Abstract
Generic entry of newer anticoagulants is expected to decrease the costs of atrial fibrillation management. However, when making switches between brand and generic medications, bioequivalence concerns are possible. The objectives of this study were to predict and compare the lifetime cost‐effectiveness of brand dabigatran with hypothetical future generics. Markov microsimulations were modified to predict the lifetime costs and quality‐adjusted life years of patients on either brand or generic dabigatran from a US private payer perspective. Event rates for generics were predicted using previously developed pharmacokinetic‐pharmacodynamic models. The analyses showed that generic dabigatran with lower‐than‐brand systemic exposure were dominant. Meanwhile, generic dabigatran with extremely high systemic exposure was not cost‐effective compared with the brand reference. Cost‐effectiveness of generic medications cannot always be assumed as shown in this example. Combined use of pharmacometric and pharmacoeconomic models can assist in decision making between brand and generic pharmacotherapies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Generic versions of medications undergo bioequivalence testing to ensure similar bioavailability and similar therapeutic effects. Failures may occur when medications have complex pharmaceutical or pharmacological properties. Projecting the impact of variations in pharmacokinetics/pharmacodynamics and incorporating these parameters into cost‐effectiveness models could inform clinical decision making.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study considered whether or not generic versions are always cost‐effective in light of bio‐in‐equivalence concerns. These analyses modeled hypothetical dabigatran generic versions using extreme values of bioequivalence thresholds and predicted event probabilities.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ With dabigatran as an example, this study showed that generic medications are not always cost‐effective when bioequivalence thresholds are considered. Although the generic dabigatran with less extreme systemic exposure is cost‐effective compared with the brand reference, generic dabigatran with extremely high systemic exposure is not cost‐effective compared with the brand reference.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ This study implies that generic medications with complex pharmaceutical or pharmacological properties should be more closely scrutinized, not only by regulatory bodies, but also once on the market by prescribers and patients. This study also is indicative of the value of multidisciplinary and translational science to inform pharmacotherapy through the combination of pharmacometric and pharmacoeconomic approaches.
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting nearly 10% of those over age 65 and 3–6 million people in the United States.1 AF increases the risk of systematic embolism, ischemic stroke, and long‐term mortality.2, 3, 4 Antithrombotic therapy with oral anticoagulants has been shown to lower the risk of stroke.5, 6 Patients with a high risk of stroke (e.g., CHA2DS2‐VASc score ≥ 2) are recommended to receive long‐term anticoagulation.1, 7, 8, 9 Anticoagulation at the same time increases the risk of major bleeding including intracranial hemorrhage (ICH) and extracranial hemorrhage (ECH).10 Thus, the risks vs. benefits of anticoagulation and varying treatment options must be considered.
Warfarin, the long‐standing mainstay of therapy, has been supplanted by direct oral anticoagulants (DOACs) as the guideline recommended treatments of choice for stroke prevention in AF.1 The first DOAC, dabigatran, (Pradaxa) was approved by the Food and Drug Administration (FDA) in 2010. Compared with warfarin, dabigatran was shown to be noninferior with regard to the prevention of stroke or systemic embolism, had less major bleeding, and fewer concerns about variability in dosing regimens and monitoring.11 The eventual market entry of generic DOACs may change market dynamics through increased utilization of cheaper generic DOACs. As it was the first approved DOAC, dabigatran is also the first expected generic, estimated to enter the market in 2021, which should substantially decrease costs for stroke prevention in AF.
Although the entry of generic dabigatran should be beneficial to patients and payers through decreased costs, pharmaceutical and pharmacological complexity can often raise concerns regarding bioequivalence (BE) vs. the reference brand product for medications with relatively narrow therapeutic indices or serious effectiveness and safety outcomes, like anticoagulants.12 Under current regulations, generic manufacturers need to demonstrate BE to the reference product by conducting studies on healthy volunteers as a surrogate for therapeutic equivalence.13, 14 Two pharmacokinetic (PK) parameters are often measured in the BE studies—maximum observed concentration (Cmax) representing the absorption rate, and area under the plasma concentration‐time curve (AUC) representing the extent of absorption.13, 14 Under FDA guidance,15 BE can be declared when the 90% confidence interval for the ratio of the population geometric means of the PK measures (such as Cmax and AUC) for generic to brand product falls within 80–125% or F = 0.80 and F = 1.25 (Figure 1). Around 98% of all small molecule formulations passed BE testing between 1996 and 2007.16
Figure 1.
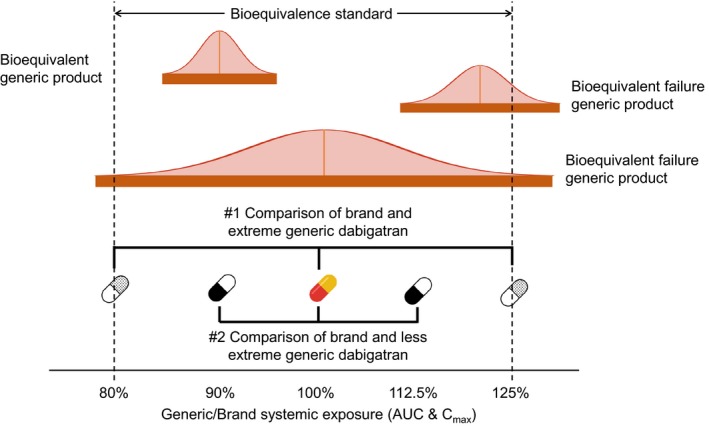
Demonstration of potential bioequivalent results and two comparisons made in this study. The solid orange bars represent the 90% confidence intervals of the bioequivalence study AUC and Cmax generic/brand ratios normally distributed around the geometric mean generic/brand ratio. Falling beyond 80–125% thresholds is a “failure” of bioequivalence. Comparison #1 compares brand to generic dabigatran with extreme systemic exposure (125% and 80%). Comparison #2 compares brand to generic dabigatran with less extreme systemic exposure (112.5% and 90%). AUC, area under the plasma concentration‐time curve; Cmax, maximum observed concentration.
The BE requirement for generic medications assumes that declarations of BE are consistent with therapeutic equivalence. However, due to batch‐to‐batch variability and between manufacturer differences, this may not always hold true. In fact, this phenomenon has been observed in examples of warfarin brand‐to‐generic comparisons. One observational study found international normalized ratios were significantly lower after switching from brand to generic warfarin.17 Another study found the number of visits with international normalized ratio values outside of therapeutic range and the number of dose changes was higher in patients on generic warfarin.18 Switching from brand to generic warfarin was associated with a higher risk of thrombotic events and hemorrhagic events.19 Alongside these detrimental studies, several other studies showed generic warfarin to likely be suitable for substitution with the brand.20, 21 Nevertheless, even a few reports of BE failures are often sufficient to increase insecurity for prescribers and patients to prescribe and use generic drugs and diminish the perception of generics overall.22, 23
BE concerns are especially relevant to anticoagulants due to relatively narrow therapeutic indices and severity of both safety and effectiveness outcomes (i.e., hemorrhage and stroke). For dabigatran, this is accentuated by a steep dose‐response curve particularly for bleeding events as well as low bioavailability.24 As dabigatran will be first to be offered as generic, these concerns raise questions regarding its known PK/pharmacodynamic (PD) profile, possible causes of variation in Cmax and AUC, and how this will impact the entire therapeutic area. Incorporating PK/PD, clinical, and cost evaluations prior to generic entry may be informative to establish the value proposition of generic formulations, especially amid situations where some uncertainty in BE and therapeutic equivalence is predicted.
Thus, the present study aimed to: (i) predict and compare the “pre‐launch” lifetime effectiveness of generic and brand dabigatran; (ii) predict and compare the “pre‐launch” lifetime overall medical cost of generic and brand dabigatran; and (iii) evaluate the cost‐effectiveness of generic dabigatran vs. the reference brand in patients with AF. This study expands upon base pharmacometric models to extrapolate parameters to clinical decision making through cost‐effectiveness models.
Materials and Methods
Economic model overview
A Markov model was adapted from Shah et al. to model the disease progression of AF (Figures 2 and 3).25 We relaxed the cohort assumption inherent with Markov models to allow variations in patient clinical characteristics, such as stroke, bleed risks, and age. The model had a cycle length of 30 days with a lifetime horizon. The simulation was conducted from the US private payer perspective. All state rewards, including probabilities, costs, and health utilities, were converted to reflect the 30‐day cycle length. The half‐cycle correction was applied to adjust for the potential overestimation of the costs and utilities. The study population represented commercially insured adult patients with AF who are eligible to receive anticoagulants. The model population consisted of 10,000 patients with AF aged 18 years or above recommended for anticoagulation with a CHA2DS2‐VASc score of 2 or above and any value of HAS‐BLED score.1, 8
Figure 2.
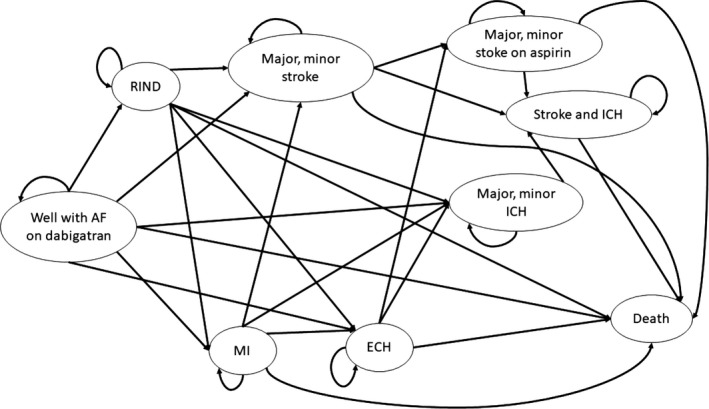
Diagram of the Markov model transition states. State transition diagram of Markov model shows all patients start with atrial fibrillation and then cycle between health states until death occurs. Major and minor stroke state were combined for demonstration purpose, so do the major and minor stroke on aspirin, as well as major and minor ICH. AF, atrial fibrillation; ECH, extracranial hemorrhage; ICH, intracranial hemorrhage; MI, myocardial infarction; RIND, reversible ischemic neurological damage.
Figure 3.
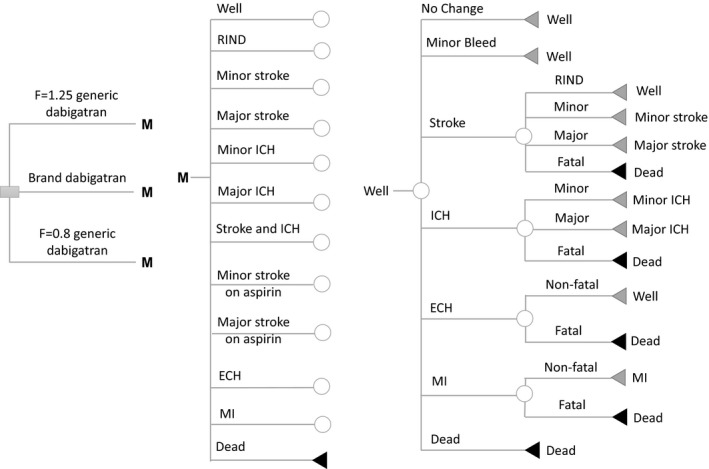
Schematic representation of Markov model shows the possible transitions for patients in well state. Ten other health states (except for death state) have similar structures of clinical event patients could encounter but different jump‐to states. Probabilities of these events depend on prescribed medications and individual stroke/bleed risk. Decision nodes (square), chance nodes (circles), and terminal nodes (triangles) are depicted. ECH, extracranial hemorrhage; ICH, intracranial hemorrhage; MI, myocardial infarction; RIND, reversible ischemic neurological damage.
We updated the prior model to expand granularity for stroke and bleed risk estimates. We replaced the CHADS2 score with CHA2DS2‐VASc score, which has been shown to perform better in risk stratification.26, 27 We also incorporated individual bleed risk using the HAS‐BLED score.28 Patients were grouped into nine exclusive subgroups based on the cross‐tabulation of their stroke risk categories (low, moderate, or high) and bleed risk categories (low, moderate, or high) in order to better classify patients in bleed and stroke risk groups. The cross‐tabulation of these scores was implemented to capture the strong correlation between the risk scores given they incorporate many of the same risk factors. The joint distribution of CHA2DS2‐VASc and HAS‐BLED scores, as well as the age distribution for the nine subgroups, were derived from IBM MarketScan Databases, which was consistent with the commercial payer perspective of the model.
Clinical outcomes considered in the model were minor bleeding, stroke, ICH, myocardial infarction (MI), ECH, and death. The model structure consisted of 12 mutually exclusive health states: (i) well with AF; (ii) reversible ischemic neurological damage; (iii) minor stroke; (iv) major stroke; (v) major ICH; (vi) minor intracranial ICH; (vii) minor stroke on aspirin; (viii) major stroke on aspirin; (ix) stroke and ICH; (x) MI; (xi) ECH, and (xii) death. Patients entered the model in the “well with AF” state on either brand or generic dabigatran and then transitioned to other health states based on the event transition probabilities. The following assumptions were made to reflect the actual treatment pattern of patients with AF and to better approximate the disease progression of patients with AF:
Twenty‐eight percent of all the ischemic strokes were transient ischemic attacks, the remaining could be one of four types: reversible, major, minor, or fatal.
ICH could be of three types: major, minor, and fatal. ECH could be either fatal or nonfatal. After experiencing ECH or ICH, patients were assumed to discontinue dabigatran and switch to aspirin for the remainder of their lives.
After experiencing two major neurological events (stroke or ICH), patients will proceed to the death state.
After experiencing two minor neurological events, patients will proceed to a major neurological event state.
For example, if patients in “major ICH” state experience a major stroke, instead of entering “major stroke” state, they will enter “death” state. If patients in “minor ICH” state experience another minor ICH, they will enter “major ICH” state instead of staying in “minor ICH" state. To estimate the NMBs, we used two different willingness‐to‐pay (WTP) threshold of US $50,000 and $100,000 per quality‐adjusted life year (QALY). Costs and utilities were discounted at a rate of 3%.
Two sets of comparisons were performed. First, we compared brand dabigatran (F = 1.0) with two hypothetical versions of generic dabigatran with worst‐case extreme values for systemic exposure based on AUC and Cmax. The two extreme generic dabigatran versions had, respectively, 80% systemic exposure of the brand (extreme lower‐bound generic, F = 0.8) and 125% systemic exposure of the brand (extreme higher‐bound generic, F = 1.25). Second, we compared brand dabigatran with two additional hypothetical dabigatran versions with less extreme values of BE, 90% and 112.5% (F = 0.9 and 1.125, respectively). Figure 1 shows the brand‐to‐generic ratio of systematic exposure for the hypothetical generic dabigatran we compared in this study.
Model input parameters
Key model input parameters are provided in Table 1 and a complete list of all input parameters, distributions, and data sources are provided in Table S1 . All costs were considered from a private payer's perspective and only direct medical costs were included. The event costs of ICH, MI, stroke, and ECH and the follow‐up costs of ICH, MI, and stroke were derived from AF‐specific events and follow‐up cost estimates. The cost for the branded dabigatran was estimated using the 2017 National Average Drug Acquisition cost compiled by the US Medicaid program.29 The cost for the generic dabigatran was calculated by multiplying the price for brand dabigatran by the reported relative cost difference between branded and generic drugs when only one generic drug is available.30 Thus, these models would be applicable to the initial period of market exclusivity for the first generic version and do not incorporate lower generic costs introduced as more generic manufacturers introduce products. To more accurately reflect a payers’ perspective in which negotiated rebates are common when there are multiple branded products in a therapeutic category, we applied a rebate of 23% to the price for both brand and generic dabigatran to represent the federally mandated rebate rate for pharmaceutical products.31 All event and follow‐up costs were inflated using the medical component of Personal Consumption Expenditures Price Index to 2017 US dollars.32 The cost for aspirin was inflated using the pharmaceutical product component of Personal Consumption Expenditures Price Index.
Table 1.
Key model input parameters
| Variable | Base case | Range | Reference |
|---|---|---|---|
| Cost in 2017 monthly (US $) | |||
| Brand dabigatran | 296.23 | — | 29, 31 |
| Generic dabigatran | 257.72 | — | 29, 30, 31 |
| Rate of ischemic stroke on brand dabigatran for different patient subgroups (%/year) | |||
| Low stroke risk subgroup (CHA2DS2‐VASc score 2–3) | 0.721 | 0.569–0.930 | IBM11 |
| Medium stroke risk subgroup (CHA2DS2‐VASc score 4) | 1.082 | 0.854–1.396 | IBM11 |
| High stroke risk subgroup (CHA2DS2‐VASc score ≥ 5) | 1.942 | 1.533–2.300 | IBM11 |
| Rate of minor bleeding on brand dabigatran for different patient subgroup (%/year) | |||
| Low bleed risk subgroup (HAS‐BLED score 0–1) | 7.361 | 6.876–7.846 | IBM11 |
| Medium bleed risk subgroup (HAS‐BLED score 2) | 9.183 | 8.577–9.788 | IBM11 |
| High bleed risk subgroup (HAS‐BLED score ≥ 3) | 13.151 | 12.029–14.018 | IBM11 |
| Rate of ICH on brand dabigatran for different patient subgroup (%/year) | |||
| Low bleed risk subgroup (HAS‐BLED score 0–1) | 0.199 | 0.167–0.298 | IBM11 |
| Medium bleed risk subgroup (HAS‐BLED score 2) | 0.248 | 0.167–0.372 | IBM11 |
| High bleed risk subgroup (HAS‐BLED score ≥ 3) | 0.355 | 0.239–0.532 | IBM11 |
| Rate of ECH on brand dabigatran for different patient subgroup (%/year) | |||
| Low bleed risk subgroup (HAS‐BLED score 0–1) | 2.050 | 1.494–2.395 | IBM11 |
| Medium bleed risk subgroup (HAS‐BLED score 2) | 2.557 | 1.864–2.9875 | IBM11 |
| High bleed risk subgroup (HAS‐BLED score ≥ 3) | 3.662 | 2.670–4.279 | IBM11 |
| Efficacy and safety of F = 1.25 generic dabigatran | |||
| HR for ischemic stroke, F = 1.25 generic vs. brand | 0.934 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for bleeding event (minor bleeding, ICH, ECH), F = 1.25 generic vs. brand | 1.211 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for MI, F = 1.25 generic vs. brand | 1.062 | NA | 34 |
| Efficacy and safety of F = 0.8 generic dabigatran | |||
| HR for ischemic stroke, F = 0.8 generic vs. brand | 1.066 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for bleeding (minor bleeding, ICH, ECH), F = 0.8 generic vs. brand | 0.842 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for MI, F = 0.8 generic vs. brand | 0.951 | NA | 34 |
| Efficacy and safety of F = 1.125 generic dabigatran | |||
| HR for ischemic stroke, F = 1.125 generic dabigatran vs. brand | 0.967 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for bleeding (minor bleeding, ICH, ECH), F = 1.125 dabigatran vs. brand | 1.1055 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for MI, F = 1.125 generic vs. brand | 1.031 | NA | 34 |
| Efficacy and safety of F = 0.9 generic dabigatran | |||
| HR for ischemic stroke, F = 0.9 generic dabigatran vs. brand | 1.033 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for bleeding (minor bleeding, ICH, ECH), F = 0.9 generic dabigatran vs. brand | 0.921 | NA | S. Kim and S. Schmidt (personal communication) |
| HR for MI, F = 0.9 generic vs. brand | 0.976 | NA | 34 |
ECH, extracranial hemorrhage; F, bioavailability ratio vs. reference brand; HR, hazard ratio; ICH, intracranial hemorrhage; MI, myocardial infarction; NA, not applicable.
Utilities were accounted in each state and with each event to estimate cumulative QALYs for each patient. State utilities (state rewards) were attached to the 12 health states. Major and minor neurological events (stroke or ICH) were associated with a permanent annual disutility of –0.61 and –0.24, respectively. The decrements in utilities (transition rewards) were calculated for the transitional events (minor bleed, reversible ischemic neurological damage, ECH, and MI) in the model. MI, ECH, and minor bleed were assigned temporary disutility of 30 days, 2 weeks, and 2 days, respectively. The detailed sources and calculation of state utilities for the 12 health states and the transition utilities for the transitional events are shown in Table S2 .
The ischemic stroke and bleeding rates (minor bleeding, ICH, and ECH) for patients on brand dabigatran within each CHA2DS2‐VASc score category were derived from IBM MarketScan databases and the RE‐LY trial.11 The difference in event rates for stroke and bleeding between generic and brand dabigatran were obtained from a PK/PD model developed by Kim et al. from the new drug application materials for brand dabigatran24 by calculating a relative event rate for each bioavailability level (e.g., F = 0.8 and F = 1.25; S. Kim and S. Schmidt, personal communication). To describe this approach briefly, a published two‐compartment PK model was applied, which was developed with data from three phase I clinical trials that included 80 healthy volunteers with 1,031 PK observations.33 The PK model described the data with first‐order absorption after an absorption lag‐time and first‐order elimination. Extrapolated trough plasma concentration (Ctrough) values were used as an exposure indicator from the PK simulations to find the corresponding probabilities of life‐threatening bleed and ischemic stroke using the exposure‐response curves digitized from the FDA clinical pharmacology review.24 To determine the impact of hypothetical bio‐in‐equivalence with respect to AUC and Cmax on the efficacy and safety profiles of dabigatran, hypothetical bio‐in‐equivalence scenarios were simulated by changing the extent (F) at which the drug is absorbed from its product. Resulting changes were then extrapolated into exposure‐response curves. R version 3.4.0 was used for data processing and result visualizations, NONMEM version 7.3 (Icon Development Solutions, Ellicott City, MD) was used for PK simulations, and WebPlotDigitizer version 3.12 (Ankit Rohatgi, San Francisco, CA) was used for digitizing the exposure‐response curves.
Relative risks for increased stroke and ICH with every decade were based on pooled analysis from clinical trials and systematic reviews. The MI rate for patients on brand dabigatran was obtained from the clinical trial. The difference between brand and generic in MI rate was approximated from a meta‐analysis comparing 150 mg dabigatran with 110 mg dabigatran, which represented a 26.7% dose difference, and we assumed a linear relationship between relative dabigatran dose vs. the brand and the occurrence of MI.34 The calculations of the event rates and the data analysis were conducted in IBM MarketScan databases and are described in detail in the Supplementary Materials.
Two cost‐effectiveness analyses were performed to compare extreme generics and more BE generics each with the brand dabigatran. We performed a series of one‐way sensitivity analysis by varying (i) cost of generic dabigatran; (ii) the event and monthly costs; (iii) the utilities; and (iv) the transition probabilities. The parameter ranges for one‐way sensitivity analysis are shown in Table S1 . Probabilistic sensitivity analysis was conducted to account for parameter uncertainty. The probability distribution reflected the nature of the data. When the simulation was initiated, a value for each input was randomly selected from its respective probability distribution. The model was run repeatedly for 100 iterations and the pooled results were summarized. Model development, implementation, and analyses were performed using TreeAge Pro 2018 (TreeAge Software, Williamstown, MA).
Results
Base case analysis
In the comparison of brand and hypothetical extreme generic dabigatran, the base case analysis showed the F = 0.8 generic dabigatran had the highest effectiveness (QALYs) among the three versions followed by the brand dabigatran (Table 2). Although the occurrence of stroke was similar across different versions of dabigatran, more variability was observed with bleeding events, which was consistent with the predicted bleed and stroke rates from the PK/PD models. The cohort on the F = 1.25 generic had an excess of roughly 2,000 more (14,112 vs. 12,193; 15.7% increase) bleeding events than the brand dabigatran and roughly 3,500 more (14,112 vs. 10,604; 33.1% increase) bleeding events than the F = 0.8 generic dabigatran (Table 2). The F = 0.8 generic dabigatran had the lowest cost among the three dabigatran treatments followed by the F = 1.25 generic. The F = 0.8 generic had both higher QALY and lower costs; thus, it dominated the other treatment options. Direct comparison between the F = 1.25 and brand dabigatran showed the brand to be more cost‐effective than the F = 1.25 generic with an incremental cost‐effectiveness ratio value of $36,483/QALY.
Table 2.
Base case comparison between brand and hypothetical extreme generic dabigatran formulations
| Treatment strategy | Therapies in ascending order of QALY | |||||||
|---|---|---|---|---|---|---|---|---|
| Cost | QALY | Stroke, N | All bleeding, N | Dominance | ||||
| Absolute | Incremental | Absolute | Incremental | Minor bleeding, N | Major bleeding, N | |||
| Generic dabigatran (F = 1.25) | $50,937 | Ref | 7.29 | Ref | 2,192 | 14,112 | Absolutely dominated | |
| 10,207 | 3,905 | |||||||
| Brand dabigatran | $53,126 | $2,189 | 7.35 | 0.06 | 2,174 | 12,193 | Absolutely dominated | |
| 8,785 | 3,408 | |||||||
| Generic dabigatran (F = 0.8) | $49,510 | −$1,527 | 7.38 | 0.09 | 2,212 | 10,604 | Dominanta | |
| 7,615 | 2,989 | |||||||
F, bioavailability ratio vs. reference brand; QALY, quality‐adjusted life years.
Dominant implies both lower costs and higher effectiveness (QALYs).
The comparison of brand and less extreme hypothetical cases of generic dabigatran versions showed consistency in that the F = 0.9 generic dabigatran was dominant with the highest effectiveness (QALYs) and the lowest cost (Table 3). The difference in the occurrence of clinical events was also less extreme. Similar to the other comparison, the occurrence of stroke event was almost the same across three dabigatran treatment groups. The cohort on the F = 1.125 dabigatran had an excess of roughly 1,000 more (13,167 vs. 12,125; 8.6% increase) bleeding events than the brand dabigatran and nearly 1,800 more (13,167 vs. 11,391; 15.6%) bleeding events than the F = 0.9 generic dabigatran (Table 3). Direct comparison between brand and F = 1.125 generic dabigatran showed the brand retained more utility than the F = 1.125 generic version but with a much higher incremental cost‐effectiveness ratio of $287,200/QALY compared with the more extreme generic (F = 1.25).
Table 3.
Comparison between brand and generic dabigatran with less extreme systemic exposure
| Treatment strategy | Therapies in ascending order of QALY | |||||||
|---|---|---|---|---|---|---|---|---|
| Cost | QALY | Stroke, N | All bleeding, N | Dominance | ||||
| Absolute | Incremental | Absolute | Incremental | Minor bleeding, N | Major bleeding, N | |||
| Generic dabigatran (F = 1.125) | $50,089 | Ref | 7.34 | Ref | 2,214 | 13,167 | Absolutely dominated | |
| 9,489 | 3,678 | |||||||
| Brand dabigatran | $52,961 | $2,872 | 7.35 | 0.01 | 2,167 | 12,125 | Absolutely dominated | |
| 8,721 | 3,404 | |||||||
| Generic dabigatran (F = 0.9) | $49,443 | −$646 | 7.37 | 0.03 | 2,203 | 11,391 | Dominanta | |
| 8,207 | 3,184 | |||||||
F, bioavailability ratio vs. reference brand; QALY, quality‐adjusted life years.
Dominant implies both lower costs and higher effectiveness (QALYs).
Sensitivity analyses
One‐way sensitivity of the comparison between brand and extreme generic dabigatran versions showed the F = 0.8 generic was the optimal treatment option under all tested conditions (Figures S1 – S4 ). Although results from direct comparison between brand and F = 1.25 dabigatran was robust to most of the parameter changes, F = 1.25 generic was more cost‐effective than the brand at a WTP threshold of $50,000/QALY in the following cases (Figures S5 – S7 ):
Monthly cost of generic dabigatran lower than $248 (Figure 4a).
Hazard ratio (HR) for ECH comparing dabigatran with warfarin was < 1.04.
MI rate for patients on warfarin < 1.06.
HR for MI comparing dabigatran with warfarin < 1.27 or > 1.85.
HR for ICH comparing dabigatran with warfarin < 0.33.
Figure 4.
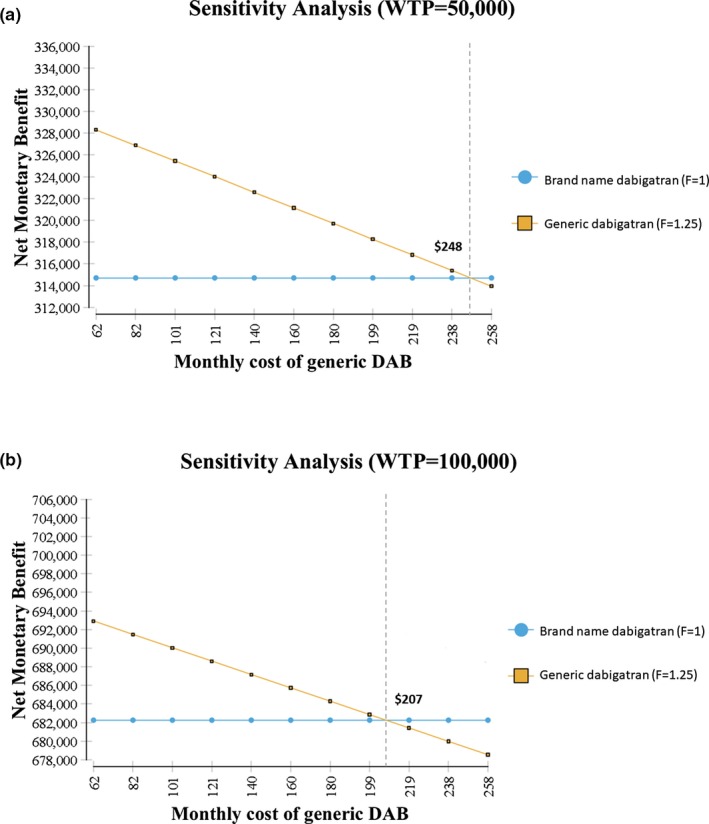
Results of one‐way sensitivity analyses for cost of generic version. Net monetary benefit of brand and generic dabigatran (DAB) with higher‐than‐brand extreme systemic exposure (a) with a willingness‐to‐pay (WTP) threshold of 50,000 (b) with a willingness‐to‐pay threshold of 100,000.
The F = 1.25 generic was also more cost‐effective than the brand at a WTP threshold of $100,000/QALY in the following situation:
Monthly cost of generic dabigatran lower than $207 (Figure 4b).
HR for ECH comparing dabigatran to warfarin was < 0.91 (Figure S7 ).
Probabilistic sensitivity analyses showed that the F = 0.8 was the most cost‐effective treatment option among the three in 100% of the iterations (Figure S15 ).
One‐way sensitivity of the comparison between brand and less extreme generic dabigatran showed F = 0.9 generic was the optimal treatment option under all tested conditions (Figures S8–S11 ). One‐way sensitivity comparisons between the brand and F = 1.125 generic version were sensitive to event costs associated with stroke and bleeding events but did not reverse the direction of the overall findings (Figure S13 ). Findings for the net monetary benefit (NMB) for generic versions were also highly sensitive to the price of the generic drug (Figure 4). As brand dabigatran was tested with a safety profile more similar to warfarin for bleeding and MI events (i.e., higher rates of these events), both generic versions were more cost‐effective than the brand (Figure S7 ). Results from the direct comparison between brand and F = 1.125 dabigatran were robust to most of the tested conditions (Figures S12–S14 ). Probabilistic sensitivity analyses showed that the F = 0.9 was the most cost‐effective option among the three in 99–100% of the iterations depending on the WTP threshold used (Figure S16 ).
Discussion
The objective of this study was to compare the cost‐effectiveness of hypothetical generic versions of dabigatran vs. the brand. Our model shows that the generic dabigatran with the lower‐than‐brand systemic exposures were the most cost‐effective options in both comparisons with the highest QALY and lowest cost. The results are robust in that in the base case analysis and across all sensitivity analyses, including probabilistic sensitivity analysis, F = 0.8 and F = 0.9 generic dabigatran were the optimal treatment strategies. When the dominant treatment strategies (F = 0.8 and F = 0.9 generic dabigatran) were excluded, and only F = 1.25 and F = 1.125 generic dabigatran were compared with the brand, our model showed different results in two comparisons. In the extreme case, brand was more cost‐effective than the F = 1.25 generic, whereas in the less extreme case, F = 1.125 generic was a preferred choice than the brand.
Note that the price of generic dabigatran was estimated from a previous study30 under the assumption that there is only one generic dabigatran available (generic costs 87% of the brand). The same study also reported the generic to brand price ratio when there are multiple generic medications available. Based on their estimations, when there are two generic dabigatrans on the market, the generic dabigatran would have a price of US $228 (i.e., 77% of the brand price). Under this scenario, the brand will no longer be cost‐effective than F = 1.25 generic dabigatran brand using a WTP threshold of $50,000/QALY but remained cost‐effective using a WTP threshold of $100,000/QALY. However, when there are three generic dabigatran available, generic dabigatran would have an estimated price of US $178 (the generic costs 60% of the brand). Under that scenario, the brand will no longer be cost‐effective than the F = 1.25 generic dabigatran using either $50,000 or $100,000 WTP threshold. Thus, these results, and the cost‐effectiveness of generic dabigatran in extreme cases of increased bioavailability, may depend on competition and pricing of generics.
Anticoagulation as a therapeutic area has experienced past and continued concerns about BE failures when generic versions of warfarin became available.20, 23 Although BE concerns with warfarin were ultimately not borne out in well‐designed studies, concerns with warfarin due to its narrow therapeutic index, interpatient variability, between manufacturer variability, and the general perceptions of generic drugs continue to make BE an important concept in the therapeutic area.1, 22, 23 However, this is a concern that is lessened with DOACs and eased given the lack of therapeutic monitoring with DOACs.1 However, DOACs still have PK/PD profiles that can be of concern, particularly for bleeding events, which have a higher incidence than ischemic events on treatment and a steep PK/PD trajectory,24 which drove the major findings in this study.
The choice of dabigatran as an example of combining pharmacometric parameters with cost‐effectiveness analysis was motivated by convenience and projections of it being the first generic DOAC. This study does not necessarily imply that dabigatran has complex pharmaceutical or pharmacological properties that may lead to BE concerns or predict future product failures. However, dabigatran does have low bioavailability and is susceptible to drug‐drug interactions.24 Paired with a relatively steep bleed risk curve with increasing bioavailability, the results of this study indicate that an increase in bioavailability of dabigatran, whether caused by formulation issues or drug‐drug interactions, can lead to excess bleed events and lack of overall cost‐effectiveness, which are concerns of the brand and future generic versions of dabigatran alike.
To the best of our knowledge, this is the first study to evaluate the cost‐effectiveness of future, hypothetical generic formulations vs. the brand using pharmacometrically derived input parameters.35 The results have important clinical implications. Such an approach, and the evidence generated, could enhance evaluation of generics by patients, providers, and healthcare payers with regard to generic medications. Combined, PK/PD models and pharmacoeconomic models can also be informative for regulatory bodies for the required evidence for generic approval.35 From a clinical standpoint, this study highlighted the clinical burden when the active ingredient of generic dabigatran was above that of the brand and demonstrated it was less of the concern when active ingredient of generic dabigatran was below that of the brand. Future studies that evaluate the cost‐effectiveness of other generic medications with more complex pharmaceutical properties may be informative to healthcare providers and decision makers.
Limitations
These results should be interpreted in light of a number of limitations. First, the assumptions of worst‐case scenarios represent extreme cases that are not feasible in the real world. For one, the BE thresholds capture a 90% confidence range and no medication would have an F exactly equal to the point estimates used in this study rather brand or generic. We chose these point estimates for simplify the modeling approaches and assumptions and to exaggerate a worst‐case scenario. Further, effectiveness and safety estimates were obtained from clinical trials and further parameterized with external information from PK/PD models and may not be generalizable to real‐world populations. Transition probabilities were further influenced by external information. To account for indirect evidence, we incorporated meaningful ranges and thorough one‐way and probabilistic sensitivity analyses to observe the robustness of results amid this uncertainty. Like past cost‐effectiveness models in AF, the model incorporates a time span much longer than the source material and does not incorporate additional incident events, such as diabetes, which would influence stroke and bleed risk scores. However, these events can generally be considered to be similar between treatment groups.
Conclusion
Predictions of cost‐effectiveness for future generic dabigatran products showed that lower‐bound generics (F = 0.80 bioavailability vs. brand) was consistently cost‐effective. Upper‐bound generics (F = 1.25 bioavailability vs. brand) were not cost‐effective, mainly due to an excess in bleeding events, although these results were sensitive to the cost of the generic version. Extensions of pharmacometric PK/PD models to cost‐effectiveness and decision analyses can be informative for multiple stakeholders and provide insight into current or future value of generic medications.
Funding
No funding was received for this work. Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
C.W. and J.B. wrote the manuscript. C.W. and J.B. designed the research. C.W., P.P., S.K., K.L., S.S., V.D., and J.B. performed the research. C.W. and P.P. analyzed the data. J.B. and S.S. contributed new reagents/analytical tools.
Supporting information
Supplementary Material. Tables S1–S2 and Figures S1–S16.
References
- 1. January, C.T. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 74, 104–132 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Pritchett, E.L. Management of atrial fibrillation. N. Engl. J. Med. 326, 1264–1271 (1992). [DOI] [PubMed] [Google Scholar]
- 3. The National Heart, Lung, and Blood Institute Working Group on Atrial Fibrillation . Atrial fibrillation: current understandings and research imperatives. J. Am. Coll. Cardiol. 22, 1830–1834 (1993). [PubMed] [Google Scholar]
- 4. Lip, G.Y. , Metcalfe, M.J. & Rae, A.P. Management of paroxysmal atrial fibrillation. Q. J. Med. 86, 467–472 (1993). [DOI] [PubMed] [Google Scholar]
- 5. [No authors listed] . Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch. Intern. Med. 154, 1449–1457 (1994). [PubMed] [Google Scholar]
- 6. Hart, R.G. , Pearce, L.A. & Aguilar, M.I. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 146, 857–867 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Wann, L.S. et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 57, 223–242 (2011). [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962 (2016). [DOI] [PubMed] [Google Scholar]
- 9. LaHaye, S.A. , Gibbens, S.L. , Ball, D.G. , Day, A.G. , Olesen, J.B. & Skanes, A.C. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Eur. Heart J. 33, 2163–2171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fang, M.C. et al . Death and disability from warfarin‐associated intracranial and extracranial hemorrhages. Am. J. Med. 120, 700–705 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connolly, S.J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Weitz, J.I. , Earl, K.M. , Leblanc, K. , Semchuk, W. & Jamali, F. Establishing therapeutic equivalence of complex pharmaceuticals: the case of dabigatran. Can. J. Cardiol. 34, 1116–1119 (2018). [DOI] [PubMed] [Google Scholar]
- 13. Nation, R.L. & Sansom, L.N. Bioequivalence requirements for generic products. Pharmacol. Ther. 62, 41–55 (1994). [DOI] [PubMed] [Google Scholar]
- 14. Meredith, P. Bioequivalence and other unresolved issues in generic drug substitution. Clin. Ther. 25, 2875–2890 (2003). [DOI] [PubMed] [Google Scholar]
- 15. Statistical approaches to establishing bioequivalence: guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). <https://www.fda.gov/media/70958/download>. Accessed August 4, 2019.
- 16. Davit, B.M. et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann. Pharmacother. 43, 1583–1597 (2009). [DOI] [PubMed] [Google Scholar]
- 17. Halkin, H. , Shapiro, J. , Kurnik, D. , Loebstein, R. , Shalev, V. & Kokia, E. Increased warfarin doses and decreased international normalized ratio response after nationwide generic switching. Clin. Pharmacol. Ther. 74, 215–221 (2003). [DOI] [PubMed] [Google Scholar]
- 18. Richton‐Hewett, S. , Foster, E. & Apstein, C.S. Medical and economic consequences of a blinded oral anticoagulant brand change at a municipal hospital. Arch. Intern. Med. 148, 806–808 (1988). [PubMed] [Google Scholar]
- 19. Ghate, S.R. et al. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann. Pharmacother. 45, 701–712 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Dentali, F. et al. Brand name versus generic warfarin: a systematic review of the literature. Pharmacotherapy 31, 386–393 (2011). [DOI] [PubMed] [Google Scholar]
- 21. Pereira, J.A. et al. Are brand‐name and generic warfarin interchangeable? Multiple n‐of‐1 randomized, crossover trials. Ann. Pharmacother. 39, 1188–1193 (2005). [DOI] [PubMed] [Google Scholar]
- 22. Dunne, S.S. & Dunne, C.P. What do people really think of generic medicines? A systematic review and critical appraisal of literature on stakeholder perceptions of generic drugs. BMC Med. 13, 173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellfritzsch, M. et al. Generic switching of warfarin and risk of excessive anticoagulation: a Danish nationwide cohort study. Pharmacoepidemiol. Drug Saf. 25, 336–343 (2016). [DOI] [PubMed] [Google Scholar]
- 24. Drug approval package: Pradaxa (dabigatran etexilate mesylate) NDA 022512. Boehringer Ingelheim Pharmaceuticals, Inc. <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022512Orig1s000TOC.cfm>. Accessed June 9, 2019.
- 25. Shah, A. , Shewale, A. , Hayes, C.J. & Martin, B.C. Cost‐effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke 47, 1555–1561 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Coppens, M. et al. The CHA2DS2‐VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur. Heart J. 34, 170–176 (2013). [DOI] [PubMed] [Google Scholar]
- 27. Olesen, J.B. , Torp‐Pedersen, C. , Hansen, M.L. & Lip, G.Y. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb. Haemost. 107, 1172–1179 (2012). [DOI] [PubMed] [Google Scholar]
- 28. Pisters, R. , Lane, D.A. , Nieuwlaat, R. , de Vos, C.B. , Crijns, H.J. & Lip, G.Y. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138, 1093–1100 (2010). [DOI] [PubMed] [Google Scholar]
- 29. Pharmacy drug pricing. National Average Drug Acquisition Costs. Medicaid Web site. <https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-National-Average-Drug-Acquisition-Cost-/a4y5-998d/data>. Accessed December 2018.
- 30. Dave, C.V. , Hartzema, A. & Kesselheim, A.S. Prices of generic drugs associated with numbers of manufacturers. N. Engl. J. Med. 377, 2597–2598 (2017). [DOI] [PubMed] [Google Scholar]
- 31. Medicaid drug rebate program. <https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/index.html>. Accessed September 10, 2019.
- 32. Bureau of Economic Analysis. U.S. Department of Commerce . Table 2.5.4. Price indexes for personal consumption expenditures by function. <https://apps.bea.gov/iTable/iTable.cfm?reqxml:id=19%26step=2#reqxml:id=19%26step=2%26isuri=1%261921=survey>. Accessed December 2018.
- 33. Dansirikul, C. , Lehr, T. , Liesenfeld, K.H. , Haertter, S. & Staab, A. A combined pharmacometric analysis of dabigatran etexilate in healthy volunteers and patients with atrial fibrillation or undergoing orthopaedic surgery. Thromb. Haemost. 107, 775–785 (2012). [DOI] [PubMed] [Google Scholar]
- 34. Douxfils, J. et al. Dabigatran etexilate and risk of myocardial infarction, other cardiovascular events, major bleeding, and all‐cause mortality: a systematic review and meta‐analysis of randomized controlled trials. J. Am. Heart Assoc. 3, e000515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt, S. , Kim, S. , Vozmediano, V. , Cristofoletti, R. , Winterstein, A.G. & Brown, J.D. Pharmacometrics physiologically based pharmacokinetics, quantitative systems pharmacology‐what's next?‐Joining mechanistic and epidemiological approaches. CPT Pharmacometrics Syst. Pharmacol. 8, 352–355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material. Tables S1–S2 and Figures S1–S16.


