Abstract
Multiple drug resistant fungi pose a serious threat to human health, therefore the development of completely new antimycotics is of paramount importance. The in vitro antifungal activity of the original, 1-amino-5-isocyanonaphthalenes (ICANs) was evaluated against reference strains of clinically important Candida species. Structure-activity studies revealed that the naphthalene core and the isocyano- together with the amino moieties are all necessary to exert antifungal activity. 1,1-N-dimethylamino-5-isocyanonaphthalene (DIMICAN), the most promising candidate, was tested further in vitro against clinical isolates of Candida species, yielding a minimum inhibitory concentration (MIC) of 0.04–1.25 µg/mL. DIMICAN was found to be effective against intrinsically fluconazole resistant Candida krusei isolates, too. In vivo experiments were performed in a severly neutropenic murine model inoculated with a clinical strain of Candida albicans. Daily administration of 5 mg/kg DIMICAN intraperitoneally resulted in 80% survival even at day 13, whereas 100% of the control group died within six days. Based on these results, ICANs may become an effective clinical lead compound family against fungal pathogens.
Keywords: multiple drug resistance; antifungal effect; 1-amino-5-isocyanonaphthalenes; 1,1-N-dimethylamino-5-isocyanonaphthalene; Candida species; C. albicans
1. Introduction
Invasive fungal infections cause 1.5 million deaths annually and may show a further increase in the following decades. Most of these infections are caused by Candida species, followed by Aspergillus and Cryptococcus neoformans [1,2,3].
Members of the Candida genus are part of the healthy human microbiome and can be found in the oral cavity, gastrointestinal and urogenital tract, however as opportunistic pathogens, these yeasts can cause a wide variety of diseases ranging from superficial infections to life threatening invasive candidiasis [4,5,6]. Incidence and mortality of the latter remained high in the last decades, despite the widespread use of echinocandins and newer generation triazoles as prophylactic and therapeutic agents [7,8]. The development of invasive candidiasis is associated with several predisposing factors, notably with immunosuppression, recent abdominal surgery, diabetes, broad spectrum antibiotic therapy and many others [9]. While risk factors are numerous, therapeutic options are very limited with only three major antifungal classes (triazoles, polyenes, echinocandins) available [10]. The most recent antifungal compounds, the echinocandins were introduced almost twenty years ago and there are few new drugs in the pipeline in the following years [11]. It should also be noted that several strains of the Candida species, such as Candida krusei exhibit intrinsic resistance to fluconazole, however despite their potential to emerge as multidrug-resistant pathogens in the hospital setting, this has not yet occurred [1]. Nevertheless, the emergence of new, drug resistant pathogenic fungi, such as Candida auris pose a serious therapeutic challenge and highlights the need for new compounds with different mechanisms of action [12,13].
Recently, we developed a novel amino-isocyanonaphthalene (ICAN) based solvatochromic fluorophore family [14], which despite their very simple structure and easy preparation, still mark a white spot on the map of chemistry. Despite their relative novelty, they already found numerous and versatile use in both analytical chemistry [15,16,17] and cell-biology [18,19,20]. During our experiments to utilize ICAN derivatives as vital stains on CaCo2, OCM-1, HuLi and HaCat cell lines [20] we noticed that they are perfectly suitable for the staining of different fungi, too. However, after testing 1-N-methylamino-5-isocyanonaphthalene (MICAN) on a commercial strain of Saccharomyces cerevisiae, a strong antimycotic effect was observed. We wondered that ICAN derivatives could be used as potential drugs against pathological fungi, and planned experiments on Candida species were started.
The aims of this study were to test the antifungal activity of 1-amino-5-isocyanonaphthalene and its derivatives on different Candida species in vitro and to test the most effective agent in vivo, in a murine model of invasive candidiasis. This discovery can lead to the development of a new original compound family, which can rival the currently approved drug classes or even top them in several fields of application. It should be noted, however that this study focuses only on the 1,5-ICAN derivatives, whereas ICANs are easy to modify and even the slightest change in the relative substitution position of the amino and isonitrile groups on the naphthalene ring can result in a completely different behavior [21].
2. Results
2.1. Antifungal Activity of the ICAN Derivatives
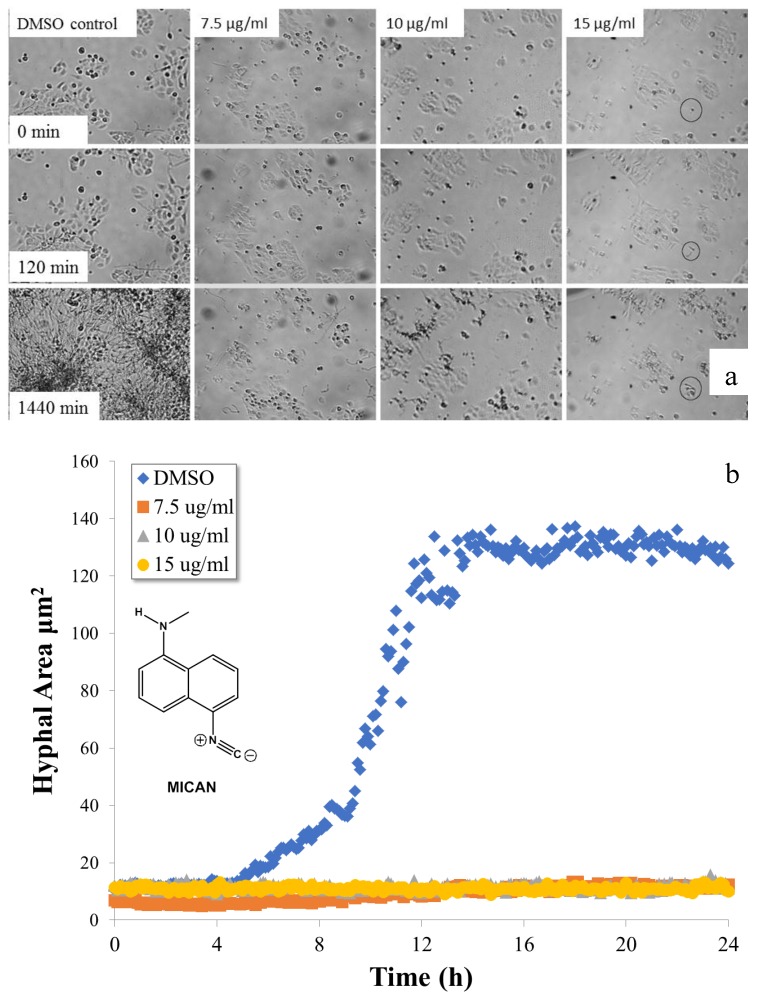
We previously carried out extensive studies to successfully utilize 1-N-methylamino-5-isocyanonaphthalene (MICAN) as a vital cell dye on different human cell lines [20]. The compound along with ICAN and 1,1-N-dimethylamino-5-isocyanonaphthalene (DIMICAN) are well tolerated by human cell cultures, their LD50 values (on HaCat cell line) were determined (MTT bio assay technique) to be 10 μg/mL for ICAN, 18 μg/mL for MICAN, and 13 μg/mL for DIMICAN. Encouraged by these results we wanted to extend their applicability to fungal staining. Since these dyes are nonpolar, they are expected to easily bind to and stain the nonpolar chitin in the cell wall of different fungal species. We tested this assumption on common yeast from a local shop, and much to our surprise after staining, the yeast cells stopped growing and died in a short time. Repeated experiments led to the same conclusion; however, the applied dye concentration was well below the LD50 determined for human cells. We assumed that these compounds may have antifungal effect, therefore Candida albicans, one of the most common human pathogenic fungi was selected for further tests. HaCat cell cultures were infected with C. albicans and were treated with different concentrations of MICAN dissolved in DMSO. The fungal growth was followed by time lapse imaging for 24 h. The results are summarized in Figure 1. As it is evident from Figure 1a,b, contrary to the untreated (DMSO control) cells, which show the typical yeast growth curve (Figure 1b), little fungal growth (~30 % of the starting C. albicans cells even germinated) was observed at even as low as 7.5 μg/mL MICAN concentrations, which is well below its LD50 value on HaCat cells. It should be noted, however, that in the case of the untreated coculture, the fungal growth exceeded 100% of the field-of-view (24 h) developing three-dimensional, multilayered hyphal mass. Additionally, the average hyphal area was 10-fold higher than in the case of the lowest MICAN concentration applied. The antifungal effect of MICAN (and DIMICAN) is also presented in the supporting video files Movie S1 and Movie S2. Despite effective fungal growth inhibitory of MICAN, the treated HaCat cells showed no sign of damage under 24 h.
Figure 1.
Hyphal growth of C. albicans in the presence of methylamino-5-isocyanonaphthalene (MICAN). Time-lapse microscopic images (a) of HaCat cells infected by C. albicans in the presence of different concentrations of MICAN and the corresponding hyphal growth curves (b) determined from the average individual hyphal area of C. albicans. (Areas were measured instead of lengths due to the hyphal ramifications).
2.2. Antifungal Susceptibility Testing–Determination of Structure-Activity Relationship
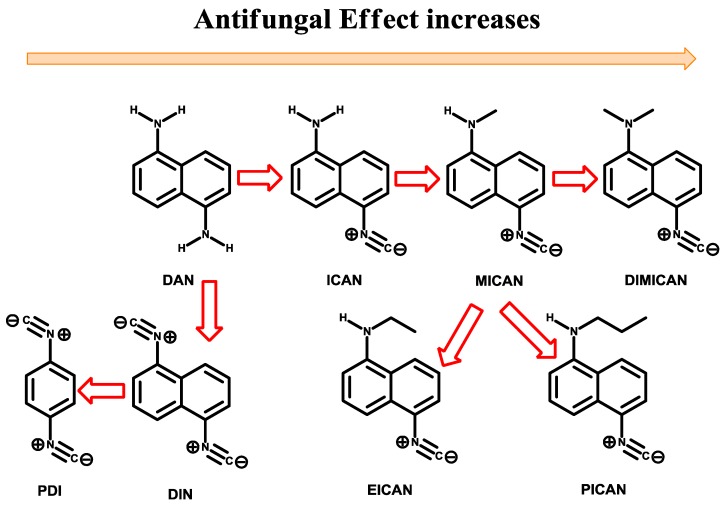
ICAN has 3 key moieties, namely the amino, naphthalene and isonitrile. To find out which ones of them are essential for the antifungal behavior and to design more efficient derivatives, comparative studies were carried out, where one of the moieties was exchanged or eliminated. The modification/synthetic strategy is summarized in Figure 2. All ICAN derivatives were tested against C. albicans SC5314, C. krusei ATCC 6258 and Candida parapsilosis ATCC 22019. Table 1 summarizes the minimum inhibitory concentrations (MICs) of the ICAN derivatives. The antifungal agents were dissolved in 100% DMSO and diluted further in RPMI-1640 (with L-glutamine but without bicarbonate) and buffered to pH 7.0 with 0.165 M morpholinopropanesulfonic acid (MOPS). MICs were read visually after 48 h incubation at 35 °C using the partial (50%) inhibition (for ICAN derivatives) (PI) and total inhibition (for amphotericin B (AMB) and ICAN derivatives) (TI) criteria. MICs were determined at least twice.
Figure 2.
The development strategy for the most effective 1-amino-5-isocyanonaphthalene (ICAN) derivative. For the full names please refer to the experimental section of this paper.
Table 1.
In vitro susceptibilities of C. albicans SC5314, C. parapsilosis ATCC 22019, and C. krusei ATCC 6258 to various ICAN derivatives determined by the Clinical & Laboratory Standards Institute (CLSI) broth microdilution method. MICs were read using the partial (MICPI) and total inhibition (MICTI) criteria. [22]. For the full names and structures of the compounds please refer to Figure 2 and the experimental section of this paper. The lowest MICs are indicated in red. For the MICTI values of AMB for these Candida strains please refer to Table 2.
| Compound | Candida Species | MICPI (µg/mL) | MICTI (µg/mL) |
|---|---|---|---|
| ICAN | C. albicans SC5314 | 1.25 | 5 |
| C. parapsilosis ATCC 22019 | 0.3 | 0.6 | |
| C. krusei ATCC 6258 | 0.6 | 2.5 | |
| MICAN | C. albicans SC5314 | 0.3 | 1.25 |
| C. parapsilosis ATCC 22019 | 0.15 | 0.75 | |
| C. krusei ATCC 6258 | 0.15 | 1.25 | |
| EICAN | C. albicans SC5314 | 0.6 | 2.5 |
| C. parapsilosis ATCC 22019 | 0.6 | 1.25 | |
| C. krusei ATCC 6258 | 0.6 | 1.25 | |
| PICAN | C. albicans SC5314 | 0.6 | 2.5 |
| C. parapsilosis ATCC 22019 | 0.6 | 1.75 | |
| C. krusei ATCC 6258 | 0.15 | 2.5 | |
| DIMICAN | C. albicans SC5314 | ≤0.04 | 0.6 |
| C. parapsilosis ATCC 22019 | 0.15 | 0.6 | |
| C. krusei ATCC 6258 | 0.15 | 0.3 | |
| DIN | C. albicans SC5314 | 0.3 | 0.6 |
| C. parapsilosis ATCC 22019 | 0.3 | 0.6 | |
| C. krusei ATCC 6258 | 0.3 | 0.6 | |
| DAN | C. albicans SC5314 | 50 | 100 |
| C. parapsilosis ATCC 22019 | 50 | 100 | |
| C. krusei ATCC 6258 | 50 | 100 | |
| PDI | C. albicans SC5314 | 50 | 100 |
| C. parapsilosis ATCC 22019 | 50 | 100 | |
| C. krusei ATCC 6258 | 50 | 100 |
Based on the data of Table 1 it can be concluded that even the simplest amino-isocyanonaphthalene molecule ICAN has good antifungal effect with MICTI values between 0.6–5 µg/mL. The starting compound 1,5-diaminonaphthalene (DAN) proved to be completely ineffective against Candida (MICTI > 100 µg/mL), therefore we can conclude that the isonitrile group is essential for the antifungal effect. Next the free amino group of ICAN was converted to isonitrile resulting 1,5-diisocyanonaphthalene (DIN), which showed similar (or higher) antifungal activity (MICTI = 0.6 µg/mL) compared to that of ICAN. However, the largely nonpolar and rigid aromatic structure of DIN significantly limits its solubility in polar solvents, such as water. We previously also encountered solubility problems with DIN, when we tried to determine its fluorescent properties [14]. Nevertheless, DIN may also become an effective antifungal drug after solving the solubility issues with proper formulation. The naphthalene core of DIN was exchanged to phenylene to obtain 1,4-phenylenediisocyanide (PDI). In contrast to DIN, PDI proved to be completely ineffective (MICTI > 100µg/mL), which means that the naphthalene core is also essential for the antifungal behavior (Figure 2.). To increase the efficacy of ICAN derivatives, the substitution of the amino group followed. The alkylation of the amino group increases the dipolarity of the molecules, however at the same time it reduces their ability to form H-bonding as an H-bond donor. Monomethylation (MICAN) led to lowered MICTI values (0.75–1.25 µg/mL), however the use of longer alkyl chains, such as ethyl and propyl (EICAN and PICAN) proved to be not so effective (MICTI = 1.25–2.5 µg/mL and 1.75–2.5 µg/mL, respectively), maybe due to steric hinderance. The lowest MIC values were obtained in the case of DIMICAN (MICPI ≤ 0.04 µg/mL and MICTI = 0.3–0.6 µg/mL), the dimethylated ICAN derivative. It should be noted that the MICs did not vary significantly for most of the compounds under investigation, only in the case of ICAN was a broader range observed (9-fold variation for ICAN (MIC = 0.6–5 µg/mL). For further tests we chose one of the most effective ICAN derivative, DIMICAN which has no cytotoxic effect [15,20]. To test the “real life” behavior of DIMICAN it was tested for in vitro susceptibility against clinical isolates collected from patients at the Clinics of the University of Debrecen. Table 2 summarizes the MICs of the new antifungal agent DIMICAN.
Table 2.
In vitro susceptibilities of Candida isolates to DIMICAN and amphotericin B (AMB), determined by CLSI broth microdilution method. MICs were determined according to the CLSI M27-A3 using the partial (MICPI) and total inhibition (MICTI) criteria. [22]. MICs were read visually after 48 h incubation at 35 °C using the partial (50%) inhibition (for DIMICAN) (PI) and total inhibition (for AMB and DIMICAN) (TI) criteria. MICs were determined at least twice. DIMICAN = 1,1-N-dimethylamino-5-isocyanonaphthalene.
| C. albicans | C. tropicalis | C. krusei | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | MICPI (µg/mL) | MICTI (µg/mL) | AMB MICTI (µg/mL) | Isolate | MICPI (µg/mL) | MICTI (µg/mL) | AMB MICTI (µg/mL) | Isolate | MICPI (µg/mL) | MICTI (µg/mL) | AMB MICTI (µg/mL) |
| ATCC 10231 | ≤0.04 | 0.3 | 0.25 | ATCC 750 | 0.15 | 1.25 | 0.5 | ATCC 6258 | 0.15 | 0.3 | 1 |
| 456 | ≤0.04 | 0.6 | 0.5 | 579 | 0.08 | 0.6 | 0.5 | 749 | 0.08 | 0.08 | 1 |
| 665 | ≤0.04 | 0.6 | 1 | 622 | 0.08 | 0.3 | 1 | 1853 | 0.08 | 0.3 | 1 |
| 685 | ≤0.04 | 0.6 | 0.25 | 1776 | 0.08 | 0.3 | 1 | 4364 | ≤0.04 | 0.15 | 1 |
| 720 | ≤0.04 | 0.6 | 0.5 | 2467 | 0.15 | 1.25 | 0.5 | 5029 | ≤0.04 | 0.08 | 1 |
| 963 | ≤0.04 | 0.6 | 0.5 | 3014 | 0.08 | 0.6 | 0.5 | 5072 | 0.08 | 0.3 | 1 |
| 1544 | ≤0.04 | 0.6 | 0.25 | 5115 | 0.08 | 1.25 | 0.5 | 25504 | 0.08 | 0.3 | 0.5 |
| 8658 | ≤0.04 | 0.6 | 1 | 5806 | 0.08 | 0.15 | 0.25 | 34987 | ≤0.04 | 0.08 | 1 |
| 3666 | ≤0.04 | 0.6 | 0.5 | ||||||||
| C. glabrata | C. parapsilosis | ||||||||||
| Isolate | MICPI (µg/mL) | MICTI (µg/mL) | AMB MICTI (µg/mL) | Isolate | MICPI (µg/mL) | MICTI (µg/mL) | AMB MICTI (µg/mL) | ||||
| ATCC 90030 | 0.15 | 0.3 | 0.5 | ATCC 22019 | 0.15 | 0.6 | 0.5 | ||||
| 14408 | ≤0.04 | 0.08 | 0.5 | 4133 | 0.08 | 0.08 | 0.25 | ||||
| 14559 | ≤0.04 | 0.3 | 0.5 | 9613 | 0.15 | 0.15 | 0.25 | ||||
| 22394 | ≤0.04 | 0.3 | 0.5 | 10252 | ≤0.04 | 0.08 | 0.5 | ||||
| 23429 | ≤0.04 | 0.3 | 1 | 18154 | 0.08 | 0.6 | 0.5 | ||||
| 23481 | 0.08 | 1.25 | 1 | 27001 | ≤0.04 | 0.3 | 0.25 | ||||
| 27643 | 0.08 | 0.3 | 0.5 | ||||||||
| 28557 | ≤0.04 | 0.6 | 0.5 | ||||||||
Amphotericin B susceptibility of the isolates was also tested, and their MICTI values were not higher than 2 µg/mL in each case. DIMICAN MICPI values were same or significantly lower compared to MICTI for all tested five Candida species. The lowest MICs were noticed in case of C. albicans.
2.3. Mouse Model of Infection and Antifungal Therapy
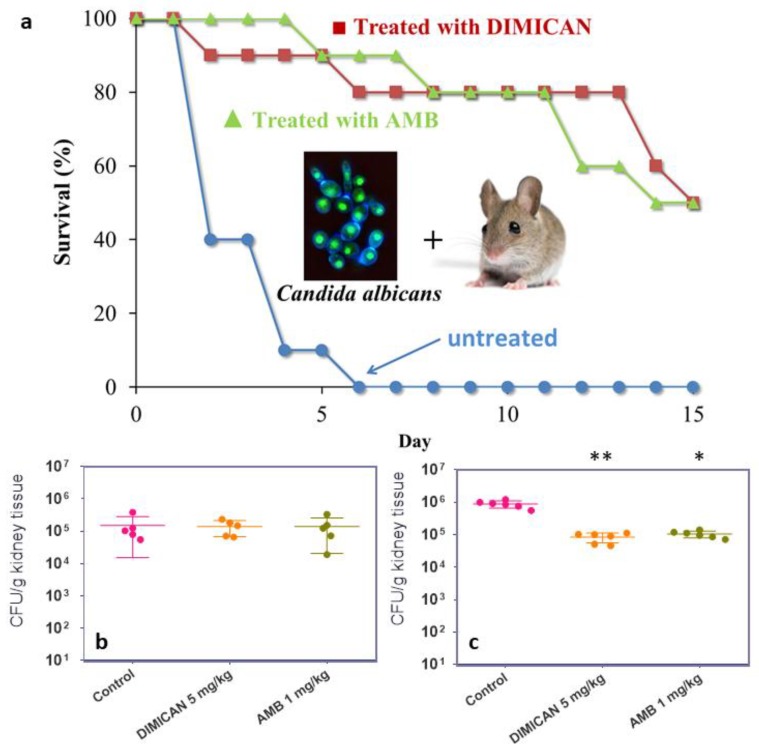
In the model of acute invasive candidiasis, neutropenic mice were infected via the lateral tail vein with C. albicans 3666 isolate (3 × 104 cfu/ mouse) to establish an acute infection. All untreated control animals died within six days. DIMICAN (5 mg/kg) prolonged the survival period of animals compared to non-treated infected controls (p < 0.05; 80 versus 0% survivors at day 7 post-infection; Figure 3a). It should be noted, however, that DIMICAN was administered as intraperitoneal injection which contained DIMICAN as an emulsion in reagent grade olive oil and water. The effect of the pure vehicle (oil/water emulsion) was tested on eight-week-old mice and aside from increased weight gain compared to the control group, no mortality was observed within five days. Proper clinical formulation may have resulted in better survival of the treated group, however it is a complex task for this new molecule and therefore exceeds the scope of this paper.
Figure 3.
In vivo antifungal activity results for DIMICAN against Candida albicans 3666 isolate. (a) Survival curves of mice infected with Candida albicans 3666 isolate. For all groups n = 10. (b) Kidney tissue burden of immunosuppressed mice infected by Candida albicans 3666 isolate, two days after infection. (c) Six days after infection (p < 0.05 (*); p <0.001 (**)).
2.4. Determination of Fungal Burden
During an independent in vivo experiment, mice were infected intravenously with C. albicans 3666 isolate. The infected mice were euthanized two or six days after infection (n = 5 and 6 mice/time point, respectively) to determine the fungal burden in the kidney. Kidney burden was analyzed using a Kruskal–Wallis test with Dunn’s posttest for multiple comparisons [23,24]. Results of fungal burden experiments are shown on Figs. 3b and c. Two days after infection AMB and DIMICAN had no effect on fungal load (p > 0.05). Six days after infection AMB and DIMICAN treatment resulted in significantly lower fungal burden compared to control: p < 0.05 (AMB); p < 0.001 (DIMICAN). It is evident in Figure 3a,b, that on day six the fungal burden increased by an order of magnitude (from ~105 to ~106) in the case of the untreated mice, while in the case of DIMICAN it remained the same as it was on day two. The same is valid for AMB. Based on these results it can be concluded that DIMICAN is at least as effective in reducing the fungal burden in the kidney as amphotericin B.
3. Discussion
It was discovered that our new, original molecule: 1-amino-5-isocyanonaphthalene (ICAN) and its derivatives exert excellent antifungal activity against a broad range of Candida species. Structure-activity studies based on minimum in vitro inhibitory concentration (MIC) determined for three Candida species, namely C. albicans, C. krusei and C. parapsilosis revealed that for the antifungal effect the naphthalene core, the isocyano- and the amino-moieties are all necessary. It was noted that alkylation especially methylation considerably increased the antifungal activity. However, 1,5-diisocyanonaphthalene (DIN) also showed low MIC (MICPI = 0.3 μg/mL, MICTI = 0.6 μg/mL), but its rigid structure and consequently its low solubility in aqueous media may limit its application. Nevertheless, proper formulation and/or substitution with solubility enhancer groups on the naphthalene core may increase its effectiveness. Based on MIC values (MICPI = 0.04–0.3 μg/mL, MICTI = 0.6 μg/mL) and its low cytotoxicity 1,1-N-dimethylamino-5-isocyanonaphthalene (DIMICAN) was selected to be tested on clinical Candida isolates. In vitro we tested it using microdilution method against Candida albicans (8 isolates), C. krusei (7 isolates), C. tropicalis (7 isolates), C. glabrata (7 isolates) and C. parapsilosis (5 isolates) to get total minimum inhibitory concentration (MICTI) values of 0.08–1.25 µg/mL. These MIC values are comparable or lower than that of amphotericin B for the same isolates. It is important to note that DIMICAN was found to be effective against intrinsically fluconazole resistant C. krusei isolates, too. The in vivo applicability of DIMICAN was tested on immunosuppressed mice and control experiments were also conducted with amphotericin B. In the model of acute invasive candidiasis, neutropenic mice were infected intravenously with C. albicans 3666 isolate (3 × 104 cfu/ mouse) to establish an acute infection, and all untreated control animals died within six days. DIMICAN (5 mg/kg) prolonged the survival period of animals compared with the survival period of non-treated infected controls, P < 0.05; 80 versus 0% survivors at day 7 post infection. Results of fungal burden in kidney revealed that six days after infection AMB and DIMICAN were efficient against Candida isolate: p < 0.05 (AMB); p < 0.001 (DIMICAN). Based on these results and the easy and versatile modification of the ICANs we hope that they have the potential to become an effective clinical lead compound family against pathogenic fungi. As potential lead compounds, ICANs, meet one or more criteria of commercial use, such as low cost, easy preparation and good chemical stability, for example after extensive toxicity and time-kill studies may be used as antimycotic coatings on medical tubes and/or devices. This simple, cheap, nontoxic and easy to prepare compound family would allow not only human treatment, but at the same time could be used as disinfectants and/or coating which is vital against the spread of highly virulent Candida strains.
4. Materials and Methods
4.1. Materials
1,5-diaminonaphthalene (DAN, Aldrich, D21200) was used as received. The synthesis of the ligands: 1-amino-5-isocyanonaphthalene (ICAN), 1-N-methylamino-5-isocyanonaphthalene (MICAN) 1-N,N-dimethylamino-5-isocyanonaphthalene (DIMICAN) and 1,5-diisocyanonaphthalene (DIN) is found in refs.: 14 and 17.
4.2. General Method for the Synthesis of Alkylated 1-amino-5-isocyanonaphthalenes
A 250 mL round-bottomed flask, equipped with a magnetic stir bar was charged with 1-amino-5-isocyanonaphthalene (1.00 g, 5.94 mmol), potassium hydroxide (0.670 g, 11.9 mmol) and absolute dimethyl formamide freshly distilled over phosphorus pentoxide (100 mL). Alkyliodide (59.4 mmol)* was added to the solution, then the flask was flushed with argon and sealed with a rubber septum. The reaction mixture was stirred at room temperature, protected from light. After two days 200 mL methylene chloride and 5% ammonia solution was added, and the solution was extracted 5 times with water, then the organic phase was dried over anhydrous magnesium sulfate. Solvent was removed on a rotary evaporator and the residue was purified on a normal-phase silicagel filled column, using methylene chloride: hexane (1:1) as eluent.
Ethyliodide (Sigma-Aldrich, Schnelldorf, Germany) was used to synthesize 1-ethyl-5-isocyanonaphthalene (EICAN) (yellow crystals, 370 mg, 32% yield) and diEICAN (light green waxy compound, 90 mg, 6.8% yield). To synthesize1-propyl-5-isocyanonaphthalene (PICAN) propyliodide (Sigma-Aldrich, Germany) was used. PICAN (light yellow waxy compound, 440 mg, 35% yield) and diPICAN (green waxy compound, 100 mg, 6.7% yield). With the excess of alkyl iodide used the ratio of the mono- and dialkylated-product formed can be controlled.
EICAN (1-N-ethylamino-5-isocyanonaphthalene)1H NMR (400 MHz, CDCl3) δ = 7.83 (d, J = 8.6 Hz, 1H), 7.50 (q, J = 8.6 Hz, 3H), 7.32 (dd, J = 8.5, 7.5 Hz, 1H), 6.66 (dd, J = 6.8, 1.6 Hz, 1H), 4.30 (s, 1H), 3.29 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H) ppm. 13C NMR (101 MHz, CDCl3) δ = 166.70 (quart. Ar-NC), 144.05 (quart. Ar), 129.06 (Ar), 124.49 (Ar), 123.52 (quart. Ar), 123.21 (Ar), 121.85 (Ar), 111.48 (Ar), 105.55 (Ar), 38.69 (Ethyl–CH2–), 14.65 (Ethyl–CH3) ppm.
PICAN (1-N-propylamino-5-isocyanonaphthalene)1H NMR (400 MHz, CDCl3) δ = 7.83 (d, J = 8.6 Hz, 1H), 7.50 (q, J = 8.6 Hz, 3H),7.32 (dd, J = 8.5, 7.5 Hz, 1H), 6.66 (dd, J = 6.4, 1.8 Hz, 1H), 4.38 (s, 1H), 3.22 (t, J = 7.0 Hz, 2H), 1.85–1.72 (m, 2H), 1.08 (t, J = 7.4 Hz, 3H) ppm. 13C NMR (101 MHz, CDCl3) δ= 166.70 (quart. Ar-NC), 144.10 (quart. Ar), 129.06 (Ar), 124.49 (Ar), 123.53 (quart. Ar), 123.19 (Ar), 121.81 (Ar), 111.36 (Ar), 105.50 (Ar), 45.96 (Propyl–CH2–), 22.51 (Propyl–CH2–), 11.81 (Propyl–CH3) ppm.
4.3. Time-lapse Microscopy and Digital Image Analysis of Cocultures of HaCaT cells and C. albicans
Human keratinocytes were grown under standard cell breeding conditions in T-25 culture vessels. At 40% of cellular monolayer confluency the cultures were treated and observed under four parallel custom-built inverted microscopes housed in a cell-breeding incubator. 1.5 × 105/mL C. albicans cells were used for inoculation. Image acquisitions were carried out at 1 frame/min frequency. Exposure-synchronized low-energy near-infrared illumination at 940 nm was used for the sake of minimal invasivity. Image sequences were preprocessed prior analysis to eliminate flickering and uneven illumination artefacts. Quantitative image analysis were done via NIH (US National Institutes of Health) ImageJ v1.39d software bundle, using hyphal separation plugins. Determination of hyphal area was preferred for precision against length, because of the frequent overlapping and ramifications in control samples.
4.4. Clinical Isolates and Antifungal Susceptibility Testing
The tested C. albicans, C. tropicalis, C. parapsilosis, C. glabrata and C. krusei ATCC strains and clinical isolates (collected from sterile body sites) are listed in Table 2. MICs were determined according to the CLSI M27-A3 [22]. Antifungal agents were dissolved in 100% DMSO and diluted further in RPMI-1640 (with L-glutamine but without bicarbonate) (Sigma, Budapest, Hungary), and buffered to pH 7.0 with 0.165 M morpholinopropanesulfonic acid (MOPS; Sigma, Budapest, Hungary). The final concentration ranges used for AMB and ICAN derivatives were 0.125 to 4 µg/mL and 0.04 to 20 µg/mL, respectively. Cell suspensions were prepared in RPMI 1640 medium and were adjusted to give a final inoculum concentration of 0.5 × 103 to 2.5 × 103 cells/mL. MICs were read visually after 48-h incubation at 35 °C using the partial (50%) inhibition (for ICAN derivatives) (PI) and total inhibition (for AMB and ICAN derivatives) (TI) criteria. MICs were determined at least twice. For amphotericin B isolates with MIC values of ≤2 mg/L were considered wild-types [25].
4.5. Mouse Model of Infection
We used 10–12 week-old female BALB/c mice for all experiments. Mice were immunosuppressed using 150 mg/kg intraperitoneal (ip) cyclophosphamide dose (4 days prior to infection) and 100 mg/kg ip cyclophosphamide dose (1 day prior to infection; 2 and 5 days after infection) [26]. All mice received ceftazidime (5 mg/day subcutaneously) from days 0 to 6 after infection. Mice were given food and water ad libitum and were monitored daily. The animals were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals, and the experiments were approved by the Animal Care Committee of the University of Debrecen, Debrecen, Hungary (permission no. 12/2014).
Mice were infected via the lateral tail vein with C. albicans 3666 bloodstream isolate. In the lethality (10 mice/group) and fungal tissue burden experiments (6 mice/group) the infectious doses were 3 × 104 and 104 cfu/ mouse, respectively. These doses led to 100% and 0% mortality, respectively by day 6 after infection among untreated mice. Inoculum density was confirmed by plating serial dilutions onto Sabouraud dextrose agar (SDA) plates.
4.6. Antifungal Therapy
AMB (Fungizone, commercial preparation) and DIMICAN treatment in a 0.5-mL bolus was started 1 h after infection. The doses were 1 mg AMB and 5 mg DIMICAN (in olive oil/water (o/w) emulsion) per kg body weight. All antifungals were administered intraperitoneally once daily for 5 consecutive days.
4.7. Determination of Fungal Burden
Infected mice were euthanized 2 and 6 days after infection (n = 6 mice/time point) to determine the fungal burden in the kidney. The organs were aseptically removed, weighed, homogenized in sterile saline, serially diluted 10-fold in sterile saline. Aliquots of 0.1 mL of the undiluted and diluted homogenates were plated onto SDA, and the colony count was determined after 48 h. Colony-forming units (CFUs) were determined after 48 h of incubation at 37 ° C and results were expressed as cfus/g tissue [26]. The lower limit of detection was 50 cfu/g tissue. Kidney burden was analyzed using a Kruskal–Wallis test with Dunn’s posttest for multiple comparisons [23,24].
4.8. Statistical Test
Survival effects were analyzed by the Kaplan–Meier log-rank test. Kidney burden was analyzed using a Kruskal–Wallis test with Dunn’s posttest for multiple comparisons [23,24].
Supplementary Materials
The following are available online. Movie S1: Time lapse video of HaCat cells infected with Candida Albicans in the presence of different concentrations of MICAN. Movie S2: Time lapse video of HaCat cells infected with Candida Albicans in the presence of different concentrations of DIMICAN.
Author Contributions
M.N., L.M., T.Z., Z.M.S., S.K., and I.P. wrote the manuscript and evaluated the data; D.R. and Z.L.N. prepared and characterized the molecules in this study; G.S.-N., A.K., Z.T., and Z.M.S. carried out the in vitro antifungal experiments and analyzed the data; L.M. and L.T. did the in vivo mice experiments and analyzed the data; S.K. and I.P. supervised the experiments and provided financial support. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the grants K-116465 and GINOP-2.3.2-15-2016-00041 given by NFKI (National Research, Development and Innovation Office, Hungary). The projects were co-financed by the European Union and the European Regional Development Fund. Furthermore, this paper was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Miklós Nagy) and by the grant Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Biotechnology thematic programme of the University of Debrecen.
Conflicts of Interest
A patent application under the number of P1900410 has been filed by the authors at the University of Debrecen.
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Footnotes
Sample Availability: Samples of the compounds ICAN, MICAN, DIMICAN and DIN are available from the authors.
References
- 1.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Solache M.A., Casadevall A. Global warming will bring new fungal diseases for mammals. MBio. 2010;1:e00061-00010. doi: 10.1128/mBio.00061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondaryk M., Kurzatkowski W., Staniszewska M. Antifungal agents commonly used in the superficial and mucosal candidiasis treatment: Mode of action and resistance development. Postep. Derm. Alergol. 2013;30:293–301. doi: 10.5114/pdia.2013.38358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odds F.C., Hanson M.F., Davidson A.D., Jacobsen M.D., Wright P., Whyte J.A., Gow N.A., Jones B.L. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 2007;56:1066–1075. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauchie M., Desmet S., Lagrou K. Candida and its dual lifestyle as a commensal and a pathogen. Res. Microbiol. 2017;168:802–810. doi: 10.1016/j.resmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Lortholary O., Renaudat C., Sitbon K., Madec Y., Denoeud-Ndam L., Wolff M., Fontanet A., Bretagne S., Dromer F. French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010) Intensive Care Med. 2014;40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano R., Sakamoto Y., Kudo K., Ohnishi M. Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: A single hospital experience. Infect. Drug Resist. 2015;8:199–205. doi: 10.2147/IDR.S80677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Ami R. Treatment of Invasive Candidiasis: A Narrative Review. J. Fungi. 2018;4:97. doi: 10.3390/jof4030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perfect J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017;16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortegiani A., Misseri G., Fasciana T., Giammanco A., Giarratano A., Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care. 2018;6:69. doi: 10.1186/s40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krysan D.J. The unmet clinical need of novel antifungal drugs. Virulence. 2017;8:135–137. doi: 10.1080/21505594.2016.1276692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rácz D., Nagy M., Mándi A., Zsuga M., Kéki S. Solvatochromic properties of a new isocyanonaphthalene based fluorophore. J. Photochem. Photobiol. A Chem. 2013;270:19–27. [Google Scholar]
- 15.Nagy M., Rácz D., Nagy Z.L., Fehér P.P., Kalmár J., Fábián I., Kiss A., Zsuga M., Kéki S. Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling. Sens. Actuators B Chem. 2018;255:2555–2567. doi: 10.1016/j.snb.2017.09.061. [DOI] [Google Scholar]
- 16.Nagy M., Kovács S.L., Nagy T., Rácz D., Zsuga M., Kéki S. Isocyanonaphthalenes as extremely low molecular weight, selective, ratiometric fluorescent probes for Mercury(II) Talanta. 2019;201:165–173. doi: 10.1016/j.talanta.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Nagy M., Rácz D., Lázár L., Purgel M., Ditrói T., Zsuga M., Kéki S. Solvatochromic Study of Highly Fluorescent Alkylated Isocyanonaphthalenes, Their π-Stacking, Hydrogen-Bonding Complexation, and Quenching with Pyridine. ChemPhysChem. 2014;15:3614–3625. doi: 10.1002/cphc.201402310. [DOI] [PubMed] [Google Scholar]
- 18.Nagy M., Rácz D., Nagy Z.L., Nagy T., Fehér P.P., Purgel M., Zsuga M., Kéki S. An acrylated isocyanonaphthalene based solvatochromic click reagent: Optical and biolabeling properties and quantum chemical modeling. Dye. Pigment. 2016;133:445–457. doi: 10.1016/j.dyepig.2016.06.036. [DOI] [Google Scholar]
- 19.Nagy M., Kéki S., Rácz D., Mathur J., Vereb G., Garda T., M-Hamvas M., Chaumont F., Bóka K., Böddi B., et al. Novel fluorochromes label tonoplast in living plant cells and reveal changes in vacuolar organization after treatment with protein phosphatase inhibitors. Protoplasma. 2018;255:829–839. doi: 10.1007/s00709-017-1190-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagy Z., Nagy M., Kiss A., Rácz D., Barna B., Könczöl P., Bankó C., Bacsó Z., Kéki S., Banfalvi G., et al. MICAN, a new fluorophore for vital and non-vital staining of human cells. Toxicol. Vitr. 2018;48:137–145. doi: 10.1016/j.tiv.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs S.L., Nagy M., Feher P.P., Zsuga M., Keki S. Effect of the Substitution Position on the Electronic and Solvatochromic Properties of Isocyanoaminonaphthalene (ICAN) Fluorophores. Molecules. 2019;24:2434. doi: 10.3390/molecules24132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wayne P. Clinical and Laboratory Standards Institute: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. CLSI Doc. M27-A3 Suppl. S. 2008;3:6–12. [Google Scholar]
- 23.Földi R., Kovács R., Gesztelyi R., Kardos G., Berényi R., Juhász B., Szilágyi J., Mózes J., Majoros L. Comparison of In Vitro and Vivo Efficacy of Caspofungin Against Candida parapsilosis, C. orthopsilosis, C. metapsilosis and C. albicans. Mycopathologia. 2012;174:311–318. doi: 10.1007/s11046-012-9554-7. [DOI] [PubMed] [Google Scholar]
- 24.Kovács R., Gesztelyi R., Berényi R., Domán M., Kardos G., Juhász B., Majoros L. Killing rates exerted by caspofungin in 50% serum and its correlation with in vivo efficacy in a neutropenic murine model against Candida krusei and Candida inconspicua. J. Med. Microbiol. 2014;63:186–194. doi: 10.1099/jmm.0.066381-0. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller M.A., Espinel-Ingroff A., Canton E., Castanheira M., Cuenca-Estrella M., Diekema D.J., Fothergill A., Fuller J., Ghannoum M., Jones R.N., et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J. Clin. Microbiol. 2012;50:2040–2046. doi: 10.1128/JCM.00248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spellberg B., Ibrahim A.S., Edwards Jr J.E., Filler S.G. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.