Abstract
Background
Meta‐analyses have shown that isometric handgrip training (IHT) can reduce brachial systolic and diastolic blood pressure (BP) by >6/4 mm Hg, respectively. However, whether IHT promotes these effects among patients with peripheral artery disease, who exhibit severe impairment in cardiovascular function, is currently unknown. This study aimed to evaluate the effects of IHT on the cardiovascular function of patients with peripheral artery disease.
Methods and Results
A randomized controlled trial with peripheral artery disease patients assigned to either the IHT or control group was conducted. The IHT group performed 3 sessions per week, for 8 weeks, of unilateral handgrip exercises, consisting of 4 sets of isometric contractions for 2 minutes at 30% of maximum voluntary contraction and a 4‐minute interval between sets. The control group received a compression ball in order to minimize the placebo effects, representing sham training. The primary outcome was brachial BP. The secondary outcomes were central BP, arterial stiffness parameters, cardiac autonomic modulation, and vascular function. The IHT program reduced diastolic BP (75 [10] mm Hg preintervention versus 72 [11] mm Hg postintervention), with no change in the control group (74 [11] mm Hg preintervention versus 74 [11] mm Hg postintervention), with this between‐group difference being significant (P=0.04). Flow‐mediated dilation improved in the IHT group (6.0% [5.7] preintervention versus 9.7% [5.5] postintervention), with no change in the control group (7.6% [5.5] preintervention versus 7.4% [5.1] postintervention), with this between‐group difference being significant (P=0.04). There was no change in other measured variables over the intervention period.
Conclusions
IHT reduced brachial diastolic BP and improved local vascular function in patients with peripheral artery disease.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02742220.
Keywords: blood pressure, cardiovascular system, intermittent claudication, peripheral vascular disease
Subject Categories: Peripheral Vascular Disease, Exercise
Clinical Perspective
What Is New?
This is the first study to analyze the effect of isometric handgrip training on cardiovascular function of peripheral artery disease patients.
This modality of training improves cardiovascular function of normotensive, prehypertensive, and hypertensive patients; however, until the present study, the effect of isometric handgrip training in peripheral artery disease patients, who exhibit severe impairment in cardiovascular function, was unknown.
This randomized controlled trial concluded that isometric handgrip training reduced brachial diastolic blood pressure and improved local vascular function in patients with peripheral artery disease.
What Are the Clinical Implications?
Isometric handgrip training, which is a simple and homebased modality of training, can be an adjunct strategy only to reduce brachial diastolic blood pressure in patients with peripheral artery disease.
Introduction
Patients with peripheral artery disease (PAD) often present with multiple health comorbidities, such as hypertension, diabetes mellitus, and dyslipidemia,1 and are at an increased risk for fatal and non‐fatal cardiovascular events.2, 3 Interventions to manage and reduce cardiovascular disease, and its complications, are therefore highly recommended for patients with PAD.4
A supervised walking program has been recommended as a first‐line strategy for patients with PAD to enhance walking capacity and cardiovascular function.5, 6, 7 However, claudication symptoms are a barrier to physical activity in these patients.8 Moreover, traveling to an exercise center for supervised exercise training can be burdensome, with many patients declining participation in center‐based exercise rehabilitation.9 In addition, a home‐based exercise program was recently recommended as exercise therapy for PAD by the American Heart Association (level of evidence, A; class of recommendation, IIA).10 Therefore, a home‐based program of exercise, using nonpainful modalities, could provide a suitable alternative strategy for patients with PAD.
Previous meta‐analyses11, 12, 13, 14 have shown that isometric handgrip training (IHT) can reduce brachial systolic and diastolic blood pressure (BP) by >6/4 mm Hg in normotensive, prehypertensive, and hypertensive patients.12, 15 In addition, improvements in cardiac autonomic function,16, 17 arterial stiffness,18 and local endothelial function19, 20 have been also been reported after IHT,17, 19, 20, 21 although these are not universal findings.22 Furthermore, all studies were conducted in normo‐ and hypertensive subjects, and whether IHT promotes effects on cardiovascular system in patients with PAD, who exhibit more impaired cardiovascular function than hypertensives, is currently unknown.
The aim of our study was to evaluate the effects of IHT on the cardiovascular function of patients with PAD. Our hypothesis was that IHT would decrease BP, with concomitant improvement in vascular function and/or autonomic cardiac modulation (measured by heart rate variability) in patients with PAD. These changes could modify the indicators of arterial stiffness.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial Design
This randomized controlled trial study, using 2 parallel groups, was preregistered in the http://www.clinicaltrials.gov database under the registration number NCT02742220 and is part of the ISOPRESS network.23 The procedures of our trial were approved by the institutional review board, in compliance with the Brazilian National Research Ethics System Guidelines. Written informed consent was obtained from each patient before participation in any phase of the study. This study follows the CONSORT (Consolidated Standards of Reporting Trials) checklist.24
Participants
Patients with PAD were recruited from hospitals in São Paulo, Brazil between March 2016 and January 2018. The inclusion criteria were: (1) age ≥45 years; (2) presence of PAD stage IIa and IIb, based on the Rutherford criteria; (3) an ankle‐brachial index <0.90; (4) for women, being postmenopausal without hormone therapy replacement; (5) absence of critical limb ischemia, pain at rest, amputated limbs, and/or ulcers; (6) absence of neurological and/or cognitive diseases; and; (7) having the capacity to perform a home‐based program of exercise. Patients who did not adhere to at least 80% of the prescribed training session, changed the type and/or dose of their antihypertensive medication, or presented with any health problems that contraindicated physical activity were excluded.
Screening
Sociodemographic information, comorbidities, and medication information were obtained through a face‐to‐face interview. Sociodemographic variables included age and sex (male or female). The clinical history included: smoking habit (ex‐ or current‐ or never‐smoker), obesity (body mass index≥30 kg/m²), diabetes mellitus (doctor‐diagnosed or use of glucose‐lowering drugs), hypertension (systolic/diastolic BP ≥140/90 mm Hg or use of antihypertensive drugs), dyslipidemia (doctor‐diagnosed or use of lipid‐lowering drugs), coronary heart disease, heart failure, and cerebrovascular disease (medical history). Body weight was measured using a calibrated scale, to the nearest 0.1 kg, with height measured using a stadiometer, to the nearest 0.01 m. The body mass index (kg/m2) was calculated from these values.
PAD severity was determined by a single evaluator using the ankle‐brachial index, in accord with previous guideline.25 Briefly, the ankle‐brachial index was measured as the highest systolic BP in the posterior tibial or dorsalis pedis artery divided by the highest systolic BP in the brachial artery. BP measurements were recorded in both limbs using a Doppler vascular monitor (Medmega DV160; Medmega Indústria Equipamentos Médico, Franca, Brazil) and a sphygmomanometer.
Randomization and Allocation
Participants were randomly assigned to either the IHT group or the control group (CG), using a block randomization number table, with numbers generated by http://www.randomizer.org, stratified by sex and baseline brachial systolic BP. This randomization was done by a researcher not involved directly in the recruitment and data collection. Allocation was concealed to the researchers and patients involved in the study.
Interventions
After screening, a familiarization of the session with the program to be performed at home was conducted to ensure understanding and comfort with the program. During this session, patients’ questions and any difficulties with performance of the program were addressed.
The IHT group performed 3 sessions per week, for 8 weeks, at home, guided by an experienced kinesiologist, who previously familiarized the patients with the exercise protocol and encouraged the patients by telephone to continue IHT training. The exercise protocol consisted of 4 sets of 2‐minute isometric handgrip holds unilateral (dominant arm), performed at 30% of maximum vonluntary contraction with a 4‐minute rest interval between repetitions. All patients used the Zona Plus (Zona Health, Inc, a Boise, ID) isometric handgrip device, which provided instructive text prompts, on a liquid crystal display, for each step of the training session, as well as visual (liquid crystal display pressure gauge) and audible signals for feedback to ensure compliance with the exercise intensity of the protocol. Adherence to the program was defined as completion of at least 80% of the prescribed training session, with >96 seconds of isometric contraction held for each repetition in each session, exported from Zona Plus at the end of the training. Patients randomized to the CG received a compression ball in order to minimize the manipulation effect of a device and the placebo effects, thus representing sham training. It was recommended that the patients perform 3 sessions per week, with 3 sets of 10 compressions, for each hand, with a 1‐minute rest interval between sets.
Patients in both groups were instructed to increase their general daily physical activity level to meet the recommendations for PAD patients, namely, 150 minutes of moderate physical activity or 75 minutes of vigorous physical activities, or an equivalent combination of moderate‐to‐vigorous physical activities per week. Patients also received a diary to record the sessions and report difficulties during training. A visit was scheduled at the fifth week to provide feedback and discuss possible problems during the training. In addition, once every 2 weeks, each patient was contacted by phone to reinforce exercise recommendations.
Outcomes
The primary outcome of the study was brachial BP. Secondary outcomes were: (1) central BP; (2) arterial stiffness parameters; (3) cardiac autonomic modulation; (4) vascular function; and (5) physical activity. All parameters were analyzed at baseline and after 8 weeks of an intervention period.
Before all cardiovascular measurements, patients were instructed to: eat a light meal before arriving at the laboratory; avoid moderate‐to‐vigorous physical activity for at least 24 hours before the visit; and avoid smoking, alcohol, and caffeine ingestion for at least 12 hours. In the laboratory, a rest period of 10 minutes in the supine position before the measures was given. All cardiovascular measurements were taken between 7:00 am to 6:00 pm, in the supine position in a quiet environment, with monitored temperature, and no interruptions. The time of the day of the preintervention measurements of each subject was maintained in postintervention evaluations.
Brachial BP (primary outcome)
Brachial BP was measured through an oscillometric device (HEM 742; Omron Healthcare, Kyoto, Japan). Three consecutive measurements were performed at a 1‐minute interval, in both arms, with the proper cuff size to arm circumference. The value used was the average of the 3 measures of the highest BP arm.26
Central BP (secondary outcome)
Central BP was obtained by pulse‐wave analysis, recorded in the radial artery, using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, Australia). The validated transfer function algorithm provided by the Sphygmocor software obtained the central values of systolic and diastolic BP (equivalent to the pressure wave measured by an invasive catheter).27 To enhance the accuracy of measurements, only those values whose quality index exceeded 90% were utilized. The value used was of the highest BP arm.
Arterial stiffness parameters (secondary outcome)
Pulse pressure (difference between systolic and diastolic BP) and augmentation index (the proportion of pulse pressure that is attributed to the reflected pulse wave) were obtained through applanation tonometry (SphygmoCor; AtCor Medical) in the radial artery in both arms, being used to analyze the highest BP arm. Carotid‐femoral pulse wave velocity was measured by applanation tonometry (Sphygmocor; AtCor Medical), following the guidelines of the Clinical Application of Arterial Stiffness, Task Force III.28 Distances between carotid artery to the suprasternal notch and femoral artery to the suprasternal notch were measured using a standard tape. Simultaneous ECG was assessed to obtain heart rate, and, according to a “foot‐to‐foot” method, the time difference between the points was measured. Then, the distance between the 2 arteries was divided by the time difference.
Cardiac autonomic modulation (secondary outcome)
Cardiac autonomic modulation was assessed though heart rate variability from the RR intervals obtained by a heart rate monitor (RS800CX; Polar Electro, Espoo, Finland). Patients remained in the supine position for 10 minutes and were stationary for at least 5 minutes; we analyzed these stationary 5 minutes period. All analyses were performed using software (Kubios HRV; Biosignal Analysis and Medical Imaging Group, University of Eastern Finland, Kuopio, Finland) by a single evaluator blinded to the group allocations, using the recommendations of the Task Force for heart rate variability (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology).29 The following time‐domain variables were examined for each recording: standard deviation of all RR intervals, root mean square of the squared differences between adjacent normal RR intervals, and the percentage of number of pairs of adjacent NN intervals differing by more than 50 ms. Additionally, frequency‐domain variables were calculated by an autoregressive method. The signals operating at frequencies between 0.04 and 0.4 Hz were considered physiologically significant with the low‐frequency component represented by oscillations between 0.04 and 0.15 Hz and the high‐frequency component represented by oscillations between 0.15 and 0.4 Hz. The power of each spectral component was normalized by dividing the power of each spectrum band by the total variance, minus the value of the very‐low‐frequency band (<0.04 Hz) and multiplying the result by 100. To interpret the results, the low‐ and high‐frequency normalized components were considered, respectively, as representative of predominantly combined parasympathetic‐sympathetic and parasympathetic modulation of the heart, and the ratio between these bands was defined as the cardiac sympathovagal balance.
Vascular function (secondary outcome)
Blood flow and flow‐mediated dilation measurements were obtained by ultrasound according to the global recommendations.30 Images of the brachial artery of trained and nontrained arms were recorded by a 2‐dimensional ultrasound machine with spectral Doppler and linear transducer (Ultra‐0122; Philips, Best, The Netherlands). The contrast resolution, depth, and gain were adjusted to optimize the longitudinal images of the lumen/arterial wall interface. Brachial artery diameter and insonation angle corrected at 60 degrees blood velocity spectra were simultaneously recorded by the pulsed‐wave mode at linear frequencies of 10 and 6.0 MHz, respectively. Baseline diameter and blood velocity waveforms were continuously recorded over 120 seconds. After that, a cuff, placed on the forearm, was inflated to a pressure >50 mm Hg above systolic BP, measured before the examination. The occlusion was maintained for 5 minutes and, after this period, rapidly released. The recordings were resumed 30 seconds before deflation and maintained for 180 seconds after. The diameter of brachial artery and postocclusion blood flow velocity were measured after the release.
All vascular variables were analyzed offline using specialized edge‐detection software (Cardiovascular Suite; Quipu, Pisa, Italy). Flow‐mediated dilation was calculated by the percentage of increase in diameter of the brachial artery postocclusion compared with their baseline values. The anterograde component of blood flow was defined as the area above and below 0 cm/s on the horizontal axis of the Doppler. The area under the shear rate curve, indicative of the vasodilation stimulus, was calculated from the moment the cuff was released until the maximum diameter was reached. Blood flow was calculated from the formula: blood flow=π (0.5×D)2×Vm, where Vm corresponds to the mean velocity of blood flow in centimeters per second, and D represents the diameter of the brachial artery.
Physical activity (secondary outcome)
Physical activity was obtained in a subgroup of the sample (IHT=25 and CG=25) through a 3‐dimensional accelerometry monitor (A300; Polar Electro), which gets patients’ steps each day of use. This monitor was used 1 week before intervention and in the last week of the intervention. The average number of steps during the days of using before and after intervention was considered.
Statistical Analysis
The data were stored and analyzed using the Statistical Package for the Social Sciences (SPSS Version 20.0; SPSS, Inc, Chicago, IL). Normality and homogeneity were checked using Shapiro–Wilk's and Levene's tests, respectively. Continuous variables were summarized as mean and SD (normally distributed data) or median and interquartile range (non‐normally distributed data), whereas categorical variables were summarized as relative frequencies. Clinical and cardiovascular baseline characteristics were compared using Mann–Whitney U tests for continuous variables and the chi‐square test for categorical variables.
To compare the effects of interventions (IHT or CG) on cardiovascular parameters, generalized estimating equations were used, followed by a post hoc pair‐wise comparison using the Bonferroni correction for multiple comparisons. When there was a significant difference between groups in preintervention variables (low frequency/high frequency ratio), preintervention values were used as adjustments.
Per‐protocol analyses were conducted in patients who adhered to at least 80% of the prescribed training session, did not changed the type and/or dose of their medications, and did not present with any health problems that contraindicated physical activity.
Intention‐to‐treat analysis was used to estimate overall effects, with all randomized patients ignoring noncompliance and dropouts. In order to do that, multiple imputations with linear regression weighted by group were applied.
The significance level was set at P<0.05 for all analyses.
Results
Recruitment and intervention periods were completed between January 2015 and December 2018. The study flowchart is shown in Figure 1. Of the 277 patients screened for possible inclusion, 129 were considered eligible, with 102 of these ultimately entered into the trial, with 50 randomized to the IHT group and 52 to the CG group. The data from all these patients were included in the intent‐to‐treat analysis. Because of multiple disease comorbidities and technical problems with the ultrasound device, some variables were not obtained, as detailed in Figure 1.
Figure 1.
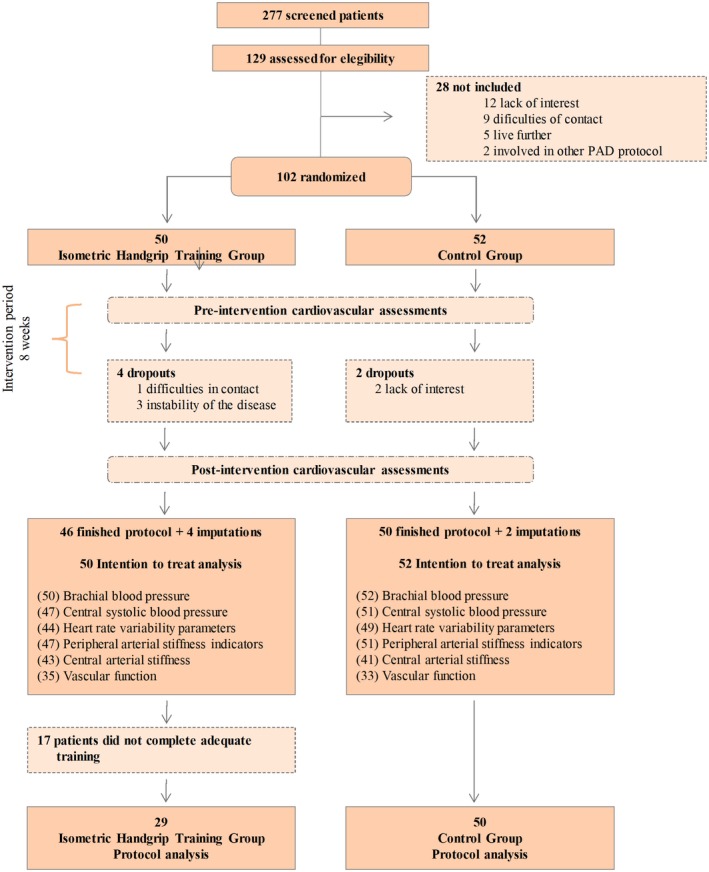
Flowchart of study. PAD indicates peripheral artery disease.
Baseline clinical characteristics were not different between the 2 groups (Table 1). Cardiovascular parameters (Table 2) were also comparable between the 2 groups in the preintervention period, with the exception of the frequency domain of heart rate variability, with patients in the IHT group presenting higher sympathovagal balance (low frequency/high frequency, P=0.041), compared with those in the CG group.
Table 1.
Characteristics of Peripheral Artery Disease Patients Included in the Sample n=102
| Variables | Control Group (n=52) | IHT (n=50) | P Value |
|---|---|---|---|
| Sex, % men | 62 | 66 | 0.684 |
| Age, y | 67 (11) | 66 (12) | 0.207 |
| Body mass index, kg/m2 | 26 (7) | 27 (5) | 0.510 |
| Ankle brachial index | 0.60 (0.24) | 0.57 (0.22) | 0.318 |
| Intima‐medial thickness, mm | 0.098 (0.038) | 0.089 (0.033) | 0.406 |
| Risk factors | |||
| Diabetes mellitus, % | 47 | 41 | 0.554 |
| Hypertension, % | 79 | 79 | 0.958 |
| Dyslipidemia, % | 89 | 89 | >0.999 |
| Obesity, % | 27 | 20 | 0.410 |
| Coronary artery disease, % | 32 | 35 | 0.773 |
| Medication | |||
| Antiplatelet, % | 84 | 83 | 0.813 |
| Inhibitor of ACE, % | 31 | 26 | 0.596 |
| Angiotensin‐receptor antagonist, % | 31 | 26 | 0.596 |
| Calcium‐channel blocker, % | 24 | 37 | 0.196 |
| Diuretic, % | 36 | 41 | 0.573 |
| Beta‐blockers, % | 38 | 52 | 0.168 |
| Statins, % | 93 | 87 | 0.485 |
| Hypoglycemics, % | 55 | 37 | 0.094 |
| Peripheral vasodilator, % | 33 | 20 | 0.136 |
| Physical capacity | |||
| Claudication onset distance, m | 117 (106) | 120 (96) | 0.598 |
| Six‐minute walk distance, m | 250 (356) | 302 (290) | 0.167 |
| Steps per daya | 5704 (3617) | 5196 (2678) | 0.575 |
| Handgrip strength, kgf | 30.0 (14.8) | 33.0 (12.5) | 0.736 |
Data presented as mean (standard deviation) or relative frequency. P, level of significance in independent t test. ACE indicates angiotensin‐converting enzyme; CG, control group; IHT, isometric handgrip training group.
Data from a subgroup analysis of 25 patients in IHT and 25 patients in the CG.
Table 2.
Cardiovascular Parameters of Patients With Peripheral Artery Disease Patients in Preintervention Moment
| Variables | n | Control Group | n | IHT | P Value |
|---|---|---|---|---|---|
| Blood pressure | |||||
| Brachial systolic BP, mm Hg | 52 | 149 (23) | 50 | 143 (22) | 0.189 |
| Brachial diastolic BP, mm Hg | 52 | 75 (11) | 50 | 74 (10) | 0.768 |
| Central systolic BP, mm Hg | 51 | 137 (23) | 47 | 133 (21) | 0.317 |
| Central diastolic BP, mm Hg | 51 | 75 (11) | 47 | 75 (10) | 0.998 |
| Heart rate variability | |||||
| RR interval, ms | 49 | 931 (164) | 44 | 949 (179) | 0.779 |
| SDNN, msa | 49 | 30.3 (23.5) | 44 | 25.9 (29.0) | 0.572 |
| RMSSD, msa | 49 | 23.3 (24.0) | 44 | 15.2 (25.2) | 0.120 |
| pNN50, %a | 49 | 2.9 (12.8) | 44 | 0.65 (7.6) | 0.084 |
| Low frequency, ms2 a | 49 | 214 (316) | 44 | 137 (434) | 0.770 |
| High frequency, ms2 a | 49 | 180 (302) | 44 | 74 (220) | 0.245 |
| LF/HFa | 49 | 0.94 (1.32) | 44 | 1.44 (2.67) | 0.041 |
| SD1, msa | 49 | 16.5 (17.1) | 44 | 10.8 (17.8) | 0.124 |
| SD2, msa | 49 | 39.6 (29.9) | 44 | 34.4 (36.5) | 0.794 |
| Shannon entropya | 49 | 3.21 (0.71) | 44 | 3.23 (0.47) | 0.908 |
| Sample entropya | 49 | 1.44 (0.72) | 44 | 1.42 (0.45) | 0.703 |
| Vascular mechanisms | |||||
| Brachial pulse pressure, mm Hga | 52 | 76 (25) | 50 | 66 (24) | 0.156 |
| Pulse pressure, mm Hga | 51 | 62 (25) | 47 | 54 (19) | 0.174 |
| Augmentation index, % | 51 | 38 (10) | 47 | 36 (12) | 0.392 |
| Pulse wave velocity, m/s | 41 | 9.4 (2.6) | 43 | 9.7 (2.7) | 0.570 |
| Brachial diameter, mm | 34 | 3.97 (0.57) | 36 | 4.01 (0.73) | 0.764 |
| Flow‐mediated dilation, %a | 33 | 5.7 (7.5) | 35 | 4.9 (6.3) | 0.267 |
| Flow‐mediated dilation, mma | 33 | 0.25 (0.29) | 35 | 0.20 (0.22) | 0.195 |
| Time to maximum diameter, sa | 33 | 63 (81) | 35 | 55 (55) | 0.535 |
| AUC shear rate×100−1 a | 32 | 172 (135) | 34 | 172 (84) | 0.700 |
| Blood flow, mL/mina | 34 | 65 (35) | 36 | 68 (47) | 0.240 |
| FMD/AUC×100−1 a | 32 | 0.05 (0.06) | 34 | 0.03 (0.03) | 0.132 |
Data presented as mean (SD). P, level of significance in independent t test or Mann–Whitney U tests. AUC indicates area under the curve of shear rate; BP, blood pressure; FMD/AUC, flow‐mediated dilation corrected by area under the curve of shear rate; IHT, isometric handgrip group; LF/HF, low frequency/high frequency ratio; pNN50, percentage of number of pairs of adjacent NN intervals differing by more than 50 ms; RMMSD, root mean square of the squared differences between adjacent normal RR intervals; SDNN, standard deviation of all RR intervals.
Data presented as median (interquartile range).
Of the 50 patients randomized to the IHT group, 74% completed training sessions weekly, with 58% training at the appropriate intensity. Therefore, of the 50 patients included in the IHT group, 29 completed the protocol as prescribed and thus were included in the per‐protocol analysis (Table 3; Figures 2 and 3).
Table 3.
Effects of IHT in Cardiovascular Parameters in Peripheral Artery Disease Patients
| Variables | Control Group (n=50) | IHT (n=29) | Interaction Effect | ||||
|---|---|---|---|---|---|---|---|
| n | Pre | Post | n | Pre | Post | ||
| Blood pressure | |||||||
| Brachial SBP, mm Hg | 50 | 149 (23) | 146 (22) | 29 | 142 (21) | 136 (23) | 0.384 |
| Brachial DBP, mm Hg | 50 | 74 (11) | 74 (11) | 29 | 75 (10) | 72 (11)b | 0.047c |
| Central SBP, mm Hg | 46 | 138 (23) | 135 (21) | 27 | 133 (21) | 127 (24) | 0.544 |
| Central DBP, mm Hg | 46 | 75 (11) | 75 (11) | 27 | 76 (11) | 73 (11) | 0.086 |
| Heart rate variability | |||||||
| RR interval, msa | 45 | 880 (244) | 893 (238) | 25 | 896 (241) | 929 (194) | 0.929 |
| SDNN, msa | 45 | 30.3 (23.5) | 29.5 (23.1) | 25 | 29.4 (30.8) | 40.3 (38.2) | 0.212 |
| RMSSD, msa | 45 | 23.3 (24) | 18.9 (26.6) | 25 | 15.1 (33) | 23.0 (27.8) | 0.461 |
| pNN50, %a | 45 | 2.9 (12.8) | 2.1 (13.3) | 25 | 1.6 (14.3) | 3.2 (16.3) | 0.584 |
| Low frequency, ms2 | 45 | 214 (316) | 159 (482) | 25 | 257 (561) | 303 (734) | 0.264 |
| High frequency, ms2 | 45 | 180 (302) | 137 (412) | 25 | 74 (504) | 136 (591) | 0.828 |
| LF/HF | 45 | 1.64 (2.08) | 2.04 (3.21) | 25 | 2.54 (2.18) | 3.08 (3.87) | 0.964 |
| SD1, msa | 45 | 16.5 (27.9) | 13.4 (18.8) | 25 | 21.4 (10.7) | 16.3 (19.6) | 0.463 |
| SD2, msa | 45 | 39.6 (29.9) | 39.6 (31.8) | 25 | 38.3 (38.9) | 51.1 (47.9) | 0.477 |
| Shannon entropya | 45 | 3.21 (0.71) | 3.12 (0.49) | 25 | 3.33 (0.52) | 3.17 (0.41) | 0.769 |
| Sample entropya | 45 | 1.44 (0.72) | 1.48 (0.62) | 25 | 1.38 (0.38) | 1.38 (0.64) | 0.562 |
| Vascular mechanisms | |||||||
| Brachial pulse pressure, mm Hg | 50 | 75 (19) | 72 (20) | 29 | 67 (16) | 65 (17) | 0.389 |
| Central pulse pressure, mm Hg a | 46 | 62 (25) | 58 (26) | 27 | 55 (22) | 53 (24) | 0.487 |
| Augmentation index, % | 46 | 38 (10) | 36 (9) | 27 | 35 (12) | 35 (10) | 0.217 |
| Pulse wave velocity, m/s | 37 | 9.6 (2.5) | 9.2 (2.7) | 26 | 9.3 (2.8) | 9.6 (2.8) | 0.251 |
| Brachial diameter, mm | 31 | 4.02 (0.48) | 4.15 (0.50) | 19 | 4.00 (0.62) | 4.11 (0.63) | 0.507 |
| Flow‐mediated dilation, % | 31 | 7.4 (4.8) | 7.7 (5.2) | 19 | 5.3 (5.2) | 9.1 (5.1)b | 0.042c |
| Flow‐mediated dilation, mma | 31 | 0.25 (0.28) | 0.26 (0.32) | 19 | 0.15 (0.20) | 0.31 (0.32) | 0.042c |
| Time to maximum diameter, s | 31 | 82 (46) | 98 (43) | 19 | 64 (39) | 93 (35) | 0.568 |
| AUC shear rate×100−1 a | 31 | 176 (135) | 203 (139) | 19 | 168 (91) | 146 (130) | 0.913 |
| FMD/AUC | 31 | 0.05 (0.05) | 0.06 (0.05) | 19 | 0.03 (0.04) | 0.08 (0.05) | 0.069 |
| Blood flow | 31 | 69 (27) | 80 (40) | 19 | 77 (34) | 66 (43) | 0.173 |
Parametric data presented as mean (SD). AUC indicates area under the curve of shear rate; DBP, diastolic blood pressure; FMD/AUC, flow‐mediated dilation corrected by area under the curve of shear rate; IHT, isometric handgrip group; LF/HF, low frequency/high frequency ratio; n, number of data for each variable analyzed; pNN50, percentage of number of pairs of adjacent NN intervals differing by more than 50 ms; RMMSD, root mean square of the squared differences between adjacent normal RR intervals; SBP, systolic blood pressure; SDNN, standard deviation of all RR intervals.
Nonparametric data presented as median (interquartile range).
Different from preintervention.
Interaction effect significant P<0.05.
Figure 2.
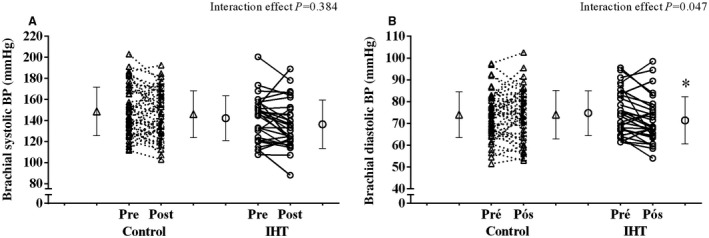
Brachial systolic (A) and diastolic (B) blood pressure responses, before and after the 8‐week isometric handgrip training (IHT) and the 8 weeks of sham training in the control group. *Different from preintervention moment.
Figure 3.
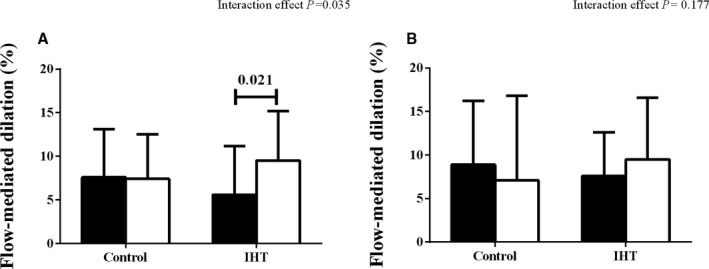
Flow‐mediated dilation in (A) trained arm and (B) nontrained arm, before (black bar) and after (white bar) 8 weeks of isometric handgrip training (IHT) and the 8 weeks of the sham training in control group.
There was no effect of IHT in brachial systolic BP (142 [21] mm Hg preintervention versus 136 [23] mm Hg postintervention), when compared with CG (149 [23] mm Hg preintervention versus 146 [22] mm Hg postintervention; P=0.384). The IHT program reduced brachial diastolic BP (75 [10] mm Hg preintervention versus 72 [11] mm Hg postintervention), with no change in the CG (74 [11] mm Hg preintervention versus 74 [11] mm Hg postintervention), with this between‐group difference being significant (P=0.047). Central systolic and diastolic BP did not change, with a significance of interaction of P=0.544 and P=0.086, respectively.
Flow‐mediated dilation of the trained arm increased in the IHT group (6.0% [5.7] preintervention versus 9.7% [5.5] postintervention), with no change in the control group (7.6% [5.5] preintervention versus 7.4% [5.1] postintervention], with this between‐group difference being significant (P=0.042). When analyzing data from both arms (number of patients, IHT=17 and CG=25; Figure 3), it was observed that the untrained arm did not improve flow‐mediated dilation (P>0.123). There was no change in other measured variables over the intervention period.
No change in general physical activity level was observed in either group over the 8‐week period of observation (P=0.509): IHT group, 5196 (2678) steps preintervention versus 6079 (3133) steps postintervention; and CG group, 5703 (3617) steps preintervention versus 5909 (3619) steps postintervention.
The intent‐to‐treat analysis (Table 4) did not reveal a significant effect of the IHT program on any of the measured outcome variables.
Table 4.
Intention‐to‐Treat Analysis of the Effect of IHT in Cardiovascular Parameters in Peripheral Artery Disease Patients
| Control Group (n=52) | IHT (n=50) | Interaction Effect | |||||
|---|---|---|---|---|---|---|---|
| n | Pre | Post | n | Pre | Post | ||
| BP | |||||||
| Brachial systolic BP, mm Hg | 52 | 149 (3) | 146 (3) | 50 | 143 (3) | 141 (3) | 0.915 |
| Brachial diastolic BP, mm Hg | 52 | 75 (2) | 74 (2) | 50 | 74 (1) | 72 (1) | 0.284 |
| Central systolic BP, mm Hg | 51 | 137 (3) | 135 (3) | 47 | 133 (3) | 130 (3) | 0.735 |
| Central diastolic BP, mm Hg | 51 | 75 (2) | 76 (2) | 47 | 75 (1) | 73 (1) | 0.377 |
| Heart rate variability | |||||||
| SDNN, ms | 49 | 38.2 (4.2) | 36.5 (4.4) | 44 | 34.6 (3.3) | 40.9 (5.5) | 0.348 |
| RMSSD, ms | 49 | 34.7 (5.3) | 38.4 (8.5) | 44 | 27.1 (3.3) | 37.6 (8.1) | 0.553 |
| pNN50, % | 49 | 9.6 (2.1) | 9.1 (2) | 44 | 7.5 (2.2) | 9.3 (2.3) | 0.138 |
| Low frequency, ms2 | 49 | 470 (125) | 1089 (203) | 44 | 449 (113) | 453 (66) | 0.269 |
| High frequency, ms2 | 49 | 825 (338) | 1520 (298) | 44 | 310 (80) | 598 (124) | 0.743 |
| LF/HF | 49 | 1.64 (0.30) | 2.16 (0.48) | 44 | 2.21 (0.30) | 2.55 (0.54) | 0.227 |
| SD1, msa | 49 | 24.6 (3.7) | 27.2 (6.0) | 44 | 19.2 (2.3) | 26.6 (5.7) | 0.557 |
| SD2, msa | 49 | 46.6 (5.0) | 47.4 (5.2) | 44 | 43.9 (4.1) | 49.9 (5.2) | 0.397 |
| Shannon entropya | 49 | 3.3 (0.1) | 3.2 (0.6) | 44 | 3.2 (0.1) | 3.2 (0.1) | 0.684 |
| Sample entropya | 49 | 1.4 (0.1) | 1.4 (0.1) | 44 | 1.4 (0.1) | 1.4 (0.1) | 0.997 |
| Vascular mechanisms | |||||||
| Pulse pressure, mm Hga | 51 | 64 (3) | 60 (3) | 47 | 58 (2) | 58 (3) | 0.115 |
| Augmentation index, % | 51 | 38 (1) | 35 (1) | 47 | 36 (2) | 36 (2) | 0.052 |
| Pulse wave velocity, m/s | 41 | 9.6 (0.5) | 9.1 (0.4) | 43 | 9.9 (0.5) | 9.7 (0.4) | 0.970 |
| Brachial diameter, mm | 34 | 3.95 (0.10) | 4.05 (0.10) | 36 | 4.01 (0.15) | 4.17 (0.15) | 0.517 |
| Flow‐mediated dilation, % | 33 | 7.69 (0.97) | 7.53 (0.89) | 35 | 5.90 (1.13) | 9.21 (1.15) | 0.057 |
| Time to maximum diameter, s | 33 | 78 (8) | 94 (7) | 35 | 67 (9) | 90 (8) | 0.713 |
| AUC shear rate×100−1 a | 32 | 199 (19) | 197 (17) | 34 | 195 (25) | 175 (28) | 0.352 |
| Blood flow, mL/min | 34 | 66 (5) | 74 (7) | 36 | 76 (7) | 70 (10) | 0.138 |
| FMD/AUC | 32 | 0.05 (0.01) | 0.05 (0.01) | 34 | 0.04 (0.01) | 0.07 (0.01) | 0.069 |
Data presented as mean (SE). AUC indicates area under the curve of shear rate; BP, blood pressure; FMD/AUC, flow‐mediated dilation corrected by area under the curve of shear rate; IHT, isometric handgrip group; LF/HF, low frequency/high frequency ratio; n, number of data for each variable analyzed; pNN50, percentage of number of pairs of adjacent NN intervals differing by more than 50 ms; RMMSD, root mean square of the squared differences between adjacent normal RR intervals; SDNN, standard deviation of all RR intervals.
Non parametric data presented as median (interquartile range).
Discussion
Our study is the first to analyze the effects of IHT on cardiovascular function in patients with PAD, and our main finding were that 8 week of home‐based program of HIT decrease brachial diastolic BP and improves vascular function in patients with PAD. However, there was no effect of IHT on systolic brachial BP, systolic and diastolic central BP, heart rate variability, or arterial stiffness parameters.
A previous study among normotensive, prehypertensive, and hypertensive individuals reported a reduction in brachial systolic BP >6 mm Hg after IHT.12 Our results were not consistent with these previous findings, in which our IHT program did not reduce brachial or central systolic BP in patients with PAD. Given that our exercise protocol and program duration were similar to a previously described program using IHT, patient‐specific factors provided a plausible explanation for the lack of reductions in systolic BP. Compared with previous research reporting a decrease in systolic BP with IHT,15 patients in our study were older, presented more comorbidities, used more types of medications, and presented with more‐advanced cardiovascular disease. Of note, a previous study similarly reported absence of an improvement in systolic BP with a program of dynamic resistance training in patients with PAD,31 indicating possible limitations of resistance training for altering systolic BP in this clinical population.
Among the autonomic and vascular parameters analyzed, only local flow‐mediated dilation was improved after the 8‐week IHT program. The absence of change in cardiac autonomic modulation and arterial stiffness with resistance training may reflect the severity of abnormality in cardiac autonomic control32, 33 and arterial stiffness34 in patients with PAD. The improvement in vascular function that was observed in the trained limb may have been mediated by the ischemia‐reperfusion phenomenon, leading to activation of a cascade of events that ultimately promote vasodilation. Given that these effects occur only in the exercised muscles, improvement in vascular function in the trained limb might be expected, as previously reported in hypertensive patients who completed a program of uni‐ or bilateral IHT.20 This raises the possible relevance of lower‐limb isometric exercise, involving larger muscle groups, as a possible intervention to promote adaptation in vascular function and possibly lowering BP.
Our 8‐week program of IHT yielded a decrease in diastolic BP of 3.4 mm Hg, on average, which is slightly lower than the previously reported range of 4 to 8 mm Hg after IHT.12, 13 Although the improvements in local flow‐mediated dilation after IHT might be related to this response, it is unlikely that this local unilateral upper‐limb adaptation was responsible for the observed reductions in diastolic BP. Although we do not have data to support or refute this hypothesis, other mechanisms could be considered, such as a decrease in systemic vascular resistance, improvement in peripheral sympathetic activity, and activation of the renin‐angiotensin system.
The strengths of this study include the large sample size, randomized allocation, and blinding. In addition, contrary to all previous studies, the CG was submitted to a sham intervention, minimizing the potential influence of the frequent contact with researchers and the placebo effect of the training. The limitations include the heterogeneity of the sample, which presented with different comorbidities and medication dose and use. However, this heterogeneity is representative of the clinical spectrum of patients with PAD. Although we verified medication use, preintervention and postintervention, we cannot guarantee that the correct use of drugs was used across the entire study duration. During the study, we encountered technical difficulties with the ultrasound equipment, and thus flow‐mediated dilation was not measured in all patients at both time points. Additionally, we were unable to collect pulse wave velocity in some patients because of the presence of arrhythmias and the lack of a quantifiable femoral pulse. There was no standardization of the time of day that the IHT was performed, which may have generated different cardiovascular responses.35 Oxidative stress and inflammation markers were not measured, and the understanding of the mechanisms systemic of BP lowering after isometric handgrip training was limited.
In conclusion, IHT reduced brachial diastolic BP and improved local vascular function in patients with PAD. From a clinical perspective, our 8‐week program of IHT could provide a simple, home‐based, adjunct strategy to reduce brachial diastolic BP in this clinical population. We note, however, that this training stimulus was insufficient to improve brachial systolic BP, central BP, cardiac autonomic modulation, and arterial stiffness parameters in patients with PAD.
Sources of Funding
This work was supported by grants from “Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP” (process#2016/16425‐9), “Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPQ” (process# 310508/2017‐7), and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES” (process# 88881.133008/2016‐01).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e013596 DOI: 10.1161/JAHA.119.013596.)
References
- 1. Farah BQ, Ritti‐Dias RM, Cucato GG, Chehuen Mda R, Barbosa JP, Zeratti AE, Wolosker N, Puech‐Leao P. Effects of clustered comorbid conditions on walking capacity in patients with peripheral artery disease. Ann Vasc Surg. 2014;28:279–283. [DOI] [PubMed] [Google Scholar]
- 2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand‐Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E III, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1–S75. [DOI] [PubMed] [Google Scholar]
- 3. Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE Jr, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. [DOI] [PubMed] [Google Scholar]
- 4. Ritti‐Dias RM, Correia MA, Andrade‐Lima A, Cucato GG. Exercise as a therapeutic approach to improve blood pressure in patients with peripheral arterial disease: current literature and future directions. Expert Rev Cardiovasc Ther. 2018;17:65–73. [DOI] [PubMed] [Google Scholar]
- 5. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola‐Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Rother J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the european society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesendorsed by: The European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 6. Chehuen M, Cucato GG, Carvalho CR, Ritti‐Dias RM, Wolosker N, Leicht AS, Forjaz CL. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: a randomized controlled trial. J Sci Med Sport. 2017;20:886–892. [DOI] [PubMed] [Google Scholar]
- 7. Grizzo Cucato G, de Moraes Forjaz CL, Kanegusuku H, da Rocha Chehuen M, Riani Costa LA, Wolosker N, Kalil Filho R, de Fatima Nunes Marucci M, Mendes Ritti‐Dias R. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. Vasa. 2011;40:390–397. [DOI] [PubMed] [Google Scholar]
- 8. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Junior JC, Wolosker N, Forjaz CL, Gardner AW, Ritti‐Dias RM. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22:70–76. [DOI] [PubMed] [Google Scholar]
- 9. Silva ATd, Fermino RC, Lopes AAdS, Alberico CO, Reis RS. Distance to fitness zone, use of facilities and physical activity in adults. Rev Bras Med Esporte. 2018;24:157–161. [Google Scholar]
- 10. Treat‐Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, Gardner AW, Hiatt WR, Regensteiner JG, Rich K; American Heart Association Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular and Stroke Nursing . Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the american heart association. Circulation. 2019;139:e10–e33. [DOI] [PubMed] [Google Scholar]
- 11. Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis. Mayo Clin Proc. 2014;89:327–334. [DOI] [PubMed] [Google Scholar]
- 12. Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis to optimize benefit. Hypertension Res. 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- 13. Cornelissen VA, Smart NA. Exercise training for blood pressure: A systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473 DOI: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin YZ, Yan S, Yuan WX. Effect of isometric handgrip training on resting blood pressure in adults: a meta‐analysis of randomized controlled trials. J Sports Med Phys Fitness. 2017;57:154–160. [DOI] [PubMed] [Google Scholar]
- 15. Farah BQ, Germano‐Soares AH, Rodrigues SLC, Santos CX, Barbosa SS, Vianna LC, Cornelissen VA, Ritti‐Dias RM. Acute and chronic effects of isometric handgrip exercise on cardiovascular variables in hypertensive patients: a systematic review. Sports (Basel). 2017;5:E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Millar PJ, Levy AS, McGowan CL, McCartney N, MacDonald MJ. Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports. 2013;23:620–626. [DOI] [PubMed] [Google Scholar]
- 17. Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–256. [DOI] [PubMed] [Google Scholar]
- 18. Cahu Rodrigues SL, Farah BQ, Silva G, Correia M, Pedrosa R, Vianna L, Ritti‐Dias RM. Vascular effects of isometric handgrip training in hypertensives. Clin Exp Hypertens. 2019. Published online January 9. DOI: 10.1080/10641963.2018.1557683. [DOI] [PubMed] [Google Scholar]
- 19. Badrov MB, Freeman SR, Zokvic MA, Millar PJ, McGowan CL. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow‐mediated dilation equally in men and women. Eur J Appl Physiol. 2016;116:1289–1296. [DOI] [PubMed] [Google Scholar]
- 20. McGowan CL, Visocchi A, Faulkner M, Verduyn R, Rakobowchuk M, Levy AS, McCartney N, MacDonald MJ. Isometric handgrip training improves local flow‐mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2007;99:227–234. [DOI] [PubMed] [Google Scholar]
- 21. Teixeira AL, Ritti‐Dias R, Antonino D, Bottaro M, Millar PJ, Vianna LC. Sex differences in cardiac baroreflex sensitivity following isometric handgrip exercise. Med Sci Sports Exerc. 2018;50:770–777. [DOI] [PubMed] [Google Scholar]
- 22. Farah BQ, Rodrigues SLC, Silva GO, Pedrosa RP, Correia MA, Barros MVG, Deminice R, Marinello PC, Smart NA, Vianna LC, Ritti‐Dias RM. Supervised, but not home‐based, isometric training improves brachial and central blood pressure in medicated hypertensive patients: a randomized controlled trial. Front Physiol. 2018;9:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farah BQ, Vianna LC, Rodrigues SLC, Correia MA, Teixeira AL, Andrade FMDd, Pedrosa RP, Moreira SR, Barros MVG, Wolosker N, Cucato GG, Ritti‐Dias RM. Effects of isometric handgrip training in patients with cardiovascular disease: rationale and design of the ISOPRESS network. Motriz. 2017;23:e101719. [Google Scholar]
- 24. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG; Consolidated Standards of Reporting Trials Group . CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:e1–e37. [DOI] [PubMed] [Google Scholar]
- 25. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 26. Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Hipertensão; Sociedade Brasileira de Nefrologia . [vi brazilian guidelines on hypertension]. Arq Bras Cardiol. 2010;95:1–51. [PubMed] [Google Scholar]
- 27. Siebenhofer A, Kemp C, Sutton A, Williams B. The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J Hum Hypertens. 1999;13:625–629. [DOI] [PubMed] [Google Scholar]
- 28. Van Bortel LM, Duprez D, Starmans‐Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, task force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–452. [DOI] [PubMed] [Google Scholar]
- 29. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 30. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gomes APF, Correia MA, Soares AHG, Cucato GG, Lima A, Cavalcante BR, Sobral‐Filho DC, Ritti‐Dias RM. Effects of resistance training on cardiovascular function in patients with peripheral artery disease: a randomized controlled trial. J Strength Cond Res. 2018;32:1072–1080. [DOI] [PubMed] [Google Scholar]
- 32. Goernig M, Schroeder R, Roth T, Truebner S, Palutke I, Figulla HR, Leder U, Voss A. Peripheral arterial disease alters heart rate variability in cardiovascular patients. Pacing Clin Electrophysiol. 2008;31:858–862. [DOI] [PubMed] [Google Scholar]
- 33. Lima AH, Soares AH, Cucato GG, Leicht AS, Franco FG, Wolosker N, Ritti‐Dias RM. Walking capacity is positively related with heart rate variability in symptomatic peripheral artery disease. Eur J Vasc Endovasc Surg. 2016;52:82–89. [DOI] [PubMed] [Google Scholar]
- 34. Husmann M, Jacomella V, Thalhammer C, Amann‐Vesti BR. Markers of arterial stiffness in peripheral arterial disease. Vasa. 2015;44:341–348. [DOI] [PubMed] [Google Scholar]
- 35. Brito LC, Pecanha T, Fecchio RY, Rezende RA, Sousa P, DA Silva‐Júnior N, Abreu A, Silva G, Mion‐Junior D, Halliwill JR, Forjaz CLM. Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. 2019;51:653–662. [DOI] [PubMed] [Google Scholar]


