Abstract
Functional outcomes at 12 months were a secondary outcome of the randomized DECRA trial of early decompressive craniectomy for severe diffuse traumatic brain injury (TBI) and refractory intracranial hypertension.
In the DECRA trial, patients were randomly allocated 1:1 to either early decompressive craniectomy or intensive medical therapies (standard care). We conducted planned secondary analyses of the DECRA trial outcomes at 6 and 12 months, including all 155 patients.
We measured functional outcome using the Glasgow Outcome Scale-Extended (GOS-E). We used ordered logistic regression, and dichotomized the GOS-E using logistic regression, to assess outcomes in patients overall and in survivors. We adjusted analyses for injury severity using the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) model.
At 12 months, the odds ratio (OR) for worse functional outcomes in the craniectomy group (OR 1.68; 95% confidence interval [CI]: 0.96-2.93; p = 0.07) was no longer significant. Unfavorable functional outcomes after craniectomy were 11% higher (59% compared with 48%), but were not significantly different from standard care (OR 1.58; 95% CI: 0.84-2.99; p = 0.16). Among survivors after craniectomy, there were fewer good (OR 0.33; 95% CI: 0.12-0.91; p = 0.03) and more vegetative (OR 5.12; 95% CI: 1.04-25.2; p = 0.04) outcomes.
Similar outcomes in survivors were found at 6 months after injury. Vegetative (OR 5.85; 95% CI: 1.21-28.30; p = 0.03) and severely disabled outcomes (OR 2.49; 95% CI: 1.21-5.11; p = 0.01) were increased.
Twelve months after severe diffuse TBI and early refractory intracranial hypertension, decompressive craniectomy did not improve outcomes and increased vegetative survivors.
Keywords: decompressive craniectomy, intensive care, outcomes, randomized trial, traumatic brain injury
Introduction
Traumatic brain injury (TBI) imposes a heavy global burden on health care systems,1 which is largely due to the 60% of patients with severe TBI who die or are severely disabled and permanently dependent.2–5 Therapies aim to reduce disability, in addition to saving lives.
In intensive care units (ICUs) therapeutic protocols for TBI aim to control intracranial pressure (ICP) below accepted thresholds to minimize secondary brain injury. Guidelines recommend early therapies to lower ICP including optimized sedation, analgesia, osmotherapy, and cerebrospinal fluid drainage via external ventricular drainage catheters.6–8 Brain tissue oxygen monitoring may also assist choice and titration of therapies.9 In some patients, however, increased ICP is refractory to these therapies, and therapeutic options then include barbiturate infusions, decompressive craniectomy surgery, or therapeutic hypothermia.7,8,10 Each option has the potential for adverse side effects, and there is controversy regarding each.
Functional outcomes after TBI may improve at least up to 12 months after TBI. Further, benefits from neurosurgery may take 12 months to become apparent,11 and complications from neurosurgery may take 12 months to improve. Twelve months may therefore be an optimal time to report long-term patient outcomes in randomized trials of neurosurgical interventions for patients with TBI.
The randomized early DECompressive CRAniectomy (DECRA) trial12 included 155 adults under the age of 60 years with diffuse TBI and intracranial hypertension refractory to usual therapies (excluding hypothermia and barbiturates), during the first 72 h after admission. Patients with mass lesions were excluded. Patients required persistent unstimulated ICP elevation above the threshold of 20 mm Hg13 after optimized sedation, normalization of arterial carbon dioxide pressure, and the use of mannitol, hypertonic saline, neuromuscular blockade, and external ventricular drainage, but before hypothermia or thiopentone infusions. Patients in both groups could receive bolus thiopentone pre-randomization, and this usually occurred during computed tomography (CT) scanning or consent procedures. DECRA compared early bi-fronto-temporo-parietal decompressive craniectomy surgery in addition to usual intensive medical care, which usually included high-dose barbiturate infusions. At 6 months, functional outcomes after craniectomy were worse.12 Functional outcomes at 12 months, both overall and in survivors, were planned secondary outcomes14 of this randomized trial.
The RESCUEicp trial11 enrolled 408 patients with severe TBI, cerebral mass lesions, or diffuse brain injury considerably later after injury, and at a higher ICP threshold (25 mm Hg). In RESCUEicp, either bi-fronto-temporal or unilateral craniectomy was permitted, whereas in DECRA all patients with craniectomy had diffuse injury and received a bi-fronto-temporo-parietal procedure. In RESCUEicp, patients had intracranial hypertension refractory to usual therapies (including hypothermia) up to 10 days after admission, whereas in DECRA patients were randomized during their first 72 h. Both trials permitted late decompressive craniectomy in the control group as a life-saving measure and this “crossover” occurred in 37.2% of standard care patients in RESCUEicp, compared with 18% in DECRA. In RESCUEicp, at 6 months mortality was lower after craniectomy (26.9%) than in standard care patients, who had a high mortality rate (48.5% compared with 19% in DECRA).12 At 12 months, the proportion of patients in RESCUEicp with at least partial independence (Glasgow Outcome Scale-Extended [GOS-E] score of 4 or better, and termed favorable outcome in this trial) was greater than in controls, but the proportions of patients in a vegetative state and all patients with severe disability were also increased. Patients with severe disability include those with lower severe (dependent on others and cannot be left alone for more than 8 h) and upper severe disability (can be left alone in the home for more than 8 h, but are not independent outside the home and cannot shop).1,15
The 12-month outcomes from the DECRA trial are presented here and are considered in comparison with those of RESCUEicp. Translation into clinical practice requires careful examination of both randomized trials.
Methods
The design, patient inclusion and exclusion criteria, procedures, assessments, outcome measures, study oversight, and statistical analysis of the DECRA trial have been previously described.12 The surgical procedure was a modification of the Kjellberg method,16 which had tested favorably in a cohort study.17 The only surgical modification entailed not dividing the sagittal sinus and falx, to minimize complications, decrease anterior herniation of the frontal lobes, and increase generalizability.18 Randomization occurred after ICU admission, when ICP had increased sufficiently to satisfy the enrollment criteria.12 Patients in the intensive medical control group could receive barbiturate infusions to control ICP, but not hypothermia. Baseline variables were well matched except for pupil reactivity at hospital admission, and outcomes at 6 months favored the intensive medical control group.
Functional outcome assessments were conducted by trained assessors, blind to treatment group, using standardized questionnaires.15,19 Functional outcome assessments at 12 months after injury, and among survivors at 6 and 12 months, were pre-planned14 and measured by the score on the GOS-E, which is scored from 1 (dead) to 8 (pre-injury function).20 Among survivors, favorable outcomes were calculated as the ratio of GOS-E scores 5–8 over 2–8, and good outcomes as GOS-E scores of 7–8 over 2–8.
Functional (GOS-E) outcomes were dichotomized as unfavorable (1–4 vs. 5–8), and analyzed using logistic regression. Outcomes (GOS-E) on a 1–8 scale were analyzed using ordinal logistic regression. Among survivors at 6 and 12 months after injury, GOS-E outcomes were analyzed using logistic regression for favorable outcomes, and for three additional dichotomies: vegetative (2 vs. 3–8), poor outcomes (2–4 vs. 5–8), and good outcomes (7–8 vs. 2–6). The last three were post hoc exploratory analyses.
Multiple covariates influence patient outcomes after TBI, but there were too many to reliably adjust for in a medium-sized randomized trial. We therefore adjusted using a single summary measure of brain injury severity—the estimated probability of unfavorable outcome from the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) core and extended algorithms.21 IMPACT outcome probabilities are derived from large databases of patients with TBI, and include the relevant covariates, that is, IMPACT core including age, Glasgow Coma Scale (GCS) motor score, pupil reactivity; and IMPACT extended, which also includes CT score, and hypoxic and hypotensive events. The single IMPACT outcome probability value provides an overall summary measure of TBI severity in both study groups (Table 1). TBI severity can be difficult to assess when covariates are many, or move in different directions. We used pupil reactivity at trial randomization for these calculations, as this measure best reflects a patient's post-resuscitation neurology, more so than does pupil reactivity at hospital admission, which we had used previously.12 Patient outcomes at 6 and 12 months post-injury were adjusted for the probability of an unfavorable outcome by using the IMPACT value as a continuous variable in the linear predictor of the appropriate regression model. The IMPACT extended model provided maximal adjustment but included missing data on hypoxia/hypertension for seven patients who were hence excluded; the IMPACT core probability of unfavorable outcome could be calculated for all 155 patients, and was the primary adjustment covariate.
Table 1.
Baseline Characteristics of 155 Patients in Decompressive Craniectomy (DC), and Standard Intensive Medical Care (Standard Care) Groups
| DC, n = 73 | Standard Care, n = 82 | P-value | |
|---|---|---|---|
| Age (years) | 0.89 | ||
| Median | 23.7 | 24.6 | |
| Interquartile range | 19.4-29.6 | 18.5-34.9 | |
| GCS motor | 0.49 | ||
| Median | 3 | 3 | |
| Interquartile range | 1-4 | 1-5 | |
| Marshall class | 0.39 | ||
| Diffuse injury II; n (%) | 17 (23) | 27 (33) | |
| Diffuse injury III/IV; n (%) | 53 (73) | 53 (65) | |
| Non-evacuated mass lesion | 3 (4) | 2 (2) | |
| Reactivity of pupils—randomization | 0.60 | ||
| One or both pupils; n (%) | 61/73 (84) | 71/82 (87) | |
| Reactivity of pupils—admission | 0.04 | ||
| One or both pupils; n (%) | 52/71 (73) | 70/80 (88) | |
| Hypoxemia | 18 (25) | 24 (29) | 0.55 |
| Hypotension | 24 (33) | 25 (30) | 0.93 |
| Probability of unfavorable outcome using IMPACT21; core % (SD) | 42 (19) | 42 (20) | 0.81 |
| Probability of unfavorable outcome using IMPACT21; extended % (SD) n = 148a | 48 (22) | 45 (22) | 0.40 |
Seven patients had missing variables for hypoxic and/or hypotensive insults, required for IMPACT extended calculation.
GSC, Glasgow Coma Scale; SD, standard deviation.
An unplanned exploratory as-treated analysis was also conducted of all patients who received a decompressive craniectomy at any time after randomization compared with all those who did not, regardless of randomized group. We wished to explore the effect on our results of the 18% of standard care patients who had received a late craniectomy as permitted in the protocol as a life-saving intervention. Owing to likely imbalance in severity, all these analyses were adjusted for the IMPACT core probability of unfavorable outcome.
Results
One hundred fifty-five patients were randomized, 73 to decompressive craniectomy and 82 to standard care. Baseline characteristics including pupil reactivity at randomization in ICU (84% and 87%) and IMPACT probability of a poor outcome (42% and 42% using IMPACT core) were similar between groups (Table 1).12 Pupil reactivity at randomization was considered the optimal indicator of post-resuscitation neurological activity.
After randomization, more standard care patients (77%) than patients with craniectomy (32%) required barbiturates to manage ICP (p < 0.001), more required high total doses of barbiturates (>30 g; 17%) compared with zero in patients with craniectomy.22 Further, the post-randomization median total dose of barbiturates received by standard care patients (6.5 g, interquartile range [IQR] 0.50–20) was substantially greater than that received by patients with craniectomy (0.00 g, IQR 0.00–1.03, p < 0.001).
As previously reported,12 short-term outcomes in the ICU in the craniectomy group compared with the standard care group had improved, including lower ICP, fewer interventions to control ICP, and fewer days receiving mechanical ventilation in the ICU. Standard care patients also often received high-dose barbiturate infusions to control their ICP.
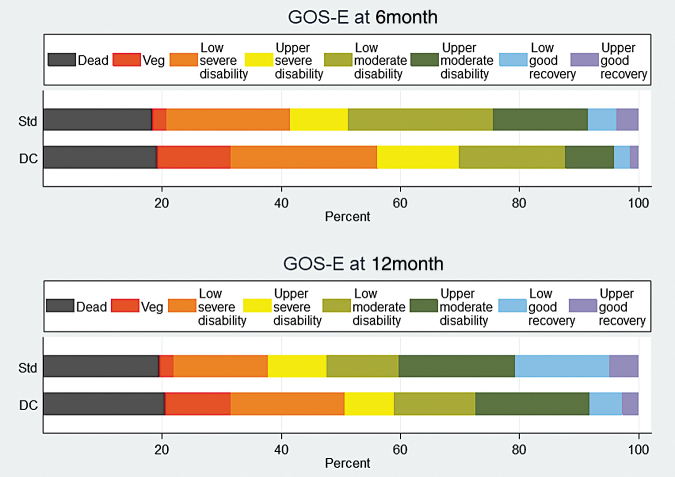
Twelve months after injury, no patients were lost to follow-up, and all 155 patients (100%) had neurological function assessed (Fig. 1). For a patient participation diagram to 12 months see the supplementary appendix published previously.22 Unlike the previously reported 6-month results, at 12 months, the functional assessment on the GOS-E (OR for a worse functional outcome in the craniectomy group 1.68; 95% CI: 0.96-2.93; p = 0.07), although concerning, was no longer significantly worse in the craniectomy group. Unfavorable outcomes occurred in 43 patients (59%) in the craniectomy group and in 39 patients (48%) in standard care (OR 1.58; 95% CI: 0.84-2.99; p = 0.16) (Table 2). After adjustment for IMPACT core score the results were similar (Table 3). In summary, 12 months after TBI some patients who had unfavorable outcomes at 6 months had improved sufficiently that the trend to worse functional outcomes after craniectomy was no longer significant.
FIG. 1.
Functional outcomes in 155 patients at 6 and 12 months measured by the Glasgow Outcome Scale-Extended (GOS-E) in decompressive craniectomy (DC), and standard intensive medical care (Standard Care) groups.
Table 2.
Extended Glasgow Outcome Scale at 12 Months in Decompressive Craniectomy (DC) and Standard Intensive Medical Care (Standard Care) Groups
| GOS-E Score: n [%] | DC, n = 73 | Standard Care, n = 82 |
|---|---|---|
| 1, Dead | 15 [21] | 16 [19] |
| 2, Vegetative | 8 [11] | 2 [3] |
| 3, Low severe disability | 14 [19] | 13 [16] |
| 4, Upper severe disability | 6 [8] | 8 [10] |
| 5, Low moderate disability | 10 [14] | 10 [12] |
| 6, Upper moderate disability | 14 [19] | 16 [19] |
| 7, Low good recovery | 4 [5] | 13 [16] |
| 8, Upper good recovery | 2 [3] | 4 [5] |
| Unfavorable score (GOS-E <5): n [%] | 43 [59] | 39 [48] |
GOS-E, Glasgow Outcome Scale-Extended.
Table 3.
Dichotomized Functional Outcomes in All Patients and in Survivors at 6 and 12 Months after Injury with and without Adjustment for the IMPACT Core Probability of a Favorable Outcome
| Functional outcome using Glasgow Outcome Scale-Extended (GOS-E) | DC vs. Standard Care |
Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|
| % with dichotomous outcome | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| 6 months after injury | |||||||
| Unfavorable, all patients (1-4/1-8) | 70% vs. 51% n = 155 |
2.21 | 1.14-4.28 | 0.02 | 2.40 | 1.18-4.91 | 0.02 |
| Vegetative survivors (2/2-8) | 15% vs. 3% n = 126 |
5.85 | 1.21-28.3 | 0.03 | 5.96 | 1.15-30.9 | 0.03 |
| Severe disability survivors (2-4/2-8) | 63% vs. 40% n = 126 |
2.49 | 1.21-5.11 | 0.01 | 2.52 | 1.18-5.37 | 0.02 |
| Good outcome survivors (7-8/2-8) | 5% vs. 10% n = 126 |
0.46 | 0.11-1.86 | 0.28 | 0.48 | 0.12 to-1.97 | 0.31 |
| 12 months after injury | |||||||
| Unfavorable, all patients (1-4/1-8) | 59% vs. 48% n = 155 |
1.58 | 0.84-2.99 | 0.16 | 1.65 | 0.83-3.28 | 0.15 |
| Vegetative survivors (2/2-8) | 14% vs. 3% n = 124 |
5.12 | 1.04-25.2 | 0.04 | 5.16 | 0.95-27.9 | 0.06 |
| Severe disability survivors (2-4/2-8) | 48% vs. 35% n = 124 |
1.74 | 0.85-3.59 | 0.13 | 1.70 | 0.80-3.62 | 0.17 |
| Good outcome survivors (7-8/2-8) | 10% vs. 26% n = 124 |
0.33 | 0.12-0.91 | 0.03 | 0.34 | 0.12-0.94 | 0.04 |
CI, confidence interval; OR, odds ratio.
Twelve months after injury, a total of 15 patients (21%) in the craniectomy group and 16 (19%) in the standard care group had died. Among the survivors at 12 months, vegetative outcomes occurred in 14% of the craniectomy group, compared with 3% of the standard care group (OR 5.12; 95% CI: 1.04-25.2; p = 0.04) (Table 3). The proportion of severe disability (dependent) survivors at 12 months was 48% in the craniectomy group, compared with 35% in the standard care group (OR 1.74; 95% CI: 0.85-3.59; p = 0.13). Good neurological outcomes in survivors at 12 months were less common in the craniectomy group (10%) than the standard care group (26%) (OR 0.33; 95% CI: 0.12-0.91; p = 0.03) (Table 3).
At 6 months after injury the previously reported unadjusted risk of an unfavorable outcome (OR 2.21; 95% CI: 1.14-4.26; p = 0.02) (DECRA 2011),12 and the adjusted risk of an unfavorable outcome using IMPACT core probability of unfavorable outcome (OR 2.40; 95% CI: 1.18-4.91; p = 0.02) were both greater in the craniectomy group compared with standard care group. Among survivors at 6 months, in the craniectomy group the proportion of patients in a vegetative state was greater (OR 5.85; 95% CI: 1.21-28.3; p = 0.03), and the proportion of patients who were dependent was also greater (63% vs. 40%; OR 2.49; 95% CI: 1.21-5.11; p = 0.01).
In an unplanned as-treated analysis, adjusted for IMPACT core, of all patients who received a decompressive craniectomy at any time compared with all those who did not, regardless of their randomized group, at 6 months unfavorable functional outcomes were increased in the craniectomy group (OR 2.04; 95% CI: 1.10-4.20; p = 0.05), whereas at 12 months the same trend was evident (OR 1.95; 95% CI: 0.97-3.99; p = 0.06).
Discussion
Some adults with severe diffuse TBI have increased ICP, which is refractory to usual therapies, leading clinicians to consider barbiturate infusions, hypothermia, or decompressive craniectomy surgery. In the DECRA trial of decompressive craniectomy compared with standard intensive medical therapies, despite immediate benefits in ICU, at 6 months the patients' functional outcomes were worse. They remained worse at 6 months after using IMPACT core to adjust for baseline balance.
The DECRA trial outcomes at 12 months were important to guide translation to patient care. At 12 months the rates of death, and severe and moderate disability in both study groups were similar. Decompressive craniectomy did not improve (and did not significantly worsen) patients' overall functional independence. However, among survivors at 12 months more patients with craniectomy were vegetative and fewer had good neurological outcomes. Our unplanned as-treated analysis confirmed that patient crossover for ethical reasons did not influence the trial findings at either time-point. These results will assist informed-consent discussions with families, prior to decompressive craniectomy surgery.
There were more independent patient outcomes at 12 compared with 6 months after injury in both study groups, and the reasons are likely multi-factorial. First, many patients with TBI improve their functional status over at least 12 months. Second, some complications including hydrocephalus requiring a shunt related to the surgeries were more common in craniectomy patients, and may have improved gradually over 12 months. Another may be the potential adverse effects of an injured brain not having the protection of overlying skull for weeks or months after injury. Other complications, relating to the unavoidable transport of critically ill patients with brain swelling or post-operative hemorrhagic complications and neurophysiological instability to and from operating theaters, may also have slowly improved over time. Cerebral white matter stretch and injury to herniating frontal lobes at the edge of a craniectomy are further potential surgical complications that may also have improved over time.
One implication from these 12-month outcome results from the DECRA trial is that therapies for ICP control should be assessed through a risk-benefit lens. In the standard care patients in DECRA, less rigorous ICP control most often using intravenous barbiturates provided similar overall outcomes, but with fewer vegetative and more good outcome survivors than decompressive surgery. The international consensus ICP treatment thresholds for clinical interventions in patients with neurotrauma may require further reconsideration.6,13,23 Further, because the standard care patients in the DECRA trial received increased medical therapies usually including barbiturate infusions, in patients with diffuse injury with early intracranial hypertension risk-benefit considerations favor these medical therapies rather than early decompressive craniectomy surgery. International consensus guidelines concerning decompressive craniectomy in the management of patients with TBI were recently published.24
Future potential modifications of the craniectomy surgery technique are unlikely to change our results: hinged craniotomy,25–27 unilateral craniectomy, or even not opening the dura at all are strategies that limit brain herniation, but were not tested in the DECRA trial. DECRA excluded patients with intracranial hematomas (mass lesions) as these patients often require craniectomy to evacuate hemorrhage, and it also did not include patients with penetrating gunshot or blast craniocerebral injury.
In comparison with DECRA, the randomized RESCUEicp decompressive craniectomy trial enrolled older patients with severe TBI with a higher ICP threshold, included both diffuse injury and mass lesions etiologies up to 10 days after injury, and found increased survival. Favorable outcomes were defined differently in the two trials. In DECRA, patients with favorable outcomes defined in the standard way when the trial was conducted (patients living independently with GOS-E scores of 5–8) were decreased after craniectomy at 6 months, and by 12 months this trend was no longer significant. In RESCUEicp after examination of overall patient severity, favorable outcomes were defined as GOS-E scores 4–8 (including patients who were independent in the home for more than 8 h, but not outside the home). These were unchanged at 6 months, and increased at 12 months. However, in these older, higher severity patients in RESCUEicp, almost all the increased survivors after decompressive craniectomy at 12 months were vegetative or had either lower or upper severe disability. In RESCUEicp there were increased favorable outcomes at 12 months after craniectomy, primarily because the upper-severe disability outcomes were increased, and were grouped as favorable. This is a valid approach, but it is a matter of opinion as to whether independent living in the home, including the inability to function independently outside or do the shopping after severe TBI, is an optimal target for patients and families. RESCUEicp did not increase fully independent outcomes.
Taken together, the DECRA and RESCUEicp trial results at 6 and 12 months inform clinicians that decompressive craniectomy surgery does not improve survival when used prior to hypothermia and/or barbiturates, but does improve survival when used after these therapies in older patients with higher baseline mortality at higher ICP thresholds. Both trials found that decompressive craniectomy did not improve functional outcomes at 12 months when independent functional outcomes were defined as scores 5–8 on the GOS-E. Both trials also found that decompressive craniectomy increased vegetative outcomes in survivors at 12 months.
Whether an increase in older patients surviving with severe disability is a desirable outcome after severe TBI and whether it is accurate to classify such an outcome as “favorable” in a randomized trial is a matter for families and clinicians, and undoubtedly for ongoing debate. However, independence both inside and outside the home (GOS-E scores 5–8) has defined “favorable outcomes” for interventional randomized trials in TBI for decades prior to RESCUEicp.
Strengths of the DECRA trial include prospective multi-center design, size, randomization with concealed allocation, use of early ventricular drainage as a pre-randomization therapy, blinded outcome assessments, and the complete (100%) follow-up rate. Inclusion of patients solely with diffuse TBI enabled clearer conclusions about a more uniform pattern of brain injury. Strengths of the outcome analysis at 12 months include the 100% follow-up rate and the verification of baseline balance for patient severity using IMPACT21 core as the adjustment covariate.
In this article, we resolved questions about pupil reactivity being unbalanced compared with other baseline variables28 by utilizing the post-resuscitation, last pre-randomization values. Overall baseline severity was then quantified using the single IMPACT probability of unfavorable outcome, and was found to be balanced.
Including patients solely with diffuse TBI may also be considered a limitation. By comparison, RESCUEicp enrolled patients with both diffuse and mass lesion injury, but both trials found similar increases in vegetative outcomes after surgery. RESCUEicp did not separately report the outcomes of the two main injury types. Other limitations may be that DECRA did not test salvage therapy for extreme or late intractable ICP elevations, nor the effect of withholding craniectomy surgery until ICP had reached higher thresholds, whereas RESCUEicp randomized patients at a higher ICP threshold of 25 mm Hg. A substantial number of standard care patients in both DECRA (18%) and RESCUEicp (37.2%) received a delayed decompressive craniectomy, by clinician direction. However, the exploratory as-treated analysis of DECRA supported the primary results. Finally, most patients in DECRA were from high-income countries, and the results are not generalizable to conditions in low-income countries with more limited facilities.24
Both trials used primary intention-to-treat analyses, because alternative per-protocol analyses may be complicated by inevitable baseline severity imbalances.
Twelve months after diffuse severe TBI, in patients who had intracranial hypertension refractory to optimized first- and second-tier therapies in their first 72 h, early decompressive craniectomy compared with standard intensive medical care did not improve survival or neurological outcomes. Exploratory analyses found that early decompressive craniectomy increased vegetative survivors and decreased survivors with good outcomes compared with standard intensive medical therapies.
Acknowledgments
We acknowledge the valuable insights and support from Professor Andrew Maas and Dr. Hester Lingsma in relation to the use of IMPACT algorithms and calculations for determining baseline TBI severity.
Contributor Information
Collaborators: for the DECRA Trial Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, P. D'Urso, T. Kossmann, P. Myles, R. Bellomo, J. Santamaria, P. Komasarof, B. Byrne, P. Simpson, S. Vallance, B. Howe, M. Kishi, P. Hwang, V. Pellegrino, S. Al-Qahtani, A. Al-Ferayan, N. Russell, A. Rishu, G. Dobb, S. Honeybul, J. Chamberlain, A. Gould, G. McEntaggart, V. Casikar, S. Nair, K. Seex, L. Weisbrodt, P. Harrigan, R. Ferch, B. McFadyen, E. Taylor, M. Hardie, J. Laidlaw, C. MacIsaac, M. Robertson, J. Presneill, T. Caf, S. Finfer, M. Briggs, R. Lee, N. Little, A. O'Connor, C. Joyce, A. Nowitzke, B. Venkatesh, M. Harward, J. Helyar, D. Dinsdale, M. Hunn, L. Andrew, D. Mackle, S. Mortimer, C. McArthur, A. Law, S. Streat, L. Newby, B. Richards, T. Withers, M. Tallott, P.V. van Heerden, S. Baker, M. Pinder, N. Knuckey, B. Roberts, M. Parr, R. Calcroft, J. Van Gelder, M. Sheridan, S. Micallef, N. Edwards, B. Brophy, M. Chapman, S. O'Connor, S. Henderson, R. Boet, N. Finnis, M. Macfarlane, and J. Mehrtens
The DECRA Trial Committees and Members
Co-Chief Investigators: D.J. Cooper, J.V. Rosenfeld; Executive Committee: D.J. Cooper (Chair), J.V. Rosenfeld, L. Murray, Y.M. Arabi, A.R. Davies, P. D'Urso, T. Kossmann, J. Ponsford, I. Seppelt, P. Reilly, R. Wolfe; Data and Safety Monitoring Committee: P. Myles (Chair), R. Bellomo, J. Santamaria, P. Komasarof, B. Byrne; Study Statistical Center: Monash University, Melbourne, Australia: R. Wolfe, P. Simpson; Study Coordinating Center: The Alfred Hospital, Melbourne, Australia; Outcome Assessors: S.Vallance, B. Howe, M. Kishi; Site Investigators (in order of patient numbers at each site recruited into the DECRA trial): Alfred Hospital, Melbourne, Australia: D.J. Cooper, J.V. Rosenfeld, L. Murray, A.R. Davies, P. Hwang, V. Pellegrino; King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Kingdom of Saudi Arabia: Y.M. Arabi, S. Al-Qahtani, A. Al-Ferayan, N. Russell, A. Rishu; Royal Perth Hospital, Perth, Australia: G. Dobb, S. Honeybul, J. Chamberlain, A. Gould, G. McEntaggart; Nepean Hospital, Sydney, Australia: I. Seppelt, V. Casikar, S. Nair, K. Seex, L. Weisbrodt; John Hunter Hospital, Newcastle, Australia: P. Harrigan, R. Ferch, B. McFadyen, E. Taylor, M. Hardie; Royal Melbourne Hospital, Melbourne, Australia: J. Laidlaw, C. MacIsaac, M. Robertson, J. Presneill, T. Caf; Royal North Shore Hospital, Sydney, Australia: S. Finfer, M. Briggs, R. Lee, N. Little, A. O'Connor; Princess Alexandra Hospital, Brisbane, Australia: C. Joyce, A. Nowitzke, B. Venkatesh, M. Harward, J. Helyar; Wellington Hospital, Wellington, New Zealand: D. Dinsdale, M. Hunn, L. Andrew, D. Mackle, S. Mortimer; Auckland Hospital, Auckland New Zealand: C. McArthur, A. Law, S. Streat, L. Newby; Gold Coast Hospital, Southport, Queensland: B. Richards, T. Withers, M. Tallott; Sir Charles Gairdner Hospital, Perth, Australia: P.V. van Heerden, S. Baker, M. Pinder, N. Knuckey, B. Roberts; Liverpool Hospital, Sydney, Australia: M. Parr, R. Calcroft, J. Van Gelder, M. Sheridan, S. Micallef; Royal Adelaide Hospital, Adelaide, Australia: N. Edwards, B. Brophy, M. Chapman, P. Reilly, S. O'Connor; Christchurch Hospital, Christchurch, New Zealand: S. Henderson, R. Boet, N. Finnis, M. Macfarlane, J. Mehrtens. The following hospitals commenced screening but did not enrol patients: Australia: Westmead Hospital, Sydney, Royal Hobart Hospital, Hobart, Wollongong Hospital, Wollongong, Flinders Medical Centre, Adelaide; New Zealand: Waikato Hospital, Hamilton; Canada: Vancouver General Hospital, Vancouver, Royal Columbian Hospital, Vancouver, Sunnybrook Medical Centre, Toronto, Hamilton General Hospital, Hamilton; U.S.A.: Baltimore Shock Trauma Center, Maryland, University of Pennsylvania, Pennsylvania.
Funding Information
The DECRA trial was supported by grants from the National Health and Medical Research Council of Australia (NHMRC 314502), the Transport Accident Commission of Victoria (Victorian Trauma Foundation and Victorian Neurotrauma Initiative), the Intensive Care Foundation (Australia), and the Western Australian Institute for Medical Research. DECRA Australian Clinical Trials registry number, ACTRN012605000009617.
Author Disclosure Statement
Dr. Cooper reports receiving consulting fees from Pressura Neuro to Monash University for an unrelated TBI drug trial.
References
- 1. Access Economics. The economic cost of spinal cord injury and traumatic brain injury in Australia. (2009). Victorian Neurotrauma Initiative, Transport Accident Commission Victoria. Canberra, ACT, Australia. https://www.spinalcure.org.au/pdf/Economic-cost-of-SCI-and-TBI-in-Au-2009.pdf (Last accessed February20, 2020)
- 2. Myburgh J.A., Cooper D.J., Finfer S.R., Venkatesh B., Jones D., Higgins A., Bishop N., and Higlett T. (2008). Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J. Trauma 64, 854–862 [DOI] [PubMed] [Google Scholar]
- 3. Murray G.D., Teasdale G.M., Braakman R., Cohadon F., Dearden M., Iannotti F., Karimi A., Lapierre F., Maas A., Ohman J., Persson L., Servadei F., Stocchetti N., Trojanowski T., and Unterberg A. (1999). The European Brain Injury Consortium survey of head injuries. Acta Neurochir. (Wien) 141, 223–236 [DOI] [PubMed] [Google Scholar]
- 4. Stocchetti N., Zanaboni C., Colombo A., Citerio G., Beretta L., Ghisoni L., Zanier E.R., and Canavesi K. (2008). Refractory intracranial hypertension and ″second-tier″ therapies in traumatic brain injury. Intensive Care Med. 34, 461–467 [DOI] [PubMed] [Google Scholar]
- 5. Maas A.I., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. (2007). Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24 (Suppl. 1), S1–S106 [DOI] [PubMed] [Google Scholar]
- 7. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J. (2017). Brain Trauma Foundation TBI Guidelines. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 8. Carney N., Totten A.M., O'Reilly C., Ullman J.S., Hawryluk G.W., Bell M.J., Bratton S.L., Chesnut R., Harris O.A., Kissoon N., Rubiano A.M., Shutter L., Tasker R.C., Vavilala M.S., Wilberger J., Wright D.W., and Ghajar J (2016). Brain Trauma Foundation. Guidelines for the Management of Severe Traumatic Brain Injury, 4th ed. https://www.braintrauma.org/uploads/13/06/Guidelines_for_Management_of_Severe_TBI_4th_Edition.pdf (Last accessed February20, 2020)
- 9. Okonkwo D.O., Shutter L.A., Moore C., Temkin N.R., Puccio A.M., Madden C.J., Andaluz N., Chesnut R.M., Bullock M.R., Grant G.A., McGregor J., Weaver M., Jallo J., LeRoux P.D., Moberg D., Barber J., Lazaridis C., and Diaz-Arrastia R.R. (2017). Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit. Care Med. 45, 1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahuquillo J., and Arikan F. (2006). Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst. Rev., CD003983. [DOI] [PubMed] [Google Scholar]
- 11. Hutchinson P.J., Kolias A.G., Timofeev I.S., Corteen E.A., Czosnyka M., Timothy J., Anderson I., Bulters D.O., Belli A., Eynon C.A., Wadley J., Mendelow A.D., Mitchell P.M., Wilson M.H., Critchley G., Sahuquillo J., Unterberg A., Servadei F., Teasdale G.M., Pickard J.D., Menon D.K., Murray G.D., and Kirkpatrick P.J. (2016). Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 375, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 12. Cooper D.J., Rosenfeld J.V., Murray L., Arabi Y.M., Davies A.R., D'Urso P., Kossmann T., Ponsford J., Seppelt I., Reilly P., and Wolfe R. (2011). Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 364, 1493–1502 [DOI] [PubMed] [Google Scholar]
- 13. (2007). Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J. Neurotrauma 24 (Suppl. 1), S55–S58 [DOI] [PubMed] [Google Scholar]
- 14. The DECRA (DECompressive CRAniectomy trial) protocol Version 29, July 4, 2007, Sections 4.7 and 7. http://www.nejm.org/doi/suppl/10.1056/NEJMoa1102077/suppl_file/nejmoa1102077_protocol.pdf (Last accessed February20, 2020)
- 15. Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 16. Kjellberg R.N., and Prieto A. (1971). Bifrontal decompressive craniotomy for massive cerebral edema. J. Neurosurg. 34, 488–493 [DOI] [PubMed] [Google Scholar]
- 17. Polin R.S., Shaffrey M.E., Bogaev C.A., Tisdale N., Germanson T., Bocchicchio B., and Jane J.A. (1997). Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery 41, 84–92; discussion 92–84. [DOI] [PubMed] [Google Scholar]
- 18. Cooper D.J., Rosenfeld J.V., Murray L., Wolfe R., Ponsford J., Davies A., D'Urso P., Pellegrino V., Malham G., and Kossmann T. (2008). Early decompressive craniectomy for patients with severe traumatic brain injury and refractory intracranial hypertension—a pilot randomized trial. J. Crit. Care 23, 387–393 [DOI] [PubMed] [Google Scholar]
- 19. Pettigrew L.E., Wilson J.T., and Teasdale G.M. (2003). Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J. Head Trauma Rehabil. 18, 252–258 [DOI] [PubMed] [Google Scholar]
- 20. Teasdale G.M., Pettigrew L.E., Wilson J.T., Murray G., and Jennett B. (1998). Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma 15, 587–597 [DOI] [PubMed] [Google Scholar]
- 21. Maas A.I., Steyerberg E.W., Marmarou A., McHugh G.S., Lingsma H.F., Butcher I., Lu J., Weir J., Roozenbeek B., and Murray G.D. (2010). IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 7, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper D.J., Rosenfeld J.V., Murray L., Arabi Y.M., Davies A.R., D'Urso P., Kossmann T., Ponsford J., Seppelt I., Reilly P., and Wolfe R. (2011). Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 364, 1493–1502. Supplementary Appendix. https://www.nejm.org/doi/suppl/10.1056/NEJMoa1102077/suppl_file/nejmoa1102077_appendix.pdf (Last accessed February20, 2020) [DOI] [PubMed] [Google Scholar]
- 23. Stocchetti N., Poole D., and Okonkwo D.O. (2018). Intracranial pressure thresholds in severe traumatic brain injury: we are not sure: prudent clinical practice despite dogma or nihilism. Intensive Care Med. 44, 1321–1323 [DOI] [PubMed] [Google Scholar]
- 24. Hutchinson P.J., Kolias A.G., Tajsic T., Adeleye A., Aklilu A.T., Apriawan T., Bajamal A.H., Barthelemy E.J., Devi B.I., Bhat D., Bulters D., Chesnut R., Citerio G., Cooper D.J., Czosnyka M., Edem I., El-Ghandour N.M.F., Figaji A., Fountas K.N., Gallagher C., Hawryluk G.W.J., Iaccarino C., Joseph M., Khan T., Laeke T., Levchenko O., Liu B., Liu W., Maas A., Manley G.T., Manson P., Mazzeo A.T., Menon D.K., Michael D.B., Muehlschlegel S., Okonkwo D.O., Park K.B., Rosenfeld J.V., Rosseau G., Rubiano A.M., Shabani H.K., Stocchetti N., Timmons S.D., Timofeev I., Uff C., Ullman J.S., Valadka A., Waran V., Wells A., Wilson M.H., and Servadei F. (2019). Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: consensus statement. Acta Neurochir. (Wien) 161, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kano T., Kurosaki S., and Wada H. (2012). Retrospective analysis of hinge technique for head trauma or stroke. Neurol. Med. Chir (Tokyo) 52, 816–821 [DOI] [PubMed] [Google Scholar]
- 26. Kenning T.J., Gooch M.R., Gandhi R.H., Shaikh M.P., Boulos A.S., and German J.W. (2012). Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy. J. Neurosurg. 116, 1289–1298 [DOI] [PubMed] [Google Scholar]
- 27. Valenca M.M., Martins C., and da Silva J.C. (2010). ″In-window″ craniotomy and ″bridgelike″ duraplasty: an alternative to decompressive hemicraniectomy. J. Neurosurg. 113, 982–989 [DOI] [PubMed] [Google Scholar]
- 28. Timmons S.D., Ullman J.S., and Eisenberg H.M. (2011). Craniectomy in diffuse traumatic brain injury. N. Engl J. Med. 365, 373; author reply, 376. [DOI] [PubMed] [Google Scholar]