Abstract
Betulinic acid (BA) is a star member of the pentacyclic triterpenoid family, which exhibits great prospects for antitumor drug development. In an attempt to develop novel antitumor candidates, 21 BA-nitrogen heterocyclic derivatives were synthetized, in addition to four intermediates, 23 of which were first reported. Moreover, they were screened for in-vitro cytotoxicity against four tumor cell lines (Hela, HepG-2, BGC-823 and SK-SY5Y) by a standard methylthiazol tetrazolium (MTT) assay. The majority of these derivatives showed much stronger cytotoxic activity than BA. Remarkably, the most potent compound 7e (the half maximal inhibitory concentration (IC50) of which was 2.05 ± 0.66 μM) was 12-fold more toxic in vitro than BA-treated Hela. Furthermore, multiple fluorescent staining techniques and flow cytometry collectively revealed that compound 7e could induce the early apoptosis of Hela cells. Structure–activity relationships were also briefly discussed. The present study highlighted the importance of introducing nitrogen heterocyclic rings into betulinic acid in the discovery and development of novel antitumor agents.
Keywords: betulinic acid, BA-nitrogen heterocyclic derivatives, antitumor, Hela, flow cytometry
1. Introduction
Natural products play a major role in the antitumor drug discovery. Over 60% of antitumor drugs are developed from natural products [1]. Pentacyclic triterpenoids are a class of pharmacologically active and structurally rich natural products with privileged motifs for further modifications and structure–activity relationship analyses [2,3,4,5]. As a lupane-type pentacyclic triterpenoid, betulinic acid (3β-hydroxy-lup-20(29)-en-28-oic acid, BA, Figure 1) is widespread in many plants. It had been demonstrated that BA possessed various bioactivities, including antitumor, anti-HIV, anti-inflammatory, antiviral and antiseptic activities [6,7,8,9,10,11]. Since minimal toxicity against normal cells and antiproliferative activity against a panel of tumors [12], it was recognized as the leading compound of antitumor agents. Moreover, BA’s continuous structural modification had been an extremely attractive hot topic worldwide. It consisted of a 30-carbon skeleton which could be modified at three positions, the secondary hydroxyl group (C-3), the hydroxyl group (C-28) and at the alkene moiety (C-20), respectively. It was reported that C-28 carboxylic acid was essential for the cytotoxicity [9,13]. For example, 20, 29-dihydro betulinic acid derivatives were synthesized with IC50 less than 0.4 μg/mL [14]. BA derivatives modified at the C-3 position [4-nitrobenzyl-oximino] had shown IC50 values 0.4 μg/mL against the U-937 cells.
Figure 1.
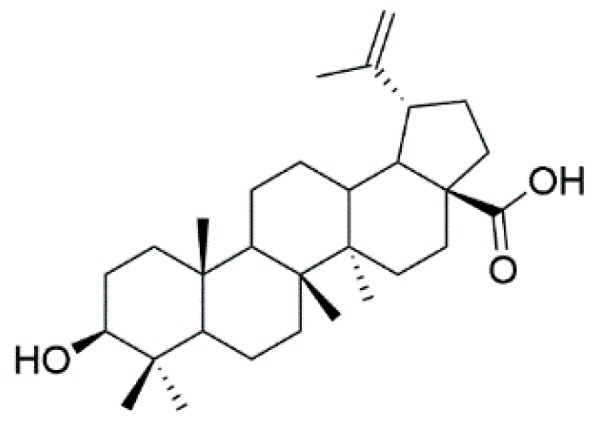
Chemical structure of BA.
In the last few years, nitrogen-containing heterocyclic derivatives had been synthesized as antitumor agents. For example, the incorporation of an imidazole scaffold at the C-28 or C-3 position of betulinic acid with ester or amide bonds could improve toxic activity significantly; and the majority of the novel compounds were particularly effective against the hepatoma HepG-2 (IC50 = 0.8, 1.7, 2.0 μM, respectively) cell line [15]. Eignerova Barbara [16] acetylated the 3 hydroxyl group of betulinic acid and piperidine, the introduced carbon chain connection on the 28 carboxyl group of which showed high and selective cytotoxicity (1.6 mM on G-361 cells). Other N-heterocyclic derivatives [17,18,19] had been reported to possess antiproliferative effects against tumor cell lines.
In the present study, a series of novel BA-nitrogen heterocyclic derivatives were designed and synthesized to introduce different nitrogen heterocycles into the 3, 28-hydroxyl of BA with the ester condensation reaction. Representative tumor cell lines were applied to evaluate the antitumor activities of these compounds. Cell morphology changes on Hela induced by compound 7e were observed by 4′,6-diamidino-2-phenylindole (DAPI) staining. Furthermore, fluorescence staining observations and flow cytometric analyses were performed to investigate the potential mechanism.
2. Results
2.1. Chemical Synthesis
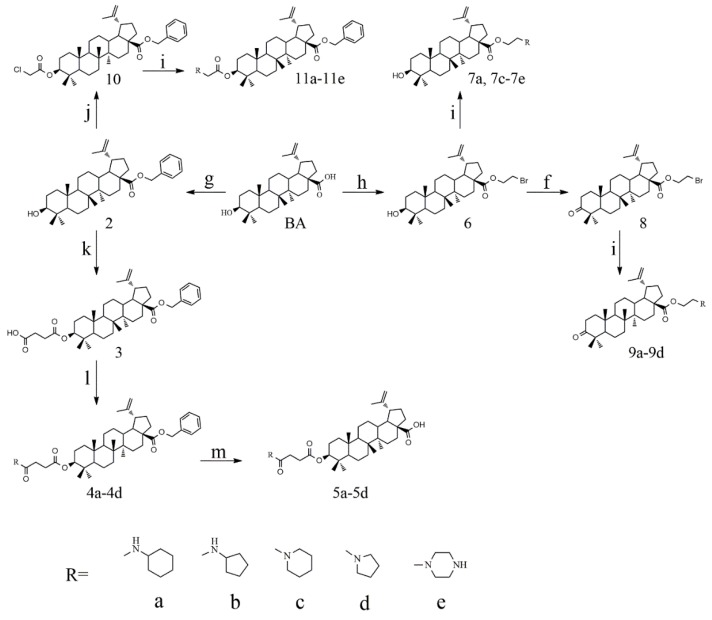
The syntheses of 21 BA-nitrogen heterocyclic derivatives were shown in Scheme 1. BA was treated with potassium carbonate solution and benzyl bromide/1, 2-dibromoethane in dimethylformamide (DMF) at 85 °C for 4 h to obtain compound 2 and 6. Then compound 2 was treated with succinic anhydride and chloroacetic acid in DCM at 80 °C for 5 h catalyzed by 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide/4-dimethylaminopyridine (CH3)2NC5H4N) (EDCI)/DMAP), and compounds 3 and 10 were obtained. By further substitution with nitrogen heterocyclic ring (R) or reduction reaction, we got the compounds 4a–4d, 5a–5d, 7a–7e and 11a–11e (Table 1). Compounds 9a–9b (Table 1) were obtained by an oxidation and substitution reaction, starting from compound 6. All BA-nitrogen heterocyclic derivatives were determined by 1H-NMR, 13C-NMR and HR-MS.
Scheme 1.
Synthesis routes of betulinic acid (BA)-nitrogen heterocyclic derivatives. Reagent: (f) chromic acid, acetone; (g) benzyl bromide, K2CO3, dimethylformamide (DMF); (h) 1, 2-dibromoethane, K2CO3, DMF; (i) nitrogen heterocyclic ring, K2CO3, DMF; (j) chloroacetic acid, 4-dimethylaminopyridine (DMAP, (CH3)2NC5H4N), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) and DCM; (k) succinic anhydride, DMAP, as well as dichloro-methane (DCM). (l) nitrogen heterocyclic ring, HoBt, where was thus used some EDCI, N,N-Diisopropylethylamine (DIPEA), DCM; (m) Pt/C, MeOH, H2.
Table 1.
The structures of 21 BA-nitrogen heterocyclic derivatives.
| Compound | Structure | Compound | Structure |
|---|---|---|---|
| 3 |
|
4a |
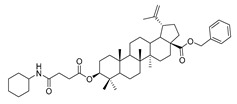
|
| 4b |
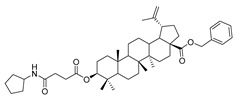
|
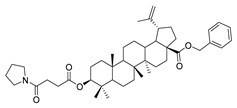
4c |
|
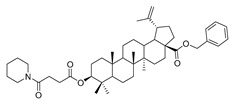
| 4d |
|
5a |
|
| 5b |
|
5c |
|
| 5d |
|
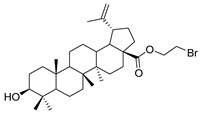
6 |
|
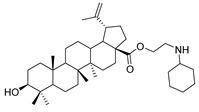
| 7a |
|
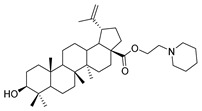
7c |
|
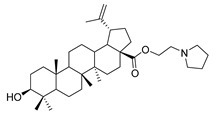
| 7d |
|
7e |
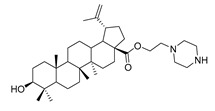
|
| 8 |
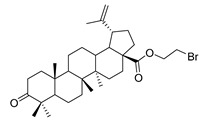
|
9a |
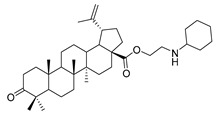
|
| 9b |
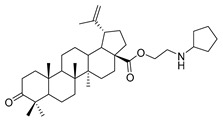
|
9c |
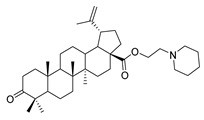
|
| 9d |
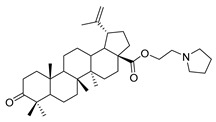
|
10 |
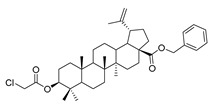
|
| 11a |
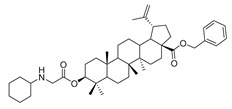
|
11b |
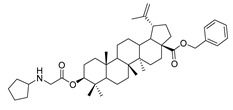
|
| 11c |
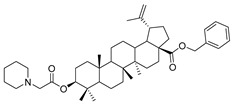
|
11d |
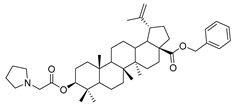
|
| 11e |
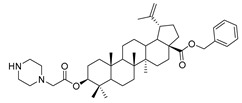
|
2.2. Cytotoxicity
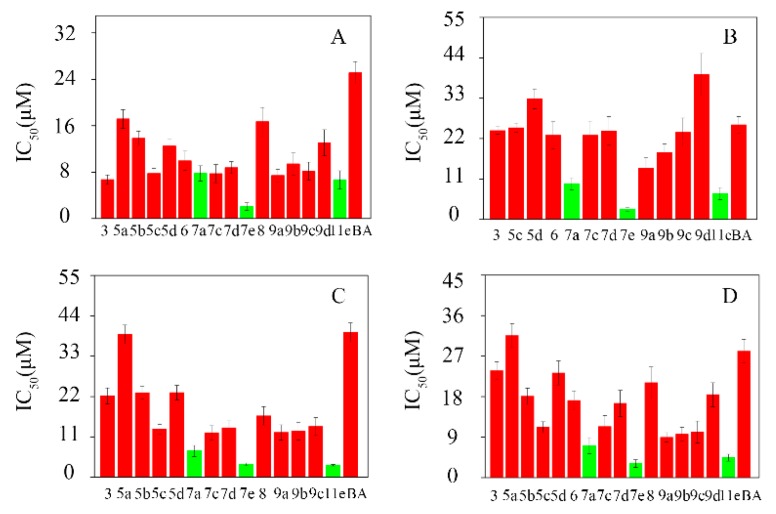
The in-vitro cytotoxicity of the BA-nitrogen heterocyclic derivatives was evaluated on four pathologic live cells (Hela, HepG-2, BGC-823 and SK-SY5Y) by MTT assays. As shown in Table 2, the IC50 of the derivatives exhibited better inhibitory activities against Hela, HepG-2, BGC-823 and SK-SY5Y compared to BA. In particular, compounds 7a, 7e and 11e showed stronger inhibitory effects against the four tumor cell lines than the rest of the compounds (Figure 2).
Table 2.
The in-vitro cytotoxicity of the BA-nitrogen heterocyclic derivatives.
| Compound | IC50 (μM) | |||
|---|---|---|---|---|
| Hela | HepG-2 | BGC-823 | SK-SY5Y | |
| 3 | 6.70 ± 0.80 | 24.19 ± 1.04 | 22.18 ± 2.17 | 23.75 ± 1.99 |
| 4a | >50 | >50 | >50 | >50 |
| 4b | >50 | >50 | >50 | >50 |
| 4c | >50 | >50 | >50 | >50 |
| 4d | >50 | >50 | >50 | >50 |
| 5a | 17.17 ± 1.61 | >50 | 38.94 ± 2.56 | 31.58 ± 2.65 |
| 5b | 13.82 ± 1.25 | >50 | 23.02 ± 1.74 | 18.07 ± 1.82 |
| 5c | 7.77 ± 0.88 | 24.96 ± 1.28 | 13.15 ± 1.32 | 11.19 ± 1.24 |
| 5d | 12.50 ± 1.27 | 32.83 ± 2.69 | 23.07 ± 1.98 | 23.27 ± 2.67 |
| 6 | 9.94 ± 1.67 | 22.92 ± 3.68 | >50 | 17.14 ± 2.15 |
| 7a | 7.78 ± 1.32 | 9.67 ± 1.69 | 7.18 ± 1.50 | 7.10 ± 1.77 |
| 7c | 7.73 ± 1.58 | 22.90 ± 3.87 | 12.14 ± 1.94 | 11.33 ± 2.47 |
| 7d | 8.78 ± 1.03 | 24.10 ± 3.91 | 13.50 ± 1.87 | 16.49 ± 2.92 |
| 7e | 2.05 ± 0.66 | 2.79 ± 0.53 | 3.52 ± 0.37 | 3.13 ± 0.84 |
| 8 | 16.70 ± 2.45 | >50 | 16.73 ± 2.51 | 21.09 ± 3.50 |
| 9a | 7.41 ± 1.08 | 13.97 ± 2.87 | 12.23 ± 2.08 | 8.94 ± 1.03 |
| 9b | 9.40 ± 1.89 | 18.19 ± 2.30 | 12.54 ± 2.38 | 9.63 ± 1.55 |
| 9c | 8.18 ± 1.55 | 23.73 ± 3.89 | 13.85 ± 2.41 | 10.12 ± 2.43 |
| 9d | 13.07 ± 2.27 | 39.42 ± 5.76 | >50 | 18.43 ± 2.68 |
| 10 | >50 | >50 | >50 | >50 |
| 11a | >50 | >50 | >50 | >50 |
| 11b | >50 | >50 | >50 | >50 |
| 11c | >50 | >50 | >50 | >50 |
| 11d | >50 | >50 | >50 | >50 |
| 11e | 6.65 ± 1.58 | 7.03 ± 1.66 | 3.28 ± 0.21 | 4.44 ± 0.78 |
| BA | 25.13 ± 1.92 | 25.74 ± 2.22 | 39.51 ± 2.59 | 28.10 ± 2.63 |
Figure 2.
The cytotoxicities of the BA-nitrogen heterocyclic derivatives to different tumor cells. (A): Hela; (B): HepG-2; (C): BGC-823; (D): SK-SY5Y.
In addition, it was observed that after introducing the nitrogen heterocycle into the 3-hydroxyl or 28-carboxyl of BA, it relatively improved their cytotoxicity. As shown in Table 2, the most promising was compound 7e, which showed higher cytotoxicity than BA. The IC50 of derivatives compound 7e were 2.05 ± 0.66 μM, 2.79 ± 0.53 μM, 3.52 ± 0.37 μM and 3.13 ± 0.84 μM against Hela, HepG-2, BGC-823 and SK-SY5Y, respectively. It was further verified that the small molecule nitrogen heterocycle could enhance BA’s bioactivity, which was in line with our previous report [20].
2.3. Cluster Analysis- Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
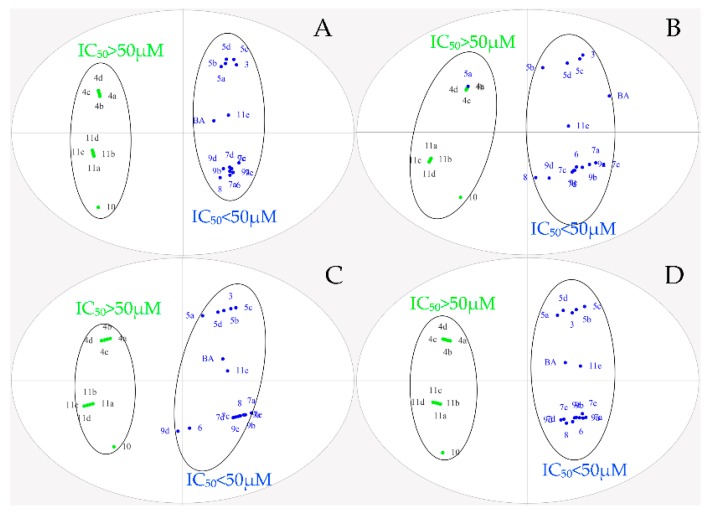
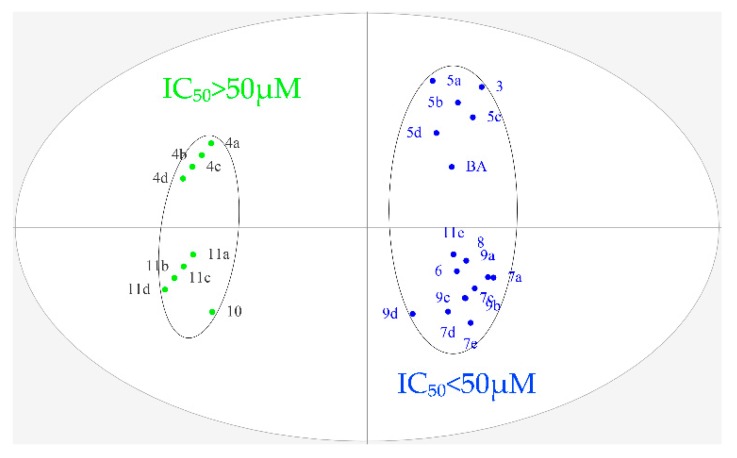
To further explore the structure–activity relationship, OPLS-DA was performed for all designed BA derivatives. Analyses revealed an antitumor activity discrimination between the different BA derivatives. As for the effect of the structure modification site and different nitrogen heterocyclic rings of BA, they were divided into two groups according to the difference in the in-vitro antitumor activity (Figure 3 and Figure 4). Through data analysis, we found that the structure modification site on BA showed a certain degree of regularity in their effect upon activity, the structural modification at positions C-3 and C-28 could improve antitumor biological activity in vitro, while the structural transformation of C-28 might have more potential to enhance cytotoxicity on the same series of tumor cells; for example, the antitumor activities of compounds 7a, 7c, 7d and 7e were stronger than compounds 5a, 5b, 5c and 5d. In addition, different nitrogen heterocyclic rings on BA also affected their activity (compound 11e > compound 11a, 11b, 11c and 11d). The cluster analysis of OPLS-DA might provide us with further directions for the further analysis of BA derivatives. All data were analyzed using SIMACA 13.0. Analysis showed no samples being outside the Hotelling T2 95% confidence ellipse that could influence the analyses, and high values of explained variation and predictive ability were obtained (Table 3). Besides, the values of explained variation and predictive ability were 0.883 and 0.978, respectively, according to OPLS-DA for the IC50 of four tumor cells shown in Figure 4.
Figure 3.
Orthogonal partial least squares discriminant analysis (OPLS-DA) of IC50 on different tumor cells. (A): Hela; (B): HepG-2; (C): BGC-823; (D): SK-SY5Y.
Figure 4.
Orthogonal partial least squares discriminant analysis (OPLS-DA) of IC50 on four tumor cells (Hela, HepG-2, BGC-823 and SK-SY5Y).
Table 3.
The evaluation of explained variation (R2X) and predictive ability (Q2 (cum)).
| Cell Types | R2X | Q2 (cum) |
|---|---|---|
| Hela | 0.742 | 0.987 |
| HepG-2 | 0.741 | 0.765 |
| BGC-823 | 0.766 | 0.910 |
| SK-SY5Y | 0.733 | 0.979 |
2.4. Morphological Analysis
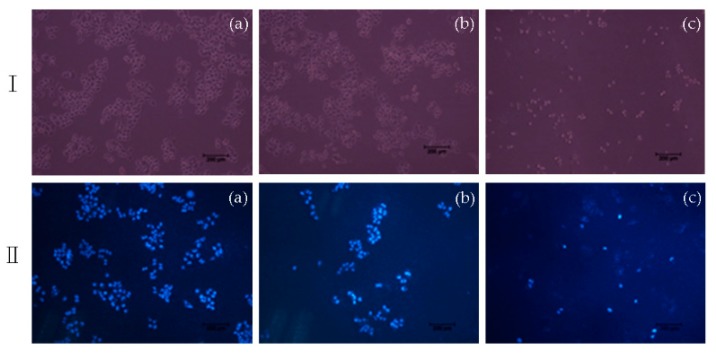
To characterize the effects of apoptosis induced by compound 7e on Hela, the nuclear morphological changes were observed with DAPI staining. After treating with compound 7e for 48 h, it can be seen from the results that the number of Hela cells was decreased sharply, and the cell space became larger significantly (Figure 5I); moreover, Hela cells showed nuclear morphological changes typical of apoptosis. As pictured in Figure 5II, in the control group, it appeared to have normal cellular morphology, the nucleus was intact, and the cells did not show the characteristics of apoptosis. When treated with BA, the number of cells was decreased, the contours of some cells became irregular, nuclear fragmentation was appeared, whereas compound 7e treatment caused a significant decrease in the number of cells, evident nuclear fragmentation, and did not see an intact nucleus. Thus, the results indicated that compound 7e could induce apoptosis in Hela cells.
Figure 5.
Cell morphology under fluorescence microscope (I) and DAPI (II) staining on Hela cells induced by compound 7e with 5 μM: (100 ×) (a) Control; (b) BA; (c) 7e.
2.5. Apoptosis Analysis Using Annexin V-FITC/Propidium Iodide (PI) Staining
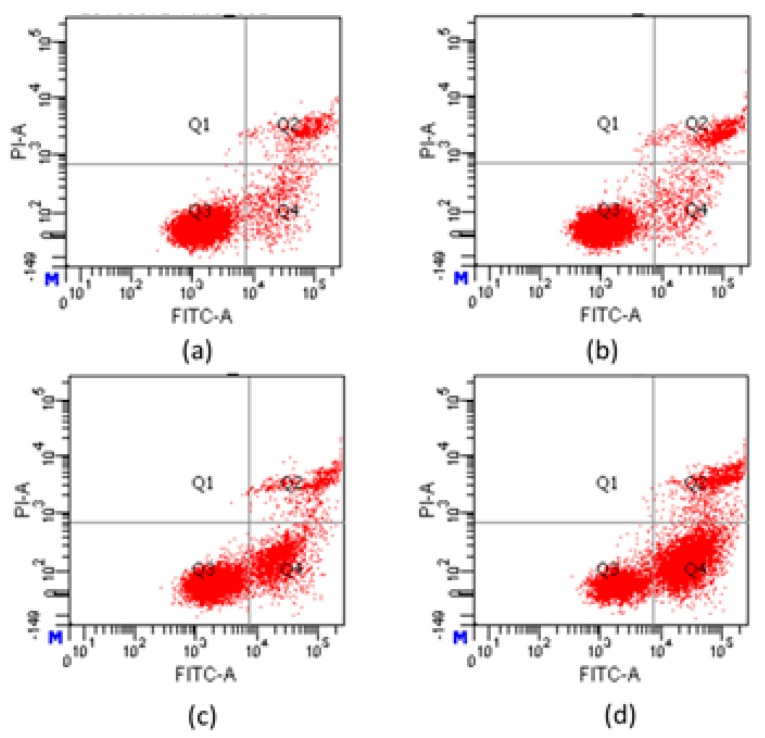
To evaluate the apoptosis induced by compound 7e and to further determine early apoptosis and secondary necrosis, apoptotic rates were analyzed by flow cytometry using an Annexin V-FITC/PI staining. As shown in Figure 6, when treated with different concentrations of compound 7e, the percentages (Q2 + Q4) of apoptotic Hela increased from 12.6% in control cells to 14.2%, 29.5% and 73.3%, respectively. Furthermore, the results indicated that compound 7e could induce Hela cells’ early apoptosis in a concentration-dependent manner. It speculated that compound 7e could induce Hela cells early apoptosis to an antitumor effect.
Figure 6.
Apoptosis analysis of Hela cells induced by compound 7e using AnnexinV-FITC/PI staining: (a) control group; (b) 1 μM; (c) 2 μM; (d) 4 μM.
3. Discussion
BA is widespread in natural plant and Chinese herbal medicine, used for the prevention and treatment of tumors, and there are large number of betulinic acid derivatives that have been synthesized [21,22,23,24]. In this report, a series of different BA-nitrogen heterocyclic derivatives were designed and synthesized to improve their biological activity and hydrophilicity. After introducing a different nitrogen heterocycle in the 3-hydroxyl/28- carboxyl of BA using the ester condensation reaction, the majority of these derivatives showed much stronger cytotoxic activity than BA.
In chemical synthesis, introducing succinic anhydride in the C-3 of BA was explored, and the reaction solvent was changed from THF to DCM. This reaction was simple, mild and controllable, with a yield of 79%, which was suitable for the synthesis of such compounds in the future. In the structure–activity relationship, we could easily find that the structural modification site of BA, and linked with different nitrogen heterocyclic rings on BA, had an effect on the antitumor activity of the BA derivatives in vitro. In general, as observation for compounds 7a, 7c, 7d, 7e and 5a–5d, structural modification at positions C-28 and C-3 could improve antitumor biological activity, and especially the structural transformation of C-28 might have more potential to enhance cytotoxicity on the same series of tumor cells; Besides, different nitrogen heterocyclic rings on BA also influenced their activity (compound 11e > compounds 11a, 11b, 11c, 11d), and the alkalinities of the different nitrogen heterocyclic rings were positively correlated with their activities, which might be likely associated with increasing bioavailability and altering an extracellular weak acidic microenvironment with further verification [25].
4. Materials and Methods
4.1. Materials and Instruments
Betulinic acid (Nanjing Jingzhu Bio-technology Co., Ltd., Nanjing, China), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI), (Bellen Chemistry Co., Ltd., Beijing, China), 4-dimethylaminopyridine (DMAP, which is (CH3)2NC5H4N), Cyclopentylamine, Cyclohexylamine, Pyrrolidine, Piperidine, Piperazine, Succinic anhydride, Benzyl bromide, Palladium, Chromium oxide, 1,2-dibromoethane (Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China), HOBt (Beijing Inno Chem Science and Technology Co., Ltd., Beijing, China) were more than 98%. All reagents were used without any further purification. Reagents of analytical reagent grade were purchased from the Beijing Chemical Plant (Beijing, China). Reactions were monitored by thin-layer chromatography (TLC) on precoated silica gel GF-254 plates (Qingdao Haiyang Chemical Co., Qingdao, China) and visualized in ultraviolet (UV) light (254 nm). Silica-gel column chromatography was performed using 200–300 mesh silica gel.
Hydrogen protonic nuclear magnetic resonance (1H-NMR) and Carbon-13 nuclear magnetic resonance (13C-NMR) assays were recorded on a Bruker AVANCE 500 NMR spectrometer (Fällanden, Switzerland). High-resolution mass spectra (HR-MS) were acquired using a Thermo Scientific TMLTQ Orbitrap XL hybrid FTMS instrument (Thermo Technologies, New York, NY, USA). Melting points were measured at a rate of 5 °C/min using an X-5 micro melting point apparatus (Beijing Tech Instrument Co., Ltd., Beijing, China). Cellular morphologies were observed using an inverted fluorescence microscope (Olympus IX71, Tokyo, Japan). Mechanisms of apoptosis were detected by flow cytometry (BD FACS Canto II, San Jose, CA, USA).
4.2. Chemical Syntheses
Benzyl lup-20(29)-en-28-oate (2). BA (3.00 g, 6.57 mmol) was dissolved in dimethylformamide (DMF) (200 mL), then benzyl bromide (1.12 g, 6.50 mmol), K2CO3 (2.72 g, 9.20 mmol) were added, and the mixture was stirred for 4 h at 85 °C. This reaction was monitored by TLC. The reaction solution was washed with water, filtered and evaporated with vacuum.
Benzyl 3β-(succinic anhydride)-lup-20(29)-en-28-oate (3). Benzyl lup-20(29)-en-28-oate (2) (3.00 g, 5.61 mmol), succinic anhydride (1.68 g, 16.83 mmol) and DMAP (1.37 g, 11.22 mmol) were dissolved in DCM, and then the mixture was refluxed and stirred for 8 h at 50 °C. After completion of the reaction, the crude product was extracted with DCM. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (10:1) as the eluent, then the product was lyophilized. White solid, 79.3% yield, 1H-NMR (500 MHz, CDCl3): δ 7.36-7.26 (m, 5H, –C6H5), 5.16–5.08 (m, 2H, –O–CH2–Ph), 4.72, 4.59 (brs, each, 1H, =CH2), 4.50–4.47 (m, 1H, –CH–O–), 2.68–2.66, 2.63–2.62 (m, each, 2H, –COO–CH2–CH2–COO–), 2.50–1.00 (28 H, methyl- and methylene- of BA), 1.67, 0.93, 0.82, 0.82, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 177.98 (–COOH), 175.97 (–COO–), 171.97 (–COO–), 150.68 (–CH=C), 109.77 (–CH=C), 81.71 (–OCOCH–), 65.86, 56.67, 55.56, 50.57, 49.56, 47.08, 42.52, 40.79, 38.49, 38.31, 37.97, 37.21, 37.07, 34.34, 32.23, 30.69, 29.69, 29.45, 29.14, 28.01, 25.61, 23.75, 21.02, 19.47, 18.29, 16.63, 16.30, 15.96, 14.78; benzene ring: 136.62, 128.62, 128.38, 128.19. m.p.: 153.6–155.4 °C. HR-MS (ESI) m/z: 647.4317 [M + H]+, calcd for: C41H59O6: 647.4233.
Compound 4a–4d. Benzyl 3β-(succinic anhydride)-lup-20(29)-en-28-oate (3) (0.30 g, 0.48 mmol), cyclohexylamine (63.36 g, 0.64 mmol)/cyclopentylamine (54.50 g, 0.64 mmol)/piperidine (54.50 g, 0.64 mmol)/pyrrolidine (48.07 g, 0.64 mmol), EDCI (122.69 g, 0.64 mmol), HoBt (86.86 g, 0.64 mmol) and DIPEA (82.72 g, 0.64 mmol) were dissolved in 10 mL dry DCM, the reaction mixture was stirred for 4 h at room temperature. After completion of the reaction, the crude product was extracted with DCM. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (8:1) as eluent, the product was lyophilized.
Benzyl 3β-4-cyclohexylamino-succinic anhydride)-lup-20(29)-en-28-oate (4a). White solid, 85.3% yield, 1H-NMR (500 MHz, CDCl3): δ 7.36–7.26 (m, 5H, –C6H5), 5.15- 5.10 (m, 2H, –O–CH2–Ph), 4.71, 4.59 (brs, each, 1H, =CH2), 4.49–4.46 (m, 1H, –CH–O–CO–), 3.03–2.98 (m, 1H, –N–CH–(CH2)2–), 2.67–2.63, 2.45–2.42 (m, each, 2H, –COO–CH2–CH2–COO–), 2.50–1.00 (38 H, methyl- and methylene- of BA and cyclohexane), 1.67, 0.93, 0.81, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 175.83 (–COO–), 172.91 (–COO–), 170.52 (–CO–NH–), 150.58 (–CH=C), 109.65 (–CH=C), 81.38 (–OCOCH–), 65.74 (–O–CH2–Ph), 56.56, 55.45, 50.48, 49.46, 48.21, 46.97, 42.40, 40.68, 38.40, 38.19, 37.88, 37.10, 36.96, 34.24, 33.14 (–N–CH2–C), 32.12, 31.52, 30.59, 30.23, 29.57, 28.00, 26.94, 25.56, 25.51, 24.83, 23.72, 20.91, 19.37, 18.18, 16.56, 16.20, 15.84, 14.66; benzene ring: 136.51, 128.51, 128.27, 128.08. m.p.: 145.5–147.8 °C. HR-MS (ESI) m/z: 728.5244 [M + H]+, calcd for: C41H59O6: 728.5176.
Benzyl 3β-(4-cyclohexylamine-succinic anhydride)-lup-20(29)-en-28-oate (4b). White solid, 82.8% yield, 1H-NMR (500 MHz, CDCl3): δ 7.36–7.26 (m, 5H, –C6H5), 5.16–5.07 (m, 2H, –O–CH2–Ph), 4.72, 4.59 (brs, each, 1H, =CH2), 4.49–4.46 (m, 1H, –CH–O–CO–), 3.04–2.99 (m, 1H, –N–CH–(CH2)2–), 2.65–2.64, 2.44–2.42 (m, each, 2H, –COO–CH2–CH2–COO–), 2.50–1.00 (36 H, methyl- and methylene- of BA and cyclopentylamine), 1.67, 0.93, 0.81, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 175.95 (–COO–), 173.04 (–COO–), 171.14 (–CO–NH–), 150.70 (–CH=C), 109.76 (–CH=C), 81.52 (–OCOCH–), 65.86, 56.67, 55.56, 51.35, 50.60, 49.57, 47.08, 42.52, 40.79, 38.51, 38.31, 37.99, 37.22, 37.07, 34.35, 33.25, 33.23, 32.24, 31.55, 30.70, 30.34, 29.69, 28.11, 25.63, 23.83, 21.02, 19.48, 19.33, 18.30, 16.67, 16.31, 15.96, 14.77, 13.88; benzene ring: 136.63, 128.62, 128.38, 128.19. m.p.: 147.2-149.6 °C. HR-MS (ESI) m/z: 714.5098 [M + H]+, calcd for: C41H59O6 714.5019.
Benzyl 3β-(4-pyrrolidine-succinic anhydride)-lup-20(29)-en-28-oate (4c). White solid, 78.8% yield, 1H-NMR (500 MHz, CDCl3): δ 7.36-7.26 (m, 5H, –C6H5), 5.13, −5.10 (m, 2H, –O–CH2–Ph), 4.71 (brs, each, 1H, =CH2), 4.59, 4.48–4.45 (m, 1H, –CH–O–CO–), 2.68–2.56 (m, 4H, –COO–CH2–CH2–COO–), 2.50–1.00 (36 H, methyl- and methylene- of BA and piperdine), 1.67, 0.93, 0.83, 0.81, 0.75 (s, each, 3H, 5 × -CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 175.95 (–COO–), 173.10 (–COO–), 169.93 (–CO–NH–), 150.69 (–CH=C), 109.76 (–CH=C), 81.19 (–OCOCH–), 65.85, 56.67, 55.58, 50.58, 49.57, 47.09, 42.52, 40.79, 38.51, 38.32, 37.98, 37.21, 37.07, 34.36, 32.24, 30.70, 29.69, 29.64, 29.56, 28.10, 26.20, 25.63, 23.80, 21.02, 19.47, 18.29, 16.63, 16.30, 15.95, 14.79; benzene ring: 136.63, 128.62, 128.37, 128.18. m.p.: 135.0–138.6 °C. HR-MS (ESI) m/z: 714.5079 [M + H]+, calcd for: C41H59O6 714.5019.
Benzyl 3β-(4- piperidine -succinic anhydride)-lup-20(29)-en-28-oate (4d). White solid, 80.8% yield, 1H-NMR (500 MHz, CDCl3): δ 7.36–7.26 (m, 5H, –C6H5), 5.13–5.10 (m, 2H, –O–CH2–Ph), 4.72 (brs, each, 1H, =CH2), 4.59, 4.48–4.45 (m, 1H, –CH–O–CO–), 2.64–2.62, 2.62–2.59 (–COO–CH2–CH2–COO), 2.50–1.00 (34 H, methyl- and methylene- of BA and pyrrolidine), 1.67, 0.93, 0.83, 0.82, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 175.94 (-COO-), 173.09 (-COO-), 169.53 (–CO–NH–), 150.68 (–CH=C), 109.75 (–CH=C), 81.09 (–OCOCH–), 65.84, 56.66, 55.58, 50.57, 49.56, 47.08, 42.51, 40.79, 38.50, 38.31, 37.98, 37.21, 37.06, 34.35, 32.23, 30.69, 29.98, 29.68, 28.19, 28.08, 26.45, 25.62, 23.79, 21.01, 19.46, 18.28, 16.68, 16.29, 15.95, 14.78; benzene ring: 136.62, 128.61, 128.37, 128.17. m.p.: 132.1–135.4 °C. HR-MS (ESI) m/z: 700.4936 [M + H]+, calcd for: C41H59O6 700.4863.
Compound 5a–5d. Compound 4a/4b/4c/4d (0.20 g, 0.46 mmol) was dissolved in dry MEOH, then suitable palladium carbon was added. The reaction mixture was stirred overnight in methanol in a hydrogen atmosphere. The reaction was monitored by TLC [petroleum ether–acetone (4:1)]. After filtering the palladium carbon and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether-acetone (8:1) as eluent, the product was lyophilized.
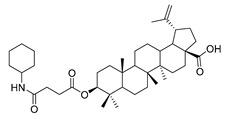
3β-(4-cyclohexylamino- succinic anhydride)-lup-20(29)-en-28-oate (5a). White solid, 85.8% yield, 1H-NMR (500 MHz, CDCl3): δ 4.72, 4.59 (brs, each, 1H, =CH2), 4.46–4.50 (m, 1H, –CH–O–CO–), 3.02–2.97 (m, 1H, –N–CH–(CH2)2–), 2.65–2.63, 2.45–2.42 (m, each, 2H, –COO–CH2–CH2–COO–), 2.50–1.00 (38 H, methyl- and methylene- of BA and cyclohexane), 1.68, 0.96, 0.92, 0.83, 0.81 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 180.79 (–COOH), 173.13 (–COO–), 170.87 (–CO–NH–), 150.61 (–CH=C), 109.80 (–CH=C), 81.58 (–OCOCH–), 56.44, 55.55, 50.92, 50.54, 49.37, 48.38, 47.06, 42.55, 40.82, 38.48, 37.99, 37.23, 37.19, 34.36, 33.18, 32.31, 31.60, 30.70, 30.35, 29.81, 28.10, 25.64, 25.58, 24.91, 23.81, 21.00, 19.47, 18.28, 16.64, 16.29, 16.15, 14.77. m.p.: 191.3–193.0 °C. HR-MS (ESI) m/z: 638.4773 [M + H]+, calcd for: C41H59O6 638.4706.
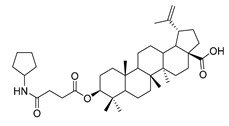
3β-(4-cyclohexylamine- succinic anhydride)-lup-20(29)-en-28-oate (5b). White solid, 87.2% yield, 1H-NMR (500 MHz, CDCl3): δ 4.73, 4.60 (brs, each, 1H, =CH2), 4.49–4.46 (m, 1H, –CH–O–CO–), 3.02–2.97 (m, 1H, –N–CH–(CH2)2–), 2.69–2.67, 2.58–2.56 (m, each, 2H, –COO–CH2–CH2–COO–), 2.50–1.00 (36 H, methyl- and methylene- of BA and cyclopentylamine), 1.68, 0.96, 0.92, 0.84, 0.82 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 181.68 (–COOH), 173.10 (–COO–), 170.17 (–CO–NH–), 150.57 (–CH=C), 109.84 (–CH=C), 81.25 (–OCOCH–), 56.50, 55.58, 50.53, 49.39, 47.06, 46.72, 42.56, 40.83, 38.52, 37.99, 37.54, 37.25, 34.37, 32.30, 31.07, 30.70, 29.83, 29.65, 29.58, 28.11, 26.18, 25.59, 24.55, 23.80, 20.99, 19.48, 18.28, 16.62, 16.29, 16.16, 14.81. m.p.: 247.3–249.6 °C. HR-MS (ESI) m/z: 624.4664 [M + H]+, calcd for: C41H59O6 624.4550.
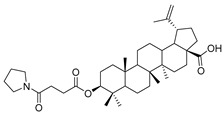
3β-(4-pyrrolidine-succinic anhydride)-lup-20(29)-en-28-oate (5c). White solid, 89.8% yield, 1H-NMR (500 MHz, CDCl3): δ 4.73, 4.60 (brs, each, 1H, =CH2), 4.50–4.47 (m,1H,–CH–O–CO–), 3.03–2.97 (m, 4H, –N–(CH2)2–(CH2)2), 2.67–2.64 (m, 4H, –COO–CH2–CH2–COO–), 2.50–1.00 (34 H, methyl- and methylene- of BA and pyrrolidine), 1.69, 0.96, 0.93, 0.84, 0.82 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 181.67 (–COOH), 173.10 (–COO–), 171.31 (–CO–NH–), 150.56 (–CH=C), 109.85 (–CH=C), 81.56 (–OCOCH–), 56.49, 55.56, 51.38, 50.55, 49.39, 47.07, 42.56, 40.84, 38.52, 38.01, 37.25, 37.20, 34.37, 33.99, 33.23, 33.22, 32.30, 31.55, 30.71, 30.35, 29.83, 28.12, 25.59, 23.84, 21.00, 19.49, 18.29, 16.66, 16.31, 16.17, 14.80. m.p.: 231.4–233.8 °C. HR-MS (ESI) m/z: 624.4611 [M + H]+, calcd for: C41H59O6 624.4550.
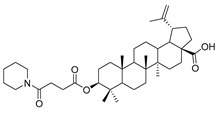
3β-(4- piperidine -succinic anhydride)-lup-20(29)-en-28-oate (5d). White solid, 88.6% yield, 1H-NMR (500 MHz, CDCl3): δ 4.73, 4.60 (brs, each, 1H, =CH2), 4.5–4.47 (m,1H,–CH–O–CO–), 3.03–2.97 (m,4H,–N–(CH2)2–(CH2)2), 2.67–2.64, 2.45–2.43 (m, each, 2H,–COO–CH2–CH2–COO–), 2.50–1.00 (32 H, methyl- and methylene- of BA and piperdine), 1.69, 0.96, 0.93, 0.84, 0.82 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 181.67 (–COOH), 173.10 (–COO–), 171.31 (–CO–NH–), 150.56 (–CH=C), 109.85 (–CH=C), 81.56 (–OCOCH–), 56.49, 55.56, 51.38, 50.55, 49.39, 47.07, 42.56, 40.84, 38.52, 38.01, 37.25, 37.20, 34.37, 33.23, 33.22, 32.30, 31.55, 30.71, 30.35, 29.83, 28.12, 25.59, 23.84, 21.00, 19.49, 18.29, 16.66, 16.31, 16.17, 14.80. m.p.: 238.2–240.7 °C. HR-MS (ESI) m/z: 624.4611 [M + H]+, calcd for: C41H59O6 624.4550.
1-bromopropane lup-20(29)-en-28-oate (6). BA (8.00 g, 17.52 mmol) was dissolved in DMF (300 mL), and then 1, 2-dibromoethane (9.80 g, 52.56 mmol) and K2CO3 (4.84 g, 35.04 mmol) were added, and the mixture was stirred for 2 h at room temperature. Reaction was monitored by TLC [petroleum ether–acetone (5:1)]. After completion of the reaction, the crude product was extracted with EtOAc. After drying, the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (50:1) as eluent, the product was lyophilized. White solid, 48.7% yield, 1H-NMR (400 MHz, CDCl3): δ 4.73, 4.60 (brs, each, 1H, =CH2), 4.42–4.38, 3.55–3.52 (m, each, 2H, –CO–CH2–CH2–Br), 3.20–3.16 (m, 1H, –(CH2)2–CH–OH), 2.50–1.00 (28 H, methyl- and methylene- of BA), 1.68, 0.96, 0.91, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.87 (–COO–), 150.58 (–CH=C), 109.82 (–CH=C), 79.11 (CH–OH), 63.48, 56.83, 55.49, 50.69, 49.56, 47.09, 42.55, 40.88, 39.00, 38.87, 38.48, 37.33, 37.13, 34.45, 32.20, 30.73, 29.82, 29.30, 28.12, 27.55, 25.67, 21.03, 19.51, 18.43, 16.27, 16.14, 15.50, 14.86. m.p.: 194.2–196.7 °C. HR-MS (ESI) m/z: 563.3105 [M + H]+, calcd for: C32H52BrO3 563.3022.
1-bromopropane 3-oxolup-20(29)-en-28-oate (8). 1-bromopropane lup-20(29)-en-28-oate (6) (2.00 g, 3.56 mmol) was dissolved in acetone (150 mL), then chromic acid was added uniformly to the acetone solution, and the mixture was stirred for 1 h at 0 °C. Reaction was monitored by TLC [petroleum ether–acetone (5:1)]. After completion of the reaction, the crude product was extracted with EtOAc. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the product was lyophilized. White solid, 90.6% yield, 1H-NMR (400 MHz, CDCl3): δ 4.74, 4.61 (brs, each, 1H, =CH2). 4.38–4.43, 3.52–3.55 (m, each, 2H, –CO–CH2–CH2–Br), 1.00–2.50 (28 H, methyl- and methylene- of BA), 1.68, 1.06, 0.98, 0.96, 0.92 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 218.28 (C=O), 175.84 (–COO–), 150.50 (–CH=C), 109.88 (–CH=C), 63.51, 56.81, 55.12, 50.05, 49.49, 47.48, 47.06, 42.61, 40.83, 39.78, 38.56, 37.11, 37.05, 34.29, 33.75, 32.14, 30.71, 29.80, 29.32, 26.76, 25.68, 21.56, 21.17, 19.78, 19.51, 16.10, 15.96, 14.77. m.p.: 140.9–142.6 °C. HR-MS (ESI) m/z: 561.2958 [M + H]+, calcd for: C32H50BrO3 561.2865.
Benzyl 3β-(2-chloroacetic acid)-lup-20(29)-en-28-oate (10). Benzyl lup-20(29)-en-28-oate (2) (3.00 g, 5.61 mmol), chloroacetic acid (1.06 g, 11.22 mmol) and DMAP (1.37 g, 11.22 mmol) was dissolved in DCM, then EDCI (2.15 g, 11.22 mmol) was added after 5 min. Reaction was monitored by TLC [petroleum ether–acetone (5:1)]. After completion of the reaction, the crude product was extracted with 10% HCl three times, washing with water three times subsequently. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether-acetone (10:1) as eluent, the product was lyophilized. White solid, 80.2% yield, 1H-NMR (400 MHz, CDCl3): δ 7.30–7.37 (m, 5H, –C6H5), 5.07–5.16 (m, 2H, –O–CH2–Ph), 4.72, 4.60 (brs, each, 1H, =CH2), 4.53–4.57 (m, 1H, –CH–O–), 4.00–4.08 (m, 2H, Cl–CH2–CO–), 1.00–2.50 (28 H, methyl- and methylene- of BA), 1.68, 0.94, 0.86, 0.85, 0.76 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.94 (–COO–), 167.27 (–COO–), 150.67 (–CH=C), 109.78 (–CH=C), 83.53 (C–OH), 65.87, 56.69, 55.53, 50.60, 49.59, 47.10, 42.55, 41.39, 40.82, 38.47, 38.32, 38.16, 37.23, 37.07, 34.35, 32.25, 30.72, 29.70, 28.07, 25.62, 23.70, 21.05, 19.49, 18.28, 16.56, 16.32, 15.98, 14.78. benzene ring: 136.64, 128.63, 128.39, 128.19. m.p.: 144.7–146.4 °C. HR-MS (ESI) m/z: 623.3882 [M + H]+, calcd for: C33H60ClO4 623.3789.
Compound 7a–7e. 1-bromopropane lup-20(29)-en-28-oate (6) (0.30 g, 0.53 mmol) and cyclohexylamine (262.35 g, 2.65 mmol)/piperidine (135.15 g, 1.59 mmol)/pyrrolidine (11.08 g, 1.59 mmol)/piperazine (54.50 g, 0.64 mmol) were dissolved in 20 mL DMF, then K2CO3 (146.50 g, 1.06 mmol) was added after 5 min. The reaction mixture was stirred at room temperature overnight. After completion of the reaction, the crude product was extracted with EtOAc. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (50:3) as eluent, the product was lyophilized.
N-propylcyclohexanamine lup-20(29)-en-28-oate (7a). White solid, 35.8% yield, 1H-NMR (400 MHz, CDCl3): δ 4.72, 4.59 (brs, each, 1H, =CH2), 4.24–4.14, 2.90–2.87 (m, each, 2H, –CO–CH2–CH2–Br), 3.20–3.16 (m, 1H, –(CH2)2–CH–OH), 2.50–1.00 (38 H, methyl- and methylene- of BA and cyclohexane), 1.68, 0.96, 0.91, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 176.17 (–COO–), 150.63 (–CH=C), 109.78 (–CH=C), 79.10 (CH–OH), 63.79, 56.76, 56.52, 55.49, 50.67, 49.54, 47.24, 45.53, 42.57, 40.83, 39.00, 38.85, 38.49, 37.32, 34.47, 33.74, 33.69, 32.41, 30.79, 29.82, 28.12, 27.54, 26.23, 25.67, 25.12, 21.02, 19.51, 18.42, 16.24, 16.21, 15.50, 14.84. m.p.: 119.0–121.8 °C. HR-MS (ESI) m/z: 582.4917 [M + H]+, calcd for: C38H64NO3 582.4808.
1-propylpiperidine lup-20(29)-en-28-oate (7c). White solid, 53.3% yield, 1H-NMR (400 MHz, CDCl3): δ 4.72, 4.59 (brs, each, 1H, =CH2), 4.28–4.20, 3.04–2.98 (m, each, 2H, –CO–CH2–CH2–N–), 3.19–3.15 (m, 1H, –(CH2)2–CH–OH), 2.50–1.00 (38 H, methyl- and methylene- of BA and pyrrolidine), 1.68, 0.96, 0.91, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 176.16 (–COO–), 150.82 (–CH=C), 109.67 (–CH=C), 79.12 (CH–OH), 61.60, 57.58, 56.67, 55.51, 54.89, 50.71, 49.55, 47.11, 42.56, 40.88, 39.01, 38.88, 38.40, 37.35, 37.18, 34.50, 32.32, 30.78, 29.80, 28.13, 27.57, 26.13, 25.70, 24.35, 21.05, 19.53, 18.46, 16.27, 16.21, 15.50, 14.84. m.p.: 138.6–140.5 °C. HR-MS (ESI) m/z: 568.4743 [M + H]+, calcd for: C37H62NO3 568.4651.
1-propylpyrrolidine lup-20(29)-en-28-oate (7d). White solid, 58.1% yield, 1H-NMR (400 MHz, CDCl3): δ 4.72, 4.59 (brs, each, 1H, =CH2), 4.25–4.21, 3.01–2.97 (m, each, 2H, –CO–CH2–CH2–N–), 3.19–3.15 (m, –OH), 2.50–1.00 (36 H, methyl- and methylene- of BA and piperdine), 1.67, 0.95, 0.91, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100MHz, CDCl3): δ 176.06 (–COO–), 150.75 (–CH=C), 109.68 (–CH=C), 79.10 (CH–OH), 62.72, 56.64, 55.50, 54.56, 54.45, 50.71, 49.56, 47.05, 42.54, 40.86, 39.00, 38.87, 38.35, 37.33, 37.12, 34.48, 32.26, 30.73, 29.79, 28.13, 27.55, 25.69, 23.68, 21.03, 19.52, 18.44, 16.27, 16.16, 15.50, 14.83. m.p.: 160.7–162.4 °C. HR-MS (ESI) m/z: 554.4544 [M + H]+, calcd for: C36H59NO3 554.4495.
1-propylpiperazine lup-20(29)-en-28-oate (7e). White solid, 30.2% yield, 1H-NMR (400 MHz, CDCl3): δ 4.71, 4.59 (brs, each, 1H, =CH2), 4.19–4.22, 2.91–3.01, (m, each, 2H, –CO–CH2–CH2–N–), 3.16–3.19 (m, 1H, –(CH2)2–CH–OH), 2.62–2.64 (m, 4H, NH–(CH2)2–), 1.00–2.50 (32 H, methyl- and methylene- of BA and piperazine). 1.67, 0.95, 0.90, 0.81, 0.74 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 176.07 (–COO–), 150.62 (–CH=C), 109.77 (–CH=C), 79.08 (CH–OH), 60.87, 57.09, 56.67, 55.49, 52.50, 50.67, 49.50, 47.10, 44.98, 42.54, 40.85, 38.99, 38.85, 38.37, 37.33, 37.15, 34.49, 32.25, 30.73, 29.77, 28.13, 27.54, 25.65, 21.03, 19.49, 18.43, 16.27, 16.22, 15.51, 14.82. m.p.: 197.9–199.2 °C. HR-MS (ESI) m/z: 569.4687 [M + H]+, calcd for: C36H61N2O3 569.4604.
Compound 9a–9d. 1-bromopropane 3-oxolup-20(29)-en-28-oate (8) (0.30 g, 0.53 mmol) and cyclohexylamine (262.35 g, 2.65 mmol)/cyclopentylamine (225.25 g, 2.65 mmol)/piperidine (135.15 g, 1.59 mmol)/pyrrolidine (113.08 g, 1.59 mmol)/piperazine (341.11 g, 3.18 mmol) were dissolved in 20 mL DMF, then K2CO3 (146.50 g, 1.06 mmol) was added after 5 min. The reaction mixture was stirred at room temperature overnight. Reaction was monitored by TLC [petroleum ether–acetone (5:1)]. After completion of the reaction, the crude product was extracted with EtOAc. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (25:1) as eluent, the product was lyophilized.
N-propylcyclohexanamine 3-oxolup-20(29)-en-28-oate (9a). White solid, 38.4% yield, 1H-NMR (400 MHz, CDCl3): δ 4.73, 4.60 (brs, each, 1H, =CH2), 2.88–2.92, 4.17–4.29 (m, each, 2H, –CO–CH2–CH2–N–), 2.47–2.52 (m, 1H, –N–CH–(CH2)2), 1.00–2.50 (38 H, methyl- and methylene- of BA and cyclohexane), 1.68, 1.06, 1.02, 0.97, 0.92 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 218.23 (C=O), 176.15 (–COO-), 150.55 (–CH=C), 109.86 (–CH=C), 63.67, 56.74, 56.59, 55.16, 50.05, 49.50, 47.49, 47.19, 45.44, 42.65, 40.81, 39.78, 38.56, 37.20, 37.06, 34.30, 33.80, 33.50, 32.33, 30.77, 29.81, 26.74, 26.18, 25.69, 25.09, 21.56, 21.19, 19.78, 19.52, 16.07, 16.03, 14.77. m.p.: 142.8–144.1 °C. HR-MS (ESI) m/z: 580.4701 [M + H]+, calcd for: C38H62NO3 580.4651.
N-propylcyclopentanamine 3-oxolup-20(29)-en-28-oate (9b). White solid, 35.2% yield, 1H-NMR (400 MHz, CDCl3): δ 4.73, 4.61 (brs, each, 1H, =CH2), 4.29–4.32, 2.93–3.05 (m, each, 2H, –CO–CH2–CH2–N–), 2.40–2.49 (m, 1H, –N–CH–(CH2)2), 1.00–2.50 (36 H, methyl- and methylene- of BA and cyclopentylamine), 1.68, 1.07, 1.02, 0.97, 0.92 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 218.23 (C=O), 176.08 (–COO–), 150.40 (–CH=C), 109.95 (–CH=C), 65.72, 59.63, 56.73, 55.14, 50.05, 49.50, 47.49, 47.05, 46.45, 42.62, 40.80, 39.78, 38.49, 37.06, 34.29, 33.78, 32.10, 30.72, 30.68, 29.85, 26.75, 25.66, 24.09, 21.56, 21.19, 19.78, 19.50, 19.34, 16.10, 15.98, 14.76, 14.27, 13.88. m.p.: 145.0–147.9 °C. HR-MS (ESI) m/z: 566.4566 [M + H]+, calcd for: C37H60NO3 566.4495
1-propylpiperidine 3-oxolup-20(29)-en-28-oate (9c). White solid, 56.6% yield, 1H-NMR (400 MHz, CDCl3): δ 4.72, 4.59 (brs, each, 1H, =CH2), 4.20–4.29, 2.98–3.07 (m, each, 2H, –CO–CH2–CH2–N–), 1.00–2.50 (38 H, methyl- and methylene- of BA and pyrrolidine), 1.68, 1.06, 1.01, 0.96, 0.92 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 218.27 (C=O), 176.10 (–COO–), 150.72 (–CH=C), 109.73 (–CH=C), 61.58, 57.56, 56.63, 55.12, 54.88, 50.05, 49.46, 47.47, 47.06, 42.60, 40.81, 39.77, 38.45, 37.13, 37.05, 34.29, 33.78, 32.23, 30.73, 29.76, 26.75, 26.09, 25.69, 24.31, 21.56, 21.17, 19.79, 19.52, 16.09, 16.00, 14.75. m.p.: 135.0–137.4 °C. HR-MS (ESI) m/z: 566.4595 [M + H]+, calcd for: C37H60NO3 566.44949.
1-propylpyrrolidine 3-oxolup-20(29)-en-28-oate (9d). White solid, 59.0% yield, 1H-NMR (400 MHz, CDCl3): δ 4.72, 4.60 (brs, each, 1H, =CH2), 4.20–4.32, 2.99–3.06 (m, each, 2H, –CO–CH2–CH2–N–),1.00–2.50 (38 H, methyl- and methylene- of BA and pyrrolidine), 1.68, 1.06, 1.01, 0.97, 0.92 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 218.28 (C=O), 176.05 (–COO–), 150.70 (–CH=C), 109.74 (–CH=C), 56.62, 55.13, 54.67, 54.56, 50.07, 49.49, 47.48, 47.03, 42.61, 40.80, 39.78, 38.43, 37.11, 37.05, 34.29, 33.78, 32.21, 30.72, 29.76, 26.76, 25.70, 23.72, 21.57, 21.17, 19.79, 19.52, 16.10, 15.97, 14.75. m.p.: 120.1–122.6 °C. HR-MS (ESI) m/z: 552.4468 [M + H]+, calcd for: C36H58NO3 552.4338.
Compound 11a–11e. Benzyl 3β-(2-chloroacetic acid)-lup-20(29)-en-28-oate (10) (0.30 g, 0.48 mmol) and cyclohexylamine (237.60 g, 2.40 mmol)/cyclopentylamine (204.00 g, 2.40 mmol)/piperidine (122.40 g, 1.44 mmol)/pyrrolidine (102.41 g, 1.44 mmol)/piperazine (248.08 g, 2.88 mmol) were dissolved in 20 mL DMF, then K2CO3 (132.68 g, 0.96 mmol) was added after 5 min. The reaction mixture was stirred at room temperature overnight. Reaction was monitored by TLC [petroleum ether–acetone (5:1)]. After completion of the reaction, the crude product was extracted with EtOAc. After drying the organic layer over anhydrous Na2SO4 and evaporating the solvent under vacuum, the crude product was separated by flash chromatography with petroleum ether–acetone (100:3) as eluent, the product was lyophilized.
Benzyl 3β-cyclohexylglycine-lup-20(29)-en-28-oate (11a). White solid, 35.1% yield, 1H-NMR(400 MHz, CDCl3): δ 7.26–7.36 (m, 5H, -C6H5), 5.07–5.19 (m, 2H, -O-CH2-Ph), 4.72, 4.59 (brs, each, 1H, =CH2), 4.50–4.54 (m, 1H, –CH–O–), 3.42–3.46 (m, 2H, –NH–CH2–CO–), 1.00–2.50 (38 H, methyl- and methylene- of BA and cyclohexane), 1.67, 0.93, 0.82, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.93 (–COO–), 172.75 (–COO–), 150.65 (–CH=C), 109.76 (–CH=C), 81.66 (C–OH), 65.84, 56.66, 56.56, 55.53, 50.57, 49.55, 48.51, 47.07, 42.51, 40.78, 38.48, 38.29, 37.97, 37.20, 37.05, 34.33, 33.37, 32.22, 31.05, 30.69, 29.82, 29.67, 28.12, 25.60, 24.96, 23.85, 21.01, 19.46, 18.28, 16.65, 16.30, 15.95, 14.76. benzene ring: 136.61, 128.60, 128.36, 128.17. m.p.: 129.1–131.4 °C. HR-MS (ESI) m/z: 686.5148 [M + H]+, calcd for: C45H68NO4 686.50701.
Benzyl 3β-cyclopentylglycine-lup-20(29)-en-28-oate (11b). White solid, 39.4% yield, 1H-NMR (400 MHz, CDCl3): δ 7.26–7.36 (m, 5H, –C6H5), 5.07–5.16 (m, 2H, –O–CH2–Ph), 4.72, 4.59 (brs, each, 1H, =CH2), 4.51–4.55 (m, 1H, –CH–O–), 3.38–3.42 (m, 2H, –NH–CH2–CO–), 1.00–2.50 (36 H, methyl- and methylene- of BA and cyclopentylamine), 1.68, 0.94, 0.83, 0.82, 0.76 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (125 MHz, CDCl3): δ 175.94 (–COO–), 172.63 (–COO–), 150.67 (–CH=C), 109.76 (–CH=C), 81.65 (C–OH), 65.85, 59.45, 56.67, 55.54, 50.59, 50.08, 49.57, 47.09, 42.52, 40.80, 38.50, 38.31, 38.03, 37.98, 37.21, 37.06, 34.35, 33.27, 33.07, 32.23, 30.70, 29.69, 28.15, 28.04, 25.62, 24.13, 23.88, 23.60, 21.03, 19.47, 18.30, 16.66, 16.57, 16.31, 15.96, 14.77. benzene ring: 136.63, 128.61, 128.37, 128.18. m.p.: 131.3–133.8 °C. HR-MS (ESI) m/z: 672.4986 [M + H]+, calcd for: C44H66NO4 672.4914.
Benzyl 3β-(2-(piperidin-1-yl)acetic acid)-lup-20(29)-en-28-oate (11c). White solid, 57.2% yield, 1H-NMR (400 MHz, CDCl3): δ 7.26–7.36 (m, 5H, –C6H5), 5.07–5.15 (m, 2H, –O–CH2–Ph), 4.71, 4.59 (brs, each, 1H, =CH2), 4.50–4.54 (m, 1H, –CH–O–), 3.17–3.21 (m, 2H, –NH–CH2–CO–), 1.00–2.50 (36 H, methyl- and methylene- of BA and piperdine), 1.67, 0.93, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.97 (–COO–), 170.59 (–COO–), 150.67 (–CH=C), 109.76 (–CH=C), 81.30 (C–OH), 65.85, 60.45, 56.67, 55.51, 54.27, 50.95, 50.57, 49.56, 47.09, 42.52, 40.79, 38.49, 38.31, 37.93, 37.20, 37.06, 34.34, 32.23, 30.69, 29.83, 29.68, 28.15, 25.91, 25.61, 24.01, 23.95, 21.02, 19.45, 18.30, 16.71, 16.30, 15.95, 14.77. Benzene ring: 136.62, 128.61, 128.36, 128.17. m.p.: 146.8–148.0 °C. HR-MS (ESI) m/z: 672.4993 [M + H]+, calcd for: C44H66NO4 672.49136.
Benzyl 3β-(2-(pyrrolidin-1-yl)acetic acid)-lup-20(29)-en-28-oate (11d). White solid, 50.7% yield, 1H-NMR (400 MHz, CDCl3): δ 7.27–7.36 (m, 5H, –C6H5), 5.07–5.16 (m, 2H, –O–CH2–Ph), 4.72, 4.59 (brs, each, 1H, =CH2), 4.52–4.56 (m, 1H, –CH–O–), 3.29–3.38 (m, 2H, –NH–CH2–CO–), 1.00–2.50 (36 H, methyl- and methylene- of BA and pyrrolidine), 1.67, 0.93, 0.83, 0.82, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.95 (–COO–), 170.79 (–COO–), 150.67 (–CH=C), 109.65 (–CH=C), 81.15 (C-OH), 65.84, 57.01, 56.67, 55.52, 54.00, 50.58, 49.57, 47.09, 42.51, 40.79, 38.51, 38.31, 37.98, 37.21, 37.07, 34.35, 32.23, 30.70, 29.69, 28.13, 25.61, 23.96, 23.91, 21.02, 19.47, 18.30, 16.70, 16.31, 15.96, 14.76. Benzene ring: 136.63, 128.61, 128.37, 128.18. m.p.: 139.8–142.6 °C. HR-MS (ESI) m/z: 658.4842 [M + H]+, calcd for: C43H64NO4 658.4757.
Benzyl 3β-(2-(piperazin-1-yl)acetic acid)-lup-20(29)-en-28-oate (11e). White solid, 32.6% yield, 1H-NMR (400 MHz, CDCl3): δ 7.29–7.36 (m, 5H, –C6H5), 5.07–5.16 (m, 2H, –O–CH2–Ph), 4.71, 4.59 (brs, each, 1H, =CH2), 4.50–4.54 (m, 1H, –CH–O–), 3.22–3.25 (m, 2H, –NH–CH2–CO–), 2.61–2.70 (m, 4H, NH–(CH2)2–), 1.00–2.50 (36 H, methyl- and methylene- of BA and pyrrolidine), 1.67, 0.93, 0.81, 0.75 (s, each, 3H, 5 × –CH3, methyl of BA); 13C-NMR (100 MHz, CDCl3): δ 175.95 (–COO–), 169.70 (–COO–), 150.68 (–CH=C), 109.77 (–CH=C), 82.04 (C–OH), 81.45, 65.86, 59.12, 56.68, 55.51, 51.31, 50.58, 50.13, 49.57, 47.09, 43.89, 42.53, 40.80, 38.48, 38.31, 37.97, 37.07, 34.34, 32.24, 30.70, 29.69, 28.20, 25.61, 23.93, 21.03, 19.47, 18.30, 16.73, 16.70, 16.30, 15.96, 14.78. benzene ring: 136.63, 128.62, 128.38, 128.19. m.p.: 143.0–145.9 °C. HR-MS (ESI) m/z: 673.4945 [M + H]+, calcd for: C43H65N2O4 673.4866.
4.3. Biology Evaluation
The human cervical cancer cell line (Hela), human hepatocellular carcinoma cell line (HepG-2), human gastric cancer cell line (BGC-823) and human neuroblastoma cell line (SY-SY5Y) were obtained from the Chinese Academy of Medical Sciences and Peking Union Medical College. Fetal bovine serum (FBS) and RPMI 1640 (DMEM) medium, penicillin and streptomycin were obtained from Thermo Technologies. 6-diamidino-2-phenylindole (DAPI) was obtained from Molecular Probes/Invitrogen Life Technologies (Carlsbad, CA, USA). The cultures of the cells were maintained in RPMI 1640 or Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 1% (v/v) penicillin/streptomycin and 10% (v/v) fetal bovine serum under a humidified atmosphere containing 5% CO2 at 37 °C.
The stock solutions of BA derivatives were dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and added at various concentrations to the cell culture. Cellular morphologies were observed using an inverted fluorescence microscope (Olympus IX71, Tokyo, Japan), a plate reader (BIORAD 550 spectrophotometer, Bio-Rad Life Science Development Ltd., Beijing, China), and a Canton 2 flow cytometer (BD, New York, NY, USA).
4.3.1. Antitumor Activity
The antitumor activity of BA derivatives was evaluated on Hela, HepG-2, BGC-823, SY-SY5Y cell lines using the MTT assay. The density of all cells was 2 × 103 cells/well plated in a 96-multiwell plate in RPMI 1640 or DMEM containing 10% FBS for 24 h at 37 °C with 5% CO2. Then, cells were treated for 48 h with the required concentrations (3.125, 6.25, 12.5, 25, 50, or 100 μM) of BA derivatives dissolved with the vehicle DMSO. Each plate contained control group, blank group and drug group. After that, 20 μL MTT in phosphate buffered saline (PBS, 5 mg/mL) was added to each well, and the plates were incubated at 37 °C for 4 h, then we removed the supernatant and adding dimethyl sulfoxide (DMSO, 150 μL) to dissolve the MTT formazan. The optical density (OD) for each well was measured on a BIORAD 550 spectrophotometer plate reader at a wavelength of 550 nm. The above tests were repeated three times in parallel. The proliferation inhibition rates of tumor cells were calculated by {1 - [OD550 (Drug group)/OD550 (Blank group)]/[OD550 (Control group) - OD550 (Blank group)]} × 100%. Compounds with concentration less than 25 μM and proliferation inhibition rates higher than 50% were rescreened. The concentrations of BA derivatives were required at 1.5625, 3.125, 6.25, 12.5, or 25 μM to calculated IC50 values for rescreened.
4.3.2. Morphological Analysis
Hela cells in the logarithmic growth phase were plated onto 6-well plates at a density of 2 × 104 cells/mL for 24 h at 37 °C in a humidified atmosphere with 5% CO2. Additionally, each group was treated with 5 μM BA and compound 7e for 48 h. Cell culture medium was discarded, and the cells were washed twice with PBS. The cells were fixed with 400 μL 4% paraformaldehyde (pH = 7.4) for 10 min and then washed twice with PBS. Then fixed cells were stained with DAPI at the concentration of 1 mg/mL for 20 min in the dark, and cell morphological changes were observed using a fluorescent inverted phase-contrast microscope at a magnification of 100 ×.
4.3.3. Apoptosis Analysis Using Annexin V-FITC/PI Staining
Hela cells in the logarithmic growth phase were plated onto 6-well plates at a density of 4 × 104 cells/mL at 37 °C in a humidified atmosphere with 5% CO2. After incubation for 24 h, Cell culture medium was discarded, and cells were treated with various concentrations (0, 1, 2, or 4 μM) of compound 7e for a further 48 h. Then, cells were collected, washed twice with cold PBS, and centrifuged at 2400 rpm for 10 min. The resulting pellet was mixed with 200 μL of binding buffer of the Annexin V-FITC kit; then, 5 μL of FITC-labeled annexin V was added and mixed gently. After incubation at 4 °C for 10 min in the dark, 5 μL of PI was added and mixed gently. Then, the cells were immediately analyzed with a flow cytometer at 488 nm [26,27].
4.4. Statistical Analysis
All results were expressed as means ± standard derivation (SD) of three independent experiments. The statistical analysis was performed by SPSS software (Version 20.0, International Business Machines Corp. New York, NY, USA) to analyze the variance. One-way analysis of variance (ANOVA) was performed to determine the significance between groups; p < 0.05 was considered to be statistically significant.
5. Conclusions
In this paper, a series of different BA-nitrogen heterocyclic derivatives were designed and synthesized. All of them were characterized by 1H-NMR, 13C-NMR (Figure S1) and were screened for cytotoxic activity employing a panel of four cell lines, including Hela, HepG-2, BGC-823 and SK-SY5Y cells, using the MTT assay. From these data analyzed with MTT, it was evident that almost all derivatives exhibited higher cytotoxicity for all tested cell lines compared to BA. Compound 7e was found to be the most likely drug candidate, showing that IC50 values were 12-fold toxic in vitro than BA-cell Hela. As shown by DAPI and Annexin V-FITC/PI staining, it was found that compound 7e mainly acted by inducing early apoptosis. Based on the above, compound 7e showed bright prospects and is valued for further study.
Acknowledgments
This study was supported by Chinese Academy of Inspection and Quarantine, Beijing Institute of Technology, and Capital Normal University.
Supplementary Materials
The following are available online. Figure S1: 1H-NMR and 13C-NMR spectra for all compounds.
Author Contributions
P.W., H.L. conceived and designed the experiments; T.X., H.C. and F.G. performed the chemistry experiments; T.X., J.Q., Q.W. and W.L. performed our biological activity experiments; T.X., Y.Y., Z.D. and S.H. performed the biological activity in vivo experiments, analyzed the pharmacological data, and elaborated the cell morphology; X.T., N.H., X.L. and Y.G. conducted data analysis and statistics; Y.Y., P.W. wrote the paper and modified the language of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81603256), Beijing Municipal Natural Science Foundation (No. 7202116, China), project of China Association of Chinese Medicine (CACM-2018-QNRC2-B08), the Fundamental Research Funds for the Central Universities (BUCM-2019-JCRC002, BUCM-2018-2020 and 2019-JYB-TD005, China), Beijing Key Laboratory for Basic and Development Research on Chinese Medicine (Beijing, 100102, China).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the 25 compounds are available from the authors.
References
- 1.Jinwoong K., Park E.J. Anti-Cancer Agents. Curr. Med. Chem. 2002;2:485–537. doi: 10.2174/1568011023353949. [DOI] [PubMed] [Google Scholar]
- 2.Wang C.M., Chen H.T., Wu Z.Y., Jhan Y.L., Shyu C.L., Chou C.H. Antibacterial and synergistic activity of pentacyclic triterpenoids isolated from Alstonia scholaris. Molecules. 2016;21:139. doi: 10.3390/molecules21020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kvasnica M., UrBAn M., Dickinson N.J., Sarek J. Pentacyclic triterpenoids with nitrogen-and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015;32:1303–1330. doi: 10.1039/C5NP00015G. [DOI] [PubMed] [Google Scholar]
- 4.Xiao S., Tian Z., Wang Y., Si L., Zhang L., Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018;38:951–976. doi: 10.1002/med.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H.M., Yin Z.Q., Zhao M.G., Jiang C.H., Zhang J., Pan K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry. 2018;151:119–127. doi: 10.1016/j.phytochem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Alakurtti S., Mäkelä T., Koskimies S., Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D.M., Xu H.G., Wang L., Li Y.J., Sun P.H., Wu X.M., Wang G.J., Chen W.M., Ye W.C. Betulinic acid and its derivatives as potential antitumor agents. Med. Res. Rev. 2015;35:1127–1155. doi: 10.1002/med.21353. [DOI] [PubMed] [Google Scholar]
- 8.Yogeeswari P., Sriram D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee R., Kumar V., Srivastava S.K., Agarwal S.K., Burman A.C. Betulinic acid derivatives as anticancer agents: Structure activity relationship. Anti-Cancer Agent. Med. 2006;6:271–279. doi: 10.2174/187152006776930846. [DOI] [PubMed] [Google Scholar]
- 10.Potze L., di Franco S., H. Kessler J., Stassi G., Paul Medema J. Betulinic acid kills colon cancer stem cells. Curr. Stem Cell Res. 2016;11:427–433. doi: 10.2174/1574888X11666151203223512. [DOI] [PubMed] [Google Scholar]
- 11.Potze L., Di Franco S., Grandela C., Pras-Raves M.L., Picavet D.I., Van Veen H.A., Van Lenthe H., Mullauer F.B., Van Der Wel N.N., Luyf A. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene. 2016;35:427. doi: 10.1038/onc.2015.102. [DOI] [PubMed] [Google Scholar]
- 12.Zuco V., Supino R., Righetti S.C., Cleris L., Marchesi E., Gambacorti-Passerini C., Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002;175:17–25. doi: 10.1016/S0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 13.Koohang A., Majewski N.D., Szotek E.L., Mar A.A., Eiznhamer D.A., Flavin M.T., Xu Z.Q. Synthesis and cytotoxicity of 2-cyano-28-hydroxy-lup-1-en-3-ones. Bioorgan. Med. Chem. Lett. 2009;19:2168–2171. doi: 10.1016/j.bmcl.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee R., Jaggi M., Rajendran P., Siddiqui M.J., Srivastava S.K., Vardhan A., Burman A.C. Betulinic acid and its derivatives as anti-angiogenic agents. Bioorgan. Med. Chem. Lett. 2004;14:2181–2184. doi: 10.1016/j.bmcl.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Santos R.C., Salvador J.A., Marín S., Cascante M. Novel semisynthetic derivatives of betulin and betulinic acid with cytotoxic activity. Bioorgan. Med. Chem. 2009;17:6241–6250. doi: 10.1016/j.bmc.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Eignerova B., Tichy M., Krasulova J., Kvasnica M., Rarova L., Christova R., Urban M., Bednarczyk-Cwynar B., Hajduch M., Sarek J. Synthesis and antiproliferative properties of new hydrophilic esters of triterpenic acids. Eur. J. Med. Chem. 2017;140:403–420. doi: 10.1016/j.ejmech.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V., Rani N., Aggarwal P., Sanna V.K., Singh A.T., Jaggi M., Joshi N., Sharma P.K., Irchhaiya R., Burman A.C., et al. Synthesis and cytotoxic activity of heterocyclic ring-substituted betulinic acid derivatives. Bioorg. Med. Chem. Lett. 2008;18:5058–5062. doi: 10.1016/j.bmcl.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Shintyapina A.B., Shults E.E., Petrenko N.I., Uzenkova N.V., Tolstikov G.A., Pronkina N.V., Kozhevnikov V.S., Pokrovsky A.G. Effect of nitrogen-containing derivatives of the plant triterpenes betulin and glycyrrhetic acid on the growth of MT-4, MOLT-4, CEM, and HepG2 tumor cells. Russ. J. Bioorgan. Chem. 2007;33:579–583. doi: 10.1134/S1068162007060076. [DOI] [PubMed] [Google Scholar]
- 19.Urban M., Sarek J., Kvasnica M., Tislerova I., Hajduch M. Triterpenoid pyrazines and benzopyrazines with cytotoxic activity. J. Nat. Prod. 2007;70:526–532. doi: 10.1021/np060436d. [DOI] [PubMed] [Google Scholar]
- 20.Fang K., Zhang X.H., Han Y.T., Wu G.R., Cai D.S., Xue N.N., Guo W.B., Yang Y.Y., Chen M., Zhang X.Y. Design, Synthesis, and Cytotoxic Analysis of Novel Hederagenin–Pyrazine Derivatives Based on Partial Least Squares Discriminant Analysis. Int. J. Mol. Sci. 2018;19:2994. doi: 10.3390/ijms19102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meira C.S., Barbosa-Filho J.M., Lanfredi-Rangel A., Guimarães E.T., Moreira D.R.M., Soares M.B.P. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016;166:108–115. doi: 10.1016/j.exppara.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Hübner D.P.G., de Brum Vieira P., Frasson A.P., Menezes C.B., Senger F.R., da Silva G.N.S., Gnoatto S.C.B., Tasca T. Anti-Trichomonas vaginalis activity of betulinic acid derivatives. Biomed. Pharmacother. 2016;84:476–484. doi: 10.1016/j.biopha.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 23.Borkova L., Hodon J., Urban M. Synthesis of Betulinic Acid Derivatives with Modified A-Rings and their Application as Potential Drug Candidates. Asian J. Org. Chem. 2018;7:1542–1560. doi: 10.1002/ajoc.201800163. [DOI] [Google Scholar]
- 24.Chen Y., Sit S.Y., Chen J., Swidorski J.J., Liu Z., Sin N., Venables B.L., Parker D.D., Nowicha-sans B., Li Z. The design, synthesis and structure-activity relationships associated with C28 amine-based betulinic acid derivatives as inhibitors of HIV-1 maturation. Bioorgan. Med. Chem. Let. 2018;28:1550–1557. doi: 10.1016/j.bmcl.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 25.Chu F., Zhang W., Guo W., Wang Z., Yang Y., Zhang X., Fang K., Yan M., Wang P., Lei H. Oleanolic acid-amino acids derivatives: Design, synthesis, and hepatoprotective evaluation in vitro and in vivo. Molecules. 2018;23:322. doi: 10.3390/molecules23020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., Ruan Y., Chen Q., Li S., Wang Q., Cai J. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur. J. Pharmacol. 2011;650:41–47. doi: 10.1016/j.ejphar.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., Gong L., Li X., Ye L., Wang B., Liu J., Qiu J., Jiao H., Zhang W., Chen J. Infrasound increases intracellular calcium concentration and induces apoptosis in hippocampi of adult rats. Mol. Med. Rep. 2012;5:73–77. doi: 10.3892/mmr.2011.597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.