Abstract
Nutrition during early life is essential for brain development and establishes the basis for cognitive and language skills development. It is well established that breastfeeding, compared to formula feeding, has been traditionally associated with increased neurodevelopmental scores up to early adulthood. We analyzed the long-term effects of a new infant formula enriched with bioactive compounds on healthy children’s language development at four years old. In a randomized double-blind COGNIS study, 122 children attended the follow-up call at four years. From them, 89 children were fed a standard infant formula (SF, n = 46) or an experimental infant formula enriched with functional nutrients (EF, n = 43) during their first 18 months of life. As a reference group, 33 exclusively breastfed (BF) were included. Language development was assessed using the Oral Language Task of Navarra-Revised (PLON-R). ANCOVA, chi-square test, and logistic regression models were performed. EF children seemed to show higher scores in use of language and oral spontaneous expression than SF children, and both SF and EF groups did not differ from the BF group. Moreover, it seems that SF children were more frequently categorized into “need to improve and delayed” in the use of language than EF children, and might more frequently present “need to improve and delayed” in the PLON-R total score than BF children. Finally, the results suggest that SF children presented a higher risk of suffering language development than BF children. Secondary analysis also showed a slight trend between low socioeconomic status and poorer language skills. The functional compound-enriched infant formula seems to be associated with beneficial long-term effects in the development of child’s language at four years old in a similar way to breastfed infants.
Keywords: infant formula, functional nutrients, breastfeeding, language development
1. Introduction
The first years of life are critical for the development of language skills, including learning to understand and speak language. Neural networks for language acquisition are fully formed before birth. In fact, infants can perceive and react to sound at 24 weeks gestation and, at 35 weeks, they begin to learn language in utero [1]. According to different authors, infants learn their mother tongue quickly and without effort from six months to three years old, when they are capable of composing full sentences. Nonetheless, this is a complex process that need further analysis, since it has been shown that maturational and experiential factors play a key role in the development of language and human speech [2,3]. The development of language depends on brain maturation, which is modulated, among other environmental factors, by differences in infant diet. In this regard, early dietary factors can influence neurodevelopmental processes [4].
Long-chain polyunsaturated fatty acids (LC-PUFAs) intake during infancy is associated with optimal brain development. There is evidence that docosahexaenoic acid (DHA) intake and status are related to infant cognitive performance, including verbal learning ability, language, reading, spelling, nonverbal intelligence, and memory [5]. Infants fed formulas supplemented with DHA and arachidonic acid (ARA) performed better in executive function, vocabulary, and intelligence at three to five years of age compared to those who were fed with the formula with no DHA and ARA [6]. Additionally, bovine milk fat globule membrane (MFGM) has been added recently to infant formulas. MFGM is rich in sialic acid as part of gangliosides and glycosylated proteins, which have also been shown to be important for optimal brain development in different studies [7,8]. Furthermore, infant formulas are currently supplemented with probiotics and prebiotics, which are known for their role in the establishment, composition, and metabolic activity of gut microbiota [9]. Moreover, a relationship between gut microbiota and the brain has been proposed, which is known as gut microbiota-brain axis [10]. The extent of how this circuit modulates neurodevelopment and how different types of feeding exert an influence is still under investigation.
We hypothesized that supplementation during the first months of life with an infant formula enriched with functional nutrients, including LC-PUFAs (DHA and ARA), MFGM, and synbiotics, among others, might have long-term beneficial effects on language development in four-year-old children.
2. Materials and Methods
2.1. Study Design and Subjects
The current analysis included 122 Spanish children born at term from COGNIS Project (A Neurocognitive and Immunological Study of a New Formula for Healthy Infants) (registered at www.ClinicalTrials.gov as NCT02094547). Detailed information on this project, including the study design, subject recruitment, and population characteristics, were described elsewhere [11,12]. Briefly, COGNIS is a prospective, randomized, and double-blind study consisting in a nutritional intervention designed to evaluate the effects of a new infant formula on psychomotor, cognitive, socioemotional, and behavioral development. A total of 220 participants met the inclusion criteria. Of them, 170 were randomized into two groups to receive infant formula during their first 18 months of life: 85 infants were fed with a standard infant formula (SF) and 85 infants received an experimental infant formula (EF) enriched with in bioactive nutrients (MFGM, Fructooligosaccharides (FOS): Inulin proportion 1:1; Bifidobacterium longum subsp. infantis CECT7210 (Bifidobacterium infantis IM1) and Lactobacillus rhamnosus LCS-742), LC-PUFAs, gangliosides, nucleotides and sialic acid). Furthermore, 50 exclusively breastfed infants (BF) were included as a reference group.
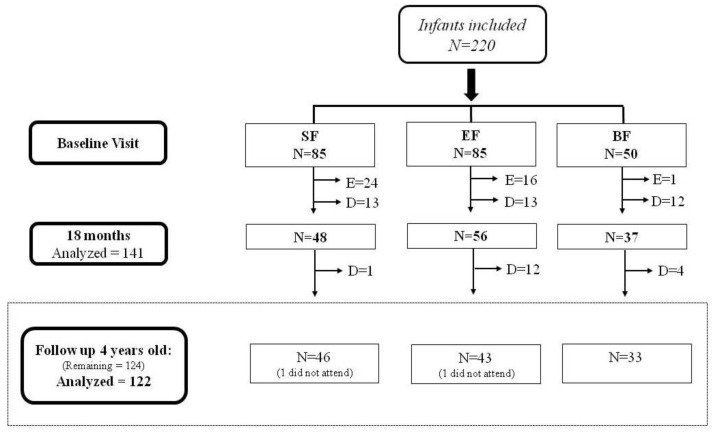
A detailed participant flowchart from the baseline visit to four years old is shown in Figure 1. Excluding dropouts, 122 infants attended visit at four years old for the analysis of language development using the Oral Language Task of Navarra-Revised (PLON-R) test (SF (n = 46), EF (n = 43) and BF (n = 33)).
Figure 1.
Participant flowchart from baseline visit to four years old. SF: Standard infant formula; EF: Experimental infant formula; BF: Breastfed infants; D = Dropouts; E = Exclusions. Up to 18 months of life, a total of 40 infants were excluded in the SF and EF groups as follows: 24 were excluded in the SF group (1 infant due to perinatal hypoxia, 1 infant had growth deficiency, 15 infants did not take the infant formula, 2 had colic of the infant, 3 were excluded due to lactose intolerance, 1 infant due to digestive surgical intervention, and 1 infant suffered hydrocephalus); 16 infants were excluded in the EF group (2 infants presented growth deficiency, 2 infants lactose intolerance, 11 infants did not take the infant formula, and 1 was excluded due to epileptic seizure). Furthermore, one infant of the BF group was excluded because he/she was not breastfed. During the follow-up visit at four years old, dropouts were considered those who did not continue to participate in the study; n = sample size.
2.2. Ethics, Consent, and Permissions
This study was performed according to the Declaration of Helsinki II Principles [13], and all procedures were approved by the University of Granada Research Ethical Committee. The Bioethical Committees for Clinical Research of the Clinical University Hospital San Cecilio and the Mother-Infant University Hospital of Granada (Spain) also approved the project and protocols. All families were informed about all procedures and signed an informed consent before involving each child in the study.
2.3. Assessments of Children Language: Oral Language Task of Navarra-Revised (PLON-R)
PLON-R is a standardized test that allows an early detection or screening of the oral language development in kindergarten children. With a focus on the language dimensions (form, content, and use), with specific activities for each dimension, the scores of each one of the dimensions are transformed into typical punctuations organized in three categories by age: “Delay” (form ≤ 25; content ≤ 22; use ≤ 28; total ≤ 27), “need to improve” (form = 36; content: 33–47; use = 39; total: 39–45), and “normal” (form ≥ 50; content ≥ 67; use ≥ 59; total ≥ 54). Higher scores are related with better language development. Moreover, this test allowed us to obtain a total punctuation regarding language development [14].
2.4. Statistical Analysis
All statistical analyses were performed using the IBM® SPSS Statistics® program, version 22.0 (SPSS Inc. Chicago, IL, USA). Normally distributed variables were presented as mean and standard deviation (SD), and non-normal variables as median and interquartile range (IQR). Categorical variables were showed as frequencies and percentages. Differences in language test scores among BF, SF, and EF groups were contrasted using analysis of variance (ANOVA) or Welch’s t-test, Kruskal–Wallis rank-sum test for non-normal continuous variables, and chi-square or Fisher test for categorical variables. In the event of significant group differences, Bonferroni-corrected post hoc comparisons were used to identify significant pair-wise group differences (corrected p-values < 0.05). In addition, variables showing significant group differences, such as maternal age and educational level, paternal age and educational level, sex of the child, and socioeconomic status, were included into analysis of covariance (ANCOVA).
Additionally, the Wald test for logistic regression was performed to calculate the odds ratios (ORs) and 95% confidence intervals (CI) of having normal or delayed values after adjustment for potential confounders. p values < 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the COGNIS Study Participants at Four Years Old
Background and baseline characteristics of 122 parents and children participating in the current study at four years old in the follow-up visit are shown in Table 1. The analysis revealed that mothers and fathers of BF children were older (p = 0.019 and p = 0.027, respectively) than parents from the SF group. Mothers from the BF group showed a higher educational level (p = 0.001) than those from EF and SF groups. Maternal IQ was higher in the BF group than mothers from the EF group (p = 0.023). Fathers from the BF group showed also a higher educational level in comparison with fathers from the SF group (p = 0.040). Furthermore, the BF group presented a higher socioeconomic status than the SF and EF groups (p < 0.001). Finally, there were significantly more girls than boys in the BF group compared to the SF group (p = 0.034). It is important to note that these differences did not exist between the SF and EF groups. Interestingly, no differences were observed in children IQ, bilingual children, and need for speech therapy at four years old.
Table 1.
Baseline characteristics of children and parents participating in the COGNIS project.
| Follow up Four Years Old | |||||
|---|---|---|---|---|---|
| Parents characteristics | SF (n = 46) | EF (n = 43) | BF (n = 33) | p 1 | |
| Maternal age (years) | 31.00 (24.00–35.00) a | 31.00 (28.00–34.00) a,b | 34.00 (31.00–38.00) b | 0.019 | |
| Maternal pBMI (kg/m2) | 24.17 (21.05–26.30) | 25.15 (22.21–28.48) | 24.46 (23.05–25.95) | 0.478 | |
| Maternal educational level | Primary | 6 (13.04%) | 9 (20.93%) | 1 (3.03%) | 0.001 |
| Secondary | 12 (26.09%) a,b | 13 (30.23%) b | 2 (6.06%) a | ||
| VT | 13 (28.26%) | 15 (34.88%) | 9 (27.27%) | ||
| University | 15 (32.61%) a | 6 (13.95%) a | 21 (63.64%) b | ||
| Maternal IQ (points) | 104.00 (95.00–112.50) a,b | 100.00 (89.00–108.00) a | 111.00 (96.00–117.00) b | 0.023 | |
| Smoking during pregnancy | No | 36 (80.00%) | 36 (83.72%) | 31 (93.94%) | 0.220 |
| Yes | 9 (20.00%) | 7 (16.28%) | 2 (6.06%) | ||
| GWG (kg) | 7.00 (3.50–10.00) | 7.00 (4.00–10.00) | 6.25 (4.50–8.50) | 0.758 | |
| Type of delivery | Vaginal | 34 (73.91%) | 31 (72.09%) | 25 (75.76%) | 0.937 |
| Cesarean | 12 (26.09%) | 12 (27.91%) | 8 (24.24%) | ||
| Postpartum Depression | No | 35 (76.09%) | 34 (80.95%) | 28 (84.85%) | 0.621 |
| Yes | 11 (23.91%) | 8 (19.05%) | 5 (15.15%) | ||
| Paternal age (years) | 32.50 ± 6.69 a | 33.54 ± 5.56 a,b | 36.24 ± 4.38 b | 0.027 | |
| Paternal educational level | Primary | 11 (25.00%) | 16 (39.02%) | 6 (18.18%) | 0.040 |
| Secondary | 16 (36.36%)a | 9 (21.95%) a,b | 4 (12.12%) b | ||
| VT | 8 (18.18%) | 9 (21.95%) | 10 (30.30%) | ||
| University | 9 (20.45%) | 7 (17.07%) | 13 (39.39%) | ||
| Paternal IQ (points) | 106.86 ± 12.38 | 104.29 ± 15.81 | 106.90 ± 12.93 | 0.659 | |
| Socioeconomic status | Low | 9 (19.57%) a,b | 10 (23.81%) b | 1 (3.03%) a | <0.001 |
| Middle-Low | 22 (47.83%) a | 25 (59.52%) a | 6 (18.18%) b | ||
| Middle-High | 12 (26.09%) a,b | 4 (9.52%) b | 17 (51.52%) a | ||
| High | 3 (6.52%) a | 3 (7.14%) a,b | 9 (27.27%) b | ||
| Place of residence | Urban | 20 (44.44%) | 11 (26.19%) | 8 (24.24%) | 0.095 |
| Rural | 25 (55.56%) | 31 (73.81%) | 25 (75.76%) | ||
| Siblings | 0 | 15 (33.33%) | 18 (42.86%) | 8 (24.24%) | 0.238 |
| ≥1 | 30 ± 66.67 | 24 ± 57.14 | 25 ± 75.76 | ||
| Gestational age (weeks) | 40.00 (39.00–41.00) | 40.00 (39.00–41.00) | 40.00 (39.00–41.00) | 0.697 | |
| Newborn and child characteristics | |||||
| Birth weight (g) | 3402.89 ± 402.49 | 3418.84 ± 482.08 | 3374.24 ± 392.58 | 0.904 | |
| Birth length (cm) | 51.00 (50.00–52.00) | 51.00 (50.00–52.00) | 51.00 (50.00–52.00) | 0.678 | |
| Birth head circumference (cm) | 35.00 (34.00–36.00) | 34.00 (34.00–35.00) | 35.00 (34.00–35.00) | 0.155 | |
| Children sex | Boy | 31 (67.39%) a | 27 (62.79%) a,b | 13 (39.39%) b | 0.034 |
| Girl | 15 (32.61%) a | 16 (37.21%) a,b | 20 (60.61%) b | ||
| Children IQ (points) | 111.50 (97.00–118.00) | 113.00 (103.00–120.00) | 115.00 (105.00–121.00) | 0.551 | |
| Children BMI/Age | Severe thinness | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.388 |
| Thinness | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | ||
| Adequate weight | 33 (71.74%) | 25 (58.14%) | 26 (78.79%) | ||
| Risk of overweight | 9 (19.57%) | 13 (30.23%) | 4 (12.12%) | ||
| Overweight | 4 (8.70%) | 4 (9.30%) | 3 (9.09%) | ||
| Obesity | 0 (0.00%) | 1 (2.33%) | 0 (0.00%) | ||
| Bilingual | 2 (4.30%) | 3 (7.00%) | 2 (6.10%) | 0.890 | |
| Speech therapy | 5 (10.90%) | 1 (2.30%) | 1 (3.00%) | 0.264 | |
Data are presented as n(%) for categorical data, mean ± SDs for parametrically distributed data, and median (IQR) for nonparametrically distributed data. 1 p-values for overall differences between COGNIS study groups. p-values were obtained from ANOVA for normally distributed variables, Kruskal–Wallis rank-sum test for non-normal continuous variables, and chi-square test for categorical variables. Values not sharing the same suffix (ab) were significantly different in the Bonferroni post hoc test. p-values < 0.05 are highlighted in bold. BF: Breastfed infants; EF: Experimental infant formula; GWG: Gestational weight gain; IQ: Intelligence quotient; pBMI: Pre-conceptional body mass index; SF: Standard infant formula; VT: Vocational training.
3.2. Effects of Infant Formulas on Language Development in COGNIS-Children at Four Years Old
Afterward, the effects of both infant formulas and breastfeeding on language development were analyzed, comparing the PLON-R scores between the study groups (Table 2). At four years old, SF children seemed to present lower scores in language content (p = 0.026) and total score of PLON-R test (p = 0.029) compared to BF children in an unadjusted model. However, after adjustment for selected confounding variables (maternal age, educational level, and IQ, paternal age and educational level, sex of the child, and socioeconomic status), these differences disappeared. Interestingly, children who received EF seemed to show higher scores in the use of language (p = 0.033) and oral spontaneous expression (p = 0.024) than children who received SF. After adjustment for the above selected confounding variables, the use of language (p = 0.035) and oral spontaneous expression (p = 0.014) remained significant.
Table 2.
Effects of infant formulas on language development at four years old.
| PLON-R Scores | SF (n = 46) | EF (n = 43) | BF (n = 33) | Punadj | F (df) | Padj | ηp2 |
|---|---|---|---|---|---|---|---|
| Form 1 | 54.85 ± 18.18 | 56.49 ± 16.92 | 59.39 ± 15.28 | 0.503 | 0.387 (2,102) | 0.680 | 0.008 |
| Phonology | 0.80 ± 0.54 | 0.81 ± 0.39 | 0.91 ± 0.29 | 0.376 | 2.006 (2,102) | 0.140 | 0.038 |
| Morphology-Syntax | 3.28 ± 1.05 | 3.40 ± 0.85 | 3.48 ± 0.87 | 0.630 | 1.285 (2,102) | 0.281 | 0.025 |
| Sentences repeat | 1.48 ± 0.75 | 1.44 ± 0.70 | 1.58 ± 0.71 | 0.717 | 0.850 (2,102) | 0.431 | 0.016 |
| Oral Spontaneous Expression | 1.8 ± 0.40 | 1.95 ± 0.30 | 1.91 ± 0.29 | 0.512 | 1.493 (2,102) | 0.230 | 0.028 |
| Content 1 | 41.50 ± 16.64 a | 42.84 ± 18.08 a,b | 51.42 ± 15.05 b | 0.026 | 0.826 (2,102) | 0.441 | 0.016 |
| Lexicon | 1.20 ± 0.58 | 1.35 ± 0.65 | 1.52 ± 0.51 | 0.062 | 1.091 (2,102) | 0.340 | 0.021 |
| Comprehension | 0.89 ± 0.31 | 0.91 ± 0.29 | 1.00 ± 0.00 | 0.161 | 1.080 (2,102) | 0.343 | 0.021 |
| Expression | 0.30 ± 0.47 | 0.44 ± 0.50 | 0.52 ± 0.51 | 0.149 | 0.707 (2,102) | 0.496 | 0.014 |
| Colors identification | 0.98 ± 0.15 | 1.00 ± 0.00 | 0.97 ± 0.17 | 0.557 | 1.442 (2,102) | 0.241 | 0.028 |
| Spatial relations | 0.85 ± 0.36 | 0.77 ± 0.43 | 0.94 ± 0.24 | 0.078 | 0.266 (2,102) | 0.767 | 0.005 |
| Opposites | 0.61 ± 0.49 | 0.65 ± 0.48 | 0.82 ± 0.39 | 0.090 | 0.539 (2,102) | 0.585 | 0.010 |
| Basic needs | 0.78 ± 0.42 | 0.72 ± 0.45 | 0.88 ± 0.33 | 0.207 | 0.048 (2,102) | 0.953 | 0.001 |
| Use 1 | 46.00 ± 11.35 a | 51.86 ± 11.15 b | 50.79 ± 10.51 a,b | 0.033 | 3.461 (2,102) | 0.035 | 0.064 |
| Oral Spontaneous Expression (picture) | 1.89 ± 0.38 | 1.93 ± 0.34 | 1.91 ± 0.29 | 0.866 | 2.376 (2,102) | 0.098 | 0.045 |
| Oral Spontaneous Expression (puzzle) | 0.4 ± 0.50 a | 0.70 ± 0.46 b | 0.67 ± 0.48 a,b | 0.024 | 4.472 (2,102) | 0.014 | 0.081 |
| PLON-R Total Score 1 | 47.07 ± 16.90 a | 51.23 ± 18.12 a,b | 57.48 ± 15.18 b | 0.029 | 1.512 (2,102) | 0.225 | 0.029 |
Data are presented as means ± SD of direct scores. 1 Data are presented as means ± SD of typical scores. Punadj is ANOVA. Values not sharing the same suffix (ab) were significantly different in a Bonferroni post hoc test. Padj is ANCOVA for the group differences using the univariate general linear model, including confounder factors: Maternal age, educational level, and IQ, paternal age and educational level, sex of the child, and socioeconomic status. F-values (F) and effect sizes (ηp2) were calculated by ANCOVA. p-values < 0.05 are highlighted in bold. BF: Breastfed infants; df: Degrees of freedom; EF: Experimental infant formula; PLON-R: Oral Language Task of Navarra-Revised; SF: Standard infant formula.
We next evaluated the association between the type of feeding and PLON-R scores categorized in each scale into normal or need to improve/delayed outcomes according to the standards test (Table 3). The analysis suggests showed that children fed with SF were more frequently categorized into delayed/need to improve/in use of language (p = 0.020) in comparison with children fed with EF. Moreover, the SF group seemed to present more frequently delayed/need to improve in PLON-R total score (p = 0.027) than children who were breastfed.
Table 3.
Association between infant formulas and PLON-R clinical clusters in children at four years old.
| PLON-R Scales | SF (n = 46) | EF (n = 43) | BF (n = 33) | Chi-Square | P 1 | |
|---|---|---|---|---|---|---|
| Form | Delayed/Need to improve | 13 (28.26) | 9 (20.93) | 4 (12.12) | 2.991 | 0.224 |
| Normal | 33 (71.74) | 34 (79.07) | 29 (87.88) | |||
| Content | Delayed/Need to improve | 37 (80.43) | 32 (74.42) | 20 (60.61) | 2.991 | 0.142 |
| Normal | 9 (19.57) | 11 (25.58) | 13 (39.39) | |||
| Use | Delayed/Need to improve | 28 (60.87) a | 14 (32.56) b | 13 (39.39) a,b | 7.786 | 0.020 |
| Normal | 18 (39.13) a | 29 (67.44) b | 20 (60.61) a,b | |||
| PLON-R Total Score | Delayed/Need to improve | 25 (54.35) a | 19 (44.19) a,b | 8 (24.24) b | 7.188 | 0.027 |
| Normal | 21 (45.65) a | 24 (55.81) a,b | 25 (75.76) b |
Data are presented as n(%). 1 p-values for overall differences between COGNIS study groups. p-values were obtained from the chi-square test for categorical variables. Values not sharing the same suffix (ab) were significantly different in the Bonferroni post hoc test. p-values < 0.05 are highlighted in bold. BF: Breastfed infants; EF: Experimental infant formula; PLON-R: Oral Language Task of Navarra-Revised; SF: Standard infant formula. Categories according to standards PLON-R: Normal (form: ≥ 50; content: ≥ 67; use: ≥ 59; total: ≥ 54) and delayed/need to improve (form: ≤ 36; content: ≤ 47; use: ≤ 39; total: ≤ 45).
Finally, in order to evaluate the influence of other confounder variables, the Wald test for logistic regression was performed (Table 4). Maternal age, educational level, and IQ, paternal age and educational level, socioeconomic status, sex of the child, and the three study groups were included in this model. The type of feeding during the first months of life (COGNIS groups) might have an effect on use of language at four years old. In fact, it seems that SF-fed children presented an increased risk of suffering problems in the use of language (OR: 8.500 (95% CI: 1.504–48.049); p = 0.015) compared to BF children. Furthermore, a marginally significant p-value was found with respect to socioeconomic status. In this matter, children who had lower socioeconomic status might present an increased risk of suffering problems in the content of language (OR: 3.583 (95% CI: 0.977–13.148); p = 0.054) and PLON-R total scores (OR: 4.821 (95% CI: 0.966–24.063); p = 0.055). No other effects of other confounder variables on language development at four years old were found in the analysis performed.
Table 4.
Effects of COGNIS groups and socioeconomic status on language outcomes at four years old.
| PLON-R Scales | SF Group * OR (95% CI) | p | Socioeconomic Status † OR (95% CI) | p |
|---|---|---|---|---|
| Form | - | - | - | - |
| Content | - | - | 3.583 (0.977–13.148) | 0.054 |
| Use | 8.500 (1.504–48.049) | 0.015 | - | - |
| Total Score | - | - | 4.821 (0.966–24.063) | 0.055 |
Model was a logistic regression (Wald method). Dichotomized scales for binary logistic regression: normal and delayed/need to improve. p-values < 0.05 are highlighted in bold. * Breastfeeding group as reference. The SF group was not included in the model of form, content, and total score. † High socioeconomic status as reference. Socioeconomic status was not included in the model of form and use. CI: Confidence interval; OR: Odds ratio; PLON-R: Oral Language Task of Navarra-Revised; SF: Standard infant formula.
4. Discussion
The present study suggests the long-term beneficial effects of a new infant formula enriched with functional nutrients on children’s language development at four years of age. In fact, children fed with the EF seemed to show better scores in use of language and oral spontaneous expression compared to those fed with the SF. Additionally, the SF group might be associated with worse scores in PLON-R total score compared to the BF group, with scores of the EF children similar to those who were breastfed. Finally, the use of language in children up to four years could be influenced by type of feeding during the first eighteen months and, to a lesser extent, by socioeconomic status. Thus, our results suggest that the addition of bioactive nutrients to the new infant formula improves children’s language development, achieving similar scores to breastfed children.
Nutrition during the first months of life helps to build brain cells, establishing connections between them and creating brain architecture for optimal neurodevelopment [15] and language acquisition [16]. One of the most accepted mechanism refers to differences in fatty acid profile of breast milk and infant formula, which might have a different effect on brain structure and function [17]. Other potential mechanisms influencing neurodevelopment could be the impact of breastfeeding on the own mother, and the special mother–infant interaction, which could shape the learning and language skills of their offspring [18]. Furthermore, neurodevelopment is the result of the dynamic interrelation between environment and genetic background. Exposure to adverse experiences can negatively affect gene expression, as well as brain development and its cognitive and behavioral functions. If this occurs during critical developmental periods, the effects could be permanent [19].
Breast milk is considered the gold standard for infant nutrition and appears to be beneficial for an adequate neurological development [20]. This effect could be caused by specific bioactive nutrients and other components present in human milk, which promote a synergistic effect and are absent or present in lower amounts in infant formulas [21,22]. Recently, it has been demonstrated that breastfed children have better language development, stimulus processing, and increased IQ during the first year of life compared to formula-fed infants [23].
LC-PUFAs, especially DHA and ARA, are found in high proportions in the structural lipids of cell membranes, particularly those of the central nervous system [24]. Interestingly, it has been reported that the LC-PUFAs content of brain cell membranes could influence gene expression within those cells [25]. DHA is considered essential for the cortical circuit maturation in the developing brain, and ARA is also critical for childhood growth, brain development and health [26]. In this matter, infants who received a supplement containing ARA (200 mg/day) and DHA (200 mg/day) from 12–24 months of age showed better cognition and language skills compared to those without supplementation [27]. An interesting issue is that the EF tested in the COGNIS study was supplemented with ARA (0.45%) and DHA (0.32%), while the SF only contained linoleic and α-linolenic acids. EF seemed to be associated with better language development at four years of age. Furthermore, higher DHA levels have been associated with larger cortical grey matter and cerebellar volumes [28], which are involved in different language tasks [29]. Several randomized clinical trials (RCTs) have also shown that infants supplemented with DHA show better language and communication skills [30,31]. On the other hand, research efforts have focused on infant formula supplementation with MFGM, which is a biologically active component derived of milk proteins present in human milk [32], including a lipid fraction consisting of LC-PUFAs (also ARA and DHA) [33]. In this regard, addition of MFGM to an infant formula has been found to improve immune system and neurodevelopment during the first months of life [8,34].
Results of the current study support the importance of the type of infant nutrition on brain development, particularly on language skills. So far, to our knowledge, the majority of studies have focused on determining the effect of a single component added to an infant formula on language development and cognitive function. In this regard, our study is pioneering because it evaluates the whole effects of several functional nutrients that help to narrow the gap between the composition of the EF and breast milk. Our current results suggest that language development in EF children is similar to BF children. However, it is difficult to ascertain whether this effect is due to a single nutrient or to the synergistic effect of all of them. Although a possible positive and direct effect of LC-PUFAs has been suggested, we do not know the potential role of other bioactive compounds, including MFGM, gangliosides, nucleotides, or sialic acid, on language development, as well as the potential interactions between them.
Furthermore, it is important to note the long-term nutritional intervention performed in the COGNIS study were carried out in the infants during their first 18 months of life, which it is established as a fundamental period of brain maturation and growth [11,35]. It is known that language development is related to brain maturation during the first two years of life, together with the maturation of the brain circuits that connect Broca’s and Wernicke’s areas (fronto-temporo-parietal language system) [36]. Previous studies have shown a greater volume of white and gray matter in the left and right parietal and left temporal lobes, as well as more activation in the right frontal and left temporal lobe in breastfed children between five and seven years old, evidencing mechanisms linked to breastfeeding and neurocognitive outcomes [20,37,38]. Therefore, the functionality and connectivity in the fronto-temporo-parietal language system could be the key for language acquisition and processing during early life [15,16,17].
It is important to note that logistic regression models seemed to show a trend of poorer language skills in children with low socioeconomic status, regardless the type of feeding received. There are different factors, such as genetic background, sex, intrauterine growth restriction, nutrition, maternal education, and socioeconomic status, among others, that have an important role during brain development [39]. Others researchers have reported that children born in families with lower socioeconomic status showed slower language development [40,41]. Furthermore, the results of the current study are in agreement with other study reporting that the beneficial effects of breastfeeding on language skills in children at five years old were reduced when social and parental factors were taken into account [42]. However, further studies should be performed in order to confirm this outcome.
The main strengths of the COGNIS study are the design, considering that is a prospective RCT with a long-term intervention and monitoring period. Moreover, several confounding factors, which may influence neurodevelopment, were taken into account in the statistical models, to provide accurate, consistent, and reliable results and conclusions. Our results could provide evidence of the long-term effects of early nutrition on language development in healthy children, while the vast majority of previous studies have focused on language pathologies. We used the PLON-R test, a highly reliable and valid instrument for assessing language development in Spanish children [14]. However, even if language tests are validated and adapted to children at four years of age, it is quite difficult to obtain significant differences in healthy children at this age, which could be a limitation of the study. Nevertheless, the statistical power at this time point on the COGNIS study reached 80%, allowing the authors to detect a minimum difference of 0.7 SD, which is enough to detect relevant differences in language development between the study groups.
5. Conclusions
The findings from this study suggest the long-term beneficial effects of a new infant formula containing LC-PUFAs, MFGM, synbiotics, and other functional nutrients on language development in healthy children at four years old. Thus, this new infant formula might promote optimal brain development in a similar way to breast milk. Furthermore, socioeconomic factors seem to be involved in language development during childhood.
Acknowledgments
The authors wish to acknowledge the parents and children who participated in the study, and also the pediatricians and technicians of the EURISTIKOS team at the Department of Pediatrics, School of Medicine, University of Granada (Spain) for their contribution.
Author Contributions
Conceptualization, C.C.; methodology, A.N.-R., A.C., M.T.M., and C.C.; formal analysis, A.N.-R. and M.T.M.; investigation, A.N.-R., E.D., and N.S.-V.; writing—original draft preparation, A.N.-R., J.A.G.-S., M.G.B.; writing—review and editing, A.N.-R., E.D., N.S.-V., E.C., J.J., M.R.-P., A.C., M.T.M., J.A.G.-S., M.G.B., and C.C.; supervision, C.C.; project administration, C.C.; funding acquisition, J.J., M.R.-P., and C.C. All authors critically revised the manuscript for important intellectual content and approved the final version. The results of this article are likely to be included in the Doctoral Thesis of A.N-R. in the context of the Clinical Medicine and Public Health Doctoral Program at the University of Granada. All authors have read and agreed to the published version of the manuscript.
Funding
This project has been funded by Ordesa Laboratories, S.L. Contract University of Granada General Foundation, No. 3349 and SMARTFOODS (CIEN) Contract University of Granada General Foundation, No. 4003, Spanish Ministry of Economy, Industry and Competitiveness; funded in part by HORIZON 2020 EU DynaHEALTH Project (GA No.633595). Natalia Sepúlveda-Valbuena has been granted with a scholarship from Fundación Carolina, Madrid, Spain.
Conflicts of Interest
Jesús Jiménez and Dra. María Rodríguez-Palmero are employees of Ordesa Laboratories, S.L.
References
- 1.Head Zauche L., Darcy Mahoney A.E., Thul T.A., Zauche M.S., Weldon A.B., Stapel-Wax J.L. The Power of Language Nutrition for Children’s Brain Development, Health, and Future Academic Achievement. J. Pediatr. Health Care. 2017;31:493–503. doi: 10.1016/j.pedhc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl P.K. Early language acquisition: Cracking the speech code. Nat. Rev. Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- 3.Werker J.F., Hensch T.K. Critical Periods in Speech Perception: New Directions. Annu. Rev. Psychol. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- 4.Pivik R.T., Andres A., Bai S., Cleves M.A., Tennal K.B., Gu Y., Badger T.M. Infant Diet-Related Changes in Syllable Processing Between 4 and 5 Months: Implications for Developing Native Language Sensitivity. Dev. Neuropsychol. 2016;41:215–230. doi: 10.1080/87565641.2016.1236109. [DOI] [PubMed] [Google Scholar]
- 5.Mulder K.A., Elango R., Innis S.M. Fetal DHA inadequacy and the impact on child neurodevelopment: A follow-up of a randomised trial of maternal DHA supplementation in pregnancy. Br. J. Nutr. 2018;119:271–279. doi: 10.1017/S0007114517003531. [DOI] [PubMed] [Google Scholar]
- 6.Colombo J., Carlson S.E., Cheatham C.L., Shaddy D.J., Kerling E.H., Thodosoff J.M., Gustafson K.M., Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 2013;98:403–412. doi: 10.3945/ajcn.112.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernell O., Domellof M., Grip T., Lonnerdal B., Timby N. Physiological Effects of Feeding Infants and Young Children Formula Supplemented with Milk Fat Globule Membranes. Nestle Nutr. Inst. Workshop Ser. 2019;90:35–42. doi: 10.1159/000490291. [DOI] [PubMed] [Google Scholar]
- 8.Timby N., Domellöf E., Hernell O., Lönnerdal B., Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014;99:860–868. doi: 10.3945/ajcn.113.064295. [DOI] [PubMed] [Google Scholar]
- 9.Cerdó T., Ruíz A., Suárez A., Campoy C. Probiotic, prebiotic, and brain development. Nutrients. 2017;9:1247. doi: 10.3390/nu9111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto-Ruiz A., García-Santos J.A., Bermúdez M.G., Herrmann F., Diéguez E., Sepúlveda-Valbuena N., García S., Miranda M.T., De-Castellar R., Rodríguez-Palmero M., et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients. 2019;11:2456. doi: 10.3390/nu11102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salas Lorenzo I., Chisaguano Tonato A., de la Garza Puentes A., Nieto A., Herrmann F., Dieguez E., Castellote A., López-Sabater M., Rodríguez-Palmero M., Campoy C., et al. The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients. 2019;11:602. doi: 10.3390/nu11030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association W.M. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Aguinaga G., Armentia M.L., Fraile A., Olangua P., Uriz N. In: PLON-R: Prueba de Lenguaje Oral Navarra—Revisada: Manual. 2nd ed. TEA Ediciones, editor. Madrid, Spain: 2005. [Google Scholar]
- 15.Mahurin Smith J. Breastfeeding and language outcomes: A review of the literature. J. Commun. Disord. 2015;57:29–40. doi: 10.1016/j.jcomdis.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Iannotti L., Jean Louis Dulience S., Wolff P., Cox K., Lesorogol C., Kohl P. Nutrition factors predict earlier acquisition of motor and language milestones among young children in Haiti. Acta Paediatr. Int. J. Paediatr. 2016;105:e406–e411. doi: 10.1111/apa.13483. [DOI] [PubMed] [Google Scholar]
- 17.Bourre J.M. In: Diet, Brain Lipids, and Brain Functions: Polyunsaturated Fatty Acids, Mainly Omega-3 Fatty Acids BT—Handbook of Neurochemistry and Molecular Neurobiology: Neural Lipids. Lajtha A., Tettamanti G., Goracci G., editors. Springer; Boston, MA, USA: 2009. pp. 409–441. [Google Scholar]
- 18.Krol K.M., Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt. Gesundh. Gesundh. 2018;61:977–985. doi: 10.1007/s00103-018-2769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchdev P.S., Boivin M.J., Forsyth B.W., Georgieff M.K., Guerrant R.L., Nelson C.A., 3rd Assessment of Neurodevelopment, Nutrition, and Inflammation from Fetal Life to Adolescence in Low-Resource Settings. Pediatrics. 2017;139:S23–S37. doi: 10.1542/peds.2016-2828E. [DOI] [PubMed] [Google Scholar]
- 20.Horta B.L., de Sousa B.A., de Mola C.L. Breastfeeding and neurodevelopmental outcomes. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21:174–178. doi: 10.1097/MCO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 21.Martin C., Ling P.-R., Blackburn G. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8:279. doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheatham C.L., Sheppard K.W. Synergistic Effects of Human Milk Nutrients in the Support of Infant Recognition Memory: An Observational Study. Nutrients. 2015;7:9079–9095. doi: 10.3390/nu7115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi H.J., Kang S.K., Chung M.R. The relationship between exclusive breastfeeding and infant development: A 6-and 12-month follow-up study. Early Hum. Dev. 2018;127:42–47. doi: 10.1016/j.earlhumdev.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Jasani B., Simmer K., Patole S.K., Rao S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017;3:CD000376. doi: 10.1002/14651858.CD000376.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaton A.E., Meldrum S.J., Foster J.K., Prescott S.L., Simmer K. Does docosahexaenoic acid supplementation in term infants enhance neurocognitive functioning in infancy? Front. Hum. Neurosci. 2013;7:774. doi: 10.3389/fnhum.2013.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauritzen L., Brambilla P., Mazzocchi A., Harsløf L.B.S., Ciappolino V., Agostoni C. DHA Effects in Brain Development and Function. Nutrients. 2016;8:6. doi: 10.3390/nu8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin A.M., Chau C.M.Y., Dyer R., Matheson J., McCarthy D., Yurko-Mauro K., Innis S.M., Grunau R.E. Developmental Outcomes at 24 Months of Age in Toddlers Supplemented with Arachidonic Acid and Docosahexaenoic Acid: Results of a Double Blind Randomized, Controlled Trial. Nutrients. 2017;9:975. doi: 10.3390/nu9090975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamino D., Studholme C., Liu M., Chau V., Miller S.P., Synnes A., Rogers E.E., Barkovich A.J., Ferriero D.M., Brant R., et al. Postnatal polyunsaturated fatty acids associated with larger preterm brain tissue volumes and better outcomes. Pediatr. Res. 2018;83:93–101. doi: 10.1038/pr.2017.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marien P., Borgatti R. Language and the cerebellum. Handb. Clin. Neurol. 2018;154:181–202. doi: 10.1016/B978-0-444-63956-1.00011-4. [DOI] [PubMed] [Google Scholar]
- 30.Agostoni C., Zuccotti G.V., Radaelli G., Besana R., Podesta A., Sterpa A., Rottoli A., Riva E., Giovannini M. Docosahexaenoic acid supplementation and time at achievement of gross motor milestones in healthy infants: A randomized, prospective, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009;89:64–70. doi: 10.3945/ajcn.2008.26590. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum S.J., D’Vaz N., Simmer K., Dunstan J.A., Hird K., Prescott S.L. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br. J. Nutr. 2012;108:1443–1454. doi: 10.1017/S0007114511006878. [DOI] [PubMed] [Google Scholar]
- 32.Lee H., Padhi E., Hasegawa Y., Larke J., Parenti M., Wang A., Hernell O., Lönnerdal B., Slupsky C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018;6:313. doi: 10.3389/fped.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingvordsen Lindahl I.E., Artegoitia V.M., Downey E., O’Mahony J.A., O’Shea C.-A., Ryan C.A., Kelly A.L., Bertram H.C., Sundekilde U.K. Quantification of Human Milk Phospholipids: The Effect of Gestational and Lactational Age on Phospholipid Composition. Nutrients. 2019;11:222. doi: 10.3390/nu11020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timby N., Hernell O., Vaarala O., Melin M., Lönnerdal B., Domellöf M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 2015;60:384–389. doi: 10.1097/MPG.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 35.Johnson M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- 36.Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- 37.Belfort M.B., Anderson P.J., Nowak V.A., Lee K.J., Molesworth C., Thompson D.K., Doyle L.W., Inder T.E. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J. Pediatr. 2016;177:133–139. doi: 10.1016/j.jpeds.2016.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby J.L., Belden A.C., Whalen D., Harms M.P., Barch D.M. Breastfeeding and Childhood IQ: The Mediating Role of Gray Matter Volume. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:367–375. doi: 10.1016/j.jaac.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan A.F., Matthews M.A. Neurodevelopmental Outcomes in Early Childhood. Clin. Perinatol. 2018;45:377–392. doi: 10.1016/j.clp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Schwab J.F., Lew-Williams C. Language learning, socioeconomic status, and child-directed speech. Wiley Interdiscip. Rev. Cogn. Sci. 2016;7:264–275. doi: 10.1002/wcs.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKean C., Reilly S., Bavin E.L., Bretherton L., Cini E., Conway L., Cook F., Eadie P., Prior M., Wake M., et al. Language Outcomes at 7 Years: Early Predictors and Co-Occurring Difficulties. Pediatrics. 2017;139:e20161684. doi: 10.1542/peds.2016-1684. [DOI] [PubMed] [Google Scholar]
- 42.Quinn P., O’Callaghan M., Williams G., Najman J., Andersen M., Bor W. The effect of breastfeeding on child development at 5 years: A cohort study. J. Paediatr. Child Health. 2001;37:465–469. doi: 10.1046/j.1440-1754.2001.00702.x. [DOI] [PubMed] [Google Scholar]