Abstract
Objectives:
We aimed to identify parameters of low-intensity vibration that initiate the greatest osteogenic response in dystrophin-deficient mice and determine vibration safety for diseased muscle in three separate studies.
Methods:
Study1: Mdx mice were randomized into seven vibration treatments and 14 d later, plasma osteocalcin and tibial osteogenic gene expression were compared among treatments. Study2: Three days of vibration was compared to other modalities known to elicit muscle injury in mdx mice. Study3: Dystrophic mice with more severe phenotypes due to altered utrophin were subjected to 7 d vibration to determine if muscle injury was induced. Muscle torque and genes associated with inflammation and myogenesis were assessed in Studies 2-3.
Results:
Two sets of parameters (45 Hz 0.6 g and 90 Hz 0.6 g) evoked osteogenic responses. 45 Hz upregulated alkaline phosphatase and tended to upregulate osteoprotegerin without altering RANKL, and 90 Hz simultaneously upregulated osteprotegerin and RANKL. Thus, subsequent muscle studies utilized 45 Hz. Vibration for 3 or 7 d was not injurious to dystrophic muscle as shown by the lack of differences between vibrated and non-vibrated mice in torque and gene expression.
Conclusions:
Results indicate that vibration at 45 Hz and 0.6 g is safe for dystrophic muscle and may be a therapeutic modality to improve musculoskeletal health in DMD.
Keywords: Bone, Duchenne Muscular Dystrophy, Mdx Mice, Dko Mice, Osteogenesis
Introduction
Bone and muscle are biomechanically and biochemically linked on many levels. As such, bone strength, geometry, and mass are largely determined by the mechanical influences of its associated muscles. In muscle diseases, such as Duchenne muscular dystrophy (DMD), decrements in bone mass are apparent in various skeletal regions as a result of muscle weakness and the consequential reductions in mechanical loading1–4. The loss of bone mass appears to compromise the strength of the bone, as indicated by the prevalence of fragility fractures, which occur after falling from standing or sitting heights1,5–8. The primary determinant of bone strength is geometry and this is altered in several skeletal regions of both patients and mouse models of DMD3,4,9,10. Exercise regimens such as running and jumping are typically prescribed to improve bone strength and geometry in healthy populations, however these activities are ill advised in patients with DMD due to their high susceptibility to fragility fractures in bone1,5,6,8 and concern for exercise-induced muscle damage11. Therefore, an alternative bone-sparing strategy is needed for DMD, one which is affective at improving bone strength and geometry, but also safe for muscle.
Low intensity vibration (i.e., less than 1 g of acceleration, where 1 g is equivalent to gravity) has been shown to have an anabolic effect on bone as well as prevent disuse-mediated bone loss12–15. However, some studies have failed to replicate these findings when utilizing similar acceleration and frequency parameters of vibration16–20. The conflicting results suggest that these parameters may need to be customized to the population of interest. That is, the optimal mechanical signal delivered by vibration to stimulate bone formation in effort to improve geometry and strength may differ depending on the underlying status of the bone and condition(s) that precipitated its decline.
Thus, prior to considering vibration as a bone-sparing modality for muscular dystrophy, our first study was designed to identify parameters of vibration that initiated the greatest osteogenic response in mdx mice. Mdx mice were selected because they have a bone phenotype across the lifespan similar to patients with DMD9,10,21. We have previously shown that cortical bone geometry and strength in mdx mice is altered 6 to 57% and that trabecular architecture is also affected by as much as 78%9. We chose circulating osteocalcin and CTX, and specific genes along the osteoblastic lineage as well as genes associated with the inhibition and activation of osteoclasts based on previous work19,22–24. In Study 1, we tested the hypothesis that mdx mice exposed to 14 daily bouts of low intensity vibration would have increase osteogenic gene expression and elevated circulating levels of osteocalcin compared to non-vibrated mdx mice.
After identifying the parameters of vibration that appeared to be most anabolic for bone of mdx mice, our next objective was to assess short-term responses of dystrophic skeletal muscle to those specific vibration-induced mechanical signals. Our previous work on wildtype mice showed that low intensity vibration training improved muscle contractility. Specifically, up to 20% improvements in strength and maximal rate of relaxation occurred following 6 wk of training, with no indication of adverse effects to muscle function25. However, the efficacy and impact of vibration training on dystrophic muscle is unknown and is critical to determine because the lack of dystrophin renders skeletal muscle vulnerable to mechanical stress26. Consequently, we performed Studies 2 and 3 to determine the extent to which low intensity vibration impacted skeletal muscle contractility and altered the expression of genes associated with inflammation and myogenesis. Study 2 tested the general hypothesis that three daily bouts of vibration would not be injurious to mdx muscle. The study was designed so that vibration-induced responses of hindlimb muscles could be directly compared with those from muscles of mdx mice that completed 3 d of voluntary wheel running or were subjected to a bout of injurious eccentric contractions. This was done in attempt to place vibration training on a continuum with other physical interventions known to elicit relatively minor and major muscle injury, that is, acute response to wheel running27 and eccentric contractions, respectively26,28. The specific hypothesis tested in Study 2 was that contractility and gene expression of muscles from vibrated mdx mice would not be different than those from control mdx mice, but would be different than those from mdx mice that wheel ran or were subjected to eccentric contraction-induced injury.
A subsequent study tested the general hypothesis that seven daily bouts of low intensity vibration would not be deleterious to muscle of dystrophic mice, even those with phenotypes substantially more severe than the mdx mouse. Mice lacking both dystrophin and utrophin (dko mice29) and mice lacking dystrophin that are haploinsufficient for utrophin (het mice30,31) as well as mdx mice, were subjected to seven daily bouts of vibration or a sham vibration intervention. The specific hypothesis tested in Study 3 was that contractility and gene expression of hindlimb muscles from vibrated dko, het, and mdx mice would not be different than those from littermates that were subjected to the sham vibration protocol. Support of hypotheses in Studies 2 and 3 would endorse the premise that low intensity vibration is not harmful to dystrophic muscle and that vibration therapy could be considered as a bone-sparing strategy in patients with DMD.
Methods
Animals
Five wk-old male mdx mice (C57B1/10ScSn-DMDmdx) were obtained from Jackson Laboratories (Bar Harbor, ME) for Study 1. For Studies 2 and 3, dystrophic mice were obtained from our breeding colony at the University of Minnesota32. Mice in this dystrophic colony had recently been backcrossed to obtain a pure C57BL/10 background (>99%) as determined by The Jackson Laboratories Genome Scanning Service. Studies 1 and 2 were conducted with male mdx mice aged 6 and 8 wk, respectively, because peak muscle pathology occurs in the mdx mouse from about 5-10 wk of age. Study 3 utilized 3 wk-old mdx, het (i.e., mdx mice haploinsufficient for utrophin), and dko (i.e., mice lacking both dystrophin and utrophin) littermates of both sexes. Relatively younger mice were selected for this study in order to determine the impact of vibration at the onset of disease pathology. This earlier timepoint was also selected to accommodate the inclusion of dko mice which have a shortened lifespan (i.e., 8 wk). All mice were housed on a 12:12-h light-dark cycle at 20-23°C and were provided food and water ad libitum. Mice in Study 1 were killed by sodium pentobarbital overdose (200 mg/kg body mass) and exsanguination. Immediately following in vivo contractility testing, mice in Studies 2 and 3 were further anesthetized with sodium pentobarbital (75 mg/kg body mass), muscles were dissected, and then mice were euthanized by exsanguination. All animal care and use procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Experimental design
The purpose of Study 1 was to determine the parameters of vibration (i.e., acceleration and frequency) that increase circulating osteocalcin levels and initiate the greatest osteogenic response in tibia of mdx mice. To do this, mdx mice were randomly assigned to one of seven vibration treatments as outlined in Table 1. At 6 wk of age, mdx mice began daily bouts of vibration (7 d/wk for 15 min/d) for 14 d. For this and all subsequent studies, non-vibrated control mice (i.e., 0 Hz at 0 g) were placed on a vibration platform while the system was turned off. As an indicator of stress, mice were weighed daily to ensure that they were eating normally and maintining body mass. Approximately 1 hr following the last bout of vibration, mice were weighted, anesthetized and plasma was collected via cardiac puncture to assess the impact vibration training had on circulating markers of bone formation and resorption. Tibial bones were removed, flash frozen in liquid nitrogen, and then stored at −80°C until the time of RNA isolation.
Table 1.
Objectives and designs of Studies 1-3.
| Study 1: Determine optimal parameters of vibration | Study 2: Compare vibration to other physical activity interventions known to elicit muscle injury | Study 3: Confirm that vibration is not injurious to muscle in more severely affected dystrophic mice | |
|---|---|---|---|
| Mouse age at beginning of the study (wk) | 6 | 8 | 3 |
| Genotype | mdx | mdx | mdx, het, dko |
| Sex | males | males | males/females |
| Treatment groups (number of mice per group) | Non-vibrated control (n=8) | Non-vibrated control (n=10) | Non-vibrated mdx (n=8) |
| 30 Hz at 0.3 g (n=6) | vibration (n=11) | Vibrated mdx (n=9) | |
| 30 Hz at 0.6 g (n=6) | wheel running (n=8) | Non-vibrated het (n=8) | |
| 45 Hz at 0.3 g (n=8) | eccentric contractions (n=8) | Vibrated het (n=12) | |
| 45 Hz at 0.6 g (n=8) | Non-vibrated dko (n=7) | ||
| 90 Hz at 0.3 g (n=6) | Vibrated dko (n=7) | ||
| 90 Hz at 0.6 g (n=6) | |||
| Duration of vibration treatment (days) | 14 | 3 | 7 |
| Outcome measures: | |||
| Stress33,37: | Body mass | Cage activity | Body mass |
| In vivo muscle function: | N/A | Yes | Yes |
| Muscle inflammation and myogenesis: | N/A | Gastrocnemius muscle | Tibialis Anterior muscle |
| Muscle damage: | CK | CK | CK |
| Bone formation: | Circulating osteocalcin, RNA from Tibial Bone | N/A | N/A |
wk: week.
N/A: not applicable.
CK: Creatine kinase activity.
The purpose of Study 2 was to determine if vibration was injurious to mdx muscle by placing it along a continuum with other physical interventions that are known to elicit minimal or major muscle injury in mice (i.e., acute wheel running and eccentric contractions, respectively). To do this, mdx mice were randomly assigned to one of four groups; control, vibration, wheel running, or eccentric contractions (Table 1). Cage activities were monitored in control, vibration, and wheel running groups to determine whether vibration was immediately stressful to mdx mice such that alterations in behavior were apparent33. Following the third daily bout of vibration (15 min/d, using 45 Hz and 0.6 g), 3 d of voluntary wheel running, or 3 d after a single bout of injurious eccentric contractions, mice were weighed, anesthetized, and plasma was collected from the retro-orbital cavity to assess creatine kinase (CK) activity and stored at −80°C. Contractility of the posterior crural muscle group (gastrocnemius, plantaris, soleus muscles) was then tested in vivo. Immediately following contractility testing, gastrocnemius muscles were excised, flash frozen in liquid nitrogen, and stored at −80°C. Subsequently, these muscles were used to determine the relative potential of low intensity vibration to increase expression of genes associated with muscle inflammation and myogenesis. The gastrocnemius muscle was selected for gene expression studies because it is the best muscle to compare across interventions. That is, the gastrocnemius muscle was the specific, major muscle that performed the eccentric contractions and it is also a highly recruited muscle during wheel running.
The purpose of Study 3 was to extend the evaluation of short-term responses of dystrophic muscle to vibration by lengthening the duration of vibration exposure to 7 d and by using dystrophic mouse models that have more severe phenotypes than mdx mice. To do this, mdx, het and dko mice were randomly assigned to vibration training or non-vibrated control groups at 3 wk of age (Table 1). Twenty-four hours after the seventh bout of vibration (15 min/d at 45 Hz and 0.6 g), mice were weighed, anesthetized, and plasma was collected from the retro-orbital cavity to assess CK, and then posterior crural muscle contractility was assessed in vivo. Immediately following testing, tibialis anterior (TA) muscles were excised, flash frozen in liquid nitrogen, and stored at −80°C until RNA was isolated to assess gene expression. The TA muscle was chosen, rather than the gastrocnemius muscle for gene analyses, to futher confirm that vibration was not injurious to skeletal muscle, especially in more severe models of DMD.
Vibration training
Our low intensity vibration device was designed after the work of Fritton et al.34 to produce vertical vibration stimuli with minor modifications that improve device performance and ensure fidelity25,35. Briefly, our vibration device has a circular-shaped platform that is driven by a centrally-mounted linear actuator (Moog CSA Engineering, Mountain View, CA) on the underside of the platform. A custom-designed program created with LabVIEW software (National Instruments, Austin, TX) controls and modulates the linear actuator based on continual accelerometer feedback. The feedback ensured that the platform produced accelerations with less than 1% error at the 45 Hz and 0.6 g settings used in this study35. Mice were placed into one of four individual compartments of the centrally-mounted cage (see 35 Figure 1A in this issue). The height of the cage was set to 5 cm to limit rearing and jumping by the mice.
Figure 1.
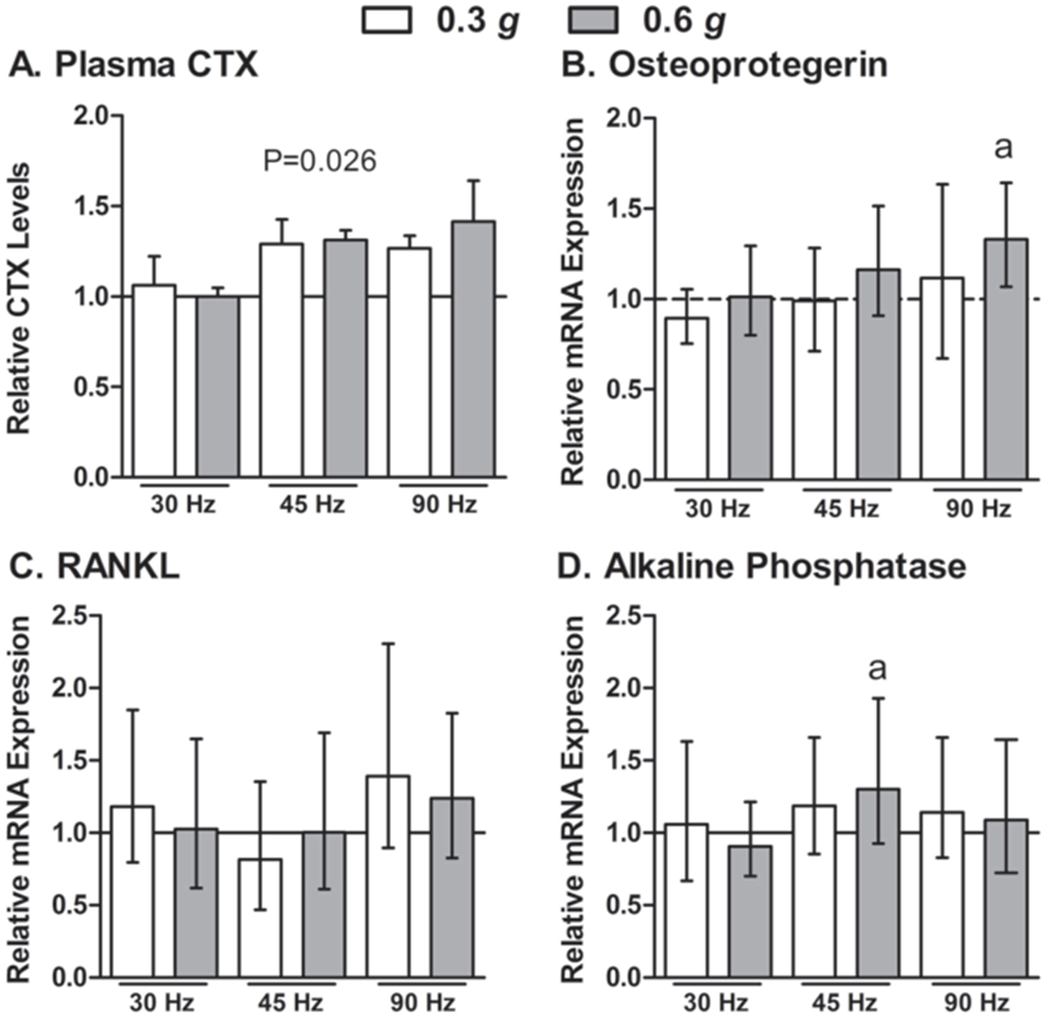
Six different permutations of low intensity vibration parameters were investigated in Study 1. A: Circulating plasma CTX values were different between groups and appeared to increase with frequency, however post-hoc tests did not detect differences between any groups. Data are mean, SE. P-value from one-way ANOVA’s is indicated above the bars. B-D: The different parameters had minimal effects on tibial bone osteogenic mRNA expression following 14 days of daily vibration exposure. The height of the bars indicate the fold change mRNA expression (i.e., delta-delta CT) above non-vibrated mdx mice (represented by the horizontal gray line) and the error bars indicate the 95% confidence intervals. aSignificantly different from non-vibrated mdx mice as determined by REST, 2009 software (P<0.05).
Wheel running
Mice were individually housed in cages containing voluntary running wheels (Single Activity Wheel Chambers, Model 80820, Lafayette Instruments Co., Lafayette, IL). One gram of resistance was applied to each wheel using resistance brakes (Model 86070-B1, Lafayette Instruments). Mice were allowed to run ad lib over the 3-d study duration, thus exceeding the 15 minute exposure to vibration. This duration of wheel running, however, is necessary to induce mild muscle injury27, thus providing a benchmark for assessing the saftety of vibration. Total distance run over the 3-d study was measured using the manufacturer’s software (Model 86065, Lafayette Instruments).This 72-hr of distance recording excluded the 30 min during which cage activity was monitored.
Eccentric contractions
In effort to compare vibration training to a relatively major muscle injury intervention, the posterior crural muscles of anesthetized mdx mice underwent a single bout of 100 eccentric contractions on day 1 as previously described36. Briefly, electrodes were percutaneously inserted on either side of the the sciatic nerve following the peroneal nerve being transected. Eccentric contractions were performed by stretching the posterior crural muscles from 19 degress of ankle plantarflexion to 19 degrees of ankle dorsiflexion, simultaneous to stimulating the sciatic nerve with a voltage between 3.0-9.0 V for a duration of 150 ms. Subsequent eccentric contractions were separated by a duration of 45 s.
Cage activity monitoring
As a more precise and immediate means of determining if vibration was stressful, as shown to occur with mild exercise in mdx mice37, cage activities were monitored in vibrated mice and compared to non-vibrated control and wheel-running mice in Study 2. Cage activity was not measured in the mice that performed the eccentric contractions in Study 2 because of the lingering effects of anesthesia. Briefly, immediately following vibration or sham vibration, mice were placed in a cage containing two parallel sets of infrared beams oriented in the transverse plane separated by a height of ~2.5 cm. As previously described38, the movement of a mouse is spacially and temporally tracked as the infrared beams are broken due the presence of the mouse. Outcome measures of interest included: active time, ambulatory distance, and stereotypic time (i.e., eating or grooming) and were measured for 30 min immediately after a bout of vibration (Med Associates Inc., St. Albans, VT)38.
Plasma osteocalcin, CTX and CK activity
Circulating levels of osteocalcin and CTX (fragments of collagen type 1) were assessed in plasma by ELISA (Biomedical Technologies Inc., Stoughton, MA and Immuno Diagnostic Systems Inc., Fountain Hills, AZ). The assays were performed in duplicate using plasma (1:10 dilution for the osteocalcin ELISA) and following manufacturers specifications. Data for the standards for each assay were fit using a 4-parameter logistic curve fit.
Plasma CK activity was determined in duplicate with plasma diluted 1:2 in PBS. Activity was measured using a kinetic assay (Creatine Kinase, C7512-300, Pointe Scientific, Inc., Canton, MI) and a Spectramax Plus 384 spectrophotometer with Soft-max Pro v5 (Molecular Devices, Sunnyvale, CA)39.
In vivo posterior crural muscle function
Contractile function of the posterior crural muscles was tested as previously reported40. After severing the peroneal nerve, the sciatic nerve was stimulated at 250 Hz for 150 ms to elicit maximal isometric torque. Torque production as a function of stimulation frequency was measured as described previously32, with the addition of measurements at 200 and 250 Hz.
RNA isolation and real-time RT-PCR
RNA was isolated from the left tibial bones of mice in Study 1 by homogenizing in liquid nitrogen using a mortar and pestle and then transferring the powdered bone to TRI reagent (Applied Biosystems, Austin, TX). Phases were separated by centrifugation and RNA was precipitated by ethanol addition and then applied to RNA clean-up spin columns (Qiagen, Valencia, CA). DNA contaminates were removed by RNase-Free DNase treatment (Promega, Madison, WI) and total RNA concentration was quantified on a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE). One microgram of cDNA was then diluted, synthesized and reverse transcribed using High Capacity cDNA Reverse Transcription (Applied Biosystems). cDNA samples were stored at −20°C until RT-PCR was performed. cDNA at 1:100 dilutions were then combined with TaqMan Universal PCR Master mix (Applied Biosystems, Carlsbad, CA) and TaqMan gene expression assays (Applied Biosystems), and were then run on a Biorad MyiQ thermocycler (Hercules, CA). Genes of interest associated with osteoblast cell activation, differentiation, signaling and bone formation included: alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), bone morphogenetic protein 2 (BMP2), and collagen type-1 α-1. Gene expression associated with the inhibition and activation of osteoclasts (i.e., Osteoprotegerin (OPG) and activator for nuclear factor ϰB ligand (RANKL), respectively) were also quantified.
The effects of short term vibration on gene expression associated with muscle inflammation (macrophage-1 antigen, (MAC-1) and the chemokine ligand 2 (MCP-1)) and myogenesis (Paired box gene-7 (Pax-7), Myogenic differentiation-1 (MyoD), and Myogenin) were assessed in gastrocnemius and TA muscles from mice in Studies 2 and 3, respectively. RNA was isolated by homogenizing muscles in TRI reagent. One mg of RNA was reverse transcribed and used for RT-PCR at a dilution of 1:100. For all analyses, triplicates were run for each mouse and the average crossing threshold for each gene of interest and the housekeeping gene (i.e., GAPDH or 18s) were used to make comparisons against non-vibrated control mice using Rest 2009 software, described below.
Statistical analyses
To examine if vibration was stressful for dystrophic mice or altered circulating osteocalcin levels in Study 1, one-way ANOVAs were used to compare body mass as well as CTX and osteocalcin levels across groups. Kruskal-Wallis ANOVA on Ranks were used when normality failed, with Dunn’s post-hoc testing. To determine optimal parameters of vibration on bone in Study 1, the combined effects of acceleration (0.3 and 0.6 g) and frequency (30, 45 and 90 Hz) were analyzed for tibial bone gene expression data using two-way ANOVAs (i.e., main effects of acceleration and frequency).
For Study 2, one-way ANOVAs were used to compare body mass, plasma CK activity, cage activities and in vivo muscle function across treatments (i.e., controls, vibration, wheel running, and eccentric contractions). When assumptions of normality or equal variance were violated, Kruskal-Wallis One Way Analysis of Variance on Ranks was performed along with Dunn’s post-hoc tests. For Study 3, two-way ANOVAs were used to analyze the effects of treatment (non-vibrated controls vs. vibrated) and genotype (mdx, het and dko) on body mass, CK activity, and in vivo muscle function. For all ANOVAs, when significant main effects or interactions were present (i.e., P<0.05), Holm-Sidak post-hoc tests were performed to determine which conditions were different from each other. These statistical analyses were carried out using SigmaStat version 3.5 (Systat Software Inc; Point Richmond, CA).
To examine the effect of vibration on gene expression profiles compared to non-vibrated control mice, in all studies, real-time RT-PCR data were analyzed with REST 2009 Software (Qiagen). Data are expressed as relative expression compared to non-vibrated mice with 95% confidence intervals41. Note that the 95% confidence intervals represent data that has an exponential distribution, and therefore the data are more heavily distributed on the lower bound making the mean closer to the lower bound than the upper bound. As such, the length of the 95% confidence interval is smaller below the mean than above.
Results
Study 1, Osteogenic responses:
In general, vibration treatment was well tolerated by mdx mice and did not appear to be stressful as indicated by the preservation of body mass (25.1±0.4 g in vibrated vs. 24.4±0.1 g in control mice at the end of the study, P=0.978). In addition, behavior of the mice did not appear to be affected during or immediately after a bout of vibration. Circulating plasma osteocalcin levels were not different among the seven groups of mice, suggesting that vibration did not alter osteoblast activity (59.2±2.2 ng/ml, P≥0.354). Circulating plasma CTX levels were elevated 29-41% in mice vibrated at 45 and 90 Hz, though post-hoc testing did not detect a signficiant difference between any of the groups (Figure 1A).
Results showed no main effects of acceleration (P≥0.156) or frequency (P≥0.181) on tibial bone mRNA expression data (data not shown), with the exception of osteoprotegerin, which had a trend toward upregulation at higher frequencies (P=0.052, Figure 1B). There were no significant differences in mRNA expression in Runx2, collagen type-I α-I, or BMP-2 between any of the six vibration groups compared to the non-vibrated group (range of fold change 0.837-1.44, P≥0.156, data not shown). Osteoprotegerin mRNA expression, however, was significantly upregulated in mdx mice vibrated at 90 Hz and 0.6 g, and trended at 45 Hz and 0.6 g (P=0.109) compared to non-vibrated mdx mice (Figure 1B). RANKL mRNA expression was 1.24 fold higher at 90 Hz and 0.6 g, and equivalent to control mdx mice at 45 Hz and 0.6 g (Figure 1C), however the ratio of osteoprotegerin and RANKL was not different from controls in either group (data not shown, P≥0.476). In addition, alkaline phosphatase mRNA expression at 45 Hz and 0.6 g was increased, but this increase was not apparent at 90 Hz at 0.6 g (Figure 1D). Due to the relative elevations of alkaline phosphatase and osteoprotegerin mRNA expression at 45 Hz at 0.6 g those parameters were used for subsequent muscle-specific studies in dystrophic mice.
Study 2, Contractility:
Mdx mice in the wheel running group voluntarily ran 17.5±2.0 km over the 3-d study and mdx mice in the eccentric group performed 100 eccentric contractions resulting in an immediate 79% loss in eccentric torque.
Following three days of normal cage activity (i.e., controls), vibration, wheel running, or a bout of eccentric contractions, peak isometric torque produced by the posterior crural muscles was significantly different between groups (Figure 2A). Vibrated mice were equivalent to both control and wheel running mice. As hypothesized, mdx mice that performed eccentric contractions had up to 37% lower isometric torque values, though post-hoc testing did not detect a significant difference between any of the groups (Figure 2A). CK activity levels were different among groups (P=0.020), with eccentric mice having 24% higher plasma CK activity levels than controls (2829.5±95.2 U/L vs 2278.9±122.1 U/L). There were no differences in CK activity levels between vibrated (2489.3±115.6 U/L), wheel running (2648.9±102.2) and control mice.
Figure 2.
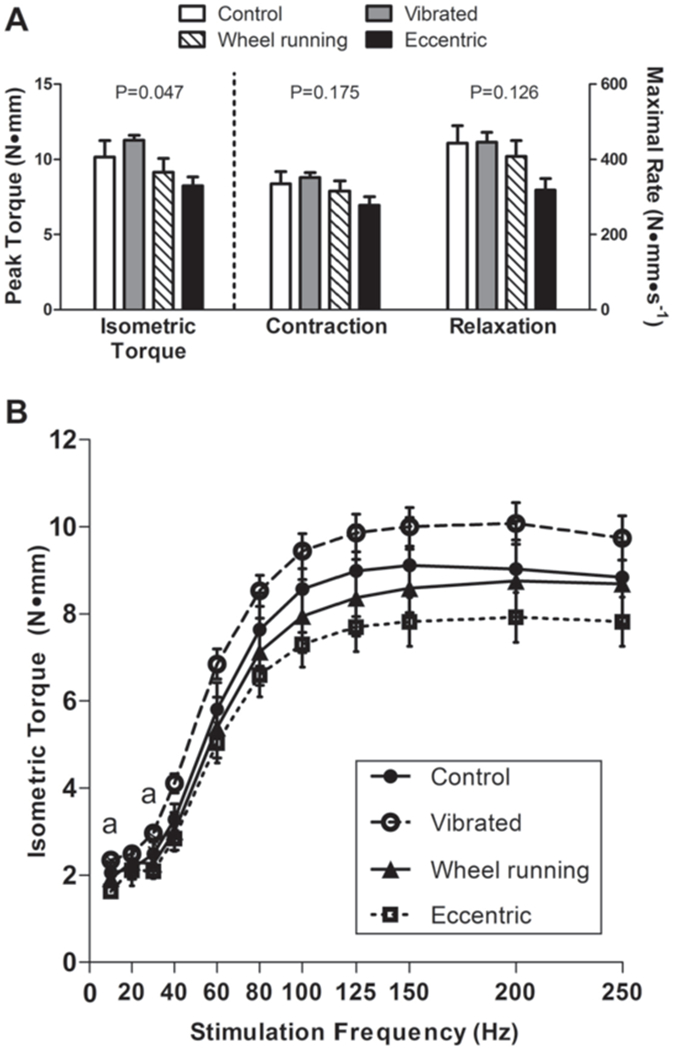
Three daily bouts of low intensity vibration on mdx mouse posterior crural muscle contractility were compared to other exercise modalities in Study 2. Data are mean, SE. A: Peak isometric torque and rates of contraction and relaxation were not detrimentally affected by vibration. Main effect P-values from one-way ANOVA’s are indicated above each set of bars. Despite the significant main effect for peak isometric torque, post-hoc testing did not detect a significant difference among any of the four groups. B: Isometric torque as a function of stimulation frequency for non-vibrated Control, Vibrated, Wheel running and Eccentrically injured mdx mice. aSignificant difference determined from post-hoc testing with torque by Vibrated mice greater than that by Eccentric mice.
Additional measures of posterior crural muscle contractile function assessed in Study 2 including maximal rates of contraction and relaxation during isometric contractions and isometric torque as a function of stimulation frequency confirm that vibration was not injurious to mdx muscle (Figure 2). Muscles of vibrated mice generated equivalent torque compared to control mice and greater torque compared to eccentrically-injured muscles at both 10 and 30 Hz (Figure 2B). Combined, these data indicate that muscle contractile function was not compromised by vibration in mdx mice, as was shown with eccentrically-injured muscle of mdx mice.
In addition, cage activity levels were not reduced during the 30 min immediately following vibration, and in fact, these mice tended to be 7% more active than non-vibrated controls (Table 2). This increase in cage activity corresponded to ~70 sec, and was primarily spent ambulating (11.6±0.5 min in vibrated mice and 10.2±0.5 min in control mice, P=0.107). Despite the increase in active time, vibrated mice traveled equivalent total distances (Table 2).
Table 2.
Comparison of 30-minute cage activity levels between mdx mice in typical mouse cages (control), following vibration, or wheel running.
| Control | Vibrated | Wheel Running | One Way ANOVA P-value | |
|---|---|---|---|---|
| Active time (min) | 17.1 (0.6) |
18.3 (0.4) |
16.0 (0.9) |
0.054 |
| Stereotypic time (min) | 10.2 (0.5) |
11.6 (0.6) |
9.2 (1.0) |
0.111 |
| Ambulatory distance (m) | 49.3 (2.5) |
55.3 (2.3) |
45.8 (5.0) |
0.157 |
Values are means (SE).
Study 3, Contractility:
Body mass did not significantly differ among the groups following 7 d of vibration exposure (14.5±0.5 g, P≥0.429).
In vivo contractile testing of the posterior crural muscles showed that vibration had no deleterious consequences on muscle function, however, by design, genotypic differences in contractile function were apparent. Peak isometric torque was not influenced by vibration, while dko mice had up to 38% lower torque and rates of contraction and relaxation compared to both mdx and het mice (Figure 3A). Similar patterns were seen for isometric torque as a function of stimulation frequency. Vibration had no effect on submaximal torques and dko mice had lower isometric torque values at all frequencies above 80 Hz (Figure 3B). CK activity was measured to assess if vibration was more injurious to models of DMD that are more susceptible to injury. There was no effect of genotype or 7 d of vibration on plasma CK activity (1354±34 U/L for all groups; P≥0.514).
Figure 3.
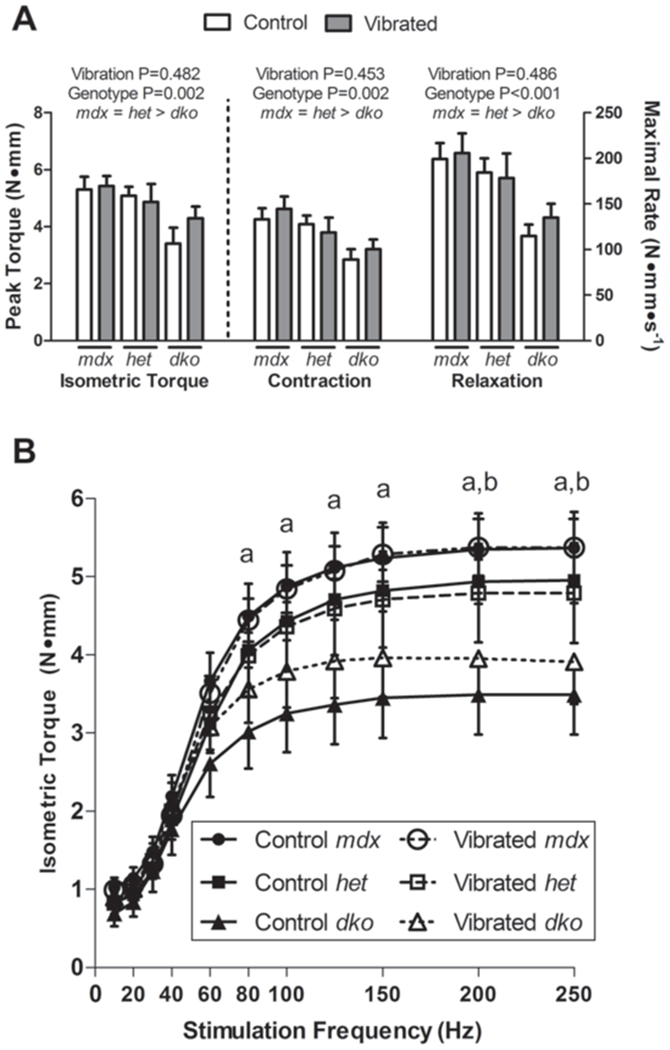
Seven daily bouts of low intensity vibration training on posterior crural muscle contractility in three mouse models of DMD which vary in their disease severity was investigated in Study 3. Data are mean, SE. A: Peak isometric torque and rates of contraction and relaxation were not different between Control and Vibrated mice. Dko mice consistently had lower contractility than both mdx and het mice. Main effect P-values from two-way ANOVA’s are indicated above each set of bars with post-hoc results in words immediately below the genotype main effects. B: Isometric torque production as a function of stimulation frequency indicated that vibration did not impact torque production at any frequency; however dko mice had lower torque production at frequencies above 80 Hz. Only main effects of genotype were detected, and therefore only post-hoc testing results are displayed: aSignificant difference between mdx and dko; bSignificant difference between het and dko.
Studies 2 and 3, Gene Expression
mRNA expression profiles were assessed in two muscles to determine if early markers of muscle inflammation and myogenesis were affected by short-term vibration training. Vibration had no impact on any of the genes assessed in the gastrocnemius muscle following 3 d of exposure (P≥0.126), however, wheel running and eccentric injured muscles had a nearly 7-fold upregulation of MAC-1 and MCP-1 mRNA expression compared to control muscles (Figure 4A). Extending vibration training out to 7 d and using the most severe model of DMD (i.e., dko mice) did not evoke changes in tibialis anterior muscle mRNA expression levels in genes associated with muscle inflammation or myogenesis (Figure 4B, P≥0.192). Het mice, however did have a small but statistically significant 1.4 fold increase in Pax-7 mRNA when vibrated (Figure 4B, P=0.044). Combined, these data suggest that vibration is not injurious to dystrophic muscle in terms of eliciting an inflammatory response and also showed little evidence for initiating myogenesis.
Figure 4.
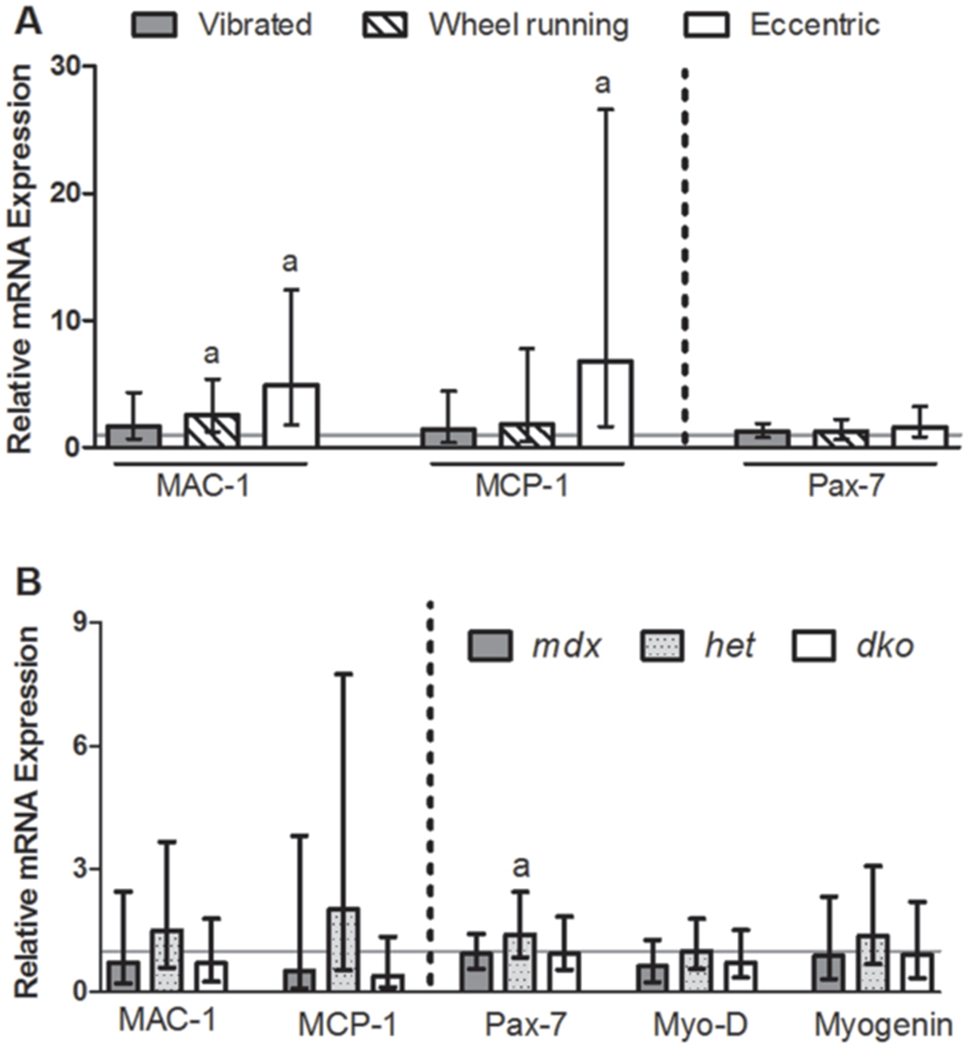
Low intensity vibration training for three or seven days (in Studies 2 and 3, respectively) did not affect muscle mRNA expression of genes associated with inflammation or myogenesis. RNA was isolated from A: gastrocnemius muscles of mdx mice three days following interventions of vibration, wheel running, or eccentric contractions and compared to those of control mdx mice (indicated by the solid gray line) and B: tibialis anterior muscle from mdx, het and dko mice that were vibrated for seven days and compared to genotype-matched, non-vibrated controls (indicated by the solid gray line). The height of the bars indicate the fold change in mRNA expression (i.e., delta-delta CT) above controls and the error bars indicate the 95% confidence intervals. aSignificantly different from respective, non-vibrated mdx mice as determined by REST, 2009 software (P<0.05).
Discussion
We had three primary findings from our project. First, low intensity vibration was well tolerated in each of the three dystrophic mouse models utilized (i.e., mdx, het and dko mice) and at two ages in the mdx mice. It also did not appear to be stressful based on preservation of cage activities and body mass. Second, in response to 14 d of low intensity vibration training, 45 Hz and 0.6 g was identified as the set of parameters that tended to be the most osteogenic in mdx mice. As such, subsequent studies on muscle responses to vibration were performed using these parameters of vibration. Third, vibration was consistently shown to be non-injurious to dystrophic muscle of various disease severity. Contractile function of the posterior crural muscles was not different between non-vibrated and vibrated dystrophic mice, and plasma CK activity and expression of genes associated with muscle inflammation also were not affected by vibration. The long term goal that this study begins to address is the potential of low intensity vibration as a therapeutic modality for DMD. The utility of vibration has received increasing attention in recent years due to its ability to improve musculoskeletal health42–44, especially in disabled children and women with low bone mass13–15,45. Theoretically, vibration would mechanically load bone to maintain bone health yet would be safe for the adjacent diseased muscle, and results of our study support this theory.
Results from our first study identified two sets of vibration parameters (i.e., 90 Hz at 0.6 g and 45 Hz at 0.6 g) that evoked an osteogenic response (Figure 1). The 45 Hz and 0.6 g setting increased both osteoprotegerin and alkaline phosphatase mRNA levels up to 30%, but did not impact RANKL (Figure 1). Similar fold-change increases in alkaline phosphatase mRNA expression have been reported following 2-4 d of a known anabolic stimulus (i.e., four-point bending19,46. The 90 Hz and 0.6 g setting upregulated osteoprotegerin and RANKL to similar extents, and therefore the increase in RANKL could potentially have negated any osteoprotegerin-induced osteogenic effects. In fact, these mice had the highest levels of ciruculating bone resorption activity as inidicated by plasma CTX levels (Figure 1A). Upregulation of RANKL has also been reported in mice vibrated for 21 d22, however in cells, vibration decreased RANKL mRNA expression up to 55% with no change in osteoprotegerin47. Consistent with our findings, others have shown no change in mRNA expression following vibration for Collagen type I22, BMP-219,22, and Runx-219. Consequently, our subsequent studies were performed utilizing 45 Hz and 0.6 g because that pair of parameters tended to increase osteoprotegerin expression with no change in RANKL and also elicited a 1.4 fold increase of alkaline phosphatase mRNA expression, which is involved with matrix maturation and mineralization.
The results of our second and third studies consistently showed that vibration was not injurious to inherently fragile dystrophic muscle. The most crucial pieces of evidence to support this statement are the functional results. That is, there was no indication that strength (submaximal or maximal isometric torque) or contractility rates (contraction or relaxation) of the posterior crural muscles were affected by 3 or 7 d of vibration treatment in dystrophic mice. This was in contrast to other interventions, such as eccentric contractions, that did cause loss of strength (Figure 2). This was substantiated in the traditional mdx mouse at two critical ages, as well as in more severe dystrophic mouse models, het and dko mice (Figure 3).
The expression profiles of genes associated with muscle inflammation and recovery from injury further support our hypothesis that vibration is not injurious. MAC-1 and MCP-1 are genes that meditate the recruitment and activation of inflammatory cells following muscle damage and these genes were not upregulated after either 3 or 7 d of vibration, but were increased by wheel running or eccentric contractions (Figure 4). These data indicated that vibration is on the low end of the continuum of exercises that cause damage in dystrophic mice. Specifically, eccentrically-injured muscles had 5-7 fold increases in MAC-1 and MCP-1 similar to previous reports48,49. Interestingly, het mice had trends toward increased MAC-1 and MCP-1 following 7 d of vibration, supporting the notion that het mice may be more sensitive to developing, and thus better models of, inflammation and fibrosis compared to mdx mice30.
Genes associated with myogenesis were also not affected by vibration, with the exception of upregulated Pax-7 gene following 7 d of vibration in het mice (Figure 4). Vibration of cultured C2C12 myoblasts has previously shown a vibration dose-dependent increase in MyoD and myogenin expression50. High intensity vibration training for two weeks in young mice induced muscle hypertrophy that was attributed to enhanced fusion and differentiation of satellite cells, and muscle fiber number and cross-sectional area51,52. Vibration-induced muscle hypertrophy has also been associated with the inhibition of muscle atrophy pathways by downregulating myostatin gene expression53. On the contrary, our previous work showed that eight weeks of low intensity vibration training in wildtype mice improved muscle functional capacity in the absence of hypertrophy25. Specifically, muscle strength improved by 14% but muscle mass, protein content, and fiber cross-sectional area were not affected25. Given these improvements in the muscle function of wildtype mice, a future goal is to determine if similar improvements are attainable in dystrophic mice and DMD patients. Reyes et al.45 has applied vibration directly to the elbow of children with motor disabilities (including patients with DMD) and reported a 65% increase in muscle force compared to a 20% decline in control patients.
The present studies thoroughly characterized dystrophic muscle function following short-term vibration training, after determining the vibration parameters that evoked the greatest osteogenic response in mouse models of DMD. The results suggest that vibration at 45 Hz and 0.6 g has the potential to have anabolic impact on bone health, while not causing injury to inherently fragile dystrophic muscle. Additional long-term treatment studies are needed to determine the efficacy of vibration to improve both bone and muscle in dystrophic mice and to improve musculoskeletal health in DMD.
Acknowledgements
The authors would like to thank Tara Mader, Kristen Baltgalvis, Gordon Warren and Angela Greising for their technical assistance. Our research has been supported by grants from the Muscular Dystrophy Association (Research Grant 114071), the National Institutes of Health grants T32-AR07612 (SAN and JAC), P30-AR0507220 (University of Minnesota Muscular Dystrophy Center), and K02-AG036827 (DAL), the Patrick and Kathy Lewis Fund (SAN), the Greg Marzolf Jr. Foundation (MDE), the University of Minnesota Undergraduate Research Opportunity (MDE and BCE), and the Lillehei Heart Institute Summer Research Scholars Program (BCE).
Footnotes
The authors have no conflict of interest.
References
- 1.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 2000;20:71–4. [PubMed] [Google Scholar]
- 2.Soderpalm AC, Magnusson P, Ahlander AC, et al. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord 2007;17:919–28. [DOI] [PubMed] [Google Scholar]
- 3.Landoll J, King W, Kissel J, Matkovic V. Forearm pQCT measurements in males with Duchenne muscular dystrophy. In. J Bone Miner Res 2008. [Google Scholar]
- 4.King W, Landoll J, Matkovic V, Kissel J. Volumetric radial and tibial bone mineral density in boys with Duchenne muscular dystrophy. In. Neurology 2009. [Google Scholar]
- 5.McDonald DG, Kinali M, Gallagher AC, et al. Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol 2002;44:695–8. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi ML, Mazzanti A, Galbiati E, et al. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos Int 2003;14:761–7. [DOI] [PubMed] [Google Scholar]
- 7.Landin LA. Epidemiology of children’s fractures. J Pediatr Orthop B 1997;6:79–83. [DOI] [PubMed] [Google Scholar]
- 8.Straathof CS, Overweg-Plandsoen WC, van den Burg GJ, van der Kooi AJ, Verschuuren JJ, de Groot IJ. Prednisone 10 days on/10 days off in patients with Duchenne muscular dystrophy. J Neurol 2009;256:768–73. [DOI] [PubMed] [Google Scholar]
- 9.Novotny SA, Warren GL, Lin AS, Guldberg RE, Baltgalvis KA, Lowe DA. Bone is functionally impaired in dystrophic mice but less so than skeletal muscle. Neuromuscul Disord 2011;21:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagaki WR, Bertran CA, Matsumura CY, Santo-Neto H, Camilli JA. Mechanical, biochemical and morphometric alterations in the femur of mdx mice. Bone 2011;48:372–9. [DOI] [PubMed] [Google Scholar]
- 11.Eagle M Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord 2002;12:975–83. [DOI] [PubMed] [Google Scholar]
- 12.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. Faseb J 2001;15:2225–9. [DOI] [PubMed] [Google Scholar]
- 13.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 2004;19:343–51. [DOI] [PubMed] [Google Scholar]
- 14.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 2004;19:360–9. [DOI] [PubMed] [Google Scholar]
- 15.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 2006;21:1464–74. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers JE, van Rietbergen B, Ito K, Huiskes R. Effects of vibration treatment on tibial bone of ovariectomized rats analyzed by in vivo micro-CT. J Orthop Res 2009;28:62–9. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen BA, Kotiya AA, Silva MJ. Constrained tibial vibration does not produce an anabolic bone response in adult mice. Bone 2009;45:750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slatkovska L, Alibhai SM, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med 2010;155:668–79, W205. [DOI] [PubMed] [Google Scholar]
- 19.Kotiya AA, Bayly PV, Silva MJ. Short-term low-strain vibration enhances chemo-transport yet does not stimulate osteogenic gene expression or cortical bone formation in adult mice. Bone 2011;48:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manske SL, Good CA, Zernicke RF, Boyd SK. High-Frequency, Low-Magnitude Vibration Does Not Prevent Bone Loss Resulting from Muscle Disuse in Mice following Botulinum Toxin Injection. PLoS One 2012;7:e36486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JE, Lentz DL, Johnson RB. Recovery from disuse osteopenia coincident to restoration of muscle strength in mdx mice. Bone 1993;14:625–34. [DOI] [PubMed] [Google Scholar]
- 22.Judex S, Zhong N, Squire ME, et al. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem 2005;94:982–94. [DOI] [PubMed] [Google Scholar]
- 23.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 2009;24:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MJ, Chang KH, Sykes MC, Talish R, Rubin C, Jo H. Low magnitude and high frequency mechanical loading prevents decreased bone formation responses of 2T3 preosteoblasts. J Cell Biochem 2009;106:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeehen JN, Novotny SA, Baltgalvis KA, Call JA, Nuckley DJ, Lowe DA. Adaptations of Mouse Skeletal Muscle to Low-Intensity Vibration Training. Med Sci Sports Exerc 2012;45:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A 1993;90:3710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irintchev A, Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res 1987;249:509–21. [DOI] [PubMed] [Google Scholar]
- 28.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve 2009;40:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 1997;90:729–38. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Rafael-Fortney JA, Huang P, et al. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci 2008;264:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Putten M, Kumar D, Hulsker M, et al. Comparison of skeletal muscle pathology and motor function of dystrophin and utrophin deficient mouse strains. Neuromuscul Disord 2012;22:406–17. [DOI] [PubMed] [Google Scholar]
- 32.Call JA, Ervasti JM, Lowe DA. TAT-muUtrophin mitigates the pathophysiology of dystrophin and utrophin double-knockout mice. J Appl Physiol 2011;111:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baltg alvis KA, Call JA, Cochrane GD, Laker RC, Yan Z, Lowe DA. Exercise Training Improves Plantarflexor Muscle Function in mdx Mice. Med Sci Sports Exerc 2012;44:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritton JC, Rubin CT, Qin YX, McLeod KJ. Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann Biomed Eng 1997;25:831–9. [DOI] [PubMed] [Google Scholar]
- 35.Novotny SA, Mehta H, Lowe DA, Nuckely DJ. Vibration platform for mice to deliver precise, low intensity mechanical signals to the musculoskeleton. J Musculoskelet Neuronal Interact 2013;13:412–417. [PubMed] [Google Scholar]
- 36.Call JA, Eckhoff MD, Baltgalvis KA, Warren GL, Lowe DA. Adaptive strength gains in dystrophic muscle exposed to repeated bouts of eccentric contraction. J Appl Physiol 2011;111:1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 2008;456:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol 2011;110:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horder M, Magid E, Pitkanen E, et al. Recommended method for the determination of creatine kinase in blood modified by the inclusion of EDTA. The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (SCE). Scand J Clin Lab Invest 1979;39:1–5. [DOI] [PubMed] [Google Scholar]
- 40.Greising SM, Call JA, Lund TC, Blazar BR, Tolar J, Lowe DA. Skeletal muscle contractile function and neuromuscular performance in Zmpste24 −/− mice, a murine model of human progeria. Age (Dordr) 2012;34:805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research 2002;30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int 2010;21:1969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil 2011;25:975–88. [DOI] [PubMed] [Google Scholar]
- 44.Mikhael M, Orr R, Fiatarone Singh MA. The effect of whole body vibration exposure on muscle or bone morphology and function in older adults: a systematic review of the literature. Maturitas 2010;66:150–7. [DOI] [PubMed] [Google Scholar]
- 45.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res 2011;26:1759–66. [DOI] [PubMed] [Google Scholar]
- 46.Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol 2005;99:1951–7. [DOI] [PubMed] [Google Scholar]
- 47.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 2010;46:1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol 2007;582:825–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren GL, Hulderman T, Mishra D, et al. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. Faseb J 2005;19:413–5. [DOI] [PubMed] [Google Scholar]
- 50.Wang CZ, Wang GJ, Ho ML, Wang YH, Yeh ML, Chen CH. Low-magnitude vertical vibration enhances myotube formation in C2C12 myoblasts. J Appl Physiol 2010;109:840–8. [DOI] [PubMed] [Google Scholar]
- 51.Ceccarelli G, Benedetti L, Pre D, Magenes G, Cusella De Angelis MG. An “in vivo” study of high frequency vibration on muscle development. In: Dössel O, Schlegel WC, (eds.). IFMBE; 2009. [Google Scholar]
- 52.Ceccarelli E, Benedetti L, Pre D, et al. High frequency vibration (HFV) induces muscle hypertrophy in newborn mice and enhances primary myoblasts fusion in satellite cells In: Bamidis PD, Pallikarakis N, (eds.). MEDICON 2010, IFMBE; 2010. [Google Scholar]
- 53.Ceccarelli G, Benedetti L, Galli D, et al. Low-amplitude high frequency vibration down-regulates myostatin and atrogin-1 expression, two components of the atrophy pathway in muscle cells. J Tissue Eng Regen Med 2012; doi: 10.1002/term.1533 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


