Abstract
The discovery of several revitalizing molecules that can stop or reduce the pathology of a wide range of diseases will be considered a major breakthrough of the present time. Available synthetic compounds may provoke side effects and health issues, which heightens the need for molecules from plants and other natural resources under discovery as potential methods of replacing synthetic compounds. In traditional medicinal therapies, several plant extracts and phytochemicals have been reported to impart remedial effects as better alternatives. Murraya koenigii (M. koenigii) belongs to the Rutaceae family, which is commonly used as a medicinally important herb of Indian origin in the Ayurvedic system of medicine. Previous reports have demonstrated that the leaves, roots, and bark of this plant are rich sources of carbazole alkaloids, which produce potent biological activities and pharmacological effects. These include antioxidant, antidiabetic, anti-inflammatory, antitumor, and neuroprotective activities. The present review provides insight into the major components of M. koenigii and their pharmacological activities against different pathological conditions. The review also emphasizes the need for more research on the molecular basis of such activity in various cellular and animal models to validate the efficacy of M. koenigii and its derivatives as potent therapeutic agents.
Keywords: Murraya koenigii, antioxidant, bioactive compounds, pharmacological activity, traditional medicine
1. Introduction
Presently, huge numbers of people in developing countries depend on medicinal plants for healthcare, skin care, economic benefits, and cultural development. For centuries, medicinal plants have been widely used in traditional medicine in countries like India, China, Germany, Thailand, etc. [1]. The World Health Organization (WHO) projected that 80% of the population relies on traditional medicine, which is clearly elucidated by the 19.4 billion USD global revenue for herbal remedies in 2010 [2]. Moreover, the demand for traditional medicinal plants is increasing; for instance, the market for medicinal plants is expanding at an annual rate of 20% in India. Likewise, in China, 30% to 50% of the total medicinal consumption is from preparations of traditional medicine [3]. Nearly 76.7% of the citizens of Thailand have reported mainly using traditional herbal medicine for their primary healthcare [4]. Around 90% of the German population uses natural remedies for certain health issues [5]. Therefore, the medicinal plants used in traditional medical treatments are significant in both developing and industrialized countries. This is clearly demonstrated by the worldwide market for traditional medicine. This market continues to gradually increase [6].
Murraya koenigii (M. koenigii) (L) Spreng (Family: Rutaceae) is usually known as “curry leaves”. The tropical and subtropical regions in the world have large distributions of M. koenigii [7]. Among the 14 global species belonging to the genus of Murraya, only two, M. koenigii and M. paniculate, are available in India. M. koenigii is more important due to its huge spectrum of traditional medicinal properties. For centuries, this plant has been used in diverse forms and holds a place of pride in Indian Ayurvedic medicine, known as “krishnanimba” [8]. Different parts of M. koenigii, such as its leaves, root, bark, and fruit, are known to promote various biological activities. Aromatic bioactive constituents in the leaves of M. koenigii retain their flavor and other qualities, even after drying [9,10,11,12,13,14]. M. koenigii leaves are slightly bitter in taste, pungent in smell, and weakly acidic. They are used as antihelminthics, analgesics, digestives, and appetizers in Indian cookery [15,16]. The green leaves of M. koenigii are used in treating piles, inflammation, itching, fresh cuts, dysentery, bruises, and edema. The roots are purgative to some extent. They are stimulating and used for common body aches. The bark is helpful in treating snakebites [17,18,19,20]. The essential oil extracted from M. koenigii leaves is reported to possess anti-oxidative, hepatoprotective [21,22,23,24], antimicrobial, antifungal [25,26,27], anti-inflammatory, and nephroprotective activities in animal models [28,29,30]. The medicinal properties of M. koenigii have been accredited to several chemical constituents of different carbazole alkaloids and other important metabolites, like terpenoids, flavonoids, phenolics, carbohydrates, carotenoids, vitamins, and nicotinic acid from different parts of the M. koenigii plant.
In recent years, greater attention has been paid to the use of M. koenigii in traditional medicines and home remedies. On the other hand, limited studies have been conducted for evaluating the pharmacological and medicinal efficacy of M. koenigii in promoting health benefits and curing disease [31,32,33,34,35,36]. This review will discuss the traditional medicinal use of M. koenigii and its bioactive compounds, highlighting their pharmacological effects. This review aims to present a well-managed summary and possible recommendation on existing studies to provide information regarding the current reports that can direct future research. Therefore, instead of discussing a few selected studies in a specific time interval, the present review will discuss and cover previous and existing major studies on M. koenigii related to the topics chosen. The details, like phytochemical screening, identification, and pharmacological activities, will be systematically categorized, compared, and summarized. We hypothesized that, through all of these efforts, a good summary on pharmacological activities that could initiate future perspectives with the utmost clarity could be produced.
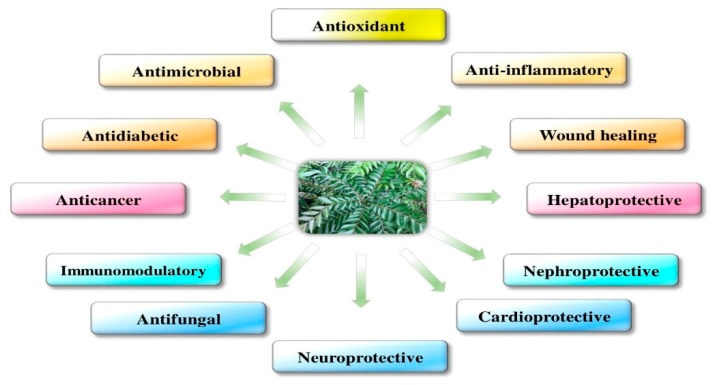
The pharmacological activities of M. koenigii are discussed in detail in Figure 1.
Figure 1.
Pharmacological activities of Murraya koenigii.
2. Murraya koenigii (M. koenigii)
2.1. Traditional Uses of M. koenigii
Essential oils and fresh leaf powder of M. koenigii are useful in seasoning food items and preparing ready-to-eat foods. Owing to the higher antimicrobial activities of the essential oil from leaf extracts [37,38], this oil can also be used as perfume and flavor agents in traditional practice. Fresh curry leaves are boiled with a coconut oil mixture until they are reduced to a black residue to produce an excellent hair tonic for retaining a normal hair tone and improving hair growth. Curry leaves have a traditional use, either whole or in parts, as antidiarrheal, antifungal, blood purifying, anti-inflammatory, and anti-depressant agents [39,40,41].
2.2. Medicinal Uses of M. koenigii
M. koenigii has numerous disease remedial activities, for instance, different parts of the plant, such as the leaves, roots, and bark, can be prepared as tonics for inducing digestion and flatulence or as antiemetics [25,42]. After decoction, the leaves become bitter to the taste and are helpful in reducing fever. The juice of the root is given to manage renal pains [43]. The leaves and roots can be given as an anthelmenticku, analgesic, cure for piles, body heat reducer, and thirst quencher and are also helpful in reducing inflammation and itching. They are also useful in managing leucoderma and blood disorders. When eaten raw, the green leaves can offer a cure for dysentery, and when they are boiled in milk, the paste has good application prospects for curing poisonous bites and eruptions [44].
2.3. Phytochemistry of M. koenigii
A wide range of phytochemicals have been isolated from the leaves, roots, and stem bark of M. koenigii. M. koenigii extracts of leaves, root, stem bark, fruits, and seeds have yielded alkaloids, flavonoids, terpenoids, and polyphenols, as shown in Table 1. The plant leaves contain a substantial amount of proximate composition; the moisture is 63.2%, protein is 8.8%, carbohydrate is 39.4%, total nitrogen is 1.15%, fat is 6.15%, total sugars are 18.92%, starch is 14.6%, and crude fiber is 6.8%. The leaves have been reported as a significant source of several vitamins, such as vitamin A (B-carotene), with a level of 6.04 ± 0.02 mg/100 g; vitamin B3, (niacin), with a level of 2.73 ± 0.02 mg/100 g; vitamin B1 (thiamin), with a level of 0.89 ± 0.01 mg/100 g; calcium, with a level of 19.73 ± 0.02 mg/100 g; magnesium, with a level of 49.06 ± 0.02 mg/100 g; and sodium, with a level of 16.50 ± 0.21 mg/100 g. The alcohol-soluble extract has a value of 1.82%, ash has a value of 13.06%, acid-insoluble ash has a value of 1.35%, cold water (20 °C) extractive has a value of 27.33%, and maximum of hot-water-soluble extractive has a value of 33.45% [15,45]. Wide ranges of carbazole alkaloids, essential oils, terpenoids, and flavonoids have numerous beneficial roles. Table 2 summarizes the major chemical constituents of M. koenigii and its pharmacological activities.
Table 1.
Phytochemical compounds identified from M. koenigii.
| Compound | Molecular Formula | Plant Part | References |
|---|---|---|---|
| Alkaloids | |||
| Mahanine | C23H25NO2 | Leaves, stem bark, and seeds | [45,46,47,48,49] |
| Mahanimbine | C23H25NO | Leaves, roots, seeds, and fruits | [47,48,49] |
| Murrayanol | C24H29NO2 | Leaves, roots, and fruits | [47,48,49] |
| Koenimbine | C19H19NO2 | Leaves, seeds, and fruits | [46,47,48,49] |
| O-Methylmurrayamine A | C19H20NO2 | Leaves | [45,46,47,48] |
| Koenigicine | C20H21NO3 | Leaves | [45,46,47,48] |
| Koenigine | C19H19NO3 | Leaves and stem bark | [47,48,49] |
| Murrayone (Coumarine) | C15H14O4 | Leaves | [45,46,47,48] |
| Mahanimbicine | C23H25NO | Leaves | [45,46,47,48] |
| Bicyclomahanimbicine | C23H25NO | Leaves | [45,46,47,48] |
| Phebalosin | C15H14O4 | Leaves | [45,46,47,48] |
| Isomahanimbine | C23H25NO | Leaves and roots | [45,46,48] |
| Koenimbidine | C20H21NO3 | Leaves and roots | [45,46,48] |
| Euchrestine B | C24H29NO2 | Leaves | [45,46] |
| Bismurrayafoline E | C48H56N2O4 | Leaves | [45,46] |
| Isomahanine | C23H25NO2 | Leaves, seeds, and fruits | [45,46,49] |
| Mahanimbinine | C23H27NO2 | Leaves and seeds | [45,46,49] |
| Girinimbilol | C18H19NO | Leaves | [45,46] |
| Pyrayafoline-d | C23H25NO2 | Leaves and stem bark | [45,46,49] |
| Glycozoline | C14H13NO | Leaves | [45,46] |
| Cyclomahanimbine | C23H25NO | Leaves | [45,46] |
| Isomurrayazoline | C23H25NO | Leaves | [45,46] |
| Mahanimboline | C23H25NO2 | Leaves | [49] |
| Mukonicine | C20H21NO3 | Leaves | [49] |
| Isolongifolene | C15H24 | Leaves | [49] |
| Mukonal | C13H9NO2 | Stems | [49] |
| Mukeic acid | C14H11NO3 | Stems | [49] |
| 9-Carbethoxy-3-methyl carbazole | C16H15NO2 | Roots and stems | [49] |
| 9-Formyl-3-methyl carbazole | C14H11NO | Roots and stems | [49] |
| Murrayazolinol | C23H25NO2 | Stems bark | [49,50] |
| Mahanimbinol | C23H27NO | Stems bark | [49,50] |
| Mukoeic acid | C14H11NO3 | Stem bark | [49,50] |
| Osthol | C15H16O3 | Stem bark | [49,50] |
| Umbelliferone | C9H6O3 | Stem bark | [49,50] |
| Murrayanine | C14H11NO2 | Stem bark | [49,50] |
| Mukoenine-A | C18H19NO | Roots and stem bark | [49,50] |
| Mukoenine-B | C23H25NO2 | Roots and stem bark | [49,50] |
| Mukoline | C14H13NO2 | Roots | [49,50] |
| Mukolidine | C14H11NO2 | Roots and stem bark | [49,50] |
| (M)-murrastifoline-F | C28H24N2O2 | Roots and stem bark | [49,50] |
| 3-Methyl-9H-carbazole-9-carbaldehyde | C14H11NO | Roots | [49,50] |
| Bismahanine | C46H48N2O4 | Roots and stem bark | [49,50] |
| Bikoeniquinone A | C27H20N2O3 | Roots and stem bark | [49,50] |
| Bismurrayaquinone | C26H16N2O4 | Roots and stem bark | [49,50] |
| 3-Methylcarbazole | C13H11N | Roots | [49] |
| Murrayafoline A | C14H13NO | Roots | [49] |
| Murrayakonine A | C37H36N2O2 | Leaves and stems | [39] |
| Murrayakonine B | C23H23NO2 | Leaves and stems | [39] |
| Murrayakonine C | C24H25NO3 | Leaves and stems | [39] |
| Murrayakonine D | C23H25NO2 | Leaves and stems | [39] |
| Girinimbine | C18H17NO | Roots, stem bark, and seeds | [49,51] |
| Murrayacine | C18H15NO2 | Stem and bark | [49,51] |
| Murrayazoline | C23H25NO | Stem and bark | [49,51] |
| Flavonoids | |||
| Quercetin | C15H10O7 | Leaves | [52] |
| Apigenin | C15H10O5 | Leaves | [52] |
| Kaempferol | C15H10O6 | Leaves | [52] |
| Rutin | C27H30O16 | Leaves | [52] |
| Catechin | C15H14O6 | Leaves | [52] |
| Myricetin | C15H10O8 | Leaves | [52] |
| 4-O-β-d-Rutinosyl-3-methoxyphenyl-1-propanone | C22H32O12 | Leaves | [53] |
| 1-O-β-d-Rutinosyl-2(R)-ethyl-1-pentanol | C19H36O10 | Leaves | [53] |
| 8-Phenylethyl-O-β-d-rutinoside | C20H30O10 | Leaves | [54] |
| Terpenoids | |||
| Blumenol A | C13H20O3 | Leaves | [53] |
| Icariside B1 | C19H30O8 | Leaves | [53] |
| Loliolide | C11H16O3 | Leaves | [53] |
| Blumenol A | C13H20O3 | Leaves | [53] |
| Icariside B1 | C19H30O8 | Leaves | [53] |
| (−)-Epiloliolide | C11H16O3 | Leaves | [55] |
| (−)-α-pinene | C10H16 | Leaves | [55] |
| (−)-β-pinene | C10H16 | Leaves | [55] |
| (+)-β-pinene | C10H16 | Leaves | [55] |
| (+)-sabinene | C10H16 | Leaves | [55] |
| Squalene | C30H50 | Leaves and bark | [56] |
| β-sitosterol | C29H50O | Leaves and bark | [56,57] |
| Polyphenols | |||
| Selin-11-en-4α-ol | C15H26O | Leaves and bark | [56] |
| 2-hydroxy-4-methoxy-3,6-dimethylbenzoic acid | C10H12O4 | Bark | [56] |
Table 2.
The major bioactive compounds of M. koenigii and its pharmacological activities.
| Serial No. | Constituent | Constituent Structure | Activity |
|---|---|---|---|
| 1 | Mahanine |
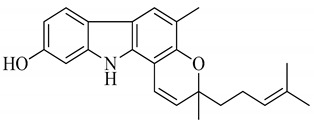
|
Cytotoxicity, anti-microbial, and anti-cancer |
| 2 | Mahanimbine |
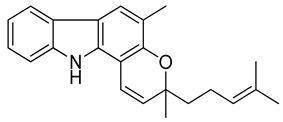
|
Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 3 | Isomahanine |
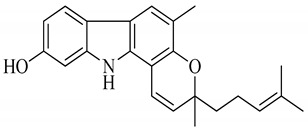
|
Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 4 | koenimbine |
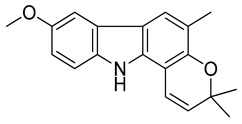
|
Cytotoxicity and anti-diarrhea |
| 5 | Girinimbine |
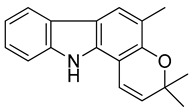
|
Anti-tumor |
| 6 | Isolongifolene |
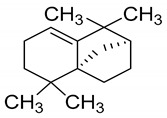
|
Anti-oxidant and neuroprotective |
| 7 | Pyrayafoline D |
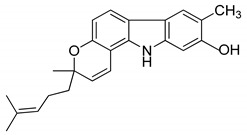
|
Anti-cancer and anti-bacterial |
| 8 | Murrayafoline |
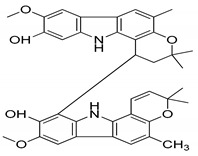
|
Cytotoxicity and anti-inflammatory |
| 9 | Murrayazoline |
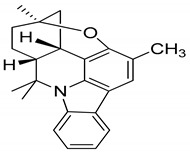
|
Cytotoxicity and anti-tumor |
| 10 | Koenoline |
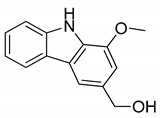
|
Cytotoxicity |
| 11 | 9-formyl-3-methyl carbazole |
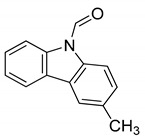
|
Anti-oxidant |
| 12 | O-Methylmurrayamine |
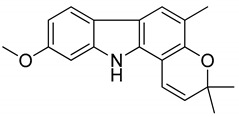
|
Anti-oxidant and neuroprotective |
| 13 | Koenine |
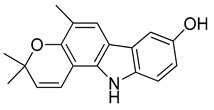
|
Anti-oxidant |
| 14 | Koenigine |
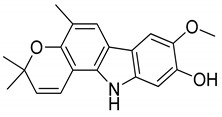
|
Anti-oxidant |
| 15 | Mukonicine |
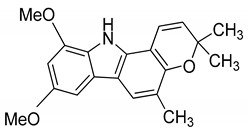
|
Anti-oxidant |
| 16 | Mahanimbinine |
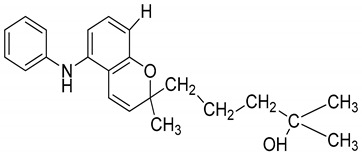
|
Anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 17 | Murrayacinine |
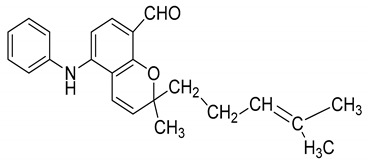
|
Anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 18 | Mahanimboline |
|
Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 19 | Mukoeic acid |
|
Anti-oxidant |
| 20 | Murrayanine |
|
Anti-oxidant |
2.4. Bioavailability Study of M. koenigii-Derived Active Constituents
An in vivo pharmacokinetic study revealed that after oral administration of the bioactive compounds at the rate of 0.1 gm/kg body weight (b.w.), the maximum systemic concentration (Cmax) of koenimbine was 1.81 ± 0.55 μM and koenidine was 2.82 ± 0.53 μM, and the time required to reach the maximum concentration was 49.8 ± 8.4 min and 240 ± 0.00 min, respectively [58]. Bhattacharya et al. demonstrated the bioavailability of mahanine—another important bioactive compound of M. koenigii—in mice through blood serum estimations based on high-performance liquid chromatography (HPLC) analysis. Mahanine, at a dose of 100 mg/kg of body weight, was found to take 60 min to reach the maximum concentration in blood serum [59].
3. Molecular Mechanism and Activities of M. koenigii
3.1. Antioxidants
Reactive oxygen species (ROS), such as singlet oxygen (O2), hydrogen peroxide (H2O2), the superoxide anion (O2•−), and the hydroxyl radical (•OH), are often generated as byproducts of cellular metabolic reactions and exogenous induction. These ROS create homeostatic imbalances, which lead to the generation of oxidative stress, which in turn, induces cell death and tissue injury [60]. ROS in elevated levels can damage biomolecules such as nucleic acids, proteins, and lipids [61]. Even though the antioxidant defense systems like enzymatic antioxidants and non-enzymatic antioxidants are functioning, uncontrolled ROS accumulation during the life cycle promotes the development of age-dependent diseases, like cancer, atherosclerosis, arthritis etc. [62]. Natural antioxidants from plant sources have been considered a promising therapy for the prevention and treatment of these diseases, especially neurodegenerative disorders, cardiovascular diseases, cancer, and other conditions. Various natural bioactive compounds, such as mahanine, mahanimbine, isolongifolene, koenimbine, girinimbine, isomahanine, koenoline, and O-methylmurrayamine, are present in M. koenigii and exhibit remarkable antioxidant properties [63,64].
The leaf extracts of M. koenigii have high antioxidant activities [65]. Rao et al. evaluated the antioxidant activities of water and an ethanol extract of M. koenigii assessed by the, α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging assay, with quercetin as a positive control. The ethanolic extract of M. koenigii showed an 80% scavenging activity, which was similar to the activities exhibited by the control antioxidant compound quercetin [66]. Gupta et al. evaluated the antioxidant activities of acetone, alcohol, and aqueous extracts of M. koenigii by the DPPH free radical scavenging assay, with ascorbic acid as a positive control. The extracts of M. koenigii exhibited activities with an half-maximum effective concentration (EC50) value of acetone of 81.81 ± 19.92 at 4.7 µg/mL, alcohol of 79.80 ± 18.68 at 4.1 µg/mL, and aqueous extract of 62.82 ± 13.62 at 4.4 µg/mL, which was comparable to the EC50 value exhibited by ascorbic acid (the positive control), which was 97.13 ± 12.64 at 2.69 µg/mL [67]. Zahin et al. also evaluated the antioxidant activities of both ethyl acetate and petroleum ether fractions of M. koenigii through DPPH radical scavenging assay, cupric reducing antioxidant capacity (CUPRAC), and ferric reducing antioxidant power (FRAP) assays, with ascorbic acid as a positive control. The benzene fraction of M. koenigii was found to be the most active free radical scavenger, exhibiting an 88.3% decrease at a concentration of 100 μg/mL, followed by ethyl acetate at 79.5% and petroleum ether at 78.7%, while positive controls of ascorbic acid and butylated hydroxytoluene (BHT) at a concentration of 100 μg/mL inhibited 93.1% and 86.5% DPPH absorption, respectively. Similarly, the antioxidant activity created by reducing activity and CUPRAC assays indicated the highest reducing potential in the benzene fraction, followed by petroleum ether and ethyl acetate. The activity was greater than that of ascorbic acid and on par with that of BHT [68].
Yogesh et al. evaluated the antioxidant activity of berry extracts of M. koenigii by DPPH free radical scavenging activity and reducing power assays. The results indicated that an M. koenigii berry extract is a powerful free radical scavenger compared to known antioxidants, such as butylated hydroxytoluene, ascorbic acid, and tannic acid [69]. Tomar et al. evaluated the total antioxidant activity of acetone and petroleum ether extracts of younger and older M. koenigii leaves by estimating the H2O2 scavenging activity. The acetone extract of old leaves was found to have a maximum activity at 151.58%, and for young leaves in petroleum ether, the value was 72.23% [70]. Waghmare et al. evaluated the antioxidant property of fruit extracts of M. koenigii with DPPH free radical scavenging activity, inhibition of nitric oxide radical (NO) and thiobarbituric acid reactive substances (TBARS) activity, and reducing power assays, and •OH was also estimated, with vitamin C as a positive control. The fruit extract of M. koenigii exhibited antioxidant activities, and the EC50 value of the extracts for the DPPH assay was 2.6 mg/mL; for the NO radical, was 2.9 mg/mL; for TBARS, was 3.1 mg/mL; for the reducing power assay, was 2.7 mg/mL; and for H2O2, was 3.3 mg/mL, which were comparable to the EC50 value of 5 mg/mL exhibited by the vitamin C positive control [71]. The antioxidant activities exhibited by the crude extracts of M. koenigii were probably due to the presence of flavonoids and phenolic derivatives. The above studies revealed that various extracts of M. koenigii display high antioxidant activity. The studies also indicated the potential for this plant to be a natural source of strong antioxidant substances that can be used in therapy for human diseases induced by ROS.
3.2. Oxidative Stress
Chemical species with one or more unpaired electrons are called free radicals. In biological systems, the term “free radicals” refers to reactive oxygen species (ROS). Major ROS include O2•−, H2O2, and •OH [72]. In addition to ROS, reactive nitrogen species (RNS), including peroxynitrite (NO3−), NO, and S-nitrosothiols, also contribute to the generation of oxidative stress. Both ROS and RNS arise as intermediates in several metabolic processes and are specifically produced as part of the cellular defense against invasive pathogens. Free radicals also regulate many processes, including cellular growth, glucose metabolism, and proliferation [73].
Apart from certain beneficial effects, free radicals induce various deleterious effects. In a non-specific manner, ROS can react on significant biomolecules, which leads to deleterious effects like a loss of enzyme activity, genetic mutations and permeability alterations in the cell membrane, and RNS-induced protein S-nitrosylation [74]. Because DNA is constantly attacked by the free radicals, around 75,000 to 100,000 DNA damage events might occur in each cell per day. •OH is the most reactive species and interacts with all biological molecules, including the C-8 position of guanine to form 8-hydroxyguanine, which is one of the most frequently found oxidized bases in DNA [75].
An increase in the free radical concentration in the body can cause subsequent oxidative and cellular damage to lipids, proteins, RNA, and DNA [76]. The leaf extract of M. koenigii has recently been shown to possess potential antioxidant activity and protection against oxidative stress induced in diabetes [77]. The aqueous leaf extract of M. koenigii has been shown to reduce lipid peroxidation and decrease cellular damage, thereby protecting the liver from ethanol-induced toxicity [31]. Khan et al. reported the antioxidant effect and preventive effect of curry leaves in cadmium-induced oxidative stress, cardiac tissue damage, and alterations in normal cardiac functions in rats [78]. Mitra et al. reported the heavy metal chelating activity of an M. koenigii leaf extract. They found that there was a significant decrease in the tissue cadmium level when the rats were pre-treated with the M. koenigii leaf extract before cadmium administration [79].
3.3. Mitochondrial Dysfunction
Mitochondria are the primary source of high-energy metabolism within the cell. Mitochondria are known as the powerhouse of the living cell. Mitochondria also regulate calcium homeostasis and play a role in scavenging free radicals and controlling programmed cell death and/or the apoptosis-signaling pathway [80]. Mitochondrial damage leads to reduced adenosine triphosphate (ATP) production, increased ROS generation, impaired calcium buffering, damage to mitochondrial DNA (mtDNA), an altered mitochondrial morphology, and alterations in mitochondrial fission and fusion. All these events eventually lead to cell death [81]. It is currently believed that the majority of ROS are generated by mitochondrial complexes I and III, likely due to the release of electrons by NADH and dihydroflavine-adenine dinucleotide (FADH2) into the electron transport chain (ETC). Mitochondrial dysfunction is a characteristic of all chronic diseases and aging. It is characterized by a loss of efficiency in the ETC, as well as reductions in the synthesis of high-energy molecules [82]. These diseases include neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis, multiple sclerosis, Huntington’s disease, cardiovascular diseases, auto-immune diseases, diabetes, and others [83,84].
Mitochondria, as essential organelles, have a noteworthy role in the viability of neuronal cells. Excess ROS formation is due to complex I inhibition inducing impairments in the mitochondrial membrane potential (MMP) and the pro-apoptotic members are believed to permeabilize the outer mitochondrial membrane due to the formation of oligomeric pores, which permits the release of apoptogenic molecules from the intermembrane space. Recent studies have evaluated the neuroprotective activities of isolongifolene and structurally similar compounds, such as girinimbine, murrayazoline, and O-methylmurrayamine A isolated from M. koenigii. By using different in vitro assays, a study reported that the above bioactive compounds exhibited the ability to restore the MMP levels and repair the mitochondrial damage [85,86,87].
3.4. Inflammation
Tissue injury, cell damage, infections due to pathogens, and alterations in biochemicals lead to a biological response called inflammation. In neurological disorders, the important components involved in inflammatory processes are believed to be mast cells, ependymal cells, microglia, astrocytes, and macrophages [88]. Microglia, a type of neuronal support cell acting as resident macrophages located throughout the brain by changing their morphology, actively respond to inflammation and participate in removing damaged neurons and pathogens. An ethanol extract from M. koenigii leaves showed significant analgesic and anti-inflammatory activity when explored using carrageenan-induced hind paw edema in albino rats [89]. Another study also confirmed the anti-inflammatory activity of an M. koenigii leaf extract in carrageenan-induced paw edema [90]. Additionally, the study recognized the analgesic activity of curry leaves with several experimental models. M. koenigii leaf extracts effectively attenuate the pain which is induced by an intraperitoneal injection of acetic acid and subplantar injection of formalin in mice, and the analgesic effect was elucidated with the writhing responses and pain responses in the late phase. Furthermore, it was reported that higher concentrations (20 and 40 mg/kg, per os (p.o.)) reduced the early-phase inflammatory responses induced by formalin [91].
Khurana et al. evaluated the in vitro and in vivo efficacy of a hydroalcoholic extract of M. koenigii curry leaves rich in carbazole alkaloids against lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 cells. The activity of inflammatory cytokines interleukin 1 beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and p65-NFκB was significantly reduced by the hydroalcoholic extract of M. koenigii. In addition, the hydroalcoholic extract of M. koenigii reduced the expression of nitrotyrosine (NT), myeloperoxidase (MPO), IL-1β, intercellular adhesion molecule 1 (ICAM-1), and cyclooxygenase (COX-2), and increased the expression of Nrf2 [92]. Iman et al. evaluated the anti-inflammatory activity of an extract of M. koenigii and its bioactive compound girinimbine against lipopolysaccharide/interferon-gamma-induced RAW 264.7 cells. The girinimbine showed reduced levels of NO overproduction and pro-inflammatory cytokine levels IL-1β and TNF-α in the peritoneal fluid. These findings strongly suggest that girinimbine could act as an anti-inflammatory agent by suppressing inflammation [85]. Another study also confirmed the anti-inflammatory activity of an M. koenigii leaf extract. Bioactive compounds like murrayakonine A, O-methylmurrayamine A, and mukolidine were reported for their efficiency in inhibiting TNF-α and IL-6 release in LPS-induced inflammation in human peripheral blood mononuclear cells (PBMCs) [39]. The above studies have shed light on the mechanism of the anti-inflammatory activity of M. koenigii leaves and their active compounds, which are comparable to nonsteroidal anti-inflammatory drugs (NSAIDs).
3.5. Apoptosis
Apoptosis is a physiological programmed cell death mechanism which is essential for the flawless growth and development of organisms. Furthermore, it is a lively physiological course causing the self-destruction of cells that comprises lethal biochemical and morphological changes in the nucleus and cytoplasm. During cellular strain like oxidative stress and DNA damage, the process of apoptosis can arise, particularly in cells with high proliferation rates and a high expression of pro-apoptotic genes [93]. Intrinsic and extrinsic pathways regulate apoptosis, but both pathways are associated and the molecules involved in those pathways can influence one another.
Previous findings have demonstrated that an M. koenigii extract and its primary active compounds regulate multiple signaling pathways, including phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT), mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK). M. koenigii and its primary active compounds exert complementary effects on oxidative stress and the alteration of proteins [94,95]. They are associated with mitochondrial-mediated apoptotic pathways. Murrayazoline and O-methylmurrayamine were shown to induce the downregulation of Akt/mTOR, suggesting downstream targeting of the cell survival pathway and an ability to potentiate the antitumor activity of D.L. Dexter (DLD-1) colon cancer cells; interestingly, this inhibition of the Akt/mTOR pathway could possibly activate the intrinsic apoptotic program [86]. Mahanine and isomahanine derived from M. koenigii leaves exert anticancer effects on oral squamous cell carcinoma cells via the induction of microtubule-associated protein 1 light chain 3, type II (LC3B-II), and cleaved caspase-3, suggesting the inhibition of autophagic flux [96]. In human leukemic cells, Mahanine was reported to induce apoptosis by interrupting signal transfer between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways in humans [59]. Recently, it was reported that girinimbine, a carbazole alkaloid isolated from M. koenigii, inhibited the growth of and induced apoptosis in human hepatocellular carcinoma cells (HepG2) [97]. In addition, Xin et al. reported that girinimbine inhibited ovarian cancer cell proliferation in a dose-dependent manner. It also inducted apoptosis and cell cycle arrest due to inhibition of the PI3K/AKT/mTOR and Wnt/b-catenin signaling pathways [98].
The alkaloid koenimbin found in M. koenigii was shown to extend pro-apoptotic activities in MCF-7 cancer cells by inhibiting glycogen synthase kinase-3 beta (GSK-3β). Koenimbin induces apoptotic cell death, phosphorylation, the accumulation of β-catenin, and the activation of nuclear factor-κB (NF-kB) in cancers. Moreover, koenimbin suppresses the expression of various anti-apoptotic genes involved in the regulation of cell proliferation and apoptosis [99]. Koenimbine has also been reported to trigger caspase activation, induce the release of cytochrome c, decrease the anti-apoptotic proteins, and increase the pro-apoptotic proteins, and all of these events lead to intrinsic apoptotic pathway activation [99]. Another study demonstrated that pyrayafoline-D and murrafoline-I isolated from M. koenigii could induce apoptosis in HL-60 cells. The same study also induced the loss of mitochondrial membrane potential and the subsequent activation of caspase-9/caspase-3, leading to the activation of apoptotic pathways [100].
4. Beneficial Pharmacological Activities of M. koenigii and Its Primary Active Derivatives
4.1. Antifungal Activity
The antifungal activity of M. koenigii has been reported in various studies. For example, the essential oil of the leaves was reported to possess antifungal activity [18]. The antifungal activity of the leaves of M. koenigii is due to the presence of phytochemical constituents of complex molecular structures and diverse action mechanisms, viz. alkaloids, terpenoids, flavonoids, phenolics, tannins, and saponins, which are known for their antimicrobial properties. Different investigations support the traditional use of the plant as an antifungal agent. In vitro antifungal activity may explain the use of curry leaves for the treatment of diarrhea, dysentery, and skin eruptions in folklore medicines [19]. Bioactive compounds of M. koenigii appreciably hold the ability of mycelial growth inhibition and thereby promote antifungal activity. The antifungal activity of M. koenigii against a wide range of pathogenic fungi has been studied. Penicillium notatum, Aspergillus flavus, Aspergillus niger, Fusarium moniliforme, Mucor mucedo, Penicillium funiculosum etc., were isolated from infected saplings and spoiled foods based on alterations of their growth characteristics, mycelial morphology, and spore morphology (Table 3) [27]. The ethanolic extract of M. koenigii exhibited notable effects on the hyphal morphology; namely, an increase in branching potential, which resulted in the development of short slender branches of hyphae with swollen tips. Such effects are usual for any antifungal compound. Bioactive compounds like girinimbine, murrayanine, marmesin-1′-O-beta-D’galactopyranoside, mahanine, murrayacine, mukoeic acid, murrayazolinine, girinimbilol, pyrafoline-D, and murrafoline-I are present in stem bark. Girinimbine, murrayanine, and marmesin-1′-O-beta-D’galactopyranoside have notable anti-fungal activity [20,101].
Table 3.
An overview of the pharmacological activities of M. koenigii and its primary bioactive compounds.
| Pharmacological Activities | Plant Parts | Extract | Bioactive Compounds | Model | Main Finding | Reference |
|---|---|---|---|---|---|---|
| In vitro studies | ||||||
| Antifungal | Leaves | Essential oil | − | Disc diffusion method | Essential oil extracted from M. koenigii exhibited activities with MIC in the range of 25.5 to 75 μg/mL against pathogenic fungi A. niger, F. moniliforme, P. notatum, M. mucedo, and P. funiculosum | [27] |
| Antibacterial | Leaves | Solvent-free microwave extraction | − | Soy agar | Minimum inhibitory concentrations (MIC) of solvent-free microwave extraction (SFME) and hydro-distilled oil from M. koenigii with values of 400 and 600 μg/mL against L. innocua SFME-essential oil at 300 μg/mL provided 92% inhibition, indicating its antibacterial potential | [37] |
| Antibacterial | Leaves | Methanol | Koenine, koenigine, and mahanine | Broth micro-dilution assay | Koenine, koenigine, and mahanine extracted from M. koenigii exhibited activities with MIC values of 3.12–12.5 µg/mL against bacterial strains S. aureus and K. pneumonia | [40] |
| Antibacterial | Leaves | Aqueous | − | Agar diffusion assay | M. koenigii-AGNPs exhibited inhibitory activity against E. coli and S. aureus, with a value of 16 mm for M. koenigii-AgNPs and 15 mm for AgNO3 solution at 100 µg/well | [18] |
| Antibacterial | Leaves | Essential oil | − | Microtiter assay | Essential oil extracts of M. koenigii treatment resulted in a reduction of biofilm formation in P. aeruginosa PAO1. M. koenigii essential oil may effectively control Pseudomonas biofilms in indwelling medical device | [19] |
| Antibacterial | Leaves | Petroleum ether, ethanol, and water | − | Colony-forming unit (CFU) assay | Ethanol extracts of M. koenigii exhibited activity half maximal inhibitory concentration ((IC50) of 400 μg/mL) against the mycobacterium smegmatis compared to petroleum ether and water extracts | [20] |
| Hepatoprotective | Leaves | Aqueous | − | Hep G2 cell line | M. koenigii leaves preventing alcohol-induced cellular damage | [31] |
| Antioxidant | Leaves | Ethanol | − | DPPH free radical scavenging assay | exhibited activities with IC50 values of 21.4–49.5 µg/mL | [21] |
| Antioxidant | Leaves | Aqueous | − | TBARS, CAT, SOD, and glutathione (GSH) assay | Carbazole alkaloids from M. koenigii extract exhibited activity with IC50 values of 120 μg/mL in an ethanol-induced hepatotoxicity in vitro model | [31] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | DPPH free radical scavenging assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 36.46 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | ABTS radical scavenging assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 11.55 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | Superoxide assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 11.47 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | H2O2 Assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 54.06 μg/mL | [64] |
| Antioxidant | Leaves | Ethanoic | − | DPPH free radical scavenging assay | The ethanoic extract of M. koenigii showed an 80% scavenging activity, which was similar to the activities exhibited by the control antioxidant compound quercetin | [66] |
| Antioxidant | Leaves | Aqueous, alcohol, and acetone | − | DPPH free radical scavenging assay | The extracts of M. koenigii exhibited activities with an EC50 value of acetone of 4.7 µg/mL, alcohol of 4.1 µg/mL, and aqueous of 4.4 µg/mL, which were comparable to the EC50 value of 2.6 µg/mL exhibited by ascorbic acid, which was the positive control | [67] |
| Antioxidant | Leaves | Petroleum ether and ethyl acetate | − | Cupric-reducing antioxidant capacity | CUPRAC assays indicated the highest reducing potential in the benzene fraction, followed by petroleum ether and ethyl acetate | [68] |
| Antioxidant | Leaves | Benzene, ethyl acetate, acetone, methanol, and ethanol | − | DPPH free radical scavenging assay | Results showed that for 100 µg/mL, the benzene fraction extracted from M. koenigii showed 88.3% free radical scavenging activity, followed by ethyl acetate (79.5%), petrol ether (78.7%), acetone (66.1%), methanol (50.7%), and ethanol (53.0%) fractions, respectively, with the positive control being ascorbic acid (93.1%) | [69] |
| Antioxidant | Fruits | Aqueous | − | DPPH free radical scavenging assay | Fruit extracted from M. koenigii exhibited activities with an EC50 value of 2.6 mg/mL | [71] |
| Cytotoxicity | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine | MTT assay | Girinimbine was shown to significantly inhibit the proliferation of HT-29 cells with an IC50 value of 4.79 ± 0.74 μg/mL. | [85] |
| Cytotoxicity | Leaves | Ethanol | Murrayazoline and O-methylmurrayamine A | MTT assay | Murrayazoline and O-methylmurrayamine A exhibited activities with IC50 values of 5.7 and 17.9 mM in both HEK-293 and HaCaT cell lines, respectively | [86] |
| Cytotoxicity | − | − | Isolongifolene | MTT assay | Isolongifolene exhibited activities at 10 µM, showing a 90% viability in SH-SY5Y cells | [87] |
| Cytotoxicity | Leaves | Methanol | − | MTT assay | M. koenigii methanolic extract exhibited activities with IC50 values >400 µg/mL in the CLS-354 cell line | [96] |
| Cytotoxicity | Leaves | Ethanol | − | MTT assay | M. koenigii ethanolic extract exhibited activities with an IC50 value of 20 µg/mL in the mouse macrophage RAW 264.7 cell line | [20] |
| Cytotoxicity | Leaves | Hexane, ethyl acetate, and methanol | − | MTT assay | Three extracts of M. koenigii exhibited were very active, with values of <1 μg/mL to 2.25 μg/mL, and were thus proved to be potent cytotoxic activity agents against HeLa cancer cells | [11] |
| Anti-inflammatory | Stems | Methanol | Murrayakonine A, murrayanine, and O-methylmurrayamine-A | Human peripheral blood mononuclear cells | In vitro experiments showed murrayakonine A (IC50 10 µM), murrayanine (IC50 9.4 µM), and O-methylmurrayamine-A (IC50 7 µM) against TNF-α, and murrayanine (IC50 8.4 µM) and methylmurrayamine-A (IC50 8.4 µM) against IL-6, respectively | [39] |
| Anticancer (Colon) | Leaves | Ethanol | O-methylmurrayamine 5.7–17.9 µM | MCF-7 cells | O-methylmurrayamine A exhibited anti-colon cancer activity through downregulation of the Akt/mTOR survival pathway and activation of the intrinsic pathway of apoptosis | [86] |
| Anticancer (Oral) | Leaves | Methanol | Mahanine 15 μM | CLS-354 cells | Mahanine increased the expression of LC3B-II, cleaved caspase-3 proteins, and the inhibition of autophagic flux | [96] |
| Anticancer (Ovarian) | Stem bark | Methanol | Girinimbine 10 µM | Ovarian cancer cell line SKOV3/ SV40 | Girinimbine was found to be mainly due to the induction of apoptosis and cell cycle arrest due to the inhibition of the PI3K/AKT/mTOR and Wnt/b-catenin signaling pathways | [98] |
| Anticancer (Breast) | Leaves | Aqueous acetone | Koenimbin 4.89 μg/mL | MCF7 breast cancer stem cells | Koenimbin induced apoptosis in MCF7 cells that was mediated by cell death and regulated the mitochondrial membrane potential by downregulating Bcl2 and upregulating Bax, due to cytochrome c release from the mitochondria to the cytosol, and significantly downregulated the Wnt/β-catenin self-renewal pathway | [98] |
| Anticancer (Prostate) | Leaves | Aqueous acetone | Koenimbin 3.73 μg/mL | Prostate cancer stem cells | Koenimbin induced apoptosis through the intrinsic signaling pathway and suppression of the translocation of cytoplasmic NF-κB into the nucleus, in addition to displaying potential for targeting PCSCs, as affirmed by the prostasphere formation and Aldefluor assay | [99] |
| Anticancer | Leaves | Methanol | Mahanine 7.5 μM | Glioma HS 683 cells | Mahanine inhibited the cell migration and invasion and inhibited cell growth was simultaneous with the suppression of p-PI3K, p-AKT, and p-mTOR | [96] |
| Anticancer (Liver) | Leaves | Methanol | Mahanine 25 μM | HepG2, HuCCT1, and KKU-100 cells | Mahanine showed potent cytotoxicity, with increased expression levels of MITF balance between the cellular stresses | [13] |
| Anticancer (Cervical) | Leaves | Methanol | Mahanine 8.6 μM | HeLa (HPV-18) and SiHa (HPV-16) cell line | Mahanine and cisplatin synergistically displayed growth inhibitory activity in cervical cancer, the inhibition of STAT3 activation, cell migration, and induced apoptosis | [14] |
| Anticancer (Lung) | Leaves | Methanol | Mahanine 15 μM | NSCLC cancer cell line A549 | Mahanine induced the impairment of mTORC2 through rictor inhibition and the destruction of NSCLC cancer cells | [22] |
| Anticancer (Colon) | Leaves | Methanol | Mahanine 0–30 μM | HCT116, HCT116, SW480, and Vero | Mahanine synergistically activated the two tumor suppressors PTEN and p53/p73 and can potentially be used in combination therapy with 5-FU for the treatment of colon carcinoma | [23] |
| Anticancer (prostate) | Leaves | Methanol | Mahanine 10 μM | PC3 and LNCaP cell line | Mahanine selectively degraded DNMT1 and DNM T3B via the ubiquitin-proteasomal pathway in a dose-dependent manner upon the inactivation of Akt signaling | [24] |
| Neuroprotective | Leaves | Methanol | Isolongifolene 10 µM | SH-SY5Y cells | Isolongifolene was effectively attenuated in oxidative stress, mitochondrial dysfunction, and apoptosis | [87] |
| Neuroprotective | Leaves | Methanol | O-methylmurrayamine A | PC12 cells | O-methylmurrayamine A possibly protects against DNA damage, apoptosis, and high levels of cell viability | [35] |
| In vivo studies | ||||||
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract resulted in a significant reduction in the level of TBARS in both the plasma (3.64 ± 0.13) and pancreas (53.40 ± 2.13) of diabetic rats | [61] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract resulted in a significant increase in the level of GSH in both the plasma (24.16 ± 1.30) and pancreas (19.52 ± 1.09) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of SOD in the pancreas (3.69 ± 0.15) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of CAT in the pancreas (12.94 ± 0.54) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of GPx in the pancreas (5.86 ± 0.22) of diabetic rats | [67] |
| Antioxidant | Leaves | Ethanol | − | Sprague Dawley rats | For 200 and 400 µg/mL b.w, the M. koenigii extract showed 80% inhibited free radical generation and 75% restored GSH levels | [10] |
| Antioxidant | Leaves | Water | − | Male albino Wistar rat | Extract exhibited the potential to reduce lipid peroxidation activity in the liver (2.44 ± 0.029) and kidney (2.34 ± 0.09) in potassium dichromate-induced Wistar rats | [38] |
| Anti-inflammatory | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine | Adult zebrafish | Girinimbine treatment significantly suppressed the IL-1β and TNF-α levels induced by peritoneal fluid mice | [85] |
| Anti-inflammatory | Leaves | Ethanol | − | Sprague Dawley rats | Oral administration of an M. koenigii extract showed the reduced formation of oedema, with values of 43.28%, 59.67%, and 62.29% induced by carrageenan, histamine, and serotonin in rats | [30] |
| Hepatoprotective | Leaves | Hydro-ethanolic | − | Male Wistar rats | M. koenigii leaves significantly decreased CCl4 -induced hepatotoxic in a time- and dose-dependent manner | [16] |
| Nephroprotective | Leaves | Aqueous | − | Male Wistar rats | M. koenigii extract treatment significantly decreased the renal functional markers, like the blood urea nitrogen and creatinine level | [28] |
| Anti-Diabetic | Leaves | Ethanol | − | Swiss albino mice | M. koenigii possesses antidiabetic activity and has antioxidant effects on STZ-NA-induced diabetes mellitus and particularly significantly decreased the HOMA-IR index | [10] |
| Anticancer (Colon) | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine 1.5–100 µg/mL | Zebrafish and Male ICR mice | Girinimbine, supplementation specifically, resulted in the induction of apoptosis, the inhibition of inflammation, and a significant increase in cell numbers in the G0/G1 phase | [85] |
| Anticancer (Breast) | Leaves | Aqueous | − | Female BALB/c mice | M. koenigii aqueous extract has potential for cytotoxicity, anti-inflammatory, and immunomodulatory effects and delays rather than inhibits tumor formation | [12] |
| Neuroprotective | Leaves | Methanol | − | Male albino mice | M. koenigii is effective in attenuating memory impairment and oxidative stress and prevents abnormal oral movements | [36] |
| Neuroprotective | Leaves | Ethanol | − | Swiss albino mice | M. koenigii supplementation resulted in an improvement of acetylcholine (ACh) and reduction in acetylcholinesterase (AChE). In addition, a significant elevation of serum biomarkers, and decline in creatinine, total cholesterol, urea nitrogen, and glucose levels, ameliorated the hepatic and renal functions in the normal ageing process | [30] |
| Neuroprotective | Leaves | Ethanol | − | Male swiss albino mice | M. koenigii leaves elevated the acetylcholine level in the brain and ultimately improved memory impairment. In vitro, it showed BACE1 inhibition and was found to be a non-competitive inhibitor | [33] |
| Neuroprotective | Leaves | Methanol | Isolongifolene 10 mg/kg b.w. | Male albino Wistar rat | Isolongifolene effectively attenuated behavioral impairment and oxidative stress, acting as an antiaging agent | [34] |
| Anti-anxiety and anti-depressant | Leaves | Aqueous | − | Swiss albino mice | M. koenigii aqueous leaf extract reduced the despair behavior in experimental animal models, suggesting an anti-depressant-like activity and also reduced spontaneous locomotor activity | [41] |
4.2. Antibacterial Activity
The unsystematic use of antibiotics promotes the development of multiple drug-resistant pathogenic strains of bacteria, which are very harmful, and there is a lack of proper treatment procedures for these ailments. Therefore, the need to search for new antimicrobials remains. Currently, in addition to antibiotics and chemically-synthesized drugs, curiosity for alternative medicines, such as natural or herbal medicines, is increasing. They may have fewer side effects or toxicity owing to their natural sources [102].
Combating microbial infections without side effects is always a tedious process. In this regard, in addition to classical antibiotics and synthetic drugs, there is an ongoing hunt for potent molecules from natural herbal medicines [102]. M. koenigii extracts have demonstrated antibacterial effects on a wide variety of microorganisms. Methanol and ethanol extracts of M. koenigii leaves were found to be effective against the bacterial strains Escherichia coli (E. coli), Staphylococcus, Streptococcus, and Proteus. Hence, M. koenigii leaves could be efficiently used as a natural remedy in everyday meals for the prevention of several bacterial infections [103]. Pyranocarbazoles isolated from M. koenigii exhibited antibacterial activity on bacterial strains of Staphylococcus aureus and Klebsiella pneumonia [40]. Green synthesized silver nanoparticles (AGNPs) from M. koenigii exhibited therapeutic efficacy against multidrug resistant MDR bacteria [103]. M. koenigii essential oil showed antibiofilm activity against Pseudomonas aeruginosa and it was reported that M. koenigii essential oil treatment revealed an 80% reduction in biofilm formation by P. aeruginosa. Microscopic analyses confirmed the drop in biofilm formation in Pseudomonas aeruginosa when treated with M. koenigii essential oil. The presence of antibiofilm substances like spathulenol (5.85%), cinnamaldehyde (0.37%), and linalool (0.04%) was reported in gas chromatography-mass spectrometry (GCMS) studies [104]. M. koenigii counteraction on uropathogenic bacteria isolated from clinical samples was reported in a different study [105]. M. koenigii was tested for its antibiotic action against Mycobacterium species, which was appreciable, like first-line anti-tuberculosis drugs. M. koenigii half maximal inhibitory concentration ((IC50) 400 μg/mL) was found to be more effective against Mycobacterium smegmatis compared to water extracts and petroleum ether. An M. koenigii ethanol extract exhibited significant synergistic antibacterial activity against Mycobacterium smegmatis and Mycobacterium bovis bacillus calmette-guerin (BCG) in combination with the anti-tuberculosis drug rifampicin [106].
4.3. Hepatoprotective Effect
Liver diseases are a worldwide concern, and accessible medical treatments have an inadequate efficacy. Since ancient times, herbs have been used when treating various disease conditions; plant extracts and natural compounds have significant applications as hepatoprotective agents. The liver is the site of drug metabolism and the detoxification site of toxic products, and so it is the organ most exposed to xenobiotics [107]. M. koenigii extended hepatoprotective activity when crude aqueous extracts were investigated against ethanol-induced hepatotoxicity in experimental animals. M. koenigii was reported to extend a protective effect in liver impairments in chronic alcoholism and was proved to be effective in maintaining the enzymatic oxidant status [108]. Water extracts of carbazole alkaloids and tannin of M. koenigii were explored for their hepatoprotective activity against ethanol-induced hepatotoxicity in a HepG2 cell line model. They exhibited excellent hepatoprotective activity, maintaining the enzymatic and non-enzymatic antioxidant level at a near normal value and also maintaining the integrity of the cells [31]. An M. koenigii hydro-ethanolic leaf extract was reported to attenuate the CCl4 hepatotoxic effects in rats. M. koenigii-pretreated rats showed a significant decrement in activity levels of hepatic markers and also maintained the level of enzymatic antioxidants [16].
4.4. Immunomodulatory Activity
The immune system makes a network and regulates processes important for maintaining the health of an organism by hindering the entry and invasion of microbes. Impairments in the immune system lead to conditions from chronic inflammation to cancer [109]. In an investigation on the humoral- and cell-mediated immune response to ovalbumin, the immunomodulatory activity of a methanolic extract of M. koenigii leaves was evaluated using a carbon clearance test. A considerable increase in the NO production indicated the increased phagocytic activity of macrophages. The M. koenigii extract holds promise as an immunomodulatory agent, which acts by stimulating humoral immunity and the phagocytic function [110]. The M. koenigii leaf extracts were reported to have certain effects in regulating mice immunology related to oxidative stress metabolism. An M. koenigii leaf extract can exhibit an immunomodulatory effect through which it can regulate the oxidative stress metabolism in diabetic mice [111].
4.5. Nephroprotective Activity
M. koenigii has been used as a nephroprotective agent in a diabetic-induced rat model [9]. The M. koenigii leaf extract was found to be efficient in maintaining normal levels of serum creatinine, blood urea nitrogen, total serum protein, serum Na+, urine output, urinary creatinine, urinary urea, total urinary protein, and urinary Na+. Furthermore, the M. koenigii extract maintained the standard pattern in in vivo antioxidants, renal myeloperoxidase (MPO) activity, and histopathology of kidneys against unilateral renal ischemia reperfusion injury. Therefore, the extract of M. koenigii was clearly demonstrated to be useful in treating kidney disorders in rats [112]. The nephroprotective activity of M. koenigii was elucidated in experimental investigations, which showed decreased levels of blood urea nitrogen (BUN), serum creatinine (Cr), and lipid peroxidation (LPO). An M. koenigii extract is efficient against cyclophosphamide-induced nephrotoxicity, which was clearly revealed through the maintenance of high levels of glutathione (GSH) and superoxide dismutase (SOD) compared to the cyclophosphamide-treated group [28]. M. koenigii protective activity has been shown to induce significant dose-dependent decreases in serum urea and creatinine levels, as well as marked increases in the levels of plasma antioxidant capacity, in diabetic rats, compared to controls. More noteworthy is the histological integrity of kidneys, which showed comparable tissue regeneration induced by the aqueous extract [9].
4.6. Antidiabetic Activity
Most prominently in developing countries, medicinal plants play a helpful role in managing diabetes mellitus due to their cost effectiveness. Diabetes mellitus, a metabolic disorder, is becoming a serious threat to human health. During the past few years, many phytochemicals responsible for anti-diabetic effects have been isolated from plants. Alkaloids present in the leaves of M. koenigii have been explored and reported to have inhibitory effects on the aldose reductase enzyme, glucose utilization, and other enzyme systems for extending anti-diabetic effects [38]. M. koenigii was tested for the α-glucosidase inhibitory property and was found to inhibit α glycosidase. Alpha-glucosidase inhibitors are widely used in the treatment of patients with type 2 diabetes [113]. A study reported that an ethanolic extract of M. koenigii showed a significant reduction in blood glucose levels, and this effect of reducing blood glucose by M. koenigii is mediated by antioxidant properties and insulin mimetic effects. In addition, M. koenigii exhibited a profound antioxidant effect by reducing the malondialdehyde (MDA) level, increasing the GSH level, and significantly decreasing the homeostatic model assessment (HOMA)-insulin resistance index. On the whole, it is evident that M. koenigii possesses antidiabetic activity and has antioxidant effects in rats [10].
4.7. Anticancer Activity (In Vivo and In Vitro)
M. koenigii possesses potential secondary metabolites that could be developed as anticancer agents. In one study, the cytotoxic activity was evaluated for three extracts: hexane, ethyl acetate, and methanol of M. koenigii leaves against the HeLa cell line. The extracts were reported as being potently cytotoxic in nature in HeLa cancer cells. These results established the potential of M. koenigii as an anticancer agent in vitro [11]. Additional evidence for the anticancer activity of M. koenigii has been obtained from rodent cancer cell lines, as well as different in vivo cancer models [12,13,14,22,23,24,114,115]. In an early study, histopathological evidence showed that M. koenigii extract treatment generated a decline in neoplasms in the colon [85]. A methanolic extract of M. koenigii was reported to have the ability to reduce proliferation in breast cancer cell lines [116]. The total alkaloid extracted from M. koenigii leaves has been shown to have promising cytotoxic activity in breast cancer cells, with an IC50 of 14.4 µg/mL [117]. The anticancer activity of mahanine and isomahanine in human oral squamous cell carcinoma CLS-354 has also been reported [96].
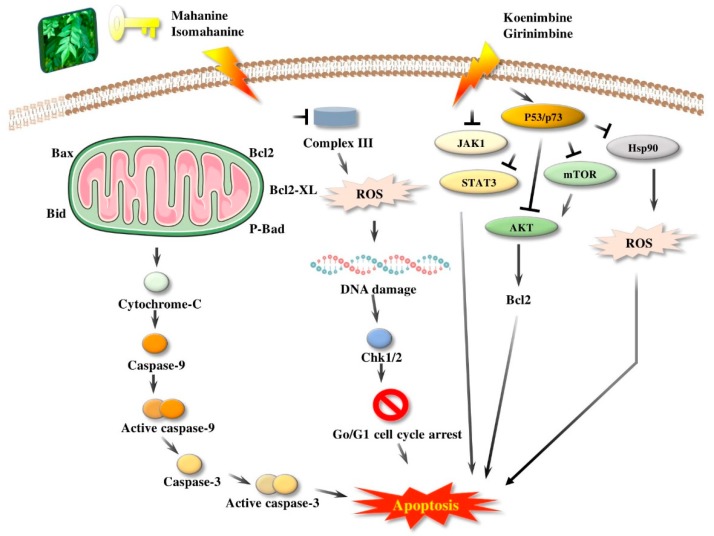
Girinimbine, another M. koenigii-derived carbazole alkaloid, showed growth inhibitory activity in human hepatocellular carcinoma and lung cancer cells in vitro [118]. Rutin, quercetin, kaempferol, and apigenin, present in leaf extracts of M. koenigii, showed the dose-dependent inhibition of endogenous 26S proteasome activity in MDA-MB-231 cells [52]. Therefore, M. koenigii contains remarkable anticancer compounds, especially mahanine, which has been reported to show anticancer activity targeting different signaling pathways [46]. Girinimbine, a carbazole alkaloid, has been found to have a good role in total leukocyte migration and result in an appreciable reduction in pro-inflammatory cytokine levels. The various activities of M. koenigii against different cancer cell lines are shown in Figure 2.
Figure 2.
Apoptosis induced by M. koenigii bioactive compounds in cancer. Bcl2: B-cell lymphoma 2; Bcl2-XL: B-cell lymphoma-extra-large; P-Bad: P plasmid araB araA araD; ROS: reactive oxygen species; Chk1/2: checkpoint kinase; Go/G1: cell cycle phase; JAK1: janus kinase 1; STAT3: signal transducer and activator of transcription 3; AKT: protein kinase B (also known as AKT); mTOR: mammalian target of rapamycin; P53/p57: tumor protein; Hsp90: heat shock protein.
4.8. Neuroprotective Activity
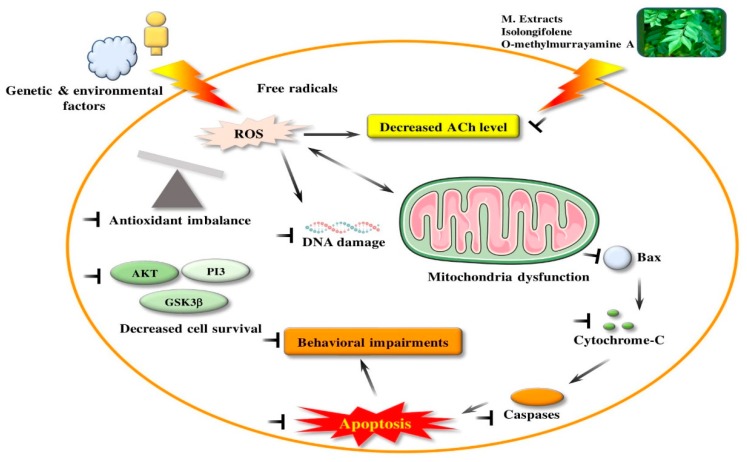
Supplementation with M. koenigii leaf extracts has been reported in the management of a wide spectrum of neurodegenerative diseases, like AD, PD, and others [30,33,34,35,41]. M. koenigii promotes neuroprotective potential against orofacial dyskinesia induced by resperine. Additionally, it stabilizes the levels of protective antioxidant enzymes like SOD, catalase (CAT), and GSH, and inhibits LPO in the forebrain regions of reserpine-treated animals. Furthermore, it has been shown to significantly inhibit reserpine-induced abnormalities in behavior. Similarly, treatment with M. koenigii significantly restored the levels of protective antioxidant enzymes (that is, SOD, CAT, and GSH) and inhibited LPO in the forebrain region when compared with reserpine, and it also inhibited catalepsy induced by haloperidol [36]. Isolongifolene (ILF), a tricyclic sesquiterpene of M. koenigii, has been reported to render neuroprotective effects against rotenone-induced mitochondrial dysfunction, oxidative stress, and apoptosis in a cellular model. Cytotoxicity, oxidative stress, and mitochondrial dysfunction were also attenuated by ILF in SH-SY5Y cells, which down-regulated Bax and caspases-3, -6, -8, and -9 expression, and up-regulated Bcl-2 expression. IFL was proved to regulate p-P13K, p-AKT, and p-GSK-3 beta expressions [87]. Preclinical studies have reported that M. koenigii leaves could enhance memory in rats [119]. The possible neuroprotective potential of amethanolic extract of M. koenigii leaves was exhibited in a two-vessel occlusion (2VO) rat model of partial global cerebral ischaemia. The Morris water maze test was implemented to assess the rats’ cognitive function postoperatively. Brain samples were histopathologically examined for viable neurons within the CA1 hippocampal region. Test findings showed that M. koenigii leaves positively improved memory and learning impairments. M. koenigii leaf extracts modestly improved memory in rats with chronic partial global cerebral ischemia [120]. The various activities of M. koenigii against neurotoxicity are shown in Figure 3.
Figure 3.
Neuroprotective effect in in vitro and in vivo studies produced by bioactive compounds from M. koenigii. PI3: phosphatidylinositol 3 kinase; GSK3β: Glycogen synthase kinase 3 beta; Ach: Acetylcholine; Bax: Bcl2-Associated X protein.
4.9. Radioprotective and Chemoprotective Activity
A methanolic extract of M. koenigii was demonstrated to render protection in chromosomal damage against radiation and cyclophasphamide in vivo. Radiation leads to a rise in all types of aberrations, like the fragmentation of chromatids and breakages in chromosomes, rings, and dicentrics. Treatment with a methanolic extract of M. koenigii before radiation significantly reduced the aberrations. M. koenigii can significantly exert bone marrow protection against radiation and cyclophasphamide [121].
4.10. Wound Healing Effect
Wound healing is a complex and multifactor process involving numerous biochemical and cellular processes which helps in the restoration of functional and anatomical continuity. M. koenigii leaves extend wound healing in male albino rats through significantly increased wound contraction and reduced epithelialization, supporting the collagen synthesis which was evident in histopathological studies [122].
5. Conclusions
The current review summarizes the medicinal uses, phytochemistry, and pharmacological properties of M. koenigii. M. koenigii is a source of several bioactive compounds, including alkaloids, polyphenol, terpenoids, and flavonoids. M. koenigii and its derivatives appear to exhibit appreciable pharmacological activities, like anticarcinogenic, proapoptotic, antiangiogenic, antimetastatic, immunomodulatory, and antioxidant properties. The molecular mechanisms underlying these activities of M. koenigii and its derivatives are due to their diversified role in combinations of cell signaling pathways at multiple levels in various diseases. M. koenigii and its derivatives mitigate oxidative stress, neurotoxicity, neuroinflammation, neuronal loss, and cognitive dysfunctions. However, like other polyphenols, to a certain extent, M. koenigii activities are limited by its bioavailability and in such conditions, enhancement of the efficiency should be conducted. Therefore, future studies need to include more experimental studies on bioavailability and efficiency enhancement in clinical investigations.
Author Contributions
R.B., writing—original draft preparation; D.V., writing—review and editing; S.-H.J., table preparation; P.G., validation; I.S.-K., interpreted the figures; D.-K.C., supervision of the review and approval of the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2018R1C1B6005129 and 2020R1A2B5B02002032)
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Handral H.K., Pandith A., Shruthi S.D. A review on Murraya koenigii: Multipotential medicinal plant. Asian J. Pharm. Clin. Res. 2012;5:5–14. [Google Scholar]
- 2.Ujowundu C.O., Okafor O.E., Agha N.C., Nwaogu L.A., Igwe K.O., Igwe C.U. Phytochemical and chemical composition of Combretum zenkeri leaves. J. Med. Plants Res. 2010;4:965–968. [Google Scholar]
- 3.Kang W., Wang Y. China Digital Governance Development Review Over the Past Two Decades. Int. J. Public Adm. Digit. Age. 2018;5:92–106. doi: 10.4018/IJPADA.2018070107. [DOI] [Google Scholar]
- 4.Zhang J., Du S., Duan X., Zhang S. Effects of ultrahigh pressure processing on the physicochemical characteristics of Taibai Kudzu starch. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2007;2017 doi: 10.3969/j.issn.1002-6819.2007.4.053. [DOI] [Google Scholar]
- 5.Phumthum M., Balslev H. Thai Ethnomedicinal Plants Used for Diabetes Treatment. OBM Integr. Complement. Med. 2018;3:1–25. doi: 10.21926/obm.icm.1803020. [DOI] [Google Scholar]
- 6.Gunjan M., Naing T.W., Saini R.S., Ahmad A., Naidu J.R., Kumar I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J. Pharm. Res. 2015;4:132–155. [Google Scholar]
- 7.Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:140–149. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 8.Ahluwalia V., Sisodia R., Walia S., Sati O.P., Kumar J., Kundu A. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest Sci. (2004) 2014;87:341–349. doi: 10.1007/s10340-013-0542-6. [DOI] [Google Scholar]
- 9.Yankuzo H., Ahmed Q.U., Santosa R.I., Akter S.F.U., Talib N.A. Beneficial effect of the leaves of Murraya koenigii (Linn.) Spreng (Rutaceae) on diabetes-induced renal damage in vivo. J. Ethnopharmacol. 2011;135:88–94. doi: 10.1016/j.jep.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Husna F., Suyatna F.D., Arozal W., Poerwaningsih E.H. Anti-Diabetic Potential of Murraya koenigii (L) and its Antioxidant Capacity in Nicotinamide-Streptozotocin Induced Diabetic Rats. Drug Res. (Stuttg) 2018;68:631–636. doi: 10.1055/a-0620-8210. [DOI] [PubMed] [Google Scholar]
- 11.Amna U., Halimatussakdiah P.W., Saidi N., Nasution R. Evaluation of cytotoxic activity from Temurui (Murraya koenigii [Linn.] Spreng) leaf extracts against HeLa cell line using MTT assay. J. Adv. Pharm. Technol. Res. 2019;10:51–55. doi: 10.4103/japtr.JAPTR_373_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeap S.K., Abu N., Mohamad N.E., Beh B.K., Ho W.Y., Ebrahimi S., Yusof H.M., Ky H., Tan S.W., Alitheen N.B. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement. Altern. Med. 2015;4:306. doi: 10.1186/s12906-015-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nooron N., Ohba K., Takeda K., Shibahara S., Chiabchalard A. Dysregulated expression of MITF in subsets of hepatocellular carcinoma and cholangiocarcinoma. Tohoku J. Exp. Med. 2017;242:291–302. doi: 10.1620/tjem.242.291. [DOI] [PubMed] [Google Scholar]
- 14.Das R., Bhattacharya K., Samanta S.K., Pal B.C., Mandal C. Improved chemosensitivity in cervical cancer to cisplatin: Synergistic activity of mahanine through STAT3 inhibition. Cancer Lett. 2014;351:81–90. doi: 10.1016/j.canlet.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari P. Curry leaf (Murraya koenigii) or Cure leaf: Review of its curative properties. J. Med. Nutr. Nutraceuticals. 2012;2:92–97. doi: 10.4103/2278-019X.101295. [DOI] [Google Scholar]
- 16.Desai S.N., Patel D.K., Devkar R.V., Patel P.V., Ramachandran A.V. Hepatoprotective potential of polyphenol rich extract of Murraya koenigii L.: An in vivo study. Food Chem. Toxicol. 2012;50:310–314. doi: 10.1016/j.fct.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 17.Gajaria T.K., Patel D.K., Devkar R.V., Ramachandran A.V. Flavonoid rich extract of Murraya koenigii alleviates in-vitro LDL oxidation and oxidized LDL induced apoptosis in raw 264.7 Murine macrophage cells. J. Food Sci. Technol. 2015;52:3367–3375. doi: 10.1007/s13197-014-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goutam M.P., Purohit R.M. Antimicrobial activity of the essential oil of the leaves of Murraya koenigii (Linn) Spreng (Indian curry leaf) Indian J. Pharm. 1974;2:48–51. [Google Scholar]
- 19.Maswada H.F., Abdallah S.A. In vitro antifungal activity of three geophytic plant extracts against three post-harvest pathogenic fungi. Pakistan J. Biol. Sci. 2013;16:1698–1705. doi: 10.3923/pjbs.2013.1698.1705. [DOI] [PubMed] [Google Scholar]
- 20.Bonde S.D., Nemade L.S., Patel M.R., Patel A.A. Murraya koenigii (Curry leaf): Ethnobotany, Phytochemistry and Pharmacology—A Review. Int. J. Pharm. Phytopharm. Res. 2011;1:23. [Google Scholar]
- 21.Ma Q.G., Xu K., Sang Z.P., Wei R.R., Liu W.M., Su Y.L., Yang J.B., Wang A.G., Ji T.F., Li L.J. Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng. Bioorg. Med. Chem. Lett. 2016;26:799–803. doi: 10.1016/j.bmcl.2015.12.091. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee P., Seal S., Mukherjee S., Kundu R., Bhuyan M., Barua N.C., Baruah P.K., Babu S.P.S., Bhattacharya S. A carbazole alkaloid deactivates mTOR through the suppression of rictor and that induces apoptosis in lung cancer cells. Mol. Cell. Biochem. 2015;405:149–158. doi: 10.1007/s11010-015-2406-2. [DOI] [PubMed] [Google Scholar]
- 23.Das R., Bhattacharya K., Sarkar S., Samanta S.K., Pal B.C., Mandal C. Mahanine synergistically enhances cytotoxicity of 5-fluorouracil through ROS-mediated activation of PTEN and p53/p73 in colon carcinoma. Apoptosis. 2014;19:149–164. doi: 10.1007/s10495-013-0907-6. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S., Amin K.S., Jagadeesh S., Baishay G., Rao P.G., Barua N.C., Bhattacharya S., Banerjee P.P. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol. Cancer. 2013;12:99. doi: 10.1186/1476-4598-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal S., Nayak A., Kar M., Banerjee S.K., Das A., Upadhyay S.N., Singh R.K., Banerji A., Banerji J. Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia. 2010;81:72–74. doi: 10.1016/j.fitote.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Ningappa M.B., Dhananjaya B.L., Dinesha R., Harsha R., Srinivas L. Potent antibacterial property of APC protein from curry leaves (Murraya koenigii L.) Food Chem. 2010;118:747–750. doi: 10.1016/j.foodchem.2009.05.059. [DOI] [Google Scholar]
- 27.Tripathi Y., Anjum N., Rana A. Chemical Composition and In vitro Antifungal and Antioxidant Activities of Essential Oil from Murraya koenigii (L.) Spreng. Leaves. Asian J. Biomed. Pharm. Sci. 2018;8:6–13. doi: 10.4066/2249-622X.65.18-729. [DOI] [Google Scholar]
- 28.Mahipal P., Pawar R.S. Nephroprotective effect of Murraya koenigii on cyclophosphamide induced nephrotoxicity in rats. Asian Pac. J. Trop. Med. 2017;10:808–812. doi: 10.1016/j.apjtm.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Rautela R., Das G.K., Khan F.A., Prasad S., Kumar A., Prasad J.K., Ghosh S.K., Dhanze H., Katiyar R., Srivastava S.K. Antibacterial, anti-inflammatory and antioxidant effects of Aegle marmelos and Murraya koenigii in dairy cows with endometritis. Livest. Sci. 2018;214:142–148. doi: 10.1016/j.livsci.2018.05.015. [DOI] [Google Scholar]
- 30.Mani V., Ramasamy K., Ahmad A., Wahab S.N., Jaafar S.M., Kek T.L., Salleh M.Z., Majeed A.B.A. Effects of the total alkaloidal extract of Murraya koenigii leaf on oxidative stress and cholinergic transmission in aged mice. Phyther. Res. 2013;27:46–53. doi: 10.1002/ptr.4676. [DOI] [PubMed] [Google Scholar]
- 31.Sathaye S., Bagul Y., Gupta S., Kaur H., Redkar R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp. Toxicol. Pathol. 2011;63:587–591. doi: 10.1016/j.etp.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Dar R.A., Shahnawaz M., Qazi P.H., Qazi H. General overview of medicinal plants: A review. J. Phytopharm. 2017;6:349–351. [Google Scholar]
- 33.Mani V., Ramasamy K., Ahmad A., Parle M., Shah S.A.A., Majeed A.B.A. Protective effects of total alkaloidal extract from Murraya koenigii leaves on experimentally induced dementia. Food Chem. Toxicol. 2012;50:1036–1044. doi: 10.1016/j.fct.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 34.Balakrishnan R., Tamilselvam K., Sulthana A., Mohankumar T., Manimaran D., Elangovan N. Isolongifolene Attenuates Oxidative Stress and Behavioral Impairment in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018;8:53–58. [Google Scholar]
- 35.Zang Y.D., Li C.J., Song X.Y., Ma J., Yang J.Z., Chen N.H., Zhang D.M. Total synthesis and neuroprotective effect of O-methylmurrayamine A and 7-methoxymurrayacine. J. Asian Nat. Prod. Res. 2017;19:623–629. doi: 10.1080/10286020.2017.1327952. [DOI] [PubMed] [Google Scholar]
- 36.Patil R., Dhawale K., Gound H., Gadakh R. Protective effect of leaves of Murraya koenigii on reserpine-induced orofacial dyskinesia. Iran. J. Pharm. Res. 2012;11:635–641. [PMC free article] [PubMed] [Google Scholar]
- 37.Erkan N., Tao Z., Vasantha Rupasinghe H.P., Uysal B., Oksal B.S. Antibacterial activities of essential oils extracted from leaves of Murraya koenigii by solvent-free microwave extraction and hydro-distillation. Nat. Prod. Commun. 2012;7:121–124. doi: 10.1177/1934578X1200700139. [DOI] [PubMed] [Google Scholar]
- 38.Patel D.K., Kumar R., Laloo D., Hemalatha S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac. J. Trop. Dis. 2012;2:139–150. doi: 10.1016/S2222-1808(12)60054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalli Y., Khajuria V., Gupta S., Arora P., Riyaz-Ul-Hassan S., Ahmed Z., Ali A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016;14:3322–3332. doi: 10.1039/C6OB00267F. [DOI] [PubMed] [Google Scholar]
- 40.Joshi T., Jain T., Mahar R., Singh S.K., Srivastava P., Shukla S.K., Mishra D.K., Bhatta R.S., Banerjee D., Kanojiya S. Pyranocarbazoles from Murraya koenigii (L.) Spreng. as antimicrobial agents. Nat. Prod. Res. 2018;32:430–434. doi: 10.1080/14786419.2017.1308363. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S., Handu S., Dubey A., Sharma P., Mediratta P., Ahmed Q. Anti-anxiety and anti-depressant like effects of Murraya koenigii in experimental models of anxiety and depression. Anc. Sci. Life. 2017;36:215–219. doi: 10.4103/asl.ASL_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adebajo A.C., Ayoola O.F., Iwalewa E.O., Akindahunsi A.A., Omisore N.O.A., Adewunmi C.O., Adenowo T.K. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine. 2006;13:246–254. doi: 10.1016/j.phymed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Tembhurne S.V., Sakarkar D.M. Hypoglycemic effects of fruit juice of Murraya koenigii (L) in alloxan induced diabetic mice. Int. J. PharmTech Res. 2009;1:1589–1593. [Google Scholar]
- 44.Sim K.M., Teh H.M. A new carbazole alkaloid from the leaves of Malayan Murraya koenigii. J. Asian Nat. Prod. Res. 2011;13:972–975. doi: 10.1080/10286020.2011.602970. [DOI] [PubMed] [Google Scholar]
- 45.Igara C., Omoboyowa D., Ahuchaogu A., Orji N., Ndukwe M. Phytochemical and nutritional profile of Murraya koenigii (Linn) Spreng leaf. J. Pharmacogn. Phytochem. 2016;5:7–9. [Google Scholar]
- 46.Samanta S.K., Kandimalla R., Gogoi B., Dutta K.N., Choudhury P., Deb P.K., Devi R., Pal B.C., Talukdar N.C. Phytochemical portfolio and anticancer activity of Murraya koenigii and its primary active component, mahanine. Pharmacol. Res. 2018;129:227–236. doi: 10.1016/j.phrs.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Ramsewak R.S., Nair M.G., Strasburg G.M., DeWitt D.L., Nitiss J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999;47:444–447. doi: 10.1021/jf9805808. [DOI] [PubMed] [Google Scholar]
- 48.Joshi B.S., Kamat V.N., Gawad D.H. On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron. 1970;26:1475–1482. doi: 10.1016/S0040-4020(01)92976-X. [DOI] [PubMed] [Google Scholar]
- 49.Gahlawat D.K., Jakhar S., Dahiya P. Murraya koenigii (L.) Spreng: An ethnobotanical, phytochemical and pharmacological review. J. Pharmacogn. Phytochem. 2014;3:109–119. [Google Scholar]
- 50.Tachibana Y., Kikuzaki H., Lajis N.H., Nakatani N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001;49:5589–5594. doi: 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava S.K., Srivastava S.D. New constituents and biological activity of the roots of Murraya koenigii. J. Indian Chem. Soc. 1993;2:607–627. [Google Scholar]
- 52.Noolu B., Gogulothu R., Bhat M., Qadri S.S.Y.H., Sudhakar Reddy V., Bhanuprakash Reddy G., Ismail A. In Vivo Inhibition of Proteasome Activity and Tumour Growth by Murraya koenigii Leaf Extract in Breast Cancer Xenografts and by Its Active Flavonoids in Breast Cancer Cells. Anticancer Agents Med. Chem. 2016;16:1605–1614. doi: 10.2174/1871520616666160520112210. [DOI] [PubMed] [Google Scholar]
- 53.Ma Q., Tian J., Yang J., Wang A., Ji T., Wang Y., Su Y. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia. 2013;87:1–6. doi: 10.1016/j.fitote.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Umehara K., Hattori I., Miyase T., Ueno A., Hara S., Kageyama C. Studies on the Constituents of Leaves of Citrus unshiu Marcov. Chem. Pharm. Bull. 1988;36:5004–5008. doi: 10.1248/cpb.36.5004. [DOI] [Google Scholar]
- 55.Mori K., Khlebnikov V. Carotenoids and Degraded Carotenoids, VIII-Synthesis of (+)-Dihydroactinidiolide, (+)- and (−)-Actinidiolide, (+)- and (−)-Loliolide as well as (+)- and (−)-Epiloliolide. Liebigs Ann. Chem. 1993;1993:77–82. doi: 10.1002/jlac.199319930113. [DOI] [Google Scholar]
- 56.Tan S.P., Ali A.M., Nafiah M.A., Amna U., Ramli S.A., Ahmad K. Terpenes and Phenolic Compounds of Murraya koenigii. Chem. Nat. Compd. 2017;53:980–981. doi: 10.1007/s10600-017-2177-y. [DOI] [Google Scholar]
- 57.ChV S. Antioxidant and Biological Activities of Three Morphotypes of Murraya koenigii L. from Uttarakhand. J. Food Process. Technol. 2013;4:1–7. doi: 10.4172/2157-7110.1000246. [DOI] [Google Scholar]
- 58.Patel O.P.S., Mishra A., Maurya R., Saini D., Pandey J., Taneja I., Raju K.S.R., Kanojiya S., Shukla S.K., Srivastava M.N., et al. Naturally Occurring Carbazole Alkaloids from Murraya koenigii as Potential Antidiabetic Agents. J. Nat. Prod. 2016;79:1276–1284. doi: 10.1021/acs.jnatprod.5b00883. [DOI] [PubMed] [Google Scholar]
- 59.Bhattacharya K., Samanta S.K., Tripathi R., Mallick A., Chandra S., Pal B.C., Shaha C., Mandal C. Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem. Pharmacol. 2010;79:361–372. doi: 10.1016/j.bcp.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Brand M.D., Affourtit C., Esteves T.C., Green K., Lambert A.J., Miwa S., Pakay J.L., Parker N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 61.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 62.Hoidal J.R. Reactive oxygen species and cell signaling. Am. J. Respir. Cell Mol. Biol. 2001;25:661–663. doi: 10.1165/ajrcmb.25.6.f213. [DOI] [PubMed] [Google Scholar]
- 63.Gill N.S., Sharma B. Study on antioxidant potential of Murraya koenigii leaves in wistar rats. Pakistan J. Biol. Sci. 2013;17:126–129. doi: 10.3923/pjbs.2014.126.129. [DOI] [PubMed] [Google Scholar]
- 64.Rehana D., Mahendiran D., Kumar R.S., Rahiman A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017;40:943–957. doi: 10.1007/s00449-017-1758-2. [DOI] [PubMed] [Google Scholar]
- 65.Kusuma I.W., Kuspradini H., Arung E.T., Aryani F., Min Y.H., Kim J.S., Kim Y.U. Biological Activity and Phytochemical Analysis of Three Indonesian Medicinal Plants, Murraya koenigii, Syzygium polyanthum and Zingiber purpurea. J. Acupunct. Meridian Stud. 2011;4:75–79. doi: 10.1016/S2005-2901(11)60010-1. [DOI] [PubMed] [Google Scholar]
- 66.Rao L.J.M., Ramalakshmi K., Borse B.B., Raghavan B. Antioxidant and radical-scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng.) Food Chem. 2007;100:742–747. doi: 10.1016/j.foodchem.2005.10.033. [DOI] [Google Scholar]
- 67.Gupta S., Paarakh P.M., Gavani U. Antioxidant activity of Murraya koenigii linn leaves. Pharmacologyonline. 2009;1:474–478. [Google Scholar]
- 68.Zahin M., Aqil F., Husain F.M., Ahmad I. Antioxidant capacity and antimutagenic potential of Murraya koenigii. BioMed Res. Int. 2013;2013:263509. doi: 10.1155/2013/263509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yogesh K., Jha S.N., Yadav D.N. Antioxidant Activities of Murraya koenigii (L.) Spreng Berry Extract: Application in Refrigerated (4 ± 1 °C) Stored Meat Homogenates. Agric. Res. 2012;1:183–189. doi: 10.1007/s40003-012-0018-6. [DOI] [Google Scholar]
- 70.Rajesh S.T., Sharmistha B., Shuchi K. Assessment of antioxidant activity of leaves of Murraya koenigii extracts and it’s comparative efficacy analysis in different solvents. J. Pharm. Sci. Res. 2017;9:288–291. [Google Scholar]
- 71.Waghmare A.N., Tembhurne S.V., Sakarar D.M. Phytochemical Analysis and In vitro Antioxidant Properties of Murraya koenigii (L.) Fruits. Am. J. Phytomedicine Clin. Ther. 2015;3:403–416. [Google Scholar]
- 72.Dexter D.T., Wells F.R., Lee A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased Nigral Iron Content and Alterations in Other Metal Ions Occurring in Brain in Parkinson’s Disease. J. Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 73.Archer S.L., Gomberg-Maitland M., Maitland M.L., Rich S., Garcia J.G.N., Weir E.K. Mitochondrial metabolism, redox signaling, and fusion: A mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 74.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;53:885–892. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Matsuzawa A., Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 76.Trachootham D., Lu W., Ogasawara M.A., Del Valle N.R., Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arulselvan P., Subramanian S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem. Biol. Interact. 2007;165:155–164. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Khan B.A., Abraham A., Leelamma S. Anti-oxidant effects of Curry leaf, Murraya koenigii and mustard seeds, Brassica juncea in rats fed with high fat diet. Indian J. Exp. Biol. 1997;35:148–150. [PubMed] [Google Scholar]
- 79.Mitra E., Ghosh A.K., Ghosh D., Mukherjee D., Chattopadhyay A., Dutta S., Pattari S.K., Bandyopadhyay D. Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 2012;50:1340–1353. doi: 10.1016/j.fct.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 80.Harman D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 81.Beal M.F. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Park. Relat. Disord. 2009;3:S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 82.Kushnareva Y., Murphy A.N., Andreyev A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002;368:545–553. doi: 10.1042/bj20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003;111:163–169. doi: 10.1172/JCI200317638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson S., Bankier A.T., Barrell B.G., De Bruijn M.H.L., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 85.Iman V., Mohan S., Abdelwahab S.I., Karimian H., Nordin N., Fadaeinasab M., Noordin M.I., Noor S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des. Dev. Ther. 2017;2017:103–121. doi: 10.2147/DDDT.S115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arun A., Patel O.P.S., Saini D., Yadav P.P., Konwar R. Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT downregulation and mitochondrial apoptosis. Biomed. Pharmacother. 2017;93:510–521. doi: 10.1016/j.biopha.2017.06.065. [DOI] [PubMed] [Google Scholar]
- 87.Balakrishnan R., Elangovan N., Mohankumar T., Nataraj J., Manivasagam T., Thenmozhi A.J., Essa M.M., Akbar M., Sattar Khan M.A. Isolongifolene attenuates rotenone-induced mitochondrial dysfunction, oxidative stress and apoptosis. Front. Biosci. (Sch. Ed.) 2018;10:248–261. doi: 10.2741/s513. [DOI] [PubMed] [Google Scholar]
- 88.Bashkatova V., Alam M., Vanin A., Schmidt W.J. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp. Neurol. 2004;186:235–241. doi: 10.1016/j.expneurol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Gupta S., George M., Singhal M., Sharma G., Garg V. Leaves extract of Murraya koenigii linn for anti-inflammatory and analgesic activity in animal models. J. Adv. Pharm. Technol. Res. 2010;1:68–77. [PMC free article] [PubMed] [Google Scholar]
- 90.Darvekar V.M., Patil V.R., Choudhari A.B., Road D. Anti-inflammatory Activity of Murraya koenigii Spreng on Experimental Animals. J. Nat. Prod. Plant Resour. 2011;1:65–69. [Google Scholar]
- 91.Mani V., Ramasamy K., Abdul Majeed A.B. Anti-inflammatory, analgesic and anti-ulcerogenic effect of total alkaloidal extract from Murraya koenigii leaves in animal models. Food Funct. 2013;4:557–567. doi: 10.1039/c3fo30356j. [DOI] [PubMed] [Google Scholar]
- 92.Khurana A., Sikha M.S., Ramesh K., Venkatesh P., Godugu C. Modulation of cerulein-induced pancreatic inflammation by hydroalcoholic extract of curry leaf (Murraya koenigii) Phyther. Res. 2019;33:1510–1525. doi: 10.1002/ptr.6344. [DOI] [PubMed] [Google Scholar]
- 93.Oben K.Z., Alhakeem S.S., McKenna M.K., Brandon J.A., Mani R., Noothi S.K., Jinpeng L., Akunuru S., Dhar S.K., Singh I.P., et al. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget. 2017;8:77436–77452. doi: 10.18632/oncotarget.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen M., Yin X., Lu C., Chen X., Ba H., Cai J., Sun J. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem. Biol. Interact. 2019;299:1–7. doi: 10.1016/j.cbi.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Yu Y., Fu X., Ran Q., Yang K., Wen Y., Li H., Wang F. Globularifolin exerts anticancer effects on glioma U87 cells through inhibition of Akt/mTOR and MEK/ERK signaling pathways in vitro and inhibits tumor growth in vivo. Biochimie. 2017;141:144–151. doi: 10.1016/j.biochi.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Utaipan T., Athipornchai A., Suksamrarn A., Jirachotikoon C., Yuan X., Lertcanawanichakul M., Chunglok W. Carbazole alkaloids from Murraya koenigii trigger apoptosis and autophagic flux inhibition in human oral squamous cell carcinoma cells. J. Nat. Med. 2017;71:158–169. doi: 10.1007/s11418-016-1045-6. [DOI] [PubMed] [Google Scholar]
- 97.Syam S., Abdul A.B., Sukari M.A., Mohan S., Abdelwahab S.I., Wah T.S. The growth suppressing effects of girinimbine on hepg2 involve induction of apoptosis and cell cycle arrest. Molecules. 2011;16:7155–7170. doi: 10.3390/molecules16087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin Q., Muer A. Girinimbine inhibits the proliferation of human ovarian cancer cells in vitro via the phosphatidylinositol-3-kinase (PI3K)/akt and the mammalian target of rapamycin (mTOR) and Wnt/β-catenin signaling pathways. Med. Sci. Monit. 2018;24:5480–5487. doi: 10.12659/MSM.910137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Ahmadipour F., Noordin M.I., Mohan S., Arya A., Paydar M., Looi C.Y., Keong Y.S., Siyamak E.N., Fani S., Firoozi M., et al. Koenimbin, a natural dietary compound of Murraya koenigii (L) spreng: Inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44+/CD24-/low): An in vitro study. Drug Des. Dev. Ther. 2015;9:1193–1208. doi: 10.2147/DDDT.S72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ito C., Itoigawa M., Nakao K., Murata T., Tsuboi M., Kaneda N., Furukawa H. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine. 2006;13:359–365. doi: 10.1016/j.phymed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Kumar N.S., Mukherjee P.K., Bhadra S., Saha B.P., Pal B.C. Acetylcholinesterase inhibitory potential of a carbazole alkaloid, mahanimbine, from Murraya koenigii. Phyther. Res. 2010;24:629–631. doi: 10.1002/ptr.3023. [DOI] [PubMed] [Google Scholar]
- 102.Deb S. Synthesis of silver nano particles using Murraya koenigii (Green Curry leaves), Zea mays (Baby corn) and its antimicrobial activity against pathogens. Int. J. PharmTech Res. 2014;6:91–96. [Google Scholar]
- 103.Qais F.A., Shafiq A., Khan H.M., Husain F.M., Khan R.A., Alenazi B., Alsalme A., Ahmad I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019;2019 doi: 10.1155/2019/4649506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sankar Ganesh P., Rai Vittal R. In vitro antibiofilm activity of Murraya koenigii essential oil extracted using supercritical fluid CO2 method against Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2015;29:2295–2298. doi: 10.1080/14786419.2015.1004673. [DOI] [PubMed] [Google Scholar]
- 105.Rath S., Padhy R.N. Monitoring in vitro antibacterial efficacy of 26 Indian spices against multidrug resistant urinary tract infecting bacteria. Integr. Med. Res. 2014;13:133–141. doi: 10.1016/j.imr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naik S.K., Mohanty S., Padhi A., Pati R., Sonawane A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement. Altern. Med. 2014 doi: 10.1186/1472-6882-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manfo F.P.T., Nantia E.A., Kuete V. Toxicological Survey of African Medicinal Plants. Elsevier; Yaoundé, Cameroon: 2014. Hepatotoxicity and Hepatoprotective Effects of African Medicinal Plants; pp. 223–256. [Google Scholar]
- 108.Shah P., Singh S.P., Kumar A. Combined effect of hydroethanolic extracts of Murraya koenigii and Phyllanthus niruri leaves on paracetamol and ethanol-induced toxicity in HepG2 cell line. Curr. Sci. 2015;109:1320. doi: 10.18520/cs/v109/i7/1320-1344. [DOI] [Google Scholar]
- 109.Kaufmann T., Simon H.U. Targeting disease by immunomodulation. Cell Death Differ. 2015;22:185–186. doi: 10.1038/cdd.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah A.S., Wakade A.S., Juvekar A.R. Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng. leaves. Indian J. Exp. Biol. 2008;46:505–509. [PubMed] [Google Scholar]
- 111.Paul S., Bandyopadhyay T.K., Bhattacharyya A. Immunomodulatory effect of leaf extract of Murraya koenigii in diabetic mice. Immunopharmacol. Immunotoxicol. 2011;33:691–699. doi: 10.3109/08923973.2011.561354. [DOI] [PubMed] [Google Scholar]
- 112.Punuru P., Sujatha D., Kumari B.P., Charisma V.V.L. Evaluation of aqueous extract of Murraya koenigii in unilateral renal ischemia reperfusion injury in rats. Indian J. Pharmacol. 2014;46:171–175. doi: 10.4103/0253-7613.129310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gul M.Z., Attuluri V., Qureshi I.A., Ghazi I.A. Antioxidant and α-glucosidase inhibitory activities of Murraya koenigii leaf extracts. Pharmacogn. J. 2012;4:65–72. doi: 10.5530/pj.2012.32.12. [DOI] [Google Scholar]
- 114.Sarkar S., Dutta D., Samanta S.K., Bhattacharya K., Pal B.C., Li J., Datta K., Mandal C., Mandal C. Oxidative inhibition of Hsp90 disrupts the super-chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int. J. Cancer. 2013;132:695–706. doi: 10.1002/ijc.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pei C., He Q., Liang S., Gong X. Mahanimbine Exerts Anticancer Effects on Human Pancreatic Cancer Cells by Triggering Cell Cycle Arrest, Apoptosis, and Modulation of AKT/Mammalian Target of Rapamycin (mTOR) and Signal Transducer and Activator of Transcription 3 (STAT3) Signalling Pathway. Med. Sci. Monit. 2018;1:6975–6983. doi: 10.12659/MSM.911013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noolu B., Ajumeera R., Chauhan A., Nagalla B., Manchala R., Ismail A. Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complement. Altern. Med. 2013;13:7. doi: 10.1186/1472-6882-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ismail A., Noolu B., Gogulothu R., Perugu S., Rajanna A., Babu S.K. Cytotoxicity and Proteasome Inhibition by Alkaloid Extract from Murraya koenigii Leaves in Breast Cancer Cells-Molecular Docking Studies. J. Med. Food. 2016;19:1155–1165. doi: 10.1089/jmf.2016.3767. [DOI] [PubMed] [Google Scholar]
- 118.Mohan S., Abdelwahab S.I., Cheah S.C., Sukari M.A., Syam S., Shamsuddin N., Rais Mustafa M. Apoptosis effect of girinimbine isolated from Murraya koenigii on lung cancer cells in vitro. Evid.-Based Complement. Altern. Med. 2013;2013:689865. doi: 10.1155/2013/689865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vasudevan M., Parle M. Antiamnesic potential of Murraya koenigii leaves. Phyther. Res. 2009;23:308–316. doi: 10.1002/ptr.2620. [DOI] [PubMed] [Google Scholar]
- 120.Mittal J. Curry Leaf (Murraya koenigii): A Spice with Medicinal Property. MOJ Biol. Med. 2017;2:236–256. doi: 10.15406/mojbm.2017.02.00050. [DOI] [Google Scholar]
- 121.Iyer D., Uma D.P. Effect of Murraya koenigii (L.) on radiation induced rate of lipid peroxidation in Swiss albino mice. Indian Drugs. 2009;46:160–162. [Google Scholar]
- 122.Nagappan T., Segaran T.C., Wahid M.E.A., Ramasamy P., Vairappan C.S. Efficacy of carbazole alkaloids, essential oil and extract of Murraya koenigii in enhancing subcutaneous wound healing in rats. Molecules. 2012;17:14449–14463. doi: 10.3390/molecules171214449. [DOI] [PMC free article] [PubMed] [Google Scholar]