5.12 | 1.36–19.31 | 0.016 | 5.12 | 1.36–19.31 | 0.016 |
| FFR | 5.76×10−7 | 0.04×10−11 to 0.08 | 0.017 | |||
| CFR | 0.91 | 0.52–1.58 | 0.732 | |||
CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; POCO, patient‐oriented cardiovascular events.
Exploratory subgroup analysis indicated that the subgroup (FFR ≤0.80) showed the qualitative interaction of sex effect (Figure 5). No other qualitative interactions were observed.
Figure 5.
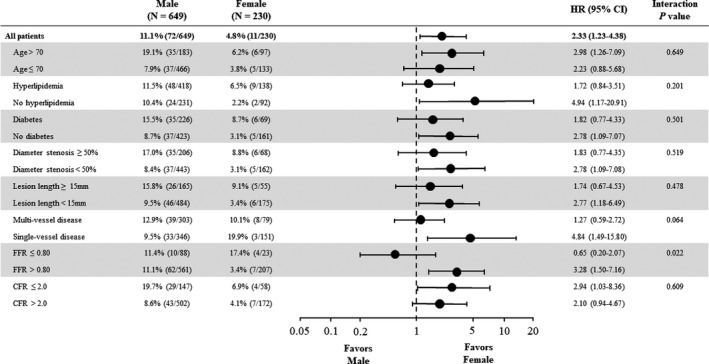
Exploratory subgroup analysis for POCO at 5 years. Exploratory subgroup analysis indicated that the subgroup (FFR ≤0.80) showed the qualitative interaction of sex effect. No other qualitative interactions were observed. The percentage of patients with an event represents the Kaplan–Meier event rate at 5 years. Horizontal lines indicate 95% CI. CFR indicates coronary flow reserve; FFR, fractional flow reserve; HR, hazard ratio; POCO, patient‐oriented composite outcome.
Discussion
This study was undertaken to investigate the sex difference in long‐term clinical outcomes of patients with revascularization deferral after FFR assessment. The important findings of the present study are the following: (1) for a nonobstructive CAD, female patients showed higher FFR values than male patients; (2) female patients with revascularization deferral showed significantly lower risk of POCO than that in male patients during 5‐year follow‐up; (3) the lower risk of POCO in female patients was confirmed by the propensity score–adjusted inverse‐probability of weighing Cox proportional hazards analysis; (4) in a total cohort with the median FFR value of 0.87, CFR, but not FFR was a significant predictor of POCO.
There is still scarce information regarding the sex difference in long‐term outcomes after FFR‐guided decision making in stable CAD patients with revascularization deferral. In the FAME substudy, an FFR‐guided PCI strategy is equally beneficial in females as it is in males.8 They showed females had similar rates of major adverse cardiac events during 2‐year follow‐up and there were no interactions between sex and treatment method for any outcome variables. From a different perspective from the previous study, our study provides the prognostic information for a longer follow‐up period (median; 5 years) and limited the analysis in patients with deferred revascularization. Although women had a significantly lower risk of POCO at 5 years, our registry data showed no statistically significant sex difference in prognosis when the analysis was limited for a 2‐year period, as was similar to the previous report.8 This longer follow‐up period might have at least in part contributed to the clinical significance of sex difference and demonstrated the better prognosis for POCO in female patients after FFR‐guided deferred revascularization. Our results, however, were obtained from the registry data and are merely hypothesis generating. Further studies are needed to clarify the sex difference in long‐term prognosis after FFR‐guided revascularization deferral. The present study might also cast a light on the sex‐specific FFR cutoff optimization in relation to the possible sex difference in long‐term prognosis for patients who undergo revascularization after FFR assessment.
Difference Between the Present Study and Previous Studies
Some of the differences between the previous studies and ours should be considered when interpreting the results of this study. First, compared with previous data, which showed females underwent about 10%—cardiac events after PCI, 5, 18, 19 Our data showed that females with deferred revascularization after FFR assessment underwent only 4.9% POCO during 5‐year follow‐up, which was longer than the previous studies and might have contributed to more prominent difference during the longer follow‐up in the current study. In particular, as suggested by the reviewer, long‐term events in male patients may have been related to lesions that did not undergo hemodynamic evaluation during initial evaluation or to lesions that were angiographically more severe in similar FFR ranges between both sexes at long‐term follow‐up. Exact reasons and/or mechanisms that can explain inconsistent results remained to be determined. The genetic difference (majority of the current cohort: Asian patients) might contribute at least to some extent as well as the difference in the baseline study population, although our female patients shared similar characteristics with the previous studies such as being older, fewer smokers, better FFR, lower CFR, smaller vessel size, and smaller cardiac mass. However, our study samples showed similar IMR, indicating no difference in microvascular responsiveness to hyperemic induction according to the sexes, and lower prevalence of multivessel disease in female patients (43.5% versus 34.3%, P=0.002). Furthermore, lower prevalence of left anterior descending artery culprit lesion location might also have impacted the results of the current study, demonstrating better prognosis in favor of deferred female patients. Another potential explanation for better prognosis in favor of female deferred patients might be as follows: our results indicated the similar microvascular function and the prevalence of increased hyperemic microvascular resistance represented by IMR. (IMR female versus male: 17.0 versus 17.3, P=0.667, prevalence of microvascular dysfunction defined by IMR >25: 21.7% versus 24.7%, P=0.42). Given the previously reported prevalence and worse outcomes in female with microvascular disease,20, 21 female patients without physiologically significant epicardial disease represented by nonischemic FFR in the present study, might have lower chance with microvascular dysfunction, which could impact the better prognosis in women in the present cohort.
It remains undetermined whether outcome in patients with FFR‐guided revascularization deferral is comparable between female and male. In our study, the analysis was limited in revascularization deferral and we adjusted any confounder for POCO using IPW analysis by considering relatively small female sample size. Therefore, there is still room for discussion regarding the difference in long‐term prognosis between female and male in patients with deferred revascularization after FFR assessment.
Differing Clinical Characteristics Based on Sex Differences
Cardiovascular disease remains the leading cause of morbidity and mortality for both female and male. However, female patients manifest differently in terms of clinical symptoms, prevalence of diagnosis, and treatment strategy. Previous reports consistently showed the differences in age and other comorbidities between both sexes. These evidences may indicate higher atherosclerotic burden in female patients with coronary heart disease, although the FFR value, which demonstrates the continuous relationship with future adverse events, has been reported to be higher compared with males for a given anatomical stenosis severity. In the present study, females showed higher FFR values than males for a comparable angiographic stenosis. These data further support the importance of functional assessment of coronary artery disease, particularly in females, since females had lower likelihood of obstructive epicardial disease than males.
Impact of Microvascular Disease on Sex Differences
For the past few decades, diagnosis and treatment practice have been focused on epicardial coronary artery stenosis, although recent emerging evidences22, 23 have established the concept that obstructive epicardial stenosis is not necessary nor required to cause ischemic symptoms of stable coronary heart disease. Recent studies reported that symptomatic women are more likely than men to present with nonobstructive CAD and coronary microvascular dysfunction, suggesting less benefit of revascularization therapy such as PCI and coronary artery bypass graft for women compared with men.20, 24 Although evidence‐based standard care should be provided equally to women and men, we need to understand the difference in pathophysiology beyond an epicardial stenosis–centered approach. Higher risk profile, more comorbidities, smaller vessel, smaller cardiac mass, different symptom manifestation, prevalence of diagnosis of epicardial coronary artery disease, and microvascular dysfunction in female patients have been consistently reported as were also observed in the present study. Sex bias was previously reported in the use of evidence‐based medical therapy,21 and whether this bias may have an impact on outcome after FFR‐guided treatment seems to be undetermined. More than 40% of patients with angina have been reported to have nonobstructive coronary artery disease, and the physiological basis of their symptoms including microvascular dysfunction remains elusive, and at the present time, no specific and evidence‐based effective therapeutic strategy has been proposed. These evidences might indicate higher atherosclerotic burden in female patients with coronary heart disease, although the FFR value, which demonstrates the continuous relationship with future adverse events, has been reported to be higher compared with males for a given anatomical stenosis severity. In contrast, microvascular dysfunction affected FFR values towards higher direction for a given epicardial stenosis. In the present study, females also showed higher FFR values than males for a comparable angiographic stenosis, which was in line with previous findings. These data further support the importance of functional assessment of coronary artery disease, particularly in females, since females had a lower likelihood of obstructive epicardial disease than males, and higher likelihood of low CFR.25, 26, 27 Furthermore, the long‐term outcomes during 5‐year follow‐up in the present study differed significantly between females and males in favor of females. Given these circumstances, our results indicate that there might be room for sex‐specific diagnostic and therapeutic optimization beyond the current 1 FFR cut‐off point for both sexes. Our results also indicated similar microvascular function represented by IMR in the present cohort. (IMR female versus male: 17.3 versus 17.0, P=0.67). Given the previously reported higher prevalence and worse outcomes in females with microvascular disease, female patients in this study population showed a lower prevalence of microvascular dysfunction. Since thermodilution CFR is calculated by the combination of resting and hyperemic Tmn, CFR values are affected by the relationship between these 2 metrics. In general, resting coronary flow has been reported to be higher in females, and this observation is attributable to lower CFR in female patients, which is consistent with the results in the present cohort. Despite similar microvascular function in females and males by IMR, CFR is lower in females. This discrepancy appears to be because of differences in resting coronary flow between the sexes. The effect of sex differences should be considered in interpretation of physiological indexes using resting coronary flow.
Clinical Implications
Previous sex‐based studies in patients undergoing PCI have reported similar outcomes after PCI in women and men.5, 8 On the other hand, in our analysis with revascularization deferral after FFR assessments, long‐term outcomes during 5‐year follow‐up differed significantly between women and men in favor of women. Because females appear to have higher FFR values for a nonobstructive CAD, it may be even more relevant to measure FFR in women to confirm hemodynamic significance. These data support the importance of functional assessment of coronary artery disease, particularly in women. Moreover, although FFR was a significant univariate predictor of prognosis in female, not FFR but CFR was a significant predictor of POCO in the overall cohort. This study underscores the need for improved research and understanding of sex‐specific differences of coronary heart disease and pathophysiology, and sex‐specific coronary flow impairment should be further studied. Our results may also suggest the potential of a more optimized approach for stable coronary heart disease in females beyond the current equal cutoff point of FFR value for revascularization decision making, although speculative.
Study Limitations
Our results should be interpreted by considering several important limitations. First, although FFR was measured in all patients and the operators were recommended to use FFR values for decision making of revascularization and not blinded to physiological indices, final decision to perform PCI was at the discretion of the operators, thereby allowing potential selection bias (especially in patients with gray zone FFR values). Final decisions for selecting the lesions for the initial physiological testing were at the discretion of operators. Culprit lesions of late revascularization may not necessarily undergo physiological assessment during the index procedures. Second, noninvasive test results were not routinely performed and not available in this study. Third, the current study was not a randomized controlled trial, and its inherent limitations of the registry studies may apply. Fourth, for randomized trials targeting ischemic heart disease, particularly for those investigating the benefit of revascularization, the enrollment of women remains low, resulting in important limitations for the powered evidence base of therapeutic guidelines. Fifth, because of the limitation that this study analyzed the data from the international multicenter registry, we could not identify the location of lesion that involved occurrence of myocardial infarction from this registry database.
Conclusions
In patients from the multinational registry with revascularization deferral after FFR assessment, long‐term outcomes during 5‐year follow‐up differed significantly between women and men in favor of women.
Disclosures
Dr Koo received an Institutional Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. Dr J. M. Lee received a Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. Dr Hahn received a Research Grant from St. Jude Medical (Abbott Vascular). Dr Escaned received personal fees from Philips 22 Volcano, Boston Scientific, and Abbott/St. Jude Medical outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Figure S1. ROC analyses of FFR values to predict revascularization. ROC analysis demonstrated the best cut‐off values of 0.80 to predict revascularization in the total cohort. FFR indicates fractional flow reserve; ROC, receiver operating curves.
Figure S2. ROC analyses of FFR values to predict revascularization. ROC analysis of the best cutoff FFR value of 0.80 to predict revascularization in male. FFR indicates fractional flow reserve; ROC, receiver operating curves.
Figure S3. ROC analyses of FFR values to predict revascularization. ROC analysis demonstrated that the best cut‐off value of 0.80 to predict revascularization in female. FFR indicates fractional flow reserve; ROC, Receiver operating curves.
Figure S4. Kaplan–Meier Curves of freedom from POCO.
(J Am Heart Assoc. 2020;9:e014458 DOI: 10.1161/JAHA.119.014458.)
References
- 1. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G; WISE Investigators . Insights from the NHLBI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender‐based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. [DOI] [PubMed] [Google Scholar]
- 2. Kang SJ, Ahn JM, Han S, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Park SW, Mintz GS, Park SJ. Sex differences in the visual‐functional mismatch between coronary angiography or intravascular ultrasound versus fractional flow reserve. JACC Cardiovasc Interv. 2013;6:562–568. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Rihal CS, Matsuo Y, Elrashidi MY, Flammer AJ, Lee MS, Cassar A, Lennon RJ, Herrmann J, Bell MR, Holmes DR, Bresnahan JF, Hua Q, Lerman LO, Lerman A. Sex‐related differences in fractional flow reserve‐guided treatment. Circ Cardiovasc Interv. 2013;6:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weintraub WS, Wenger NK, Kosinski AS, Douglas JS Jr, Liberman HA, Morris DC, King SB III. Percutaneous transluminal coronary angioplasty in women compared with men. J Am Coll Cardiol. 1994;24:81–90. [DOI] [PubMed] [Google Scholar]
- 5. Mikhail GW, Gerber RT, Cox DA, Ellis SG, Lasala JM, Ormiston JA, Stone GW, Turco MA, Joshi AA, Baim DS, Colombo A. Influence of sex on long‐term outcomes after percutaneous coronary intervention with the paclitaxel‐eluting coronary stent: results of the “TAXUS Woman” analysis. JACC Cardiovasc Interv. 2010;3:1250–1259. [DOI] [PubMed] [Google Scholar]
- 6. Duvernoy CS, Smith DE, Manohar P, Schaefer A, Kline‐Rogers E, Share D, McNamara R, Gurm HS, Moscucci M. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry. Am Heart J. 2010;159:677–683.e671. [DOI] [PubMed] [Google Scholar]
- 7. Mehilli J, Kastrati A, Bollwein H, Dibra A, Schuhlen H, Dirschinger J, Schomig A. Gender and restenosis after coronary artery stenting. Eur Heart J. 2003;24:1523–1530. [DOI] [PubMed] [Google Scholar]
- 8. Kim HS, Tonino PA, De Bruyne B, Yong AS, Tremmel JA, Pijls NH, Fearon WF; FAME Study Investigators . The impact of sex differences on fractional flow reserve‐guided percutaneous coronary intervention: a FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc Interv. 2012;5:1037–1042. [DOI] [PubMed] [Google Scholar]
- 9. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 10. Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, Hahn JY, Doh JH, Nam CW, Shin ES, Hur SH, Koo BK. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11:1423–1433. [DOI] [PubMed] [Google Scholar]
- 11. Echavarria‐Pinto M, Escaned J, Macias E, Medina M, Gonzalo N, Petraco R, Sen S, Jimenez‐Quevedo P, Hernandez R, Mila R, Ibanez B, Nunez‐Gil IJ, Fernandez C, Alfonso F, Banuelos C, Garcia E, Davies J, Fernandez‐Ortiz A, Macaya C. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128:2557–2566. [DOI] [PubMed] [Google Scholar]
- 12. Mejia‐Renteria H, Lee JM, Lauri F, van der Hoeven NW, de Waard GA, Macaya F, Perez‐Vizcayno MJ, Gonzalo N, Jimenez‐Quevedo P, Nombela‐Franco L, Salinas P, Nunez‐Gil I, Del Trigo M, Goto S, Lee HJ, Liontou C, Fernandez‐Ortiz A, Macaya C, van Royen N, Koo BK, Escaned J. Influence of microcirculatory dysfunction on angiography‐based functional assessment of coronary stenoses. JACC Cardiovasc Interv. 2018;11:741–753. [DOI] [PubMed] [Google Scholar]
- 13. Hamaya R, Yonetsu T, Kanaji Y, Usui E, Hoshino M, Yamaguchi M, Hada M, Kanno Y, Murai T, Hirao K, Kakuta T. Diagnostic and prognostic efficacy of coronary flow capacity obtained using pressure‐temperature sensor‐tipped wire‐derived physiological indices. JACC Cardiovasc Interv. 2018;11:728–737. [DOI] [PubMed] [Google Scholar]
- 14. Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 15. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. [DOI] [PubMed] [Google Scholar]
- 16. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 17. Lipworth B, Jackson C. Leukotriene antagonists in asthma. N Engl J Med. 2011;365:272–273; author reply 273‐274 [DOI] [PubMed] [Google Scholar]
- 18. Abbott JD, Vlachos HA, Selzer F, Sharaf BL, Holper E, Glaser R, Jacobs AK, Williams DO; National Heart, Lung, and Blood Institute Dynamic Registry . Gender‐based outcomes in percutaneous coronary intervention with drug‐eluting stents (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol. 2007;99:626–631. [DOI] [PubMed] [Google Scholar]
- 19. Solinas E, Nikolsky E, Lansky AJ, Kirtane AJ, Morice MC, Popma JJ, Schofer J, Schampaert E, Pucelikova T, Aoki J, Fahy M, Dangas GD, Moses JW, Cutlip DE, Leon MB, Mehran R. Gender‐specific outcomes after sirolimus‐eluting stent implantation. J Am Coll Cardiol. 2007;50:2111–2116. [DOI] [PubMed] [Google Scholar]
- 20. Kelsey SF, James M, Holubkov AL, Holubkov R, Cowley MJ, Detre KM. Results of percutaneous transluminal coronary angioplasty in women. 1985–1986 National Heart, Lung, and Blood Institute's Coronary Angioplasty Registry. Circulation. 1993;87:720–727. [DOI] [PubMed] [Google Scholar]
- 21. Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM; Euro Heart Survey Investigators . Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. [DOI] [PubMed] [Google Scholar]
- 22. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 23. Crea F. Coronary microvascular obstruction—a puzzle with many pieces. N Engl J Med. 2015;372:1464–1465. [DOI] [PubMed] [Google Scholar]
- 24. Cowley MJ, Mullin SM, Kelsey SF, Kent KM, Gruentzig AR, Detre KM, Passamani ER. Sex differences in early and long‐term results of coronary angioplasty in the NHLBI PTCA Registry. Circulation. 1985;71:90–97. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC, Tremmel JA. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ROC analyses of FFR values to predict revascularization. ROC analysis demonstrated the best cut‐off values of 0.80 to predict revascularization in the total cohort. FFR indicates fractional flow reserve; ROC, receiver operating curves.
Figure S2. ROC analyses of FFR values to predict revascularization. ROC analysis of the best cutoff FFR value of 0.80 to predict revascularization in male. FFR indicates fractional flow reserve; ROC, receiver operating curves.
Figure S3. ROC analyses of FFR values to predict revascularization. ROC analysis demonstrated that the best cut‐off value of 0.80 to predict revascularization in female. FFR indicates fractional flow reserve; ROC, Receiver operating curves.
Figure S4. Kaplan–Meier Curves of freedom from POCO.


