Abstract
Background: Lethal apnea is a significant cause of acute mortality following a severe traumatic brain injury (TBI). TBI is associated with a surge of adenosine, which also suppresses respiratory function in the brainstem.
Methods and Materials: This study examined the acute and chronic effects of caffeine, an adenosine receptor antagonist, on acute mortality and morbidity after fluid percussion injury.
Results: We demonstrate that, regardless of preinjury caffeine exposure, an acute bolus of caffeine given immediately following the injury dosedependently prevented lethal apnea and has no detrimental effects on motor performance following sublethal injuries. Finally, we demonstrate that chronic caffeine treatment after injury, but not caffeine withdrawal, impairs recovery of motor function.
Conclusions: Preexposure of the injured brain to caffeine does not have a major impact on acute and delayed outcome parameters; more importantly, a single acute dose of caffeine after the injury can prevent lethal apnea regardless of chronic caffeine preexposure.
Keywords: adenosine, caffeine, apnea, traumatic brain injury, fluid percussion injury, rat
Introduction
Severe traumatic brain injury (TBI) causes over 50,000 deaths per year in the United States,1 half of which occur before hospital admission.2 Apnea and impaired respiration are significant risk factors for lethal outcome, with apnea duration directly related to injury severity.3 For first responders, airway management and stabilization for transport to a trauma center are essential to minimize mortality and secondary injury4; accordingly, endotracheal intubation and mechanical ventilation are widely practiced. However, worldwide studies have demonstrated that prehospital intubation does not have a protective effect, and may even be deleterious.5–8 In contrast, once a patient is admitted, intubation is an essential component of successful critical care. While it is still not entirely clear why prehospital intubation can be detrimental, hypotheses center on the difficulty of field intubation, mechanical ventilation, and respiratory monitoring. An effective treatment to prevent apnea in the critical period after TBI could save thousands of lives each year.
TBI is associated with a massive surge of adenosine in the brain, which typically acts as an endogenous neuroprotective response.9 Although this adenosine surge limits the spatial and temporal extent of the injury,10 the resulting excess in adenosine needs to be metabolized effectively to avoid excessive postinjury depression. In the adult brain, the metabolic clearance of adenosine is largely under the control of astrocytes expressing equilibrative nucleoside transporters and the adenosine removing enzyme adenosine kinase (ADK).11,12 Astroglial ADK thereby forms a “metabolic sink” for adenosine. Three lines of evidence support a critical role of adenosine and defects in its metabolic clearance for the development of lethal apnea: (i) lethal apnea following a severe TBI shares a similar etiology with sudden infant death syndrome (SIDS),13,14 a condition that was mimicked by the genetic deletion of ADK in mice: 35% of all ADK-deficient pups died within the first 4 days of life and most cases of sudden death were accompanied by prolonged apnea.15 (ii) Severe TBI is associated with a surge in adenosine, and high CSF levels of adenosine correlated with lethal apnea in human subjects.16 Conversely, an acute bolus of caffeine given immediately after severe TBI in rats prevented lethal apnea.17 (iii) A combination of a seizure with pharmacological disruption of metabolic adenosine clearance triggered lethal outcome with periods of irregular breathing, prolonged apnea, and respiratory secretions in normal mice.18 Conversely, a single acute dose of caffeine attenuated this outcome. Thus, excessive levels of adenosine in the brain constitute a candidate mechanism to trigger lethal apnea following a severe TBI.
Adenosine controls neuronal excitability largely by stimulation of inhibitory Gi or Go containing G protein-coupled adenosine A1 receptors (A1Rs) and facilitatory Gs containing G protein-coupled A2A receptors (A2ARs),19,20 whereby increased activation of the A1R mediates the neuroprotective activity of adenosine.21–24 In addition, adenosine regulates respiratory processes in the brainstem through both A1 and A2AR activation, and can thereby cause respiratory dysfunction when receptors are overstimulated.25,26 In the brainstem, the A2AR is expressed in regions of the medulla oblongata containing GABAergic neurons.27 Several lines of evidence suggest that A2AR activation may suppress respiration by GABAergic inhibition of arousal-promoting neurons in the brainstem.28,29 It is important to note that, while A2ARs are abundantly expressed in brainstem, the expression of this receptor subtype in the seizure-generating limbic system is low20; thus, A2AR antagonists might uniquely be suited to exert major effects on brainstem physiology, while sparing seizure-generating brain areas. A2AR antagonists (including caffeine) might therefore exhibit respiration-promoting activities in the absence of increased seizure risk and are consequentially of translational value. In line with this, cerebrospinal fluid levels of caffeine are positively correlated to improved clinical outcome after TBI.30 Furthermore, the nonselective adenosine receptor antagonists theophylline and caffeine are clinically used in the treatment of apnea of prematurity.31
The methylxanthines caffeine and theophylline are in widespread chronic use in the human population. Therefore, it is fair to assume that a large proportion of TBI victims are under chronic caffeination at the time point of injury. Caffeine consumption exerts a rapid influence on the brain function by adenosine A1 and A2A receptor antagonism.32 Chronic caffeine consumption has an added influence on adenosine signaling, causing regional and dose-dependent changes in A1 and A2A receptor expression,33–35 modulating the influence of an injury-induced adenosine surge. Clinical TBI management usually leads to a default withdrawal of caffeine after hospitalization. The behavioral effects of caffeine withdrawal are well documented, including headache, drowsiness, and impaired concentration.36 Caffeine withdrawal is associated with increased cerebral blood flow, likely by A2A receptor signaling37; however, the physiology of caffeine withdrawal and how it might influence outcome after TBI are not well understood.
Our prior study in caffeine-naive (rats never exposed to caffeine at the time of injury) rats demonstrated that a single bolus of caffeine administered intraperitoneally, immediately following a severe TBI, restored regular breathing, preventing lethal injury-induced apnea.17 To investigate whether caffeine-based therapeutic interventions can be translated to human populations with varied caffeine preexposure histories, we examined the efficacy of acute, postinjury caffeine administration under a range of potentially confounding caffeine consumption conditions that included dose, chronic consumption, and withdrawal. We also evaluated injury severity, to demonstrate potential bidirectional outcome measures. We utilized the lateral fluid percussion injury (FPI) model of TBI in rats with acute lethal apnea as our primary outcome measure. We used rotarod performance at 24 hours and 7 days after TBI to demonstrate whether caffeine administration influenced early recovery of motor function. Our results suggest that an acute bolus of caffeine given immediately following TBI may prevent lethal outcome in a broad range of circumstances. Furthermore, acute caffeine treatment does not impair recovery of motor function during the first week after TBI. Together, these results justify future long-term studies to fully characterize the behavioral, motor, and histological effects of caffeine rescue following severe TBI.
Methods
Animals
Procedures were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care according to protocols approved by the Legacy Institutional Animal Care and Use Committee and the US Army Medical Research and Materiel Command (USAMRMC) Animal Care & Use Review Office, and guidelines from the National Institute of Health. Male Sprague-Dawley rats (Charles River, Wilmington, MA) were used for all studies. Rats were allowed 5–7 days to acclimate before any experimental procedure.
Experimental design
Severe FPI was set at the median lethal pressure, resulting in baseline mortality of 50% in caffeine-naive rats, to explore both prosurvival and antisurvival effects of different caffeine treatment paradigms. The experiments presented in this study were performed in three independent cohorts, described in Tables 1–3 and Figure 1. Within a cohort, rats were randomly distributed among preinjury caffeine consumption, injury level, and postinjury caffeine level. To ensure that baseline injury mortality remained constant throughout each cohort, we calibrated the magnitude of “severe” injury to the level resulting in 40–50% mortality in designated caffeine-naive calibration-only rats, confirmed throughout the execution of each individual cohort. Mild and moderate injury levels were scaled accordingly within each cohort. To blind the experimenter, each rat within a cohort was assigned a unique ID number upon receipt for reference in all further testing and randomly assigned to an experimental group before FPI.
Table 1.
Postinjury Bolus Experimental Matrix and Injury Parameters (Mean ± SD)
| Caffeine bolusa |
Shamb |
Mild |
Moderate |
Severe |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (sec) | Dose (mg/kg) | n | Weight (g) | FPI (atm) | n | Weight (g) | FPI (atm) | n | Weight (g) | FPI (atm) | n | Weight (g) | FPI (atm) |
| 10 | 0.0 | 8 | 400 ± 12 | 0 ± 0 | 8 | 373 ± 17 | 0.60 ± 0.03 | 8 | 373 ± 9 | 1.90 ± 0.05 | 12 | 371 ± 16 | 3.01 ± 0.07 |
| 12.5 | — | — | — | — | — | — | — | — | — | 12 | 380 ± 12 | 2.98 ± 0.05 | |
| 25.0 | 6 | 404 ± 7 | 0 ± 0 | 8 | 378 ± 15 | 0.62 ± 0.03 | 8 | 373 ± 16 | 1.90 ± 0.08 | 16 | 368 ± 11 | 2.98 ± 0.04 | |
| 50.0 | — | — | — | — | — | — | — | — | — | 12 | 375 ± 14 | 3.03 ± 0.05 | |
| 90 | 25.0 | — | — | — | — | — | — | — | — | — | 14 | 378 ± 12 | 3.01 ± 0.07 |
All rats in this study received caffeine in their drinking water (0.3 g/L) for 3 weeks before FPI.
Postinjury bolus delivered IP in 1 mL/kg saline.
Heavy rats were assigned to the Sham FPI group.
FPI, fluid percussion injury.
Table 2.
Chronic Caffeine Consumption Experimental Matrix and Injury Parameters (Mean ± SD)
| Caffeinea |
Severe FPI |
|||
|---|---|---|---|---|
| Preinjuryb | Postinjuryc | n | Weight (g) | FPI (atm) |
| 0 g/L | N/A4 | 9 | 364 ± 15 | 3.25 ± 0.04 |
| 0 g/L | 4 | 375 ± 02 | 3.27 ± 0.05 | |
| 0.3 g/L | 5 | 362 ± 09 | 3.25 ± 0.02 | |
| 0.3 g/L | N/Ad | 5 | 371 ± 16 | 3.23 ± 0.04 |
| 0 g/L | 3 | 352 ± 20 | 3.21 ± 0.08 | |
| 0.3 g/L | 6 | 356 ± 27 | 3.21 ± 0.04 | |
Chronic caffeine: administered ad libitum in drinking water.
Preinjury caffeine exposure for 3 weeks.
Postinjury caffeine exposure from FPI until sacrifice (1 week).
N/A Indicates rats that died as a result of the FPI.
Table 3.
Preinjury Caffeine Bolus Experimental Matrix and Injury Parameters (mean ± SD)
| Preinjury |
Severe FPI |
|||
|---|---|---|---|---|
| Chronic caffeinea | Acute | n | Weight (g) | FPI (atm) |
| 0 g/L | None | 22 | 367 ± 20 | 2.80 ± 0.24 |
| Bolusb | 20 | 368 ± 14 | 2.88 ± 0.21 | |
| 0.3 g/L | None | 20 | 366 ± 18 | 2.80 ± 0.26 |
| Bolusb | 20 | 370 ± 16 | 2.83 ± 0.23 | |
| Withdrawc | 20 | 365 ± 18 | 2.84 ± 0.24 | |
Chronic caffeine: administered ad libitum in drinking water for 3 weeks before FPI.
Bolus: 25 mg/kg, administered IP in 1 mL/kg saline 1 hour before FPI.
Withdraw: 36-hour caffeine withdrawal before FPI.
FIG. 1.
Experimental design. Summary of the assignment of three different experimental cohorts of animals to different caffeination paradigms before or after a FPI. FPI, fluid percussion injury,
All rats in Cohort 1 (Table 1) were caffeine preconditioned for 3 weeks before FPI. Following FPI, rats were treated with a single bolus of caffeine or saline, and then returned to their home cages with plain drinking water for the 24-hour and 1-week assessments.
Rats in Cohort 2 (Table 2) were either caffeine preconditioned for 3 weeks (0.3 g/L) or caffeine naive (0 g/L) at the time of FPI. Following injury, surviving rats were assigned to receive either chronic caffeine (0.3 g/L) or plain drinking water during the 1-week postinjury follow-up.
Rats in Cohort 3 (Table 3) were assigned to one of five experimental groups: (i) caffeine naive (0 g/L chronic caffeine), no pre-FPI caffeine bolus; (ii) caffeine naive, single caffeine bolus (25 mg/kg) 1 hour before FPI; (iii) caffeine preconditioned (0.3 g/L), no pre-FPI caffeine bolus; (iv) caffeine preconditioned, single caffeine bolus (25 mg/kg) 1 hour before FPI; or (v) caffeine preconditioned for 3 weeks and caffeine withdrawn for 36 hours before FPI. All rats in Cohort 3 received plain drinking water in their home cages during the 1-week postinjury follow-up.
Caffeine dosing
Caffeine (Sigma-Aldrich, St. Louis, MO) was prepared on the day of use. For the acute treatment of rats, caffeine was prepared in sterile saline at 0–50 mg/mL, and administered intraperitoneally (IP) at 1 mL/kg. In Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations (Institute of Medicine- Consensus Study Report 2001) from the Institute of Medicine, an acute dose of caffeine given as a bolus in the amounts of 200–600 mg can be effective in maintaining cognitive and physical performance in humans, regardless of habitual caffeine consumption. Therefore, we selected a nominal dose of 25 mg/kg of caffeine in rats, equivalent to a dose of 340 mg in an 85 kg human,38,39 and within a safe range in mice and rats.40,41 Studies on the effects of chronic human caffeine consumption report average consumption of around 300 mg/day, or 3.4 mg/kg/day, resulting in plasma caffeine concentration of 11.45 μM.42,43 To replicate this dose of caffeine in rats, we selected a dose of 0.3 g/L in water for the chronic caffeine consumption (ad libitum consumption, replaced every other day), which results in plasma concentration of 11.8 μM methylxanthines and to caffeine tolerance in rats.44
Lateral FPI
Rats were anesthetized with isoflurane (2% isoflurane at 2 mL/min in 2:1 N2O:O2) in an induction chamber, and then affixed into a stereotactic frame fitted with a ventilated non-rebreathing face mask; all anesthetic gasses were exhausted from the room. A 5 mm trephine hole was drilled centered at bregma—4.5 mm anterior-posterior and +2.8 mm medial-lateral.17 TBI was produced by a fluid-percussion device (Custom Design and Fabrication, Richmond, VA). A 21–23 ms fluid pulse was applied to the exposed dura, measured by an external pressure transducer, digitized by a PowerLab A/D converter (ADInstruments, Colorado Springs, CO), and then recorded using Scope (ADInstruments). For each of our three studies (detailed in Tables 1–3), FPI severity was calibrated using caffeine-naive rats to determine the peak pressure resulting in 40–50% mortality, considered “severe” injury. Mild and moderate injuries were scaled accordingly (Tables 1–3 for average values for each cohort). Sham-injured controls were prepared using identical manipulations without impact.
Mortality and apnea assessment
All experiments were done by two investigators, with one investigator handling the animals and the other investigator timing respiratory activity by visual inspection. The duration of apnea was measured in seconds from the time of FPI until the first acute inspiratory effort, or “gasp.” Immediately following FPI, rats were placed in dorsal recumbency (on their back) for continuous observation of respiratory activity, which allows an observer to clearly distinguish between apnea (no breathing), shallow respiratory activity, the postapnea gasp, and regular breathing. Monitoring continued until regular spontaneous ventilation was apparent, or until the complete cessation of inspiratory effort for at least 5 minutes.
Motor function assessment
Using an accelerating rotarod, rats were assessed for motor performance 1 day before FPI and following FPI at 24 hours and 7 days by an individual blinded to the experimental condition. Rats were placed on the rotarod rotating at a constant rate of 2 rpm. Acceleration began at time 0 and reached a top speed of 30 rpm at 96 seconds. The time for each trial was recorded at the point that the rat fell off the rod or rotated with the rod for two revolutions. The rotarod test consisted of three trials, each a maximum of 120 seconds and a rest period of 2 minutes between trials. Recovery of rotarod performance was calculated as the difference between the normalized 7-day performance and 24-hour performance.
Statistics
All statistics were performed using StatView (SAS Institute, Cary, NC) or Prism 5 (GraphPad, San Diego, CA). The distributions of weight and FPI pressure for each group were compared by ANOVA (StatView), and were not different among groups (Tables 1–3). Correlation between caffeine treatment and mortality was assessed using logistic regression test (StatView). Rotarod performance was assessed only for those rats that survived the procedure, and was evaluated by repeated measures ANOVA. Recovery of rotarod performance was assessed by t-test or one-way ANOVA as appropriate.
Results
Acute administration of caffeine after severe FPI dose dependently prevents lethal outcome in caffeine-preconditioned rats
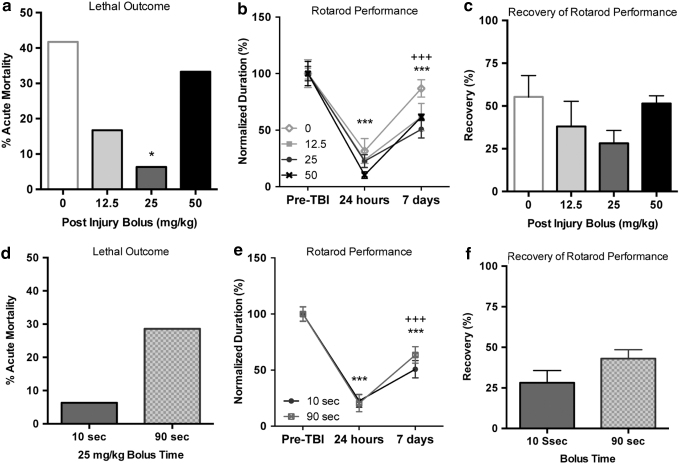
It is fair to assume that few adults in the human population are caffeine naive at the time point of a TBI. To create a more realistic scenario, to study the protective effects of an acute caffeine bolus following a TBI, we combined chronic caffeination of rats before a TBI with a post-TBI caffeine bolus, in contrast to our prior work, which was conducted in caffeine-naive animals.17 After a 3-week caffeine preconditioning period (0.3 g/L caffeine, ad libitum), rats were exposed to a severe FPI. At 10 seconds after injury, a single bolus of caffeine (0, 12.5, 25, or 50 mg/kg caffeine in saline) was administered IP (Table 1), followed by plain drinking water (i.e., caffeine withdrawal) for the 1-week follow-up period. Acute mortality was defined as death that occurred without sustained restoration of regular respiration. Acute mortality in the rats receiving vehicle (0 mg/kg caffeine) was 41.7%; mortality decreased to 16.7%, 6.3%, and 33.3% in the animals that received a bolus of 12.5, 25, or 50 mg/kg caffeine, respectively (Fig. 2a). The reduced mortality was significant for the group receiving 25 mg/kg caffeine (p < 0.05).
FIG. 2.
Caffeine bolus after severe FPI prevents acute lethal outcome. (a) Caffeine protection is dose dependent, with maximum protection at 25 mg/kg (*p < 0.05). (b) Motor function in the rotarod at 24 hours after severe FPI was significantly impaired when compared to baseline, preinjury function (***p < 0.001). Rotarod performance was still impaired at 7 days after injury compared to baseline (***p < 0.001), but spontaneous recovery over the 24-hour time point was measured (+++p < 0.001). There was no significant difference among the different caffeine concentrations tested. (c) Recovery of function at 7 days was not significantly influenced by bolus size. A delayed bolus did not improve acute mortality (d), rotarod performance (e), or recovery of rotarod function (f).
We evaluated rotarod performance at 24 hours and 7 days after FPI, normalizing results to preinjury baseline values. At 24 hours after severe injury, performance on the rotarod dropped to 10–30% of preinjury performance (Fig. 2b). By 7 days after severe injury, rats spontaneously recovered to 50–87% of preinjury performance. Repeated measures ANOVA demonstrated that the caffeine bolus dose delivered after the injury did not significantly affect rotarod performance (F[3,30], p = 0.169), a significant influence of recovery time (F[2,60], p < 0.0001), and no interaction between dose and recovery time was found. Post hoc evaluation of the recovery time demonstrated that performance at 24 hours and 7 days following FPI was significantly impaired (p < 0.0001), but found significant recovery at 7 days when compared to the 24-hour performance (p < 0.0001). To more closely look at the influence of a postinjury caffeine bolus on recovery of rotarod performance, we evaluated postinjury recovery of rotarod performance (Fig. 2c), but found no significant difference in recovery rates between the different bolus sizes (F[3.30], p = 0.2179).
All rats experienced a period of apnea following severe FPI. Of the rats that died, some experienced a short transient period of respiration, while others had no spontaneous respiratory activity. The fraction of rats with complete cessation of respiration after FPI was 60% of the vehicle-treated rats (0 mg/kg caffeine), and 50%, 0%, and 75% in the rats that received 12.5, 25, or 50 mg/kg caffeine (not significant by logistic regression), whereas a chi-square test revealed statistical significance (p < 0.01) between the 25 and 0 mg caffeine groups. To uncover any negative influence of a late caffeine bolus, we delayed delivery of a caffeine bolus (25 mg/kg) to 90 seconds in a group of rats (Table 1). Delayed treatment did not result in a reduction in acute lethal outcome (Fig. 2d), nor did it influence rotarod deficits at 24 hours or recovery at 7 days (Fig. 2e, f) suggesting general safety of postinjury administration of an acute dose of caffeine.
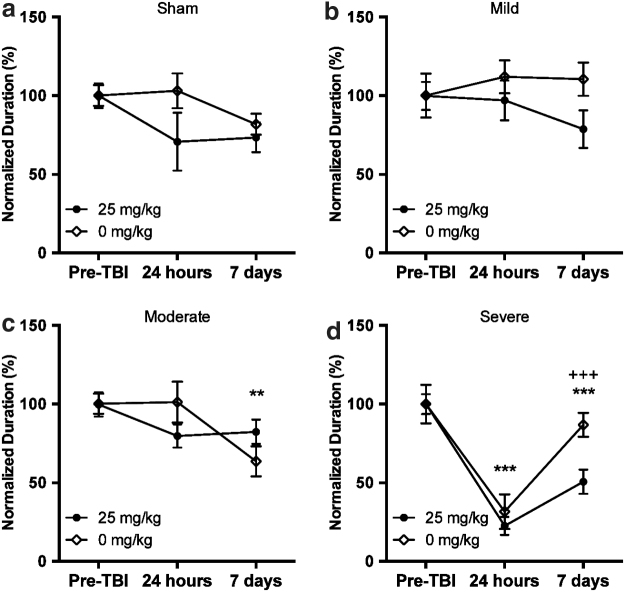
Acute caffeine treatment after mild or moderate injury does not have negative consequences
A limitation of safety studies in severely injured rats is an experimental bias toward improved outcome. To assess possible negative consequences of postinjury caffeine in a more critical manner, we evaluated graded injury levels to assess bidirectional effects of treatment. We examined the effect of a 25 mg/kg bolus of caffeine administered 10 seconds after sham, mild, or moderate injury (Table 1). After sham or mild injury, there was no significant effect of injury or postinjury caffeine bolus on rotarod performance at either 24 hours or 7 days after FPI (Fig. 3a, b). After moderate injury, we found a significant effect of time after injury (F[2,28], p < 0.01), but no significant effect of caffeine (Fig. 3c); post hoc tests showed a significant reduction in function at 7 days after FPI.
FIG. 3.
Influence of a caffeine bolus after nonlethal injury. A 25 mg/kg caffeine bolus after sham (a), mild (b), or moderate (c) injury does not influence rotarod performance at 24 hours or 7 days after injury. (d) Rotarod performance after severe injury presented for reference. One-way ANOVA with Tukey's multiple comparison post-hoc test (**P < 0.01, ***P < 0.001, +++P < 0.0001 for significance).
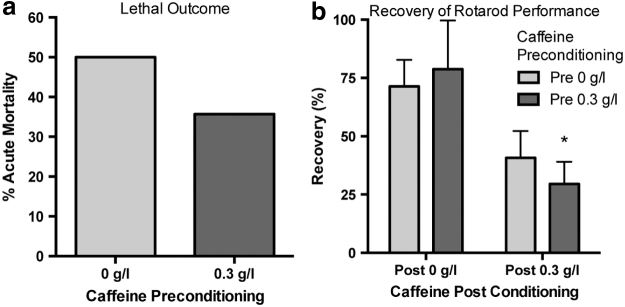
Chronic caffeine after injury impairs spontaneous recovery of rotarod performance
Assuming that human TBI victims are chronically caffeinated at the time of injury, as a matter of standard care, TBI is followed by default caffeine withdrawal. Rats were caffeine preconditioned (0 g/L or 0.3 g/L caffeine) for 3 weeks before severe FPI (Table 2). We found no change in acute mortality after severe FPI associated with preinjury caffeine conditioning (Fig. 4a). Following the acute monitoring period, surviving rats were randomly assigned to receive either chronic caffeine or plain drinking water for the duration of 1-week recovery period (Table 2). Rotarod performance was assessed at 24 hours and 7 days following severe injury. At 24 hours, all rats had significant impairment in their rotarod performance, regardless of caffeine consumption; there was no difference between groups in performance based on caffeine consumption. At 7 days after injury, however, we found significant impairment in the recovery of rotarod performance in rats that received caffeine in their drinking water for the week-long recovery period (F[1,14], p = 0.0073, Fig. 4b). Therefore, chronic caffeination after the injury impairs recovery, irrespective of the preinjury caffeination status.
FIG. 4.
Chronic caffeine consumption after injury is detrimental to recovery of spontaneous motor function. Three weeks of caffeine preconditioning (0.3 g/L, ad libitum) does not influence acute mortality after severe injury (a) Rats that survived the acute injury were randomly assigned to receive either plain water (Post 0 g/L) or caffeine water (0.3 g/L). (b) There was no difference in rotarod performance at 24 hours among the treatment groups. However, recovery of rotarod performance at 7 days was significantly impaired in the rats that received caffeine postinjury (F[1,14], p = 0.0073). One-way ANOVA with Tukey's multiple comparison post-hoc test (*P < 0.05 for significance).
Preinjury caffeine and withdrawal
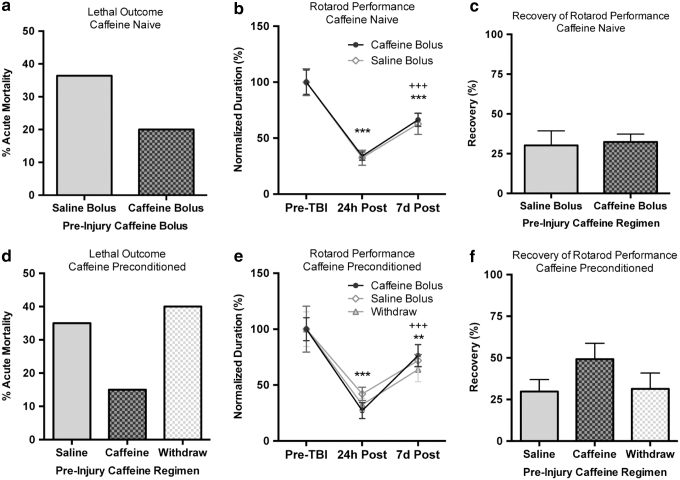
With growing availability of products that quickly deliver high amounts of caffeine (such as energy drinks, caffeine gum, and caffeine pills), there is potential for unusually high plasma levels of caffeine at the time of injury. Conversely, regular caffeine consumers may be in a state of withdrawal at the time of injury. With this in mind, we investigated whether an acute bolus of caffeine 1 hour before injury affects injury severity or recovery in caffeine-naive rats. We also examined the influence of an additional bolus of caffeine versus 36 hours of caffeine withdrawal preinjury in caffeine-preconditioned rats (Table 3). Acute mortality was not significantly reduced by a preinjury caffeine bolus in caffeine-naive rats (Fig. 5a). Rotarod performance was significantly impaired at 24 hours and 7 days after injury (F[2,48], p < 0.0001), but there was no influence of caffeine bolus in the caffeine-naive rats (Fig. 5b). While there was significant recovery of rotarod function by 7 days following injury, there was no influence of caffeine on the recovery rate (p = 0.8239, Fig. 5c). In caffeine-preconditioned rats, neither a preinjury caffeine bolus nor 36 hours of caffeine withdrawal had a significant influence on acute mortality (Fig. 5d). Rotarod performance was significantly impaired in the caffeine-preconditioned rats (F[2,62], p < 0.0001), but there was no influence of caffeine bolus or withdrawal in the caffeine-preconditioned rats (Fig. 5e). Again, there was a significant recovery of rotarod performance by 7 days postinjury, but no significant difference in the recovery among the three groups (F[2,31], p = 0.2328, Fig. 5f).
FIG. 5.
Preinjury caffeine consumption patterns (including withdrawal) do not influence outcome after severe injury. (a) In caffeine-naive rats, a preinjury caffeine bolus (1 hour before FPI) does not significantly reduce acute mortality. (b) Motor function at 24 hours after severe FPI was significantly impaired when compared to baseline, preinjury function (***p < 0.001) in caffeine-naive rats. Rotarod performance was still impaired at 7 days after injury compared to baseline (***p < 0.001), but spontaneous recovery over the 24-hour time point was measured (+++p < 0.001). There was no significant difference between a 25 mg/kg caffeine bolus and saline. (c) Recovery of function at 7 days was not significantly influenced by the caffeine bolus in caffeine-naive rats. (d) In caffeine-preconditioned rats, a preinjury caffeine bolus (1 hour before FPI) does not significantly reduce acute mortality, and 36 hours of preinjury caffeine withdrawal have no influence on acute mortality. (e) Motor function at 24 hours after severe FPI was significantly impaired when compared to baseline, preinjury function (**p < 0.01, ***p < 0.001) in caffeine-preconditioned rats. Rotarod performance was still impaired at 7 days after injury compared to baseline (***p < 0.001), but spontaneous recovery over the 24-hour time point was measured (++p < 0.01). There was no significant influence of caffeine bolus or withdrawal. (f) Recovery of function at 7 days was not significantly influenced by the caffeine bolus or withdrawal in caffeine-preconditioned rats.
Discussion
This is the first comprehensive and systematic assessment of the effects of peri-TBI caffeine exposure on acute mortality and short-term functional outcome graded across several severity levels of the FPI. This study is of relevance as caffeine is widely consumed in the human population, and it can therefore be assumed that a TBI happens within the context of various exposure levels and histories to prior caffeine exposure; in addition, TBI may lead by default to caffeine withdrawal in victims who are chronic caffeine users. Yet surprisingly, little is known about the effects of acute or chronic caffeine on the outcome of a TBI, warranting the systematic study undertaken here. Several aspects of our study deserve a more detailed discussion:
Caffeine-based resuscitation
Respiratory deficits are a significant source of mortality and morbidity after TBI. In the clinical as well as in the experimental setting, injury to the brain is associated with a massive micromolar surge in adenosine and high CSF levels of adenosine correlate with lethal apnea in human TBI subjects.16 In support of these findings, clinical and experimental evidence have demonstrated a clear role for methylxanthines in the preservation of respiration,45,46 although the precise mechanisms controlling respiration remain poorly understood. Recent studies highlight the role of the pre-Bötzinger Complex (preBotC) in establishing and maintaining respiratory rhythms, demonstrating a link between purinergic signaling and respiratory activity.47 In particular, ATP release in the pre-BotC has been shown to initially increase respiration, and that the subsequent respiratory depression is dependent on ATP hydrolysis, suggesting a role for adenosine.48 Further studies have specifically demonstrated that the activation of adenosine A1R depressed respiration.49 The pre-BotC is located in the ventral medulla, an anatomic location that may make it vulnerable to injury at high injury levels. In cats, severe FPI has been linked to a brain stem injury based on acute physiologic changes.50 We therefore hypothesized that acute lethal outcome following a severe TBI is due to prolonged apnea based on overstimulation of adenosine receptors in the brainstem. In support of our hypothesis, a single acute bolus of caffeine (25 mg/kg) in rats when given 10 seconds after a severe TBI almost completely prevented lethal apnea under conditions in which 40% of all animals succumbed to lethal apnea.17
Related conditions
Respiratory suppression has also been demonstrated in related lethal conditions, in which adenosine has been implicated. In Sudden Unexpected Death in Epilepsy (SUDEP), a seizure-induced adenosine surge in combination with deficient metabolic clearance of adenosine has been implicated in lethal outcome and the postseizure administration of caffeine was found to be beneficial.18 Interestingly, SIDS is likewise characterized by respiratory suppression, and perinatal ADK knockout mice that have deficits in the metabolic clearance of adenosine have prolonged periods of apnea and succumb to perinatal death.15 ADK primarily regulates physiological adenosine levels under baseline conditions51 and might become a limiting factor under conditions of increased metabolic clearance needs. These conditions further support a critical role for adenosine homeostasis in the control of respiratory function. Consequently, respiratory function and survival may critically depend on the status of the adenosine system, which in turn may also depend on external factors such as habitual caffeine use.
Role of prior caffeination status
In this study, we demonstrate that the protective effects of caffeine are maintained in caffeine-preconditioned rats, an important consideration as most adults are habitual caffeine consumers. Under the most severe injury conditions, we found that a single bolus of caffeine given before or after the injury consistently reduced mortality irrespective of the subjects' caffeination history. These findings suggest that an acute caffeine bolus given as soon as possible after a TBI might have life-rescuing value in emergency medicine in a wide range of subjects. Importantly, an acute bolus of caffeine is still able to significantly reduce apnea with lethal outcome in chronically caffeinated subjects. Our data need to be carefully considered, although, since the effects of a TBI might also depend on other lifestyle choices, which are known to influence adenosine homeostasis in the brain. For example, sleep deprivation52–55 as well as exercise56 lead to an increase of adenosine in the brain. These additional factors need to be considered to fully understand the role of adenosine in a “real-life scenario” of TBI.
Role of chronic caffeination
Chronic caffeine consumption causes regional changes in brain adenosine receptor expression.44 The combination of chronic antagonism and altered receptor expression may have significant influence on the efficacy of caffeine as a therapeutic agent acting through A1R antagonism. An “effect inversion” has been attributed to caffeine, in which chronic caffeine consumption results in opposite outcomes to acute caffeine treatment.57 The widespread chronic caffeine consumption in most parts of the world made it necessary to demonstrate that an acute bolus of caffeine can still prevent lethal outcome in caffeine-preconditioned rats. Because an effect inversion was not demonstrated in this study and adenosine receptor densities were not assessed, the possibility exists that the caffeine doses used here to mimic human caffeine consumption were not sufficient to induce caffeine-preconditioning effects through changes in adenosine receptor densities. Alternatively, chronic caffeine consumption is also associated with alterations in N-methyl d-aspartate (NMDA)58 and serotonin59 receptors and function. Damage to glutamatergic neurons is well documented in modeled TBI, and given the role of NMDA receptors in cognition, these results highlight a potential role for caffeine in the variable recovery of cognitive function after TBI.60 These results demonstrate the need for an expanded repertoire of behavioral tests and long-term studies designed to evaluate cognitive and psychiatric deficits, and long-term recovery after TBI, with and without caffeine as a potential confounding factor.
Safety of caffeine-based therapeutic interventions
In this study, we show maximum therapeutic effectiveness of a dose of 25 mg/kg caffeine given 10 seconds after an injury. Importantly, deviation from the optimal dose in either direction is without adverse effects, as is a delayed administration of caffeine. These findings show the general safety and applicability of caffeine-based resuscitation after TBI: even suboptimal doses or delayed delivery will not worsen outcome and may still have some benefits. As shown in our current data, evaluation of caffeine treatment after mild and moderate injury demonstrated that unneeded treatment in the case of an isolated TBI does not have negative consequences, demonstrating the general safety of caffeine-based interventions. While adenosine is an effective agent to suppress epileptic seizures across a wide frequency range, irrespective of seizure-related adenosine fluctuations,61 methylxanthines are known proconvulsants, in particular, in methylxanthine-naive subjects.62 Importantly, none of our caffeine-treated animals developed acute seizures as a result of treatment, supporting further the safety of our approach. Since epileptogenesis depends on adenosine receptor-independent functions of adenosine,63 we do not anticipate that a caffeine-based therapeutic intervention will worsen posttraumatic epileptogenesis; in support of this, our prior work in caffeine-naive rats demonstrated a reduction in epileptiform bursting in caffeine-rescued rats after a severe TBI.17 Further studies are also warranted to better understand the long-term effects of acute caffeine rescue and adjuvant therapies on a wider range of outcome parameters, including learning and memory, executive function, psychosocial interaction, as well as expanded motor function.
Therapeutic implications
Hypoxia and hypotension are commonly associated with TBI and correlate to poor outcome.5,64 In the most acute prehospital phase (10 minutes after TBI), apnea is a common cause of mortality and, in survivors, likely aggravates morbidity through hypoxia and impaired cerebral autoregulation.3 Although there are currently insufficient data available to establish treatment guidelines in head-injured patients, prehospital management has been directed at patient stabilization for transport, in particular maintenance of respiration and oxygenation to minimize secondary injury.4,65,66 Intubation and mechanical ventilation by emergency personnel before transport have become the standard of care to prevent the effects of hypoxia, yet recent retrospective studies suggest that this may not provide a universal benefit.6–8 Considering the inadequacy of current prehospital TBI management, there is a clear need for more reliable and effective approaches for treating TBI victims.
Understanding the influence of caffeine consumption before TBI provides insight into the variable resilience of individuals to TBI, whereas postinjury treatment with caffeine and/or specific adenosine receptor modulators may represent a therapeutic opportunity. Treatment initiated after TBI suggested improved motor function with a nonspecific adenosine receptor agonist, but a slight decrease in motor function after an A1 receptor antagonist.67 In contrast, 15 minutes post-TBI treatment with caffeinol, a mix of caffeine (3.3 mg/kg) and ethanol (0.65 g/kg), resulted in improved working memory and reduced contusion volume, although this was not replicated with delayed treatment.68 Acute mortality was not reported for these focal injury (cortical contusion) studies, and treatment modality (immediate intrahippocampal infusion in mice) and time point (15 minutes post-injury in rats) suggest that neither treatment would be likely to influence acute mortality. However, they do demonstrate the potential of caffeine to have further protective effects not explored in this study. The dose–response results presented here after diffuse injury (fluid percussion) demonstrate significant, 75% reduction in acute mortality after 25 mg/kg treatment in rats without impairment of motor function, demonstrating that quality of life may be maintained with caffeine rescue after diffuse injury. Further studies examining a broader range of injury modalities and behavioral outcomes are necessary to establish the general safety of caffeine treatment. In addition, as the principle targets of caffeine are the adenosine A1 and A2A receptors, mechanistic studies should be performed to determine whether improved efficacy against acute mortality and improved behavioral outcome can be achieved with specific receptor antagonists.
Head injuries rarely occur in isolation and, apart from lethal apnea, hemorrhagic shock is a significant factor in mortality and morbidity after TBI,69 and may influence both the safety and efficacy of caffeine-based therapy. Systemic A1 receptor activation slows heart rate and lowers blood pressure,70 while A2A activation increases heart rate and lowers blood pressure.71 An endogenous surge of plasma adenosine has been measured in hemorrhagic shock in caffeine-naive rats, with caffeine preconditioning amplifying this increase.72 Acute treatment with caffeine restored blood pressure in modeled hemorrhagic shock72; similarly, A1 receptor antagonists were effective for the restoration of cardiac function in hypoxia.73 The potential cardiovascular effects of caffeine warrant further safety and efficacy studies of caffeine-based therapy in the context of polytrauma.
Conclusions
Treatment during the acute time frame following an injury is critical in the determination of survival and recovery after TBI. We show here that treatment initiated at the earliest possible time after injury is most beneficial, perhaps in a time frame that can only be achieved in a laboratory setting. However, rodent metabolism is significantly higher than in humans, which may shorten the effective treatment window compared to humans. Furthermore, translating the acute caffeine dose of 25 mg/kg in rodents to human equivalent by body surface area39 results in a dose of ∼4 mg/kg, well below the toxic dose of caffeine estimated to be 150 mg/kg in humans. Caffeine is stable at room temperature, making it a good candidate for emergency kits. Intramuscular delivery may provide a possible route of administration in a human trauma setting.
Acknowledgments
This research was made possible by a cooperative agreement that was awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), under Contract Number: W81XWH-10-1-0757. The TATRC Contracting Officer's Representative (COR) is Dr. Brenda Bart-Knauer (Brenda.Bart-Knauer@TATRC.ORG) and the TATRC Project Officer is Cheryll Quirin (Cheryll.Quirin@TATRC.ORG). The views, opinions, and findings contained in this research are those of the company and do not necessarily reflect the views of the Department of Defense, and should not be construed as an official DoD/Army policy unless so designated by other documentation. No official endorsement should be made. In addition, we acknowledge generous funding from Dale Rice for Project Q and the NIH (NS065957 and NS103740).
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1. Faul M, Xu L, Wald MM, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hispitalizations, and Deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010 [Google Scholar]
- 2. Bruns J Jr., Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;44 Suppl 10:2–10 [DOI] [PubMed] [Google Scholar]
- 3. Atkinson JL. The neglected prehospital phase of head injury: Apnea and catecholamine surge. Mayo Clin Proc. 2000;75:37–47 [DOI] [PubMed] [Google Scholar]
- 4. Stiver SI, Manley GT. Prehospital management of traumatic brain injury. Neurosurg Focus. 2008;25:E5. [DOI] [PubMed] [Google Scholar]
- 5. Davis DP. Prehospital intubation of brain-injured patients. Curr Opin Crit Care. 2008;14:142–148 [DOI] [PubMed] [Google Scholar]
- 6. Bukur M, Kurtovic S, Berry C, et al. Pre-hospital intubation is associated with increased mortality after traumatic brain injury. J Surg Res. 2011;170:e117–e121 [DOI] [PubMed] [Google Scholar]
- 7. Stiell IG, Nesbitt LP, Pickett W, et al. The OPALS Major Trauma Study: Impact of advanced life-support on survival and morbidity. CMAJ. 2008;178:1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Elm E, Schoettker P, Henzi I, et al. Pre-hospital tracheal intubation in patients with traumatic brain injury: Systematic review of current evidence. Br J Anaesth. 2009;103:371–386 [DOI] [PubMed] [Google Scholar]
- 9. Gomes CV, Kaster MP, Tome AR, et al. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399 [DOI] [PubMed] [Google Scholar]
- 10. Kochanek PM, Vagni VA, Janesko KL, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575 [DOI] [PubMed] [Google Scholar]
- 11. Boison D. Adenosine kinase: Exploitation for therapeutic gain. Pharmacol Rev. 2013;65:906–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fedele DE, Koch P, Brüstle O, et al. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett. 2004;370:160–165 [DOI] [PubMed] [Google Scholar]
- 13. Tao JX, Qian S, Baldwin M, et al. SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia. 2010;51:2344–2347 [DOI] [PubMed] [Google Scholar]
- 14. Hirsch LJ, Donner EJ, So EL, et al. Abbreviated report of the NIH/NINDS workshop on sudden unexpected death in epilepsy. Neurology. 2011;76:1932–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boison D, Scheurer L, Zumsteg V, et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark RS, Carcillo JA, Kochanek PM, et al. Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans. Neurosurgery. 1997;41:1284–1292; discussion 1292–1293 [DOI] [PubMed] [Google Scholar]
- 17. Lusardi TA, Lytle NK, Szybala C, et al. Caffeine prevents acute mortality after TBI in rats without increased morbidity. Exp Neurol. 2012;234:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): Role of impaired adenosine clearance. Epilepsia. 2010;51:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fredholm BB, Chen JF, Masino SA, et al. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412 [DOI] [PubMed] [Google Scholar]
- 20. Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534 [DOI] [PubMed] [Google Scholar]
- 21. Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: Neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;193:535–587 [DOI] [PubMed] [Google Scholar]
- 22. Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fredholm BB. Adenosine and neuroprotection. Int Rev Neurobiol. 1997;40:259–280 [PubMed] [Google Scholar]
- 24. Pignataro G, Maysami S, Studer FE, et al. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23 [DOI] [PubMed] [Google Scholar]
- 25. Barraco RA, Janusz CA, Schoener EP, et al. Cardiorespiratory function is altered by picomole injections of 5'-N-ethylcarboxamidoadenosine into the nucleus tractus solitarius of rats. Brain Res. 1990;507:234–246 [DOI] [PubMed] [Google Scholar]
- 26. Liu C, Cao Y, Malhotra A, et al. Sleep fragmentation attenuates the hypercapnic (but not hypoxic) ventilatory responses via adenosine A1 receptors in awake rats. Respir Physiol Neurobiol. 2011;175:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaidi SI, Jafri A, Martin RJ, et al. Adenosine A2A receptors are expressed by GABAergic neurons of medulla oblongata in developing rat. Brain Res. 2006;1071:42–53 [DOI] [PubMed] [Google Scholar]
- 28. Wilson CG, Martin RJ, Jaber M, et al. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir Physiol Neurobiol. 2004;141:201–211 [DOI] [PubMed] [Google Scholar]
- 29. Coleman CG, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem. 2006;96:1750–1759 [DOI] [PubMed] [Google Scholar]
- 30. Sachse KT, Jackson EK, Wisniewski SR, et al. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2008;28:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121 [DOI] [PubMed] [Google Scholar]
- 32. Fredholm BB, Battig K, Holmen J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133 [PubMed] [Google Scholar]
- 33. Hawkins M, Dugich MM, Porter NM, et al. Effects of chronic administration of caffeine on adenosine A1 and A2 receptors in rat brain. Brain Res Bull. 1988;21:479–482 [DOI] [PubMed] [Google Scholar]
- 34. Green RM, Stiles GL. Chronic caffeine ingestion sensitizes the A1 adenosine receptor-adenylate cyclase system in rat cerebral cortex. J Clin Invest. 1986;77:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johansson B, Georgiev V, Lindstrom K, et al. A1 and A2A adenosine receptors and A1 mRNA in mouse brain: Effect of long-term caffeine treatment. Brain Res. 1997;762:153–164 [DOI] [PubMed] [Google Scholar]
- 36. Evans SM, Griffiths RR. Caffeine withdrawal: A parametric analysis of caffeine dosing conditions. J Pharmacol Exp Ther. 1999;289:285–294 [PubMed] [Google Scholar]
- 37. Pelligrino DA, Xu HL, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis. 2010;20 Suppl 1:S51–S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661 [DOI] [PubMed] [Google Scholar]
- 39. Sharma V, McNeill JH. To scale or not to scale: The principles of dose extrapolation. Br J Pharmacol. 2009;157:907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W, Dai S, An J, et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151:1198–1207 [DOI] [PubMed] [Google Scholar]
- 41. Al Moutaery K, Al Deeb S, Ahmad Khan H, et al. Caffeine impairs short-term neurological outcome after concussive head injury in rats. Neurosurgery. 2003;53:704–711; discussion 711–702 [DOI] [PubMed] [Google Scholar]
- 42. McLellan TM, Kamimori GH, Voss DM, et al. Caffeine effects on physical and cognitive performance during sustained operations. Aviat Space Environ Med. 2007;78:871–877 [PubMed] [Google Scholar]
- 43. Tikuisis P, Keefe AA, McLellan TM, et al. Caffeine restores engagement speed but not shooting precision following 22 h of active wakefulness. Aviat Space Environ Med. 2004;75:771–776 [PubMed] [Google Scholar]
- 44. Svenningsson P, Nomikos GG, Fredholm BB. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci. 1999;19:4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spitzer AR. Evidence-based methylxanthine use in the NICU. Clin Perinatol. 2012;39:137–148 [DOI] [PubMed] [Google Scholar]
- 46. Nantwi KD. Recovery of respiratory activity after C2 hemisection (C2HS): Involvement of adenosinergic mechanisms. Respir Physiol Neurobiol. 2009;169:102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ballanyi K, Panaitescu B, Ruangkittisakul A. Control of breathing by “nerve glue”. Sci Signal. 2010;3:pe41. [DOI] [PubMed] [Google Scholar]
- 48. Lorier AR, Huxtable AG, Robinson DM, et al. P2Y1 receptor modulation of the pre-Botzinger complex inspiratory rhythm generating network in vitro. J Neurosci. 2007;27:993–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vandam RJ, Shields EJ, Kelty JD. Rhythm generation by the pre-Botzinger complex in medullary slice and island preparations: Effects of adenosine A(1) receptor activation. BMC Neurosci. 2008;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shima K, Marmarou A. Evaluation of brain-stem dysfunction following severe fluid-percussion head injury to the cat. J Neurosurg. 1991;74:270–277 [DOI] [PubMed] [Google Scholar]
- 51. Etherington LA, Patterson GE, Meechan L, et al. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–135 [DOI] [PubMed] [Google Scholar]
- 53. Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bjorness TE, Kelly CL, Gao T, et al. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scharf MT, Naidoo N, Zimmerman JE, et al. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86:264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dworak M, Diel P, Voss S, et al. Intense exercise increases adenosine concentrations in rat brain: Implications for a homeostatic sleep drive. Neuroscience. 2007;150:789–795 [DOI] [PubMed] [Google Scholar]
- 57. Jacobson KA, von Lubitz DK, Daly JW, et al. Adenosine receptor ligands: Differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Georgiev V, Johansson B, Fredholm BB. Long-term caffeine treatment leads to a decreased susceptibility to NMDA-induced clonic seizures in mice without changes in adenosine A1 receptor number. Brain Res. 1993;612:271–277 [DOI] [PubMed] [Google Scholar]
- 59. Shi D, Nikodijevic O, Jacobson KA, et al. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell Mol Neurobiol. 1993;13:247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dikmen SS, Corrigan JD, Levin HS, et al. Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil. 2009;24:430–438 [DOI] [PubMed] [Google Scholar]
- 61. Boison D, Huber A, Padrun V, et al. Seizure suppression by adenosine-releasing cells is independent of seizure frequency. Epilepsia. 2002;43:788–796 [DOI] [PubMed] [Google Scholar]
- 62. Boison D. Methylxanthines, seizures and excitotoxicity. Handb Exp Pharmacol. 2010;200:251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Williams-Karnesky RL, Sandau US, Lusardi TA, et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Inv. 2013;123:3552–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jeremitsky E, Omert L, Dunham CM, et al. Harbingers of poor outcome the day after severe brain injury: Hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54:312–319 [DOI] [PubMed] [Google Scholar]
- 65. The Brain Trauma Foundation, The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Resuscitation of blood pressure and oxygenation. J Neurotrauma. 2000;17:471–478 [DOI] [PubMed] [Google Scholar]
- 66. Gabriel E, Ghajar J, Jagoda A, et al. Guidelines for Prehospital Management of Traumatic Brain Injury. New York, NY: Brain Trauma Foundation; 2000 [DOI] [PubMed] [Google Scholar]
- 67. Varma M, Dixon C, Jackson E, et al. Administration of adenosine receptor agonists or antagonists after controlled cortical impact in mice: Effects on function and histopathology. Brain Res. 2002;951:191. [DOI] [PubMed] [Google Scholar]
- 68. Dash PK, Moore AN, Moody MR, et al. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma. 2004;21:1573–1583 [DOI] [PubMed] [Google Scholar]
- 69. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222 [DOI] [PubMed] [Google Scholar]
- 70. Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761–845 [DOI] [PubMed] [Google Scholar]
- 71. Schindler CW, Karcz-Kubicha M, Thorndike EB, et al. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. Br J Pharmacol. 2005;144:642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Conlay LA, Evoniuk G, Wurtman RJ. Endogenous adenosine and hemorrhagic shock: Effects of caffeine administration or caffeine withdrawal. Proc Natl Acad Sci U S A. 1988;85:4483–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cummings J, Kaplan JL, Gao E, et al. Antagonism of the cardiodepressant effects of adenosine during acute hypoxia. Acad Emerg Med. 2000;7:618–624 [DOI] [PubMed] [Google Scholar]