Abstract
Background
Blood immunoreactive biomarkers, such as C-reactive protein (CRP), and metabolic abnormalities have been associated with schizophrenia. Studies comprehensively and bidirectionally probing possible causal links between such blood constituents and liability to schizophrenia are lacking.
Methods
To disentangle putative causal links between CRP blood levels and schizophrenia in both directions, we conducted multiple univariable Mendelian-randomization (MR) analyses, ranging from fixed-effect to inverse variance-weighted (IVW), weighted-median, MR Egger and generalized summary-data-based Mendelian-randomization (GSMR) models. To prioritize metabolic risk factors for schizophrenia, a novel multivariable approach was applied: multivariable Mendelian-randomization–Bayesian model averaging (MR-BMA).
Results
All forward univariable MR analyses consistently showed that CRP has a protective effect on schizophrenia, whereas reverse MR analyses consistently suggested absent causal effects of schizophrenia liability on CRP blood levels. Using MR-BMA, as the top protective factors for schizophrenia we prioritized leucine and as the prime risk-factor triglycerides in medium very-low-density lipoprotein (VLDL). The five best-performing MR-BMA models provided one additional risk factor: triglycerides in large VLDL; and two additional protective factors: citrate and lactate.
Conclusions
Our results add to a growing body of literature hinting at metabolic changes—in particular of triglycerides—independently of medication status in schizophrenia. We also highlight the absent effects of genetic liability to schizophrenia on CRP levels.
Keywords: C-reactive protein, blood metabolites, schizophrenia, Mendelian randomization
Key Messages
Using unprecedentedly large datasets, we confirm a protective effect of C-reactive protein on liability to schizophrenia and for the first time highlight the absence of reverse causal effects.
Of the blood constituents investigated, triglycerides were the prime schizophrenia liability-increasing constituents. Leucine was the top protective factor for schizophrenia liability.
These results deepen our understanding of the complex relationships between blood biomarkers and schizophrenia.
Background
Schizophrenia (SCZ) is a debilitating psychiatric disorder, affecting 1% of the population worldwide. Identification of robust biomarkers may aid in the diagnostic work-up, thus potentially enabling optimized personalized treatment.
Immunoreactive biomarkers such as C-reactive protein (CRP) blood levels have been found to be increased in SCZ patients compared with controls,1 suggesting that CRP may be a peripheral biomarker of this disorder.2,3 CRP levels depend on disease status in psychosis, e.g. elevated CRP levels in SCZ patients are largely normalized after recovery from psychotic episodes, whereas elevated CRP levels remain high in treatment-resistant psychosis patients.4–6 Moreover, elevated CRP levels in childhood/adolescence increase the odds of developing psychosis in adulthood.6 However, in contrast to these findings, recently published Mendelian-randomization (MR) studies show that CRP may play a protective rather than a risk-increasing role in schizophrenia7–9—a discrepancy that has not yet been disentangled.
Metabolic abnormalities (such as higher glucose, lipids and insulin levels) are common in subjects at high risk for schizophrenia10 and antipsychotic-naïve first-episode psychosis patients,10–15 pointing to disturbances in metabolic and growth-hormone-signalling pathways in psychosis unrelated to antipsychotic treatment.16 Our understanding of the genetic determinants underlying blood metabolic variations was deepened by nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry detection techniques, enabling the identification and quantification of the hundreds of blood metabolites.17 Moreover, genetic overlap between metabolic traits and schizophrenia has been detected: three metabolic genetic loci were found to be significantly associated with schizophrenia (p < e-5).18 However, a study comprehensively dissecting the causal links between blood metabolic profiles and CRP on the one hand and SCZ on the other is currently lacking. This might be due to challenges inherent in such research, such as state-dependent effects of medication and the nature of high dimensionality of data.
MR is a powerful approach to disentangle putative causal effects of a risk factor (or exposure) on an outcome using genetic variants as instrumental variables.19 Common MR approaches comprise inverse variance-weighted (IVW), weighted-median20 and MR Egger,21 and include sensitivity analyses testing whether certain assumptions are violated (such as pleiotropy). Compared with standard MR, a novel MR approach—generalized summary-data-based Mendelian randomization (GSMR)22—has obvious advantages: it is on average more powerful than MR Egger, while accounting for linkage disequilibrium between SNPs. In addition, it is more robust for detecting instrumental outliers using the Heterogeneity in Dependent Instruments (HEIDI) test. To our knowledge, GSMR has not been applied to disentangle the possible causal links between CRP and schizophrenia. Here, all the above-mentioned MR tools were used to investigate possible causal links between CRP and schizophrenia in both forward and reverse directions, i.e. to disentangle whether CRP is a biomarker influencing the risk of schizophrenia (forward direction) or CRP levels are altered as a consequence of liability to schizophrenia (reverse direction).
The nature of high-dimensional correlated blood metabolites is not appropriate to fit into univariable MR. Multivariable Mendelian randomization (MV-MR) is an extension of univariable MR that allows detecting causal effects of multiple risk factors jointly.23 MV-MR takes pleiotropy into account; the assumption of multivariable MR is that genetic variants influence a set of multiple measured risk factors rather than a specific single risk factor. However, standard MV-MR has limited ability to handle high-throughput blood metabolites. To deal with the nature of high-throughput metabolites, we used MR Bayesian model averaging (MR-BMA, a novel approach based on MV-MR).24 MR-BMA is designed to perform multiple MV-MR individual models, and the models are averaged by a Bayesian framework. MR-BMA determines the best model based on the highest posterior probability (PP) from the models that randomly select different sets of risk factors.
In sum, univariable MR was conducted to detect whether CRP increases or decreases the risk of schizophrenia or whether CRP levels are influenced by schizophrenia liability. In addition, we performed the first multivariate Mendelian-randomization study using MR-BMA to prioritize blood metabolites as potential risk or protective factors for schizophrenia.
Methods
Univariable MR analyses of CRP with schizophrenia
MR analyses were conducted using the largest available CRP GWAS (n = 204 402 participants, published in 2018)25 and SCZ GWAS (n = 105 318) summary statistics data.26 We selected 52 independent SNPs significantly (p < 5e-8) associated with CRP as instrumental variables (for selection details, see Supplementary Methods, available as Supplementary data at IJE online). The GSMR analyses were performed using the ‘GSMR’22 package in R (see Supplementary Methods, available as Supplementary data at IJE online). The fixed-effects IVW, weighted-median and MR Egger models (see Supplementary Methods, available as Supplementary data at IJE online) were performed using the ‘TwosampleMR’ package.27 Effect estimates were reported in β values when the outcome was continuous (i.e. CRP levels) and converted to odds ratios (ORs) when the outcome was dichotomous (i.e. SCZ status). We then performed additional sensitivity analyses, including the strength of instruments estimated by F-statistics, horizontal pleiotropic effects estimated by the intercept of MR Egger, statistics (this validates the suitability of instruments in MR Egger)28 residual heterogeneity estimated by Cochran’s Q test29 and leave-one-out analyses to evaluate whether any single instrumental variable was driving the results. We used default options in GSMR with HEIDI testing for the detection of instrumental outliers. The significance threshold was Bonferroni-corrected for the four models that we applied (p = 0.05/4 = 0.012).
Besides testing causal effects of CRP on schizophrenia in forward MR analyses, we also investigated the reverse hypothesis (so whether liability to schizophrenia impacts CRP levels) using the 106 SNPs independently and significantly (p < 5e-8) associated with schizophrenia as instrumental variables (see Supplementary Methods, available as Supplementary data at IJE online).
MR-BMA analyses of blood metabolites with schizophrenia
For MR-BMA, we used the currently largest and deep phenotyping circulating metabolites GWAS (n = 24 295)30 wherein summary statistics for 47 out of 123 metabolites were first selected as candidate blood biomarkers (for selection details, see Supplementary Methods, available as Supplementary data at IJE online). Second, we selected 129 independently and significantly associated SNPs as instrumental variables for MR-BMA analyses (see Supplementary Methods, available as Supplementary data at IJE online). Finally, the beta and standard error (only for the SCZ GWAS) coefficients of these 129 genetic variants from 47 blood metabolites and SCZ GWASs were extracted. We used the ‘TwoSampleMR’ package to harmonize the SNP information.27
MR-BMA was used for multiple regular multivariable MRs with a subset of randomly selected risk factors, with (i) the multivariable models ranking option: different multivariable models were prioritized based on PPs of Bayesian model averaging (BMA); (ii) risk factors prioritizing options based on marginal inclusion probability (MIP) (the sum of PP out of all models where the risk factor is present); and (iii) the instrument outliers detecting option based on the Q-statistic (the contribution of each instrument on heterogeneity, which was calculated as the squared differences of predicted and observed associations with outcomes) and influential observations that were qualified by Cook’s distance (Cd)31 to detect the instruments distorting the association with the outcome and the accuracy. represents the causal effects estimated from a multivariable model. The overall direct causal effect of a single risk factor on outcome was estimated based on MACE, model-averaged causal estimate (MACE) from all individual models containing the same risk factor, where λ represents the causal effect of a single risk factor on the outcome in a specific individual MV-MR model.
We ran MR-BMA including all (n = 129) available genetic variants on the number of dimension (d = 47) metabolite associations using prior probability as p = 0.1 (reflecting that an a priori model size of a maximum of 0.1 × 47 ≈ 5 risk factors may have true causal effects on the outcome) and prior variance σ2 = 0.25. As we assumed that a small subset of metabolites was causally related to schizophrenia and that the set of true causal risk factors is randomly distributed, we allowed for models with up to 12 risk factors and with 100 000 iterations in the shotgun stochastic search (an approach that identifies variables of high PP over models based on a Bayesian framework; for more details, see Supplementary Methods, available as Supplementary data at IJE online).32
The models were averaged by PP and ranked, and the best individual model was identified (PP > 0.01). The risk factors of blood metabolites were prioritized by their MIPs from MR-BMA. Then, invalid instruments with unmeasured pleiotropic effects (the association between instruments with outcome, but not mediated by the risk factors) were investigated by influential observations such as heterogeneity (estimated by the Q-statistic) and Cook’s distance (Cd). In the end, the MR-BMA analysis was re-run after removing invalid instruments. The best individual models were identified based on PP > 0.01. To further validate our results, the best multivariable individual models identified from MR-BMA were repeated in standard multivariable MR analysis using the ‘MendelianRandomization’ package33 (Supplementary Methods, available as Supplementary data at IJE online). Finally, despite low power due to a limited number of significant SNPs in each of the metabolite genome-wide analyses, standard univariable MR analyses were performed to be able to show causal effects per metabolic trait on schizophrenia (see Supplementary Methods, available as Supplementary data at IJE online).
Results
Univariable MR analysis of CRP with schizophrenia
All models except MR Egger suggested that CRP has a protective effect on schizophrenia (Table 1A, Figure 1A and Supplementary Figure 2A, available as Supplementary data at IJE online). The results show that high CRP has a significant negative effect on schizophrenia in fixed-effect IVW [OR = 0.91, confidence interval (CI) = 0.865–0.958, p = 3.2E-4) and weighted-median (OR = 0.909, CI = 0.843–0.981, p = 0.012) models. Although MR Egger was not significant (p = 0.165), overall horizontal pleiotropic effects were absent (for intercept of MR Egger: β = 0.0007, p = 0.864). The statistics of MR Egger were larger than 90% ( = 98.6%), suggesting the absence of substantial bias in the causal estimates due to uncertainty in the genetic associations. In addition, the F-statistics as a proxy for the instrument strength indicated that the results do not suffer from weak-instrument bias: these were 28.22 and 29.47, before and after exclusion of outliers, respectively. The Cochran’s Q test in the fixed-effect IVW model (p = 7.0E-16) and MR Egger model (p = 3.8E-16) suggested that there was heterogeneity in the instrumental variables, which may be caused by true causality rather than by violation of instrument variable assumptions. The leave-one-out sensitivity analysis (Supplementary Figure 2A, available as Supplementary data at IJE online) showed that no SNPs altered the pooled β coefficient, indicating the stability and reliability of our results. The HEIDI test detected seven SNPs as pleiotropic outliers. The forward GSMR result demonstrated consistent negative causal effects of CRP on SCZ (OR = 0.885, CI = 0.843–0.931, p = 1.6E-6).
Table 1.
The forward and reverse univariable MR analyses results of CRP with SCZ liability (A) Forward MR analyses of CRP on SCZ liability
| Forward model (using CRP SNPs as instruments) | N | OR (95% CI)* | P |
|---|---|---|---|
| IVW | 52 | 0.910 (0.865–0.958) | 3.2E-4 |
| Weighted median | 52 | 0.909 (0.843–0.981) | 0.011 |
| MR Egger | 52 | 0.902 (0.782–1.040) | 0.161 |
| GSMR | 45 | 0.885 (0.843–0.931) | 1.6E-6 |
Figure 1.
Scatter plots of MR analyses using several models to investigate causal relationships between CRP (C-reactive protein) and SCZ (schizophrenia). The four models applied in the current manuscript are all denoted. (A) Forward models assessing the effect of CRP on SCZ liability. Lines in dashed, dotted and dotdash represent β for fixed effect IVW, weighted median, and MR Egger models using 52 instruments. The longdash line is β from GSMR model with 45 instruments removing outlier detected by HEIDI test. The scatters in grey are instrument outliers by HEIDI (Heterogeneity in Dependent Instruments) test. (B) Reverse models assessing the effects of genetic liability to SCZ on CRP. Lines in dashed, dotted and dotdash represent β for fixed effect IVW, weighted median, and MR Egger models using 106 instruments. The longdash line is β from GSMR model with 105 instruments removing outlier detected by HEIDI test. The scatters in blue are instrument outliers by HEIDI (Heterogeneity in Dependent Instruments) test. IVW, inverse variance-weighted model; GSMR, generalized summary-data-based Mendelian-randomization model.
The reverse MR analyses for the fixed-effect IVW, weighted-median and MR Egger models successfully extracted 106 instrumental variables (Table 1B, Figure 1B and Supplementary Figure 2B, available as Supplementary data at IJE online). No significant causal effects of SCZ liability on CRP were detected. Nonetheless, horizontal pleiotropic effects were absent in MR Egger (for the intercept of MR Egger: β = 0.0003, p = 0.920). The small value (20.1%) suggests that bias due to measurement errors in MR Egger estimation was large, warranting caution in interpreting MR Egger results. On the other hand, the large F-statistics (235.36 and 236.33, respectively) indicated that causal estimations did not suffer from weak-instrument bias. The strong evidence of heterogeneity in the instruments may be due to the pleiotropy or violation of NO Measurement Error (NOME) assumption, with significant estimates by Cochran’s Q for both the fixed-effect IVW model (Q-statistic = 313.645, df = 105, p = 1.2E-22) and MR Egger model (Q-statistic = 313.15, df = 104, p = 6.9E-23). The leave-one-out sensitivity analysis (Supplementary Figure 3B, available as Supplementary data at IJE online) showed that no single instrumental variable significantly altered the IVW-model estimates. The HEIDI test detected 1 SNP as a pleiotropic instrument outlier, resulting in 105 SNPs as instrumental variables in GSMR. Consistently with the other reverse MR models, no significant causal effect of schizophrenia liability on the CRP level was estimated from GSMR (β = –0.010, CI = –0.020–0.000, p = 0.056).
(B). Reverse MR analyses of SCZ liability on CRP
| Reverse model (using SCZ SNPs as instruments) | N | β (95% CI) | P |
|---|---|---|---|
| IVW | 106 | –5.3E-4 (–0.013 to 0.012) | 0.933 |
| Weighted median | 106 | –0.011 (–0.031 to 0.009) | 0.297 |
| MR Egger | 106 | –0.005 (–0.095 to 0.085) | 0.914 |
| GSMR excluding outliers | 105 | –0.010 (–0.020 to 0.000) | 0.056 |
N, number of SNPs used as instrumental variables in MR analyses; OR, odds ratio, indicating the change of odds for SCZ per 1-unit increase in CRP levels (mg/L); β, effect size, indicating the change of CRP (mg/L) per 1-standard deviation increase in SCZ odds; IVW, fixed-effects inverse variance-weighted model.
MR-BMA results: causal links between metabolic blood constituents and schizophrenia liability
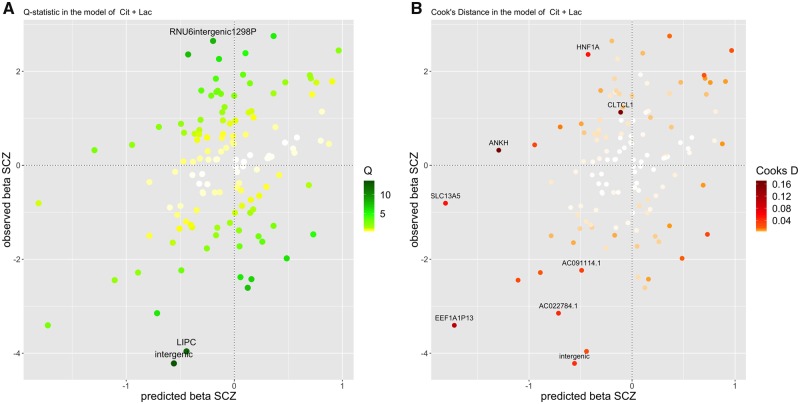
When including all selected instruments (n = 129) in both the NMR and the SCZ summary data, the prioritized metabolites for schizophrenia with respect to their MIPs (Supplementary Table 2, available as Supplementary data at IJE online) were: total cholesterol in LDL (LDL.C, MIP = 0.425) with a negative effect (MACE = –0.113), compared with other metabolites’ MIPs all being <0.3. The four best models identified based on PP > 0.01 (Supplementary Table 2, available as Supplementary data at IJE online) showed protective effects of citrate (Cit, MACE –0.198), Lactate (Lac,MACE = 0.015) and leucine (Leu, MACE = 0.01) and risk-increasing effects of triglycerides in medium very-low-density lipoprotein (VLDL) (M.VLDL.TG, MACE = –0.057) on SCZ. As an example, for the best individual model from MR-BMA, we provide a graph showing good predictability for the observed SCZ associations (Figure 2). In the best individual models based on the minimum Q-statistic >10 (in Supplementary Table 3, available as Supplementary data at IJE online, and Figure 2A) and Cook's distance >0.1 (Supplementary Table 4, available as Supplementary data at IJE online, and Figure 2B), the most heterogeneous variant was rs193084249 (minimum Q-statistic = 13.216) and it was the most influential variant in the best models (minimum Cook's distance = 0.019).
Figure 2.
Diagnostic plot of the best individual MR-BMA model (including Citrate (Cit) and Lactate (Lac)), showing predicted associations with SCZ (fitted β values on SCZ, x-axis) against the observed associations with SCZ (β values from the SCZ summary statistics, y-axis). Each dot represents an instrument variable (n = 129 genetic variants). The color code shows: (A) the Q-statistic for outliers and (B) Cook's distance for influential points. Any genetic variants with Q-statistic > 8 or Cook's distance > 0.03 (4/129) are marked by a label indicating the gene region. One variant was detected as an outlier (with minimum Q-statistic > 10 and minimum Cook's distance > 0.01 in all the models with posterior probability (PP) > 0.01): the intergenic variant rs193084249, denoted as ‘intergenic’ in the graph. (A) the Q-statistic: Cit + Lac. (B) Cook's distance: Cit + Lac. Cit, citrate; Lac, lactate, SCZ, schizophrenia.
After removing the outlier rs193084249 (n = 128), the top two prioritized protective metabolites were Leu (MIP = 0.343, MACE = –0.064) and Lac (MIP = 0.276, MACE = –0.064) and the top two risk-increasing metabolites were triglycerides in medium VLDL (M.VLDL.TG; MIP = 0.215, MACE = 0.035) and triglycerides in large VDLD L.VLDL.TG (MIP = 0.134, MACE = 0.02; Table 2A). The best final individual model (with the highest PP) consisted of the top prioritized protective factor Leu (λ = –0.180) and the top risk factor M.VLDL.TG (λ = 0.139). Besides this top model with the highest PP, four additional best individual models were identified and prioritized based on their PP >0.01; they were thus ranked by decreasing PP (Table 2B and Supplementary Figure 4, available as Supplementary data at IJE online). The second-best individual model consisted of Cit (λ = –0.157, MACE = –0.017) and Lac (λ = –0.283). The third-best individual model consisted of Lac only (λ = –0.229). The fourth-best individual model consisted of M.VLDL.TG only (λ = 0.1). The fifth-best individual model consisted of L.VLDL.TG (λ = 0.145) and Leu (λ = –0.186).
Table 2.
Ranking of risk and protective factors for schizophrenia (A) according to their marginal inclusion probability (MIP) and (B) the best 10 individual models according to their posterior probability (PP). A negative causal estimate (MACEλ) indicates a protective effect as suggested by the model, whereas a positive value indicates a risk factor, as suggested by the model (A) Model averaging
| Risk/protective factor | Marginal inclusion probability (MIP) | Model-averaged causal estimate MACE | |
|---|---|---|---|
| 1 | Leu | 0.343 | –0.064 |
| 2 | Lac | 0.276 | –0.064 |
| 3 | M.VLDL.TG | 0.215 | 0.035 |
| 4 | L.VLDL.TG | 0.134 | 0.02 |
| 5 | Cit | 0.131 | –0.017 |
| 6 | AcAce | 0.129 | –0.024 |
| 7 | LDL.D | 0.128 | 0.016 |
| 8 | XS.VLDL.TG | 0.099 | 0.013 |
| 9 | S.VLDL.TG | 0.096 | 0.012 |
| 10 | Ile | 0.092 | –0.007 |
(B) Individual averaging
| Risk factors | Posterior probability (PP) | Model-specific causal estimates λ | Model-specific standard error(s) of λ | |
|---|---|---|---|---|
| 1 | Leu, M.VLDL.TG | 0.023 | –0.180, 0.139 | 0.056, 0.036 |
| 2 | Cit, Lac | 0.020 | –0.157, –0.283 | 0.050, 0.083 |
| 3 | Lac | 0.018 | –0.229 | 0.090 |
| 4 | M.VLDL.TG | 0.015 | 0.100 | 0.035 |
| 5 | L.VLDL.TG, Leu | 0.013 | 0.145, –0.186 | 0.039, 0.057 |
| 6 | L.VLDL.C, Leu | 0.010 | 0.132, –0.176 | 0.036, 0.056 |
| 7 | AcAce, Leu, M.VLDL.TG | 0.009 | –0.195, 0.210, 0.182 | 0.076, 0.056, 0.039 |
| 8 | L.VLDL.C | 0.009 | 0.093 | 0.035 |
| 9 | S.VLDL.TG | 0.009 | 0.083 | 0.031 |
| 10 | Cit | 0.008 | –0.125 | 0.051 |
MACE is the model-averaged causal effect and λ is the causal-effect estimate for a specific model. In Table 2A, blood biomarkers are ranked by decreasing marginal inclusion probability (MIP). In Table 2B, individual models are ranked by decreasing posterior probability (PP).
AcAce, acetoacetate; Cit, citrate; Lac, lactate; Leu, leucine; L.VLDL.C, total cholesterol in large VLDL; L.VLDL.TG, triglycerides in large VLDL; M.VLDL.TG, triglycerides in medium VLDL; Serum.TG, serum total triglycerides; S.VLDL.C, total cholesterol in small VLDL; S.VLDL.TG, triglycerides in small VLDL. The best individual multivariable models were identified by PP >0.01 (in bold).
The results of the univariable MR analyses with all 47 metabolites on SCZ liability are shown in Supplementary Table 5A, available as Supplementary data at IJE online. The biomarkers passing the nominal significance level (p < 0.05) in this analysis were (by decreasing level of significance): M.VLDL.TG, VLDL.D, XS.VLDL.TG, S.VLDL.TG, IDL.TG, M.VLDL.C and serum TG (serum total triglycerides) with positive causal effects and alanine (Ala) with a protective effect. The risk factors M.VLDL.TG and L.VLDL.TG consistently showed causal effects on SCZ liability. Confirming the MR-BMA analyses, standard MV-MR analysis was conducted using the top three multivariable prioritized models (Leu + M.VLDL.TG, Cit + Lac and L.VLDL.TG + Leu). In line with the univariable and MR-BMA analyses, these standard MV-MR models suggest causal effects of M.VLDL.TG and L.VLDL.TG on SCZ liability (see Supplementary Table 5B, available as Supplementary data at IJE online).
Discussion
Our aim was to investigate possible causal links between CRP and SCZ liability and to prioritize metabolic risk factors for SCZ. Our MR results based on the most recent and largest GWASs confirm previous evidence based on smaller studies hinting that CRP is protective for SCZ.7–9 Moreover, we provide new and consistent evidence showing the absence of reverse causality, i.e. our findings suggest that liability to SCZ does not lead to CRP alterations. Finally, this first multivariable MR study prioritizes triglycerides as risk-increasing and three metabolites as protective for SCZ, namely leucine, citrate and lactate.
Protective effects of CRP on schizophrenia were previously revealed by multiple MR studies.7,9 apparently contradicting clinical observations.34 For example, higher CRP levels, in an age-dependent manner,35 were found not only to be associated with schizophrenia status, but also to be positively correlated with negative symptoms, cognitive impairment and risk of metabolic syndrome in SCZ patients.36,37 A relatively high proportion of schizophrenia patients seem to have elevated CRP blood levels.1,38 It is important to note, however, that MR is designed to estimate a ‘lifetime effect’ of a risk factor. The GWAS of CRP used for the current analyses was mainly conducted in middle-aged participants. One may speculate that genetic mechanisms underlying CRP blood-level variation in childhood differ from those determining CRP blood-level concentration at older age.39 Following this line of reasoning, our findings do not necessarily contradict meta-analytical evidence showing that childhood central nervous system (CNS) viral infections (that may result in elevated CRP in childhood) increase the risk of adult psychotic illness.6,40 With regard to genetic overlap between CRP and schizophrenia, although a small negative genetic correlation (rg = –0.07, p-value = 0.015) has been found,25 no association between polygenic risk scores (PRS) of schizophrenia with risk of infection and absent interaction effects of SCZ PRS and infection on the risk of schizophrenia have been found.41 We thus speculate that the increased incidence of early-childhood infections in schizophrenia patients are not fully attributed to genetic mechanisms. As early childhood is a sensitive period for the effects of infection on neurocognitive development, early-childhood infections may constitute an environmental factor increasing the odds of psychosis in adolescence. Nonetheless, the underlying mechanisms of high CRP levels decreasing the risk of SCZ remain unknown. Our reasoning was that reverse causality of CRP on schizophrenia might exist, which had not been tested using MR. However, our results render this notion unlikely: no causal effects of liability to schizophrenia on CRP levels were detected by reverse MR analyses. This insignificant reverse causal result is robust because more instruments for schizophrenia in reverse MR analysis were used than instruments for CRP in forward MR models and the big sample size of the GWASs.
Studies show that schizophrenia patients have a unique blood metabolic profile compared with healthy controls,42 suggesting that blood metabolites can be used as potential biomarkers. Identification of metabolic biomarkers for schizophrenia may increase diagnostic accuracy, facilitate the discovery of drug targets and deepen the understanding of its aetiology. We successfully identified two potential triglycerides in VLDL as risk factors for schizophrenia. Our results of triglycerides being associated with schizophrenia are in line with a longitudinal study showing that triglyceride blood levels are significantly higher in schizophrenia patients and remained stable over a 5-year follow-up period.43 In addition, higher triglycerides are significantly positively associated with suicidal behaviour in schizophrenia patients44 independently of antipsychotic medication.
The three prioritized protective factors were leucine, citrate and lactate, respectively. Increased leucine blood and cerebrospinal-fluid levels have been observed in unmedicated SCZ patients.45 However, those results need further validation due to the small sample sizes. Another study argued that altered leucine levels in SCZ may be confounded by medication status.46 The second protective factor prioritized by MR-BMA was citrate, which has strong clinical support: a modest but significant negative genetic correlation between citrate levels and schizophrenia was previously unraveled.47 In addition, lower citrate levels were observed in SCZ.48 We found no studies reporting on blood-lactate levels associated with SCZ-disease status. However, lactate is a (waste) product of glycolytic metabolism from muscles, playing an important role in brain-energy metabolism, especially during acute neural activation. An animal study showed lactate-transport glycogen-maintained adenosine triphosphate (ATP) is a possible defence mechanism for neurons in an exhausted brain.49 In addition, lactate plays a neuroprotective role against excitotoxicity, oxidative stress and damage from trauma.50,51 Possibly, lactate may in the same manner protect against vulnerability to SCZ.
Limitations
This study has several limitations. First, although there is no cohort overlap between the meta-analysis of CRP (n = 204 402) and schizophrenia (n = 105 318), both population-based studies were obtained from mostly European studies and might therefore have some sample overlap, resulting in inflation of test results.52 However, the fact that we found similar results using both CRP GWASs renders substantial inflation unlikely. In addition, MR estimates the lifetime effects of exposures.39 The genetic instruments of CRP effects may vary over time and CRP may only affect schizophrenia risk in a specific period (e.g. childhood). Furthermore, regarding the reverse-causality analysis, the current findings merely suggest that liability to schizophrenia does not lead to CRP-level alterations. To investigate possible causal effects of liability to schizophrenia-associated clinical features (e.g. outcome) on CRP-level changes would require longitudinal designs using individual-level data from SCZ patients. Furthermore, the SNPs significantly associated with blood constituents explained a relatively small proportion of the variance in these traits; future, even better-powered GWASs may thus yield more powerful genetic instruments. Finally, MR-BMA is a relatively new multivariable MR method and we hope that future techniques will be used to replicate these findings.
Conclusions
We provide consistent evidence for a protective effect of CRP on schizophrenia without reverse causal effects, suggesting that CRP is not influenced by schizophrenia liability. Multiple potential biomarkers for schizophrenia such as triglycerides, leucine, citrate and lactate were detected. These findings add to a growing body of literature hinting at metabolic changes—in particular related to triglycerides—independently of medication status in SCZ and may be used to further investigate the effects of interventions targeting the above-mentioned constituents.
Funding
We thank the following sources for funding or research: the German Ministry for Education and Research (National Genome Research Net-Plus 01GS0820), the German Research Foundation (DFG; HI865/2–1) and the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements nos 245009 and 262055. Part of this work was initiated in the ECNP Nutrition Network.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Miller BJ, Culpepper N, Rapaport MH.. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses 2014;7:223–30. [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez-Blanco L, Maria P-P, Garcia-Alvarez L. et al. Elevated C-reactive protein as a predictor of a random one-year clinical course in the first ten years of schizophrenia. Psychiatry Res 2018;269:688–91. [DOI] [PubMed] [Google Scholar]
- 3. Fond G, Lancon C, Auquier P, Boyer L.. C-Reactive protein as a peripheral biomarker in schizophrenia: an updated systematic review. Front Psychiatry 2018;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howren MB, Lamkin DM, Suls J.. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71:171–86. [DOI] [PubMed] [Google Scholar]
- 5. Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM.. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 2018;23:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB.. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2015;2:258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G.. Inflammatory biomarkers and risk of schizophrenia: a 2-sample Mendelian randomization study. JAMA Psychiatry 2017;74:1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoshita M, Numata S, Tajima A. et al. Retraction: a significant causal association between C-reactive protein levels and schizophrenia. Sci Rep 2018;8:46947.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prins BP, Abbasi A, Wong A. et al. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium Mendelian randomization study. PLoS Med 2016;13:e1001976.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cordes J, Bechdolf A, Engelke C. et al. Prevalence of metabolic syndrome in female and male patients at risk of psychosis. Schizophr Res 2017;181:38–42. [DOI] [PubMed] [Google Scholar]
- 11. Ryan MC, Collins P, Thakore JH.. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry 2003;160:284–89. [DOI] [PubMed] [Google Scholar]
- 12. Chen DC, Du XD, Yin GZ. et al. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia: relationships with clinical phenotypes and cognitive deficits. Psychol Med 2016;46:3219–230. [DOI] [PubMed] [Google Scholar]
- 13. Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH.. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med 2007;24:481–85. [DOI] [PubMed] [Google Scholar]
- 14. Thakore JH, Mann JN, Vlahos I, Martin A, Reznek R.. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes Relat Metab Disord 2002;26:137–41. [DOI] [PubMed] [Google Scholar]
- 15. Guest PC, Wang L, Harris LW. et al. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naive schizophrenia patients. Mol Psychiatry 2010;15:118–19. [DOI] [PubMed] [Google Scholar]
- 16. van Beveren NJM, Schwarz E, Noll R. et al. Evidence for disturbed insulin and growth hormone signaling as potential risk factors in the development of schizophrenia. Transl Psychiatry 2014;4:e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suhre K, Gieger C.. Genetic variation in metabolic phenotypes: study designs and applications. Nat Rev Genet 2012;13:759–69. [DOI] [PubMed] [Google Scholar]
- 18. Hebebrand J, Peters T, Schijven D. et al. The role of genetic variation of human metabolism for BMI, mental traits and mental disorders. Mol Metab 2018;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies NM, Holmes MV, Davey Smith G.. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bowden J, Smith GD, Haycock PC, Burgess S.. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowden J, Smith GD, Burgess S.. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu ZH, Zheng ZL, Zhang FT. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun 2018;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess S, Thompson SG.. Multivariable Mendelian Randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuber V, Colijn JM, Klaver C, Burgess S.. Selecting causal risk factors from high-throughput experiments using multivariable Mendelian randomization. bioRxiv 2018;396333.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ligthart S, Vaez A, Vosa U. et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet 2018;103:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pardinas AF, Holmans P, Pocklington AJ. et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 2018;50:381–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemani G, Zheng J, Elsworth B. et al. The MR-base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowden J, Del Greco MF, Minelli C, Smith GD, Sheehan NA, Thompson JR.. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I-2 statistic. Int J Epidemiol 2016;45:1961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burgess S, Scott RA, Timpson NJ, Smith GD, Thompson SG, Consortium E-I.. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 2015;30:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kettunen J, Demirkan A, Wurtz P. et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 2016;7:11122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook RD. Influential observations in linear-regression. J Am Stat Assoc 1979;74:169–74. [Google Scholar]
- 32. Hans C, Dobra A, West M.. Shotgun Stochastic search for ‘Large p’ regression. J Am Stat Assoc 2007;102:507–16. [Google Scholar]
- 33. Yavorska OO, Burgess S.. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017;46:1734–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orsolini L, Sarchione F, Vellante F. et al. Protein-C reactive as biomarker predictor of schizophrenia phases of illness? A systematic review. Curr Neuropharmacol 2018;16:583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puzianowska-Kuznicka M, Owczarz M, Wieczorowska-Tobis K. et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 2016;13:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E.. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 2007;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Bernardo M, Kirkpatrick B.. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features (vol 198, pg 212, 2012). Psychiatry Res 2013;210:1329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacomb I, Stanton C, Vasudevan R. et al. C-reactive protein: higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Front Immunol 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Labrecque JA, Swanson SA.. Interpretation and potential biases of mendelian randomization estimates with time-varying exposures. Am J Epidemiol 2019;188:231–38. [DOI] [PubMed] [Google Scholar]
- 40. Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB.. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res 2012;139:161–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benros ME, Trabjerg BB, Meier S. et al. Influence of polygenic risk scores on the association between infections and schizophrenia. Biol Psychiatry 2016;80:609–16. [DOI] [PubMed] [Google Scholar]
- 42. He Y, Yu Z, Giegling I. et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl Psychiatry 2012;2:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solberg DK, Bentsen H, Refsum H, Andreassen OA.. Lipid profiles in schizophrenia associated with clinical traits: a five year follow-up study. BMC Psychiatry 2016;16:299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeynep BT. The relationship between serum lipid levels and lifetime suicide attempts in patients with schizophrenia. Med Sci 2018;7:724–996. [Google Scholar]
- 45. Bjerkenstedt L, Edman G, Hagenfeldt L, Sedvall G, Wiesel FA.. Plasma and muscle amino-acids in relation to cerebrospinal-fluid monoamine metabolites in schizophrenic-patients and healthy controls. Int J Neurosci 1987;32:707–08. [DOI] [PubMed] [Google Scholar]
- 46. De Luca V, Viggiano E, Messina G. et al. Peripheral amino acid levels in schizophrenia and antipsychotic treatment. Psychiatry Investig 2008;5:203–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duncan LE, Shen H, Ballon JS, Hardy KV, Noordsy DL, Levinson DF.. Genetic correlation profile of schizophrenia mirrors epidemiological results and suggests link between polygenic and rare variant (22q11.2) cases of schizophrenia. Schizophr Bull 2018;44:1350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xuan JK, Pan GH, Qiu YP. et al. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res 2011;10:5433–43. [DOI] [PubMed] [Google Scholar]
- 49. Matsui T, Omuro H, Liu YF. et al. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc Natl Acad Sci USA 2017;114:6358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jourdain P, Allaman I, Rothenfusser K, Fiumelli H, Marquet P, Magistretti PJ.. L-Lactate protects neurons against excitotoxicity: implication of an ATP-mediated signaling cascade. Sci Rep 2016;6:21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berthet C, Lei HX, Thevenet J, Gruetter R, Magistretti PJ, Hirt L.. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 2009;29:1780–89. [DOI] [PubMed] [Google Scholar]
- 52. Burgess S, Davies NM, Thompson SG.. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 2016;40:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.