Abstract
Variable adherence to antiretroviral therapy (ART) can maintain HIV viral suppression, but our understanding of the ART adherence continuum remains limited. In a clinical cohort of adult persons living with HIV treated with a tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC)-based regimen, data on 3-month self-reported adherence and dried blood spots (DBS) for TFV diphosphate (TFV-DP) and FTC triphosphate (FTC-TP) were collected. Among 521 participants in whom DBS were available upon enrollment, 333 were virologically suppressed (<20 copies/mL). Only 145 (44%) of them reported 100% 3-month adherence, and 69 (21%) had drug concentrations in the highest adherence categories (TFV-DP ≥1,850 fmol/punch and quantifiable FTC-TP). These findings demonstrate a wide range of ART adherence and drug exposure associated with viral suppression, indicating that modern regimens are pharmacologically forgiving. Additional research is needed to understand the biologic effects of variable adherence and drug exposure beyond plasma virologic suppression.
Keywords: HIV, pharmacology, antiretroviral therapy, adherence, tenofovir diphosphate, emtricitabine triphosphate, dried blood spots
Antiretroviral therapy (ART) leads to HIV suppression and prevents HIV disease progression and transmission. Historically, high rates of ART adherence (>90%–95%) were required to achieve and sustain viral suppression, leading to the perception that viral suppression was equivalent to high adherence.1 However, modern ART has become more potent and pharmacologically forgiving, resulting in durable viral suppression with adherence rates as low as 80%–85%.2 While advantageous, this gap between perfect and suppressive ART adherence has allowed for permissiveness to missed doses in clinical practice, which may not be detected if viral suppression is maintained, but could have deleterious consequences such as enhanced residual inflammation, immune activation, coagulopathy, subclinical viral replication, and/or virologic failure.3–5 Thus, an undetectable HIV viral load (VL) can no longer be used as surrogate marker for perfect adherence.
Recently, drug concentrations of phosphorylated antiretroviral anabolites in dried blood spots (DBS) have been used to assess adherence and exposure to ART.6–9 In particular, tenofovir diphosphate (TFV-DP, the phosphorylated anabolite of tenofovir [TFV]) in DBS is a measure of cumulative TFV adherence and exposure, given its long half-life of 17 days in red blood cells, which are abundant in DBS.7,9 This unique pharmacology informs about TFV disoproxil fumarate (TDF) intake over the preceding 8 weeks, similar to how glycosylated hemoglobin A1c informs about glucose in individuals with diabetes mellitus.9 This adherence biomarker has been recently found to be strongly associated with viral suppression in a clinical cohort of persons living with HIV (PLWH) on TDF-including ART, where the adjusted odds ratio of viral suppression in individuals who had TFV-DP ≥1,850 fmol/punch was 73.5 (95% confidence interval 25.7–210.5) compared with those with <350 fmol/punch.8 Of note, drug concentrations in this study of PLWH were higher than the estimates for daily dosing in HIV-uninfected participants, possibly due to the effects of other antiretrovirals (i.e., pharmacologic boosters) and/or factors such as race and body mass index.8 In addition to TFV-DP, emtricitabine triphosphate (FTC-TP, the phosphorylated anabolite of emtricitabine [FTC]) can also be quantified in DBS. This anabolite is informative of recent dosing due to its shorter half-life of 35 h in this matrix,6 and is also predictive of viral suppression.10 Thus, coupling both TFV-DP and FTC-TP in PLWH, individuals who had TFV-DP ≥1,850 fmol/punch and quantifiable FTC-TP would represent the highest tier of adherence/exposure. Based on this premise, we aimed to assess the spectrum of adherence and exposure in PLWH with suppressed plasma HIV-RNA using novel pharmacologic adherence measures.
We enrolled a prospective clinical cohort of adult PLWH taking any TDF/FTC-based regimen at the University of Colorado Hospital (UCH), in Aurora, Colorado, as previously described.8 Briefly, participants were required to have blood drawn for routine HIV VL at the time of their clinic visit, and could complete up to three visits in a 48-week period of time.8 After informed consent, 4–6 mL of whole blood in EDTA was collected for DBS, which was used for drug concentration analysis, using previously validated methods.7–9 HIV VL was assayed at the UCH clinical laboratory and viral suppression was defined as an HIV VL <20 copies/mL. Three-month, 30-day, and 3-day self-reported adherence were assessed using a visual analog scale ranging from 0% to 100% adherence.8 Approval by the Colorado Multiple Institutional Review Board (COMIRB #13-2104) was obtained before any study procedure.
In this analysis, we performed a cross-sectional evaluation of the participants with available drug concentrations in DBS at the enrollment visit. TFV-DP was dichotomized to <1,850 or ≥1,850 fmol/punch according to our previous findings in this cohort.8 FTC-TP was dichotomized as quantifiable or below the limit of quantification.6,10 Simple proportions were used to estimate the percentage of participants on TDF/FTC, who sequentially matched the following adherence categories: 1) were virologically suppressed <20 copies/mL; 2) met criteria (1) and reported 100% adherence in last 3 months, and; 3) met both criteria (1) and (2) and also had TFV-DP ≥1,850 fmol/punch with quantifiable FTC-TP. Data are presented as n (%) or median (interquartile range [IQR]). Statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC) and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA).
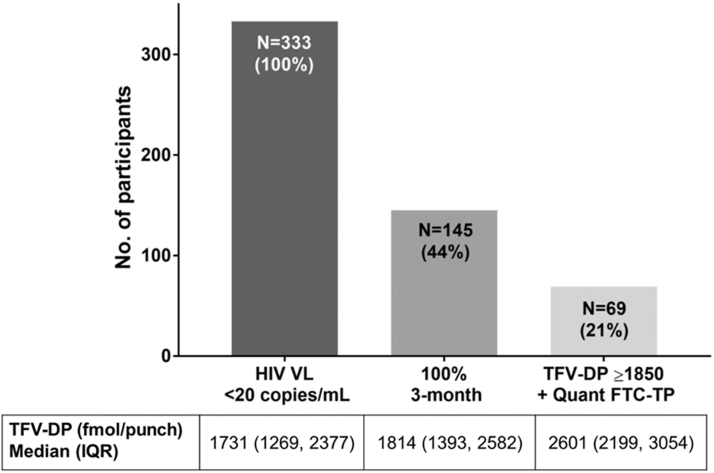
From a total of 807 participants enrolled in the cohort, DBS samples from 521 participants were assayed upon enrollment. The demographics, clinical characteristics, and the assay strategy of these participants have been described elsewhere.8 Among the 521 participants with available drug concentrations, 347 (66%) were virologically suppressed at the enrollment visit.8 Participants not prescribed FTC (n = 14) were excluded from further analysis. From a total of 333 virologically suppressed PLWH on both TDF and FTC (35% on an NNRTI, 32% on an INSTI, 24% on a boosted PI, and 9% on a multiclass regimen), the number of participants substantially decreased between each adherence category (Fig. 1). Only 145 (44%) of those 333 with HIV VL <20 copies/mL reported 100% adherence in the last 3 months. This proportion was similar for 30-day (142, 41%) and 3-day (141, 41%) self-reported adherence. However, only 69 (21%) had both a TFV-DP concentration in DBS ≥1,850 fmol/punch (i.e., high cumulative adherence) and quantifiable FTC-TP (i.e., dosing within 48 h), as noted in the Figure 1. The median (IQR) concentration of TFV-DP in DBS among this group was 2,601 (2,199–3,054) fmol/punch. This was similar when we included participants who reported >85% adherence (2,504 [2,102–3,216]). Comparatively, the median (IQR) TFV-DP concentration in DBS among the 188 (56%) virologically suppressed participants reporting <100% adherence in the previous 3 months was 1,658 (1,206–2,295) fmol/punch.
FIG. 1.
Proportion of study participants according to their antiretroviral therapy adherence category. 3-month, 3-month self-reported adherence; FTC-TP, emtricitabine triphosphate; HIV VL, human immunodeficiency virus viral load; IQR, interquartile range; Quant, quantifiable; TFV-DP, tenofovir diphosphate.
In this study, we identified that only a small proportion of virologically suppressed PLWH on chronic TDF/FTC-based ART had high adherence and exposure based on pharmacologic measures. These findings highlight the wide range of adherence that can result in viral suppression in the modern ART era, and also illustrate the notion that an undetectable HIV VL should not be extrapolated to perfect adherence or high drug exposure. While this observation reflects the pharmacological potency of newer ART regimens, PLWH with “suppressive”—but imperfect—adherence could theoretically be at risk for virologic breakthrough and HIV transmission if transient episodes of viremia occur during periods of nonadherence.11 In addition, variable adherence—despite virologic suppression in plasma—may increase residual inflammation, immune activation, or subclinical viral replication.3,4 This emphasizes the ongoing need to identify and modify some potential behavioral and/or social factors associated with imperfect adherence, including limited access to care or ART, lack of engagement in mental health care, or ongoing substance use. Furthermore, only a small proportion (21%) of our participants had TFV-DP concentrations in the category associated with the highest odds of suppression coupled with objective evidence of recent dosing (TFV-DP ≥1,850 fmol/punch + quantifiable FTC-TP), which highlights the pharmacological forgiveness and potency of modern ART. These findings could also reflect an overestimation of self-reported adherence, or be driven by intrinsic biological and clinical factors (i.e., gender, race, body mass index, concomitant ART, and drug-drug interactions) that could influence the pharmacokinetics of TFV-DP8 and/or FTC-TP independent of adherence.
The strengths of our study include a large sample size in the setting of a “real-world” clinical cohort and the novel pharmacologic measures of adherence. Limitations include the use of self-report as the only nonpharmacologic adherence measure, the lack of existing TFV-DP benchmarks that are associated with 100% adherence in PLWH, and the focus on TDF. Furthermore, additional independent factors (i.e., nonpharmacological and/or adherence related), such as gender,12 pre-ART viral set point,13 or duration of infection, could lead to different rates of virologic suppression among diverse populations, which could also explain the “disconnect” between adherence/exposure and suppression observed in our study. Parsing out these effects will be indispensable to better understand the future clinical applications of these adherence biomarkers. Future research to expand these results using different study populations, new antiretrovirals (i.e., TAF), and multiple adherence measures is required.
Acknowledgments
We would like to thank the study participants and the personnel at the Colorado Antiviral Pharmacology Laboratory for their invaluable assistance and support of this study. We would also like to thank Dr. Steven Johnson, director of the University of Colorado Hospital HIV program, and the medical assistants (Nancy Olague, Brittany Limon, Ariel Cates, Maureen Sullivan and Missy Sorrell) at the University of Colorado Hospital-Infectious Disease Group Practice for their invaluable contributions and support of this study.
Funding Statement
This work was supported by the National Institutes of Health [K23 AI104315 to J.C.M.; R01 AI122298 to P.L.A.]. P.L.A. and J.J.K. have received research funding from Gilead Sciences, paid to their institution. The other authors reported no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the article have been disclosed.
Author Disclosure Statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.J.K and P.L.A. have received research support from Gilead Sciences, paid to their institution. Other authors reported no conflicts of interest.
References
- 1. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30 [DOI] [PubMed] [Google Scholar]
- 2. Viswanathan S, Detels R, Mehta SH, Macatangay BJ, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015;19:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016;63:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li JZ, Gallien S, Ribaudo H, Heisey A, Bangsberg DR, Kuritzkes DR. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014;28:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: Evolving concepts. Infect Disord Drug Targets 2011;11:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo-Mancilla J, Seifert S, Campbell K, et al. Emtricitabine-triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother 2016;60:6692–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018;62:e01710–e01717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castillo-Mancilla J, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with HIV infection. Clin Infect Dis 2019;68:1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013;29:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frasca K, Morrow M, Coyle RP, et al. Emtricitabine triphosphate in dried blood spots is a predictor of viral suppression in HIV infection and reflects short-term adherence to antiretroviral therapy. J Antimicrob Chemother 2019 [Epub ahead of print]; DOI: 10.1093/jac/dky559 [DOI] [PMC free article] [PubMed]
- 11. Morrow M, Mawhinney S, Coyle RP, et al. Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019 [Epub ahead of print]; DOI: 10.1093/infdis/jiz144 [DOI] [PMC free article] [PubMed]
- 12. Saunders P, Goodman A, Smith C, et al. Does gender or mode of HIV acquisition affect virological response to modern antiretroviral therapy (ART)? HIV Med 2016;17:18–27 [DOI] [PubMed] [Google Scholar]
- 13. Hatano H, Vogel S, Yoder C, et al. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS 2000;14:1357–1363 [DOI] [PubMed] [Google Scholar]