Abstract
Activity-based therapy is routinely integrated in rehabilitation programs to facilitate functional recovery after spinal cord injury (SCI). Among its beneficial effects is a reduction of hyperreflexia and spasticity, which affects ∼75% of the SCI population. Unlike current anti-spastic pharmacological treatments, rehabilitation attenuates spastic symptoms without causing an active depression in spinal excitability, thus avoiding further interference with motor recovery. Understanding how activity-based therapies contribute to decrease spasticity is critical to identifying new pharmacological targets and to optimize rehabilitation programs. It was recently demonstrated that a decrease in the expression of KCC2, a neuronal Cl– extruder, contributes to the development spasticity in SCI rats. Although exercise can decrease spinal hyperexcitability and increase KCC2 expression on lumbar motoneurons after SCI, a causal effect remains to be established.
Activity-dependent processes include an increase in brain-derived neurotrophic factor (BDNF) expression. Interestingly, BDNF is a regulator of KCC2 but also a potent modulator of spinal excitability. Therefore, we hypothesized that after SCI, the activity-dependent increase in KCC2 expression: 1) functionally contributes to reduce hyperreflexia, and 2) is regulated by BDNF. SCI rats chronically received VU0240551 (KCC2 blocker) or TrkB-IgG (BDNF scavenger) during the daily rehabilitation sessions and the frequency-dependent depression of the H-reflex, a monitor of hyperreflexia, was recorded 4 weeks post-injury. Our results suggest that the activity-dependent increase in KCC2 functionally contributes to H-reflex recovery and critically depends on BDNF activity. This study provides a new perspective in understanding how exercise impacts hyperreflexia by identifying the biological basis of the recovery of function.
Keywords: chloride homeostasis, KCC2, neuroplasticity, rehabilitation, spinal cord injury
Introduction
Activity-based therapies promote sensorimotor recovery after spinal cord injury (SCI) and are routinely integrated in rehabilitation programs in the clinic. Among its beneficial effects is a decrease in spasticity that reduces incapacitating symptoms and significantly improves the quality of life of individuals with SCI.1,2 Spasticity is a debilitating condition affecting ∼75% of persons with SCI.3–5 Currently available anti-spastic drugs have serious side effects (sedation, dizziness, deep long-lasting depression of spinal excitability) that significantly reduce muscle activity and interfere with motor recovery.6–9 There is therefore a critical need to identify new targets to diminish spasticity without hindering motor output.
Evidence-based clinical practice highlights the beneficial effect of rehabilitation programs on spastic symptoms,1,2 suggesting the involvement of a potent activity-dependent mechanism. To date, the most compelling findings indicate a critical role for brain-derived neurotrophic factor (BDNF) in promoting activity-dependent plasticity. BDNF is released in an activity-dependent manner10 and its contribution to functional plasticity in the healthy and injured spinal cord has been extensively studied.11,12 Exercise increases BDNF serum levels in individuals with SCI.13 While the consequences of this activity-dependent increase remains to be determined in humans, experiments performed in animal models suggest that it is associated with an enhanced response of lumbar motor pools to descending drive, normalization of motoneuronal electrophysiological properties, reduction in allodynia, and improvement in spinal reflex modulation and locomotor recovery.14–17
BDNF acts as a regulator of the K+-Cl– co-transporter KCC2 in various disease models including neuropathic pain and hyperalgesia.18–22 After chronic SCI, KCC2 is downregulated in spinal neurons causing an increase in intracellular chloride concentration ([Cl–]i). Consequently, gamma-aminobutyric acid (GABA)-mediated responses become less hyperpolarizing, and lead to a reduction in post-synaptic inhibition and increased spinal reflex excitability.23–25 Exercise increases both BDNF and KCC2 levels in the lumbar spinal cord after a chronic SCI in animals that display less spasticity and better reflex modulation.17,26 Although it is generally accepted that activity-based therapies increase KCC2 expression in lumbar motoneurons,26–28 the potential contribution to functional recovery remains to be determined.
Mechanisms of activity-dependent regulation of KCC2 and subsequent shift in EGABA include TrkB activation by BDNF.29–32 In the hippocampus, the regulation of KCC2 following neonatal status epilepticus is dependent on BDNF and accompanied by increased KCC2 expression, enhanced neuronal Cl– extrusion, and hyperpolarized EGABA.33,34 We therefore hypothesized that the beneficial effect of rehabilitation on the modulation of spinal reflexes relies on a BDNF-dependent increase in KCC2 expression in motoneurons and the restoration of endogenous inhibition.
Adult rats received a spinal transection (T12), and were exercised on motorized bikes for 4 weeks. During this exercise period, animals were treated with the specific KCC2 blocker VU0240551 or TrkB-IgG to chelate BDNF. During a terminal experiment, H-reflexes were recorded and analyzed as a measure of hyperreflexia and spasticity. Our data illustrate that preventing KCC2 activity during exercise impedes reflex recovery after SCI and that the upregulation of KCC2 expression triggered by exercise requires BDNF to restore reflex modulation. These results strongly suggest the presence of activity-dependent mechanisms involved in the regulation of chloride co-transporters in the spinal cord and demonstrate their involvement in functional recovery after SCI. This further lends support for chloride co-transporters as effective targets to improve motor recovery after SCI.
Methods
Experimental design
In an SCI rat model of complete thoracic transection injury (T12), we investigated the role of KCC2 in motor recovery after a chronic SCI and its dependence on the activity-dependent regulation of BDNF in the lumbar spinal cord by using VU0240551, a selective inhibitor of KCC2, or chelating the endogenously released BDNF with the fusion protein TrkB-IgG. Both were delivered intrathecally during the rehabilitation program. Rats were randomly assigned to one of the following groups: 1) intact (n = 11); 2) SCI sedentary control rats receiving the vehicle (SCI, n = 14); 3) SCI rats receiving the KCC2 blocker VU0240551 (SCI+VU0240551, n = 8); 4) SCI rats receiving the BDNF scavenger TrkB-IgG (SCI+TrkB-IgG, n = 9); 5) SCI bike-trained rats receiving the vehicle (SCI+Ex, n = 14); 6) SCI bike-trained rats receiving VU0240551 (SCI+Ex+VU0240551, n = 14); and 7) SCI-bike-trained rats receiving TrkB-IgG (SCI+Ex+TrkB-IgG, n = 11). Hyperreflexia was assessed 4 weeks after SCI and the spinal cord tissue was harvested for immunohistochemistry or western blot analysis.
All procedures complied with Animal Research: Reporting of In Vivo Experiments (ARRIVE), were conducted in compliance with the guidelines of the National Institutes of Health for the care and use of laboratory animals, and were approved by Drexel University Institutional Animal Care and Use Committee.
Surgical procedures and postoperative care
Adult female Sprague-Dawley rats (225–250 g, Charles Rivers Laboratories) were used in this study and the time line for procedures is illustrated in Figure 1A. Rats were housed by pairs in cages in a 12-h light-dark cycle and controlled room temperature with ad libitum access to food. All animals but the intact group underwent a complete spinal cord transection at T12 under aseptic conditions as described previously.26
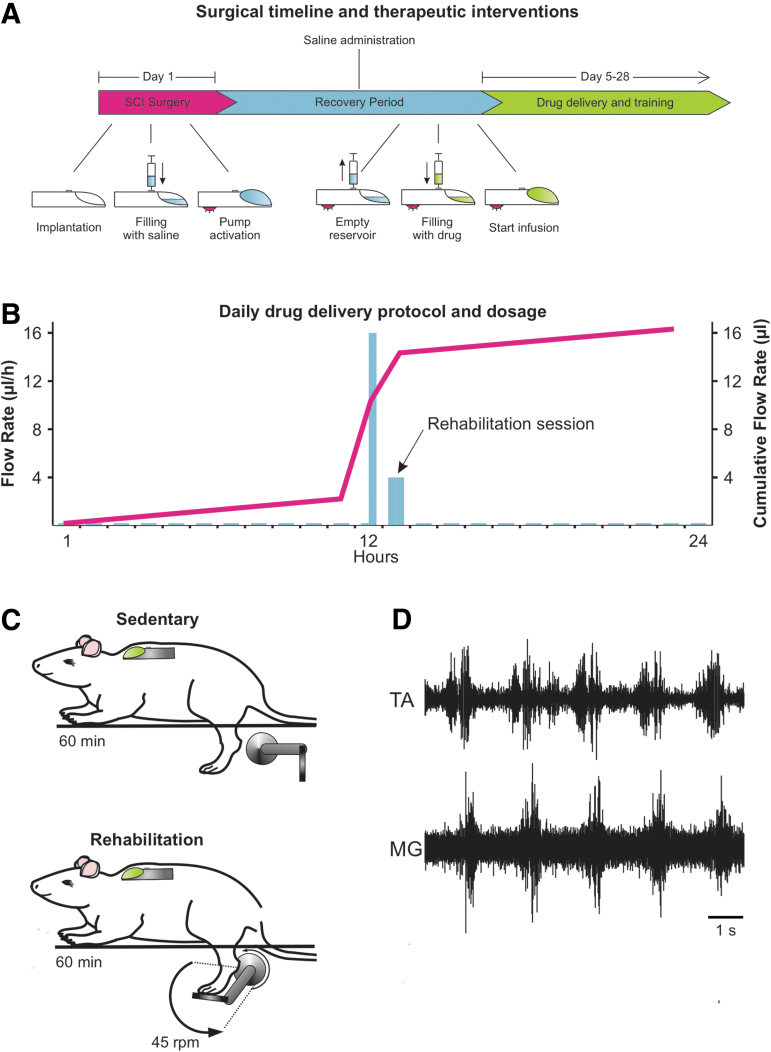
FIG 1.
Experimental time line. (A) The surgical procedure took place on day 1. During the procedure, a complete SCI was performed at the thoracic level (T12). An intrathecal catheter was also implanted subdurally and fitted to a programmable microinfusion pump (iPrecio©) to deliver drugs to the lumbar enlargement of the spinal cord. During the post-SCI recovery period (∼4 days), saline was continuously delivered at a steady flow rate (3 μL/h) to prevent blockage of the tip of the cannula. The iPrecio© Management System calculates the time at which the reservoir needs to be emptied from saline and re-filled with drug so as to reach the tip of the cannula 30 min prior to the first rehabilitation session. The saline remaining in the reservoir was then replaced through the subcutaneous port with the vehicle (10% DMSO in saline) for SCI controls, VU0240551 (30 μM) or TrkB-IgG (10 μg/mL in phosphate-buffered saline). (B) The pumps were programmed on a variable 24-h delivery schedule. Delivery started 30 min before the exercise session at a flow rate of 16 μL/h for 30 min followed by a 4 μL/h maintenance dose during the 60-min training session. Drug delivery was tapered off during the remaining 22.5 h to 0.2 μL/h, the lowest delivery flow to prevent clogging of the catheter. (C) Exercised groups received a 60-min bicycling session during which the hindlimbs go through a complete range of motion at a rate of 45 rpm. Sedentary animals are also seated on the apparatus for 60 min. (D) Example of EMG activity recorded during cycling. DMSO, dimethyl sulfoxide; EMG, electromyogram; SCI, spinal cord injury.
Briefly, rats were anesthetized with isoflurane (1–4%) and their backs were shaved, cleansed, and disinfected. Body temperature was maintained at ∼37°C throughout the surgical procedure and post-surgical recovery. A laminectomy (T10–T11) was performed, the dura slit opened, and a 2-mm cavity created by aspiration. A second laminectomy was also performed (L2) and the proximal end of an intrathecal catheter (Alzet®, Durect Corporation) was threaded rostrally in the subdural space over an ∼10 mm distance. The catheter was secured and glued onto a dry, clean transverse process and the distal end coupled to a programmable microinfusion pump (iPrecio©, Durect Corporation).35 The pump reservoir was filled with saline and the drug delivery program activated. Back muscles were sutured in layers leaving the distal end of the catheter exiting between two sutures. The pump was positioned subcutaneously (s.c.) just below the shoulder blades and sutured to back muscles. The remaining portion of tubing was coiled for stress relief and the skin closed with staples. The completeness of the lesion was recognized by the retraction of the rostral and caudal portions of the spinal cord and inspection of the ventral floor of the spinal canal during the surgery and confirmed post-mortem. The animals were given saline (5 mL/day s.c. for 3 days) to avoid dehydration, prophylactic cefazolin (160 mg/kg/day s.c. for 7 days) and slow-release buprenorphine (0.05 mg/kg, s.c., single dose) as an analgesic for pain control. Bladders were expressed manually twice a day until the end of the study.
Drug delivery
KCC2 activity was blocked using VU0240551 (Sigma-Aldrich). VU0240551 was re-suspended in dimethyl sulfoxide (DMSO) as a 50-mM stock solution, and was later diluted in saline to the appropriate concentration. Recombinant human TrkB Fc chimera protein (TrkB-IgG; R&D Company), used to chelate the endogenously released BDNF, was diluted in phosphate-buffered saline (PBS). Parameters for drug delivery (dose, volume, time to reach maximal effect at the time of training) were consistent with previous reports.28,36–38 The specific delivery protocol and dosage are described in Figure 1B.
Rehabilitation program
Beginning on day 5, exercised groups received a 60-min bicycling session. Animals were seated in a support harness with the hindlimbs hanging while the feet were secured to pedals with surgical tape (Fig. 1C). The custom-built motor-driven apparatus moves the hindlimbs through a complete range of motion during pedal rotation at a rate of 45 rpm17,26,39 and triggers rhythmic activity in flexors and extensors (Fig. 1D).39 The rehabilitation program took place 5 days a week until completion of the study.
Electrophysiological recordings and analysis
H-reflex recordings were performed 4 weeks post-SCI as described previously.17,26 Rats were anesthetized with ketamine (60 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally (i.p.). The tibial nerve was dissected free and mounted on a bipolar hook electrode for stimulation. Skin flaps were used to form a pool of mineral oil to prevent desiccation of the nerve. Bipolar wire electrodes (Cooner Wire) were inserted into the interosseus muscles for electromyogram (EMG) recordings and the ground electrode inserted into the skin of the back. H-reflexes were evoked via an isolated pulse stimulator (A-M Systems) that delivered single bipolar pulses (100 μsec) to the tibial nerve, and H- and M-waves were recorded in the interosseus muscle in response to a range of increasing stimulus intensities. The intensity that elicited the maximal H-reflex amplitude (below the activation threshold for group Ib–II afferents, ∼1.2–1.4 MT) was then used to determine the properties of the M-wave and H-reflex as well as to evoke frequency-dependent depression (FDD). FDD was estimated using three series of 20 consecutive stimulations delivered at 0.3, 5, and 10 Hz. The experiment was first completed on the left leg, and the protocol then was performed on the right leg.
EMG recordings were amplified (A-M Systems) and bandpass filtered (10–5 kHz), and the signal was digitized (10 kHz) using a 1401 interface (Cambridge Electronic Design, CED) and fed to a computer running Signal 5 software (CED). Properties of the M-wave and H-reflex (n = 8–13 animals/group, Table 1) are presented as mean ± standard error of the mean (SEM). For the analysis of the FDD, the first five responses to a train of stimulation were discarded to allow reflex stabilization. The 15 remaining responses were then averaged. Peak-to-peak amplitude of the M and H responses were measured and the H-reflex amplitude was normalized to Mmax. The change in H-reflex peak-to-peak response at 5 Hz and 10 Hz was calculated as a percentage of the response obtained at 0.3 Hz. FDD data (n = 8–13 animals/group) are presented as mean ± SEM.
Table 1.
Properties of the M-Wave and H-Reflex: Latency, Amplitude, and Threshold
| |
N = |
Motor threshold |
Mmax |
Hmax |
M latency |
H latency |
H-reflex threshold |
Hmax |
|---|---|---|---|---|---|---|---|---|
| (mA) | (mV) | (mV) | (msec) | (msec) | (x MT) | (xMT) | ||
| Intact | 11 | 0.013 ± 0.003 | 9.71 ± 1.40 | 3.38 ± 0.78 | 2.46 ± 0.11 | 9.35 ± 0.50 | 1.76 ± 0.19 | 1.76 ± 0.19 |
| SCI | 11 | 0.019 ± 0.002 | 6.79 ± 0.53 | 2.71 ± 0.34 | 2.38 ± 0.08 | 8.84 ± 0.18 | 0.94 ± 0.02*** | 1.38 ± 0.13 |
| SCI + Ex | 9 | 0.017 ± 0.002 | 5.22 ± 0.57 | 2.29 ± 0.34 | 2.19 ± 0.08 | 8.80 ± 0.16 | 1.09 ± 0.05 | 1.49 ± 0.08 |
| SCI + VU0240551 | 8 | 0.019 ± 0.004 | 7.77 ± 1.33 | 2.59 ± 0.68 | 2.22 ± 0.08 | 8.65 ± 0.19 | 1.11 ± 0.11* | 1.58 ± 0.08 |
| SCI + Ex + VU0240551 | 13 | 0.019 ± 0.003 | 7.64 ± 0.99 | 3.96 ± 0.65 | 2.50 ± 0.09 | 8.69 ± 0.18 | 1.10 ± 0.04* | 1.37 ± 0.08 |
| SCI + TrkBIgG | 9 | 0.018 ± 0.003 | 6.77 ± 0.58 | 2.13 ± 0.31 | 2.49 ± 0.06 | 8.95 ± 0.13 | 1.05 ± 0.05* | 1.52 ± 0.08 |
| SCI + Ex + TrkBIgG | 11 | 0.024 ± 0.005 | 6.59 ± 1.25 | 3.35 ± 0.75 | 2.37 ± 0.08 | 8.84 ± 0.17 | 0.99 ± 0.04** | 1.42 ± 0.18 |
P < 0.001, **p < 0.01, *p < 0.05 vs. intact.
Values are mean/±/SEM.
MT, motor threshold, SCI, spinal cord injury; SEM, standard error of the mean.
After completion of the terminal experiment, rats were overdosed with Euthasol (390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin, i.p.) and the animal was either transcardiacally perfused with cold saline followed by 4% paraformaldehyde in PBS or fresh tissue was extracted for western blotting.
Extraction of fresh tissue and western blotting
Blocks of spinal tissue (L4–L5) were lysed in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris buffer pH 6.8, 1% Triton-X, 0.1% sodium dodecyl sulfate [SDS], 1 mM dithiothreitol [DTT], 0.5% deoxycholate, 150 mM NaCl) containing protease and phosphatase inhibitors (Roche), 2 mM phenylmethylsulphonyl fluoride (PMSF), and 1 mM NaF. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce). Samples (30 μm of total protein) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (10–16% gels for BDNF, 4–20% gradient TGX gels [Biorad] for KCC2/NKCC1). Antibodies were targeted against KCC2 (1:1000, Millipore, 07-4432), BDNF (1:1000; Santa Cruz Biotechnologies, sc-546), or mouse T4 monoclonal antibody against NKCC (1:1000, Developmental Studies Hybridoma Bank), followed by incubation with HRP-conjugated secondary antibody (Jackson ImmunoResearch). The optical densities of protein bands corresponding to BDNF (18 to 27 kDa), KCC2 (∼140 kDa), and NKCC1 (145 kDa) were further quantified using Image J.17,26 Values for each sample were averaged, normalized to actin, and combined for each group. Western blot data (n = 6–11/group) is presented as a ratio of the intact group.
Tissue preparation and immunohistochemistry
Spinal cord tissue was post-fixed overnight at 4°C, transferred to 30% sucrose for cryoprotection, and cut transversally (40 μm sections) using a cryostat. Free-floating sections were permeated in PBS containing 5% donkey serum and 0.1% Triton X-100 for 1 h at room temperature and were incubated with antibodies targeted against KCC2 (1:1000; Millipore) and ChAT (1:100, Millipore) for 24 h. After brief washes in PBS, spinal cord sections were incubated in secondary antibody conjugated to fluorescein isothiocynate (FITC) and rhodamine (1:400; Jackson ImmunoResearch) for 2 h at room temperature.
Stacked images of motoneurons (40 μm sections) were acquired with a spectral confocal and multi-photon system (Leica TCS SP2). Only motoneurons showing a clear nucleus were scanned. The fluorescence intensity of KCC2 immunolabeling on the plasma membrane of motoneurons (identified by ChAT+, typical large size, and location within the ventral horn) was measured by averaging the integrated area of the density curve obtained by drawing three lines across each motoneurons (six data points) using ImageJ software.26,40 A minimum of 15 lumbar motoneurons were averaged per group.
Statistical analysis
Significant differences for the properties of the M-wave and H-reflex as well as immunoblotting data were determined by either using a one-way analysis of variance (ANOVA) followed by the Holm-Sidak multiple comparisons post hoc test, or a Kruskal-Wallis one-way ANOVA on ranks followed by Dunn's method if the sample variables did not fit a normal distribution (Shapiro-Wilk) or were not equally variant (Brown-Forsythes). Significant effects on the amplitude of the H-reflex by stimulation frequency and treatment group for the FDD were determined by a two-way ANOVA followed by the Holm-Sidak post hoc test, and the possible interaction of these factors with the variable was determined. Linear regression analysis was used to correlate NKCC1 to KCC2 and KCC2 to BDNF protein levels obtained by western blotting. All data are presented as mean ± SEM. Statistical analysis was performed using Sigma Plot software 14.0 and statistically significant levels were set to p < 0.05.
Results
KCC2 activity is required for H-reflex recovery after chronic SCI
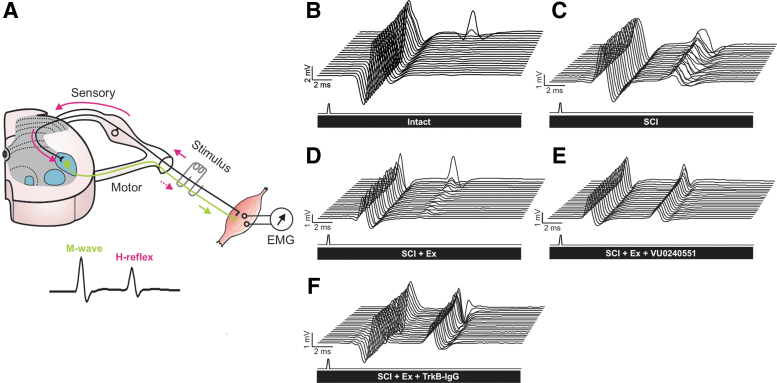
SCI induces a reduction in KCC2 expression in the lumbar enlargement of the spinal cord that is associated with the establishment and maintenance of spasticity after SCI.24 Our earlier findings illustrate that motor training prevents the injury-induced decrease in KCC2 expression on lumbar motoneurons and reduces spasticity,26 but a causal relationship remained to be established. We therefore hypothesized that the decrease in hyperreflexia triggered by exercise requires KCC2 activity in spinal motoneurons. We developed an intrathecal catheter delivery method to assess the effect of pharmacological inhibition of KCC2 on activity-dependent H-reflex recovery after SCI. KCC2 activation was specifically prevented/decreased during the daily rehabilitation sessions (Fig. 1B) for 4 weeks post-SCI, at which time the properties of the H-reflex as well as its modulation were assessed. Because of its monosynaptic nature, the location of the neuroplasticity triggered by the injury and/or inhibition is limited to primary afferent from group Ia fibers, α-motoneuron, and their synaptic connection. In response to a tibial nerve stimulation, two successive responses can be recorded from the interosseous muscle: the M-wave results from the direct activation of motor axons, whereas the H-reflex is evoked by the activation of Ia afferents that form a monosynaptic connection with motoneurons (Fig. 2A).
FIG. 2.
Representative recordings of H-reflexes evoked by a train of stimulation to the tibial nerve in the interosseus muscle. (A) The stimulation of the tibial nerve evokes a volley in Ia afferents (solid pink arrows) that monosynaptically excite alpha motoneurons. The M-wave (green arrow) precedes the H-reflex (dotted pink arrow) and is due to the direct activation of motor axons. (B–F) Typical EMG recordings over a series of 20 stimulations to the tibial nerve illustrating that during a 10-Hz stimulation train, the depression of the H-reflex is impaired after SCI (C) as compared with intacts (B) but is substantially restored in exercised animals (D). However, the exercised groups that received VU0240551 (E) or TrkB-IgG (F) during the daily rehabilitation session exhibited a very modest depression as compared with exercised animals. Overall, blocking KCC2 or BDNF activity in exercised animals (E–F) yields responses similar to non-exercised SCI (C). EMG, electromyogram; SCI, spinal cord injury.
The recruitment curve was used to determine the amplitude of the maximal M-wave (Mmax), the response of all motor units with supramaximal stimulation of axons of the tibial nerve, and of the maximal H-reflex amplitude (Hmax). To assess the relative proportion of motoneurons recruited through the monosynaptic reflex loop versus the activation of the entire motor pool, the Hmax/Mmax ratio was also calculated. The Hmax/M ratio was also used to evaluate the relative activation of the motor pool required to reach maximal reflex amplitude. Neither SCI nor VU0240551 affected the M-wave or H-reflex latency, the amplitude of the maximal M-wave (Mmax) and H-reflex (Hmax), the stimulation intensity at which Hmax is evoked, or the stimulation threshold to evoke a M-wave (Table 1). However, rehabilitation prevented the decrease in the stimulation intensity required to evoke a H-reflex after SCI. More importantly, blocking KCC2 activity prevented the effect of exercise with H-reflex thresholds similar to unexercised SCI but significantly lower than intact. Together, these results suggest that the decrease in H-reflex excitability triggered by exercise after a chronic SCI requires KCC2 activity.
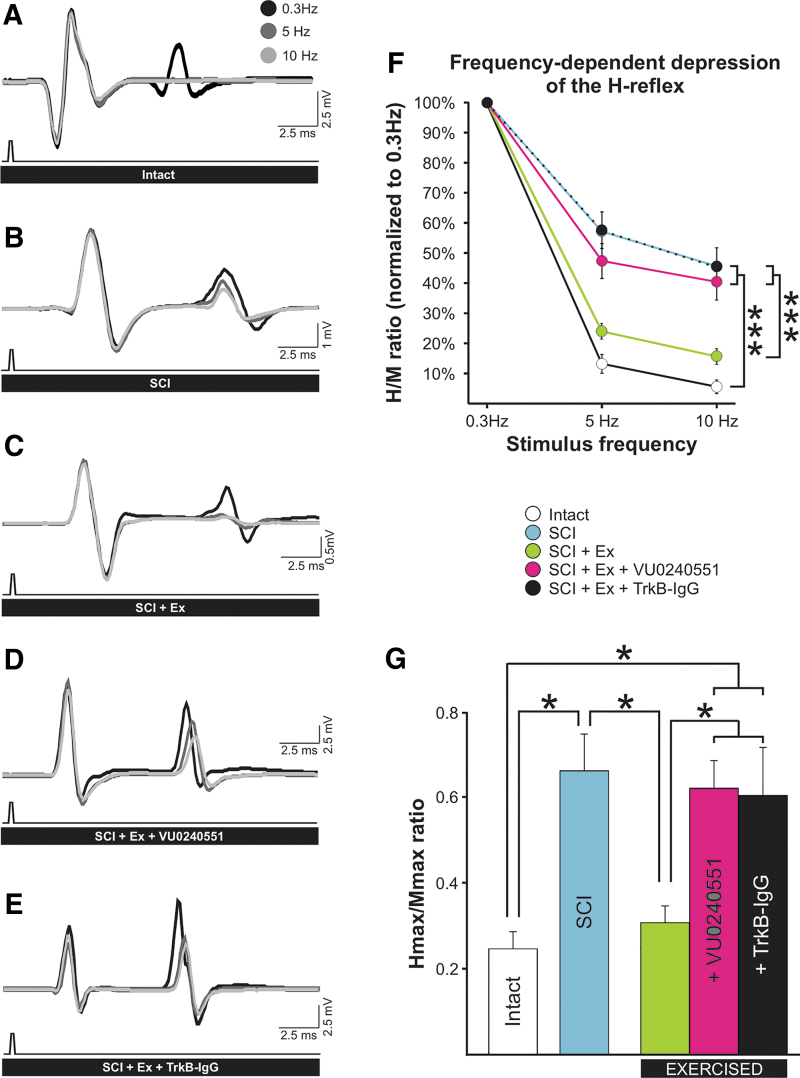
After SCI, the excitability of the spinal cord gradually increases as a result of the injury. The transition to a state of hyperreflexia is well established by 1 month after SCI41,42 and the reduction in the low frequency-dependent depression (FDD) of the H-reflex is widely accepted as a reliable correlate of spasticity.24,43,44 The improvement in FDD observed in exercised animals after SCI is characterized by the presence of a clear depression following a series of stimulations at 10Hz (Fig. 2D) that is similar to intact animals (Fig. 2B), while the depth of the modulation is much shallower in an animal not undergoing a rehabilitation program (Fig.2C). VU0240551 prevented the activity-dependent recovery triggered by exercise and the FDD was very modest (Fig. 2E). If some level of H-reflex modulation is still present after SCI regardless of exercise or VU0240551, as all groups displayed a decrease in H-reflex amplitude as the stimulation frequency increased, there was a remarkable difference in the depth of modulation across groups (Fig. 3A–E). Figure 3F shows the averaged FDD for each group at each frequency expressed as a percentage of the response at 0.3 Hz, a frequency at which there is no or little depression of the H-reflex.
FIG. 3.
The activity-dependent recovery of the FDD in exercised animals is prevented by blocking KCC2 activity or scavenging BDNF after SCI. (A–E) Averaged H-reflex traces (n > 15) evoked by the stimulation of the tibial nerve at 0.3 Hz (black), 5 Hz (dark gray), and 10 Hz (light gray). Increasing stimulus frequency from 0.3 Hz to 10 Hz decreased H-reflex amplitude in all animal groups. (F) There was a statistically significant difference across stimulation frequency (p < 0.001) and across experimental groups (p < 0.001), and the interaction between frequency and groups was also significant (p < 0.001). H-reflexes were significantly smaller at 5 Hz and 10 Hz as compared with 0.3 Hz in all groups. The FDD drastically decreased after SCI (blue; see also B) with the amplitude of the H-reflex increasing from 13 ± 3% in intact animals to 57 ± 7% in SCI at 5 Hz (p < 0.001) and from 6 ± 2% to 46 ± 6% at 10 Hz (p < 0.001). Rehabilitation prevented this decrease with values not significantly different from uninjured animals at 5Hz (24 ± 2%; p = 0.358) and 10 Hz (6 ± 2%; p = 0.653) (green; see also C), but displayed considerably more depression than SCI at both stimulation frequencies (p < 0.001).
VU0240551 prevented the recovery of FDD (pink; see also D) observed in exercised animals. SCI+Ex+VU0240551 had FDDs similar to SCI at 5 Hz (47 ± 6%; p = 0.317) and 10 Hz (40 ± 6%; p = 0.650), but the depression was significantly lower than in exercised animals in which KCC2 activity was not blocked (p = 0.002 and p < 0.001 respectively). Scavenging BDNF also prevented the activity-dependent recovery of the FDD (black; see also E) at 5 Hz and 10 Hz (p < 0.001) with values similar to sedentary SCI controls at 5 Hz (p = 0.951) and 10 Hz (p = 0.986) and SCI+Ex+VU0240551 (p = 0.309 and p = 0.778, respectively). Data are presented as mean ± SEM expressed as a percentage of the response obtained at 0.3 Hz. ***P < 0.001. Two-way ANOVA analysis with Holm-Sidak post hoc test, n = 9–11 animals/group. (G) The Hmax/Mmax ratio was also significantly different across groups (p < 0.001). Post hoc analysis revealed that SCI increases the Hmax/Mmax ratio (p < 0.027). Exercise restored Hmax/Mmax, which was lower than SCI values (p = 0.045) but similar to intact (p = 0.986). VU0240551 prevented the activity-dependent decrease in the Hmax/Mmax, which was not different from SCI (p = 0.980) but were significantly higher than exercised SCI (p = 0.041) and intact (p = 0.021). Scavenging BDNF also prevented the activity-dependent decrease in the Hmax/Mmax ratio (p = 0.033) with values similar to SCI (p = 0.974) and SCI+Ex+VU0240551 (p = 0.858). For illustration purposes, significance is illustrated for 10 Hz only. Hmax/Mmax ratios are presented as mean ± SEM. *P < 0.05. One-way ANOVA analysis with Holm-Sidak post hoc test (n = 9–11 animals/group). ANOVA, analysis of variance; BDNF, brain-derived neurotrophic factor; FDD, frequency-dependent depression; SCI, spinal cord injury; SEM, standard error of the mean.
Higher H-reflex amplitude depicts less depression of the reflex. A two-way ANOVA revealed statistically significant differences across stimulation frequency (F[4,215] = 284.923, p < 0.001) and across experimental groups (F[4,215] = 17.183, p < 0.001) with a significant interaction between frequency and groups (F[8,215] = 4.393, p < 0.001). Post hoc comparisons suggested that 5 and 10 Hz values were different from 0.3 Hz values in all groups (p < 0.001). The FDD drastically decreased after SCI with the amplitude of the H-reflex increasing from 13 ± 3% to 57 ± 7% at 5 Hz and from 6 ± 2% to 46 ± 6% at 10 Hz. Rehabilitation prevented this decrease with values not significantly different from uninjured animals at 5 Hz and 10 Hz (24 ± 2% and 6 ± 2%, respectively), but considerably lower (i.e., more depression) than SCI. Blocking KCC2 prevented the recovery of FDD observed in exercised animals. VU0240551 significantly decreased the depression observed in exercised animals, which had a FDD similar to SCI at 5 Hz and 10 Hz (47 ± 6% and 40±6%). These results suggest that exercise failed to return H-reflex modulation after a chronic SCI when KCC2 could not be activated during training.
The Hmax/Mmax ratio, which estimates the fraction of motoneurons recruited relative to the activation of the entire motor pool, was also significantly different across groups (F[4,52] = 5.234, p < 0.001). Blocking KCC2 during rehabilitation sessions prevented the activity-dependent decrease in the Hmax/Mmax ratio with SCI+Ex+VU0240551 animals displaying similar values to SCI but significantly higher than exercised and intact (Fig. 3G). This further supports that KCC2 activity is required for the activity-dependent decrease in spinal hyperexcitability.
BDNF activity is required for H-reflex recovery after chronic SCI
Because BDNF-TrkB signaling is known as a potent regulator of KCC2 in neurons,18–20,45 we sought to further consider a causal relationship between the upregulation of BDNF and KCC2. If such a link exists after chronic SCI, preventing BDNF action, specifically during the training session, should prevent both the increase in KCC2 expression and the exercise-dependent recovery of the H-reflex modulation. We first examined the effect of the BDNF-sequestering TrkB-IgG on H-reflex properties.
Similar to what we observed when blocking KCC2 activity, chelating endogenous BDNF did not modify any features of the H-reflex but for the stimulation threshold to initiate a response (Table 1). TrkB-IgG prevented rehabilitation to restore the stimulation intensity required to initiate an H-reflex close to intact levels, and this group displayed values similar to the SCI group and significantly lower than intact. This suggests that the decrease in H-reflex excitability triggered by exercise after SCI not only requires KCC2 activity, but also BDNF signaling. Scavenging BDNF also prevented the activity-dependent decrease in the Hmax/Mmax ratio with values similar to SCI and SCI+Ex+VU0240551 (Fig. 3G).
Similar to VU0240551 (Fig. 2E), TrkB-IgG prevented the activity-dependent recovery triggered by exercise and the FDD was very modest (Figs. 2F and 3E). Overall, scavenging BDNF significantly prevented the activity-dependent recovery of the FDD (Fig. 3F) (5 and 10 Hz, p < 0.001) and H-reflex amplitude values were no different from unexercised SCI or exercised animals that had received VU0240551. This provides evidence that rehabilitation fails to reduce hyperexcitability in spinal networks after SCI when BDNF activity was prevented. Blocking BDNF or KCC2 activity yielded very comparable results.
Blocking KCC2 or scavenging BDNF after SCI does not affect hyperreflexia in unexercised animals
To exclude any confounding factor and verify that the results observed are not simply due to the effect of the drugs on spinal networks, rather than blocking the exercise-dependent recovery of the H-reflex modulation, we assessed if there was any effect of blocking KCC2 or BDNF activity in unexercised SCI rats. When comparing SCI animals that received VU0240551 or TrkB-IgG with animals that received the vehicle, none of the general features of the M-wave and H-reflex were significantly altered 4 weeks post-SCI (Table 1). These results suggest that, at this dose, drugs alone had no effect on spinal excitability when no rehabilitation program was implemented. Similarly, the modulation of the H-reflex was not different after SCI whether a drug treatment was used or not (not shown). The FDD of SCI animals that received VU0240551 or TrkB-IgG was not significantly different from that of SCI animals receiving the vehicle, with all groups displaying a very modest depression at both 5 Hz (respectively 41 ± 5%, 47 ± 6%, 57 ± 7% %) and 10 Hz (30 ± 5%, 32 ± 5%, 46 ± 6%). This confirms that blocking KCC2 or BDNF activity had no effect after SCI in untrained animals when KCC2 and BDNF levels are already low, and suggests that VU0240551 and TrkB-IgG do not impact motor recovery unless combined with training. No further analysis was carried out on these control groups as the objective of the study was to investigate if KCC2 is critical to the beneficial effect of activity-based therapies.
BDNF signaling is required for the activity-dependent increase in KCC2 expression
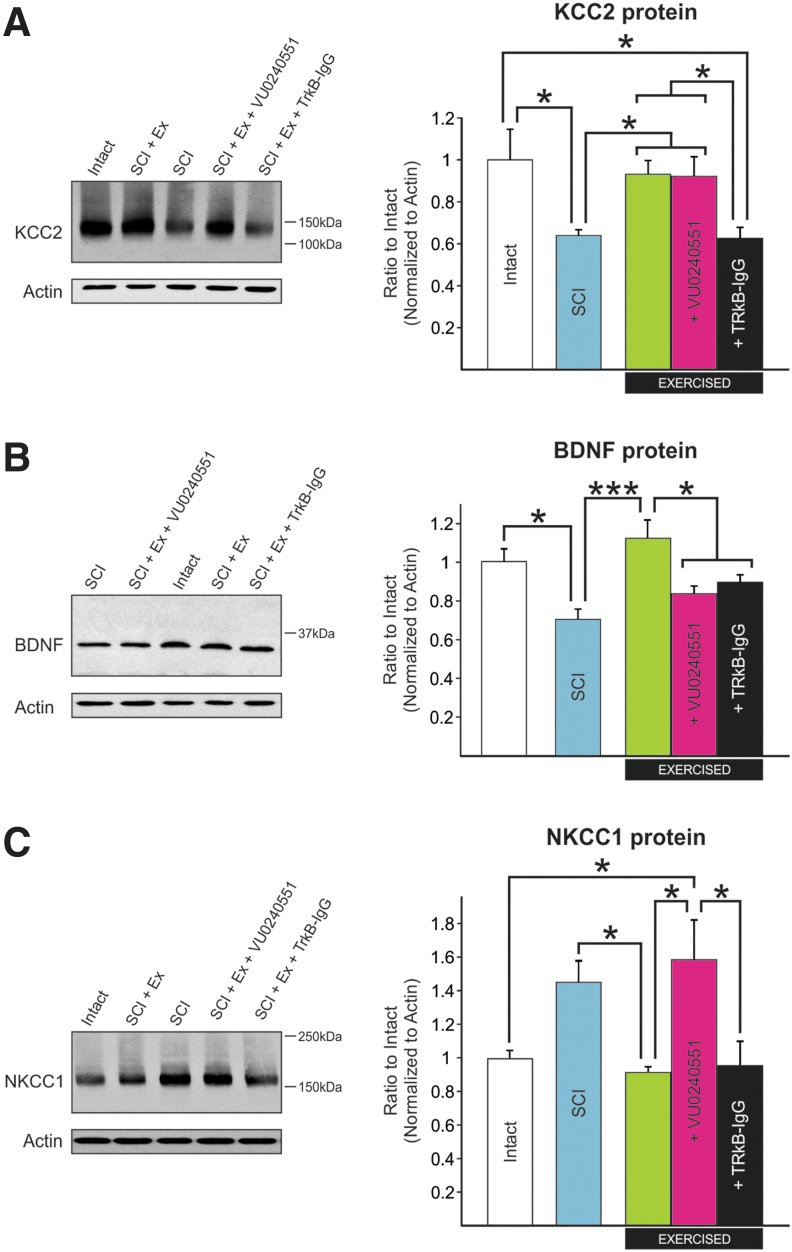
We then performed western blot analysis on tissue from the lumbar enlargement to evaluate the effect of blocking KCC2 or BDNF activity. One-way ANOVA confirmed differences in KCC2 protein expression in the lumbar enlargement across groups (F[4,92] = 5.742, p < 0.001). Further post hoc analysis confirmed earlier reports that KCC2 expression is modulated after SCI,24,26 decreasing to 64 ± 3% of intact values (Fig. 4A). Interestingly, the injury-induced downregulation in KCC2 expression was prevented by the rehabilitation program, whether KCC2 activity was blocked or not, with KCC2 expression levels not different from intact. This suggests that in animals undergoing a rehabilitation program, VU0240551 only affected KCC2 activity, but did not affect its protein synthesis, as KCC2 protein levels were unchanged. On the contrary, scavenging BDNF prevented the activity-dependent increase in KCC2 expression with values similar to unexercised animals (63 ± 5%) and lower than intact or exercised animals, suggesting that BDNF activity is required to restore KCC2 expression induced by exercise.
FIG. 4.
The activity-dependent modulation of KCC2, NKCC1, and BDNF protein expression in the lumbar enlargement of SCI rats requires KCC2 and BDNF activity. Western blot analysis shows that (A) one-way ANOVA confirmed differences in KCC2 protein expression across groups (p < 0.001). Further post hoc analysis showed that KCC2 expression is modulated after SCI, decreasing to 64 ± 3% of intact values (p = 0.015). This decrease was prevented by a rehabilitation program whether KCC2 was blocked (p < 0.034) or not (p < 0.021) as both SCI-Ex and SCI+Ex+VU0240551 displayed KCC2 expression levels similar to intact (93 ± 7%, p = 0.898 and 92 ± 10%, p = 0.944, respectively). Scavenging BDNF prevented the activity-dependent increase in KCC2 expression with values similar to sedentary animals (63 ± 5%, p = 0.989), and lower than intact (p = 0.015) and SCI+Ex (p = 0.022). One-way ANOVA (p < 0.001) with Holm-Sidak post hoc test, n = 6–11/group).
(B) One-way ANOVA analysis confirmed a difference in BDNF protein expression between groups (p < 0.001). The decrease in BDNF expression after a chronic SCI as compared with intact (p = 0.024) was prevented by exercise. SCI-Ex displayed BDNF expression levels higher than SCI (p < 0.001) and similar to intact (p = 0.425). Blocking KCC2 activity prevented the beneficial effect of exercise on BDNF levels with values significantly lower than exercised animals receiving the vehicle (p = 0.011). Scavenging BDNF during training yielded lower levels of BDNF in the lumbar enlargement (p = 0.043). (C) NKCC1 protein expression in the lumbar enlargement was significantly different between groups (p = 0.003). NKCC1 levels tended to increase after SCI. Although this increase did not reach statistical significance (p = 0.068), rehabilitation returned NKCC1 expression levels close to intact (93 ± 4%, p = 0.964) with significantly less NKCC1 in the lumbar enlargement than sedentary SCI animals (147 ± 14%, p = 0.036). Blocking KCC2 prevented the activity-dependent decrease in NKCC1 expression and SCI+Ex+VU0240551 displayed values similar to sedentary SCI animals (161 ± 24%, p = 0.930) but higher than SCI-Ex (p = 0.012). Scavenging BDNF did not prevent the activity-dependent decrease in NKCC1 expression (97 ± 16%, p = 0.969). One-way ANOVA analysis with Holm-Sidak post hoc test (n = 6–11/group). One-way ANOVA analysis (p < 0.001) with Holm-Sidak post hoc test (n = −11/group). Protein levels are presented as mean ± SEM. *P < 0.05, ***p < 0.001 versus intact. ANOVA, analysis of variance; BDNF, brain-derived neurotrophic factor; SCI, spinal cord injury.
One-way ANOVA also confirmed a difference in BDNF protein expression between groups (F[4,66] = 6.120, p < 0.001). Exercise prevents the decrease in BDNF expression after a chronic SCI (Fig. 4B). Animals that followed a rehabilitation program had higher BDNF expression levels as compared with unexercised SCI, and scavenging BDNF during training yielded lower levels. Surprisingly, blocking KCC2 activity prevented the beneficial effect of exercise on BDNF levels with values significantly lower than exercised animals receiving the vehicle, suggesting a mechanism by which KCC2 can regulate BDNF levels.
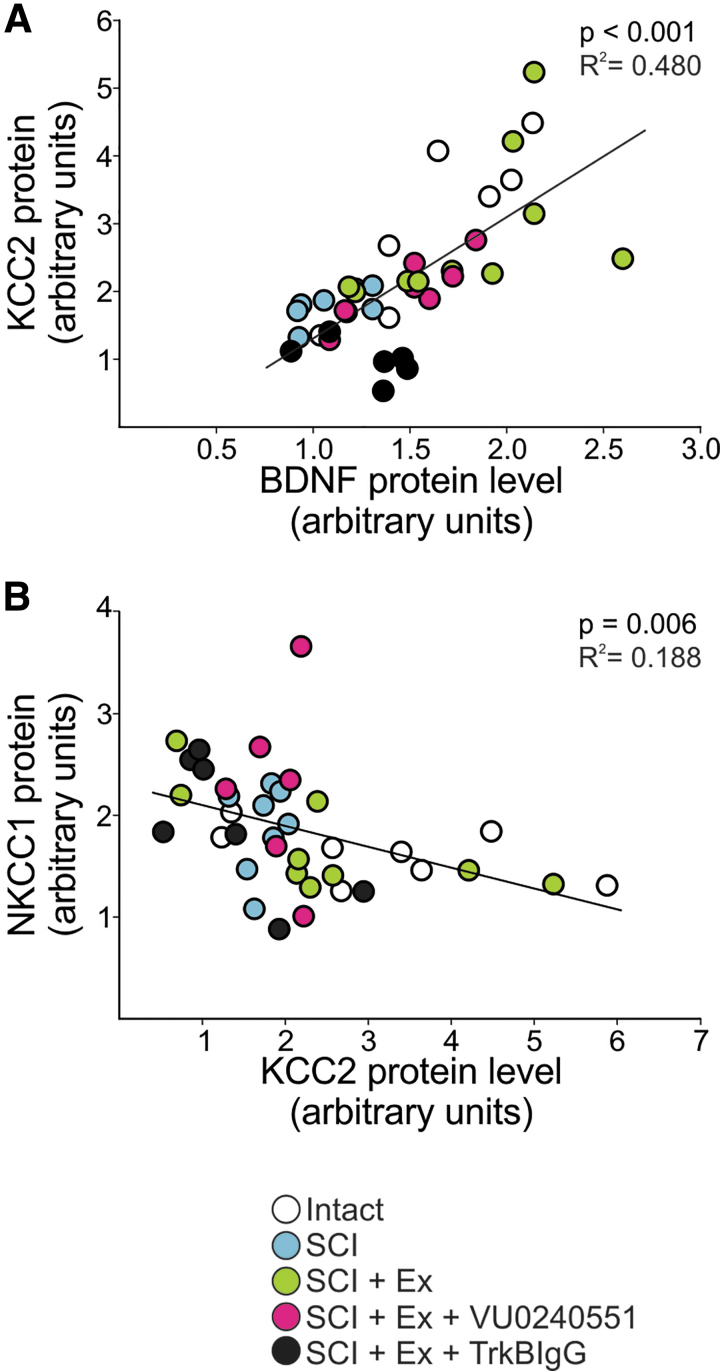
A regression analysis showed that there is a significant linear relationship between BDNF and KCC2 expression in the lumbar enlargement of the spinal cord (F[1,36] = 33.284, p < 0.001) with an R2 of 0.480 (Fig. 5A). Data from SCI animal that received the vehicle or TrkB-IgG are clustered in the lower-left quadrant with low expression levels of both BDNF and KCC2, whereas intact and SCI animals that followed a rehabilitation program are grouped in the top-right quadrant displaying higher levels of both proteins. This further supports a role for BDNF in regulating KCC2.
FIG. 5.
Relationship between the expression of NKCC1, KCC2, and BDNF in the lumbar spinal cord. (A) A linear regression analysis showed that there is a significant positive relationship between BDNF and KCC2 expression level in the lumbar spinal cord (p < 0.001, R2 = 0.480). SCI and SCI+Ex+TrkB-IgG groups are clustered in the lower-left quadrant with low expression levels of BDNF and KCC2, whereas intact and SCI+Ex are grouped in the top-right quadrant displaying higher levels of both proteins. (B) A linear regression analysis illustrates a significant negative relationship between NKCC1 and KCC2 expression level in the lumbar spinal cord (p = 0.006, R2 = 0.188) with low levels of KCC2 expression associated with higher levels of NKCC1. BDNF, brain-derived neurotrophic factor; SCI, spinal cord injury.
Although the shift in chloride homeostasis and impaired FDD after chronic SCI was shown to depend on a decrease in KCC2 expression,24 the inwardly directed Na+-K+-Cl– co-transporter isoform 1, NKCC1, is also upregulated after SCI.46–48 The relative expression of KCC2 and NKCC1 critically determine [Cl–]i and GABA-mediated responses. NKCC1 is responsible to bring Cl– in the cell, in opposition to KCC2, so that a simultaneous decrease in KCC2 and increase in NKCC1 after SCI26 would act synergistically to depolarize ECl-.
We therefore measured changes in NKCC1 protein expression in the lumbar enlargement and found a significant difference between groups (F[4,26] = 5.735, p = 0.002) (Fig. 4C). Rehabilitation returned NKCC1 expression levels close to intact values (93 ± 4%) with significantly less NKCC1 in the lumbar enlargement than unexercised SCI animals (147 ± 14%). VU0240551 prevented the restoration of NKCC1 expression levels following a rehabilitation program with values not different from unexercised SCI animals (161 ± 24%) but higher than exercised. Scavenging BDNF did not affect NKCC1 expression (97 ± 16%). This suggests that NKCC1 is also modulated in an activity-dependent manner after SCI and may contribute to the shift in chloride homeostasis induced by exercise. A regression analysis was also carried out to address the expression of NKCC1 in the lumbar spinal cord as a function of KCC2. We observed a significant negative relationship between NKCC1 and KCC2 expression levels in the lumbar spinal cord (F[1,30] = 8.352, p < 0.001) with an R2 of 0.188 (Fig. 5B). Consistent with their opposing directionality in chloride transport, this supports previous findings suggesting that NKCC1 and KCC2 protein levels are reciprocally regulated. The low, but significant, correlation coefficient suggests that this reciprocal regulation of KCC2 and NKCC1 is perhaps disrupted when drug is delivered as suggested by the vertical clustering of SCI+Ex+VU09240551 in Fig. 5B (pink). However, the statistical power of running a regression analysis of our groups independently is not sufficient to assess the presence/absence of a regression within each group and would warrant further investigation.
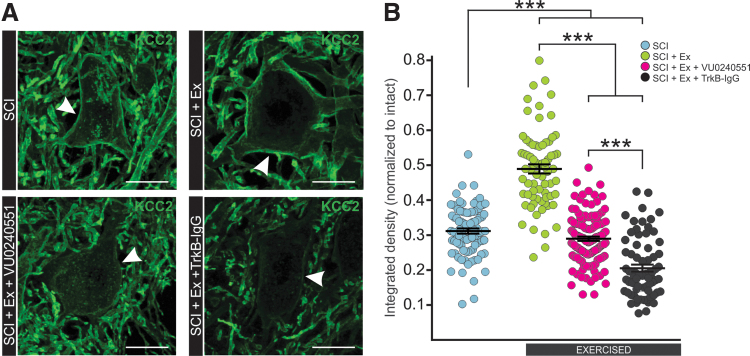
As hyperreflexia and spasticity are specifically associated with a decrease in KCC2 in motoneuronal membrane in the lumbar enlargement,24 we sought to investigate if the BDNF-dependent increase in KCC2 expression involved in activity-dependent recovery occurs at this specific location. In the ventrolateral spinal cord, KCC2 labeling is particularly strong around the motoneuronal membrane (Fig. 6A). A Kruskal-Wallis test revealed differences in KCC2 expression in motoneuronal membranes between groups (H[3, n = 360] = 180.023, p < 0.001). Further post hoc analysis using Dunn's method revealed that exercise-increased KCC2 expression around the motoneuronal membrane and less cytoplasmic clusters are visible in the soma, suggesting a decrease in the internalization of KCC2 into vesicles. Blocking KCC2 or BDNF activity during exercise prevented this effect as the activity-dependent restoration of KCC2 around the motoneuronal membrane was not observed (Fig. 6B).
FIG. 6.
Blocking KCC2 or BDNF activity during exercise prevents the activity-dependent increase in KCC2 levels in lumbar motoneuronal membrane. (A) Digital images showing KCC2 expression in the membrane around the motoneuronal soma (white arrows) and also in dendrites in the ventral horn of the lumbar enlargement. (B) Quantification of the integrated density of KCC2 around the motoneuronal membrane reveals that exercise increases KCC2 immunoreactivity (p < 0.001) and that blocking KCC2 or BDNF activity prevents this activity-dependent increase after SCI (p < 0.001). Data are presented as mean ± SEM. Kruskal-Wallis ANOVA on ranks (p < 0.001) followed by Dunn's method, n > 15 motoneurons/group. ***P < 0.001, scale bar = 25 μm. ANOVA, analysis of variance; BDNF, brain-derived neurotrophic factor; SCI, spinal cord injury.
Discussion
Rehabilitation approaches that promote repetitive motor activity are widely used in the clinic and are a critical component of successful functional recovery in individuals with SCI. Activity-based therapies, interventions that target the repetitive activation of the neuromuscular system below the level of the spinal cord lesion to retrain the nervous system,49,50 have revealed potential to alleviate spasticity, but the molecular pathways involved in functional recovery remain elusive. We show that KCC2 activity is required for the beneficial effect of exercise on spasticity and relies on BDNF activity-dependent plasticity.
Functional relevance of KCC2 to activity-based therapy-induced decrease in spasticity
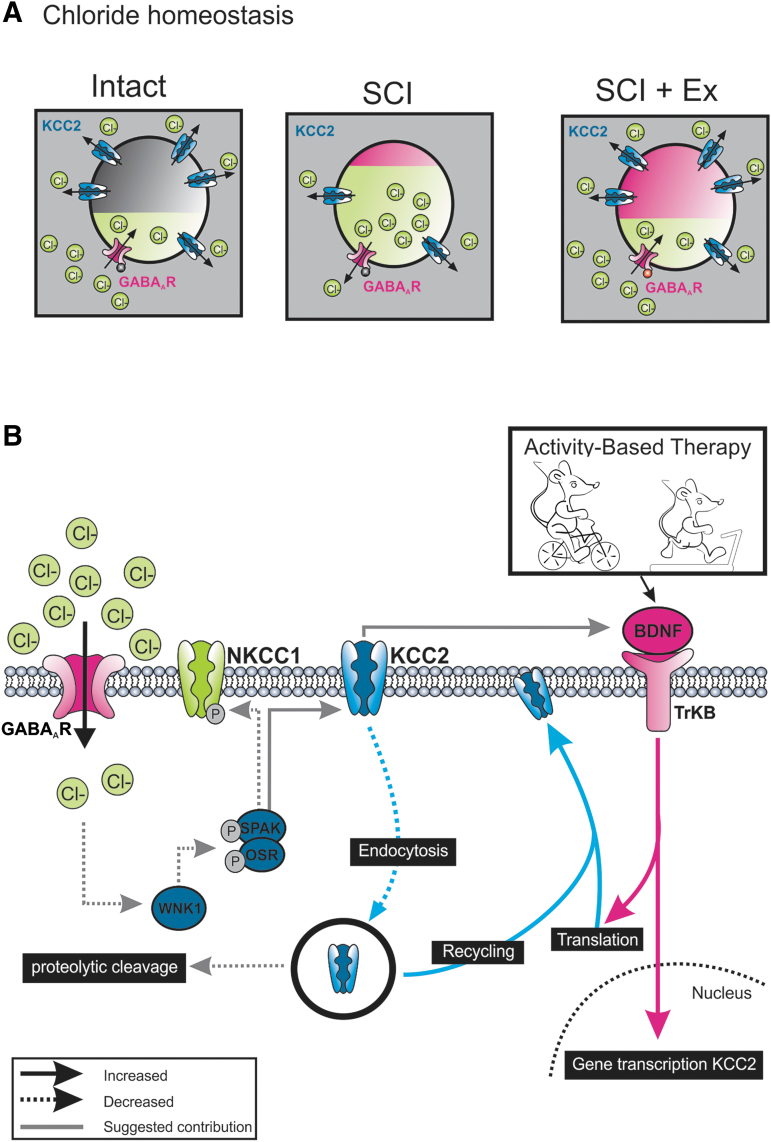
SCI markedly increases the expression of gephyrin, glutamic acid decarboxylase (GAD)67, glycine receptors (GlyR), and GABAA receptors (GABAAR) in the lumbar spinal cord below the injury.51–54 Despite this augmentation, spinal pathways are clearly hyperreflexive, and SCI-induced plasticity in inhibitory systems has detrimental consequences on motricity, in particular locomotor movements.55–57 Under physiological conditions, KCC2 extrudes Cl– from neurons to maintain low levels of [Cl–]i and ensures GABAergic synaptic transmission remains hyperpolarizing (Fig. 7A). A condition that negatively affects KCC2 function leads to an accumulation of [Cl–]i, which undermines GABAA-mediated inhibition. In spinal motoneurons, genetic (KCC2 knockouts) or pharmacological manipulations (acutely blocking KCC2) that decrease KCC2 expression or activity elicit a ∼10–20 mV depolarizing shift in ECl–, which increases neuronal excitability and impairs post-synaptic inhibition.58–60 Similarly, chronic SCI decreases motoneuronal KCC2 expression and contributes to spinal hyperexcitability and spasticity.24,61 In addition to a less hyperpolarized response in response to GABAA activation, the increase in GABAAR expression after SCI also contributes to increasing spinal excitability.
FIG. 7.
Chloride homeostasis and activity-based therapies. (A) Neuronal GABA receptor-initiated responses are highly dependent on chloride homeostasis. In healthy mature neurons, KCC2 maintains low intracellular Cl– concentration leading to an inflow of Cl– following GABAR activation (left). Whereas SCI decreases KCC2 expression and shifts chloride equilibrium (middle), activity-based therapies restore inhibition through an increase in KCC2 expression after SCI (right). This overall mitigates the hyperexcitability observed after SCI. (B) Rehabilitation increases KCC2 synthesis and post-translational mechanisms responsible for KCC2 trafficking to the membrane (increased insertion rate to the membrane and/or decreased internalization by endocytosis). Among the numerous regulators of KCC2 (not illustrated), BDNF contributes to activity-dependent shuttling of KCC2 to the motoneuronal membrane. The regulation of calpains by TrkB activation could contribute to decrease the proteolytic cleavage of KCC2 and alters its ability to extrude Cl–. Our results also suggest the presence of a retroactive feedback loop through which KCC2 can regulate BDNF expression and the activity-dependent decrease in the activation of the WNK1, due to higher levels of [Cl–]i that favor the activation of KCC2 and inactivation of NKCC1. BDNF, brain-derived neurotrophic factor; GABA, gamma-aminobutyric acid; SCI, spinal cord injury.
Rehabilitation programs can both restore KCC2 expression on lumbar motoneurons (Fig.7A) and improve reflex recovery after SCI,26 but the causal relationship linking a shift in chloride homeostasis to motor recovery remains unclear. To evaluate the functional role of KCC2 in rehabilitation-dependent motor recovery (vs. spontaneous), we restricted the delivery of the KCC2 blocker to the daily exercise period by taking advantage of its relatively short therapeutic window.37 The lack of effect of VU0240551 in unexercised animals suggests a low target availability and confirms that our observations are not solely relying on the effect of the drug, but on its interaction with activity-based therapy. While blocking KCC2 did not affect its expression in the lumbar enlargement, confocal analysis distinctly illustrated a decrease in expression on motoneuronal membrane as well as the increased presence of clusters in the cytoplasm. This supports findings suggesting that VU0240551 affects post-translational mechanisms, including preventing shuttling to the membrane.62 Discrepancies between western blot data, performed on lumbar spinal cord (Fig. 4), and immunohistochemistry performed specifically on motoneurons (Fig. 6), may also arise from expression of KCC2 (and blocking its activity) in lumbar interneurons24,63 or spinal dorsal horn neurons.23 However, if the effect of rehabilitation and of VU0240551 certainly impacted KCC2 activity/expression in spinal interneurons and dorsal horn neurons, it is unlikely to have affected our H-reflex data.
The loss of supraspinal control and increased gain of afferent feedback after SCI increases spinal reflex excitability resulting in permanent undesirable effects such as inappropriate timing of activation of muscles during movement and failure to adjust reflex excitability to meet task requirements. To estimate hyperreflexia, we have used the FDD, which is indicative of the function of the spinal inhibitory system. In rodents the magnitude of the FDD is dependent on functional GABAAR-mediated spinal inhibition,64,65 and is also associated with spasticity.24 Under neuropathological conditions, a re-emergence of depolarizing GABAA-mediated responses is associated with a progressive decrease in KCC2 expression.18 19,66–69 Similarly, the impairment of FDD has been attributed to a decrease in KCC2 expression and consequent changes to post-synaptic GABAAR function in lumbar motoneurons after SCI.24 In agreement with earlier studies, rehabilitation decreased spinal hyperexcitability.17,26 However, the activity-dependent recovery of the FDD was prevented by VU0240551, indicating that KCC2 activity is critical to the beneficial effect of exercise. Our data further suggest that rehabilitation increases KCC2 synthesis as well as post-translational mechanisms responsible for KCC2 trafficking to the membrane. Such mechanisms include increased insertion rate to the membrane and/or decreased internalization by endocytosis (Fig.7B).62
Activity-dependent modulation of KCC2 is BDNF-dependent
KCC2 is dynamically modulated in an activity-dependent manner by multiple signaling pathways, the most prevalent being BDNF signaling onto TrkB receptors.45,70 The polarity of BDNF regulation of KCC2 differs depending on the developmental stage and the integrity of the central nervous system (CNS). While BDNF reduces GABAergic inhibition through KCC2 downregulation in mature neurons,18–20,24,71 it promotes an increase in KCC2 expression in embryonic neurons or following an axotomy in the adult.29,67,72
Activity-based therapies increase KCC2 and BDNF levels in the lumbar spinal cord after SCI.17,26–28 Increased BDNF level is associated with the normalization of motoneuronal properties15 and locomotor recovery,16,73,74 and is positively correlated to the restoration of reflex modulation, suggesting a linear relationship with hyperreflexia.17 Interestingly, BDNF upregulates KCC2 in spinal dorsal horn neurons and attenuates central sensitization through reinstating GABAergic inhibition.71,75 To identify BDNF-TrkB as an activity-dependent regulator of KCC2 after SCI, we used TrkB-IgG, a chimeric molecule comprising the extracellular domain of TrkB that sequesters BDNF by competing with endogenous TrkB. TrkB-IgG delivered during training yielded lower levels of BDNF as compared with exercised animals that received the vehicle, confirming that TrkB-IgG treatment was effective at scavenging BDNF. Decreasing BDNF availability not only prevented the rehabilitation-induced recovery of the FDD, but more importantly the increase in KCC2 motoneuronal expression triggered by exercise. This indicates that BDNF contributes to decrease hyperreflexia after SCI through regulating KCC2 expression and restoring chloride homeostasis after SCI.
In the hippocampus, enhanced membrane insertion and retention of KCC2 depends on BDNF-TrkB signaling in response to increased network activity.33,34 The results presented here provide evidence that a similar mechanism is at play in the spinal cord. Chronic SCI recapitulates an earlier developmental state in which BDNF promotes KCC2 upregulation and restores GABA-mediated inhibition. Our results suggest that the activity-dependent activation of BDNF-TrkB alters chloride extrusion and the global inhibitory action of GABAA-mediated responses as depicted by the restoration of the FDD in exercised animals. Among several possibilities, TrkB activation can regulate calpains.76 Interestingly, calpain-dependent proteolytic cleavage of KCC2 alters its ability to extrude Cl– in the hippocampus and spinal cord77,78 and contributes to the development of spasticity after SCI.79,80 This suggests that activity-dependent plasticity may be affecting this pathway in motoneurons (Fig.7B).
It is also worth noting that not only does chelating BDNF decrease KCC2 expression, but blocking KCC2 also decreases BDNF levels in exercised animals, suggesting the presence of a retroactive feedback loop through which KCC2 can also regulate BDNF expression (Fig.7B).
Reciprocal regulation of KCC2 and NKCC1
Activity-dependent plasticity not only increases KCC2 but also decreases the expression of the chloride intruder NKCC126 (Fig. 4C). VU0240551 prevented the effect of exercise on both KCC2 and NKCC1, so that their expression levels in the lumbar spinal cord maintained a negative correlation. Because NKCC1 staining did not reveal strong enough staining on motoneuronal membrane, and is strongly expressed in small glial cells surrounding motoneurons81 and primary afferent terminals,82 identifying if its expression was specifically downregulated in motoneurons was not possible.
Scavenging BDNF did not affect NKCC1 expression, suggesting that BDNF is not responsible for the reciprocal regulation of KCC2 and NKCC1. NKCC1 and KCC2 activity is regulated in a reciprocal fashion through the WNK-SPAK/OSR1 pathway, providing a coordinated control over [Cl–]i.83,84 Decreasing WNK1 activity triggers a hyperpolarizing shift in GABA responses by enhancing KCC2-mediated Cl− extrusion85 and reduces allodynia and hyperalgesia in spinal dorsal horn neurons.86 Moreover, the WNK1 pathway is activated by lower levels of [Cl–]i, a hallmark of SCI. Anecdotal reports suggest the involvement of WNK-SPAK/OSR1 in activating NKCC1 at the lesion site after SCI48 but warrants further studies. Whether activity-based therapies decrease WNK1 activity remains to be determined, but our results strongly support this possibility (Fig.7B).
Therapeutic significance
Inhibitory synapses constitute ∼30% of all synapses in the spinal cord and are critical for the optimal function of neural circuits by regulating network oscillations, contributing to neuronal processing, and limiting the extent of excitatory drive. Decreased spinal reflex inhibition in individuals with SCI87–90 contributes to spasticity and co-contraction of flexor and extensor muscles. Available anti-spastic medication have serious side effects including a profound depression of spinal excitability, which significantly reduces muscle activity and interferes with motor recovery.6–9 While supplying BDNF can improve spasticity, long-term administration has shown serious therapeutic drawbacks and warrants more scrutiny.91,92 Given the role of KCC2 in regulating the strength of inhibitory synaptic transmission, facilitating Cl– extrusion by directly targeting KCC2 signaling pathway has emerged as a promising alternative to restore endogenous inhibition, rather than actively depress excitability93 and a viable option to increase GABAergic transmission in vivo.63,94,95 This critically identifies this pathway as a potential therapeutic target to improve hyperreflexia after a chronic SCI for individuals with comorbidities that delay the onset of physical therapy.
Acknowledgments
We are in debt to late Professor Emerita Marion Murray and Dr. Michel Lemay for their significant criticisms, comments, input, and exchange of ideas in earlier versions of the article.
Funding Information
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (RO1 NS083666) and the Craig H. Neilsen Foundation (189758).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Dietz V. (2001). Spinal cord lesion: effects of and perspectives for treatment. Neural Plast. 8, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petropoulou K.B., Panourias I.G., Rapidi C.A., and Sakas D.E. (2007). The importance of neurorehabilitation to the outcome of neuromodulation in spasticity. Acta Neurochir Suppl. 97, 243–250 [DOI] [PubMed] [Google Scholar]
- 3. Maynard F.M., Karunas R.S., and Waring W.P. 3rd (1990). Epidemiology of spasticity following traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 71, 566–569 [PubMed] [Google Scholar]
- 4. Skold C., Levi R., and Seiger A. (1999). Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 80, 1548–1557 [DOI] [PubMed] [Google Scholar]
- 5. Holtz K.A., Lipson R., Noonan V.K., Kwon B.K., and Mills P.B. (2017). Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 98, 1132–1138 [DOI] [PubMed] [Google Scholar]
- 6. Dario A., and Tomei G. (2004). A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf. 27, 799–818 [DOI] [PubMed] [Google Scholar]
- 7. Adams M.M., and Hicks A.L. (2005). Spasticity after spinal cord injury. Spinal Cord 43, 577–586 [DOI] [PubMed] [Google Scholar]
- 8. Elbasiouny S.M., Moroz D., Bakr M.M., and Mushahwar V.K. (2010). Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil. Neural Repair 24, 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angeli C., Ochsner J., and Harkema S. (2012). Effects of chronic baclofen use on active movement in an individual with a spinal cord injury. Spinal Cord 50, 925–927 [DOI] [PubMed] [Google Scholar]
- 10. Lu B. (2003). BDNF and activity-dependent synaptic modulation. Learn. Mem. 10, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyce V.S., and Mendell L.M. (2014). Neurotrophins and spinal circuit function. Front. Neural Circuits 8, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyce V.S., and Mendell L.M. (2014). Neurotrophic factors in spinal cord injury. Handb. Exp. Pharmacol. 220, 443–460 [DOI] [PubMed] [Google Scholar]
- 13. Leech K.A., and Hornby T.G. (2017). High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. J. Neurotrauma 34, 1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutchinson K.J., Gomez-Pinilla F., Crowe M.J., Ying Z., and Basso D.M. (2004). Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127, 1403–1414 [DOI] [PubMed] [Google Scholar]
- 15. Beaumont E., Kaloustian S., Rousseau G., and Cormery B. (2008). Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 62, 147–154 [DOI] [PubMed] [Google Scholar]
- 16. Ying Z., Roy R.R., Zhong H., Zdunowski S., Edgerton V.R., and Gomez-Pinilla F. (2008). BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155, 1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Côté M.-P., Azzam G.A., Lemay M.A., Zhukareva V., and Houle J.D. (2011). Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma 28, 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., Kokaia Z., Airaksinen M.S., Voipio J., Kaila K., and Saarma M. (2002). BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 159, 747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivera C., Voipio J., Thomas-Crusells J., Li H., Emri Z., Sipila S., Payne J.A., Minichiello L., Saarma M., and Kaila K. (2004). Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., and De K.Y. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 21. Miletic G., and Miletic V. (2008). Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain 137, 532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrini F., Trang T., Mattioli T.A., Laffray S., Del'Guidice T., Lorenzo L.E., Castonguay A., Doyon N., Zhang W., Godin A.G., Mohr D., Beggs S., Vandal K., Beaulieu J.M., Cahill C.M., Salter M.W., and De Koninck Y. (2013). Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat. Neurosci. 16, 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu Y., Zheng J., Xiong L., Zimmermann M. and Yang J. (2008). Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J.Physiol. 586, 5701–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boulenguez P., Liabeuf S., Bos R., Bras H., Jean-Xavier C., Brocard C., Stil A., Darbon P., Cattaert D., Delpire E., Marsala M., and Vinay L. (2010). Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 16, 302–307 [DOI] [PubMed] [Google Scholar]
- 25. Gackiere F., and Vinay L. (2015). Contribution of the potassium-chloride cotransporter KCC2 to the strength of inhibition in the neonatal rodent spinal cord in vitro. J. Neurosci. 35, 5307–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Côté M.-P., Gandhi S., Zambrotta M., and Houle J.D. (2014). Exercise modulates chloride homeostasis after spinal cord injury. J. Neurosci. 34, 8976–8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chopek J.W., Sheppard P.C., Gardiner K., and Gardiner P.F. (2015). Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J. Neurophysiol. 113, 1369–1376 [DOI] [PubMed] [Google Scholar]
- 28. Tashiro S., Shinozaki M., Mukaino M., Renault-Mihara F., Toyama Y., Liu M., Nakamura M., and Okano H. (2015). BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via upregulation of KCC2. Neurorehabil. Neural Repair 29, 677–689 [DOI] [PubMed] [Google Scholar]
- 29. Aguado F., Carmona M.A., Pozas E., Aguilo A., Martinez-Guijarro F.J., Alcantara S., Borrell V., Yuste R., Ibanez C.F., and Soriano E. (2003). BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development 130, 1267–1280 [DOI] [PubMed] [Google Scholar]
- 30. Ludwig A., Uvarov P., Soni S., Thomas-Crusells J., Airaksinen M.S., and Rivera C. (2011). Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J. Neurosci. 31, 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaila K., Price T.J., Payne J.A., Puskarjov M., and Voipio J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 15, 637–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaila K., Ruusuvuori E., Seja P., Voipio J., and Puskarjov M. (2014). GABA actions and ionic plasticity in epilepsy. Curr. Opin. Neurobiol. 26, 34–41 [DOI] [PubMed] [Google Scholar]
- 33. Khirug S., Ahmad F., Puskarjov M., Afzalov R., Kaila K., and Blaesse P. (2010). A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus. J. Neurosci. 30, 12028–12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puskarjov M., Ahmad F., Khirug S., Sivakumaran S., Kaila K., and Blaesse P. (2015). BDNF is required for seizure-induced but not developmental up-regulation of KCC2 in the neonatal hippocampus. Neuropharmacology 88, 103–109 [DOI] [PubMed] [Google Scholar]
- 35. Tan T., Watts S.W., and Davis R.P. (2011). Drug delivery: enabling technology for drug discovery and development. iPRECIO Micro Infusion Pump: programmable, refillable, and implantable. Front. Pharmacol. 2, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gomez-Pinilla F., Huie J.R., Ying Z., Ferguson A.R., Crown E.D., Baumbauer K.M., Edgerton V.R., and Grau J.W. (2007). BDNF and learning: evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 148, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin T.M., and Delpire E. (2011). Inhibition of KCC2 in mouse spinal cord neurons leads to hypersensitivity to thermal stimulation. Anesth. Analg. 113, 1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ford A., Castonguay A., Cottet M., Little J.W., Chen Z., Symons-Liguori A.M., Doyle T., Egan T.M., Vanderah T.W., De Koninck Y., Tosh D.K., Jacobson K.A., and Salvemini D. (2015). Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J. Neurosci. 35, 6057–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houle J.D., Morris K., Skinner R.D., Garcia-Rill E., and Peterson C.A. (1999). Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve 22, 846–856 [DOI] [PubMed] [Google Scholar]
- 40. Liabeuf S., Stuhl-Gourmand L., Gackiere F., Mancuso R., Sanchez Brualla I., Marino P., Brocard F., and Vinay L. (2017). Prochlorperazine increases KCC2 function and reduces spasticity after spinal cord injury. J. Neurotrauma 34, 3397–3406 [DOI] [PubMed] [Google Scholar]
- 41. Bennett D.J., Gorassini M., Fouad K., Sanelli L., Han Y., and Cheng J. (1999). Spasticity in rats with sacral spinal cord injury. J. Neurotrauma 16, 69–84 [DOI] [PubMed] [Google Scholar]
- 42. Yates C., Charlesworth A., Allen S.R., Reese N.B., Skinner R.D., and Garcia-Rill E. (2008). The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord 46, 798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson F.J., Reier P.J., Lucas C.C., and Parmer R. (1992). Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J. Neurophysiol. 68, 1473–1486 [DOI] [PubMed] [Google Scholar]
- 44. Grey M.J., Klinge K., Crone C., Lorentzen J., Biering-Sorensen F., Ravnborg M., and Nielsen J.B. (2008). Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp. Brain Res. 185, 189–197 [DOI] [PubMed] [Google Scholar]
- 45. Ferrini F., and De Koninck Y. (2013). Microglia control neuronal network excitability via BDNF signalling. Neural. Plast. 2013, 429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cramer S.W., Baggott C., Cain J., Tilghman J., Allcock B., Miranpuri G., Rajpal S., Sun D., and Resnick D. (2008). The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol. Pain 4, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hasbargen T., Ahmed M.M., Miranpuri G., Li L., Kahle K.T., Resnick D., and Sun D. (2010). Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann. N.Y. Acad. Sci. 1198, 168–172 [DOI] [PubMed] [Google Scholar]
- 48. Lee H.K., Ahmed M.M., King K.C., Miranpuri G.S., Kahle K.T., Resnick D.K., and Sun D. (2014). Persistent phosphorylation of NKCC1 and WNK1 in the epicenter of the spinal cord following contusion injury. Spine J14, 777–781 [DOI] [PubMed] [Google Scholar]
- 49. Behrman A.L., and Harkema S.J. (2000). Locomotor training after human spinal cord injury: a series of case studies. Phys.Ther. 80, 688–700 [PubMed] [Google Scholar]
- 50. Jones M.L., Harness E., Denison P., Tefertiller C., Evans N., and Larson C.A. (2012). Activity-based therapies in spinal cord injury: clinical focus and empirical evidence in three independent programs. Top Spinal Cord Inj. Rehabil. 18, 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tillakaratne N.J., Mouria M., Ziv N.B., Roy R.R., Edgerton V.R., and Tobin A.J. (2000). Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J. Neurosci. Res. 60, 219–230 [DOI] [PubMed] [Google Scholar]
- 52. Tillakaratne N.J., De Leon R.D., Hoang T.X., Roy R.R., Edgerton V.R., and Tobin A.J. (2002). Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 22, 3130–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Edgerton V.R., Leon R.D., Harkema S.J., Hodgson J.A., London N., Reinkensmeyer D.J., Roy R.R., Talmadge R.J., Tillakaratne N.J., Timoszyk W., and Tobin A. (2001). Retraining the injured spinal cord. J.Physiol. 533, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khristy W., Ali N.J., Bravo A.B., de Leon R., Roy R.R., Zhong H., London N.J., Edgerton V.R., and Tillakaratne N.J. (2009). Changes in GABA(A) receptor subunit gamma 2 in extensor and flexor motoneurons and astrocytes after spinal cord transection and motor training. Brain Res. 1273, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hart B.L. (1971). Facilitation by strychnine of reflex walking in spinal dogs. Physiol. Behav. 6, 627–628 [DOI] [PubMed] [Google Scholar]
- 56. Robinson G.A., and Goldberger M.E. (1986). The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp. Brain Res. 62, 387–400 [DOI] [PubMed] [Google Scholar]
- 57. De Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1999). Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J. Neurophysiol. 81, 85–94 [DOI] [PubMed] [Google Scholar]
- 58. Hubner C.A., Stein V., Hermans-Borgmeyer I., Meyer T., Ballanyi K., and Jentsch T.J. (2001). Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30, 515–524 [DOI] [PubMed] [Google Scholar]
- 59. Jean-Xavier C., Pflieger J.F., Liabeuf S., and Vinay L. (2006). Inhibitory postsynaptic potentials in lumbar motoneurons remain depolarizing after neonatal spinal cord transection in the rat. J. Neurophysiol. 96, 2274–2281 [DOI] [PubMed] [Google Scholar]
- 60. Bos R., Sadlaoud K., Boulenguez P., Buttigieg D., Liabeuf S., Brocard C., Haase G., Bras H., and Vinay L. (2013). Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. U S A 110, 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Modol L., Mancuso R., Ale A., Francos-Quijorna I., and Navarro X. (2014). Differential effects on KCC2 expression and spasticity of ALS and traumatic injuries to motoneurons. Front. Cell Neurosci. 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee H.H., Walker J.A., Williams J.R., Goodier R.J., Payne J.A., and Moss S.J. (2007). Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 [DOI] [PubMed] [Google Scholar]
- 63. Chen B., Li Y., Yu B., Zhang Z., Brommer B., Williams P.R., Liu Y., Hegarty S.V., Zhou S., Zhu J., Guo H., Lu Y., Zhang Y., Gu X., and He Z. (2018). Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell 174, 521–535, e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee-Kubli C.A., and Calcutt N.A. (2014). Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain 155, 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jolivalt C.G., Lee C.A., Ramos K.M., and Calcutt N.A. (2008). Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain 140, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Coull J.A., Boudreau D., Bachand K., Prescott S.A., Nault F., Sik A., De K.P., and De K.Y. (2003). Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 [DOI] [PubMed] [Google Scholar]
- 67. Payne J.A., Rivera C., Voipio J., and Kaila K. (2003). Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26, 199–206 [DOI] [PubMed] [Google Scholar]
- 68. Nabekura J., Ueno T., Okabe A., Furuta A., Iwaki T., Shimizu-Okabe C., Fukuda A., and Akaike N. (2002). Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J, Neurosci. 22, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yan Y., Dempsey R.J., and Sun D. (2001). Na+-K+-Cl- cotransporter in rat focal cerebral ischemia. J. Cereb. Blood Flow Metab. 21, 711–721 [DOI] [PubMed] [Google Scholar]
- 70. Fiumelli H., and Woodin M.A. (2007). Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr. Opin. Neurobiol. 17, 81–86 [DOI] [PubMed] [Google Scholar]
- 71. Huang Y.J., Lee K.H., and Grau J.W. (2017). Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Exp. Neurol. 288, 38–50 [DOI] [PubMed] [Google Scholar]
- 72. Carmona M.A., Pozas E., Martinez A., Espinosa-Parrilla J.F., Soriano E., and Aguado F. (2006). Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cereb. Cortex 16, 47–63 [DOI] [PubMed] [Google Scholar]
- 73. Boyce V.S., Tumolo M., Fischer I., Murray M., and Lemay M.A. (2007). Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J. Neurophysiol. 98, 1988–1996 [DOI] [PubMed] [Google Scholar]
- 74. Boyce V.S., Park J., Gage F.H., and Mendell L.M. (2012). Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 35, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huie J.R., Garraway S.M., Baumbauer K.M., Hoy K.C. Jr., Beas B.S., Montgomery K.S., Bizon J.L., and Grau J.W. (2012). Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience 200, 74–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zadran S., Jourdi H., Rostamiani K., Qin Q., Bi X., and Baudry M. (2010). Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J. Neurosci. 30, 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou H.Y., Chen S.R., Byun H.S., Chen H., Li L., Han H.D., Lopez-Berestein G., Sood A.K., and Pan H.L. (2012). N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl- cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 287, 33853–33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Puskarjov M., Ahmad F., Kaila K., and Blaesse P. (2012). Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J. Neurosci. 32, 11356–11364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brocard C., Plantier V., Boulenguez P., Liabeuf S., Bouhadfane M., Viallat-Lieutaud A., Vinay L., and Brocard F. (2016). Cleavage of Na(+) channels by calpain increases persistent Na(+) current and promotes spasticity after spinal cord injury. Nat. Med. 22, 404–411 [DOI] [PubMed] [Google Scholar]
- 80. Plantier V., and Brocard F. (2017). [Calpain as a new therapeutic target for treating spasticity after a spinal cord injury]. Med. Sci. (Paris) 33, 629–636 [DOI] [PubMed] [Google Scholar]
- 81. Kanaka C., Ohno K., Okabe A., Kuriyama K., Itoh T., Fukuda A., and Sato K. (2001). The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104, 933–946 [DOI] [PubMed] [Google Scholar]
- 82. Stil A., Liabeuf S., Jean-Xavier C., Brocard C., Viemari J.C., and Vinay L. (2009). Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience 164, 809–821 [DOI] [PubMed] [Google Scholar]
- 83. Kahle K.T., Rinehart J., and Lifton R.P. (2010). Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim. Biophys. Acta 1802, 1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alessi D.R., Zhang J., Khanna A., Hochdorfer T., Shang Y., and Kahle K.T. (2014). The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci. Signal 7, re3. [DOI] [PubMed] [Google Scholar]
- 85. Friedel P., Kahle K.T., Zhang J., Hertz N., Pisella L.I., Buhler E., Schaller F., Duan J., Khanna A.R., Bishop P.N., Shokat K.M., and Medina I. (2015). WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci. Signal 8, ra65. [DOI] [PubMed] [Google Scholar]
- 86. Kahle K.T., Schmouth J.F., Lavastre V., Latremoliere A., Zhang J., Andrews N., Omura T., Laganiere J., Rochefort D., Hince P., Castonguay G., Gaudet R., Mapplebeck J.C., Sotocinal S.G., Duan J., Ward C., Khanna A.R., Mogil J.S., Dion P.A., Woolf C.J., Inquimbert P., and Rouleau G.A. (2016). Inhibition of the kinase WNK1/HSN2 ameliorates neuropathic pain by restoring GABA inhibition. Sci. Signal 9, ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boorman G.I., Lee R.G., Becker W.J., and Windhorst U.R. (1996). Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr. Clin. Neurophysiol. 101, 84–92 [DOI] [PubMed] [Google Scholar]
- 88. Mazzocchio R., and Rossi A. (1997). Involvement of spinal recurrent inhibition in spasticity. Further insight into the regulation of Renshaw cell activity. Brain 120 ( Pt. 6), 991–1003 [DOI] [PubMed] [Google Scholar]
- 89. Katz R. (1999). Presynaptic inhibition in humans: a comparison between normal and spastic patients. J. Physiol. Paris 93, 379–385 [DOI] [PubMed] [Google Scholar]
- 90. Harkema S.J. (2008). Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 57, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weishaupt N., Blesch A., and Fouad K. (2012). BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 238, 254–264 [DOI] [PubMed] [Google Scholar]
- 92. Fouad K., Bennett D.J., Vavrek R., and Blesch A. (2013). Long-term viral brain-derived neurotrophic factor delivery promotes spasticity in rats with a cervical spinal cord hemisection. Front. Neurol. 4, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doyon N., Ferrini F., Gagnon M., and De Koninck Y. (2013). Treating pathological pain: is KCC2 the key to the gate? Expert Rev. Neurother. 13, 469–471 [DOI] [PubMed] [Google Scholar]
- 94. Gagnon M., Bergeron M.J., Lavertu G., Castonguay A., Tripathy S., Bonin R.P., Perez-Sanchez J., Boudreau D., Wang B., Dumas L., Valade I., Bachand K., Jacob-Wagner M., Tardif C., Kianicka I., Isenring P., Attardo G., Coull J.A., and De Koninck Y. (2013). Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 19, 1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ferrini F., Lorenzo L.E., Godin A.G., Quang M.L., and De Koninck Y. (2017). Enhancing KCC2 function counteracts morphine-induced hyperalgesia. Sci. Rep. 7, 3870. [DOI] [PMC free article] [PubMed] [Google Scholar]