Abstract
This research was designed to investigate the metabolite profiling, phenolics, and flavonoids content as well as the potential antioxidant and antibacterial, properties of orange-yellow resin from Zuccagnia punctata Cav (ZpRe). Metabolite profiling was obtained by a ultrahigh resolution liquid chromatography orbitrap MS analysis (UHPLC-ESI-OT-MS-MS). The antioxidant properties were screened by four methods: 2,2-diphenyl-1-picrylhydrazyl assay (DPPH), trolox equivalent antioxidant activity assay (TEAC), ferric-reducing antioxidant power assay (FRAP), and lipid peroxidation in erythrocytes (LP)). The antibacterial activity was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) rules. The resin displayed a strong DPPH scavenging activity (IC50 = 25.72 µg/mL) and showed a percentage of inhibition of LP close to that of the reference compound catechin (70% at 100 µg ZpRe/mL), while a moderated effect was observed in the FRAP and TEAC assays. The resin showed a content of phenolic and flavonoid compounds of 391 mg GAE/g and 313 mg EQ/g respectively. Fifty phenolics compounds were identified by ultrahigh resolution liquid chromatography orbitrap MS analysis (UHPLC-PDA-OT-MS) analysis. Thirty-one compounds are reported for the first time, updating the knowledge on the chemical profile of this species. The importance of the biomolecules identified support traditional use of this endemic plant. Furthermore, additional pharmacological data is presented that increase the potential interest of this plant for industrial sustainable applications.
Keywords: xanthene’s derivatives, trichothecene, dipping in dichloromethane, biomolecules antioxidants, vedelianin derivatives
1. Introduction
The resins are nonvolatile products of plants, which include surface resins, naturally secreted by plants or internal resins, which can be obtained or collected from incisions. Their chemical composition includes flavonoids, terpenoids, and fatty substances that in some cases are protective barrier for the plant against the attack of some herbivores and other insects [1]. A limited number of families including Fabaceae, Burseraceae, and Pinaceae stand out for their high resin production [1]. Argentina’s Andean region is the habitat of arid and semiarid land species belonging to the genera Larrea, Zuccagnia, and Bulnesia recognized for their high production of resins or exudates, of which there is a lack of knowledge about their potential as sources of biomolecules of pharmacological and industrial interest. Chemical studies carried out in the last decades have reported a limited number of metabolites. Plants exudates are known to possess several biological activities including antimicrobial, antioxidant, anthelmintic, and nematicidal [1,2]. Zuccagnia punctata is used in Argentina, to treat injuries and bruises, as a disinfectant of wounds, a repellent of insects, for roof construction in rural areas, and as a vegetable fuel for cooking food. Medicinal plants form a primary means for treatment of various diseases in many parts of the world. In Argentine Z. punctata is “the medicinal plant”, it has the largest number of studies of chemistry and biological activity, including antimicrobial, antioxidant, anti-mutagenic, anti-inflammatory, and cytoprotective activities [3].
Principally their bioactivities evidenced have been associated on the basis of the major component (chalcones) [4,5,6,7,8,9].Recently, a selective and reliable characterization of the botanical phenolic profile of Z.punctata collected in the northwestern regions of Argentina by liquid chromatography coupled to diode array detector and quadrupole-time of flight mass spectrometry (LC-DAD-Q-TOF-MS) system was reported, highlighting the identification of the major constituent of an ethanolic extract as 4′-hydroxy-2′methoxydihydrochalcone together with other chalcones, flavanones, and caffeic acid derivatives [10].
The interest on the Andean plants is remarkable, because they represent a source very little explored that can offer extracts or biomolecules promising for the study and development of new drugs of pharmacological interest.
In the last decades, several extracts, decoctions, and infusions of medicinal plants and fruits native to Argentina and Chile have been analyzed using the more accurate and reliable quadrupole orbitrap spectrometer (Q-OT-MS), updating significantly the chemical composition in most of the species reported [11,12,13,14,15,16,17,18,19].
In this work, the antioxidant and antibacterial effects complemented with the exhaustive polyphenolic profile of Zuccagnia punctata resin are reported, showing the presence of unique bioactive molecules of pharmacological and industrial interest.
2. Materials and Methods
2.1. Chemicals
Ultra-pure water (<5 µg/L TOC, (Total Organic Carbon) was obtained from a water purification system Arium 126 61316-RO, plus an Arium 611 UV unit (Sartorius, Goettingen, Germany). Methanol (HPLC grade) and formic acid (puriss. p.a. for mass spectrometry) from J.T. Baker (Phillipsburg, NJ, USA) were obtained. Folin-Ciocalteu (FC) reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric chloride hexahydrate, 2,4,6-tris(2-pyridyl)-s-triazine, trolox, quercetin, gallic acid, DMSO, and HPLC standards (caffeic acid phenethyl ether (CAPE), galangin, morusin, naringenin, pinocembrin, rhamnetin and shikonin, with purity higher than 95% by HPLC) were purchased from Sigma-Aldrich Chem. Co. (St. Louis, MO, USA) or Extrasynthèse (Genay, France). Cefotaxime was from Argentia® (Bristol-Myers Squibb, Buenos Aires, Argentina). Mueller-Hinton broth was provided by Laboratorio Britania (Buenos Aires, Argentina).
2.2. Plant Material
The aerial parts of Zuccagnia punctata Cav. (Fabaceae, Caesalpinoideae) were collected in February 2015, on Iglesia district, province of San Juan (Argentina) at an altitude of 1800 m above sea level. A voucher specimen has been deposited at the herbarium of the Botanic Museum of Córdoba (CORD 1125), Universidad Nacional de Córdoba, Argentina.
2.3. Z. punctata Orange-Yellow Resin (ZpRe)
The orange-yellow resin was obtained by dipping fresh aerial parts (500 g; 4L of dichloromethane grade HPLC, 1 min; the extraction procedure was done three times), filtered and evaporated under reduced pressure to yield a semisolid yellow-orange resin (10 ± 1% yield w/w). The ZpRe was stored in a freezer at −40 °C until its use to bioassays, phenolics, and flavonoids identification/quantification as well as in ultrahigh resolution liquid chromatography orbitrap MS analysis (UHPLC-PDA-OT-MS) analysis.
The main chalcones of Z.punctata: 2′,4′-dihydroxychalcone, 2′,4′-dihydroxy-3′-methoxychalcone and caffeic acid derivatives, 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl caffeic acid ester and 1-methyl-3-(4′-hydroxyphenil)-propyl caffeic acid ester were isolated and characterized by analysis of their spectroscopic data (1H and 13C NMR), which agree with those reported in the literature [3,6].
2.4. UHPLC-DAD-MS Instrument
An UHPLC-high-resolution MS machine Thermo Dionex Ultimate 3000 system with PDA detector controlled by Chromeleon 7.3 software (Thermo Fisher Scientific, Waltham, MA, USA) hyphenated with a Thermo Q-Exactive MS focus (Thermo, Bremen, Germany) was used [16].
2.5. LC Parameters and MS Parameters
Liquid chromatography was performed using an UHPLC C-18 column (150 × 4.6 mm Acclaim, ID, 2.5 µm; Thermo Fisher Scientific, Bremen, Germany) at 25 °C, hyphenated with a Thermo Q-Exactive MS focus (Thermo, Bremen, Germany) was used. The detection wavelengths were 330,280, 254, and 354 nm, and photodiode array detectors were set from 200–800 nm. Solvent delivery was performed at 1 mL/min using ultra-pure water supplemented with 1% formic acid (A) and acetonitrile with 1% acid formic (B) and a program starting with 5% B at zero time, then maintained 5% B for 5 min, then changing to 30% B within 10 min, then maintaining 30% B for 15 min, then going to 70% B for 5 min, then maintaining 70% B for 10 min, and finally returning to 5% B in 10 min. and keeping this condition for twelve additional minutes to achieve column stabilization before next injection of 20 µL. For the analysis, 5 mg of the resin was dissolved in 2 mL of methanol, filtered through a 200-µm PTFE (polytetrafluoroethylene) filter, and 20 µL was injected in the instrument. Standards and the resin dissolved in methanol were kept at 10 °C during storage in the autosampler. The HESI II and Orbitrap spectrometer (Thermo, Bremen, Germany) parameters were optimized as previously reported [16,17]. Additionally, relevant experimental parameters have been reported recently in detail [18] The Q-Exactive 2.3 SP 2, Xcalibur 2.4 and Trace Finder 3.3 (Thermo Fisher Scientific, Bremen, Germany) were used for UHPLC mass spectrometer control and data processing, respectively.
2.6. Total Phenolic (TP) and Flavonoid (F) Content
The total phenolics and flavonoid content of ZpRe was determined by employing total phenols assay by Folin–Ciocalteu reagent and flavonoids by AlCl3 assay, both in microplate [18]. The total phenolic was expressed as milligrams of gallic acid equivalents (GAE) per gram of extracts (mg GAE/g ZpRe). Flavonoids were expressed as milligrams of quercetin equivalents (QE) per gram of extracts (mg QE/g ZpRe). The values were obtained using a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA), and are showed as the mean ± standard deviation (SD).
2.7. Antioxidant Activity
2.7.1. 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity Assay
The Capacity of ZpRe to 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging (DPPH) was run by the following procedure: DPPH solution (20 mg/L) in methanol was mixed with ZpRe solution at concentrations of 1, 5, 10, 50, and 100 µg/mL [18]. The reaction progress absorbance of the mixture was monitored at 515 nm using a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA). The percentage of the DPPH decoloration was proportional to the five antioxidant concentrations, and the concentration responsible for a decrease in the initial DPPH concentration by 50% was defined and calculated as EC50 value, which is showed as the mean ± SD.
2.7.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
The FRAP assay was run in microplate, as previously reported methodology [18,20]. Briefly, FRAP reagent and a methanolic solution of ZpRe resin (1 mg/mL) were mixed; simultaneously, a calibration curve was prepared by mixing FRAP reagent and Trolox solutions, at concentrations between 0 and 1 mmol/L. The absorbance values of mixtures were obtained in a Multiskan FC Microplate Photometer Results were obtained by linear regression from the FRAP-Trolox calibration plot and are showed in equivalent milligrams Trolox/g ZpReresin.
2.7.3. Trolox Equivalent Antioxidant Activity Assay (TEAC)
TEAC assay was run in microplate, as following the previously reported methodology [18,21]. Briefly, a ZpRe methanolic solution was mixed with 200 µL of ABTS, measuring their absorbance at 734 nm after 4 min. Results were obtained by linear regression from a calibration curve constructed with Troloxand are showed expressed as equivalent milligrams Trolox/g ZpRe resin.
2.7.4. Lipid Peroxidation in Erythrocytes
The ability of the ZpReresin at three concentrations (100, 250, and 500 µg/mL) and of catechin at a single concentration (100 µg/mL) to inhibit lipoperoxidation in erythrocytes (LP), induced by tert-Butyl hydroperoxide, was determined. Relevant technical aspects of the trial have been reported recently in detail [15,18]. The values obtained are expressed as percentages of lipid oxidation inhibition.
2.8. Antibacterial Activity
2.8.1. Microorganisms
For antibacterial evaluation, were used strains from the American Type Culture Collection (ATCC, Rockville, MD, USA) and clinical isolates from Laboratorio de Microbiología, Hospital Marcial Quiroga, San Juan, Argentina (MQ).The panel comprised the following bacteria: Staphylococcus aureus methicillin-sensitive ATCC 29213, Staphylococcus aureus methicillin-resistant ATCC 43300, Staphylococcus aureus methicillin-resistant-MQ1, Staphylococcus aureus methicillin-resistant-MQ2, Streptococcus agalactiae-MQ3, Streptococcus pyogenes-MQ4, and Escherichia coli ATCC 25922.
2.8.2. Antibacterial Susceptibility Testing
Minimum inhibitory concentration (MIC) of ZpRe and antibiotic Cefotaxime (Argentia®, Buenos Aires, Argentina) was carried outby broth microdilution techniques, in according to CLSI [22]. The ZpRe was tested from 0.98 to 3000 µg/mL.using an inoculum of each bacterium adjusted to 5 × 105 cells with colony forming units (CFU)/mL. The absorbances at 620 nm were determined in a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA).
2.9. Statistical Analysis
The Duncan’s test from InfoStat edition 2016 software (Universidad Nacional de Córdoba, Argentina) was run to determine potential significant differences (p < 0.05) in the carried out assays
3. Results
3.1. UHPLC-PDA-OT-MS Analysis of the Orange-Yellow Resin From San Juan Province, Argentina
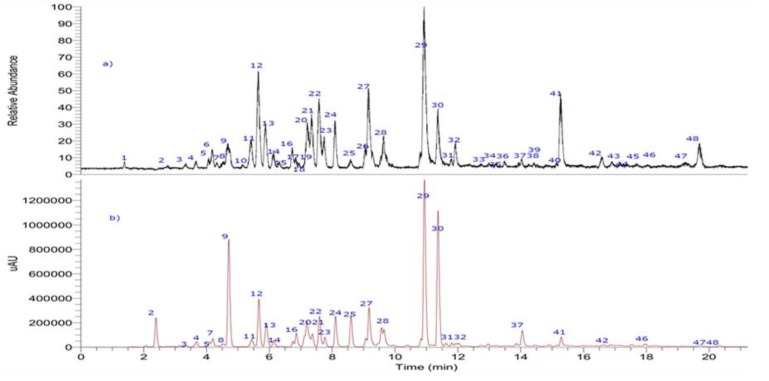
Fifty-one compounds were detected in ZpRe by UHPLC-PDA-OT-MS analysis, combining full mass spectra and MSn experiments, of which fifty were tentatively identified including flavonoids, chalcones, caffeic acid derivatives, coumaric acid esters, naphthoquinone, xanthene’s derivatives, trichocethenes; vedelianin derivatives, and others. Several phenolics compounds from ZpRe were rapidly identified using available standards. Thirty-one not previously reported updated the chemical composition of this species. The identification of unknown phenolic compounds xanthene’s characteristics of this bioactive plant was possible from comprehensive analysis of the full scan mass spectra, base peaks chromatograms, and data-dependent scan experiment, since the orbitrap provided high-resolution and accurate mass product ion spectra from precursor ions that are unknown before and within a single run.
The molecular formula was obtained through high resolution accurate mass analysis (HRAM) and matching with the isotopic pattern. The acquisition of the data in the UHPLC-PDA-OT-MS analysis was developed using electrospray negative mode, because compounds with a phenolic OH lose easily the proton in electrospray ionization, giving very good and diagnostic parent ions and fragments.
The metabolome identification of the 50 tentatively identified compounds is developed below, highlighting the relevant information of the 31 compounds that are new reports for the species (See Figure 1, Figure 2 and Figure 3, Table 1, and Supplementary Material Figure S1 for some representative compounds and spectra S1 and S2).
Figure 1.
UHPLC-MS (total ion current) chromatograms of ZpRe resin.
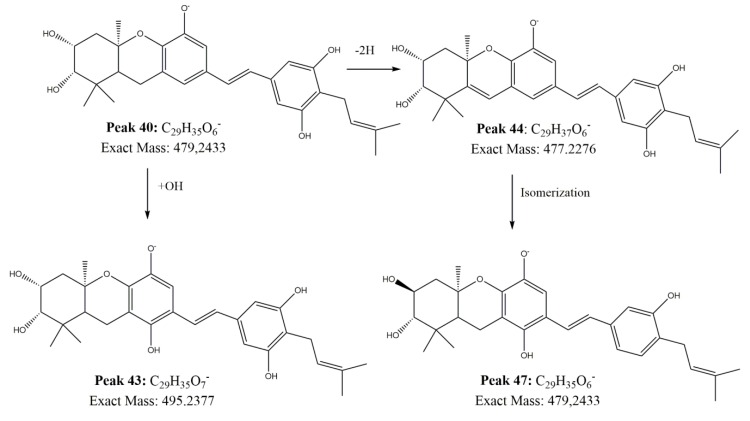
Figure 2.
Proposed biosynthesis and structures of Vedelianin and some derivatives in Z punctata.
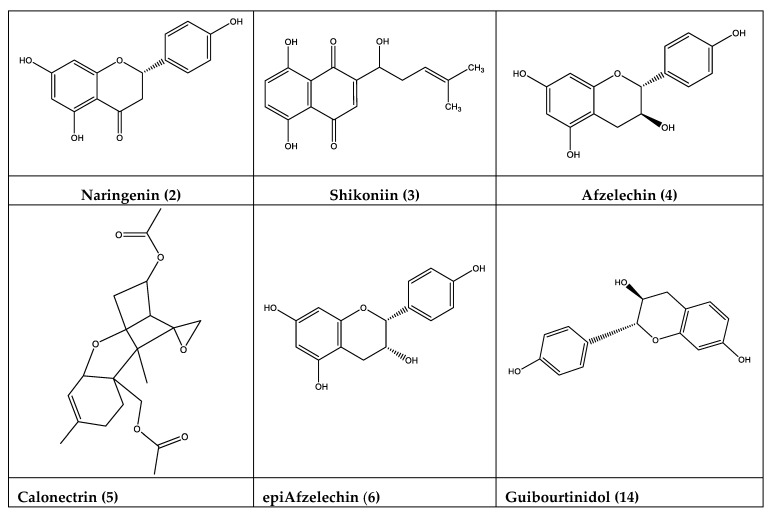
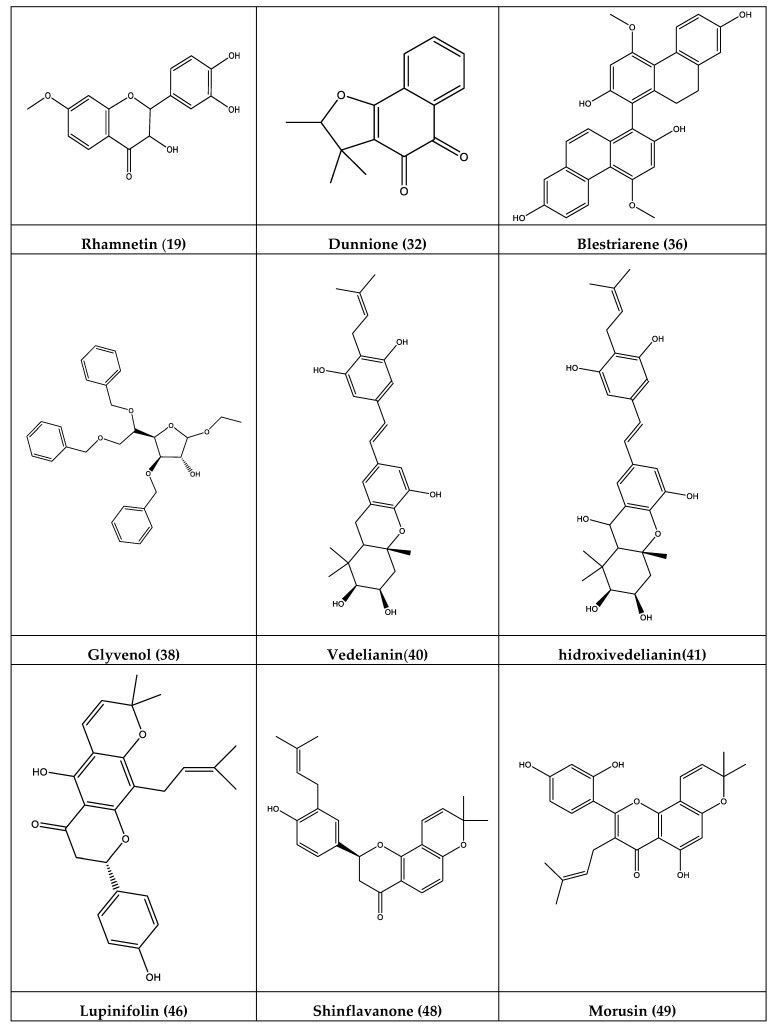
Figure 3.
Structures of some newly reported compounds in ZpRe resin.
Table 1.
Resolution UHPLC-PDA-Q-Orbitrap identification of biomolecules from ZpRe resin.
| Peak | Retention Time (min) | UV Max | Tentative Identification | Elemental Composition [M − H]− | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy(δ ppm) | MSnIons |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.33 | unknown | C16H15O5 | 85.00343 | ||||
| 2 | 2.77 | 279 | Naringenin a | C15H11O5 | 271.06110 | 271.0601 | 3.67 | |
| 3 | 3.35 | 279–367 | Shikoniin a | C16H15O5 | 287.09325 | 287.0923 | 3.40 | |
| 4 | 3.65 | 279 | Afzelechin a | C15H13O5 | 273.07575 | 273.0766 | 3.22 | |
| 5 | 4.05 | - | Calonectrin a | C19H25O6 | 349.16456 | 349.1654 | 2.58 | 85.00342 |
| 6 | 4.18 | 279 | EpiAfzelechin a | C15H13O5 | 273.07575 | 273.0766 | 3.10 | |
| 7 | 4.28 | 287 | Naringenin enantiomer a | C15H11O5 | 271.06195 | 271.0611 | 3.67 | 151.0394:109.0286 |
| 8 | 4.31 | 287 | 3,7-dihydroxiflavanone | C15H11O4 | 255.06519 | 255.0661 | 3.67 | 151.0394:109. 0286 |
| 9 | 4.61 | 287 | 7,8-dihydroxiflavone | C15H11O4 | 255.06519 | 255.0661 | 3.11 | 237.0553 |
| 10 | 5.12 | 287 | 5-hydroxy-4′,7-dimethoxyflavanone | C17H15O5 | 299.09140 | 299.0929 | 2.95 | 285.0403;179.0345, 135.0444 |
| 11 | 5.38 | 287 | 3,7,8-trihydroxydihydroflavanone | C15H11O5 | 271.06010 | 271.0610 | 3.33 | 253.0503;225.0552; 197.0603;151.0029 |
| 12 | 5.67 | 234-292-325 | 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl caffeic acid ester | C19H19O6 | 343.11761 | 343.1187 | 2.49 | 179.0344;161.0236; 135.0443 |
| 13 | 5.88 | 236-277-312 | 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl caffeic acid ester isomer a | C19H19O6 | 343.11761 | 343.1183 | 2.22 | 257.0818;179.0345; 151.0393;135.0444; 107.0494 |
| 14 | 5.88 | 236-277-312 | Guibourtinidol a | C15H13O4 | 257.08084 | 257.0816 | 3.10 | 179.0345;151.0393; 135.0444;107.0494 |
| 15 | 6.14 | 279–367 | 1-methyl-3-(3′,4′-dihydroxyphenil)-propyl caffeic acid ester isomer a | C19H19O6 | 343.11853 | 343.1187 | 2.66 | 287.0818;151.0393; 119.0495;107.0494 |
| 16 | 6.14 | 279–367 | Shikoniin isomer a | C16H15O5 | 287.09140 | 287.0923 | 3.18 | 151.0393;119.0495; 107.0494 |
| 17 | 6.27 | 285 | 7,4′-dihydroxy-5-methoxy-flavanone | C16H13O5 | 285.07575 | 285.0766 | 3.19 | 149.9952;119.0495 |
| 18 | 6.73 | 287 | Dihydroxyflavanone | C15H11O4 | 255.06519 | 255.0669 | 3.05 | 237.0553;209.0604; 195.0400 |
| 19 | 6.91 | 251–349 | Rhamnetin a | C16H11O7 | 315.04993 | 315. 0511 | 3.18 | 185.0034,146.93796 |
| 20 | 7.18 | 246-324-237 | 3,7-dihydroxyflavone | C15H9O4 | 253.05029 | 253.0495 | 2.99 | 208.0524;223.0326; 195.0447; 180.0576 |
| 21 | 7.31 | 277 314 | 1-methyl-3-(4′-hydroxyphenil)-propyl caffeic acid ester | C19H19O5 | 327.12357 | 327.1227 | 2.64 | 135.0443 |
| 22 | 7.55 | 242, 291–324 | 2-methyl-3-(3-hydroxy-4′-methoxyphenyl)-propyl caffeic acid ester | C20H21O6 | 357.13409 | 357.1332 | 2.32 | 343.1104;193.0500; 179.0343;161.0237;135.0440 |
| 23 | 7.70 | 249-285-323 | 1-methyl-3-(4′-hydroxyphenil)-propyl caffeic acid ester isomer a | C19H19O5 | 327.12380 | 327.1227 | 3.01 | 179.0344;163.0394; 135.0443;119.0494 |
| 24 | 8.04 | 235-343 | Pinocembrin | C15H11O4 | 255.06601 | 255.0651 | 3.23 | 227.0907;213.0503; 164.0109;151.0029; 123.0080 |
| 25 | 8.54 | 239–306 | 2′hydroxy-4-methoxychalcone a | C16H13O3 | 253.08592 | 253.0866 | 3.02 | |
| 26 | 9.00 | 291 | Pinocembrin isomer a | C15H11O4 | 255.06599 | 255.0651 | 3.17 | 227.0709;213.0553; 164.0109;145.0642; 123.0080 |
| 27 | 9.14 | 267-315-360 | Galangin(3,5,7-trihydroxyflavone) | C15H9O5 | 269.04579 | 269.0453 | 3.22 | 213.0551;169.0653 |
| 28 | 9.39 | 242-268-310-357 | Caffeic acid phenetyl esther | C17H15O14 | 283.09649 | 283.0794 | 3.38 | |
| 29 | 9.61 | 271 | 4′-terbutyloxyphenyl p-coumaric acid ester | C19H19O4 | 311.12866 | 311.1286 | 2.91 | 163.0394;119.0490 |
| 30 | 9.67 | 231-308 347 |
1-methyl-3-(4′-hydroxyphenyl)-propyl p-coumaric acid ester | C19H19O4 | 311.12779 | 311.1289 | 2.91 | 179.0344;163.0394; 134.0366;119.0490 |
| 31 | 9.88 | 231-308-347 | 1-methyl-3-(4′-hydroxyphenyl)-propyl p-coumaric acid ester isomer a |
C19H19O4 | 311.12866 | 311.1289 | 2.81 | 179.0344;163.0394; 135.0444;119.0494 |
| 32 | 10.80 | 277–312 | Dunnione a | C15H13O3 | 241.08592 | 241.0866 | 2.98 | |
| 33 | 10.88 | 287 | Flavanone * | C15H11O3 | 239.07027 | 239.0709 | 2.91 | 197.0603;169.0653; 153.0186;135.0080; 121.0280 |
| 34 | 10.90 | 232–346 | 2′,4′-dihydroxychalcone | C15H11O3 | 239.07027 | 239.0710 | 197.0603;169.0653; 153.0186;135.0080; 121.0280 |
|
| 35 | 11.37 | 232–345 | 2′,4′-dihydroxy-3′-methoxychalcone | C16H13O4 | 269.08167 | 269.0808 | 3.08 | |
| 36 | 11.77 | 285 | Blestriarene B a | C30H23O6 | 479.14957 | 479.14891 | 1.36 | |
| 37 | 11.91 | 280–323 | 4′-terbutyloxyphenyl p-coumaric acid ester isomer a | C19H19O4 | 311.12779 | 311.1286 | 2.71 | 179.0344;161.0237; 135.0442 |
| 38 | 12.72 | 283 | Glyvenol a | C29H33O6 | 477.22754 | 477.2271 | 0.78 | |
| 39 | 12.95 | 293 | 1-methyl-3-(3′,4′-dihydroxyphenil)-propyl caffeic acid ester isomer a | C19H19O6 | 343.11761 | 343.1183 | 179.0344; 161.0238; 135.0444: 109.0286 |
|
| 40 | 13.19 | 280 | Vedelianin a | C29H35O6 | 479.24882 | 479.2433 | 1.17 | |
| 41 | 13.47 | 280 | Hidroxivedelianin a | C29H35O7 | 495.23773 | 495.2381 | 0.82 | 161.0238;135.0443; 109.0286 |
| 42 | 13.93 | 3,7-dimethyl-2-octaenyl caffeic acid ester | C19H21O4 | 313.14344 | 313.1443 | 2.91 | ||
| 43 | 14.04 | 267–357 | hidroxivedelianin isomer a | C29H35O7 | 495.23785 | 495.2377 | 0.25 | 479.2432;239.0710; 179.0345;161.0238; 135.0442 |
| 44 | 14.42 | 285 | Vedelianin reduced a | C29H33O6 | 477.22717 | 477.2276 | 1.10 | |
| 45 | 15.26 | 285–320 | 3,7-dimethyl-2,6-octadienyl caffeic acid ester (geranyl Caffeate) | C19H23O4 | 315.16993 | 315.1600 | 3.10 | 178.0265;134.0364; 133. 0289 |
| 46 | 16.58 | 289-320 | Lupinifolin | C25H25O6 | 405.16965 | 405.1754 | −1.27 | |
| 47 | 16.58 | 289 | Vedelianin isomer a | C29H35O6 | 479.24882 | 479.2433 | 1.17 | |
| 48 | 16.89 | 287 | Shinflavanone a | C25H25O4 | 389.17474 | 389.1756 | 2.37 | 371.1654 |
| 49 | 17.39 | 285 | Morusin a | C25H23O6 | 419.1502 | 419.14891 | 2.21 | 363.0873;179.0344; 151.0354;109.0286 |
| 50 | 19.23 | 291 | 8-C-Prenyl-6″,6″-dimethylpyrano [2″,3″:7,6] flavanone a | C25H25O3 | 373.19782 | 373.1806 | 2.09 | |
| 51 | 19.68 | 287 | Shinflavanone isomer a | C25H25O4 | 389.17474 | 389.1757 | 2.52 |
a New reports for the species Zuccagnia punctata.
3.1.1. Flavonoids
Peak 2 with a [M − H]− ion at m/z: 271.06010 was identified as naringenin (C15H11O5−) [23], peak 3 as shikoniin (C9H7O4−; m/z: 287.09238) [24]; peak 4 as afzelechin (C15H13O5−; m/z: 273.07575) [25]; peak 6 as epiafzelechin (C15H13O5−; m/z: 273.07660) [26]; peak 7 showing a [M − H]− ion at m/z: 271.06110 was tentatively identified as an naringenin enantiomer (C15H11O5−); peak 8 was identified as 3,7-dihydroxiflavanone [10]; Peak 9 was tentatively identified as 7,8-dihydroxiflavone [10]; peak 10 was proposed as 5-Hydroxy-4′,7-dimethoxyflavanone [27]; peak 11, was identified as 3,7,8 trihydroxydihydroflavanone [10]; peak 14 with a [M − H]− ion at m/z: 271.06010 (C15H13O4−)was identified as guibourtinidol (2R,3S)-4′,7-Dihydroxyflavan-3-ol), (C15H13O4−) [28]; peak 17 was assigned to 7,4′-dihydroxy-5methoxy flavanone [10]; peak 18 is agree to dihydroxyflavanone [10]; peak 19 with a [M − H]− ion at m/z: 315.0511 was identified as rhamnetin (C16H11O7−) [29]; peak 20 was identified as 3,7-dihydroxyflavone [10]; peak 24 and 26 were identified as dihydroxyflavanone pinocembrin [3,10]; and its pinocembrin isomer; showing both compounds similarly MS/MS compared with authentically reference compounds; peak 27 was identified as 3,5,7-trihydroxyflavone (galangin) compared with compound of reference standard [3]; peak 33 was tentatively proposed as flavanone supported by UV signal at 287nm and MS/MS fragmentation (C15H11O3−; m/z: 239.07097); Peak 48 with a [M − H]− ion at m/z: 389.17566 was identified as shinflavanone (C25H25O4−; UV signals 287 nm) [30]; while peak 49 with a [M − H]− ion at m/z: 419.1502 was identified as prenylated flavonoid morusin (C25H23O6−) [31]; peak 50 was tentatively identified as 8-C-Prenyl-6″,6″-dimethylpyrano [2″,3″:7,6] flavanone (C25H25O3−; m/z: 373.18060); peak 51 with [M − H]− ion at m/z: 389.17572 and UV signals (287 nm) was identified as 3,7-dihydroxyflavone (C25H25O4).
3.1.2. Chalcones
Peaks 25 with a [M − H]− ion at m/z: 253.08669 was identified as 2′-hydroxy-4-methoxychalcone (C16H13O3−), while peaks 34, and 35 were identified as characteristics chalcones reported to Z. punctata 2′,4′-dihydroxychalcone and 2′,4′-dihydroxy-3′-methoxychalcone; both chalcones were determined by MS/MS experiment and compared with authentic reference compounds previously isolated [3].
3.1.3. Caffeic Acid Derivatives
Peak 12 was identified as 1-methyl-3-(3′,4′-dihydroxyphenil)-propyl caffeic acid ester by their MS/MS properties compared with authentic reference compounds previously isolated [7,10]; while peaks 13 and 15 with a [M − H]− ion at m/z: 343.11838 and 343.11870 respectively, were tentatively identified as 1-methyl-3-(3′,4′-dihydroxyphenil)-propyl caffeic acid ester isomers (C19H19O6−); peaks 21 was identified as 1-methyl-3-(4′-hydroxyphenil)-propyl caffeic acid ester by their MS/MS compared with authentically reference compounds previously isolated [7,10]; in the same way, peak 22 was assigned to 2-methyl-3-(3-hydroxy-4′-methoxyphenyl)-propyl caffeic acid ester, while peak 23 is supported by its mass properties as an isomer of compound 21; peak 28 was tentatively identified as recognized caffeic acid phenetyl ether; peak 42 was identified as 3,7-dimethyl-2,6-octadienyl caffeic acid ester; and peak 45 was identified as 3,7-dimethyl-2,6-octadienyl caffeic acid ester.
3.1.4. Coumaric Acid Esters
Peak 29 was identified as 4′-terbutyloxyphenyl p-coumaric acid ester [10], while peak 30 was tentatively proposed to 1-methyl-3-(4′-hydroxyphenyl)-propyl p-coumaric acid ester [10]; peak 31 by identical MS/MS properties and UV signals (C19H19O4−; m/z: 311.1289; 231–308–347 nm) was identified as a isomer of compound 30; and peak 37 was proposed supported by MS/MS fragmentation as isomer of compound 29.
3.1.5. Xanthene’s Derivatives, Trichothecenes; Vedelianin Derivatives, and Others
Peak 5 with a [M − H]− ion at m/z: 349.16456 was identified as trichothecene calonectrin (C19H25O6−) [32]. Peak 16 with a [M − H]− ion at m/z: 287.09232was identified as naphthoquinone derivative, shikoniin isomer (C16H15O5−).
Peak 32 with a [M − H]−ion at m/z: 349.16456 (C15H13O3−), was identified as dunnione. Peak 36 was identified as blestriarene B, with a [M − H]− ion at m/z: 479.14891 (C30H23O6−) [33].
Peak 38 with a [M − H]− ion at m/z: 477.22717 (C29H33O6−) was identified as glyvenol (tribenoside).
Peaks 40, 41, 43, 44, and 47 are structurally related, were thus assigned based on their mass properties and characteristic UV signals as the hexahydroxanthene derivative vedelianin (peak 40; C29H35O6−; m/z: 479.24338) [34] and some of its derivatives as follows: Peak 41 with a [M − H]− ion at m/z: 495.23813 (C29H35O7−)was identified as hidroxivedelianin; peak 43 with a [M − H]− ion at m/z: 495.23773 (C29H35O7−)was tentatively assigned to hidroxivedelianin isomer, peak 44, was identified as a reduced vedelianin (C29H33O6−; m/z: 477.22769); while peak 47 (C29H35O6−; m/z: 479.24338) was assigned to other vedelianin isomer, supported by identical MS/MS. A proposed biosynthesis and structures of vedelianin and some derivatives in Z punctata are showed in Figure 2. Peak 46 was tentatively identified as lupinifolin [35].
3.2. Total Phenolic and Flavonoid Contents and Antioxidant, and Antibacterial Activities
The orange-yellow resin from Z.punctata (ZpRe) displayed a stronger DPPH scavenging activity with an EIC50 25.72 µg/mL, as well as an outstanding inhibition of lipid peroxidation in erythrocytes (70% percent at 100 µg ZpRe/mL), this was comparable to the value shown by the reference compound catechin (74% at 100µg/mL) (Table 2) and to the values shown recently by others Andean species [14,15,18]. Phenolic antioxidant compounds acting as free radical scavengers can delay or inhibit lipid oxidation processes. This protection of polyunsaturated fatty acids against free radical damage may explain or supports phenolic compounds as a valuable natural product with potential to improve human health [36] Regarding FRAP and ABTS results, the ZpRe exhibited moderate effect in both trials. The ZpRe presented a high content of TP, with values of 391 mg GAE/g ZpRe, where as approximately eighty percent correspond to flavonoids (313 mg QE/g ZpRe).
Table 2.
Antioxidant assays and total phenolic and flavonoids content of ZpRe from Z. punctata.
| Phenolics Content | ZpRe |
| Total phenolics (mg GAE/g ZpRe) | 391.40 ± 2.18 |
| Flavonoids (mg QE/g ZpRe) | 313.18 ± 3.10 |
| Antioxidant Assay | |
| DPPH (EC50 in µg ZpRe/mL) | 25.72 ± 1.51 |
| FRAP (mg TE/g ZpRe) | 1.74 ± 0.13 |
| TEAC (mg TE/g ZpRe) | 1.25 ± 0.01 |
| Percentage LP (at 100 µg ZpRe/mL) | 70.14 ± 2.26 |
| Percentage LP (at 100 µg catechin/mL) | 74.14 ± 1.25 |
No significant differences were found between the three samples. ANOVA (analysis of variance) followed by Dunett’s comparison test was used (significance p < 0.05). DPPH: (2,2-diphenyl-1-picrylhydrazyl; TEAC: trolox equivalent antioxidant activity assay; FRAP: ferric-reducing antioxidant power assay.
Results of antibacterial activity are depicted in Table S1 (Supplementary Material).The ZpRe showed activity against Gram-positive bacteria, including Staphylococcus aureus methicillin-resistant ATCC 43300, S. aureus methicillin-resistant-MQ-1, S. aureus methicillin-resistant-MQ-2, S. aureus methicillin-sensitive ATCC 25923 and Streptococcus pyogenes (MICs values were between 125 and 250 µg/mL). However, the ZpRe resin was not active against most of the other strains tested (MIC values >250 µg/mL).
The antioxidant, antimicrobial, and other biological activities have been associated, by several authors, with the content of flavonoids and chalcones and some specific flavonoids such as pinocembrin [3,4,5,6,7,8,9]. In a previous study, the quantification of selected markers performed by HPLC-UV method, showed that the resin contains on average 3.18; 3.20; 16.04; and 12.84 g of pinocembrin (24), galangin (27), 2′,4′-dihydroxychalcone (34) and 2′,4′-dihydroxy-3′methoxychalcone (35) respectively, each quantified in 100 g of ZpRe [8].
However, the full UHPLC-MS identification of thirty one biomolecules for the first time in this species (peaks 2–7, 13–16, 19, 23, 25, 26, 31–33, 36–41, 43, 44, 46–51), some of them showing a broad spectra of pharmacological properties, including antioxidant and antimicrobial, canprovide additional and relevant support for the activities displayed by ZpRe resin. Figure 3 and Figure S2 show the structures of some newly reported compounds in the resin of this plant.
Pharmacological activities of naringenin (2), as therapeutic agent to treat different diseases, such as cancer, diabetes, cardiovascular diseases and neurological disorders, oxidative stress and inflammation have been extensively reported [37,38]. Additionally, antibacterial activity against Salmonella thypi, Staphylococcus aureus, and Escherichia coli ATCC as well as their antinociceptive and anti-inflammatory effect in mice model, have been also reported [39,40,41,42]. Furthermore, the free radical scavenging and antioxidant properties havebeen associated with improvements experienced by rats with diabetes type I treated with naringenin [43,44]. The protective effect against metabolic diseases of naringenin is supported by its ability to scavenging some free radicals, by its ability to induce antioxidant enzymes and targeting on phosphoinositide 3-kinase/protein Kinase B/nuclear factors [38]. These mechanisms are involved in the neuroprotective effect recently reported by Chandran et al. [45].
Shikonin (3) have demonstrated a broad spectrum of relevant biological activities such as, antioxidant, anti-inflammatory, antithrombotic, antimicrobial, wound healing effects, as well as neuroprotective effects against cerebral ischemia/reperfusion injury associated or supported to its antioxidant properties [46,47,48,49,50,51]. On the other hand, shikonin inhibited the proliferation of three human pancreatic cancer cell lines, and potentiated synergistically the cytotoxic effect of the gemcitabine a chemotherapeutic drug [52].
Additionally, afzelechin (4), epiafzelechin (6), and some catechol derivatives have been extensively reported as antioxidant compounds. Moreover, in relation to the antioxidant properties of afzelechin (4) its neuroprotective effect related to glutamate-induced neurotoxicity in HT22 cells has been informed [53,54,55,56].
Compound 25 (2-hydroxy-4–methoxychalcone) has been reported as antiangiogenic, antitumoral, glutathione S-transferase inhibitor, and as therapeutic agent to treat atherosclerosis. In addition, several epidemiological studies support the idea that regular consumption of fruits and vegetables rich in flavonoids reduces the risk of cardiovascular diseases [57,58,59].
In the other hand, the strong antibacterial activity of blestriarene B (36) and its antibacterial activity against Streptococcus mutans and Staphylococcus aureus (with MICs values of 12.5 and 6.25 µg/mL respectively) has been also reported [33].
Vedelianin (40) has been recently reported as a potent antiproliferative agent against several cancer cell lines [60,61]. The potential of morusin (49) against human colon rectal cancer and human cell lung cancer has been informed by Chang Lee et al. [62] and Park et al. [63]. Also, the cytotoxic activity of lupinifolin (46) in the cell line P-388 [35] was reported.
The tribenoside glyvenol, (38) was used clinically for hemorrhoidal disease associated with coagulation, inflammation, and wounds. Kikkawa et al. (2010) [64] reported that tribenoside interacts with epidermal cells and regulates the expression and localization of laminins to help reconstruct basement membranes in wound healing of hemorrhoids. Evidence exists to recommend the use of tribenoside as a fast, effective and safe option for the local treatment of low-grade hemorrhoids [65].
Regarding guibourtinidol (14), recently, the hepatoprotective activity and powerful antioxidant properties of Cassia abbreviata root extract, rich in (epi)-catechin, (epi)-afzelechin, (epi)-guibourtinidol, and (ent)-cassiaflavan monomers as well as their dimers and trimers has been reported [66].
Caffeic acid phenethyl ester (CAPE, 28) is a bioactive compound of propolis and the exudate extract. It is known that CAPE possesses antioxidant, anti-inflammatory, anticancer, and cytotoxic properties and is a versatile therapeutically active polyphenol and an effective adjuvant of chemotherapy [67,68,69,70,71].
4. Conclusions
Fifty phenolics compounds were identified by ultrahigh resolution liquid chromatography orbitrap MS analysis (UHPLC-PDA-OT-MS). Thirty-one are reported for the first time, updating the knowledge of the chemical profile of this species. The importance of the biomolecules identified support its traditional use. Herein, more scientific data on bioactivity and chemistry is showed for this plant that increase its potential for sustainable applications and industrial interest that Z. punctata offers, a species that grows in semi-arid Andean areas.
Acknowledgments
J.G. and S.M. held fellowships from CONICET, Argentina. G.E.F. and B.L. are researchers from CONICET, Argentina.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/2/123/s1. Figure S1: (a–h). Full orbitrap MS spectra and structures of representative compounds 3, 4, 14, 28, 30, 40, 43, and 48. Figure S2: Structures of newly reported compounds to ZpRe. Table S1. Antimicrobial activity of ZpRe.
Author Contributions
M.J.S., G.E.F., B.L., and A.T. conceived and designed the experiments; J.G., B.L., and S.M. performed the antioxidant, antimicrobial, and total phenolic and flavonoid content experiments; M.J.S. and J.B. analyzed the data of HPLC/MS. All authors wrote the paper, and read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CICITCA-UNSJ, PME-2015-0200 and PIO CONICET-SECITI Nº 022, Argentina. M.J.S. and J.B. received financial support from Fondecyt, Chile (Grant 1180059).
Conflicts of Interest
The authors do not have any conflicts of interest.
References
- 1.Lima Licá I.C., Dos Santos Soares A.M., Silva de Mesquita L.S., Malik S. Biological properties and pharmacological potential of plant exudates. Food Res. Int. 2018;105:1039–1053. doi: 10.1016/j.foodres.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 2.Modak B., Salina M., Rodilla J., Torres R. Study of the chemical composition of the resinous exudate isolated from Heliotropium sclerocarpum and evaluation of the antioxidant properties of the phenolic compounds and the resin. Molecules. 2009;14:4625–4633. doi: 10.3390/molecules14114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agüero M.B., Gonzalez M., Lima B., Svetaz L., Sánchez M., Zacchino S., Feresin G.E., Schmeda-Hirschmann G., Palermo J.A., Wunderlin D.A., et al. Argentinean Propolis from Zuccagniapunctata Cav. (Caesalpinieae) Exudates: Phytochemical Characterization and Antifungal Activity. J. Agric. Food Chem. 2010;58:194–201. doi: 10.1021/jf902991t. [DOI] [PubMed] [Google Scholar]
- 4.Zampini I.C., Vattuone M.A., Isla M.I. Antibacterial activity of Zuccagnia punctata Cav. ethanolic extracts. J. Ethnopharmacol. 2005;102:450–456. doi: 10.1016/j.jep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Zampini I.C., Villarini M., Moretti M., Dominici L., Isla M.I. Evaluation of genotoxic and antigenotoxic effects of hydroalcoholic extracts of Zuccagnia punctata Cav. J. Ethnopharmacol. 2007;115:330–335. doi: 10.1016/j.jep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Svetaz L., Tapia A., López S.N., Furlán R., Petenatti E., Pioli R., Schmeda-Hirschmann G., Zacchino S.A. Antifungal chalcones and new cafeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004;52:3297–3300. doi: 10.1021/jf035213x. [DOI] [PubMed] [Google Scholar]
- 7.Svetaz L., Aguero M.B., Alvarez S., Luna L., Feresin G., Derita M., Tapia A., Zacchino S. Antifungal activity of chalcones from Zuccagnia punctata Cav. acting against clinically important fungi and studies of mechanism of action. Planta Med. 2007;73:1074–1080. doi: 10.1055/s-2007-981561. [DOI] [PubMed] [Google Scholar]
- 8.Butassi E., Svetaz L., Ivancovich J.J., Feresin G., Tapia A., Zacchino S. Synergistic antifungal fixed-ratio combinations of Zuccagnia punctata Cav. and Larrea nitida Cav., using Mixed-Effects Loewe (MixLow) method. Phytomedicine. 2015;22:666–678. doi: 10.1016/j.phymed.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Butassi E., Svetaz L.A., Sortino M.A., Quiroga A.D., Carvalho V.S.D., Cortés J.C.G., Ribas J.C., Zacchino S.A. Approaches to the mechanism of antifungal activity of Zuccagnia punctata-Larrea nitida bi-herbal combination. Phytomedicine. 2019;54:291–301. doi: 10.1016/j.phymed.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Solorzano E.R., Bortolini C., Bogialli S., Di Gangi I.M., Favaro G., Maldonado L., Pastore P. Use of a LC-DAD-QTOF system for the characterization of the phenolic profile of the argentinean plant Zuccagnia punctata and of the related propolis: New biomarkers. J. Funct. Foods. 2017;33:425–435. doi: 10.1016/j.jff.2017.04.003. [DOI] [Google Scholar]
- 11.Cornejo A., Salgado F., Caballero J., Vargas R., Simirgiotis M., Areche C. Secondary metabolites in Ramalina terebrata detected by UHPLC/ESI/MS/MS and identification of parietin as tau protein inhibitor. Int. J. Mol. Sci. 2016;17:1303. doi: 10.3390/ijms17081303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quispe C., Bórquez J., Villalobos M., Simirgiotis M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018:1–12. doi: 10.1155/2018/6123850. [DOI] [Google Scholar]
- 13.Simirgiotis M.J., Quispe C., Mocan A., Villatoro J.M., Areche C., Bórquez J., Sepúlveda B., Echiburu-Chau C. UHPLC high resolution orbitrapmetabolomic fingerprinting of the unique species Ophryosporus triangularis meyen from the atacama desert, Northern Chile. Rev. Bras. Farmacogn. 2017;27:179–187. doi: 10.1016/j.bjp.2016.10.002. [DOI] [Google Scholar]
- 14.Luna L., Simirgiotis M.J., Lima B., Bórquez J., Feresin G.E., Tapia A. UHPLC-MS metabolome fingerprinting: The isolation of main compounds and antioxidant activity of the andean species Tetraglochin ameghinoi (Speg.) Speg. Molecules. 2018;23:793. doi: 10.3390/molecules23040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez J., Simirgiotis M.J., Lima B., Paredes J.D., Villegas Gabutti C.M., Gamarra-Luques C., Bórquez J., Luna L., Wendel G.H., Maria A.O., et al. Antioxidant, Gastroprotective, Cytotoxic Activities and UHPLC-PDA-Q Orbitrap Mass Spectrometry Identification of Metabolites in Baccharis grisebachii Decoction. Molecules. 2019;24:1085. doi: 10.3390/molecules24061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simirgiotis M.J., Quispe C., Areche C., Sepulveda B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC-Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules. 2016;21:245. doi: 10.3390/molecules21030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simirgiotis M.J., Quispe C., Bórquez J., Schmeda-Hirschmann G., Avendaño M., Sepúlveda B., Winterhalter P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crop. Prod. 2016;85:159–166. doi: 10.1016/j.indcrop.2016.02.054. [DOI] [Google Scholar]
- 18.Gómez J., Simirgiotis M.J., Lima B., Gamarra-Luques C., Bórquez J., Caballero D., Feresin G.E., Tapia A. UHPLC–Q/Orbitrap/MS/MS Fingerprinting, Free Radical Scavenging, and Antimicrobial Activity of Tessaria absinthiodes (Hook. &Arn.) DC. (Asteraceae) Lyophilized Decoction from Argentina and Chile. Antioxidants. 2019;8:593–609. doi: 10.3390/antiox8120593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cifuentes F., Palacios J., Nwokocha C.R., Bórquez J., Simirgiotis M.J., Norambuena I., Chiong M., Paredes A. Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat. Antioxidants. 2019;8:591. doi: 10.3390/antiox8120591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzie I.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2013. CLSI document M100-S23 (ISBN 1-56238-865-7 [Print]; ISBN 1-56238-866-5 [Electronic]) [Google Scholar]
- 23.Parejo I., Jauregui O., Sanchez-Rabaneda F., Viladomat F., Bastida J., Codina C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum vulgare) Using Liquid Chromatography−Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004;52:3679–3687. doi: 10.1021/jf030813h. [DOI] [PubMed] [Google Scholar]
- 24.Liao M., Yana P., Liua X., Dua Z., Jiaa S., Aybekc R., Lid A., Kaisac S., Jianga H. Spectrum-effect relationship for anti-tumor activity of shikonins and shikonofurans in medicinal Zicao by UHPLC-MS/MS and chemometric approaches. J. Chromatogr. B. 2019;1136:121924. doi: 10.1016/j.jchromb.2019.121924. [DOI] [PubMed] [Google Scholar]
- 25.Drewes S.E., Taylor C.W., Cunningham A. B. (+)-Afzelechin 3-Rhamnoside from Cassipourea gerrardzi. Phytochemlstry. 1992;31:1073–1075. doi: 10.1016/0031-9422(92)80083-Q. [DOI] [Google Scholar]
- 26.King F.E., Clark-Lewis J.W., Forbes W.F. The Chemistry of Extractives from Hardwoods. Part XXV, (–)-epiAfzelechin, a New Member of the Catechin Series. J. Chem. Soc. 1955:2948–2956. doi: 10.1039/JR9550002948. [DOI] [Google Scholar]
- 27.Narasimhachari N., Von Rudloff E. Gas-liquid chromatography of some flavonoid compounds and hydroxydiphenyls. Can. J. Chem. 1962;40:1960–1961. doi: 10.1139/v62-171. [DOI] [Google Scholar]
- 28.Nel R.J.J., Mthembua M., Coetzee J., Van Rensburga H., Malana E., Ferreira D. The novelavan-3-ol, (2R,3S)-guibourtinidol and its diastereomers. Phytochemistry. 1999;52:1153–1158. doi: 10.1016/S0031-9422(99)00348-9. [DOI] [Google Scholar]
- 29.Zhang L., Zhu C.C., Zhao Z.X., Lin C.Z. Simultaneous determination of seven flavonoids in Nerviliafordii with HPLC. ActaPharm. Sin. B. 2011;46:1237–1240. [PubMed] [Google Scholar]
- 30.Kitagawa I., Chen W., Hori K., Harada E., Yasuda N., Yoshikawa M., Ren J. Chemical studies of chinese licorice-roots. I. Elucidaton of Five New Flavonoid Constituents from the roots of Glycyrrhiza glabra L. Collected in Xinjiang. Chem. Pharm. Bull. 1994;42:1056–1062. doi: 10.1248/cpb.42.1056. [DOI] [PubMed] [Google Scholar]
- 31.Nomura T., Fukai T., Yamada S., Katayanagi M. Studies on the constituents of the cultivated Mulberry tree. I. Three new prenylflavones from the root bark of Morusalba L. Chem Pharm. Bull. 1978;26:1394–1402. doi: 10.1248/cpb.26.1394. [DOI] [Google Scholar]
- 32.Gardner D., Glen A.T., Turner W.B. Calonectrin and 15-deacetylcalonectrin, new trichothecanes from Calo-nectrianivalis. J. Chem. Soc. Perkin Trans. 1972;1:2576–2578. doi: 10.1039/p19720002576. [DOI] [PubMed] [Google Scholar]
- 33.Yamaki M., Bai L., Inoue K., Takagi S. Biphenanthrenes from Bletillastriata. Phytochemistry. 1989;28:3503–3505. doi: 10.1016/0031-9422(89)80373-5. [DOI] [Google Scholar]
- 34.Thoison O., Hnawia E., Guérite-Voegelein F., Sévenet T. Vedelianin, a hexahydroxanthene derivative isolated from Macarangavedeliana. Phytochemistry. 1992;31:1439–1442. doi: 10.1016/0031-9422(92)80315-6. [DOI] [Google Scholar]
- 35.Mahidol C., Prawat H., Ruchirawat S., Lihkitwitayawuid K., Lin L., Coriell G.A. Prenylatedflavanones from Derris reticulata. Phytochemistry. 1997;45:825–829. doi: 10.1016/S0031-9422(97)00001-0. [DOI] [Google Scholar]
- 36.Kiokias S., Varzakas T., Oreopoulou V. In vitro activity of vitamins, flavanoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008;48:78–93. doi: 10.1080/10408390601079975. [DOI] [PubMed] [Google Scholar]
- 37.Joshi R., Kulkarni Y.A., Wairkar S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018;215:43–56. doi: 10.1016/j.lfs.2018.10.066. [DOI] [PubMed] [Google Scholar]
- 38.Karim N., Jiac Z., Zhenga X., Cuib S., Chen W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018;79:35–54. doi: 10.1016/j.tifs.2018.06.012. [DOI] [Google Scholar]
- 39.Agus S., Achmadi S.S., Mubarik N.R. Antibacterial activity of naringenin-rich fraction of pigeon pea leaves toward Salmonella thypi. Asian. Pac. J. Trop. Biomed. 2017;7:725–728. doi: 10.1016/j.apjtb.2017.07.019. [DOI] [Google Scholar]
- 40.Wang L., Wen Q., Zenga X., Hana Z., Brennan C.S. Influence ofnaringenin adaptation and shockon resistance of Staphylococcus aureus and Escherichia coli to pulsed electric fields. Food Sci. Technol. 2019;107:308–317. [Google Scholar]
- 41.Nair M.S., Ma F., Lauc P., Upadhyayad I., Venkitanarayanan K. Inactivation of Escherichia coli O157:H7 in apple cider by resveratrol and naringenin. Food Microbiol. 2020;86:103327. doi: 10.1016/j.fm.2019.103327. [DOI] [PubMed] [Google Scholar]
- 42.Xue N., Wu X., Wu L., Li L., Wang F. Antinociceptive and anti-inflammatory effect of Naringenin in different nociceptive and inflammatory mice models. Life Sci. 2019;217:148–154. doi: 10.1016/j.lfs.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Wojnar W., Zych M., Kaczmarczyk-Sedlak I. Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed. Pharmacother. 2018;108:974–984. doi: 10.1016/j.biopha.2018.09.092. [DOI] [PubMed] [Google Scholar]
- 44.Mato E.P.M., Essop M.F., Owira P.M.O. Effects of naringenin on renal expression of organic cation transporter 1 and 2 proteins and metformin disposition in diabetic rats. J. Funct. Foods. 2019;59:1–7. doi: 10.1016/j.jff.2019.05.021. [DOI] [Google Scholar]
- 45.Chandran A.M.K., Christina H., Das S., Mumbrekar K.D., Rao B.S.S. Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ. Toxicol. Pharmacol. 2019;71:103224. doi: 10.1016/j.etap.2019.103224. [DOI] [PubMed] [Google Scholar]
- 46.Andújar I., Ríos J.L., Giner R.M., Recio M.C. Pharmacological Properties of Shikonin—A Review of Literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 47.Kourounakisa A.P., Assimopoulou A.N., Papageorgiou V.P., Gavalasa A., Kourounakisa P.N. Alkannin and Shikonin: Effect on Free Radical Processes and on Inflammation. A Preliminary Pharmacochemical Investigation. Arch. Pharm. Pharm. Med. Chem. 2002;6:262–266. doi: 10.1002/1521-4184(200208)335:6<262::AID-ARDP262>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 48.Assimopoulou A.N., Boskou D., Papageorgiou V.P. Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates. Food Chem. 2004;87:433–438. doi: 10.1016/j.foodchem.2003.12.017. [DOI] [Google Scholar]
- 49.Assimopoulou A.N., Papageorgiou V.P. Radical scavenging activity of Alkanna tinctoria root extracts and their main constituents, hydroxynaphthoquinones. Phytother Res. 2005;19:141–147. doi: 10.1002/ptr.1645. [DOI] [PubMed] [Google Scholar]
- 50.Jin R., Bai Y. Theoretical investigation of the radical scavenging activity of shikonin and acylshikonin derivatives. J. Mol. Model. 2012;18:1401–1408. doi: 10.1007/s00894-011-1170-9. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z., Liu T., Gan L., Wang T., Yuan X., Zhang B., Chen H., Zheng Q. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 2010;643:211–217. doi: 10.1016/j.ejphar.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Zhou Y., Jia G., Han B., Liu J., Teng Y., Lv J., Song Z., Li Y., Ji L. Shikonin suppresses tumor growth and synergizes with gemcitabine in a pancreatic cancer xenograft model: Involvement of NF-kB signaling pathway. Biochem. Pharmacol. 2014;88:322–333. doi: 10.1016/j.bcp.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 53.Li G., Min B., Zheng C., Lee J., Oh S., Ahn K., Lee H. Neuroprotective and Free Radical Scavenging Activities of Phenolic Compounds from Hovenia dulcis. Arch Pharm. Res. 2005;28:804–809. doi: 10.1007/BF02977346. [DOI] [PubMed] [Google Scholar]
- 54.Li D., Li X., Peng Z., Wang B. Flavanol Derivatives from Rhizophora stylosa and Their DPPH Radical Scavenging Activity. Molecules. 2007;12:1163–1169. doi: 10.3390/12051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruby K., Chauhan R., Sharma S., Dwivedi J. Polypharmacological activities of Bergenia species. Int. J. Pharm. Sci. Rev. Res. 2012;13:100–110. [Google Scholar]
- 56.Fu C., Wang H., Ling N.W., Song L., Huang D. Antioxidant Activity and Proanthocyanidin Profile of Selliguea feei Rhizomes. Molecules. 2013;18:4282–4292. doi: 10.3390/molecules18044282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y.S., Lim S.S., Shin K.H., Kim Y.S., Ohuchi K., Jung S.H. Anti-angiogenic and Anti-tumor Activities of 2 -Hydroxy-4-Methoxychalcone. Biol. Pharm. Bull. 2006;29:1028–1031. doi: 10.1248/bpb.29.1028. [DOI] [PubMed] [Google Scholar]
- 58.Özaslan M.S., Hatice Y.D., Aslan E., Beydemir S., Küfrevioglu Ö.I. Evaluation of chalcones as inhibitors of glutathione S-transferase. J. Biochem. Mol. Toxicol. 2018;32:e22047. doi: 10.1002/jbt.22047. [DOI] [PubMed] [Google Scholar]
- 59.Liu C.S., Chang C.C., Du Y.C., Chang F.R., Wu Y.C., Chang W.C., Hsieh T.J. 2-Hydroxy-4′-Methoxychalcone Inhibits Proliferation and Inflammation of Human Aortic Smooth Muscle Cells by Increasing the Expression of Peroxisome Proliferator–Activated Receptor Gamma. J. Cardiovasc. Pharmacol. 2012;59:339–351. doi: 10.1097/FJC.0b013e3182440486. [DOI] [PubMed] [Google Scholar]
- 60.Topczewski J.J., Wiemer D.F. First total synthesis of (+)-vedelianin, a potent antiproliferative agent. Tetrahedron Lett. 2011;52:1628–1630. doi: 10.1016/j.tetlet.2011.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Según P.A., Ogbolea O.O., Ismail F.M.D., Nahar L., Evans A.R., Ajaiyeoba E.O., Sarker S.D. Bioassay-guided isolation and structure elucidation of cytotoxic stilbenes and flavonols from the leaves of Macaranga barteri. Fitoterapia. 2019;134:151–157. doi: 10.1016/j.fitote.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Chang Lee J., Won S., Chao C., Wu F., Liu H., Ling P., Lin C., Su C. Morusin induces apoptosis and suppresses NF-jB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008;372:236–242. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 63.Park H., Min T., Chi G., Choi Y., Park S. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018;505:194–200. doi: 10.1016/j.bbrc.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 64.Kikkawa Y., Takaki S., Matsuda Y., Okabe K., Taniguchi M., Oomachi K., Samejima T., Katagiri F., Hozumi K., Nomizu M. The Influence of Tribenoside on Expression and Deposition of Epidermal Laminins in HaCaT Cells. Biol. Pharm. Bull. 2010;33:307–310. doi: 10.1248/bpb.33.307. [DOI] [PubMed] [Google Scholar]
- 65.Lorenc Z., Gökçe Ö. Tribenoside and lidocaine in the local treatment of hemorrhoids: An overview of clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2742–2751. [PubMed] [Google Scholar]
- 66.Sobeh M., Mahmoud M.F., Abdelfattah M.A.O., Cheng H., El-Shazly A.M., Wink M. A proanthocyanidin-rich extract from Cassia abbreviate exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018;213:38–47. doi: 10.1016/j.jep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Natarajan K., Singh S., Burke T.R., Grunberger D., Aggarwal B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-KB (tumor necrosis factor/okadaic acid/ceramide/phorbol ester/hydrogen peroxide) Proc. Natl. Acad. Sci. USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L., Jin Y., Chen H., Sun C., Fu W., Zheng L., Lu M., Chen P., Chen G., Zhang Y., et al. Discovery of caffeic acid phenethyl ester derivatives as novel myeloid differentiation protein 2 inhibitors for treatment of acute lung injury. Eur. J. Med. 2018;14:361–375. doi: 10.1016/j.ejmech.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 69.Tambuwala M.M., Kesharwani P., Shukla R., Thompson P.D., McCarron P.A. Caffeic acid phenethyl ester (CAPE) reverses fibrosis caused by chronic colon inflammation in murine model of colitis. Eur. J. Med. 2018;143:361–375. doi: 10.1016/j.prp.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 70.Guan Y., Chen H., Zhong Q. Nanoencapsulation of caffeic acid phenethyl ester in sucrose fatty acid esters to improve activities against cancer cells. J. Food Eng. 2019;246:125–133. doi: 10.1016/j.jfoodeng.2018.11.008. [DOI] [Google Scholar]
- 71.Murtaza G., Karim S., Akram M.R., Khan S.A., Azhar S., Mumtaz A., Bin Asad M.H.H. Caffeic Acid Phenethyl Ester and Therapeutic Potentials. Bio. Med. Res. Int. 2014:1–9. doi: 10.1155/2014/145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.