Abstract
Background
Increased left ventricular (LV) mass is characterized by increased myocardial wall thickness and/or ventricular dilatation that is associated with worse outcomes. We aim to comprehensively compare sex‐stratified associations between measures of LV remodeling and increasing LV mass in the general population.
Methods and Results
Participants were prospectively recruited in the National Heart Center Singapore Biobank to examine health and cardiovascular risk factors in the general population. Cardiovascular magnetic resonance was performed in all individuals. Participants with established cardiovascular diseases and abnormal cardiovascular magnetic resonance scan results were excluded. Global and regional measures of LV remodeling (geometry, function, interstitial volumes, and wall stress) were performed using conventional image analysis and novel 3‐dimensional machine learning phenotyping. Sex‐stratified analyses were performed in 1005 participants (57% males; 53±13 years). Age and prevalence of cardiovascular risk factors were well‐matched in both sexes (P>0.05 for all). Progressive increase in LV mass was associated with increased concentricity in either sex, but to a greater extent in females. Compared with males, females had higher wall stress (mean difference: 170 mm Hg, P<0.0001) despite smaller LV mass (42.4±8.2 versus 55.6±14.2 g/m2, P<0.0001), lower blood pressures (P<0.0001), and higher LV ejection fraction (61.9±5.9% versus 58.6±6.4%, P<0.0001). The regions of increased concentric remodeling corresponded to regions of increased wall stress. Compared with males, females had increased extracellular volume fraction (27.1±2.4% versus 25.1±2.9%, P<0.0001).
Conclusions
Compared with males, females have lower LV mass, increased wall stress, and concentric remodeling. These findings provide mechanistic insights that females are susceptible to particular cardiovascular complications.
Keywords: cardiovascular magnetic resonance imaging, left ventricular hypertrophy, myocardial wall stress
Subject Categories: Magnetic Resonance Imaging (MRI), Hypertrophy, Mechanisms
Clinical Perspective
What Is New?
Progressive increase in left ventricular (LV) mass was associated with increased concentricity, to a greater extent in females than males.
Compared with males, females had higher regional wall stress (corresponded to regions of increased concentricity) and increased extracellular volume fraction despite smaller LV mass, lower blood pressures, and higher LV ejection fraction.
What Are the Clinical Implications?
These findings may explain why females are predisposed to cardiovascular complications, particularly when LV pressures are increased.
The findings have important clinical implications, particularly in Asians because of the smaller LV mass and volumes compared with other ethnicities.
Introduction
Increased left ventricular (LV) mass is a well‐established predictor of incident cardiovascular disease and deaths.1 The pathological process of increased LV mass is characterized by increased myocardial wall thickness and/or ventricular dilatation that is associated with adverse consequences of myocardial fibrosis and cardiac dysfunction.2
Sex has a profound impact on LV remodeling in health and disease. Previous studies had examined sex‐related differences in cardiac remodeling in specific cardiovascular conditions, such as hypertension and aortic valve disease.3, 4, 5 In the general population, sex‐related differences in cardiac remodeling have not been well defined.
Recent development in cardiovascular magnetic resonance (CMR) has advanced our ability to detect regional geometric changes and wall stress using 3‐dimensional (3D) machine learning phenotyping.6, 7 The aim of the study is to compare the sex‐stratified associations between measures of LV remodeling and mass in the general population.
Methods
The data that support the findings of the study are available from the corresponding author upon reasonable request.
Study Population
This study consisted of adults (>18 years) who were prospectively recruited in the National Heart Center Singapore Biobank to examine health and cardiovascular risk in the general population. Individuals with either established cardiovascular conditions (heart failure, atrial fibrillation, ischemic heart and/or strokes) or abnormal CMR (moderate to severe valvular abnormalities, cardiomyopathies, myocardial infarction, and/or regional wall motion abnormalities) were excluded. Informed consent was obtained from all participants. The study was approved by the institutional review board.
CMR Imaging and Analysis
CMR (Siemens Aera 1.5 T; Siemens Healthineers, Erlangen, Germany) was performed in all participants. Balanced steady‐state free precision cine images were acquired in the long axis (2‐, 3‐, and 4‐chamber) planes and right ventricular (RV) long‐axis view aligned with the tricuspid inflow and RV outflow tract. Short‐axis cines extending from the atrioventricular ring to the apex covered the entire left and right ventricles (acquired voxel size: 1.6×1.3×8.0‐mm slice thickness; 2‐mm gap; 30 phases per cardiac cycle). In those who consented to gadolinium administration, myocardial fibrosis was assessed using late gadolinium‐enhanced imaging and myocardial T1 mapping. Late gadolinium‐enhanced imaging was performed ≈8 minutes after administration of 0.1 mmol/kg of gadobutrol (Gadovist; Bayer Pharma AG, Germany). An inversion‐recovery fast gradient echo sequence was used; and the inversion time was optimized to achieve appropriate nulling of the myocardium. Myocardial T1 mapping based on the Modified Look‐Locker inversion‐recovery sequence was used to assess diffuse myocardial fibrosis. Extracellular volume fraction (ECV) was estimated from the native and 15‐minute postcontrast T1 map, analyzed using the T1 mapping module (CVI42, Circle Cardiovascular Imaging, Calgary, Canada).
LV measures of geometry and function (volumes, wall thickness, ejection fraction, myocardial mass, and multidirectional myocardial strain) were analyzed using CVI42 (Circle Cardiovascular Imaging) at our NHRIS CMR Core Laboratory, according to standardized analysis protocols.8, 9, 10 Volumes in the interstitial and myocyte compartments were estimated from the product of myocardial volume (LV mass/1.05 g/mL) and ECV or [100−ECV], respectively.
Regional myocardial remodeling was assessed using an atlas‐based approach as previously described.6 A fully automated machine learning segmentation algorithm11 comprising cine images of 100 participants from the local cohort was used (Tensorflow r1.13, Google Brain, Mountain View, CA; https://github.com/UK-Digital-Heart-Project/4Dsegment), complementing the reference atlas from the UK Digital Heart Project. The final segmentations of all the participants in the study were subsequently co‐registered to an average template surface mesh. This makes the data anatomically consistent between each individual and provides a smooth interpolation of cardiac shape.
Wall thickness was calculated at over >40 000 points at end‐diastole, measured as the distance between the endo‐ and epicardial surfaces perpendicular to a midwall plane. Regional concentric remodeling was defined as chamber volume reduction because of inward displacement of the endocardium. Conversely, regional eccentric remodeling was defined as cavity dilatation because of outward endocardial expansion. Regional wall stress was determined at each point in the LV from the 3D Gaussian radius of curvature, wall thickness, and peripheral systolic blood pressure, measured before CMR.7
Statistical Analysis
Mean±SD or median (interquartile range) was used to present continuous variables, and percentages for categorical data. Comparison of continuous data was performed using either parametric Student t test or nonparametric Mann–Whitney U test, and χ2 test for categorical data. In the sex‐stratified associations between global measures of LV remodeling and mass, the forward selection multiple linear regression was used to adjust for the effects of clinically important confounders: age, systolic blood pressure, height, and weight. Statistical analyses were performed using GraphPad Prism Version 8 (GraphPad Software, Inc, San Diego) and SPSS Version 24 (IBM Corp., Armonk, NY).
The association between 3D phenotypic parameters (wall thickness and wall stress) and mass was assessed using sex‐specific regression models, adjusted for the same confounders. The regression modeling applied threshold‐free cluster enhancement and permutation to derive P values associated with each regression coefficient following adjustment to control for false discovery rate.12 Regions where association between 3D phenotypes and mass was significant (P<0.05) were reported as a percentage of the total LV surface.12 Sex‐related differences in concentricity and wall stress relative to mass were expressed as the ratio of the standardized beta coefficients between males and females. Statistical analyses were performed on RStudio version 1.1.463 (Boston, MA).
Results
Data from 1005 participants (57% males; 53±13 years) were analyzed (Table). Age and proportions of participants with cardiovascular risk factors were similar in both sexes (P>0.50 for all), except higher blood pressures and larger body sizes in males (Table). Most participants (79%) did not have left ventricular hypertrophy on CMR, using Asian thresholds.8 Of the 728 participants (72%) with gadolinium‐enhanced imaging, 14.6% had nonischemic fibrosis: the proportions were similar in either sex (P=0.182; Table).
Table 1.
Characteristics of the Study Population Stratified by Sex
| All Participants (n=1005) | Stratified by Sex | |||
|---|---|---|---|---|
| Males (n=570) | Females (n=435) | P Value | ||
| Clinical characteristics | ||||
| Age, y | 53.6±13.1 | 53.3±13.1 | 54.0±13.1 | 0.427 |
| Hyperlipidemia, n (%) | 300 (29.9) | 171 (30.0) | 129 (29.7) | 0.906 |
| Diabetes mellitus, n (%) | 115 (11.4) | 66 (11.6) | 49 (11.3) | 1.000 |
| Hypertension, n (%) | 689 (68.6) | 415 (72.8) | 274 (63.0) | 0.945 |
| Height, cm | 165.0±8.8 | 170.5±6.3 | 157.8±5.8 | <0.0001 |
| Weight, kg | 69.8±15.5 | 76.2±14.4 | 61.4±12.7 | <0.0001 |
| Body mass index, kg/m2 | 25.5±4.5 | 26.1±4.3 | 24.6±4.6 | 0.039 |
| Systolic blood pressure, mm Hg | 130.1±15.2 | 132.4±15.1 | 127.2±14.8 | <0.0001 |
| Diastolic blood pressure, mm Hg | 79.2±10.5 | 81.6±10.5 | 76.0±9.6 | <0.0001 |
| Cardiovascular magnetic resonance imaging | ||||
| Indexed LV EDV, mL/m2 | 73.2±13.1 | 76.5±13.6 | 68.7±10.8 | <0.0001 |
| Indexed LV ESV, mL/m2 | 29.5±9.2 | 31.9±9.5 | 26.4±7.8 | <0.0001 |
| Indexed LV SV, mL/m2 | 43.6±7.4 | 44.6±7.9 | 42.4±6.5 | <0.0001 |
| LV ejection fraction, % | 60.0±6.4 | 58.6±6.4 | 61.9±5.9 | <0.0001 |
| Indexed LV mass, g/m2 | 49.9±13.6 | 55.6±14.2 | 42.4±8.2 | <0.0001 |
| LV mass/EDV ratio | 0.69±0.15 | 0.73±0.15 | 0.63±0.12 | <0.0001 |
| Maximum wall thickness, mm | 8.5±1.9 | 9.3±1.8 | 7.4±1.4 | <0.0001 |
| Circumferential strain, % | −21.6±3.2 | −20.6±3.2 | −22.9±2.9 | <0.0001 |
| Longitudinal strain, % | −18.7±3.1 | −17.9±3.0 | −19.8±2.9 | <0.0001 |
| Radial strain, % | 44.4±12.4 | 41.2±11.4 | 49.0±12.3 | <0.0001 |
| Nonischemic late gadolinium enhancementa, n (%) | 106 (14.6) | 70 (16.0) | 36 (12.4) | 0.182 |
| Native myocardial T1a, ms | 1016±29 | 1008±26 | 1027±29 | <0.0001 |
| Extracellular volume fractiona, % | 25.9±2.8 | 25.1±2.9 | 27.1±2.4 | <0.0001 |
| Myocyte volumea, mL/m2 | 36.8±10.2 | 40.6±10.6 | 30.9±5.7 | <0.0001 |
| Interstitial volumea, mL/m2 | 12.8±4.0 | 13.7±4.4 | 11.5±2.7 | <0.0001 |
| Interstitial volume/myocyte volumea | 0.35±0.04 | 0.33±0.04 | 0.37±0.04 | <0.0001 |
EDV indicates end‐diastolic volume; ESV, end‐systolic volume; LV, left ventricular volume; SV, stroke volume.
Based on 728 participants who had gadolinium contrast imaging performed (females, n=290; males, n=438).
Sex‐Related Differences in Global Left Ventricle Geometry and Function
An increase in LV mass was associated with increased concentricity, wall thickness, and LV end‐diastolic volumes (Figure 1). In females, a steeper increase in concentricity (P=0.014 for difference in regression slopes) and wall thickness (P=0.010) with increasing mass was observed. Furthermore, females had smaller LV end‐diastolic volumes than males. Although the progressive increase in mass was associated with reduced LVEF and strain in both sexes, LV function was higher in females (Figure 1). Despite a significantly lower LV mass, females had higher ECV compared with males. Similar findings were observed with native myocardial T1 values and interstitial:myocyte ratio. For the same myocyte volume, females had higher interstitial volume compared with males (Table; Figure 2).
Figure 1.
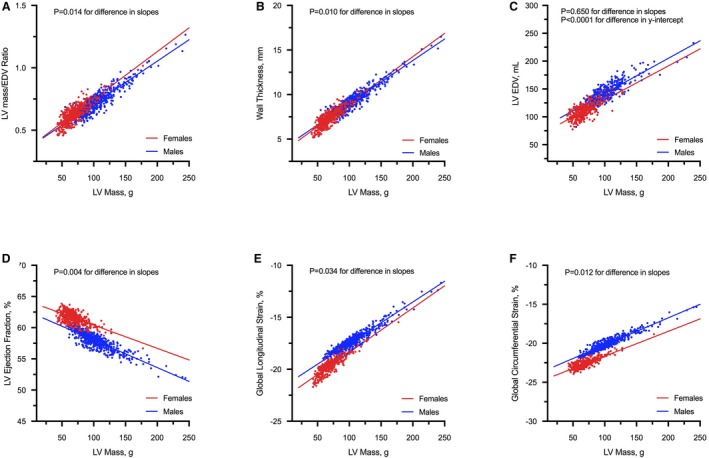
Association between LV geometry, function, and mass. There was a positive association between concentricity (A), wall thickness (B), and cardiac volumes (C) with LV mass in both sexes. In females, an increase in LV mass was associated with a steeper increase in concentric remodeling (mediated by relative increased wall thickness and smaller LV volumes) compared with males. With increasing mass, there was a reduction in LV ejection fraction (D), and global myocardial strain (E and F) in both sexes, with higher LV function in females. All analyses were adjusted for the effects of age, systolic blood pressure, and body size. EDV indicates end diastolic volumes; LV, left ventricular.
Figure 2.
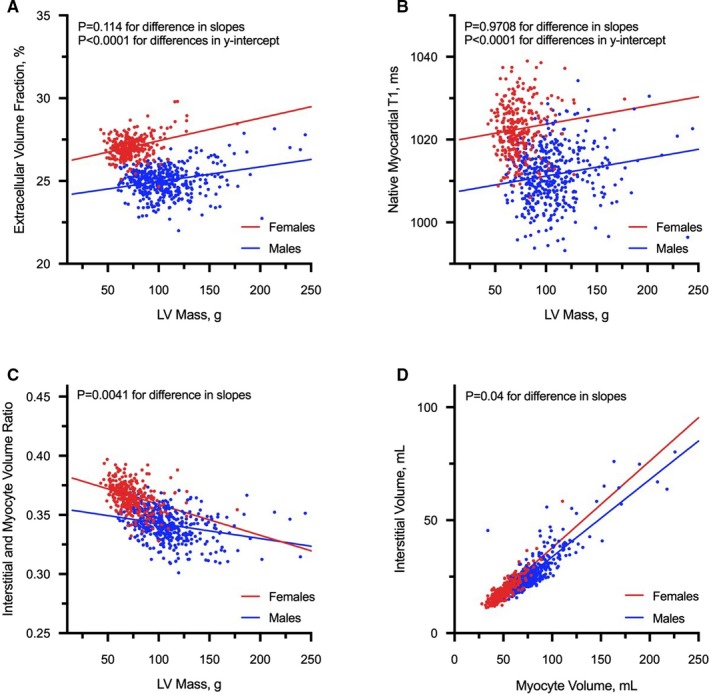
Association between measures of extracellular volume and LV mass. With increasing left ventricular mass, females had consistently higher extracellular volume fraction (A), native myocardial T1 (B) and interstitial:myocyte ratio (C) compared with males. For the same myocyte volume, females have higher interstitial volume compared with males (D). All analyses were adjusted for the effects of age, systolic blood pressure, and body size. LV indicates left ventricular.
Sex‐Related Differences in Regional Concentricity and Wall Stress
The intraclass correlation between manual and atlas‐based measures was 0.97 for LV end‐diastolic volumes and mass. 3D analyses confirmed the findings observed with global LV measures. Compared with males, females had smaller ventricles and increased concentric remodeling with increasing mass. The distribution of increased concentric remodeling was not uniform and localized at the basal and midventricular myocardium (79% of the ventricular surface; Figure 3). Females had relatively higher wall stress compared with males (mean difference: 170 mm Hg [22.6 kPa], P<0.0001), predominantly at the basal anterior walls and septum; and corresponded to similar regions of increased concentric remodeling (Figure 3).
Figure 3.
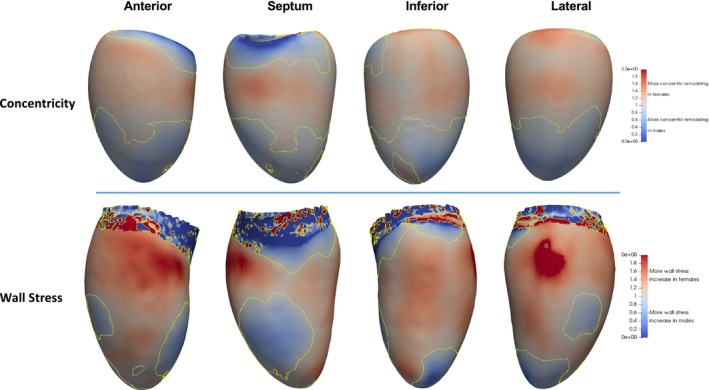
Regional concentric remodeling and wall stress. Compared with males, females had regions of higher wall stress at the anterior walls and septum, corresponding to similar regions of increased concentric remodeling. All analyses were adjusted for the same potential confounders as global LV measures. LV indicates left ventricular.
Discussion
In the general population, increasing LV mass is positively associated with concentricity, but to a greater extent in females that is mediated by increased wall thickness and smaller LV volumes than males. Despite more favorable profiles of smaller hearts, lower SBP, and higher LV function, females have higher regional myocardial wall stress that corresponded to regions of increased concentric remodeling; and elevated ECV that has been associated with diffuse myocardial fibrosis.
In pressure and volume overloaded conditions, females develop more concentric remodeling and preserved LV function compared with males.3, 4, 5, 13, 14 Our study extends previous findings to the general population and reports that the increased concentric remodeling in females is mediated by increased wall thickness and smaller ventricles. These smaller ventricles may partially explain the higher LV function in females compared with males, at a given LV mass.15 LV function is reduced progressively with increasing mass in both sexes that could be the response to elevated wall stress. Consistent with previous studies, we have shown that females have an expanded interstitium assessed using several T1 measures (ECV, native T1, and interstitial:myocyte ratio) that may represent a fundamental physiological sex‐related difference in myocardial composition and pathological expansion from myocardial fibrosis, thereby conferring an increased risk in females.16, 17, 18
Myocardial contraction combined with elastance (stiffness) mediate wall stress, resulting in either concentric or eccentric remodeling. Therefore, a knowledge of wall stress will provide valuable mechanistic insights in the different cardiovascular conditions. Assessing myocardial wall stress is challenging because it is heterogeneous across the LV; and robust methodologies of estimating regional wall stress are lacking. Using atlas‐based models, assessment of regional wall stress in large imaging data sets has recently been demonstrated.7 This approach is less dependent on any geometric assumptions as computation is performed across ≈40 000 vertices in the entire LV.
For the first time, we demonstrated that females have paradoxically higher regional wall stress despite smaller hearts compared with males. One of the possible reasons is the greater LV elastance (stiffness) in females,19 which can be partly explained by the increased interstitial volumes relative to myocyte volumes as demonstrated in our study. As a consequence, females are more susceptible to further increase in LV pressures. This may account for their greater predisposition to complications such as heart failure with preserved ejection fraction.20 The higher regional wall stress at the anterior walls and septum observed in females may suggest a mechanism for asymmetric basal septal hypertrophy commonly seen in elderly females with hypertension.21 Our findings have important clinical implications, particularly in Asians because of the smaller LV mass and volumes compared with other ethnicities.
Study Limitations
The cross‐sectional study cannot determine the causal relationships between wall stress and adverse features of cardiac remodeling. We used the Laplacian model of wall stress that assumed uniform mechanical properties of the myocardium.
Conclusions
We have demonstrated the role of atlas‐based models in assessing regional wall stress in large imaging data sets. Females have smaller hearts, higher regional wall stress, and increased concentric remodeling. These findings may explain why females are predisposed to cardiovascular complications, particularly when LV pressures are increased.
Sources of Funding
The study was supported by the National Medical Research Council.
Disclosures
None.
Acknowledgments
We thank the radiographers at the Department of Cardiovascular Magnetic Resonance, National Heart Center Singapore for their assistance in conducting the study.
(J Am Heart Assoc. 2020;9:e014781 DOI: 10.1161/JAHA.119.014781.)
References
- 1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 2. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. [DOI] [PubMed] [Google Scholar]
- 3. Kostkiewicz M, Tracz W, Olszowska M, Podolec P, Drop D. Left ventricular geometry and function in patients with aortic stenosis: gender differences. Int J Cardiol. 1999;71:57–61. [DOI] [PubMed] [Google Scholar]
- 4. Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. [DOI] [PubMed] [Google Scholar]
- 5. Rohde LE, Zhi G, Aranki SF, Beckel NE, Lee RT, Reimold SC. Gender‐associated differences in left ventricular geometry in patients with aortic valve disease and effect of distinct overload subsets. Am J Cardiol. 1997;80:475–480. [DOI] [PubMed] [Google Scholar]
- 6. de Marvao A, Dawes TJW, Shi W, Minas C, Keenan NG, Diamond T, Durighel G, Montana G, Rueckert D, Cook SA, O'Regan DP. Population‐based studies of myocardial hypertrophy: high resolution cardiovascular magnetic resonance atlases improve statistical power. J Cardiovasc Magn Reson. 2014;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Marvao A, Dawes TJW, Shi W, Durighel G, Rueckert D, Cook SA, O'Regan DP. Precursors of hypertensive heart phenotype develop in healthy adults: a high‐resolution 3D MRI study. JACC Cardiovasc Imaging. 2015;8:1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le TT, Tan RS, De Deyn M, Goh EP, Han Y, Leong BR, Cook SA, Chin CW. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson. 2015;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai J, Bryant JA, Le TT, Su B, de Marvao A, O'Regan DP, Cook SA, Chin CW. Fractal analysis of left ventricular trabeculations is associated with impaired myocardial deformation in healthy Chinese. J Cardiovasc Magn Reson. 2017;19:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chin C, Semple S, Malley T, White AC, Mirsadraee S, Weale PJ, Prasad S, Newby DE, Dweck MR. Optimization and comparison of myocardial T1 techniques at 3T in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2014;15:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duan J, Bello G, Schlemper J, Bai W, Dawes TJW, Biffi C, de Marvao A, Doumond G, O'Regan DP, Rueckert D. Automatic 3D bi‐ventricular segmentation of cardiac images by a shape‐refined multi‐task deep learning approach. IEEE Trans Med Imaging. 2019;38:2151–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biffi C, de Marvao A, Attard MI, Dawes TJW, Whiffin N, Bai W, Shi W, Francis C, Meyer H, Buchan R, Cook SA, Rueckert D, O'Regan DP. Three‐dimensional cardiovascular imaging‐genetics: a mass univariate framework. Bioinformatics. 2018;34:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, Karp RB. Sex‐associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. [DOI] [PubMed] [Google Scholar]
- 14. Piro M, Bona Della R, Abbate A, Biasucci LM, Crea F. Sex‐related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. [DOI] [PubMed] [Google Scholar]
- 15. Voigt JU, Cvijic M. 2‐ and 3‐dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12:1849–1863. [DOI] [PubMed] [Google Scholar]
- 16. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age‐related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced T1 mapping: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosmini S, Bulluck H, Captur G, Treibel TA, Abdel‐Gadir A, Bhuva AN, Culotta V, Merghani A, Fontana M, Maestrini V, Herrey AS, Chow K, Thompson RB, Piechnik SK, Kellman P, Manisty C, Moon JC. Myocardial native T1 and extracellular volume with healthy ageing and gender. Eur Heart J Cardiovasc Imaging. 2018;19:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schelbert EB, Miller CA. Myocardial tissue characteristics undoubtedly differ by gender but not age. Eur Heart J Cardiovasc Imaging. 2018;19:611–612. [DOI] [PubMed] [Google Scholar]
- 19. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005;112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 20. Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, Kannel WB, D'Agostino RB, Lee DS, Levy D. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelshiker MA, Mayet J, Unsworth B, Okonko DO. Basal septal hypertrophy. Curr Cardiol Rev. 2013;9:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]


