Abstract
Nonalcoholic fatty liver disease (NAFLD) is present in approximately 25% of the population worldwide. It is characterized by the accumulation of triacylglycerol in the liver, which can progress to steatohepatitis with different degrees of fibrosis, stages that lack approved pharmacological therapies and represent an indication for liver transplantation with consistently increasing frequency. In view that hepatic steatosis is a reversible condition, effective strategies preventing disease progression were addressed using combinations of natural products in the preclinical high-fat diet (HFD) protocol (60% of fat for 12 weeks). Among them, eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:5n-3, DHA), DHA and extra virgin olive oil (EVOO), or EPA plus hydroxytyrosol (HT) attained 66% to 83% diminution in HFD-induced steatosis, with the concomitant inhibition of the proinflammatory state associated with steatosis. These supplementations trigger different molecular mechanisms that modify antioxidant, antisteatotic, and anti-inflammatory responses, and in the case of DHA and HT co-administration, prevent NAFLD. It is concluded that future studies in NAFLD patients using combined supplementations such as DHA plus HT are warranted to prevent liver steatosis, thus avoiding its progression into more unmanageable stages of the disease.
Keywords: extra virgin olive oil, hydroxytyrosol, n-3 polyunsaturated fatty acids, nonalcoholic fatty liver disease
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is considered the hepatic expression of the metabolic syndrome, which is characterized by (i) the accumulation of triacylglycerol (TG) in the cytoplasm of hepatocytes; (ii) a 25% prevalence in the population worldwide; (iii) the manifestation of a spectrum of liver alterations including simple hepatic steatosis, steatosis with inflammation (steatohepatitis, NASH), and different degrees of fibrosis; (iv) the absence of approved pharmacological therapies; and (v) the estimation that 20%–30% of NAFLD patients may progress to NASH [1]. The latter inflammatory condition is estimated to evolve to cirrhosis in 7%–25% of the patients [1,2] and represents an indication for liver transplantation with a consistently increasing frequency [3]. In this scenario, the diminution in the energy intake with concomitant physical activity is the first strategy for the adequate handling of NAFLD. However, when these lifestyle changes are inefficient, pharmacological management, dietary interventions, or the use of different therapeutic agents has become the second line of attack [4].
Considering that hepatic steatosis is a reversible condition, effective therapies are required to avoid the progression of steatosis to NASH and fibrosis, otherwise causing chronic liver disease involving irreversible modifications [5,6]. Furthermore, NAFLD is a multifactorial illness that includes complex metabolic changes, not only in the liver but also in adipose tissue and muscle, which suggest that monotherapies are unlikely to elicit successful responses [4]. In view of these considerations, new therapeutic approaches were evaluated using the combination of bioactive compounds, a strategy that is characterized by (i) the use of lower doses of compounds than monotherapies with shorter supplementation periods to minimize possible side effects; and (ii) the involvement of compounds with protective effects that are exerted through different or similar mechanisms of action, thus allowing synergistic or additive actions and a more efficient control of the damaging effects [4,7]. For example, (i) preservation of liver tissue regeneration post-hepatectomy can be obtained by a L-3,3′,5-triiodothyronine (T3) plus methylprednisolone treatment [8]; (ii) high-fat diet (HFD)-induced liver steatosis can be diminished by n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA) and extra virgin olive oil (EVOO) [9]; (iii) combined T3 and fish oil supplementation suppresses ischemia-reperfusion inflammatory liver injury [10]; whereas (iv) resveratrol and enalapril improved glucose and lipid profiles by decreasing lipogenic gene expression [11]. Interestingly, an inverse correlation between serum free thyroxine (T4) levels and hepatic steatosis was established in overweight and obese patients [12] or with elevated serum thyroid-stimulating hormone concentrations in overweight/obese children [13], while higher baseline levels of T3 and T4 predict more weight loss, but not weigh regain, in overweight/obese patients with normal thyroid function subjected to weight loss diets [14]. Collectively, these evidences point to the concept that natural products, drugs, and thyroid hormones constitute hormetic agents or hormetins, which favor beneficial effects by acting at low dosages through one or more pathways of maintenance and repair, thus conferring resistance to subsequent, otherwise harmful, conditions of increased stress [15], including metabolic stress [16]. The aim of this article is to discuss recent data concerning the use of natural product co-administration in the prevention of NAFLD development.
2. Material and Methods
The review includes several literature searches that considered the metabolic and beneficial effects of n-3 LCPUFA, particularly eicosapentaenoic acid (C20:5n-3, EPA) and/or docosahexaenoic acid (C22:6n-3, DHA), EVOO, hydroxytyrosol (HT), n-3 LCPUFA plus EVOO, or n-3 LCPUFA plus HT using in vivo and in vitro models. Study searches were performed using the PubMed database from the National Library of Medicine—National Institutes of Health. Emphasis was placed on the participation of n-3 LCPUFA, EVOO, and HT as potential hepatoprotective compounds in liver steatosis models.
3. Influence of Energy Intake and Diet Composition on Liver Steatosis Development
Diet has a relevant role in the development and progression of NAFLD, since a high energy intake and consumption of specific nutrients have a direct impact on the abnormal accumulation of TG in the liver, a hallmark of NAFLD [4]. High intake of nutrients that include saturated fatty acids (FA) such as palmitic acid (C16:0) [17,18,19] and trans FA of industrial origin [20] decrease FA oxidation (FAO), stimulate the synthesis and secretion of TGs, and trigger lipotoxic effects in the liver [4,17,18]. Moreover, high intake of n-6 PUFA, especially linoleic acid (C18:2n-6), and low consumption of n-3 LCPUFA (EPA and DHA) also appear to favor the development of hepatic steatosis [21]. Furthermore, the consumption of fructose has significantly increased worldwide with the growth of processed foods using high fructose corn syrup [22]. In the liver, fructose metabolism is different from glucose metabolism and proceeds without regulation, thus providing excess acetyl units that promotes a hepatic prolipogenic state [23], with further ATP depletion, oxidative stress, n-3 LCPUFA depletion, and development of a proinflammatory state [24,25]. Moreover, derangements in liver iron and copper homeostasis are related to the development of NAFLD. An increase in liver iron levels is associated with advanced lesions in patients with NAFLD [26], a condition in which the levels of hepatic n- 3 LCPUFA are diminished in relation to the development of oxidative stress, triggering de novo lipogenesis over FAO [27]. Unlike iron, the content of hepatic copper is decreased in NAFLD patients, a situation that favors TGs and cholesterol biosynthesis [28,29], with the concomitant oxidative stress enhancement due to diminution in the antioxidant potential of the liver and the induction of iron overload [30].
4. Diminution of Liver Steatosis by Natural Products Co-Administration
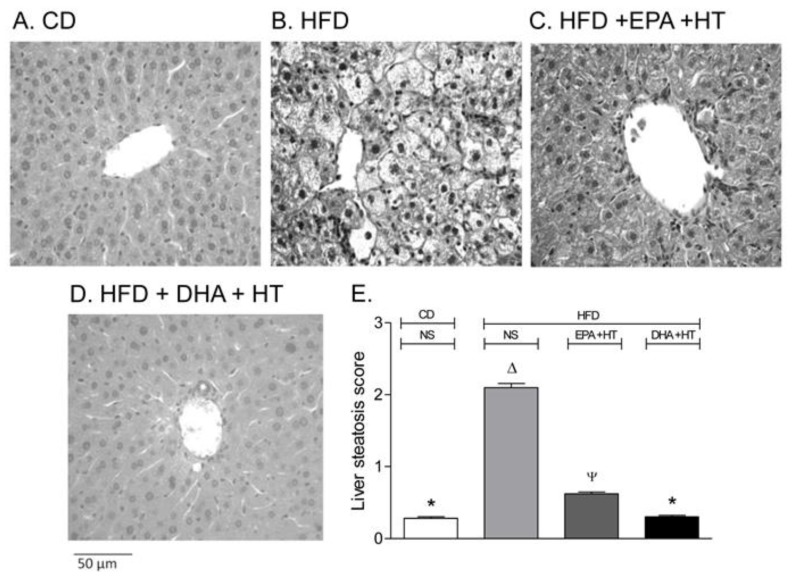
Mice subjected to HFDs comprising 60% of the total calories as saturated fat, mainly from lard, for 12 weeks is considered as a suitable experimental approach for liver steatosis development, similar to that found in NAFLD patients [31]. Under these conditions, fatty liver with a steatosis score of around 2 is developed (Figure 1A,B), which corresponds to 33% to 66% of hepatocytes infiltrated with fat [32]. Concomitantly, HFD did not alter serum transaminase levels or induce overt hepatic inflammatory hallmarks; however, liver oxidative stress and inflammatory cytokine expression were significantly enhanced, thus inducing a proinflammatory state [33]. HFD-induced liver steatosis is diminished by 66% to 83% with EPA plus DHA [34,35,36], DHA plus EVOO [37], or EPA plus hydroxytyrosol (HT) supplementations (Figure 1C) [38,39,40]. The attenuation of HFD-triggered hepatic steatosis by these combinations is comparable to the sum of effects elicited by the separate supplementations, thus reaching additive responses [37,38,39,40].
Figure 1.
Liver Histological assessment in mice subjected to (A) control diet (CD), (B) high-fat diet (HFD) without supplementation (NS), (C) HFD supplemented with eicosapentaenoic acid (EPA) plus hydroxytyrosol (HT) and (D) HFD, supplemented with docosahexaenoic acid (DHA) plus HT. Weaning male C57BL/6J mice (n = 7 per experimental group) were allowed free access to a CD (10% fat, 20% protein, and 70% carbohydrate, with a caloric values of 3.85 KcaL/g; Rodent Diet, Product data D12450B and D12492, Research Diet Inc., USA) or HFD (60% fat, 20% protein, and 20% carbohydrate, with a caloric values of 5.24 Kcal/g; Rodent Diet, Product data D12492, Research Diet Inc., USA) for 12 weeks. Animals subjected to CD (not shown) or HFD were simultaneously supplemented with EPA (50 mg/kg/day) plus HT (5 mg/kg/day) or DHA (50 mg/kg/day) plus HT (5 mg/kg/day) through gavage. Liver samples were fixed in phosphate-buffered formalin, embedded in paraffin, stained with haematoxylin-eosin, and analyzed by optical microscopy in blind fashion describing the presence of steatosis, graded according to Brunt et al. [32]. (E) Liver steatosis scores (mean ± SEM; n = 7). a,b,c,d Groups sharing the same lyrics are not significantly different among them according to a two‐way ANOVA and Bonferroni’s post-test (p < 0.05). Adapted from Echeverría et al. [38] and Soto-Alarcón et al. [56].
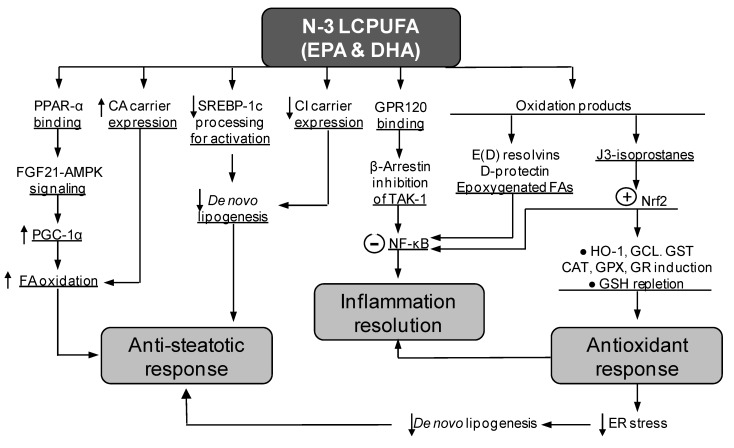
In the case of the co-administration of EPA and DHA, the partial anti-steatotic effects are elicited either when the supplementation is carried out along with the HFD for 12 weeks [33,35] (Figure 1C) or when animals given HFD for 12 weeks are subjected to a control diet plus EPA and DHA for 8 additional weeks [35]. These findings indicate that EPA + DHA supplementation partially prevents fatty liver development, an effect that is associated with different molecular mechanisms triggered by the n-3 LCPUFA (Figure 2). EPA is a precursor of DHA [41], which contributes to the enhancement in liver DHA availability [33]. Compared with EPA, DHA exhibits a greater chemical reactivity [7] associated with the formation of active derivatives (Figure 2) [42,43,44] and affords more beneficial effects than EPA [45,46]. DHA binding to peroxisome proliferator-activated receptor-α (PPAR-α) leads to PPAR-α activation with enhanced binding capacity to DNA, promoting the expression of genes encoding for proteins involved in different aspects of FA metabolism [47]. In the liver, these include FA uptake through membranes, intracellular trafficking and activation, FAO, and phospholipid remodeling [48]. FAO is associated with upregulation of the hepatic energy sensing cascade involving fibroblast growth factor 21 (FGF21), AMPK-activated protein kinase (AMPK), and PPAR-γ coactivator-1α (PGC-1α) (Figure 2) [40], as PPAR-α also controls FGF21 expression [48]. Furthermore, elevated liver FAO is likely to be subsidized by the increased transcription of the carnitine/acylcarnitine carrier (CAC) gene elicited by n-3 LCPUFA (Figure 2), a component of the carnitine cycle catalyzing the transport of fatty acyl units into mitochondria to undergo oxidation [49]. Importantly, a recent report identified 25 genes that are dysregulated during steatosis progression to NASH, including the significant loss of those encoding for PPAR-α and PGC-1α, which drastically disturb mitochondrial function [50]. Furthermore, progression of steatosis to NASH decreases the content of liver LCPUFA by 59%, 78%, and 89% compared with control values in mice subjected to a Western diet (40% energy as fat) for 4, 10, and 24 weeks, respectively [51]. Besides supporting liver FAO, n-3 LCPUFA promote the decline of hepatic de novo lipogenesis through at least three mechanisms of action, namely, (i) diminution of the nuclear availability of the lipogenic transcription factor sterol regulatory element binding protein-1c (SREBP-1c) via AMPK-mediated serine-365 phosphorylation of nascent SREBP-1c, resulting in inhibition of the intramembrane proteolysis of the nascent SREBP-1c (Figure 2; [52]); (ii) DHA-dependent downregulation of the expression of SREBP-1c and target lipogenic enzymes through interaction with G-protein-coupled receptor 40 (GPR40) [53]; and (iii) n-3 LCPUFA-dependent repression of the citrate carrier (CIC) expression secondary to SREBP-1c downregulation, thus decreasing the transport of mitochondrial acetyl-CoA units as citrate outside mitochondria for FA synthesis (Figure 2) [49]. These findings support the contention that HFD-induced hepatic steatosis is partly decreased by n-3 LCPUFA in association with a change in the prolipogenic pattern of the liver imposed by HFD, through the establishment of a significantly lower SREBP-1c/PPAR-α ratio [54]. Notably, liver SREBP-1c upregulation, PPAR-α downregulation, and depletion of n-3 LCPUFA are also observed in obese patients with NAFLD, the respective SREBP-1c/PPAR-α ratios being inversely correlated with the n-3 LCPUFA levels and directly associated with insulin resistance [55].
Figure 2.
Molecular mechanisms of the n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA) EPA and DHA explaining antisteatotic and antioxidant responses and inflammation prevention or resolution in the liver. Abbreviations: AMPK, AMP-activated protein kinase; CA carrier, carnitine/acylcarnitine carrier; CAT, catalase; CI carrier, citrate carrier; ER, endoplasmic reticulum; FA, fatty acids; FGF21, fibroblast growth factor 21; GCL, glutamate-cysteine ligase; GPR120, G-protein receptor 120; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GST, glutathione-S-transferase; HO-1, heme oxygenase-1; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PPAR-α, peroxisome proliferator-activated receptor-α; SREBP-1c, sterol regulatory element binding protein-1c; TAK-1, transforming growth factor-β-activated kinase-1.
Finally, the antisteatotic effect of n-3 LCPUFA supplementation is also related to their high susceptibility to undergo spontaneous lipid peroxidation with formation of J3-isoprostanes, which promote the activation of transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) [44]. Nrf2 activation triggers antioxidant defenses against the oxidative stress prevailing NAFLD [33,57], which may avoid (i) further n-3 LCPUFA depletion that favors steatosis development; and (ii) the induction of endoplasmic reticulum (ER) stress through protein oxidation/unfolding that upregulates lipogenic SREBP-1c and PPAR-γ expression [58]. Supporting the importance of the actions of n-3 LCPUFA on hepatic steatosis development, alterations in gut microbiota from fat-1 mice protect the liver against high-fat/high-sucrose diet-induced NAFLD [59]. These fat-1 transgenic mice encode an n-3 FA desaturase that converts n-6 to n-3 LCPUFA, thus endogenously increasing the levels of n-3 LCPUFA [59] and their antisteatotic signaling (Figure 2). In this respect, n-3 LCPUFA are considered as prebiotics influencing the composition of gut microbiota, which is altered following HFD-feeding (dysbiosis), thus representing potential agents able to restore eubiosis in the intestinal flora that may abrogate the pathological changes induced by HFD [60].
Besides, the antioxidant effects of n-3 LCPUFA allow the abrogation of the redox activation of nuclear factor-κB (NF-κB) that promotes inflammation expansion [61]. N-3 LCPUFA-induced anti-inflammatory responses are linked to the production of several oxidation products including EPA- and DHA-derived E-series and D-series of resolvins, DHA-derived D-protectins, and epoxygenated FA, acting as NF-κB downregulators (Figure 2) [62]. In this respect, EPA supplementation increases the hepatic levels of EPA and DHA, resulting in increased levels of resolvins RvE1/2 and RvD1/2 [39], whereas DHA elicited similar results in RvD1/2 availability [63]. The activation of NF-κB with induction of the inflammasome NOD-like receptor protein 3 (NLRP3) components is also a target of the n-3 LCPUFA-dependent actions limiting inflammation, which may involve (i) interference of NF-κB activation by activated PPAR-α [64]; (ii) inhibition of NLRP3 activation [65,66]; and (iii) a DHA-G-protein receptor 120 (GPR120) interaction [66] (Figure 2).
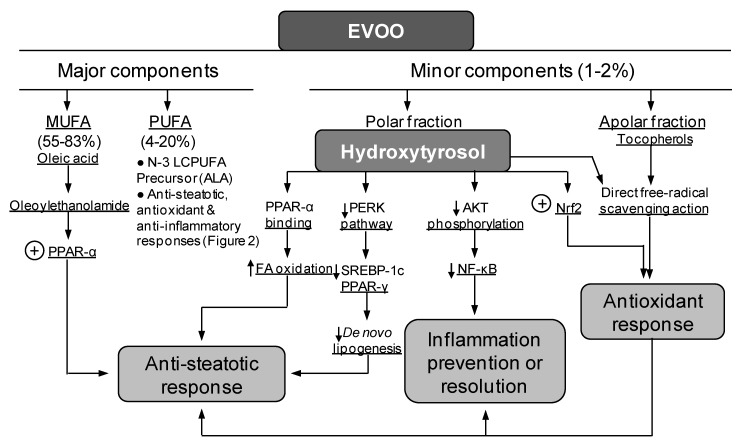
In addition to EPA and DHA co-administration, the combined DHA and EVOO [37] or EPA plus HT [37,38,39,40] administration also elicited a diminution of HFD-induced liver steatosis. The study using DHA plus EVOO revealed that HFD induced fat accumulation in more than 60% of the hepatocytes with 160% increase in the content of TG, both of which were diminished by 10%–20% and 47%, respectively, by DHA plus EVOO [37]. Mechanistically, the partial antisteatotic effect of DHA plus EVOO mainly relies on the processes set in by DHA (Figure 2), with EVOO having a secondary role at the dosages used [37]. Nonetheless, EVOO oil improves the postprandial glycemic and lipid profiles in patients with impaired fasting glucose [67]. Additionally, acute high-polyphenols EVOO intake is able to modify the transcriptome of peripheral blood mononuclear cells through the modulation of different pathways associated with the pathophysiology of cardio-metabolic disease and cancer [68]. In this context, the beneficial effects of EVOO on the liver are exerted through its major (monounsaturated FA (MUFA) and polyunsaturated FA (PUFA)) and minor (HT and tocopherols) constituents (Figure 3) [69,70,71]. Similarly to n-3 LCPUFA [46], EVOO attenuate diet-induced risks factors for metabolic syndrome, by favoring the relative abundance of prebiotic microbiota [72]. At the dosage used (50 mg/kg/day), EVOO supplementation alone did not modify liver PPAR-α signaling in control and HFD groups, whereas in mice subjected to HFD, it elicited minor changes in parameters related to de novo lipogenesis, oxidative stress, and inflammation [37]. A comparable situation occurs with the combined administration of EPA and HT, in which attenuation in HFD-induced steatosis development is achieved, an effect characterized by the additivity of EPA and HT effects that mainly depend on HT [37,38,39,40]. Although HT exerts beneficial outcomes associated with its significant direct free radical scavenging action [73,74] (Figure 3), alternate mechanisms of action involve (i) antioxidant responses via Nrf2 activation [75]; (ii) antisteatotic effects underlying PPAR-α [76] and FGF21 [77] upregulation and ER stress downregulation; [78] (iii) improvement of mitochondrial oxidative function through AMPK/PGC-1α signaling to favor FAO [40,75,79]; and (iv) prevention or resolution of inflammatory processes due to NF-κB deactivation [76,80].
Figure 3.
Protective molecular mechanisms associated with extra virgin olive oil (EVOO) in the liver through its major and minor components. Abbreviations: ALA, α-linolenic acid (C18:3n-3); MUFA, mono unsaturated fatty acids; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; PERK, double-stranded RNA-dependent protein kinase (PKR)-like endoplasmic reticulum kinase; PPAR-α(γ), peroxisome proliferator-activated receptor-α(γ); PUFA, polyunsaturated fatty acids; SREBP-1c, sterol regulatory element binding protein-1c.
5. Suppression of Liver Steatosis Development by the Co-Administration of Docosahexaenoic Acid and Hydroxytyrosol
The partial antisteatotic effect of the co-administration of natural products during HFD feeding, namely, EPA and DHA, DHA and EVOO, and EPA and HT [33,34,35,36,37,38,39,40], indicate that the threshold required to obtain effective functional responses is not attained. This may reflect that the interaction of the underlying mechanisms (Figure 2 and Figure 3) is inadequately exerted in relation to either the intracellular levels of the agents that are actually achieved or differences in their potency to trigger significant responses. In the case of the supplementation with DHA and HT, however, these factors seem to be overcome, since (i) liver DHA levels are higher than those of EPA under normal conditions [9,37,38,40]; (ii) DHA administration to control and HFD-fed mice reaches higher than basal DHA levels in the liver [37]; (iii) DHA is more reactive [7] and beneficial than EPA [45,46,81]; and (iv) the in vivo HT supplementation represents a greater HT dosage (5 mg/kg/day) [38,39,40] than that given as EVOO (Figure 3), thus inhibiting steatosis development (Figure 1D). Accordingly, under conditions of combined DHA and HT administration, an additive antisteatotic effect is attained, since the hepatic steatosis score induced by HFD is diminished by 64%, 38%, and 100% by DHA, HT, and DHA plus HT supplementation, respectively [56]. The above outcome of DHA plus HT involves the normalization of key hepatic metabolic functions that are deranged by HFD, namely, the upregulation of lipogenic SREBP-1c signaling and the downregulation of the pro-FAO action of PPAR-α, thus leading to basal SREBP-1c/PPAR-α ratios avoiding fat accumulation. Moreover, the pro-inflammatory status induced by HFD is also abrogated in relation to the regularization of the NF-κB signaling, which is reinforced by the significant enhancement in liver resolvin availability [56]. These two actions of the DHA and HT co-administration are associated with the normalization of the HFD-induced oxidative stress status, which is achieved through upregulation of the Nrf2 signaling and direct interception of free radicals (Figure 2 and Figure 3), thus avoiding DHA lipid peroxidation and NF-κB redox activation [56].
6. Conclusions
The preclinical studies discussed in this review suggest that the combined supplementation with natural products, including EPA and DHA, DHA and EVOO, or EPA plus HT, has a positive impact on NAFLD by diminishing hepatic fat deposition (Figure 1C) and the development of a proinflammatory state by HFD [34,35,36,37,38,39,40], with DHA and HT co-administration preventing liver steatosis development completely (Figure 1D) [56]. Although interventions with joined DHA and HT in human NAFLD are not available at present time, most, but not all, trials with n-3 LCPUFA show improvement in hepatic lipid deposition in adult and paediatric NAFLD patients within a 1 to 2 year time period, without diminution or exacerbation of NASH [54,82,83], DHA being more potent than EPA regarding the suppression of hepatic lipogenesis [84]. In relation to HT, (i) a study in healthy volunteers revealed that a dose of 15 mg/day for 3 weeks exerted positive effects on human health by diminishing parameters related to oxidative stress, with improvement in lipid and plasma profile [85]; (ii) a combined HT and vitamin E protocol improved hepatic steatosis and oxidative stress in children with NAFLD [86]; whereas (iii) the plasma levels of tissue inhibitor of metalloproteinases 1 (TIMP-1) in the group of patients receiving HT (15 mg/day for 63 days) were significantly lower than those levels found in the control group after the epirubicin-cyclophosphamide chemotherapy [87]. Recently, several meta-analyses established that n-3 LCPUFA supplementation (especially DHA) is useful in the dietary management of patients with NAFLD (adults and children), but additional trials are needed to better understand the effects of n-3 LCPUFA on histological outcomes in patients with NASH [88,89,90,91]. Additionally, new omics techniques have proven the beneficial effects of DHA (alone or co-administrated with other products) on hepatocyte lipidome [92]. These antecedents and the molecular mechanisms discussed for DHA and HT co-administration warrant future studies in NAFLD patients, to attained liver steatosis resolution, thus avoiding its progression into more unmanageable stages of the disease. The combined supplementation with DHA and HT may be also of importance in the prevention of metabolic dysregulations associated with the metabolic syndrome, type-2 diabetes, and cardiovascular disease, along with human autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and type-1 diabetes, due to the effective preventive effects demonstrated for n-3 LCPUFA in the area [93].
Acknowledgments
We would like to University of Chile and University of toronto by support this research. The authors also thank Richard Bazinet and Raphael Chouinard-Watkins for his comments and English editing.
Author Contributions
Conceptualization, R.V. and L.A.V.; writing original draft, R.V. and L.A.V.; writing review and editing, R.V. and L.A.V. All the authors have approved the final version of the article. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Fund for Scientific and Technological Development (Chile) FONDECYT grant 11140174 for financial support (to RV).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Tirosh O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell. Longev. 2018;2018:2548154. doi: 10.1155/2018/2548154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton M.R., Burns J.M., Pedersen R.A., Watt K.D., Heimbach J.K., Dierkhising R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Rodas M.C., Valenzuela R., Videla L.A. Relevant Aspects of Nutritional and Dietary Interventions in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2015;16:25168–25198. doi: 10.3390/ijms161025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vizuete J., Camero A., Malakouti M., Garapati K., Gutierrez J. Perspectives on Nonalcoholic Fatty Liver Disease: An Overview of Present and Future Therapies. J. Clin. Transl. Hepatol. 2017;5:67–75. doi: 10.14218/JCTH.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Videla L.A. Combined docosahexaenoic acid and thyroid hormone supplementation as a protocol supporting energy supply to precondition and afford protection against metabolic stress situations. IUBMB Life. 2019;71:1211–1220. doi: 10.1002/iub.2067. [DOI] [PubMed] [Google Scholar]
- 8.D’Espessailles A., Dossi C., Intriago G., Leiva P., Romanque P. Hormonal pretreatment preserves liver regenerative capacity and minimizes inflammation after partial hepatectomy. Ann. Hepatol. 2013;12:881–891. doi: 10.1016/S1665-2681(19)31293-1. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela R., Espinosa A., Llanos P., Hernandez-Rodas M.C., Barrera C., Vergara D., Romero N., Pérez F., Ruz M., Videla L.A. Anti-steatotic effects of an n-3 LCPUFA and extra virgin olive oil mixture in the liver of mice subjected to high-fat diet. Food Funct. 2016;7:140–150. doi: 10.1039/C5FO01086A. [DOI] [PubMed] [Google Scholar]
- 10.Mardones M., Valenzuela R., Romanque P., Covarrubias N., Anghileri F., Fernández V., Videla L.A., Tapia G. Prevention of liver ischemia reperfusion injury by a combined thyroid hormone and fish oil protocol. J. Nutr. Biochem. 2012;23:1113–1120. doi: 10.1016/j.jnutbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 11.De Almeida Pinheiro T., de Almeida Pinheiro T., Feltenberger J.D., Andrade J.M.O., Neves Ferreira E.C., De Farias Lelis D., Guimaraes A.L.S., de Paula A.M.B., Caldeira A.P., Sousa Santos S.H. Effects of Resveratrol and ACE Inhibitor Enalapril on Glucose and Lipid Profiles in Mice. Protein Pept. Lett. 2017;24:854–860. doi: 10.2174/0929866524666170728153600. [DOI] [PubMed] [Google Scholar]
- 12.Ittermann T., Haring R., Wallaschofski H., Baumeister S.E., Nauck M., Dörr M., Lerch M.M., Meyer zu Schwabedissen H.E., Rosskopf D., Völzke H. Inverse association between serum free thyroxine levels and hepatic steatosis: Results from the Study of Health in Pomerania. Thyroid. 2012;22:568–574. doi: 10.1089/thy.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacifico L., Bonci E., Ferraro F., Andreoli G., Bascetta S., Chiesa C. Hepatic steatosis and thyroid function tests in overweight and obese children. Int. J. Endocrinol. 2013;2013:381014. doi: 10.1155/2013/381014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G., Liang L., Bray G.A., Qi L., Hu F.B., Rood J., Sacks F.M., Sun Q. Thyroid hormones and changes in body weight and metabolic parameters in response to weight loss diets: The POUNDS LOST trial. Int. J. Obes. (Lond.) 2017;41:878–886. doi: 10.1038/ijo.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattan S.I. Hormesis in aging. Ageing Res. Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hayes D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007;61:147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- 17.Naguib G., Morris N., Yang S., Fryzek N., Haynes-Williams V., Huang W.A., Norman-Wheeler J., Rotman Y. Dietary fatty acid oxidation is decreased in non-alcoholic fatty liver disease: A palmitate breath test study. Liver Int. 2019 doi: 10.1111/liv.14309. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luukkonen P.K., Sädevirta S., Zhou Y., Kayser B., Ali A., Ahonen L., Lallukka S., Pelloux V., Gaggini M., Jian C., et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández E.Á., Kahl S., Seelig A., Begovatz P., Irmler M., Kupriyanova Y., Nowotny B., Nowotny P., Herder C., Barosa C., et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017;127:695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oteng A.B., Loregger A., van Weeghel M., Zelcer N., Kersten S. Industrial Trans Fatty Acids Stimulate SREBP2-Mediated Cholesterogenesis and Promote Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019;63:e1900385. doi: 10.1002/mnfr.201900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela R., Videla L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011;2:644–648. doi: 10.1039/c1fo10133a. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J.L., Diehl A.M., Johnson R.J., Abdelmalek M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jegatheesan P., De Bandt J. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients. 2017;9:230. doi: 10.3390/nu9030230. [DOI] [Google Scholar]
- 24.Siddiqui R.A., Xu Z., Harvey K.A., Pavlina T.M., Becker M.J., Zaloga G.P. Comparative study of the modulation of fructose/sucrose-induced hepatic steatosis by mixed lipid formulations varying in unsaturated fatty acid content. Metabolism (Lond.) 2015;12:41. doi: 10.1186/s12986-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ter Horst K.W., Serlie M.J. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients. 2017;9:981. doi: 10.3390/nu9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson J.E., Klintworth H., Kowdley K.V. Iron metabolism in Nonalcoholic Fatty Liver Disease. Curr. Gastroenterol. Rep. 2012;14:8–16. doi: 10.1007/s11894-011-0234-4. [DOI] [PubMed] [Google Scholar]
- 27.Barrera C., Valenzuela R., Rincón M.Á., Espinosa A., Echeverria F., Romero N., Gonzalez-Mañan D., Videla L.A. Molecular mechanisms related to the hepatoprotective effects of antioxidant-rich extra virgin olive oil supplementation in rats subjected to short-term iron administration. Free Radic. Biol. Med. 2018;126:313–321. doi: 10.1016/j.freeradbiomed.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Aigner E., Strasser M., Haufe H., Sonnweber T., Hohla F., Stadlmayr A., Solioz M., Tilg H., Patsch W., Weiss G., et al. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am. J. Gastroenterol. 2010;105:1978–1985. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 29.Aigner E., Theurl I., Haufe H., Seifert M., Hohla F., Scharinger L., Stickel F., Mourlane F., Weiss G., Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680–688. doi: 10.1053/j.gastro.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Al-Othman A.A., Rosenstein F., Lei K.Y. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc. Soc. Exp. Biol. Med. 1993;204:97–103. doi: 10.3181/00379727-204-43640. [DOI] [PubMed] [Google Scholar]
- 31.Lau J.K., Zhang X., Yu J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2017;241:36–44. doi: 10.1002/path.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 33.Valenzuela R., Espinosa A., González-Mañán D., D’Espessailles A., Fernández V., Videla L.A., Tapia G. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS ONE. 2012;7:e46400. doi: 10.1371/journal.pone.0046400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tapia G., Valenzuela R., Espinosa A., Romanque P., Dossi C., Gonzalez-Mañán D., Videla L.A., D’Espessailles A. N-3 long-chain PUFA supplementation prevents high fat diet induced mouse liver steatosis and inflammation in relation to PPAR-α upregulation and NF-κB DNA binding abrogation. Mol. Nutr. Food Res. 2014;58:1333–1341. doi: 10.1002/mnfr.201300458. [DOI] [PubMed] [Google Scholar]
- 35.Dossi C.G., Tapia G.S., Espinosa A., Videla L.A., D’Espessailles A. Reversal of high-fat diet-induced hepatic steatosis by n-3 LCPUFA: Role of PPAR-α and SREBP-1c. J. Nutr. Biochem. 2014;25:977–984. doi: 10.1016/j.jnutbio.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Soni N.K., Nookaew I., Sandberg A.S., Gabrielsson B.G. Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 2015;14:74. doi: 10.1186/s12944-015-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Rodas M.C., Valenzuela R., Echeverría F., Rincón-Cervera M.Á., Espinosa A., Illesca P., Muñoz P., Corbari A., Romero N., Gonzalez-Mañan D., et al. Supplementation with Docosahexaenoic Acid and Extra Virgin Olive Oil Prevents Liver Steatosis Induced by a High-Fat Diet in Mice through PPAR-α and Nrf2 Upregulation with Concomitant SREBP-1c and NF-kB Downregulation. Mol. Nutr. Food Res. 2017;61:1700479. doi: 10.1002/mnfr.201700479. [DOI] [PubMed] [Google Scholar]
- 38.Echeverría F., Valenzuela R., Bustamante A., Álvarez D., Ortiz M., Soto-Alarcon S.A., Muñoz P., Corbari A., Videla L.A. Attenuation of High-Fat Diet-Induced Rat Liver Oxidative Stress and Steatosis by Combined Hydroxytyrosol-(HT-) Eicosapentaenoic Acid Supplementation Mainly Relies on HT. Oxid. Med. Cell. Longev. 2018;2018:5109503. doi: 10.1155/2018/5109503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echeverría F., Valenzuela R., Espinosa A., Bustamante A., Álvarez D., Gonzalez-Mañan D., Ortiz M., Soto-Alarcon S.A., Videla L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019;63:35–43. doi: 10.1016/j.jnutbio.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Echeverría F., Valenzuela R., Bustamante A., Álvarez D., Ortiz M., Espinosa A., Illesca P., Gonzalez-Mañan D., Videla L.A. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: Attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food Funct. 2019;10:6170–6183. doi: 10.1039/C9FO01373C. [DOI] [PubMed] [Google Scholar]
- 41.Lee H., Park W.J. Unsaturated fatty acids, desaturases, and human health. J. Med. Food. 2014;17:189–197. doi: 10.1089/jmf.2013.2917. [DOI] [PubMed] [Google Scholar]
- 42.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calder P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016;69(Suppl. 11):7–21. doi: 10.1159/000448262. [DOI] [PubMed] [Google Scholar]
- 44.Gao L., Wang J., Sekhar K.R., Yin H., Yared N.F., Schneider S.N., Sasi S., Dalton T.P., Anderson M.E., Chan J.Y., et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007;282:2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 45.Depner C.M., Traber M.G., Bobe G., Kensicki E., Bohren K.M., Milne G., Jump D.B. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in LDLR-/-mice. PLoS ONE. 2013;8:e83756. doi: 10.1371/journal.pone.0083756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song J., Li C., Lv Y., Zhang Y., Amakye W.K., Mao L. DHA increases adiponectin expression more effectively than EPA at relative low concentrations by regulating PPARγ and its phosphorylation at Ser273 in 3T3-L1 adipocytes. Nutr. Metab. 2017;14:52. doi: 10.1186/s12986-017-0209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Videla L.A., Pettinelli P. Misregulation of PPAR Functioning and Its Pathogenic Consequences Associated with Nonalcoholic Fatty Liver Disease in Human Obesity. PPAR Res. 2012;2012:107434. doi: 10.1155/2012/107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Iacobazzi V., Infantino V., Palmieri F. Transcriptional Regulation of the Mitochondrial Citrate and Carnitine/Acylcarnitine Transporters: Two Genes Involved in Fatty Acid Biosynthesis and β-oxidation. Biology. 2013;2:284–303. doi: 10.3390/biology2010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desterke C., Chiappini F. Lipid Related Genes Altered in NASH Connect Inflammation in Liver Pathogenesis Progression to HCC: A Canonical Pathway. Int. J. Mol. Sci. 2019;20:5594. doi: 10.3390/ijms20225594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xavier A., Zacconi F., Gainza C., Cabrera D., Arrese M., Uribe S., Sing-Long C., Andia M.E. Intrahepatic fatty acids composition as a biomarker of NAFLD progression from steatosis to NASH by using 1H-MRS. RSC Adv. 2019;9:42132. doi: 10.1039/C9RA08914D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng X., Dong Q., Bridges D., Raghow R., Park E.A., Elam M.B. Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase. Biophys. Acta. 2015;1851:1521–1529. doi: 10.1016/j.bbalip.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 53.On S., Kim H.Y., Kim H.S., Park J., Kang K.W. Involvement of G-Protein-Coupled Receptor 40 in the Inhibitory Effects of Docosahexaenoic Acid on SREBP1-Mediated Lipogenic Enzyme Expression in Primary Hepatocytes. Int. J. Mol. Sci. 2019;20:2625. doi: 10.3390/ijms20112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valenzuela R., Ortiz M., Hernández-Rodas M.C., Echeverría F., Videla L.A. Targeting n-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease. Curr. Med. Chem. 2019 doi: 10.2174/0929867326666190410121716. in press. [DOI] [PubMed] [Google Scholar]
- 55.Pettinelli P., Del Pozo T., Araya J., Rodrigo R., Araya A.V., Smok G., Csendes A., Gutierrez L., Rojas J., Korn O., et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: Correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta. 2009;1792:1080–1086. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Soto-Alarcón S.A., Ortiz M., Orellana P., Echeverría F., Bustamante A., Espinosa A., Illesca P., Gonzalez-Mañán D., Valenzuela R., Videla L.A. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevents liver steatosis and related parameters in mice subjected to high-fat diet: A molecular approach. Biofactors. 2019;45:930–943. doi: 10.1002/biof.1556. [DOI] [PubMed] [Google Scholar]
- 57.Videla L.A., Rodrigo R., Orellana M., Fernandez V., Tapia G., Quiñones L., Varela N., Contreras J., Lazarte R., Csendes A., et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. (Lond.) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 58.Zheng Z., Zhang C., Zhang K. Role of unfolded protein response in lipogenesis. World J. Hepatol. 2010;2:203–207. doi: 10.4254/wjh.v2.i6.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bidu C., Escoula Q., Bellenger S., Spor A., Galan M., Geissler A., Bouchot A., Dardevet D., Morio B., Cani P.D., et al. The Transplantation of ω3 PUFA-Altered Gut Microbiota of fat-1 Mice to Wild-Type Littermates Prevents Obesity and Associated Metabolic Disorders. Diabetes. 2018;67:1512–1523. doi: 10.2337/db17-1488. [DOI] [PubMed] [Google Scholar]
- 60.Costantini L., Molinari R., Farinon B., Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017;18:2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bieghs V., Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013;34:446–452. doi: 10.1016/j.it.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 62.De Roos B., Mavrommatis Y., Brouwer I.A. Long-chain n-3 polyunsaturated fatty acids: New insights into mechanisms relating to inflammation and coronary heart disease. Br. J. Pharmacol. 2009;158:413–428. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Videla L.A., Vargas R., Valenzuela R., Muñoz P., Corbari A., Hernandez-Rodas M.C. Combined administration of docosahexaenoic acid and thyroid hormone synergistically enhances rat liver levels of resolvins RvD1 and RvD2. Prostaglandins Leukot. Essent. Fatty Acids. 2019;140:42–46. doi: 10.1016/j.plefa.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Videla L.A. Liver NF-κB and AP-1 activation and PPAR-α expression are negatively correlated in obese patients: Pro-inflammatory implications. Clin. Nutr. 2010;29:687–688. doi: 10.1016/j.clnu.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Williams-Bey Y., Boularan C., Vural A., Huang N.N., Hwang I.Y., Shan-Shi C., Kehrl J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sui Y.H., Luo W.J., Xu Q.Y., Hua J. Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation. World J. Gastroenterol. 2016;22:2533–2544. doi: 10.3748/wjg.v22.i8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Amore S., Vacca M., Cariello M., Graziano G., D’Orazio A., Salvia R., Sasso R.C., Sabbà C., Palasciano G., Moschetta A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta. 2016;1861:1671–1680. doi: 10.1016/j.bbalip.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Carnevale R., Loffredo L., Del Ben M., Angelico F., Nocella C., Petruccioli A., Bartimoccia S., Monticolo R., Cava E., Violi F. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin. Nutr. 2017;36:782–787. doi: 10.1016/j.clnu.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez S., Bermudez B., Montserrat-de la Paz S., Jaramillo S., Varela L.M., Ortega-Gomez A., Abia R., Muriana F.J. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta. 2014;1838:1638–1656. doi: 10.1016/j.bbamem.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Soto-Alarcon S.A., Valenzuela R., Valenzuela A., Videla L.A. Liver Protective Effects of Extra Virgin Olive Oil: Interaction between Its Chemical Composition and the Cell-signaling Pathways Involved in Protection. Endocr. Metab. Immune Disord. Drug Targets. 2018;18:75–84. doi: 10.2174/1871530317666171114120552. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Z., Shi A., Wang Q., Zhou J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients. 2019;11:3005. doi: 10.3390/nu11123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Echeverría F., Ortiz M., Valenzuela R., Videla L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017;18:930. doi: 10.3390/ijms18050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marković A.K., Torić J., Barbarić M., Jakobušić C.B. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2019;24:2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu L., Liu Z., Feng Z., Hao J., Shen W., Li X., Sun L., Sharman E., Wang Y., Wertz K., et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010;21:1089–1098. doi: 10.1016/j.jnutbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Pirozzi C., Lama A., Simeoli R., Paciello O., Pagano T.B., Mollica M.P., Di Guida F., Russo R., Magliocca S., Canani R.B., et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016;30:108–115. doi: 10.1016/j.jnutbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 77.López de Las Hazas M.C., Martin-Hernández R., Crespo M.C., Tomé-Carneiro J., Del Pozo-Acebo L., Ruiz-Roso M.B., Escola-Gil J.C., Osada J., Portillo M.P., Martinez J.A., et al. Identification and validation of common molecular targets of hydroxytyrosol. Food Funct. 2019;10:4897–4910. doi: 10.1039/c9fo01159e. [DOI] [PubMed] [Google Scholar]
- 78.Giordano E., Davalos A., Nicod N., Visioli F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014;58:954–962. doi: 10.1002/mnfr.201300465. [DOI] [PubMed] [Google Scholar]
- 79.Zheng A., Li H., Xu J., Cao K., Li H., Pu W., Yang Z., Peng Y., Long J., Liu J., et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015;113:1667–1676. doi: 10.1017/S0007114515000884. [DOI] [PubMed] [Google Scholar]
- 80.Lopez S., Montserrat-de la Paz S., Lucas R., Bermudez B., Abia R., Morales J.C., Muriana F.J.G. Effect of metabolites of hydroxytyrosol on protection against oxidative stress and inflammation in human endothelial cells. J. Funct. Foods. 2017;29:238–247. doi: 10.1016/j.jff.2016.12.033. [DOI] [Google Scholar]
- 81.Jacobson T.A., Glickstein S.B., Rowe J.D., Soni P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J. Clin. Lipidol. 2012;6:5–18. doi: 10.1016/j.jacl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 82.Parker H.M., Johnson N.A., Burdon C.A., Cohn J.S., O’Connor H.T., George J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Hepatology. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Caldwell S. NASH Therapy: Omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin. Mol. Hepatol. 2017;23:103–108. doi: 10.3350/cmh.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelley N.S. Treatment of Nonalcoholic Fatty Liver Disease with Long-Chain n-3 Polyunsaturated Fatty Acids in Humans. Metab. Syndr. Relat. Disord. 2016;14:417–430. doi: 10.1089/met.2016.0051. [DOI] [PubMed] [Google Scholar]
- 85.Colica C., Di Renzo L., Trombetta D., Smeriglio A., Bernardini S., Cioccoloni G., Costa de Miranda R., Gualtieri P., Sinibaldi Salimei P., De Lorenzo A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017;2017:2473495. doi: 10.1155/2017/2473495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nobili V., Alisi A., Mosca A., Crudele A., Zaffina S., Denaro M., Smeriglio A., Trombetta D. The Antioxidant Effects of Hydroxytyrosol and Vitamin E on Pediatric Nonalcoholic Fatty Liver Disease, in a Clinical Trial: A New Treatment? Antioxid. Redox Signal. 2019;31:127–133. doi: 10.1089/ars.2018.7704. [DOI] [PubMed] [Google Scholar]
- 87.Ramirez-Tortosa C., Sanchez A., Perez-Ramirez C., Quiles J.L., Robles-Almazan M., Pulido-Moran M., Sanchez-Rovira P., Ramirez-Tortosa M. Hydroxytyrosol Supplementation Modifies Plasma Levels of Tissue Inhibitor of Metallopeptidase 1 in Women with Breast Cancer. Antioxidants. 2019;8:393. doi: 10.3390/antiox8090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo X.F., Yang B., Tang J., Li D. Fatty acid and non-alcoholic fatty liver disease: Meta-analyses of case-control and randomized controlled trials. Clin. Nutr. 2018;37:113–122. doi: 10.1016/j.clnu.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Musa-Veloso K., Venditti C., Lee H.Y., Darch M., Floyd S., West S., Simon R. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr. Rev. 2018;76:58–6021. doi: 10.1093/nutrit/nuy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu W., Li S., Li J., Wang J., Zhang R., Zhou Y., Yin Q., Zheng Y., Wang F., Xia Y., et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract. 2016;2016:1459790. doi: 10.1155/2016/1459790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen L.H., Wang Y.F., Xu Q.H., Chen S.S. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018;37:516–521. doi: 10.1016/j.clnu.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 92.Ghini V., Di Nunzio M., Tenori L., Valli V., Danesi F., Capozzi F., Luchinat C., Bordoni A. Evidence of a DHA Signature in the Lipidome and Metabolome of Human Hepatocytes. Int. J. Mol. Sci. 2017;18:359. doi: 10.3390/ijms18020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X., Bi X., Wang S., Zhang Z., Li F., Zhao A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019;10:2241. doi: 10.3389/fimmu.2019.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]