Abstract
Background
Clopidogrel nonresponsiveness is a prognostic marker after percutaneous coronary intervention. Prasugrel and ticagrelor provide a better platelet inhibition and represent the first‐line antiplatelet treatment in acute coronary syndrome. We sought to assess the prognostic impact of high platelet reactivity (HPR) and the potential clinical benefit of a “tailored” escalated or changed antiplatelet therapy in patients with chronic total occlusion.
Methods and Results
From Florence CTO‐PCI (chronic total occlusion‐percutaneous coronary intervention) registry, platelet function assessed by light transmission aggregometry, was available for 1101 patients. HPR was defined by adenosine diphosphate test ≥70% and optimal platelet reactivity by adenosine diphosphate test <70%. The endpoint of the study was long‐term cardiac survival. Patients were stratified according to light transmission aggregometry results: optimal platelet reactivity (82%) and HPR (18%). Means for the adenosine diphosphate test were 44±16% versus 77±6%, respectively. Three‐year survival was significantly higher in the optimal platelet reactivity group compared with HPR patients (95.3±0.8% versus 86.2±2.8%; P<0.001). With the availability of new P2Y12 inhibitors, a deeper platelet inhibition (46±17%) and similar survival to the optimal platelet reactivity group were achieved in patients with HPR on clopidogrel therapy after escalation. Conversely, HPR on clopidogrel therapy “not switched” was associated with cardiac mortality (hazard ratio 2.37; P=0.003) after multivariable adjustment.
Conclusions
HPR on treatment could be a modifiable prognostic marker by new antiaggregants providing a deeper platelet inhibition associated with clinical outcome improvement in complex chronic total occlusion patients. A “tailored” antiplatelet therapy, also driven by the entity of platelet inhibition, could be useful in these high risk setting patients.
Keywords: antiplatelet therapy, chronic total occlusion, platelet reactivity
Subject Categories: Stent, Revascularization, Percutaneous Coronary Intervention, Pharmacology
Clinical Perspective
What Is New?
In the “individualized” medicine era, the availability of more P2Y12 receptor inhibitors could ideally allow clinicians to escalate/de‐escalate and change antiplatelet therapy.
Indeed, current guidelines contemplate the possibility of switching therapy according to specific clinical scenarios, but data supporting long‐term benefit are missing.
Data from our study suggest that a therapeutic approach, “tailored” either on platelet reactivity assessment or global view of atherothrombotic risk, could be helpful in the “real‐world” subset of patients with chronic total occlusions.
What Are the Clinical Implications?
The clinical decision‐making of a “tailored” antiplatelet therapy in patients with high atherothrombotic risk, by switching and/or escalating drugs, based on platelet test results and clinical aspects could lead to a survival benefit.
In this setting, the achievement of more effective platelet inhibition after the guided escalation (as confirmed by platelet reactivity test) and the decision of a prolonged dual antiplatelet therapy, in patients with extensive coronary artery disease treated by a complex percutaneous revascularization, could be the links for survival improvement.
Introduction
Chronic total occlusion (CTO) is a severe expression of advanced coronary artery disease.1, 2, 3, 4Generally patients affected are older and present with several morbidities. Patients who undergo percutaneous coronary intervention (PCI) for CTO are at high risk of thrombotic events.5, 6 Antiplatelet therapy could play a leading role in reducing clinical event rates. Clopidogrel nonresponsiveness is a well‐known marker of cardiac death and risk of stent thrombosis after PCI.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 The majority of evidence has been obtained in the clinical setting of acute coronary syndrome (ACS). New P2Y12 antagonists prasugrel and ticagrelor have demonstrated in the past decade a more reliable pharmacodynamic effect and a deeper platelet inhibition.19, 20, 21, 22 For these reasons, guidelines indicate them as first‐line antiplatelet drugs in ACS patients. Notwithstanding, some randomized controlled trials have been unable to show the clinical superiority of a strategy of platelet function monitoring to adjust therapy in patients undergoing PCI either in the stable coronary artery disease or ACS setting.23, 24, 25, 26, 27 On the other hand, the TROPICAL‐ACS (Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment For Acute Coronary Syndromes) trial demonstrated the efficacy and safety of early de‐escalation of antiplatelet treatment from prasugrel to clopidogrel guided by platelet function testing.27 Currently, the availability of more P2Y12 receptor antagonists with their own pharmacodynamic and pharmacokinetic profiles can enable clinicians to individualize antiplatelet therapy, balancing clinical circumstances and personal bleeding/thrombotic risk for each subject.28, 29No data are available on the long‐term impact of a “tailored” antiplatelet therapy, based on platelet function assessment, in patients undergoing CTO‐PCI on clopidogrel and new antiplatelet therapy. The objective of the study was to assess the prognostic implication of platelet hyperreactivity in CTO patients, either before or after the introduction of new P2Y12 inhibitors that allowed escalation or change in antiplatelet therapy in nonresponder patients.
Methods
Data Sharing
Our study data cannot be made available because of institutional review board restrictions. However, study materials supporting the findings of this study and the methods used in the analyses will be provided by the corresponding author upon reasonable request.
Study Design and Population
From the Florence CTO‐PCI (chronic total occlusion‐percutaneous coronary intervention) registry, we retrospectively identified consecutive patients who underwent CTO‐PCI between 2004 and 2017. Details of the Florence CTO‐PCI registry have been previously published.5, 30 CTO was defined as a coronary obstruction with Thombolysis in Myocardial Infarction flow grade 0 with an estimated duration >3 months. The indication for CTO‐PCI was supported by demonstration of viable myocardium in the territory of the occluded vessel by echographic or scintigraphic provocative tests when needed. Complete revascularization was based on post‐PCI angiographic evaluation.5, 30 Inclusion criteria of the study were the following: (1) patients undergoing CTO‐PCI attempt; and (2) the availability of platelet function assessment. The only exclusion criterion was concomitant anticoagulant therapy (Figure 1).
Figure 1.
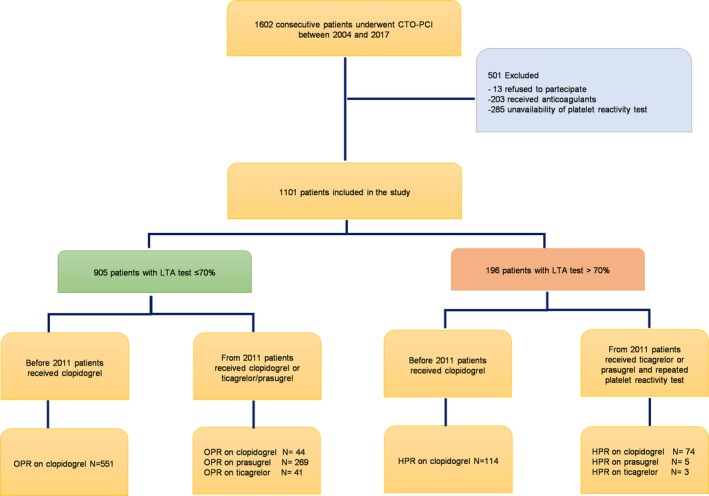
Flow‐chart of the study. CTO indicates chronic total occlusion; HPR, high platelet reactivity; LTA, light transmission aggregometry; OPR, optimal platelet reactivity; PCI, percutaneous coronary intervention.
Treatment
Platelet function was assessed by light transmission aggregometry (LTA) (APACT4, Helena Laboratories, Milan, Italy), performed on platelet‐rich plasma using arachidonic acid and adenosine diphosphate (ADP) as agonists of platelet aggregation. Blood samples anticoagulated with 0.109 M sodium citrate (ratio 9:1) were obtained 12 to 18 hours after clopidogrel (600 mg) or prasugrel (60 mg) or ticagrelor (180 mg) loading dose and before CTO‐PCI. Platelet‐rich plasma, obtained by centrifuging whole blood for 10 minutes at 200g, was then stimulated with 10 μmol/L ADP. Patients with platelet aggregation by 10 μmol ADP ≥90th percentile of controls were considered abnormal. HPR was defined as residual platelet aggregation by ADP ≥70%.10, 13, 14, 15, 16, 17Optimal platelet reactivity (OPR) was defined when LTA <70%. From 2011, therapy of nonresponders on clopidogrel (preprocedural LTA ≥70%) was escalated to prasugrel or ticagrelor, while a change between prasugrel and ticagrelor was made if HPR with the same LTA threshold (≥70%) was found in patients on new P2Y12 receptor antagonist treatment. A second platelet inhibition test was then performed within the following 7 to 30 days. All patients were treated for at least 12 months with aspirin (100 mg daily indefinitely) and clopidogrel (75 mg daily) and from 2011 with prasugrel (5 or 10 mg daily as appropriate) or ticagrelor (90 mg bid) in those with ACS and concurrent CTO and/or high anatomic coronary complexity. A prolonged dual antiplatelet therapy (DAPT) beyond 12 months was allowed after the assessment at 1 year of the ischemic/bleeding risk in patients with complex and extended coronary disease who received complex PCI procedures according to institutional protocol. Other drugs such as β‐blockers, angiotensin‐converting enzyme inhibitors, and statins were used in accordance with standard and recommended practice. All patients had clinical examination at 6 to 12 months and yearly thereafter. All other possible information derived from hospital re‐admission or by the referring physician, relatives, or municipality live registries were entered into the prospective database. The study conformed to the principles of the Helsinki Declaration and all subjects gave written consent to participate.
End Point
The primary end point of the study was long‐term cardiac survival: all deaths were considered cardiac unless otherwise documented.31 All other outcome end points were explorative.
Statistical Analysis
Patients were divided mainly in 2 groups according to the platelet reactivity results. Discrete data are summarized as frequencies, while continuous as mean±SD or median and interquartile range. The χ2 test or Fisher exact test when appropriate were used for comparison of categorical variables, while the unpaired 2‐tailed Student t test or Mann‐Whitney rank‐sum test were used to test differences among continuous variables. A paired t test was used to test the difference between paired data. Survival curves were generated using the Kaplan‐Meier method, and the difference between groups was assessed by a log‐rank test. The univariable and multivariable analyses to evaluate the independent contribution of clinical and angiographic variables to the primary end point were performed by the Cox proportional hazards model. The variables that reached the highest significance at the univariable analysis were considered in the final multivariable model in order to avoid overfitting. Hazard ratios (HR) and their 95% CI were calculated. All tests were 2‐tailed. In order to minimize the bias because of the nonrandomized nature of the study and the possibility of overfitting, a propensity score analysis was performed using a logistic regression model from which the probability for HPR was calculated for each patient; variables introduced into the propensity score model were age (years), male sex, diabetes mellitus, previous coronary artery bypass graft, previous myocardial infarction (MI), chronic kidney disease, left ventricular ejection fraction <0.40, ACS, left anterior descending artery CTO, and 3‐vessel disease. Model discrimination was assessed with the C‐statistic and goodness‐of‐fit with Hosmer and Lemeshow test. Thereafter, a Cox multivariable analysis was performed using the propensity score as a continuous covariate. A P<0.05 was considered significant. Analyses were performed using the software packages SPSS 19 (SPSS Inc., Chicago, IL).
Results
Study Population
Between 2004 and 2017, 1602 consecutive patients underwent a CTO‐PCI attempt in our institution. Out of these, 488 (30%) were excluded from the study analysis because of concomitant anticoagulant therapy or the absence of data concerning platelet inhibition assessment. Inclusion criteria were met for 1101 patients (Figure 1). HPR by ADP was found in 196 patients (18%) (Table S1) while LTA revealed OPR in 905 subjects (82%). Table 1 summarizes baseline, clinical, and angiographic characteristics of the overall population, OPR, and HPR groups by ADP LTA test. Overall, 32% of patients were older than 75 years, 27% had diabetes mellitus, half of the patients had a history of MI, almost one fourth presented with ACS, and more than one third had a moderate/severe left ventricular dysfunction with left ventricular ejection fraction <0.40. Chronic kidney disease, defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2 calculated by Cockroft‐Gault equation, was found in 9% of patients. The large majority of subjects had multivessel disease and 3‐vessel disease was revealed in more than half of the study cohort. Successful CTO‐PCI and completeness of revascularization were achieved in 81% and 70% of cases, respectively. There were no significant differences in baseline clinical characteristics between OPR and HPR groups except older age and diabetes mellitus, which were more frequent in patients with HPR, while more subjects in the OPR group achieved a complete revascularization. At discharge, 318 patients (29%) received new P2Y12 antiplatelet therapy (86% prasugrel, 14% ticagrelor). In this group the prevalence of diabetes mellitus (34% versus 24%; P<0.001), previous PCI (52% versus 42%; P=0.002), second‐generation drug‐eluting stents (90% versus 41%; P<0.001), successful CTO‐PCI (88% versus 78%; P<0.001), and completeness of revascularization (75% versus 68%; P<0.001) was higher.
Table 1.
Baseline Characteristics
| All patients (n=1101) | OPR (n=905) | HPR (n=196) | P Value | |
|---|---|---|---|---|
| Age, y | 68.8±10.4 | 68.4±10.3 | 70.5±10.1 | 0.010 |
| ≥75 y | 351 (32) | 274 (30) | 77 (39) | 0.014 |
| Male sex, (%) | 940 (85) | 780 (86) | 160 (82) | 0.102 |
| Hypertension, (%) | 695 (63) | 564 (62) | 131 (67) | 0.235 |
| Hypercholesterolemia, (%) | 684 (62) | 557 (61) | 127 (65) | 0.395 |
| Diabetes mellitus, (%) | 296 (27) | 230 (25) | 66 (34) | 0.018 |
| CKD, (%) | 100 (9) | 76 (12) | 24 (17) | 0.126 |
| Previous MI, (%) | 554 (50) | 451 (50) | 103 (53) | 0.490 |
| Previous PCI, (%) | 492 (45) | 413 (46) | 79 (40) | 0.174 |
| Previous CABG, (%) | 154 (14) | 119 (13) | 35 (18) | 0.085 |
| ACS, (%) | 256 (23) | 207 (23) | 49 (25) | 0.523 |
| LVEF (%) | 45.3±12.6 | 45.2±12.9 | 45.4±12.6 | 0.858 |
| LVEF <0.40, (%) | 372 (34) | 305 (34) | 67 (34) | 0.905 |
| Multivessel disease, (%) | 936 (85) | 762 (84) | 174 (89) | 0.104 |
| Three‐vessel disease, (%) | 585 (53) | 479 (53) | 106 (54) | 0.769 |
| CTO vessel | ||||
| LAD, (%) | 330 (30) | 271 (30) | 59 (30) | 0.440 |
| RCA, (%) | 468 (42) | 379 (42) | 89 (45) | |
| Second generation DES, (%) | 508 (56) | 417 (56) | 91 (60) | 0.358 |
| Successful CTO PCI, (%) | 889 (81) | 738 (81) | 151 (77) | 0.147 |
| Complete revascularization, (%) | 772 (70) | 651 (72) | 121 (62) | 0.005 |
| Platelet Reactivity: ADP Test Results by LTA | |
|---|---|
| Overall population | |
| P2Y12 antagonist responders | n=905 |
| Mean, (%) | 44±16 |
| Median, (%) | 51 [15–68] |
| HPR on clopidogrel therapy | n=196 |
| Mean, (%) | 77±6 |
| Median, (%) | 77 [71–90] |
| HPR group on clopidogrel | |
| “not switched” | n=114 |
| Mean, (%) | 78±6 |
| Median, (%) | 76 [71–92] |
| “switched” | n=82 |
| Mean, (%) | 46±17 |
| Median, (%) | 49 [19–74] |
Values are mean±SD, number of patients (%) and median (%) [25th–75th percentiles]. ACS indicates acute coronary syndrome; ADP, adenosine diphosphate; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CTO, chronic total occlusion; DES, drug‐eluting stent; HPR, high platelet reactivity; LAD, left anterior descending artery; LTA, light transmission aggregometry; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OPR, optimal platelet reactivity; PCI, percutaneous coronary intervention; RCA, right coronary artery.
Platelet Reactivity
Results of platelet reactivity stimulated by ADP and measured by LTA are listed in Table 1. Among 196 patients who were clopidogrel nonresponders, antiplatelet therapy for 82 patients (42%) belonging to new DAPT era was escalated to prasugrel and ticagrelor; thereafter they underwent a second platelet function assessment (Table 1). Data of the latter platelet inhibition test were available for 75 subjects (90%): HPR was found in only 6 patients (8%) of this subgroup on prasugrel treatment, promptly changed to ticagrelor. A significant difference was found between paired data of LTA tests of the HPR group before and after escalation to new P2Y12 inhibitors (P<0.001). No significant baseline, clinical, or angiographic differences resulted among patients previously on treatment with clopidogrel and subsequently escalated to new P2Y12 inhibitors and those who received prasugrel or ticagrelor from the beginning. A prolonged DAPT beyond 12 months was adopted in most of the patients (72%) with a median time of 28 months. After 2 years, 67% of the patients were on DAPT (86% in the HPR group).
One‐Year Outcome
In Table 2 are summarized clinical outcomes. Clinical follow‐up rate at 1 year was 100%. Overall 1‐year cardiac mortality was 3% and the MI rate was 1.5%. No significant differences were found in the definite/probable stent thrombosis rate according to the Academic Research Consortium definition31 and 1‐year all‐cause death; furthermore, 1‐year cardiac death rate was numerically higher in the HPR group. The composite end point of 1‐year coronary events (cardiac death, nonfatal MI and definite/probable stent thrombosis) was significantly lower in the OPR cohort (4.6% versus 8.1%; P=0.045). Conversely, in the HPR subgroup of patients “not switched,” 1‐year cardiac mortality was significantly increased compared with the OPR group (7.0% versus 2.7%; P=0.010).
Table 2.
Clinical Outcomes
| OPR (n=905) | HPR (n=196) | P Value | |
|---|---|---|---|
| One‐year outcome | |||
| All‐cause death | 37 (4.1) | 12 (6.1) | 0.211 |
| Cardiac death | 24 (2.7) | 10 (5.1) | 0.072 |
| Nonfatal myocardial infarction | 13 (1.4) | 4 (2.1) | 0.529 |
| Stroke | 4 (0.5) | 0 (0) | 0.355 |
| CTO‐vessel repeated PCI | 95 (10.5) | 21 (10.8) | 0.918 |
| CABG | 10 (1.1) | 1 (0.5) | 0.448 |
| MACCE | 146 (16) | 36 (18) | 0.445 |
| Definite/probable stent thrombosis | 5 (1.0) | 2 (2.2) | 0.366 |
| Composite of coronary eventsa | 42 (4.6) | 16 (8.1) | 0.045 |
| Long‐term survival | |||
| Cardiac survival | |||
| 1 y | 97.6±0.5 | 94.9±1.6 | <0.001 |
| 3 y | 95.3±0.8 | 86.2±2.8 | |
| All‐cause death | |||
| 3 y | 86±1.5 | 75±3.7 | 0.001 |
Values are number of events (%) or mean±SE for survival analyses. CABG indicates coronary artery bypass grafting; CTO, chronic total occlusion; HPR, high platelet reactivity; MACCE, major acute cardiovascular and cerebrovascular events; OPR, optimal platelet reactivity; PCI, percutaneous coronary intervention.
Composite of cardiac death, nonfatal myocardial infarction, and stent thrombosis.
Thrombolysis in Myocardial Infarction major bleedings were numerically higher in patients receiving new P2Y12 inhibitors (prasugrel and ticagrelor) when compared with patients treated with clopidogrel, but this difference was not significant (2.6% versus 1.9% respectively; P=0.528).
Long‐Term Outcome
The 3‐year cardiac survival (median follow‐up 3 years [interquartile range 2.0–4.0]) was significantly higher in the OPR group as compared with the HPR group (95.3±0.8% versus 86.2±2.8%; P<0.001) (Table 2). Survival was numerically higher in the OPR group with a statistical trend difference, also excluding patients with incomplete coronary revascularization (97.4±0.7% OPR versus 94.1±0.2% HPR; P=0.097). When the 3‐year cardiac survival was analyzed according to platelet reactivity in patients on clopidogrel therapy “not switched,” patients with OPR showed a significant increase in survival as compared with the HPR group (95.3±0.8% versus 83.2±3.8%; P<0.001) (Figure 2). Conversely, cardiac survival was similar in patients with OPR as compared with those with HPR whose therapy had been escalated to new antiplatelet therapy (95.3±0.8% versus 90.7±3.9%; P=0.172) (Figure 2). In a further analysis, after the inclusion of discontinuation time of DAPT as censoring event together with death and loss to follow‐up, the between‐group survival difference in OPR and HPR “not switched” groups was similar (95.2±0.8% versus 83.0±4.4%, respectively; P<0.001); notably, survival curves of patients in OPR and HPR “switched” groups were found to be very close (95.2±0.8% versus 92.4±3.5%, respectively; P=0.410), probably supporting the potential effect of a “tailored” DAPT in reducing the prognostic differences between these subsets of patients (Figure 2B).
Figure 2.
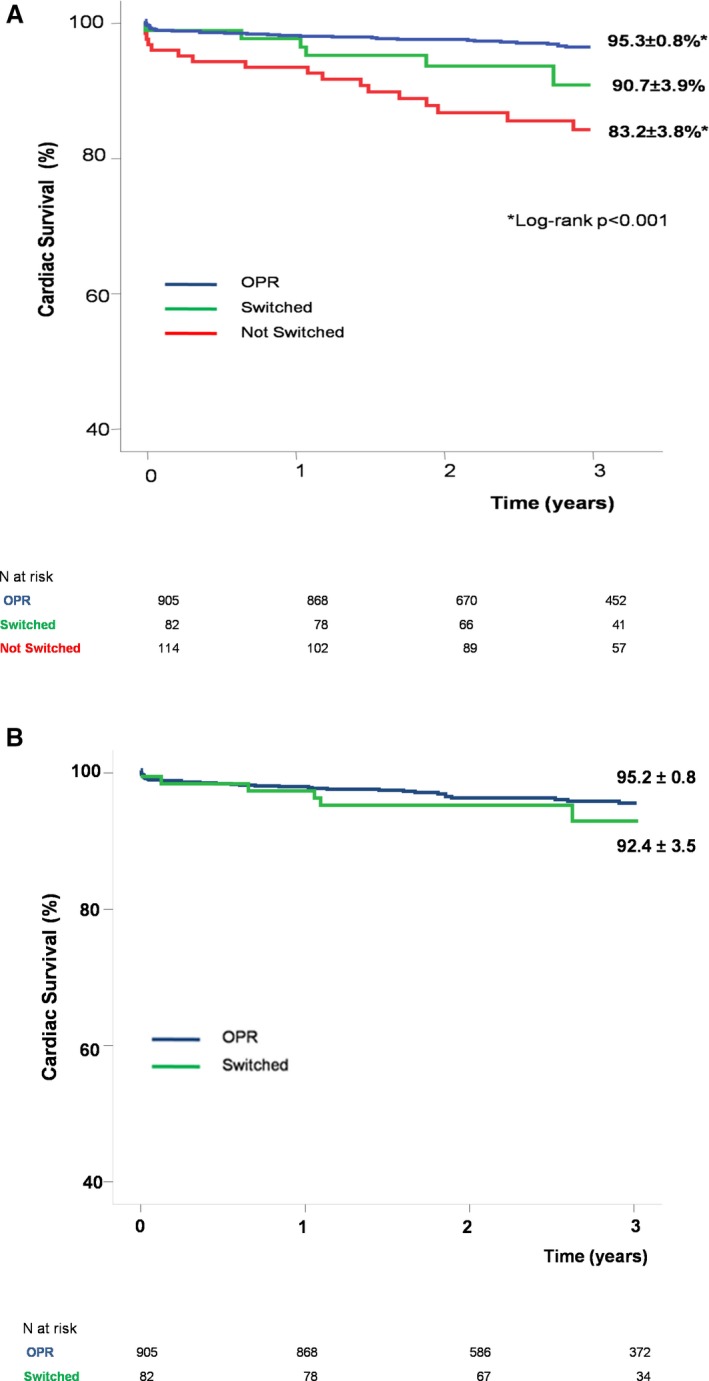
Survival analysis according to platelet reactivity. A, Cardiac survival curves demonstrated a long‐term benefit in the OPR group compared with the HPR subgroup in which antiplatelet therapy was “not switched.” Conversely, after a “tailored” antiplatelet therapy by escalation and/or change, no more significant differences in survival curves were detected between the HPR “switched” subgroup and the OPR group. B, Survival analysis including discontinuation time of DAPT as censoring event together with death and loss to follow‐up in OPR and HPR “switched” groups. DAPT indicates dual antiplatelet therapy; HPR, high platelet reactivity; OPR, optimal platelet reactivity.
Up to 3 years, refractory congestive heart failure represented the most frequent cause of death (51% of patients in OPR group versus 35% in HPR cohort); fatal MI occurred in 13% in the OPR group versus 22% in the HPR cohort and possible stent thrombosis in 19% of OPR patients versus 26% of the HPR group, respectively. Other indeterminable deaths were derived from municipality live registries and were classified as cardiac death per protocol definition. Overall fatal MI and stent thrombosis occurred numerically higher in the HPR than in the OPR group (5.6% versus 1.3%).
Table 3 reports univariable and multivariable analyses. At univariable analysis, HPR on clopidogrel therapy “not switched” to new P2Y12 inhibitors was independently related to long‐term cardiac mortality (hazard ratio 3.46; P<0.001) and remained significantly associated (hazard ratio 2.37; P=0.003) after multivariable adjustment (Table 3). Conversely, HPR was not significantly associated with long‐term cardiac mortality after escalation of therapy to new P2Y12 inhibitors (P=0.436). HPR in patients whose therapy was “not switched” remained significantly associated with the primary end point after propensity score adjustment (hazard ratio 3.01, 95% CI 1.69–5.36; P<0.001) (C‐statistic 0.64; P=0.327 for Hosmer‐Lemeshow test).
Table 3.
Unadjusted and Adjusted Predictors Associated with Long‐Term Cardiac Mortality
| Unadjusted Hazard Ratio (95% CI) | P Value | Multivariable Adjusted Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Age (per y) | 1.08 (1.05–1.11) | <0.001 | 1.07 (1.04–1.10) | <0.001 |
| Male sex | 0.42 (0.24–0.74) | 0.003 | ||
| Diabetes mellitus | 3.39 (2.04–5.64) | <0.001 | 2.86 (1.70–4.80) | <0.001 |
| Previous MI | 1.68 (0.99–2.85) | 0.051 | ||
| Previous CABG | 2.54 (1.46–4.41) | 0.001 | ||
| Chronic kidney disease | 4.51 (2.57–7.92) | <0.001 | ||
| ACS | 1.70 (0.99–2.90) | 0.053 | ||
| LVEF <0.40 | 7.06 (3.88–12.85) | <0.001 | 5.27 (2.87–9.65) | <0.001 |
| Left anterior descending artery CTO | 1.81 (1.09–3.02) | 0.022 | ||
| Three‐vessel disease | 1.67 (0.98–2.84) | 0.058 | ||
| Successful CTO‐PCI | 0.33 (0.20–0.56) | <0.001 | ||
| Complete Revascularization | 0.20 (0.12–0.34) | <0.001 | 0.31 (0.18–0.54) | <0.001 |
| HPR on clopidogrel not “switched” | 3.46 (1.97–6.07) | <0.001 | 2.37 (1.33–4.20) | 0.003 |
| HPR on clopidogrel “switched” | 1.39 (0.60–3.25) | 0.436 | ||
| New P2Y12 antagonist therapy | 0.84 (0.46–1.52) | 0.578 | ||
| Year index | 0.99 (0.85–1.16) | 0.980 | ||
| Second generation DES | 0.90 (0.56–1.46) | 0.697 |
ACS indicates acute coronary syndrome; CABG, coronary artery bypass graft; CTO, chronic total occlusion; DES, drug‐eluting stent; HPR, high platelet reactivity; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
The main findings of the study can be summarized as follows: (1) HPR to ADP in patients undergoing CTO‐PCI was associated with long‐term cardiac mortality; (2) HPR on clopidogrel treatment could be successfully overcome by switching to new P2Y12 receptor inhibitors as shown by platelet function laboratory tests; (3) HPR of nonresponders, whose therapy had been effectively escalated to prasugrel and ticagrelor or changed between these drugs, was no longer significantly related to long‐term cardiac mortality.
To our knowledge, this was the first study to assess the long‐term prognosis of patients undergoing CTO‐PCI and managed with a “tailored” antiplatelet therapy based on platelet function testing in the new antiplatelet era. Several observational studies and randomized controlled trials have explored the impact of platelet hyperreactivity on cardiovascular event rates in different clinical settings, often with conflicting results.12, 22, 23, 24, 25, 26, 27 In particular, results of previous randomized controlled trials that did not establish clinical improvements after treatment adjustments based on platelet function testing had a strong impact driving clinical practice guidelines that do not currently recommend routine assessment of platelet reactivity. The GRAVITAS (Gauging Responsiveness with a VerifyNow P2Y12 Assay: Impact on Thrombosis and Safety) study showed the inability of a double dose of clopidogrel to completely overcome HPR and improve outcomes; furthermore, the population was underpowered and the follow‐up time was short (6 months). TRIGGER‐PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study failed to demonstrate a 6‐month survival benefit in patients with HPR switched to prasugrel for a very low observed ischemic event rate in a low‐risk population that was even underpowered. The ARCTIC (Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy) trial extended the follow‐up time to 12 months and included 27% of ACS but only 9.3% of patients were discharged home on prasugrel in the monitoring group. In the ANTARCTIC (Tailored Antiplatelet Therapy Versus Recommended Dose of Prasugrel) trial, patients included were older >75 years and all presented with ACS: in this high‐risk population, platelet function monitoring did not improve 1‐year ischemic or safety outcomes. More recently, in TROPICAL‐ACS, guided de‐escalation of antiplatelet treatment was noninferior to standard treatment with prasugrel after PCI in terms of net clinical benefit at 1 year. All these randomized controlled trials have been conducted with different platelet function assays and thresholds; hypothetically, the results obtained with 1 of these tests could not be transferred to the others.
In our study, platelet aggregation was assessed by LTA, a laboratory assay considered as a gold standard past years but currently replaced by other tests (VerifyNow, VASP, and Multiplate) because of the lack of standardization between institutions.32 HPR to ADP was found in 18% of the study population, mainly older and diabetic patients, consistently with previous data.11, 17, 18 The clinical benefit demonstrated by prasugrel in diabetic patients21, the earlier availability of this agent, and the better compliance of patients explain the prevalence of this prescription; ticagrelor was mainly prescribed in case of contraindications to prasugrel therapy. In the HPR cohort of our study, 82 patients (42%) belonging to the new DAPT era received a “tailored” antiplatelet therapy with drugs whose major effectiveness had been proved. This latter era was also characterized by the predominant use of second‐generation drug‐eluting stents (mainly everolimus eluting stents) whose superior safety and effectiveness have been widely confirmed; however, no significant statistical associations were found between long‐term cardiac mortality and first/second generation drug‐eluting stents or year of the index procedure (Table 3). The high anatomical complexity and the extended coronary multivessel disease, together with a more pronounced atherothrombotic risk in the large majority of patients, led to the preferred prolongation of DAPT beyond 12 months, in agreement with the results of contextual studies that showed a benefit in this subset of patients.33, 34, 35, 36 Patients presented with ACS, history of prior MI, prior percutaneous or surgical revascularization, diabetes mellitus, lack of optimal risk factors control, residual cardiovascular risk, multivessel coronary disease, complex PCI procedures, and any other condition at increased ischemic risk were the preferred candidates for a prolonged DAPT strategy. The indication was then confirmed at 1 year re‐evaluation in the absence of major bleeding complications. The duration of DAPT was prescribed at the physician's discretion as an integral part of a clinical decision‐making process, consistent with a “tailored” therapy approach.
Our study cohort was very representative of a “real world” population presenting with advanced coronary artery disease: 50% had a history of MI, mean left ventricular ejection fraction was 45.3±12.6%, 85% had multivessel disease, and 53% had 3‐vessel disease. Notwithstanding, completeness of revascularization was achieved in 70% of patients. Of course, complete coronary revascularization was a strong point in our population: its prognostic impact has been widely acknowledged.30, 37, 38
The lack of 1‐year cardiac survival benefit, according to platelet reactivity between OPR and HPR groups (Table 2), could be explained by the inclusion of an “escalated therapy” cohort in HPR group; of note, in support of this hypothesis, 1‐year cardiac mortality of the HPR patients with “not switched” therapy was significantly higher when compared with the OPR group. The achievement of more effective platelet inhibition after the guided escalation, as confirmed by the platelet reactivity test, could be the main drive and the link with an associated improvement in terms of survival. Indeed, no significant prognostic association was detected between the HPR group after escalation or change of antiplatelet therapy and OPR group. Probably, the “tailored” antiplatelet therapy of these patients, through the reversal of nonresponsiveness, allowed achievement of a clinical outcome comparable to that of OPR patients.
Study Limitations
The study had several limitations; first, data were derived from a single‐center registry and was a retrospective analysis. Despite the use of multivariable analysis, it remains unknown whether residual confounders may have affected the outcome in the present analyses. Another limitation was the number of “switched” patients, which made type II errors possible. Furthermore, a prolonged DAPT beyond 12 months was adopted in most patients at the clinician's discretion; different decisions could have affected the results, although the “clinical decision‐making” process is part of a “tailored” therapy approach. It must be acknowledged that this study did not show a cause‐and‐effect relationship, but only an association. Thus, the results of this study should be considered only as hypothesis generating. Furthermore, platelet inhibition tests were not available during follow‐up. The use of LTA to assess platelet reactivity could be currently considered out of date.
Conclusions
In conclusion, data of our “real world” registry, in this setting of increased atherothrombotic risk patients, suggest a potential clinical benefit of a “tailored” antiplatelet therapy also based on platelet function assays. In an era of individualized medicine, further clinical investigations are needed to assess and balance the thrombotic and bleeding risk with a “tailored” antiplatelet therapy.
Disclosures
Marcucci reports lecture and consultant fees from Bayer, Pfizer, Sanofi, Boehringer Ingelheim, AMGEN, Daichi Sankyo, and Werfen. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics
Acknowledgments
We are grateful to Paola Baldini and Fabio Torrini, (A.R. CARDOnlus Foundation, Florence, Italy) for their secretarial assistance.
(J Am Heart Assoc. 2020;9:e014676 DOI: 10.1161/JAHA.119.014676.)
References
- 1. Hoebers LP, Claessen BE, Dangas GD, Råmunddal T, Mehran R, Henriques JP. Contemporary overview and clinical perspectives of chronic total occlusions. Nat Rev Cardiol. 2014;11:458–469. [DOI] [PubMed] [Google Scholar]
- 2. Mehran R, Claessen BE, Godino C, Dangas GD, Obunai K, Kanwal S, Carlino M, Henriques JP, Di Mario C, Kim YH, Park SJ, Stone GW, Leon MB, Moses JW, Colombo A; Multinational Chronic Total Occlusion Registry . Long‐term outcome of percutaneous coronary intervention for chronic total occlusions. J Am Coll Cardiol Intv. 2011;4:952–961. [DOI] [PubMed] [Google Scholar]
- 3. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, Sparkes JD, Wright GA, Strauss BH. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991–997. [DOI] [PubMed] [Google Scholar]
- 4. Azzalini L, Jolicoeur EM, Pighi M, Millán X, Picard F, Tadros VX, Fortier A, L'Allier PL, Ly HQ. Epidemiology, management strategies, and outcomes of patients with chronic total coronary occlusion. Am J Cardiol. 2016;118:1128–1135. [DOI] [PubMed] [Google Scholar]
- 5. Valenti R, Vergara R, Migliorini A, Parodi G, Carrabba N, Cerisano G, Dovellini EV, Antoniucci D. Predictors of reocclusion after successful drug‐eluting stent‐ supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61:545–550. [DOI] [PubMed] [Google Scholar]
- 6. Isaaz K, Gerbay A, Terreaux J, Khamis H, Tammam K, Richard L, Cerisier A, Lamaud M, Da Costa A. Restenosis after percutaneous coronary intervention for coronary chronic total occlusion. The central role of an optimized immediate post‐procedural angiographic result. Int J Cardiol. 2016;224:343–347. [DOI] [PubMed] [Google Scholar]
- 7. Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. [DOI] [PubMed] [Google Scholar]
- 8. Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US. Platelet reactivity in patients and recurrent events post‐stenting: results of the PREPARE POST‐STENTING Study. J Am Coll Cardiol. 2005;46:1820–1826. [DOI] [PubMed] [Google Scholar]
- 9. Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–1834. [DOI] [PubMed] [Google Scholar]
- 10. Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, Moschi G, Gori AM, Abbate R, Antoniucci D. Impact of platelet reactivity after clopidogrel administration on drug‐eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312–2317. [DOI] [PubMed] [Google Scholar]
- 11. Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, Ernst A, Sawhney NS, Schatz RA, Teirstein PS. Prognostic significance of post‐clopidogrel platelet reactivity assessed by a point‐of‐care assay on thrombotic events after drug‐eluting stent implantation. Eur Heart J. 2008;29:992–1000. [DOI] [PubMed] [Google Scholar]
- 12. Finn MT, Redfors B, Karmpaliotis D, Kirtane AJ, Green P, McAndrew T, Liu M, Cloney MB, Witzenbichler B, Weisz G, Stuckey TD, Brodie BR, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Mehran R, Stone GW. Adverse events in patients with high platelet reactivity following successful chronic total occlusion PCI: the Assessment of Dual AntiPlatelet Therapy with Drug‐Eluting Stents (ADAPT‐DES) study. Am Heart J. 2019;211:68–76. [DOI] [PubMed] [Google Scholar]
- 13. Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, Buonamici P, Gensini GF, Vergara R, Abbate R, Antoniucci D. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug‐eluting stents. J Am Coll Cardiol. 2008;52:734–739. [DOI] [PubMed] [Google Scholar]
- 14. Migliorini A, Valenti R, Marcucci R, Parodi G, Giuliani G, Buonamici P, Cerisano G, Carrabba N, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long‐term clinical outcome after drug‐eluting stenting for unprotected left main coronary disease. Circulation. 2009;120:2214–2221. [DOI] [PubMed] [Google Scholar]
- 15. Valenti R, Marcucci R, Capodanno D, De Luca G, Migliorini A, Gori AM, Parodi G, Giusti B, Carrabba N, Paniccia R, Cantini G, Marrani M, Gensini GF, Abbate R, Antoniucci D. Residual platelet reactivity to predict long‐term clinical outcomes after clopidogrel loading in patients with acute coronary syndromes: comparison of different cutoff values by light transmission aggregometry from the responsiveness to clopidogrel and stent thrombosis 2‐acute coronary syndrome (RECLOSE 2‐ACS) study. J Thromb Thrombolysis. 2015;40:76–82. [DOI] [PubMed] [Google Scholar]
- 16. Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point‐of‐care assay: a 12‐month follow‐up. Circulation. 2009;119:237–242. [DOI] [PubMed] [Google Scholar]
- 17. Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, Buonamici P, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long‐term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223. [DOI] [PubMed] [Google Scholar]
- 18. Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, Patti G, Breet NJ, DiSciascio G, Cuisset T, Dangas G. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta‐analysis of individual participant data. J Am Coll Cardiol. 2011;58:1945–1954. [DOI] [PubMed] [Google Scholar]
- 19. Jernberg T, Payne CD, Winters KJ, Darstein C, Brandt JT, Jakubowski JA, Naganuma H, Siegbahn A, Wallentin L. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non‐responders compared with clopidogrel in aspirin‐treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166–1173. [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; Investigators PLATO , Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 21. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON‐TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 22. Valenti R, Marcucci R, Comito V, Marrani M, Cantini G, Migliorini A, Parodi G, Gensini GF, Abbate R, Antoniucci D. Prasugrel in Clopidogrel Nonresponders Undergoing Percutaneous Coronary Intervention: the RECLOSE‐3 Study (REsponsiveness to CLOpidogrel and StEnt Thrombosis). JACC Cardiovasc Interv. 2015;8:1563–1570. [DOI] [PubMed] [Google Scholar]
- 23. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ; GRAVITAS Investigators . Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 24. Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug‐eluting stents: results of the TRIGGER‐PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol. 2012;59:2159–2164. [DOI] [PubMed] [Google Scholar]
- 25. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Boueri Z, Belle L, Van Belle E, Rousseau H, Aubry P, Monségu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint‐Etienne C, Barthélémy O, Beygui F, Silvain J, Vicaut E, Montalescot G; ARCTIC Investigators . Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. [DOI] [PubMed] [Google Scholar]
- 26. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo‐Silberman S, Saint‐Etienne C, Delarche N, Bellemain‐Appaix A, Range G, El Mahmoud R, Carrié D, Belle L, Souteyrand G, Aubry P, Sabouret P, du Fretay XH, Beygui F, Bonnet JL, Lattuca B, Pouillot C, Varenne O, Boueri Z, Van Belle E, Henry P, Motreff P, Elhadad S, Salem JE, Abtan J, Rousseau H, Collet JP, Vicaut E, Montalescot G; ANTARCTIC investigators . Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open‐label, blinded‐endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–2022. [DOI] [PubMed] [Google Scholar]
- 27. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann FJ, Koltowski L, Mehilli J, Huczek Z, Massberg S; TROPICAL‐ACS Investigators . Guided de‐escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL‐ACS): a randomised, open‐label, multicentre trial. Lancet. 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 28. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, Gurbel PA, Hochholzer W, De Luca L, Bonello L, Aradi D, Cuisset T, Tantry US, Wang TY, Valgimigli M, Waksman R, Mehran R, Montalescot G, Franchi F, Price MJ. International expert consensus on switching platelet P2Y12 receptor‐inhibiting therapies. Circulation. 2017;136:1955–1975. [DOI] [PubMed] [Google Scholar]
- 29. Sibbing D, Gross L, Trenk D, Jacobshagen C, Geisler T, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Parma R, Felix SB, Neumann FJ, Hausleiter J, Baylacher M, Koltowski L, Mehilli J, Huber K, Huczek Z, Aradi D, Massberg S; TROPICAL‐ACS Investigators . Age and outcomes following guided de‐escalation of antiplatelet treatment in acute coronary syndrome patients undergoing percutaneous coronary intervention: results from the randomized TROPICAL‐ACS trial. Eur Heart J. 2018;39:2749–2758. [DOI] [PubMed] [Google Scholar]
- 30. Valenti R, Migliorini A, Signorini U, Vergara R, Parodi G, Carrabba N, Cerisano G, Antoniucci D. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. 2008;29:2336–2342. [DOI] [PubMed] [Google Scholar]
- 31. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 32. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, ten Berg J, Janssen P, Angiolillo DJ, Siller‐Matula JM, Marcucci R, Patti G, Mangiacapra F, Valgimigli M, Morel O, Palmerini T, Price MJ, Cuisset T, Kastrati A, Stone GW, Sibbing D. Bleeding and stent thrombosis on P2Y12 inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36:1762–1771. [DOI] [PubMed] [Google Scholar]
- 33. Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, Fitzgerald D, Hirsh J, Husted S, Kvasnicka J, Montalescot G, García Rodríguez LA, Verheugt F, Vermylen J, Wallentin L, Priori SG, Alonso Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Fernández Burgos E, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Morais J, Deckers J, Ferreira R, Mazzotta G, Steg PG, Teixeira F, Wilcox R; Expert Consensus Document on the Use of Antiplatelet Agents . The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European Society of Cardiology. Eur Heart J. 2004;25:166–181. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry‐Ribaudo L, Booth J, Topol EJ; CHARISMA Investigators . Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 35. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines . 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 36. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators . Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagaraja V, Ooi SY, Nolan J, Large A, De Belder M, Ludman P, Bagur R, Curzen N, Matsukage T, Yoshimachi F, Kwok CS, Berry C, Mamas MA. Impact of incomplete percutaneous revascularization in patients with multivessel coronary artery disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e004598 DOI: 10.1161/JAHA.116.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, Brilakis ES. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta‐analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421–1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics


