Abstract
Background
Routine molecular surveillance for imported drug-resistant malaria parasites to the USA and European Union is an important public health activity. The obtained molecular data are used to help keep chemoprophylaxis and treatment guidelines up to date for persons traveling to malaria endemic countries. Recent advances in next-generation sequencing (NGS) technologies provide a new and effective way of tracking malaria drug-resistant parasites.
Methods
As part of a technology transfer arrangement between the CDC Malaria Branch and the Istituto Superiore di Sanità (ISS), Rome, Italy, the recently described Malaria Resistance Surveillance (MaRS) protocol was used to genotype 148 Plasmodium falciparum isolates from Eritrea for kelch 13 (k13) and cytochrome b (cytb) genes, molecular markers associated with resistance to artemisinin (ART) and atovaquone/proguanil (AP), respectively.
Results
Spanning the full-length k13 gene, seven non-synonymous single nucleotide polymorphisms (SNPs) were found (K189N, K189T, E208K, D281V, E401Q, R622I and T535M), of which none have been associated with artemisinin resistance. No mutations were found in cytochrome b.
Conclusion
All patients successfully genotyped carried parasites susceptible to ART and AP treatment. Future studies between CDC Malaria Branch and ISS are planned to expand the MaRS system, including data sharing, in an effort to maintain up to date treatment guidelines for travelers to malaria endemic countries. 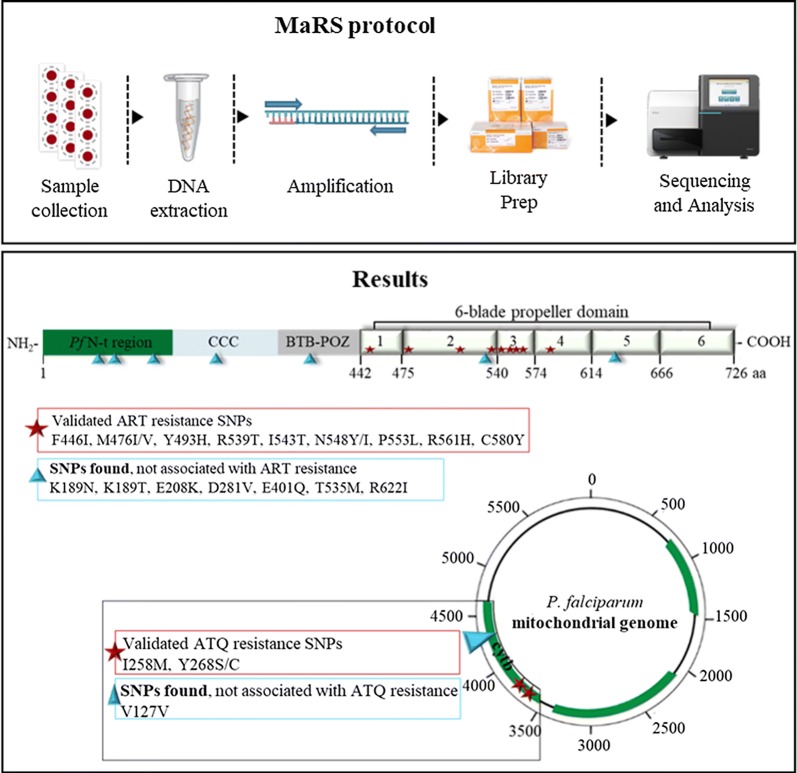
Keywords: Plasmodium falciparum, Drug resistance, Next-generation sequencing, Molecular surveillance
Background
In the last decade, total malaria cases have been reduced by 40% worldwide [1, 2] leading to a dramatic reduction in mortality in children, especially in sub-Saharan Africa. This was achieved through the deployments of vector control measures, accurate diagnosis, and treatment of uncomplicated Plasmodium falciparum malaria with artemisinin-based combination therapies (ACTs). Despite this overall progress, thousands of travel-related malaria cases are imported into the European Union (EU) and the USA [3, 4]. The European Centre for Disease Prevention and Control (ECDC) estimate that an average of 8000 malaria cases are imported to the EU annually, most of which are travelers returning home after visiting friends and relatives in Africa [4]. In the USA, according to the Centers for Disease Control and Prevention (CDC), approximately 1700 malaria cases are imported annually to the country [3].
Both the EU and the USA, recommend the use of atovaquone/proguanil (AP) for chemoprophylaxis [5–7]. In the USA, AP is used as a primary treatment choice for imported uncomplicated P. falciparum cases, while in the EU, two ACTs, artemether-lumefantrine (AL) and dihydroartemisinin/piperaquine (DHA/PPQ) are licensed as treatment options for uncomplicated P. falciparum malaria [8–10]; AP can be used if an ACT is not available [10].
Single nucleotide polymorphisms (SNPs) in the cytochrome b (cytb) gene, in the mitochondrial genome of P. falciparum, confer resistance to AP. The putative I258M and Y268S/C SNPs have been associated with AP resistance [11, 12]. In comparison, resistance to artemisinin derivatives, given as part of ACTs, such as AL, are reported with parasites carrying SNPs in the kelch 13 (k13) gene. Specifically, the F446I, N458Y, M476I, Y493H, R539T, I543T, P553L, R561H and C580Y SNPs are confirmed to confer resistance to artemisinin. [13]. The k13 gene provides a validated molecular marker to detect, track and monitor the emergence and spread of artemisinin-resistant SNPs [14–16]. While a number of other k13 SNPs have been described in Africa, none have yet been confirmed to be associated with artemisinin resistance [17–19], except in a case described by Lu et al. [20] in which was shown that a P. falciparum imported into China from Equatorial Guinea was resistant to artemisinin in vitro.
Molecular surveillance of imported malaria cases is an important public health activity that can aid in the detection of drug-resistant P. falciparum parasites [21, 22]. Recent advancements in next-generation sequencing are providing a new way to rapidly detect and characterize drug-resistant malaria parasites, including minor parasite populations in mixed infection P. falciparum cases [23, 24]. These molecular data can be used to help keep chemoprophylaxis and treatment guidelines up to date for persons traveling to malaria endemic countries.
Towards this end, as part of a technology transfer training agreement between the CDC and the Istituto Superiore di Sanità, Rome, Italy, we used the recently published Malaria Resistance Surveillance (MaRS) [24] protocol to genotype SNPs in the kelch 13 and cytochrome b genes from 148 P. falciparum-positive samples from Eritrea.
Methods
Samples
A total of 148 P. falciparum dried blood spots (DBS) were used in this study; these were originally collected as part of a cross-sectional study between November 2013 and November 2014 in two regions (Barentu and Agordat) of Eritrea endemic for falciparum malaria, as previously described by Menegon et al. [25]. Samples were initially analyzed for P. falciparum infection using molecular diagnosis at the Gezira University (Wad Medani, Sudan) in a collaboration existing between Gezira University and the Eritrean Ministry of Health.
DNA extraction
DNA was extracted using the QIAmp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) using the recommended guidelines in the original MaRS protocol [24].
Plasmodium falciparum species confirmation and sample quality assurance
The previously described real-time PET-PCR method [26] was used to confirm P. falciparum infections and assess the quality of DNA from the filter blood spot samples. This method is based on self-quenching photo-induced electron transfer (PET) fluorogenic primers used in a multiplex real-time PCR to detect Plasmodium spp. and P. falciparum. Samples with a Cq value > 40 were considered P. falciparum-negative.
Kelch 13 and cytochrome b PCR enrichment
The following primers were used: k13 gene (forward: 5′-CTA TGA CGT ATG ATA GGG AAT CTG G-3′ and reverse: 5′-CTG GGA ACT AAT AAA GAT GGG CC-3′); cytochrome b gene (forward: 5′-CTA TTA ATT TAG TTA AAG CAC AC-3′ and reverse: 5′-ACA GAA TAA TCT CTA GCA CCA-3′). Previously described PCR conditions were used [24]. Plasmodium falciparum kelch13 wild-type strains HB3, 7G8 and P. falciparum kelch13 C580Y mutant strain MRA1236 (ATCC) were used as controls. DNA isolated from whole blood of individuals without malaria was used as a negative control.
PCR product purification, normalization, pooling, library prep and sequencing
Briefly, PCR products were purified and normalized using the SequalPrep Kit (Cat # A1051001; Thermo Fisher Scientific, Waltham, USA). PCR products were pooled in 10.0 µl volumes for each sample. Library prep was performed using the Illumina Nextera XT kit (Cat # FC-131-1096 and FC‐131‐1002; Illumina, San Diego, USA). Pooled fragments were assessed for correct size using the Agilent D5000 ScreenTape Station (Cat # G2940C, # 5067-4626, and # G2940CA; Agilent Technologies, Santa Clara, USA) and DNA concentration checked using the Qubit 3.0 Flurometer (Cat #Q33216 and #Q32853; Life Technologies Corporation, Carlsbad, USA). Sequencing was performed using the MiSeq v2 reagents using the 500 cycle kit (Cat # MS-102-2003; Illumina MiSeq reagents v2, San Diego, USA).
Data analysis
The next-generation sequencing analysis toolkit (NeST) (https://github.com/CDCgov/MaRS) was used to call non-synonymous and synonymous single nucleotide polymorphisms (SNPs) in k13 and cytb genes, respectively. All SNPs were also confirmed using the Geneious Prime software (www.geneious.com).
MaRS protocol details
Details about the laboratory and data analysis protocols can be found at https://github.com/CDCgov/MaRS. This protocol is based on collating and mapping SNPs associated with antimalarial drug resistance by using a targeted amplicon deep sequencing (TADS) approach.
Results
Sequencing outcome using dried blood spots
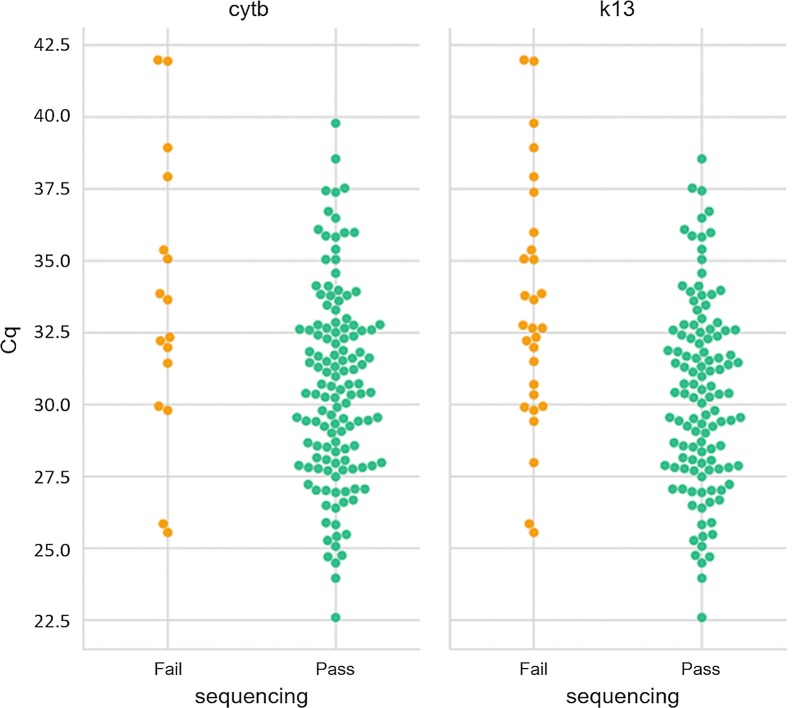
PET-PCR was performed on all 148 samples in duplicate. A total of 144 out of 148 screened samples were positive for both the genus Plasmodium and P. falciparum based on the Cq values (mean of 30.9). Out of the positive samples, successful sequencing results were obtained for 82.6% (119/144) and 91.7% (132/144) samples for the k13 and cytb genes, respectively, as shown in Fig. 1.
Fig. 1.
Sequencing outcome for kelch 13 and cytochrome b genes. Sequencing pass (green dots) or fail (red dots) is shown on the x-axis and Cq values for each sample on the y-axis. Successful sequencing data were obtained for 82.6% (119/144) and 91.7% (132/144) samples for the k13 and cytb genes, respectively
Read depth for SNPs associated with malarial drug-resistant k13 and cytb
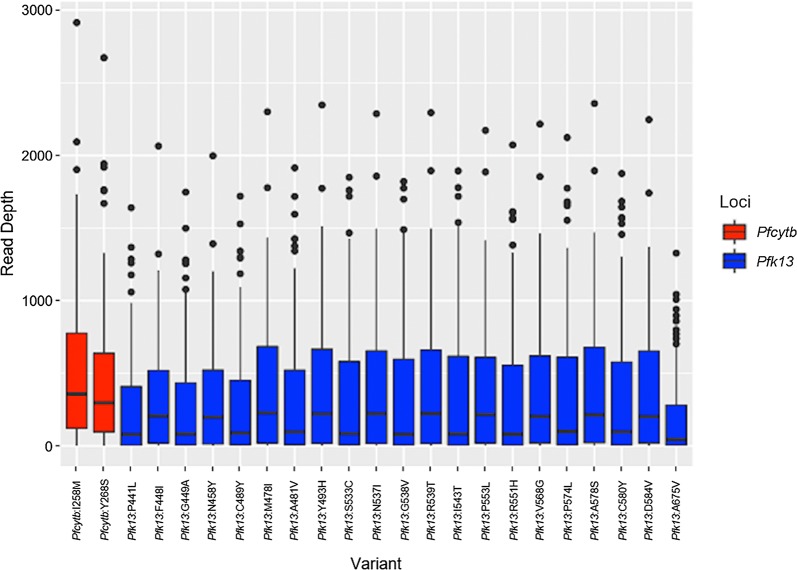
Read depth at 2 SNPs in the cytb and 21 SNPs (9 validated SNPs and 12 candidate SNPs based on the 2018 WHO artemisinin report) in k13 for 119 and 132 samples, respectively, were analyzed (Fig. 2). Median read depth across SNPs in cytb was 310.5× coverage. The median read depth across SNPs in k13 was 126X coverage.
Fig. 2.
Read depth for SNPs associated with malarial drug resistance in the Plasmodium falciparum cytb and k13. Middle bar, median; upper box hinge, 75th percentile; lower box hinge, 25th percentile; upper whisker, largest value no further than 1.5 × IQR (inter-quartile range or distance from first and third quartiles) from the hinge; lower whisker, smallest value at most 1.5 × IQR from the hinge; dots, outlying samples
Kelch 13
Spanning the full-length k13 gene in the 82.6% (119/144) successfully sequenced samples, seven non-synonymous SNPs were detected (Table 1). Of the seven non-synonymous mutations detected, five were found outside the propeller domain region: K189N, K189T, E208K, D281V and E401Q. Of these, the most frequent SNP was K189T found in 59.7% (71/119) of samples at an allele frequency of 100%, except for one sample in which the polymorphism was identified as minor allele at an allele frequency of 43.0%. Inside the k13 propeller domain, two non-synonymous SNPs were identified: R622I and T535M. R622I was detected as a major allele at an allele frequency of 100% and T535M as a minor allele at an allele frequency of less of 50% (Table 1).
Table 1.
Prevalence of polymorphisms in kelch 13 and cytochrome b genes in the analyzed falciparum isolates. K13 protein consists of a Plasmodium-specific N-terminal region (Pf N-t region), coiled-coil-containing domain (CCC), a Broad-complex, Tramtrack, Bric-a-Brac/Poxvirus and Zincfinger domain (BTB/POZ) and a C-terminal Kelch-repeat propeller domain (KREP)
| Target gene | Codon position | Domain location | Reference sequence | Mutant sequence | Type | Allele frequency (%) | n/N | ||
|---|---|---|---|---|---|---|---|---|---|
| Amino acid | Nucleotide | Amino acid | Nucleotide | ||||||
| Pfk13 | 189 | Pf N-t region | K | AAA | N | AAT | NS | 100 | 4/119 |
| 189 | Pf N-t region | K | AAA | T | ACA | NS | 100 | 70/119 | |
| 189 | Pf N-t region | K | AAA | T | ACA | NS | 43 | 1/119 | |
| 208 | Pf N-t region | E | GAA | K | AAA | NS | 100 | 10/119 | |
| 281 | CCC | D | GAT | V | GTT | NS | 100 | 2/119 | |
| 401 | BTB/POZ | E | GAG | Q | CAG | NS | 100 | 4/119 | |
| 622 | KREP | R | AGA | I | ATA | NS | 100 | 1/119 | |
| 535 | KREP | T | ACG | M | ATG | NS | 20 | 1/119 | |
| Pfcytb | 127 | – | V | GTG | V | GTG | Syn | 100 | 2/132 |
Note: The boldface highlights the nucleotide base change
Abbreviations: n, number of samples containing mutant allele; N, total number of successfully sequenced samples; NS, non-synonymous mutation
Cytochrome b
A total of 91.7% (132/144) were successfully sequenced for cytb. Of these, 98.5% (130/132) carried the wild-type 258I and 268Y alleles. Two samples were identified with the V127V synonymous mutation (Table 1).
Discussion
Active surveillance of imported malaria cases is an important public health activity in the USA and the EU [27, 28]: it plays a pivotal role in estimating the incidence in imported malaria cases, preventing malaria reintroduction, and providing chemoprophylaxis guidelines to travelers to malaria endemic regions. Historically, anti-malaria drug resistance genotyping was performed using traditional low throughput sequencing methodologies, such as Sanger sequencing [29]. However, currently, next-generation sequencing and accompanying standardized bioinformatics tools provide advanced and rapid protocols for monitoring molecular marker genes involved in P. falciparum drug resistance [24].
Based on the WHO guidelines, routine malaria surveillance is critical for the intervention in all malaria endemic and non-endemic countries, with the goal to reduce the incidence of malaria, eliminate the disease and prevent its re-establishment through detection and characterization of malaria parasites [30]. To achieve this goal, laboratory and analysis protocols related to malaria molecular surveillance activities need to be standardized, have appropriate quality assurance systems in place, and provide information in a timely fashion. The recent advances in next-generation sequencing (NGS), including high throughput and decreasing costs, meet these requirements and are making this technology more suitable for routine molecular surveillance of P. falciparum drug resistance genes in public health laboratories [24]. Notably, with this approach, lots of samples can be analyzed simultaneously, allow focusing on the full length of the genes, rather than only some regions. Toward this end, as part of a technology transfer arrangement between the CDC and the Istituto Superiore di Sanità, Rome, the recently developed Malaria Resistance Surveillance (MaRS) protocol was used to genotype Pfk13 and Pfcytb genes using dried blood spot samples available for the present study. The k13 and cytb genes were genotyped since AP, AL and DHA/PPQ are used for treatment of malaria cases imported into Italy.
Our results are in agreement with previously published reports on the k13 gene from Africa, where a large number of non-artemisinin resistant associated SNPs have been reported. Notably, the K189T mutation, which is widely prevalent in African artemisinin-sensitive P. falciparum parasites [31] as well as in South East Asian parasites [32] was observed. We found no evidence of the putative I258M and Y268S/C SNPs associated with AP resistance [11, 12], suggesting that the use of AP for prophylaxis for travelers to Eritrea remains effective.
Conclusions
The MaRS protocol used in this technology transfer training provides a standardized, high-throughput laboratory and analysis system for characterizing and tracking possible polymorphisms in molecular marker genes linked to drug resistance. The goal of this study was to evaluate the possibility to set up MaRS system also in Italy and to maintain active collaboration between the two institutes.
Acknowledgments
We thank Peter McElroy, Michael Aidoo and Venkatachalam Udhayakumar for reviewing this manuscript and we want to thank Barbara J. Marston for reviewing and approving this study under the human subject’s protocol 2017-228.
Abbreviations
- NGS
next-generation sequencing
- CDC
Centers for Disease Control and Prevention
- ISS
Istituto Superiore di Sanità
- MaRS
Malaria Resistance Surveillance
- k13
kelch 13 gene
- cytb
cytochrome b gene
- ART
artemisinin
- ATQ
atovaquone
- AP
atovaquone/proguanil
- SNPs
single nucleotide polymorphisms
- ACT
artemisinin-based combination therapy
- ECDC
European Centers for Disease Control and Prevention
- AL
artemether/lumefantrine
- DHA
dihydroartemisinin
- PPQ
piperaquine
- DBS
dried blood spot
- PET
photo-induced electron transfer
- PCR
polymerase chain reaction
- NeST
next-generation sequencing analysis toolkit
- IQR
inter-quartile range
Authors’ contributions
ML, CS and ET designed the study and wrote the manuscript. ML and JK performed the experiments. ML, DP, SS, SR and ET performed the data analysis and figure generation. MM, EP, AN, AT and BN provided the samples, reviewed and approved the study. NL contributed to initiating this collaboration and assisted with editing of the final manuscript. All authors read and approved the final manuscript.
Funding
This study was made possible through support from the Advanced Molecular Detection (AMD) initiative at the CDC. We also acknowledge partial support by the Bioinformatics Fellowship Program administered by the Association of Public Health Laboratories (APHL) and funded by the CDC. SES is currently supported in part by the Bioinformatics Fellowship Program administered by the APHL and funded by the CDC. JK is currently supported in part by the CDC Foundation. DP is employed by Williams Consulting LLC. Williams Consulting LLC provided support in the form of salary for DP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘Authors’ contributions’ section.
Availability of data and materials
The datasets generated during the present study are available in the PRJNA428490 repository, https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP128900.
Ethics approval and consent to participate
Original ethical approval for drug resistance studies was obtained from the Ethics Committee of the Eritrean Ministry of Health (OSM 29/377/13). The present study was approved by the Office of the Associate Director of Science, Center for Global Health, Center for Disease Control and Prevention as research not involving human subjects (protocol 2017-228). In May 2017, the Department of Infectious Diseases, Istituto Superiore di Sanità, Rome, Italy; the Malaria Branch laboratory at the Centers for Disease Control and Prevention (CDC), Atlanta, USA and the University of Gezira, Wad Medani, Sudan agreed to work together on generating molecular data related to the original study entitled “Genetic basis of malaria drug resistance in Eritrea”. For all samples, identifying information was removed so that data cannot be linked or re-linked with identifiable human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mariangela L’Episcopia, Email: mariangela.lepiscopia@iss.it.
Julia Kelley, Email: xfi8@cdc.gov.
Dhruviben Patel, Email: yyr4@cdc.gov.
Sarah Schmedes, Email: obf2@cdc.gov.
Shashidahar Ravishankar, Email: shashidhar.ravishankar@gatech.edu.
Michela Menegon, Email: michela.menegon@iss.it.
Edvige Perrotti, Email: edvige.perrotti@iss.it.
Abduselam M. Nurahmed, Email: peace.abdu@gmail.com
Albadawi A. Talha, Email: badawiat@gmail.com
Bakri Y. Nour, Email: bakrinour@gmail.com
Naomi Lucchi, Email: frd9@cdc.gov.
Carlo Severini, Email: carlo.severini@iss.it.
Eldin Talundzic, Email: wrj5@cdc.gov.
References
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Malaria Report. 2018. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/.
- 3.Mace KE, Arguin PM, Tan KR. Malaria surveillance—United States, 2015. MMWR Surveill Summ. 2018;67:1–28. doi: 10.15585/mmwr.ss6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ECDC. European Centre for Disease Prevention and Control. Annual epidemiological report for 2017. Surveillance report. Stockholm; 2019. https://www.ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2017.
- 5.CDC. Centers for Disease Control and Prevention: Malaria. Guidelines for Treatment of Malaria in the United States; 2015. https://www.cdc.gov/malaria/resources/pdf/treatment_guidelines_101819.pdf.
- 6.LaRocque RC, Rao SR, Lee J, Ansdell V, Yates JA, Schwartz BS, et al. Global TravEpiNet: a national consortium of clinics providing care to international travelers—analysis of demographic characteristics, travel destinations, and pretravel healthcare of high-risk US international travelers, 2009–2011. Clin Infect Dis. 2012;54:455–462. doi: 10.1093/cid/cir839. [DOI] [PubMed] [Google Scholar]
- 7.Shellvarajah M, Hatz C, Schlagenhauf P. Malaria prevention recommendations for risk groups visiting sub-Saharan Africa: a survey of European expert opinion and international recommendations. Travel Med Infect Dis. 2017;19:49–55. doi: 10.1016/j.tmaid.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Cordel H, Cailhol J, Matheron S, Bloch M, Godineau N, Consigny PH, et al. Atovaquone-proguanil in the treatment of imported uncomplicated Plasmodium falciparum malaria: a prospective observational study of 553 cases. Malar J. 2013;12:399. doi: 10.1186/1475-2875-12-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. http://apps.who.int/medicinedocs/documents/s21839en/s21839en.pdf. [PubMed]
- 10.Lalloo DG, Shingadia D, Bell DJ, Beeching NJ, Whitty CJM, Chiodini PL, et al. UK malaria treatment guidelines 2016. J Infect. 2016;72:635–649. doi: 10.1016/j.jinf.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plucinski MM, Huber CS, Akinyi S, Dalton W, Eschete M, Grady K, et al. Novel mutation in cytochrome b of Plasmodium falciparum in one of two atovaquone-proguanil treatment failures in travelers returning from same site in Nigeria. Open Forum Infect Dis. 2014;1:ofu059. doi: 10.1093/ofid/ofu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessl JJ, Ha KH, Merritt AK, Lange BB, Hill P, Meunier B, et al. Cytochrome b mutations that modify the ubiquinol-binding pocket of the cytochrome bc1 complex and confer anti-malarial drug resistance in Saccharomyces cerevisiae. J Biol Chem. 2005;280:17142–17148. doi: 10.1074/jbc.M500388200. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Artemisinin resistance and artemisinin-based combination therapy efficacy. Geneva: World Health Organization; 2018. https://apps.who.int/medicinedocs/documents/s23555en/s23555en.pdf.
- 14.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra N, Prajapati SK, Kaitholia K, Bharti RS, Srivastava B, Phookan S, et al. Surveillance of artemisinin resistance in Plasmodium falciparum in India using the kelch13 molecular marker. Antimicrob Agents Chemother. 2015;59:2548–2553. doi: 10.1128/AAC.04632-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2015;211:1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2015;211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A Worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–993. doi: 10.1056/NEJMc1612765. [DOI] [PubMed] [Google Scholar]
- 21.Russo G, L’Episcopia M, Menegon M, Souza SS, Dongho BGD, Vullo V, et al. Dihydroartemisinin-piperaquine treatment failure in uncomplicated Plasmodium falciparum malaria case imported from Ethiopia. Infection. 2018;46:867–870. doi: 10.1007/s15010-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawe L, Menegon M, Ramatlho P, Muthoga CW, Mutukwa N, Vurayai M, et al. Molecular surveillance of Plasmodium falciparum drug resistance markers in clinical samples from Botswana. Am J Trop Med Hyg. 2018;99:1499–1503. doi: 10.4269/ajtmh.18-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talundzic E, Plucinski MM, Biliya S, Silva-Flannery LM, Arguin PM, Halsey ES, et al. Advanced molecular detection of malarone resistance. Antimicrob Agents Chemother. 2016;60:3821–3823. doi: 10.1128/AAC.00171-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talundzic E, Ravishankar S, Kelley J, Patel D, Plucinski M, Schmedes S, et al. Next-generation sequencing and bioinformatics protocol for malaria drug resistance marker surveillance. Antimicrob Agents Chemother. 2018;62:e02474–e02517. doi: 10.1128/AAC.02474-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menegon M, Nurahmed AM, Talha AA, Nour BY, Severini C. Molecular surveillance of antimalarial drug resistance related genes in Plasmodium falciparum isolates from Eritrea. Acta Trop. 2016;157:158–161. doi: 10.1016/j.actatropica.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Talundzic E, Maganga M, Masanja IM, Peterson DS, Udhayakumar V, Lucchi NW. Field evaluation of the photo-induced electron transfer fluorogenic primers (PET) real-time PCR for the detection of Plasmodium falciparum in Tanzania. Malar J. 2014;13:31. doi: 10.1186/1475-2875-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mace KE, Arguin PM, Lucchi NW, Tan KR. Malaria surveillance—United States, 2016. MMWR Surveill Summ. 2019;68:1–35. doi: 10.15585/mmwr.ss6805a1. [DOI] [PubMed] [Google Scholar]
- 28.ECDC. European Centre for Disease Control and Prevention. Rapid risk assessment: hospital-acquired malaria infections in the European Union. Stockholm; 2018. https://ecdc.europa.eu/sites/portal/files/documents/2018-04-30-RRA-Hospital-acquired-Malaria-European-Union-with%20erratum-1.pdf.
- 29.Nsanzabana C, Ariey F, Beck HP, Ding XC, Kamau E, Krishna S, et al. Molecular assays for antimalarial drug resistance surveillance: a target product profile. PLoS One. 2018;13:e0204347. doi: 10.1371/journal.pone.0204347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. Malaria surveillance, monitoring and evaluation: a reference manual. Geneva: World Health Organization; 2018. https://apps.who.int/iris/handle/10665/272284.
- 31.Ocholla H, Preston MD, Mipando M, Jensen AT, Campino S, MacInnis B, et al. Whole-genome scans provide evidence of adaptive evolution in Malawian Plasmodium falciparum isolates. J Infect Dis. 2014;210:1991–2000. doi: 10.1093/infdis/jiu349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaw MT, Emran NA, Lin Z. Updates on k13 mutant alleles for artemisinin resistance in Plasmodium falciparum. J Microbiol Immunol Infect. 2018;51:159–165. doi: 10.1016/j.jmii.2017.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the present study are available in the PRJNA428490 repository, https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP128900.