Abstract
Pragmatic clinical trials (PCTs) have an established presence in clinical research and yet have only recently garnered attention within the landscape of genomic medicine. Using the PRagmatic‐Explanatory Continuum Indicator Summary 2 (PRECIS‐2) as a framework, this paper illustrates the application of PCT principles to The Integrating Pharmacogenetics In Clinical Care (I‐PICC) Study, a trial of pharmacogenetic testing prior to statin initiation for cardiovascular disease prevention in primary care. The trial achieved high engagement with providers (85% enrolled of those approached) and enrolled a representative sample of participants for which statin therapy would be recommended. The I‐PICC Study has a high level of pragmatism, which should enhance the generalizability of its findings. The PRECIS‐2 may be useful in the design and evaluation of PCTs of genomic medicine interventions, contributing to the generation of evidence that can bridge the gap between genomics innovation and clinical adoption.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Pragmatic clinical trials (PCTs) offer researchers a means to study the performance of precision medicine interventions under real‐world conditions. Although PCTs have an established presence in clinical research, they have only recently received attention within the landscape of genomic medicine.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Using the PRagmatic‐Explanatory Continuum Indicator Summary 2 (PRECIS‐2) as a framework, this paper illustrates the application of PCT principles to a trial of preemptive pharmacogenetic testing.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Our characterization of the pragmatic design of the Integrating Pharmacogenetics In Clinical Care (I‐PICC) Study presents a feasible model for the conduct of a precision medicine PCT.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ PCTs have become recognized as a valuable tool for generating high quality and generalizable evidence about the effectiveness of genomic interventions. As calls for more real‐world evidence about these interventions are made, PCTs are poised to play an increasingly important role in supporting their adoption into the routine practice of medicine.
In 2011, the National Human Genome Research Institute proposed a vision for moving genomics innovations into the routine practice of medicine.1 This vision included the building of scientific evidence through a continuum of discovery, application, and outcomes measurement.2 Highlighted by the potential to optimize patient health, genomics research has quickly led to significant biological discoveries and healthcare applications across a wide spectrum of disease.3 The development of operational infrastructures to support the infusion of these innovations into the clinic has led a variety of precision medicine interventions to the cusp of widespread adoption.4 Despite these strides, however, uptake by healthcare systems, providers, and payers has been slow.5 Many barriers to uptake have been well‐described3, 4; principal among these is the lack of rigorous evidence for clinical effectiveness.6, 7 To continue the translation of potentially impactful genomic medicine innovations to clinical adoption, more evidence about their real‐world outcomes is needed.8, 9
Pragmatic clinical trials (PCTs) offer researchers a means to study the performance of precision medicine interventions under real‐world conditions.8, 10 Proposed by Schwartz and Lellouch,11 PCTs are conducted in the context of usual care and aim to inform decision makers about the benefits, drawbacks, and costs of adopting an intervention into practice.12 This approach differs from the traditional explanatory trial, carried out under ideal conditions with a goal of explaining a causal relationship between an intervention and a biological or clinical outcome.10 The classic pharmacologic explanatory trial example is a randomized placebo‐controlled trial with well‐defined inclusion and exclusion criteria, often including a run‐in period during which participants who are nonadherent or intolerant of the intervention are withdrawn. Such an approach seeks to minimize variability with respect to intervention response but may also limit the inclusion of some participants, including those with comorbidities or concomitant medication use. Explanatory and pragmatic trials address different questions and yet are equally important in building evidence to support the efficacy (“Does this work in an ideal setting?”) and effectiveness (“Does this work in real‐world practice?”) of a new intervention.13 Moreover, these approaches are not fully dichotomous but, rather, end points of a continuum, along which a trial's degree of pragmatism is measured by the congruence between its conduct and the real‐world setting where its results are intended to apply.14 The type of evidence desired determines whether the researcher designs a trial as purely explanatory, pragmatic, or somewhere in between.15 This fit‐for‐purpose design, coupled with randomization, makes the PCT a useful option for generating generalizable and rigorous outcomes data.16 In addition, the embedding of PCTs into routine clinical practice may result in greater efficiency and lower costs compared with some explanatory trial designs.17, 18 These characteristics allow for the ready transition of PCTs into existing healthcare infrastructures and make them particularly appealing to comparative effectiveness research19 and the evidence‐based mission of “learning healthcare systems.”20
As calls for more real‐world evidence are made to support the clinical adoption of genomics innovations in health care, PCTs are poised to play an increasingly important role in precision medicine outcomes research.7, 8 Thus, it is timely for precision medicine researchers, clinicians, and policymakers to become familiar with the underlying principles and design features of PCTs. Here, we illustrate the application of PCT principles to precision medicine by describing aspects of the Integrating Pharmacogenetics in Clinical Care (I‐PICC) Study (http://ClinicalTrials.gov NCT02871934), a randomized PCT of pharmacogenetic testing in primary care settings across the Veterans Affairs Boston Healthcare System (VABHS). The I‐PICC Study is examining patient outcomes after immediate vs. delayed genotyping for rs4149056 in SLCO1B1, a common variant with a well validated association with statin‐associated muscle symptoms (SAMS).
METHODS
I‐PICC Study overview
The I‐PICC Study has been approved by the VABHS Institutional Review Board, and all participants provided informed consent. A complete description of the protocol has been previously reported.21 Figure 1 outlines the study timeline, detailing important study milestones and design modifications. Study participants include primary care and women's health providers and their patients across all 8 sites of the VABHS, which cares for > 50,000 military veterans annually. No financial or other incentives are offered to providers or patients for their participation. Patients are eligible if they: (i) are aged 40–75 years, (ii) have no history of statin use, (ii) meet at least one criteria for elevated cardiovascular disease (CVD) risk per the American College of Cardiology/American Heart Association (ACC/AHA) guidelines,22 (iv) have received care at VABHS for a minimum of 6 months, and (v) are receiving clinical care from an enrolled provider. Eligible patients provide verbal consent to participate in the study and are only enrolled upon their provider's signing of an order for SLCO1B1 genotyping on an existing clinical blood sample collected through routine care. Once enrolled, patients are randomly assigned at the point of care to have their providers receive the results through the electronic health record (EHR) at baseline (pharmacogenetics+) or after 12 months (pharmacogenetics−). Most outcomes are collected through the VA Corporate Data Warehouse, a repository of administrative and clinical data from the nationally deployed EHR (the Computerized Patient Record System). The Corporate Data Warehouse includes diagnoses, laboratory values, prescription data, and clinical notes from inpatient and outpatient environments. Additional outcomes derive from a brief patient end‐of‐study telephone survey. The trial's primary outcome is change in low‐density lipoprotein cholesterol. Secondary outcomes are in concordance with Clinical Pharmacogenetics Implementation Consortium guidelines for simvastatin therapy,23 concordance with ACC/AHA guidelines for statin use, and incidence of SAMS. The I‐PICC Study is testing the hypotheses that SLCO1B1 genotyping leads to a reduction in SAMS while maintaining adequate CVD prevention.
Figure 1.
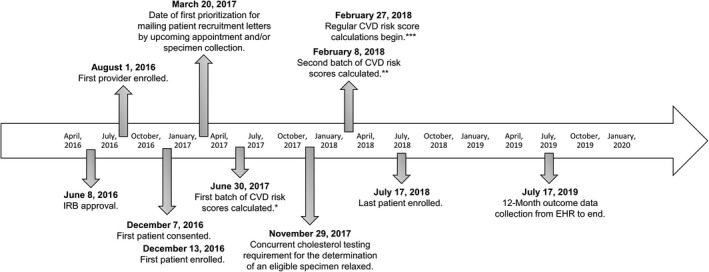
Study timeline for the Integrating Pharmacogenetics in Clinical Care Study. Risk scores are 10‐year CVD risk scores as calculated using American College of Cardiology/American Heart Association Pooled Cohort Risk Assessment Equations.22 *10‐year CVD risk scores calculated for 1,455 patients and added to potentially eligible patient database (not including African American women). **10‐year CVD risk scores calculated for 1,019 additional patients between February 8, 2018, and February 27, 2018, and added to potentially eligible patient database (including patients from all demographic backgrounds). ***First date of regular 10‐year CVD risk score calculations begin on a daily basis for potentially eligible patients using the birthday parity method as described by Vassy et al.21 CVD, cardiovascular disease; EHR, electronic health record; IRB, Institutional Review Board.
The I‐PICC Study as a PCT
The purpose of the I‐PICC Study is to generate evidence for the clinical utility of preemptive pharmacogenetic testing in the initiation of statin therapy. A pragmatic design was appropriate for the I‐PICC Study, given its aim to determine the impact of SLCO1B1 genotyping in a real‐world primary care context. To aid researchers in the design and evaluation of PCTs, Loudon et al.15 proposed the PRagmatic‐Explanatory Continuum Indicator Summary 2 (PRECIS‐2), a revised version of the original PRECIS tool.13 The face and content validity of the PRECIS‐2 have been assessed by experienced trialists and, in the retrospective assessment of trial protocols spanning the explanatory‐pragmatic continuum, the tool achieves good interrater reliability and moderate discriminant validity in distinguishing pragmatic from explanatory designs.24 Moreover, the PRECIS‐2 has demonstrated relative stability over time25 and has been used to characterize the design elements of ongoing pragmatic trials within the United States National Institutes of Health (NIH) Health Care Systems Research Collaboratory.26 With usual care as the comparator, the tool guides researchers through nine domains on which a trial's level of pragmatism can be assessed. Many of these domains are overlapping and as a whole are intended to describe the representativeness of a trial to clinical practice.27 Table 1 lists and briefly describes each domain. Design choices are considered for each domain and scored on a five‐point Likert continuum ranging from very explanatory (1) to very pragmatic (5). A thorough discussion and examples of how to use the PRECIS‐2 can be found in Loudon et al.15 The application of the PRECIS‐2 yields a granular assessment of pragmatism across multiple trial dimensions, visualized using a PRECIS‐2 wheel.15 The PRECIS tools have been successfully utilized by researchers rating their own trials as well as external reviewers during trial planning, trial conduct, and post hoc, as a mechanism for the assessment of pragmatism in completed trials.28, 29
Table 1.
PRECIS‐2 domains and pragmatism assessment for the design of the I‐PICC Study
| Domain | Domain description | Assessment of pragmatism | Rationale for PRECIS‐2 scoring of I‐PICC Study | Score |
|---|---|---|---|---|
| Eligibility | Specifies inclusion and exclusion criteria for the trial and frames the target population(s) for which its results are intended to apply | Are participants in the trial similar to those who would receive the intervention if it were available in usual care? | All primary care and women's health providers with prescribing privileges, except residents, are eligible for participation. Patient eligibility criteria are overall rather inclusive of the majority of patients for whom preemptive SLCO1B1 testing would be relevant if it were available in usual care. | 4 |
| Recruitment | Outlines the steps for the identification, consent, and enrollment of participants into the trial | How much extra effort is made to recruit participants into the trial above what would occur in usual care? | The trial leverages available data, informatics, and clinical resources at VABHS to recruit and enroll participants in the context of primary care. Recruitment effort is minimally greater than what occurs in usual care, including use of the EHR to consent providers and enroll patients and the sending of a recruitment letter and brief consent telephone call (< 5 minutes) to patients. | 4 |
| Setting | Context under which the trial is carried out, including factors such as geographic location and clinical infrastructure of the study site(s) | How different is the setting of the trial and the usual care setting? | The trial embeds SLCO1B1 genetic testing into eight VABHS women's health and primary care sites across eastern Massachusetts. The resources, clinical infrastructure, and reach of primary care services at VABHS are comparable to those found in other typical large healthcare systems. | 4 |
| Organization | Structure and delivery of the intervention, including the clinical resources required to provide the intervention | How different are the resources, provider expertise, and organization of care delivery in the intervention arm of the trial and usual care? | Beyond the intervention, there is no difference between the delivery of care in the trial and usual care. No specialized training is administered to providers, no additional clinical staff is required, and study staff integrated the intervention into the EHR and primary care setting using available data, informatics, and clinical resources. | 5 |
| Flexibility in delivery | How the trial intervention is delivered to study participants | How different is the flexibility in how the intervention is delivered and the flexibility likely in usual care? | No specified protocol is used for the delivery of SLCO1B1 test results. Providers receive structured results and clinical interpretation and prescription recommendations via the EHR per standard practice, are encouraged to communicate results to patients, and are provided with a standardized results letter template to do so. Nonetheless, the decision whether and how results are used and delivered to each patient is left entirely to the provider. | 5 |
| Flexibility in adherence | How closely study participants are monitored for compliance to the trial intervention and the measures used to maintain or improve adherence | How different is the flexibility in how participants must adhere to the intervention and the flexibility likely in usual care? | Participant adherence to the study intervention is not monitored or required. Provider adherence to CPIC guidelines for statin prescribing is encouraged but not protocolized (secondary outcome). Patients may adhere or not to any clinical recommendation associated with his or her SLCO1B1 test result. | 5 |
| Follow‐up | The rigor of measurement and amount of contact between the study staff and trial participants for the purposes of event tracking and data collection | How different is the intensity of follow‐up of participants in the trial and the likely follow‐up in usual care? | The intensity of participant follow‐up is minimally greater than what might occur in usual care. Providers receive no contact beyond notification of their patients' SLCO1B1 results. The 12‐month patient outcomes are collected observationally through the EHR, except for a brief end‐of‐study telephone survey (< 5 minutes). The majority of participant data are collected through the EHR via structured clinical data, chart review, and tracking of providers' use of study features in the EHR. | 4 |
| Primary outcome | The main variable to be measured for use in assessing the effect of the study intervention | To what extent is the trial's primary outcome relevant to participants? | The primary outcome is change in LDL‐C (12‐month LDL‐C minus baseline LDL‐C). LDL‐C is a clinically relevant biomarker, and well‐validated proxy for CVD risk. As a surrogate, it is not immediately relevant to patients, but is considered exceptionally meaningful to providers and policymakers. | 3 |
| Primary analysis | The approach used for the analysis of final results | To what extent are all data included in the analysis of the primary outcome? | The primary outcome will be analyzed using an intention‐to‐treat approach. No participant data will be excluded on the basis of intervention compliance or recruitment volume thresholds (i.e., a provider who signed only one order). | 5 |
Adapted from Ford and Norrie 201610 and Loudon et al. 201515. Domain scores range from 1 (very explanatory) to 5 (very pragmatic).
CPIC, Clinical Pharmacogenetics Implementation Consortium; CVD, cardiovascular disease; EHR, electronic health record; I‐PICC, Integrating Pharmacogenetics in Clinical Care; LDL‐C, low‐density lipoprotein cholesterol; PRECIS‐2, PRagmatic‐Explanatory Continuum Indicator Summary 2; VABHS, Veterans Affairs Boston Healthcare System.
To illustrate our characterization of pragmatism in the I‐PICC Study, we mapped its design elements onto the nine domains of the PRECIS‐2.15 In our assessment, we defined usual care as standard CVD risk management and statin prescribing as it would occur in a typical large healthcare system primary care setting, without SLCO1B1 genotyping. This mapping enables a determination of the extent to which our intentions of conducting a PCT have been met and informs the potential for generalizability of the I‐PICC Study results. We referenced the CONSORT Extension for Pragmatic Trials32 to guide our description of the I‐PICC Study as a PCT of genomic medicine.
RESULTS
Figure 2 illustrates the I‐PICC Study design, highlighting the intersections of clinical and research informatics resources, study staff, and provider‐patient clinical relationships that enable the SLCO1B1 genotyping intervention. Enrollment of 47 providers and 408 patients was completed in July 2018.
Figure 2.
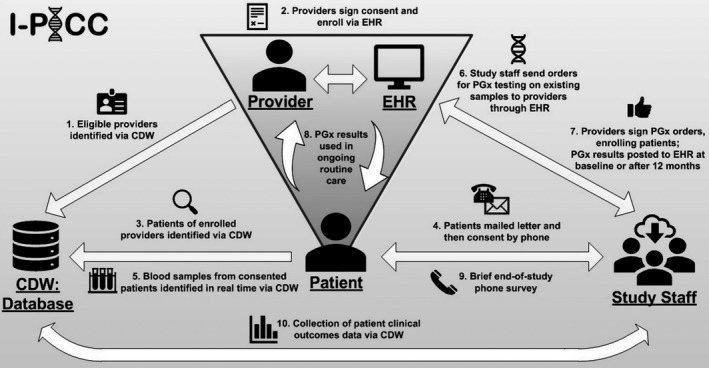
The Integrating Pharmacogenetics in Clinical Care (I‐PICC) Study design and workflow. Study staff, with clinical trial management software support, interface directly with providers, patients, the electronic health records (EHRs), and the corporate data warehouse (CDW) to recruit and enroll participants, introduce SLCO1B1 pharmacogenetic (PGx) testing and its results, and track patient clinical outcomes within the context of routine care.
Table 1 describes our evaluation of the pragmatism of the I‐PICC Study design using the nine PRECIS‐215 domains, further visualized in Figure 3. Overall, we characterized the I‐PICC Study design as rather pragmatic considering our ratings across all domains (median 4; range 3–5). Below, we describe the key domains of Eligibility, Recruitment, and Organization in greater detail and present their related outcomes.
Figure 3.
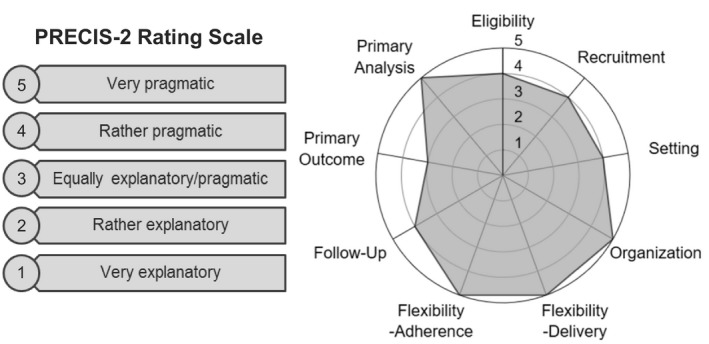
PRagmatic‐Explanatory Continuum Indicator Summary 2 (PRECIS‐2) rating scale (left) and mapping of the Integrating Pharmacogenetics in Clinical Care study design to the PRECIS‐2 wheel (right).
Eligibility (PRECIS‐2 score 4)
The I‐PICC Study eligibility criteria have few exclusions for the two target populations: providers and patients. The trial design seeks to model preemptive SLCO1B1 testing, where providers may order SLCO1B1 genotyping in anticipation of initiating statin therapy for statin‐naïve patients. All VABHS primary care and women's health providers are eligible, being clinicians who commonly manage CVD risk and initiate statin therapy for CVD risk reduction. To capture the patient population for whom preemptive SLCO1B1 genotyping might be most clinically relevant, VABHS patients of participating providers are eligible if they meet ACC/AHA recommendations for statin therapy22 and have no history of statin use.
The characteristics of relevant VABHS patient samples (Table 2) illustrate the generalizability of the I‐PICC Study to the larger VABHS patient population. The 408 patient enrollees are generally similar to the overall sample of VABHS patients who were eligible during the study period (n = 6,245), all patients who consented to participate but were not enrolled in the study by their providers (n = 492), and patients who declined study participation (n = 433). More broadly, enrollees are also generally representative of the overall age‐matched VABHS primary care population during study recruitment (n = 20,959), 56% of whom had a history of statin use and were therefore not eligible for this study of preemptive testing.
Table 2.
Patient characteristics in the I‐PICC study
| All VABHS patients aged 40–75 yearsa | I‐PICC eligible patientsb | I‐PICC declined patients | I‐PICC consented, not enrolled patientsc | I‐PICC enrolled patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (40–75 years): | 63.1 | 9.4 | 63.3 | 8.2 | 65.3 | 7.1 | 63.7 | 8.1 | 64.1 | 7.8 |
| n | % | n | % | n | % | n | % | n | % | |
| 20,959 | 6,245 | 433 | 492 | 408 | ||||||
| Sex | ||||||||||
| Female | 1,406 | 6.7 | 293 | 4.7 | 18 | 4.2 | 25 | 5.1 | 25 | 6.1 |
| Race | ||||||||||
| American Indian/Alaskan Native | 88 | 0.4 | 32 | 0.5 | 5 | 1.2 | 3 | 0.6 | 1 | 0.3 |
| Asian | 92 | 0.4 | 15 | 0.2 | 0 | 0.0 | 1 | 0.2 | 1 | 0.3 |
| Black/African American | 2,287 | 10.9 | 826 | 13.2 | 62 | 14.3 | 67 | 13.6 | 50 | 12.3 |
| Native Hawaiian/Pacific Islander | 43 | 0.2 | 11 | 0.2 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 |
| White | 17,548 | 83.7 | 5,053 | 80.9 | 347 | 80.1 | 407 | 82.7 | 341 | 83.6 |
| Multiracial | 107 | 0.5 | 30 | 0.5 | 2 | 0.5 | 2 | 0.4 | 4 | 1.0 |
| Unknown/declined | 794 | 3.8 | 278 | 4.5 | 16 | 3.7 | 11 | 2.2 | 11 | 2.7 |
| Ethnicity | ||||||||||
| Hispanic or Latino | 527 | 2.5 | 150 | 2.4 | 13 | 3.0 | 7 | 1.7 | 8 | 2.0 |
| Smoking status | ||||||||||
| Smoker | 7,140 | 34.1 | 2,537 | 40.6 | 147 | 33.9 | 192 | 39.0 | 137 | 33.6 |
| ACC/AHA risk criteriad | ||||||||||
| Existing CVD | 6,339 | 30.2 | 1,038 | 16.6 | 98 | 22.6 | 102 | 20.7 | 98 | 24.0 |
| LDL‐C ≥190 mg/dL | 1,944 | 9.3 | 242 | 3.9 | 17 | 3.9 | 12 | 2.4 | 11 | 2.7 |
| Diabetes | 6,329 | 30.2 | 1,205 | 19.3 | 111 | 25.6 | 101 | 20.5 | 98 | 24.0 |
| 10‐year CVD risk ≥ 7.5% | 16,764 | 80.0 | 5,871 | 94.0 | 413 | 95.4 | 463 | 94.1 | 367 | 90.0 |
Patient samples derived during the study recruitment and enrollment period between August 1, 2016, and July 17, 2018. Individual patients may be included in multiple samples.
ACC/AHA, American College of Cardiology/American Heart Association; CVD, cardiovascular disease; I‐PICC, Integrating Pharmacogenetics in Clinical Care; LDL‐C, low‐density lipoprotein cholesterol; VABHS, Veterans Affairs Boston Healthcare System.
This sample matched on age and existence of an active relationship with a primary care provider during period of study recruitment and enrollment. Of these, 11,766 (56%) had been prescribed a statin prior to the study period.
Eligible sample based on I‐PICC Study eligibility criteria (age, no history of statin use, ACC/AHA risk criteria,22 receiving care at VABHS for at least 6 months, and active relationship with a primary care provider) at any time during the period of study recruitment and enrollment.
By design, patients consent to be considered for enrollment but are only enrolled if/when their provider signs an order for a pharmacogenetic test to be performed on an existing clinical blood sample.
Patients may satisfy any one of multiple ACC/AHA risk criteria.
Recruitment (PRECIS‐2 score 4)
The I‐PICC Study used a rather pragmatic recruitment strategy by leveraging the highly integrated VABHS clinical and research informatics infrastructures,33 recruiting and consenting patients by telephone, and subsequently enrolling them at the point‐of‐care. By eliminating the need for an in‐person study visit, this strategy minimized the burden of study participation and enabled consent and enrollment procedures embedded within but not disruptive of clinical care.
As a result, the I‐PICC Study achieved high participation rates from eligible providers and eligible patients. Study staff distributed informed consent documentation to 55 of 76 (73%) potentially eligible providers between August 1, 2016, and July 17, 2018. Of these, 47 (85%) were enrolled. Three providers withdrew from the study at some point after enrollment. No demographic information was collected directly from enrolled providers during their study participation.
The patient recruitment strategy was modified during the I‐PICC Study to enhance yield by (i) prioritizing the distribution of recruitment letters by upcoming primary care or laboratory appointments, (ii) eliminating the requirement that a lipid profile be collected concurrently with a patient's eligible blood specimen, and (iii) using phased introduction of the 10‐year CVD risk score calculation into the patient eligibility algorithm. These modifications generally increased weekly patient enrollment over time (Figure 4).
Figure 4.
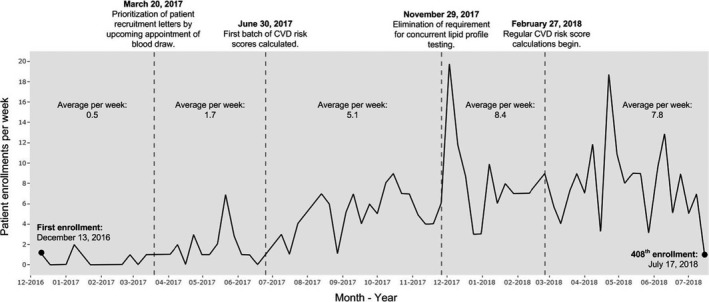
Average patient enrollment per week between December 13, 2016, and July 17, 2018, for the Integrating Pharmacogenetics in Clinical Care (I‐PICC) Study. Time segments are separated by modifications to the I‐PICC Study recruitment design and workflow after first patient enrollment. Risk scores are 10‐year CVD risk scores as calculated using American College of Cardiology/American Heart Association Pooled Cohort Risk Assessment Equations.22 Regular CVD risk score calculations refer to the daily generation of 10‐year CVD risk scores using the birthday parity method as described by Vassy et al.21 CVD, cardiovascular disease.
In total, letters were prioritized by upcoming appointment over the next 6 months and mailed in batches to 2,238 potentially eligible participants. There were 5,395 consent telephone calls (median of 2 calls per patient; range 1–9) administered to participants who were sent a recruitment letter during the study's recruitment and enrollment period. Overall, 900 of 2,238 patients (40%) receiving a recruitment letter consented to be in the study. These procedures yielded one consented patient per every 2.5 letters sent and 6.0 telephone calls made and one enrolled patient per every 5.5 letters sent and 13.2 telephone calls made. The I‐PICC Study recruitment letter (Supplementary Material S1) and telephone script (Supplementary Material S2) are available as supplemental materials.
Organization (PRECIS‐2 score 5)
Beyond the SLCO1B1 genetic testing itself, clinical care delivered during the I‐PICC Study is indistinguishable from usual care at VABHS. Providers order and receive SLCO1B1 results through the EHR as they would any other laboratory test. Because specific simvastatin dosing recommendations from the Clinical Pharmacogenetics Implementation Consortium23 are provided with each SLCO1B1 result, the additional clinical guidance or education needed for implementation is minimal.
The I‐PICC Study intervention further conforms to the VABHS existing organizational structure by leveraging extant blood samples collected as a part of routine care for SLCO1B1 genotyping. We used the study staff to identify blood samples from consented patients in relative real time and forward SLCO1B1 genotyping laboratory orders to providers to sign. The provider's signature of the SLCO1B1 test order enrolls and randomizes the patient. In all, 514 SLCO1B1 genotyping orders were sent to 41 enrolled providers. Of these, 425 (83%) were signed (408 genotyped samples, 17 inadequate samples) by 39 providers, each of whom enrolled a median 7 patients (range 1–61; Figure 5). The remaining consented patients did not have an eligible clinical blood specimen drawn before the enrollment target of 408 was met.
Figure 5.
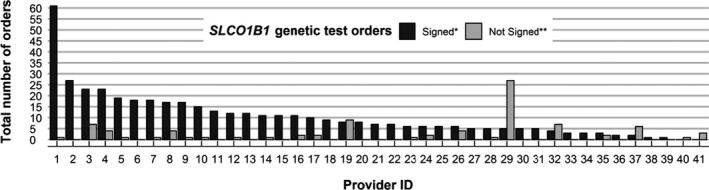
Provider engagement in the Integrating Pharmacogenetics in Clinical Care Study SLCO1B1 genetic testing intervention. Five hundred fourteen total SLCO1B1 genetic test orders were distributed by study staff to 41 enrolled providers between December 13, 2016, and July 17, 2018. *There were 425 (83%) orders (including orders on both adequate and inadequate specimens) that were signed. **Eighty‐nine (17%) orders were declined or not acted upon within seven days of collection, the maximum timeframe in which the Veterans Affairs Boston Healthcare System laboratory retains clinical specimens for assessment.
DISCUSSION
Guided by the PRECIS‐2 framework, we assessed the pragmatism of the I‐PICC Study, a PCT of a genomic medicine application. Parsing the I‐PICC Study into defined elements allowed us to develop a detailed characterization of its level of pragmatism and, by extension, its potential for generalizability to its target population of patients and providers at VABHS. This assessment will enhance our understanding of the representativeness of the results of the I‐PICC Study to clinical practice and its ability to inform the clinical adoption of SLCO1B1 pharmacogenetic testing. Although the I‐PICC Study specifically aims to determine the clinical outcomes of integrating SLCO1B1 testing into routine primary care, it also illustrates the potential value that PCTs might have for evidence generation for precision medicine and CVD prevention more broadly.
Despite calls for comparative effectiveness research in genomic medicine,7, 8 the use of PCTs to develop this evidence base remains sparse.34, 35 At present, the majority of genomic and precision medicine research has occurred within the early phases of the translational continuum (T0/T1), focusing primarily on gene discovery, support for analytic and clinical validity, and the development of health applications.2, 9 Although growing, very few research initiatives have been dedicated to the development of evidence for clinical effectiveness (T2–T4), such as the clinical utility, implementation, and population health effects of genomics interventions, to support the uptake of potentially impactful applications into routine medical practice.2, 9 Most outcome data from the clinical implementation of pharmacogenetic interventions derive from observational or single‐arm research or demonstration projects.34, 36, 37 Although these approaches do provide important process, cost, and clinical outcomes data to inform the potential uptake of pharmacogenetic testing, there remains some concern about their ability to rigorously demonstrate clinical effectiveness.37 Pragmatic trials, like the I‐PICC Study, have been recognized as a valuable tool for generating high quality and generalizable evidence about the effectiveness of genomic interventions and may prove beneficial in closing this gap.7, 8 The principles of PCTs challenge researchers to be inclusive of diverse populations, to focus on patient‐centered outcomes, and to consider strategies that allow for the post‐trial implementation of their interventions, increasing the likelihood that beneficial interventions will be taken up into clinical care.6, 8, 9, 15
Design elements of the I‐PICC Study, particularly within the domains of Eligibility, Recruitment, and Organization, yielded a representative sample of study participants and enabled delivery of SLCO1B1 genotyping at the point‐of‐care. Often, trials recruit or enroll subjects who are different from the overall population of potentially eligible participants in terms of demographic, health, and other factors, which, if not considered or reported, may limit a trial's generalizability and usefulness to decision makers,38 particularly in genomic medicine research.9 Following the CONSORT recommendations of Zwarenstein and colleagues,32 we present a detailed report of these recruitment and enrollment outcomes of the I‐PICC Study. Our exploration of the characteristics and representativeness of the I‐PICC Study's enrolled population provides a comprehensive metric for comparison to other clinical populations and may improve the study's usefulness for stakeholder decision making regarding SLCO1B1 genetic testing upon study completion.14, 38, 39
As we have previously described, pragmatic recruitment of our target population was made possible through the use of a clinical trial management system integrated with clinical data and the EHRs.21 This platform allowed us to mechanize the real‐time tracking of participants, their biospecimens, and their SLCO1B1 results. Integration with the EHR was particularly valuable for engaging provider‐participants, who could consent to their own study participation, enroll their consented patients into the study, and view their patients' study results, all within the EHR without interrupting their clinical workflow. Although upfront effort was required to develop the clinical informatics platform, effort to engage enrolled providers during the study was minimal. In addition, as evidenced in Figure 4, phased deployment of patient eligibility criteria and priority recruitment by upcoming appointment were instrumental to increasing point‐of‐care patient enrollment over the course of the study.
Key challenges for obtaining informed consent from patient‐participants in both genomic medicine research40 and PCTs41 have been described. Informed consent for genomic medicine studies are usually more involved as they often entail less rigorously tested interventions and must address specific issues associated with how, when, and in what form potentially sensitive genetic information should be integrated into the EHR or delivered to patients.40 Lengthy informed consent procedures may limit a trial's degree of pragmatism and have the potential to bias the engagement of some groups.41 Most PCTs outside genomic medicine, on the other hand, use highly vetted interventions, physician oversight, or interventions whose probability of harm is minimal.42 The I‐PICC Study navigated the sometimes competing demands of informed consent and pragmatism by consenting a pool of eligible patients through letters and telephone calls, offloading the clinical encounter, but ultimately allowing providers to enroll individual patients at the point of care by signing an order for SLCO1B1 testing on an existing clinical blood sample. Our ability to automate the real‐time detection of eligible blood specimens from consented patients enabled the delivery of the genomic medicine intervention directly to a potentially relevant clinical moment. As a result, the I‐PICC Study presents a valuable example of the successful delivery of an actionable genetic test to providers at the point‐of‐care, overcoming one barrier to the adoption of many genomic medicine interventions into clinical practice.36 Moreover, engagement by providers in the I‐PICC Study aligns well with the notion that primary care providers are generally supportive of clinical research, particularly when the intervention is relevant and participation is minimally burdensome.43 High provider participation in the I‐PICC Study suggests that, at least at the ordering stage, the intervention's intended users are interfacing with and willing to engage with it (Figure 5). Whether the return of SLCO1B1 results is acted upon by providers and leads to improvement in patient outcomes remains unknown but will be addressed by the trial's outcomes. One risk of applying a pragmatic design to study a precision medicine intervention is that the broad eligibility criteria, a strength of PCTs, may dilute the effect of the intervention.44 If so, examining the reasons the providers and patients did not act on the information will be critical for interpreting the trial's results.
This report is intended both to illustrate the concepts of pragmatism to precision medicine researchers and stakeholders and to evaluate the I‐PICC Study as a PCT of precision medicine. The examination of the trial's PRECIS‐2 scores and its recruitment and enrollment metrics inspire some degree of confidence that the ultimate results of the I‐PICC Study will generalize to the target patient population within VABHS, that is, patients who are statin‐naïve but nonetheless meet guidelines for consideration of statin therapy. Its findings may be less applicable to SLCO1B1 genetic testing for patients who are previous statin users or known statin‐intolerant, the subjects of a prior RCT.45 Whether the I‐PICC Study experience generalizes to patient populations outside VABHS involves other considerations,46 including patient characteristics, provider practice patterns, and organizational health system factors. Clinicians and policymakers should consider these elements in determining whether the trial's results will be applicable to their specific contexts.39
In conclusion, the I‐PICC Study is a feasible model of a precision medicine PCT. Many of its features, such as the use of an integrated EHR, are translatable to other healthcare systems with similar informatics and clinical resources. Through this description of the I‐PICC Study's pragmatic design we have demonstrated the integration of a precision medicine PCT into an existing framework of clinical care. The details of our experiences in designing and carrying out the I‐PICC Study may be informative to others looking to conduct trials that can generate evidence to bridge the gaps among genomics innovation, real‐world clinical adoption, and improved patient outcomes.
Funding
This work is supported by Veterans Affairs (VA) Career Development Award IK2‐CX001262 and by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) at VA Boston Healthcare System. Boston Heart Diagnostics provided genotyping for this study but had no role in its design or analysis and publication of study data. The views expressed here do not necessarily represent those of the United States government or the Department of Veterans Affairs.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
C.A.B. and J.L.V. wrote the manuscript. S.J.M., N.M., A.J.Z., and J.L.V. designed the research. C.A.B., S.J.M., N.M., L.M., S.A., S.A.L., and J.L.V. performed the research. C.A.B. and C.H. analyzed the data.
Supporting information
Material S1. I‐PICC Study Patient Recruitment Letter.
Material S2. I‐PICC Study Patient Recruitment Phone Script.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT02871934. Identifier: NCT02871934.
References
- 1. Green, E.D. & Guyer, M.S. Charting a course for genomic medicine from base pairs to bedside. Nature 470, 204–213 (2011). [DOI] [PubMed] [Google Scholar]
- 2. Khoury, M.J. , Gwinn, M. , Yoon, P.W. , Dowling, N. , Moore, C.A. & Bradley, L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet. Med. 9, 665 (2007). [DOI] [PubMed] [Google Scholar]
- 3. McCarthy, J.J. , McLeod, H.L. & Ginsburg, G.S. Genomic medicine: a decade of successes, challenges, and opportunities. Sci. Transl. Med. 5, 189sr4 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Manolio, T. Implementing genomics and pharmacogenomics in the clinic: the National Human Genome Research Institute's genomic medicine portfolio. Atherosclerosis 253, 225–236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manolio, T.A. et al Implementing genomic medicine in the clinic: the future is here. Genet. Med. 15, 258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips, K.A. et al Making genomic medicine evidence‐based and patient‐centered: a structured review and landscape analysis of comparative effectiveness research. Genet. Med. 19, 1081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Academies of Sciences, Engineering, and Medicine . An evidence framework for genetic testing (The National Academies Press, Washington DC: 2017). <http://nationalacademies.org/hmd/Reports/2017/an-evidence-framework-for-genetic-testing.aspx>. [PubMed] [Google Scholar]
- 8. Khoury, M.J. , Rich, E.C. , Randhawa, G. , Teutsch, S.M. & Niederhuber, J. Comparative effectiveness research and genomic medicine: an evolving partnership for 21st century medicine. Genet. Med. 11, 707 (2009). [DOI] [PubMed] [Google Scholar]
- 9. Roberts, M.C. , Kennedy, A.E. , Chambers, D.A. & Khoury, M.J. The current state of implementation science in genomic medicine: opportunities for improvement. Genet. Med. 19, 858–863 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford, I. & Norrie, J. Pragmatic trials. N. Engl. J. Med. 375, 454–463 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Schwartz, D. & Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Clin. Epidemiol. 62, 499–505 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Tunis, S.R. , Stryer, D.B. & Clancy, C.M. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 290, 1624–1632 (2003). [DOI] [PubMed] [Google Scholar]
- 13. Thorpe, K.E. et al A pragmatic‐explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J. Clin. Epidemiol. 62, 464–475 (2009). [DOI] [PubMed] [Google Scholar]
- 14. Treweek, S. & Zwarenstein, M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials 10, 37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loudon, K. , Treweek, S. , Sullivan, F. , Donnan, P. , Thorpe, K.E. & Zwarenstein, M. The PRECIS‐2 tool: designing trials that are fit for purpose. BMJ 350, h2147 (2015). [DOI] [PubMed] [Google Scholar]
- 16. Chalkidou, K. , Tunis, S. , Whicher, D. , Fowler, R. & Zwarenstein, M. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin. Trials. 9, 436–446 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Vickers, A.J. & Scardino, P.T. The clinically‐integrated randomized trial: proposed novel method for conducting large trials at low cost. Trials 10, 14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Califf, R.M. & Sugarman, J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin. Trials 12, 436–441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiore, L.D. & Lavori, P.W. Integrating randomized comparative effectiveness research with patient care. N. Engl. J. Med. 374, 2152–2158 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Weinfurt, K.P. et al Pragmatic clinical trials embedded in healthcare systems: generalizable lessons from the NIH Collaboratory. BMC Med. Res. Methodol. 17, 144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vassy, J.L. et al The Integrating Pharmacogenetics in Clinical Care (I‐PICC) study: protocol for a point‐of‐care randomized controlled trial of statin pharmacogenetics in primary care. Contemp. Clin. Trials 75, 40–50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stone, N.J. et al 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129 (25 suppl. 2), S1–S45 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Ramsey, L.B. et al The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin‐induced myopathy: 2014 update. Clin. Pharmacol. Therapeut. 96, 423–428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loudon, K. et al The PRECIS‐2 tool has good interrater reliability and modest discriminant validity. J. Clin. Epidemiol. 88, 113–121 (2017). [DOI] [PubMed] [Google Scholar]
- 25. Lipman, P.D. , Loudon, K. , Dluzak, L. , Moloney, R. , Messner, D. & Stoney, C.M. Framing the conversation: use of PRECIS‐2 ratings to advance understanding of pragmatic trial design domains. Trials 18, 532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson, K.E. et al Use of PRECIS ratings in the National Institutes of Health (NIH) health care systems research collaboratory. Trials 17, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Groenwold, R.H.H. & Dekkers, O.M. Designing pragmatic trials‐what can we learn from lessons learned? J. Clin. Epidemiol. 90, 3–5 (2017). [DOI] [PubMed] [Google Scholar]
- 28. Forbes, G. , Loudon, K. , Treweek, S. , Taylor, S.J.C. & Eldridge, S. Understanding the applicability of results from primary care trials: lessons learned from applying PRECIS‐2. J. Clin. Epidemiol. 90, 119–126 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Jordan, A.E. , Perlman, D.C. , Smith, D.J. , Reed, J.R. & Hagan, H. Use of the PRECIS‐II instrument to categorize reports along the efficacy‐effectiveness spectrum in an hepatitis C virus care continuum systematic review and meta‐analysis. J. Clin. Epidemiol. 93, 66–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer, J.A. et al A dynamic application of PRECIS‐2 to evaluate implementation in a pragmatic, cluster randomized clinical trial in two nursing home systems. Trials 19, 453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sajobi, T.T. et al A comparison of meta‐analytic methods for synthesizing evidence from explanatory and pragmatic trials. System. Rev. 7, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zwarenstein, M. et al Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 337, a2390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Avolio, L. et al Implementation of the department of veterans Affairs' first point‐of‐care clinical trial. J. Am. Med. Inform. Assoc. 19, e170–e176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weitzel, K.W. et al The IGNITE network: a model for genomic medicine implementation and research. BMC Med. Genom. 9, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith, D.M. et al CYP2D6‐guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet. Med. 21, 1842–1850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottesman, O. et al The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet. Med. 15, 761 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Volpi, S. et al Research directions in the clinical implementation of pharmacogenomics: an overview of us programs and projects. Clin. Pharmacol. Ther. 103, 778–786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothwell, P.M. External validity of randomised controlled trials: "To whom do the results of this trial apply?" Lancet 365, 82–93 (2005). [DOI] [PubMed] [Google Scholar]
- 39. Zwarenstein, M. , Treweek, S. & Loudon, K. PRECIS‐2 helps researchers design more applicable RCTs while CONSORT Extension for Pragmatic Trials helps knowledge users decide whether to apply them. J. Clin. Epidemiol. 84, 27–29 (2017). [DOI] [PubMed] [Google Scholar]
- 40. Hartzler, A. et al Stakeholder engagement: a key component of integrating genomic information into electronic health records. Genet. Med. 15, 792–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalkman, S. et al Series: Pragmatic trials and real world evidence: Paper 4. Informed consent. J. Clin. Epidemiol. 89, 181–187 (2017). [DOI] [PubMed] [Google Scholar]
- 42. Lantos, J.D. , Wendler, D. , Septimus, E. , Wahba, S. , Madigan, R. & Bliss, G. Considerations in the evaluation and determination of minimal risk in pragmatic clinical trials. Clin. Trials 12, 485–493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Worsley, S.D . et al Series: pragmatic trials and real world evidence: Paper 2. Setting, sites, and investigator selection. J. Clin. Epidemiol. 88, 14–20 (2017). [DOI] [PubMed] [Google Scholar]
- 44. Oude Rengerink, K. et al Series: pragmatic trials and real world evidence: Paper 3. Patient selection challenges and consequences. J. Clin. Epidemiol. 89, 173–180 (2017). [DOI] [PubMed] [Google Scholar]
- 45. Peyser, B. et al Effects of delivering SLCO1B1 pharmacogenetic information in randomized trial and observational settings. Circ. Genom. Precis. Med. 11, e002228 (2018). [DOI] [PubMed] [Google Scholar]
- 46. Schmidt, A.F. , Klungel, O.H. , Nielen, M. , de Boer, A. , Groenwold, R.H. & Hoes, A.W. Tailoring treatments using treatment effect modification. Pharmacoepidemiol. Drug Saf. 25, 355–362 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Material S1. I‐PICC Study Patient Recruitment Letter.
Material S2. I‐PICC Study Patient Recruitment Phone Script.


