Abstract
Pregnancy complications are associated with oxidative stress induced by accumulation of trophoblastic ROS in the placenta. We employed the human trophoblast HTR8/SVneo cell line to determine the effect of curcumin pre-treatment on H2O2-induced oxidative damage in HTR8/Sveo cells. Cells were pretreated with 2.5 or 5 μM curcumin for 24 h, and then incubated with 400 μM H2O2 for another 24 h. The results showed that H2O2 decreased the cell viability and induced excessive accumulation of reactive oxygen species (ROS) in HTR8/Sveo cells. Curcumin pre-treatment effectively protected HTR8/SVneo cells against oxidative stress-induced apoptosis via increasing Bcl-2/Bax ratio and decreasing the protein expression level of cleaved-caspase 3. Moreover, curcumin pre-treatment alleviated the excessive oxidative stress by enhancing the activity of antioxidative enzymes. The antioxidant effect of curcumin was achieved by activating Nrf2 and its downstream antioxidant proteins. In addition, knockdown of Nrf2 by Nrf2-siRNA transfection abolished the protective effects of curcumin on HTR8/SVneo cells against oxidative damage. Taken together, our results show that curcumin could protect HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway.
Keywords: curcumin, HTR8/SVneo cells, Nrf2, oxidative stress
1. Introduction
Intrauterine growth retardation (IUGR) and preeclampsia (PE) are detrimental pregnancy complications that could cause significant increased perinatal morbidity and mortality [1]. Normal placental development during early pregnancy depends entirely on the differentiation, proliferation, and invasion of trophoblast cells [2]. IUGR refers to impaired growth and development of the fetus or fetal organs, and these consequences are associated with the dysfunction of placental trophoblast cells [3].
Oxidative stress arose from the production of reactive oxygen species (ROS) reduces the antioxidant capacity of cells, which, in turn, results in cell damage and eventually cell death [4,5]. During normal pregnancy, the metabolisms of the mother and fetus are enhanced because of higher energy and oxygen requirements [6]. This could consequently accelerate the accumulation of ROS, and eventually induce excessive oxidative stress in the trophoblast cells. Nevertheless, cells have developed an antioxidant defense system to protect against oxidative stress. The system consists of antioxidant enzymes such as glutathione (GSH), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT), which could scavenge ROS to prevent possible cellular damage [7]. Previous studies have demonstrated that antioxidants could attenuate the oxidative damage via enhancing the activities of these antioxidant enzymes [8,9]. Thus, antioxidants might protect placental trophoblast cells from excessive oxidative stress during pregnancy. It is necessary to explore antioxidants with prophylactic or therapeutic potential for placental dysfunction.
Curcumin, a major constituent derived from the root of curcuma longa, has antioxidant, anti-inflammatory and antimicrobial properties [10,11]. Numerous studies have indicated that curcumin is an effective antioxidant both in vivo and in vitro [12,13,14]. Several studies have shown that curcumin treatment could attenuate cell apoptosis, decrease the level of lipid peroxidation, and increase the activity of SOD [15,16,17,18]. The underlying mechanism is associated with the function of NFE2-related factor-2 (Nrf2) [19]. Recent studies have suggested that Nrf2 plays some functionally significant roles in protecting against oxidative stress and apoptotic damage [20,21,22]. A previous study has implicated that curcumin treatment is able to up-regulate the expression of Nrf2, NADP(H) quinine oxidoreductase 1 (NQO1) and heme oxygenase-1 (HO-1) [17]. In addition, Woo et al. has found that curcumin protects retinal pigment epithelial cells against oxidative stress via increasing the expression of HO-1 [23]. Yu et al. has reported that the regulation of Nrf2/HO-1 pathway is vital for protecting placenta against oxidative stress [24]. Our recent study has shown that daily curcumin supplementation could improve maternal placental function and fetal growth in mice with IUGR [25]. However, data are lacking in elaborating the potential molecular mechanism of curcumin against placental dysfunction under oxidative stress.
Therefore, we used human trophoblast HTR8/Sveo cell line as an in vitro model of placental trophoblast cells. The objective of this study was to investigate whether curcumin exerts antioxidant protection on HTR8/Sveo cells against H2O2-induced oxidative damage and to explore the potential molecular mechanism.
2. Materials and Methods
2.1. Chemicals and Reagents
Curcumin and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich Chemicals (St. Louis, MO, USA). Phosphate-Buffered Saline (PBS), Dulbecco’s Modified Eagle’s Medium (DMEM/F-12), fetal bovine serum (FBS), trypsin and penicillin-streptomycin were purchased from Gibco, Invitrogen (Carlsbad, CA, USA).
2.2. Cell Culture
Human trophoblast HTR8/SVneo cell line was obtained from the FuHeng Cell Center (Shanghai, China). Cells were cultured in DMEM/F-12 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified incubator containing 5% CO2 at 37 °C. Cells in the logarithmic growth phase were used in subsequent experimentation. Curcumin were dissolved in DMSO and stored at −20 °C. The final concentration of DMSO in the medium was kept less than 0.1%, which has been shown nonlethal to the cells [26]. Cells in the H2O2-treated group were treated with 400 μM H2O2 alone for 24 h. Cells in the curcumin + H2O2-treated groups were pre-treated with 2.5 μM or 5 μM curcumin for 24 h, respectively, and then they were treated with 400 μM H2O2 for another 24 h.
2.3. Cell Viability Assay
Cell viability was determined following treatment with curcumin and H2O2, with a Cell Counting kit-8 assay (Dojindo Molecular Technologies, Inc. Shanghai, China). HTR8/SVneo cells were cultured in 96-well culture plates for 24 h. Then, after different concentration of curcumin and H2O2 treatment for 24 h, a total of 10 μL CCK-8 reagent was added in and incubated for 2 h in a 5% CO2 incubator at 37 °C. Finally, the optical density values were acquired with a microplate reader at 450 nm.
2.4. Analysis of the Contents of CAT, GSH-Px and ROS
After experimental treatment, cells were lysed with RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and centrifugated at 12,000× g for 10 min at 4 °C. Protein quantification was performed using a Bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Shanghai, China). The activities of CAT, GSH-Px and the content of ROS were determined using the corresponding assay kits (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. All results were normalized to protein concentration in each sample.
2.5. Analysis of Apoptosis by Annexin V-Alexa Fluor 647/Propidium Iodide (PI) Staining
The cell apoptosis in each group treated with 2.5 or 5 μM curcumin for 24 h and 400 μM H2O2 for another 24 h were determined by an Annexin V-Alexa Fluor 647/propidium iodide double-stain assay, according to the manufacturer’s protocol (Annexin V-Alexa Fluor 647/PI Apoptosis Assay Kit, FMSAV647-100, FcMACS, Nanjing, China). Briefly, adherent cells (1 × 106) of the four experimental groups were collected by trypsin and resuspended in 100 μL binding buffer containing 5 μL Annexin V/Alexa Fluor 647 and 10 μL 20 μg/mL PI for 15 min at room temperature in dark. The flow cytometric analyses were performed on flow cytometry (Becton Dickinson). FlowJo software was used for data acquisition and analysis.
2.6. Quantitative Real-Time PCR
Total RNA was isolated from the cells with TRIzol reagent (TaKaRa, Dalian, China) and processed for quantitative real-time PCR. Total RNA was reverse transcribed into cDNA with PrimeScript reverse transcriptase reagent kit (TaKaRa, Dalian, China). Real-time PCR analysis was performed using a QuantStudio 5 Real-Time PCR System (Thermo Scientific, Wilmington, USA) and a TB Premix Ex Taq Kit (TaKaRa, Dalian, China). The reaction program was as follows: 95 °C for 30 s, 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The melting curve was used to verify the amplification of a single product. The primers were synthesized by Sangon Biotech (Sangon Biotech Co., Ltd., Shanghai, China), and the primer sequences used in this study were shown in Table 1. All samples were measured in triplicate, and the data were analyzed using the 2−△△Ct method.
Table 1.
The primer sequences for real-time PCR.
| Genes (GenBank) | Primer Sequences (5’-3’) | Product Size (bp) |
|---|---|---|
| N rf 2 | F: CTTGGCCTCAGTGATTCTGAAGTG | 124 |
| (NM_006164.5) | R: CCTGAGATGGTGACAAGGGTTGTA | |
| HO-1 | F: CAGGAGCTGCTGACCCATGA | 195 |
| (NM_002133.3) | R: AGCAACTGTCGCCACCAGAA | |
| GCLC | F: GAAGTGGATGTGGACACCAGATG | 128 |
| (NM_001498.4) | R: TTGTAGTCAGGATGGTTTGCGATAA | |
| GCLM | F: GGAGTTCCCAAATCAACCCAGA | 71 |
| (NM_002061.4) | R: TGCATGAGATACAGTGCATTCCAA | |
| NQO1 | F: GGATTGGACCGAGCTGGAA | 140 |
| (NM_000903.3) | R: AATTGCAGTGAAGATGAAGGCAAC | |
| Bcl-2 | F: ATAACGGAGGCTGGGTAGGT | 127 |
| (NM_000657.2) | R: TTTATTTCGCCGGCTCCACA | |
| Bax | F: GCCCTTTTGCTTCAGGGGATG | 76 |
| (NM_138763.4) | R: CAGCTGCCACTCGGAAAAAG | |
| SLC2A1 | F: TGAGCATCGTGGCCATCTTT | 298 |
| (NM_006516.3) | R: CCGGAAGCGATCTCATCGAA | |
| SLC2A3(NM_006931.3) | F: GCACATAGCTATCAAGTGTGCTT R: CCTGCCTTACTGCCAACCTA |
97 |
| GAPDH | F: GACAGTCAGCCGCATCTTCT | 104 |
| (NM_002046.7) | R: GCGCCCAATACGACCAAATC |
Nrf2, nuclear factor-erythroid 2-related factor 2; HO-1, home oxygenase-1; GCLC, glutamate-cysteine ligase catalytic; GCLM, glutamate-cysteine ligase modifier; NQO1, NAD(P)H quinone dehydrogenase 1; Bcl-2, Bcl-2 apoptosis regulator; Bax, Bcl-2 associated X, apoptosis regulator; SLC2A1, solute carrier family 2 member 1; SLC2A3, solute carrier family 2 member 3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
2.7. Nrf2 Immunofluorescence
The nuclear translocation of Nrf2 was determined by immunofluorescence. Briefly, HTR8/SVneo-cells were treated as previously described. Then, the cells were washed 3 times with PBS, fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, blocked with 1% BSA and then incubated with anti-Nrf2 antibody (Proteintech, 16396-1-AP, Wuhan, China, 1:200 dilution) overnight at 4 °C. The bound antibody was detected by Cy3 conjugated Goat Anti-rabbit IgG (Servicebio, GB21303, Wuhan, China). The nuclei were stained with 4’,6-diamidino-2-phenylindole staining solution (C1005, Beyotime Biotechnology, Shanghai, China). The coverslips were inverted on glass slides and then examined under a Pannoramic 250 (3D HISTECH) using the same parameter settings among the different treated groups.
2.8. Western Blotting
Cells were washed twice with PBS, and then suspended in 100 μL RIPA (Beyotime Biotechnology, Shanghai, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF, Beyotime Biotechnology, Shanghai, China), followed by centrifugation at 12,000× g for 10 min at 4 °C. Nucleoprotein was extracted with Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Wilmington, DE, USA). The protein concentrations were measured by a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). The lysate (10 μg protein/lane) was resolved in 4%–20% SDS-PAGE and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). After the membranes were blocked with 5% bovine serum albumin (BSA) for 2 h at room temperature, target proteins were immunodetected using specific antibodies. The membranes were incubated with specific primary antibodies against Nrf2, HO-1, NQO1, Bcl-2, Bax and Cleaved-Caspase 3 (Proteintech, 16396-1-AP; Zen-Bioscience, 380753; Proteintech, 11451-1-AP; Affinity, AF6139; Proteintech, 50599-2-lg; Affinity, AF7022) overnight at 4 °C. Histone H3 (Proteintech, 17168-1-AP) was applied as a loading control for nucleoprotein. β-Actin (Proteintech, 660091-1) was utilized as a loading control for total protein. After three washes in TBST for 10 min each, the membranes were incubated with secondary antibody (1:2000, AS003, ABclonal Biotechnology Co., Ltd., Wuhan, China) for 60 min at room temperature. Finally, the blots were washed in TBST for three times and protein bands were detected using an enhanced chemiluminescence (ECL) kit (Thermo Scientific, Wilmington, DE, USA). Signals were visualized using Luminescent Image Analyzer LAS4000 (FuJI Film, Tokyo, Japan). The protein expressions were estimated by quantifying the intensities of the bands using ImageJ software.
2.9. Small Interfering RNA (siRNA) Transfection
The human-specific siRNAs targeting Nrf2 were designed and synthesized by GenePharma (Shanghai, China). For transfection, the HTR8/SVneo cells were seeded in 6-well culture plates and siRNA-Nrf2 were transfected into the cells using the Lipofectamine™ 2000 Transfection reagent (Thermo Scientific, Wilmington, DE, USA) prior to treatment with curcumin and H2O2 according to the manufacturer’s instructions. The target sequences used in this study were shown in Table 2.
Table 2.
Primer sequences of Nrf2 siRNA.
| Name | Primer Sequences (5–3 Orientation) |
|---|---|
| siRNA-Nrf2 | Sense: GGUUGAGACUACCAUGGUUTT Antisense: AACCAUGGUAGUCUCAACCTT |
| siRNA-NC | Sense: UUCUCCGAACGUGUCACGUTT Antisense: ACGUGACACGUUCGGAGAATT |
2.10. Statistical Analysis
All the data are presented as mean ± SEM for at least three independent experiments. The Shapiro–Wilk test was used to estimate the normality distribution of the data. Statistical differences were determined by one-way ANOVA followed by Tukey’s test (Graph Pad Software Inc. San Diego, CA, USA). Differences were considered to be significant at p < 0.05.
3. Results
3.1. Curcumin Protected against H2O2-Induced Cytotoxicity in HTR8/SVneo Cells
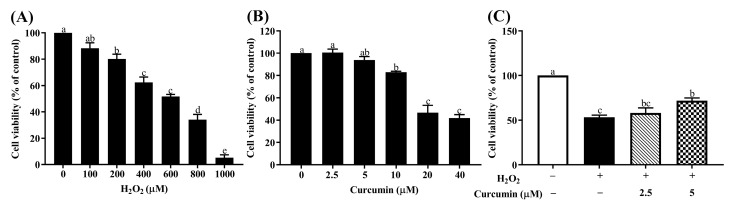
Firstly, to achieve the optimized oxidative stress conditions, we examined HTR8/SVneo cells treated with different concentrations of H2O2 (100, 200, 400, 600, 800 and 1000 μM) for 24 h. Cell viabilities were analyzed using a CCK-8 assay. As shown in Figure 1A, a dose-dependent increase in cytotoxicity of HTR8/SVneo cells was observed in response to H2O2. The IC50 value of H2O2 concentration was 400 μM which resulted in 50% inhibition of HTR8/SVneo cells. Thus, 400 μM H2O2 treatment for 24 h was chosen to perform subsequent experiments. Secondly, to evaluate cell viability in different concentrations of curcumin and to determine its non-cytotoxic concentration, HTR8/SVneo cells were pretreated with different concentrations of curcumin (0, 2.5, 5, 10, 20 and 40 μM) for 24 h. As shown in Figure 1B, 10 μM curcumin has cytotoxicity to HTR8/SVneo cells, thus we chose 2.5 and 5 μM curcumin for the following experiments. Finally, to assess the cytoprotective effect of curcumin, HTR8/SVneo cells were pretreated with 2.5 and 5 μM curcumin for 24 h, followed by 400 μM H2O2 for another 24 h. As shown in Figure 1C, the 5 μM curcumin pretreatment group significantly increased the cell viability was significantly increased in the 5 μM curcumin pre-treatment group compared with the H2O2 treatment group.
Figure 1.
Effects of curcumin on H2O2-induced cell viability in HTR8/SVneo cells measured by the CCK-8 assay; (A) Cell viability of HTR8/SVneo cells treated with different concentrations of H2O2; (B) cell viability of HTR8/SVneo cells treated with different concentrations of curcumin; (C) cell viability of HTR8/SVneo cells pretreated with 2.5 or 5 μM curcumin against H2O2-induced oxidative stress. Data are presented as the mean ± SEM (n = 3) a, b, c Means with different letters are significantly different (p < 0.05).
3.2. Curcumin Increased H2O2-Induced CAT, GSH-Px Activity and Reduced the Level of Intracellular ROS in HTR8/SVneo Cells under Oxidative Stress
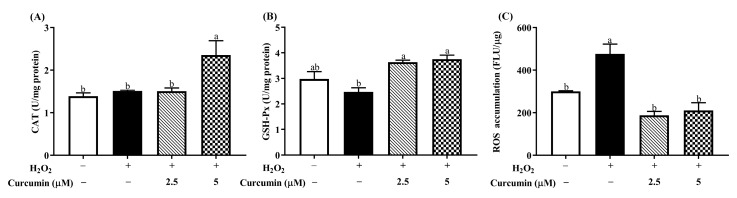
To evaluate the effects of curcumin on the activity of antioxidant enzymes in HTR8/SVneo cells, the activities of CAT and GSH-Px were assessed. As shown in Figure 2A,B, the activities of CAT and GSH-Px showed no significant difference (p > 0.05) between the control and H2O2 treatment group. Whereas, pre-treatment with 5 μM curcumin followed by 400 μM H2O2 up-regulated (p < 0.05) the activities of CAT and GSH-Px in HTR8/SVneo cells. To further prove the protective of curcumin against oxidative stress, we measured the intracellular level of ROS by using fluorescent probe DCFH-DA. As shown in Figure 2C, the accumulation of ROS in HTR8/SVneo cells was significantly increased (p < 0.05) in the H2O2 group compared with the control group, while pre-treatment with 2.5 and 5 μM curcumin remarkably reduced (p < 0.05) the H2O2-induced ROS accumulation in HTR8/SVneo cells.
Figure 2.
Effect of curcumin on the activity of antioxidant enzymes and ROS generation. Cells were pretreated with 2.5 or 5 μM curcumin for 24 h, and then incubated with 400 μM H2O2 for another 24 h. The activity of cellular CAT (A), GSH-Px (B) and ROS accumulation (C) were measured. ROS production was determined using the DCFH-DA assay. Data are presented as the mean ± SEM (n = 3). a, b Means with different letters are significantly different (p < 0.05).
3.3. Curcumin Inhibited H2O2-Induced Apoptosis of HTR8/SVneo Cells
To further evaluate the inhibitory effect of curcumin on H2O2-induced apoptosis in HTR8/SVneo cells, apoptotic rates were measured by Annexin V-FITC/PI double staining using flow cytometry. As shown in Figure 3, the percentage of apoptotic cells was 4.56% ± 1.04% in the control group, whereas that of the H2O2 treatment group was 14.13% ± 1.56% (p < 0.05). However, pre-treatment with 5 μM curcumin markedly decreased apoptotic rates to 6.00% ± 1.00% (p < 0.05) in HTR8/SVneo cells treated with 400 μM H2O2.
Figure 3.
Effects of curcumin on H2O2-induced apoptosis in HTR8/SVneo cells measured by flow cytometry. (A) Control, the control group; H2O2, H2O2 treatment group; 2.5 μM Cur + H2O2, 2.5 μM curcumin pre-treatment followed by the treatment of 400 μM H2O2; 5 μM Cur + H2O2, 5 μM curcumin pre-treatment followed by the treatment of 400 μM H2O2. (B) The apoptosis rates of HTR8/SVneo cells after different treatments. Data are presented as the mean ± SEM (n = 3). a, b Means with different letters are significantly different (p < 0.05).
3.4. Curcumin Regulated mRNA Expression of Multiple Antioxidant Genes and Nutrient Transporter Genes in HTR8/SVneo Cells under Oxidative Stress
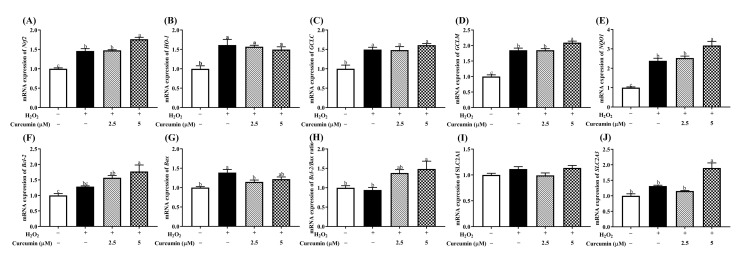
Since curcumin has been shown to exert antioxidant effect by inducing several antioxidant defense enzymes in several cell lines [14,23]. Thus, we performed RT-qPCR to detect whether the protective function of curcumin against the oxidative stress induced by H2O2 was associated with antioxidant-associated factors in HTR8/SVneo cells. Our results showed that H2O2 stimulation alone significantly increased the mRNA levels of Nrf2, HO-1, GCLC, GCLM and NQO1 (p < 0.05), whereas pre-treatment of 5 μM curcumin further up-regulated (p < 0.05) the expression of Nrf2, GCLM and NQO1. H2O2 treatment alone increased (p < 0.05) the transcription level of Bax, and pre-treatment with 5 μM curcumin increased (p < 0.05) the expression of Bcl-2. The expression of Bcl-2/Bax was also up-regulated (p < 0.05) in both curcumin pre-treatment groups (Figure 4F–H). In addition, H2O2 treatment alone had no effect (p > 0.05) on the mRNA expression of solute carrier family 2 member 1 (SLC2A1) and solute carrier family 2 member 3 (SLC2A3), while pre-treatment with 5 μM curcumin increased (p < 0.05) the gene expression of SLC2A3 (Figure 4I,J).
Figure 4.
Effects of curcumin on the mRNA expression of Nrf2 (A), HO-1 (B), GCLC (C), GCLM (D), NQO1 (E), Bcl-2 (F), Bax (G), Bcl-2/Bax ratio (H), SLC2A1 (I) and SLC2A3 (J) gene expression. Cells were pre-treated with 2.5 or 5 μM curcumin for 24 h, and then treated with 400 μM H2O2 for another 24 h. GAPDH was used as a housekeeping gene. Data are presented as the mean ± SEM (n = 3). a, b, c Means with different letters are significantly different (p < 0.05). No letters or the same letters mean the no significantly difference.
3.5. Curcumin Increased Nrf2, HO-1 and NQO1 Protein Expression and Nrf2 Translocation in HTR8/SVneo Cells under Oxidative Stress
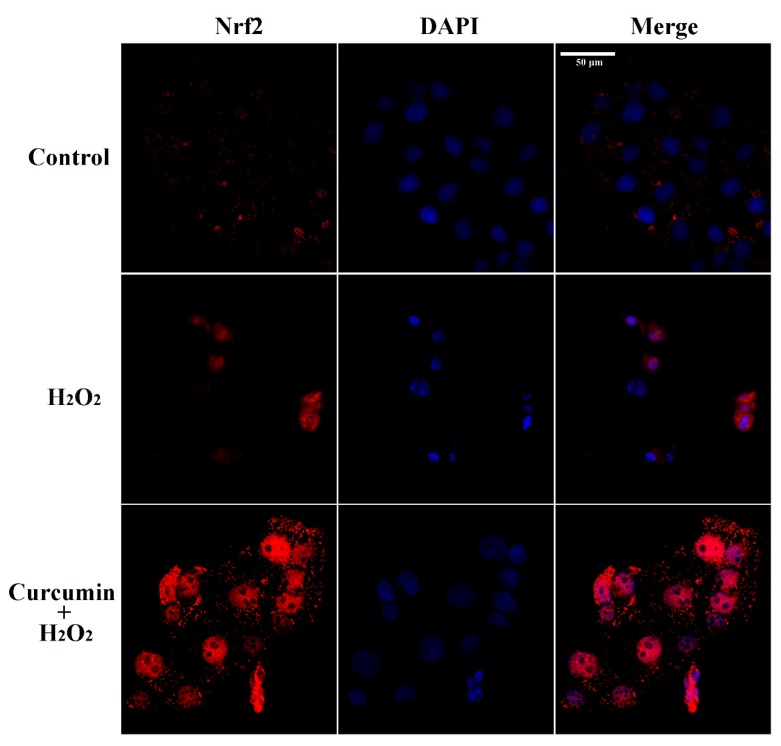
As shown in Figure 5, H2O2 stimulation alone up-regulated (p < 0.05) the protein expression of Nrf2 and NQO1, while it did not affect (p > 0.05) the protein expression of HO-1. Pre-treatment with 2.5 or 5 μM curcumin up-regulated (p < 0.05) the protein expression of nuclear Nrf2, total-Nrf2, HO-1 and NQO1. In addition, immunofluorescence staining showed that both H2O2 and curcumin stimulated Nrf2 nuclear translocation in HTR8/SVneo cells (Figure 6). However, we observed that pre-treatment with curcumin induced more Nrf2 nuclear translocation compared with the H2O2 treatment group. Furthermore, pre-treatment with curcumin significantly increased the Bcl-2/Bax ratio and decreased the expression of active caspase-3 in H2O2-treated HTR8/SVneo cells (Figure 5A,E,F).
Figure 5.
Effects of curcumin on nuclear Nrf2, total-Nrf2, HO-1, NQO1, Bcl-2, Bax and cleaved-caspase 3 protein expression. The bands (A) and relative protein expression of nuclear Nrf2 (B), total-Nrf2 (C), HO-1 (D), NQO1 (E), Bcl-2/Bax (F) and cleaved-caspase 3 (G) were measured by Western blotting. Cells were pre-treated with 2.5 or 5 μM curcumin for 24 h, and then treated with 400 μM H2O2 for another 24 h. Histone H3 was used as a loading control for nucleoprotein. β-actin was used as a loading control for total protein. Data are presented as the mean ± SEM (n = 3). a, b, c Means with different letters are significantly different (p < 0.05).
Figure 6.
Effects of curcumin on Nrf2 nuclear translocation in HTR8/SVneo cells. Immunofluorescence staining of Nrf2 in HTR8/SVneo cells treated with H2O2 or pre-treated with 5 μM curcumin for 24 h and H2O2 for another 24 h. Blue and red colors indicate localization of DAPI (nuclei) and Nrf2, respectively. Scale bar = 50 μm.
3.6. Nrf2 Knockdown Attenuated the Protective Effect of Curcumin on HTR8/SVneo Cells under Oxidative Stress
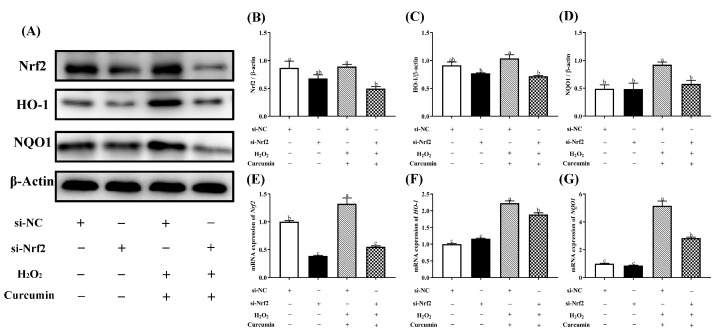
To further elucidate the role of Nrf2 in the cytoprotective effects of curcumin against oxidative stress, we transfected HTR8/SVneo cells with a Nrf2 siRNA for 24 h, then pre-treated with 5 μM curcumin followed by the treatment of 400 μM H2O2. As shown in Figure 7, after the transfection with si-Nrf2 in curcumin and H2O2 treatment group, we observed markedly decreased (p < 0.05) mRNA and protein expression level of Nrf2, HO-1 and NQO1 compared with the curcumin + H2O2 transfected with si-NC group. In addition, Nrf2 silencing abolished (p < 0.05) the upregulation of cell viability caused by curcumin pretreatment (Figure 8A). The activation of CAT and GSH-Px were significantly decreased (p < 0.05) with knockdown of Nrf2 compared with the curcumin + H2O2 transfected with si-NC group (Figure 8C).
Figure 7.
Silencing Nrf2 gene abolished the prevention made by curcumin. HTR8/SVneo cells transfected with siRNA against Nrf2 or control siRNA for 24 hours, followed by 24 h pre-treatment with 5 μM curcumin, and then treated with 400 μM H2O2 for another 24 h. The bands (A) and relative protein expression of Nrf2 (B), HO-1 (C) and NQO1 (D) were measured by Western blotting. The mRNA expression of Nrf2 (E), HO-1 (F) and NQO1 (G) were measured by RT-PCR. Data are presented as the mean ± SEM (n = 3). a, b, c Means with different letters are significantly different (p < 0.05).
Figure 8.
Silencing Nrf2 gene abolished the prevention made by curcumin. HTR8/SVneo cells transfected with siRNA against Nrf2 or control siRNA for 24 hours, followed by 24 h pre-treatment with 5 μM curcumin, and then treated with 400 μM H2O2 for another 24 h. The cell viability (A), CAT (B) and GSH-Px (C) activity of cells were determined respectively. Data are presented as the mean ± SEM (n=3). a, b, c, d Means with different letters are significantly different (p < 0.05).
4. Discussion
Placental trophoblast cells play important roles in the pregnancy and the development of the fetus. Excessive accumulation of trophoblastic ROS induced by greater maternal and fetal metabolism has been considered as an important factor that leads to placental dysfunction [27]. In the present study, we found for the first time that curcumin, a natural antioxidant known for its cytoprotective and anti-apoptotic actions [28,29], ameliorates H2O2-induced oxidative stress and cell apoptosis in human trophoblast HTR8/SVneo cells by activating the Nrf2 signaling pathway.
It is well established that H2O2 could be used to stimulate oxidative stress in vitro [30,31,32]. In the present study, we found that treatment with 400 μM H2O2 resulted in decreased cell viability in HTR8/SVneo cells, which is consistent with a previous study [1]. Curcumin has been reported to be toxic at a high dose, while it exerts a strong antioxidant effect at a low dose [23]. In the present study, pre-treatment of more than 10 μM curcumin for 24 h can induce obvious cell death in HTR8/SVneo cells, so we chose 2.5 and 5 μM curcumin as the optimal concentrations of curcumin for the subsequent experiments. Similar with the previous findings, pre-treatment with curcumin had the ability to enhance the viability of HTR8/SVneo cells after treatment of H2O2 [23,33]. Moreover, the result of 5 μM curcumin pre-treatment showed a better effect than 2.5 μM, suggesting curcumin might exert the protective effect in a dose-dependent manner in HTR8/SVneo cells. These results indicate that low dose curcumin could protect HTR8/SVneo cells from death induced by H2O2.
ROS are oxygen free radicals, and the accumulation of ROS could cause oxidative stress and cell apoptosis [33]. Excessive oxidative stress induced by trophoblastic ROS is a pivotal factor for IUGR [27]. Our data showed that the exposure of HTR8/Sveo cells to H2O2 resulted in increased intracellular ROS accumulation, but it was significantly alleviated by pre-treatment with either 2.5 μM or 5 μM curcumin. Consistent with our result, a previous study has also found that curcumin is able to reduce ROS accumulation [34]. This effect might be related with augmented antioxidant defense system promoted by curcumin pre-treatment. In addition, CAT and GSH-Px are the main members of antioxidant defense system. CAT converts hydrogen peroxide into water and oxygen [35]. GSH-Px is a vital antioxidant enzyme that catalyzes the reduction of hydroperoxides at the expense of reduced GSH [36]. In agreement with a previous finding that curcumin could improve the antioxidant capacity, curcumin pre-treatment enhanced the activities of CAT and GSH-Px in the HTR8/Sveo cells treated with H2O2 [37]. The antioxidant property of curcumin has been reported to be mainly associated with its free radical scavenging activity [15]. Thus, our results indicate that pre-treatment with curcumin could reduce oxidative stress in HTR8/Sveo cells treated with H2O2.
Oxidative stress can induce excessive cell apoptosis through either mitochondria-dependent or independent pathway [38]. Equally, the apoptosis rate can also reflect the degree of oxidative stress [39]. We observed that apoptosis was sharply promoted in the H2O2 treatment group. Supportively, H2O2 treatment reduced the protein expression level of Bcl-2/Bax ratio. Bcl-2 is the core molecule which plays a considerable role of resistance in apoptosis, whereas Bax is a promoting apoptosis protein. Bcl-2/Bax ratio has been reported to be an important determinant factor of apoptosis [40]. Ample pieces of evidence have also proven that H2O2 treatment could increase cell apoptosis [32,41]. However, pre-treatment with curcumin inhibited cell apoptosis, which was demonstrated by declined apoptosis rate, reduced protein expression of cleaved-caspase 3 and increased expression level of Bcl-2/Bax ratio. These results were consistent with the previous studies, which have also affirmed the anti-apoptotic effects of curcumin against oxidative stress-induced apoptosis [42]. Moreover, apoptosis is an essential regulatory cell process that occurs via caspase-independent pathway or caspase-dependent pathway [43]. Therefore, the decline of cleaved-caspase 3 protein expression level suggests that pre-treatment with curcumin can suppress apoptosis in HTR8/Sveo cells through inhibiting caspase-dependent pathway. Further functional investigation should be performed to estimate whether curcumin could reduce apoptosis in trophoblast cells via inhibiting caspase-independent pathway.
Nrf2 is a vital transcription factor that regulates cell survival and maintains redox homeostasis [44,45]. Nrf2 signaling pathway is also a crucial antioxidant pathway, which is responsible for regulating the expression of antioxidant enzymes against oxidative stress in cells [46,47,48]. The transcription of HO-1 and NQO1, the downstream genes of Nrf2, are primarily under the control of Nrf2 in maintaining cell redox balance [49,50]. Under oxidative stress, Nrf2 could translocate into the nucleus to activate the expression of its downstream antioxidant enzymes [32]. Similar with the previous studies, the Nrf2 signaling was activated by H2O2 treatment in order to compensate for the oxidative damage [32,51]. Nrf2 nuclear translocation was also observed in the H2O2 treatment group. In addition, previous researchers have made great effort to prove that curcumin activates Nrf2 and HO-1 signaling leading to protection against oxidative stress in different cell types [17,19,52,53]. In accordance with these findings, pre-treatment with curcumin induced a more obvious Nrf2 nuclear translocation than the H2O2 treatment alone. Combined with the results of more effectively enhanced mRNA and protein expression of Nrf2, HO-1 and NQO1 induced by pre-treatment with curcumin, our data suggested that curcumin exerted its antioxidant effect against oxidative stress in HTR8/SVneo cells by promoting the activation of Nrf2 signaling pathway. The enhanced activation of Nrf2 signaling could also explain for the increased activity of CAT and decreased accumulation of ROS. In addition, up-regulation of nutrient transporters (SLC2A3) can improve the transfer of nutrients (including amino acids, fatty acids, and glucose) from mother to fetus, which plays an important role in placental development and fetal growth [54,55]. In our previous in vivo experiment, we have proven that curcumin has beneficial effects on nutrient transport and placental development, which could be applied for alleviating IUGR of mice. Furthermore, we found that knockdown of Nrf2 by Nrf2-siRNA transfection markedly diminished the curcumin-induced up-regulation of Nrf2, HO-1 and NQO1. Similarly, a previous study has found that knockdown of Nrf2 could decrease the expression level of HO-1 and NQO1 [56]. Here, we have shown that knockdown of Nrf2 decreased the protective effect of curcumin pre-treatment on the cell viability against oxidative stress, suggesting that Nrf2 is a key factor in maintaining the survival of HTR8/SVneo cells. Since Nrf2 controls the transcription of antioxidant enzymes, the increased activities of CAT and GSH-Px by pre-treatment with curcumin were also reduced after knockdown of Nrf2. Collectively, our data strongly proves that the antioxidant effect of curcumin on HTR8/SVneo cells against oxidative stress is achieved by activation of Nrf2 signaling pathway.
5. Conclusions
In conclusions, this is the first study demonstrating that pre-treatment with curcumin could alleviate H2O2-induced oxidative stress in human trophoblast HTR8/SVneo cells by improving the antioxidant capacity and activating Nrf2 signaling pathway.
Author Contributions
Conceptualization, L.Q. and T.W.; validation, T.W.; formal analysis, L.Q.; investigation, L.Q.; resources, J.Z., L.Z. and T.W.; data curation, L.Q.; writing—original draft preparation, L.Q.; writing—review and editing, L.Q., J.J., J.Z., L.Z. and T.W.; visualization, L.Q. and J.J.; supervision, T.W.; project administration, T.W.; funding acquisition, T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China (NO.31572418).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li J., Ding Z., Yang Y., Mao B., Wang Y., Xu X. Lycium barbarum polysaccharides protect human trophoblast HTR8/SVneo cells from hydrogen peroxideinduced oxidative stress and apoptosis. Mol Med Rep. 2018;18:2581–2588. doi: 10.3892/mmr.2018.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter A.M., Enders A.C., Pijnenborg R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015;370:20140070. doi: 10.1098/rstb.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo S., Borghesi A., Tzialla C., Stronati M. IUGR and infections. Early Hum. Dev. 2014;90(Suppl. 1):S42–S44. doi: 10.1016/S0378-3782(14)70014-3. [DOI] [PubMed] [Google Scholar]
- 4.Cap M., Vachova L., Palkova Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Med. Cell. Longev. 2012;2012:976753. doi: 10.1155/2012/976753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray R., Shah A.M. NADPH oxidase and endothelial cell function. Clin. Sci. (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Cuevas I., Parra-Llorca A., Sanchez-Illana A., Nunez-Ramiro A., Kuligowski J., Chafer-Pericas C., Cernada M., Escobar J., Vento M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017;12:674–681. doi: 10.1016/j.redox.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- 8.Mehta J., Rayalam S., Wang X. Cytoprotective Effects of Natural Compounds against Oxidative Stress. Antioxidants. 2018;7:147. doi: 10.3390/antiox7100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiokias S., Proestos C., Oreopoulou V. Effect of Natural Food Antioxidants against LDL and DNA Oxidative Changes. Antioxidants. 2018;7:133. doi: 10.3390/antiox7100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin K., Orhan C., Tuzcu Z., Tuzcu M., Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012;50:4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Anand P., Thomas S.G., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Sung B., Tharakan S.T., Misra K., Priyadarsini I.K., Rajasekharan K.N., et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Xu L., Zhang L., Ying Z., Su W., Wang T. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 2014;144:1211–1218. doi: 10.3945/jn.114.193573. [DOI] [PubMed] [Google Scholar]
- 14.Wu J., Ibtisham F., Niu Y.F., Wang Z., Li G.H., Zhao Y., Nawab A., Xiao M., An L. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2 / ARE signaling pathway in chicken fibroblasts cells. J. Therm. Biol. 2019;79:112–119. doi: 10.1016/j.jtherbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Hou X., Ahmad H., Zhang H., Zhang L., Wang T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014;145:57–65. doi: 10.1016/j.foodchem.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z., Wu B., Shen G., Li X., Wu Q. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Mol. Med. Rep. 2018;17:103–108. doi: 10.3892/mmr.2017.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 18.Gao S., Duan X.X., Wang X., Dong D.D., Liu D., Li X., Sun G.F., Li B. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem. Toxicol. 2013;59:739–747. doi: 10.1016/j.fct.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 21.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., Leon R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Nakai K., Fujii H., Kono K., Goto S., Kitazawa R., Kitazawa S., Hirata M., Shinohara M., Fukagawa M., Nishi S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014;27:586–595. doi: 10.1093/ajh/hpt160. [DOI] [PubMed] [Google Scholar]
- 23.Woo J.M., Shin D.Y., Lee S.J., Joe Y., Zheng M., Yim J.H., Callaway Z., Chung H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012;18:901–908. [PMC free article] [PubMed] [Google Scholar]
- 24.Yu M., Chen L., Peng Z., Wang D., Song Y., Wang H., Yao P., Yan H., Nussler A.K., Liu L., et al. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins. 2017;9:188. doi: 10.3390/toxins9060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi L., Jiang J., Zhang J., Zhang L., Wang T. Maternal curcumin supplementation ameliorates placental function and fetal growth in mice with intrauterine growth retardation. Biol. Reprod. 2020 doi: 10.1093/biolre/ioaa005. [DOI] [PubMed] [Google Scholar]
- 26.Dhanasekaran S. Augmented cytotoxic effects of paclitaxel by curcumin induced overexpression of folate receptor-α for enhanced targeted drug delivery in HeLa cells. Phytomedicine. 2019;56:279–285. doi: 10.1016/j.phymed.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Myatt L., Cui X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 28.Dai C., Li D., Gong L., Xiao X., Tang S. Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules. 2016;21:1061. doi: 10.3390/molecules21081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchiani A., Rozzo C., Fadda A., Delogu G., Ruzza P. Curcumin and curcumin-like molecules: from spice to drugs. Curr. Med. Chem. 2014;21:204–222. doi: 10.2174/092986732102131206115810. [DOI] [PubMed] [Google Scholar]
- 30.Jin X., Wang K., Liu H., Hu F., Zhao F., Liu J. Protection of Bovine Mammary Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Cell Damage by Resveratrol. Oxidative Med. Cell. Longev. 2016;2016:2572175. doi: 10.1155/2016/2572175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettaib J., Talarmin H., Droguet M., Magne C., Boulaaba M., Giroux-Metges M.A., Ksouri R. Tamarix gallica phenolics protect IEC-6 cells against H2O2 induced stress by restricting oxidative injuries and MAPKs signaling pathways. Biomed. Pharmacother. 2017;89:490–498. doi: 10.1016/j.biopha.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang S., Yu R., Zhong J., Liu P., Liu Z. Rhein from Rheum rhabarbarum Inhibits Hydrogen-Peroxide-Induced Oxidative Stress in Intestinal Epithelial Cells Partly through PI3K/Akt-Mediated Nrf2/HO-1 Pathways. J. Agric. Food Chem. 2019;67:2519–2529. doi: 10.1021/acs.jafc.9b00037. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Wu G., Qin X., Ma Q., Zhou Y., Liu S., Tan Y. Expression of Nodal on Bronchial Epithelial Cells Influenced by Lung Microbes Through DNA Methylation Modulates the Differentiation of T-Helper Cells. Cell Physiol. Biochem. 2015;37:2012–2022. doi: 10.1159/000438561. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J.F., Bai K.W., He J.T., Niu Y., Lu Y., Zhang L.K., Wang T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018;96:867–879. doi: 10.1093/jas/sky009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Flohé L. Glutathione Peroxidases. Encycl. Biol. Chem. 2013;1830:399–404. [Google Scholar]
- 37.Zhong W., Qian K., Xiong J., Ma K., Wang A., Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. Biomed Pharmacother. 2016;83:302–313. doi: 10.1016/j.biopha.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 38.Sinha K., Das J., Pal P.B., Sil P.C. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 39.Namazi N.S., Saberi S.F., Falak R., Karimi M.Y., Davoodzadeh M.G., Rangbar A., Hosseini A. Phosphodiesterase 4 and 7 inhibitors produce protective effects against high glucose-induced neurotoxicity in PC12 cells via modulation of the oxidative stress, apoptosis and inflammation pathways. Metab. Brain Dis. 2018:1–14. doi: 10.1007/s11011-018-0241-3. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui W.A., Ahad A., Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 41.Mou K., Pan W., Han D., Wen X., Cao F., Miao Y., Li P. Glycyrrhizin protects human melanocytes from H2O2-induced oxidative damage via the Nrf2dependent induction of HO1. Int. J. Mol. Med. 2019;44:253–261. doi: 10.3892/ijmm.2019.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bucolo C., Drago F., Maisto R., Romano G.L., D’Agata V., Maugeri G., Giunta S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J. Cell Physiol. 2019;234:17295–17304. doi: 10.1002/jcp.28347. [DOI] [PubMed] [Google Scholar]
- 43.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 45.Tao S., Justiniano R., Zhang D.D., Wondrak G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013;1:532–541. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong J.Y., Cha H.J., Choi E.O., Kim C.H., Kim G.Y., Yoo Y.H., Hwang H.J., Park H.T., Yoon H.M., Choi Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019;16:145–155. doi: 10.7150/ijms.27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Yao W., Shi H., Liu H., Li Y., Gao Y., Liu R., Xu L. Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis. J. Ethnopharmacol. 2016;185:361–369. doi: 10.1016/j.jep.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 48.Ndisang J.F. Synergistic Interaction Between Heme Oxygenase (HO) and Nuclear-Factor E2- Related Factor-2 (Nrf2) against Oxidative Stress in Cardiovascular Related Diseases. Curr. Pharm. Des. 2017;23:1465–1470. doi: 10.2174/1381612823666170113153818. [DOI] [PubMed] [Google Scholar]
- 49.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 51.Sun W., Meng J., Wang Z., Yuan T., Qian H., Chen W., Tong J., Xie Y., Zhang Y., Zhao J., et al. Proanthocyanidins Attenuation of H2O2-Induced Oxidative Damage in Tendon-Derived Stem Cells via Upregulating Nrf-2 Signaling Pathway. Biomed. Res. Int. 2017;2017:7529104. doi: 10.1155/2017/7529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng C., Zhong P., Zhao Y., Kanchana K., Zhang Y., Khan Z.A., Chakrabarti S., Wu L., Wang J., Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell. Cardiol. 2015;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Lu C., Xu W., Zhang F., Shao J., Zheng S. Nrf2 Knockdown Disrupts the Protective Effect of Curcumin on Alcohol-Induced Hepatocyte Necroptosis. Mol. Pharm. 2016;13:4043–4053. doi: 10.1021/acs.molpharmaceut.6b00562. [DOI] [PubMed] [Google Scholar]
- 54.Kwan S.T.C., King J.H., Yan J., Wang Z., Jiang X., Hutzler J.S., Klein H.R., Brenna J.T., Roberson M.S., Caudill M.A. Maternal Choline Supplementation Modulates Placental Nutrient Transport and Metabolism in Late Gestation of Mouse Pregnancy. J. Nutr. 2017;147:2083–2092. doi: 10.3945/jn.117.256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosario F.J., Kanai Y., Powell T.L., Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S., Yi X., Su X., Jian Z., Cui T., Guo S., Gao T., Li C., Li S., Xiao Q. Ginkgo biloba extract protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J. Cell. Mol. Med. 2019;23:5193–5199. doi: 10.1111/jcmm.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]