Abstract
The interaction between the entomopathogenic fungus Beauveria bassiana (Balsamo) and the parasitoid Coptera haywardi (Oglobin), as potential biological control agents for Anastrepha obliqua (Macquart) fruit flies, was evaluated under laboratory and semi-protected field cage conditions. The effects of the parasitoids and fungus were individually and jointly assessed in Plexiglas cages. Application of B. bassiana dry conidia to soil produced 40% mortality in A. obliqua adults. However, mortality was lower (21.2%) on evaluation under field cage conditions. According to the multiple decrement life table analysis, the probability of death of A. obliqua was 88% when C. haywardi parasitoids and B. bassiana conidia were used in conjunction, 89% when only C. haywardi parasitoids were released and 23% when only B. bassiana conidia were applied. These results demonstrate that no synergistic, additive or antagonistic interaction took place with the simultaneous use of these natural enemies, since the presence of B. bassiana had no effect on the C. haywardi parasitism. These results indicate that the parasitoid is a better natural enemy for the control of A. obliqua, and show that, although the two biological control agents can be used simultaneously, their joint application will not produce increased control.
Keywords: parasitoid, entomopathogen, multiple biological control, fruit fly, intraguild interaction
Natural enemies reduce the density of their hosts or prey in various ways depending on ecological conditions and the interactions that take place with their environment. For example, in some cases, the effect of entomopathogens can be considered greater than that of predators and/or parasitoids (Baverstock et al. 2009).
The diversity and density of natural enemies determine their effect in terms of regulating host populations (Bianchi et al. 2006). A classic proposal in biological control is to combine the action of two or more natural enemies with the aim of increasing the overall efficacy of control. However, regulation of pest populations through multiple agents depends on the intraguild interactions presented during the action of the natural enemies. These interactions are based on the division or overlap of the ecological niches of each species (Pedersen and Mills 2004). Ferguson and Stiling (1996) state that these interactions can be synergistic, additive or non-additive in nature, and their characterization is therefore important in order to define strategies that can optimize the multiple use of natural enemies (Roy and Pell 2000, Straub et al. 2008).
The ideal scenario is one in which a synergistic or additive interaction takes place between the natural enemies selected, since this should translate into a greater mortality in the pest population. However, if an antagonistic interaction is presented, possibly as a result of interference between the regulatory organisms, suppression of the pest population could be lower than that estimated, which would be detrimental for the purposes of biocontrol (Ferguson and Stiling 1996, Roy and Pell 2000).
The most frequently cited cases of antagonism are those of the interaction between entomopathogenic fungi and parasitoids (Baverstock et al. 2009, Martins et al. 2014). Most entomopathogenic fungi are considered to be generalists and opportunists. For this reason, they can easily be antagonistic for many insects, including the parasitoids. Technical adjustments using specific criteria, such as the time of application and dose management are important for the integrated use of fungi and parasitoids (Brodeur and Rosenheim 2000). Different studies have produced encouraging results in management involving entomopathogenic fungi and an insect (parasitoid or predator) as control agents. The use of the parasitoid Encarsia formosa Gahan (Hymenoptera: Aphelinidae) with the fungus Beauveria bassiana (Balsamo) Vuillemin has been shown to be effective for the control of the greenhouse whitefly Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae) (Labbé et al. 2009). Applying this same fungus along with the predators Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) and Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) produced improved control of the aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae), compared to that of any single natural enemy (Zhu and Jun 2012).
Fruit flies (Diptera: Tephritidae) are a group of pest species of great economic importance. For efficient control, an area-wide integrated pest management approach is recommended (Vargas et al. 2003). In this integrated management, biological control appears to be the most commonly researched strategy (Dias et al. 2018); however, its application is limited by the need for a thorough analysis and evaluation of the availability and associated application costs of potential natural enemies (Montoya and Toledo 2010). Anastrepha obliqua (Macquart) is a polyphagous species that is prominent in the Neotropical region for its wide distribution. It is the main pest of mango (Mangifera indica L.) and hog plum fruits (Spondias spp.) in different countries of Latin America (Hernández-Ortiz 1992, Aluja 1996, Castañeda et al. 2015, López et al. 2020).
Parasitoids are the most common natural enemies used for the biological control of fruit flies (Wharton and Gilstrap 1983, Ovruski et al. 2000). One group of interest are the pupal parasitoid species, which were the first parasitoids of fruit flies to be described, but have been little used (Sivinski et al. 1997). Fruit fly pupal parasitoids can represent a key mortality factor because they attack the final immature stage of the pest, prior to adult emergence. This group includes the solitary endoparasitoid Coptera haywardi (Oglobin), which is highly specific for Tephritidae species and has a high capacity for locating and discriminating hosts (Sivinski et al. 1998, Guillén et al. 2002, Cancino et al. 2012). Mass rearing of this species has been developed (Cancino and Montoya 2008) and different studies under both laboratory and field cage conditions have shown that it can present high percentages of parasitism of Anastrepha flies (e.g., Cancino 2012, López-Arriaga et al. 2014).
Another group of organisms used as an alternative for the biocontrol of fruit fly pupae are the entomopathogenic fungi. Beauveria bassiana is a generalist fungus and natural inhabitant of the soil that is effective in the control of pests such the European spruce bark beetle Ips tipographus (L.) (Coleoptera: Scolitidae) (Kreutz et al. 2004), cabbage maggot Delia radicum L. (Diptera: Anthomyiidae) (Meadow et al. 2000) and coffee berry borer (Hypothenemus hampei Ferrari) (Coleoptera: Scolitidae) (Campos-Almengor 2008), among others. Several studies have demonstrated that certain strains of this fungus are pathogenic to fruit flies (De la Rosa et al. 2002, Ekesi et al. 2002, Dimbi et al. 2003, Konstantopoulou and Mazomenos 2005, Sookar et al. 2008, Hernández Díaz-Ordaz et al. 2010), which gives it great potential for use as an additional component in the integrated management of these pests (Ortu et al. 2009).
Considering that these two natural enemies of the fruit fly occupy different ecological niches, and act upon two different developmental stages of the pest, their combined use for the control of A. obliqua is theoretically feasible. The population of non-parasitized pupae could subsequently be infected by the fungus in the adult stage, producing a greater overall suppression of the pest population. A previous study showed that C. haywardi females have a low susceptibility to B. bassiana and their fecundity is unaffected (Martínez-Barrera et al. 2019), suggesting the possibility for simultaneous use of both organisms in the control of A. obliqua.
Our aim in this study was to characterize the type of interaction of the parasitoid C. haywardi with the fungus B. bassiana in the context of their use as biocontrol agents of A. obliqua.
Materials and Methods
Study Site
The study was carried out in two phases: The first was conducted under laboratory conditions (24 ± 1°C, 60–80% RH, and 12:12 (L:D) h photoperiod) in the Methods Development Moscafrut Program in Detection and Control Laboratory (SADER-SENASICA), located in Metapa de Domínguez, Chiapas, Mexico. This phase was used to determine the optimum method of B. bassiana application in order to cause the maximum infection of A. obliqua adults. The second phase was carried out under field cage conditions, in the municipality of Cacahoatan, Chiapas (15°0′15.0″ N: 92°9′28.0″ W; 549 masl in elevation), in a mixed orchard of tomato (Solanum lycopersicum L.), rambutan (Nephelium lappaceum L.), banana (Musa paradisiaca L.), avocado (Persea americana Mill) and coconut (Cocos nucifera L.). The experiments were run in cubic Plexiglas cages kept under an aluminum roof for protection against the rain. During the experimental period, the temperature ranged from 19 to 30°C, and relative humidity ranged from 65 to 85% HR. These conditions were recorded using a data logger (HOBO U12-011) located inside a randomly chosen cage.
Biological Material
The formulation of the B. bassiana strain Bb-Et was used. This was produced in the Laboratory of Beneficial Organisms, Fungi, Insects, and Nematodes, located in Tuxtla Chico in Chiapas, Mexico, and was formulated with Celite 400 in package of 250 g (at a total concentration of 2.0 × 109 conidia/g), and with a viability > 85% (4.25 × 1011 CFU-colony-forming unit). Adults of C. haywardi were obtained from a colony of ~260 generations in the Laboratory of Biological Control of the Moscafrut Program in Metapa de Dominguez, Chiapas, Mexico. The environmental conditions in the laboratory were temperature 24 ± 2°C, RH 60–80%, and photoperiod 12:12 (L:D) h. Anastrepha obliqua larvae were provided by the Moscafrut facility (SADER-SENASICA), where they had been reared following the procedures described by Artiaga-López et al. (2004) and Orozco-Davila et al. (2017).
Selection of Method for Application of B. bassiana to Adults of A. obliqua
Two methods of fungus application were evaluated in plastic containers (4 × 2 cm: diameter × height) containing 90 g of soil. The first was direct application of 0.8 g of dry conidia/kg soil (~1.6 × 109 conidia), which was weighed on an analytical balance and directly distributed evenly on the soil using a plastic fork. The second method consisted of spraying 5 ml of a stock solution at 1% (5.0 × 109 conidia/g in 100 ml sterile distilled water) over the soil. In the control, 5 ml of distilled water without B. bassiana was applied by spraying over the soil. One hour after application, 20 A. obliqua larvae of 8–9 d of age were placed in each container. The soil was moistened with 2 – 3 ml of distilled water daily, according to its moisture content, until emergence of the flies. The containers of each treatment were maintained in 11 × 11 × 16.5 cm (height by width by depth) plastic containers until emergence of the flies. Five replicates were performed per treatment.
Emerged adults were transferred to similar containers (11 × 11 × 16.5 cm) and provided with water and food (mixture of hydrolyzed yeast with sugar at a 1:3 ratio). The number of dead flies was recorded daily over a period of 15 consecutive days or until the last individual had died. These dead individuals were collected and subjected to a process of disinfection, initially by rinsing in a 1% solution of sodium hypochlorite for 5 s, and then washing with sterile distilled water. The disinfected dead flies were then placed in moist chambers, formed by filter paper moistened with sterile distilled water in Petri dishes (11 cm diameter). These conditions allowed fungus growth, and through observation of the mycelium, the cause of death was confirmed as infection by B. bassiana. The treatment with the highest mortality was selected as the most effective inoculation method for subsequent testing.
Interaction of B. bassiana and C. haywardi
The interaction between the parasitoid and the entomopathogenic fungus was evaluated in four different treatments:
1) Release of C. haywardi and application of B. bassiana (Ch + Bb),
2) Release of C. haywardi and no application of B. bassiana (Ch),
3) No release of C. haywardi and application of B. bassiana (Bb),
4) No release of C. haywardi and no application of B. bassiana (Control).
Each treatment was placed in a cubic Plexiglas cage of 27 dm3. Three walls of the cage were made of plexiglass, two sides had a mesh for ventilation and the sixth wall a finer mesh for the introduction and removal of materials. These cages were placed on a metal bench and the legs of the bench were covered with glue to avoid predation by ants. A tray with soil and the exposed larvae was placed inside each cage. Five replicates were conducted per treatment.
The soil used in the different assays of this study was characterized for composition and pH in the Soil Analysis Laboratory of the Faculty of Agricultural Sciences of the Universidad Autónoma de Chiapas, Mexico. The texture was sandy loam, comprising 3.10% organic material, 47.12% sand, 33.00% silt, 19.88% clay, and the pH was 6.32. This soil was obtained from a mango orchard in the municipality of Tuxtla Chico in Chiapas, Mexico (14°53′00.3″ N, 92°13′23.2″ W; elevation 169 masl).
The soil was exposed to direct sunlight for 24 h in order to dehydrate it, then passed through a No. 10 sieve and packed into 1 kg aluminum bags for sterilization in an autoclave (AESA Model CV300, Mexico) at 15 psi for 15 min (Trevors 1996). Finally, the soil was placed in a drying oven at 95°C for 24 h until reaching 1–2% humidity. Moisture content was determined by weight loss using an OHAUS moisture analyzer (Model MB45, Switzerland) at 100°C for 10 min. Subsequently, 130–150 ml of distilled water were applied per kg of soil (weight/volume) in order to bring the soil moisture content up to a uniform 14% (Wilson et al. 2017).
One kg of soil was placed in a plastic tray (5 × 18 × 25 cm: height by width by depth) and 100 third instar A. obliqua larvae (~8–9 d of age) were added. Each tray was placed inside a cage, and the soil moisture maintained by spraying 2–4 ml of distilled water every day, according to the soil moisture content. Replicates were established every week, each replicate consisted of four cages, which were located at random on the experimental bench. A mango branch of ~35 cm in length with foliage was placed inside the cage to create semi-natural conditions. Four days after the introduction of larvae, 50 C. haywardi adults (25♀:25♂) of between 3 and 4 d in age were released inside the cage and provided with water and honey (honey was used as food in a mixture with tissue paper (1:1).
From day 11 to 14 after larvae introduction, 0.8 g of B. bassiana formulation was uniformly applied over 1 kg of soil and homogenized with a plastic fork (Wilson et al. 2017). Every day, the emerged flies and parasitoids were collected, transferred to plastic containers (11 × 11 × 16.5 cm) and provided with water and food. A daily record of mortality was kept for 15 consecutive days. The dead insects were collected, disinfected as described above and transferred to moist chambers for confirmation of fungal infection. Those that were found to be negative for B. bassiana infection were classified as “death by other causes”.
Twenty-five days after the release of the parasitoids, the cages were moved to the laboratory, where the number of live and dead parasitoids was recorded. The dead individuals were disinfected and placed in a moist chamber. The soil was then sieved in order to collect the remaining parasitized pupae, which were maintained until emergence of the adult parasitoid offspring in order to determine the percentage of parasitism. The pupae were placed on the bottom of Petri dishes (diameter: 5 cm) along with 10 g of the soil used in the test, and stored in a plastic container (10 × 8 cm: diameter × height). Offspring parasitoid emergence was recorded daily, and the adults separated into another container and provided with water and food. Finally, those pupae from which no flies or parasitoids emerged were classified as dry mass, deformed (pupae of unusual size or of gelatinous content) or with possible parasitism. These pupae were classified as “death by other causes.” Five replicates were conducted for each treatment.
Data Analysis
The emergence of A. obliqua was analyzed using a Generalized Linear Model (GLM) with Poisson response and a means comparison carried out using a Tukey test. Parasitism, expressed in percentages, was analyzed using a t test, and sex ratio (♀: ♂) was evaluated using a GLM with binomial response. Time to emergence was analyzed through a Welch t test.
The data of A. obliqua mortality by B. bassiana and by other causes were evaluated using a GLM with binomial response and overdispersion correction. The different characteristics (dry mass, deformed, and possible parasitism) observed in the dissection of the pupae that did not emerge were evaluated using a GLM with negative binomial response. Where differences were found among treatments, means comparisons were conducted using a Tukey test. The analyses were performed with the statistical program Rx64 3.2.4 (R Core Team 2017).
The mortality data were analyzed using a multiple decrement life table (Carey 1993) in order to determine the effects of the parasitoid and the entomopathogenic fungus as individual causes of A. obliqua induced mortality, in addition to their interaction. The total number of treated insects in this table was 500, corresponding to the five replicates conducted. Recording of mortality began from the A. obliqua third instar larval stage (8–9 d old).
The probability of A. obliqua death was estimated when exposure to the two natural enemies was separate and sequential. In Table 3, analysis of the multiple decrement life table was conducted in four sections. In section 1, the total number of deaths (Dx), where those deaths caused by parasitism of C. haywardi (Dx1), infection by the fungus B. bassiana (Dx2) and other causes (Dx3) (unviable pupae and natural mortality in adults) in the different fruit fly life stages as larvae, pupae and adult were described (raw and independent data). The second section describes the probability of dying from a given cause, from one life stage to the next, including all of the causes: total (aqx), those caused by C. haywardi parasitism (aqx1), B. bassiana infection (aqx2) and other causes (aqx3). Subsequently, a column with the fraction of surviving individuals (alx) is included, considering all causes of death. The complementary part describes the death fraction in two groups. The first includes all causes of death, starting with the total (adx), followed by death from parasitism (adx1), fungal infection (adx2), and other causes (adx3). The second group was the causes of death were considered separately in the same evaluated order: by parasitism (qx1), fungal infection (qx2), and other causes (qx3).
Table 3.
Multiple decrement life table of Anastrepha obliqua
| Stage | Kx Number beginning stage | Dx Total deaths | Number of death from | Total aqx | Cause-specific probability of death from specified causes in the presence of all causes | Fraction living | Fraction death of | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Including all causes | Separated causes | ||||||||||||||||
| Parasitism Dx1 | Infection Dx2 | Other causes Dx3 | Parasitism aqx1 | Infection aqx2 | Other causes aqx3 | alx | adx Total | Parasitism adx1 | Infection adx2 | Other causes adx3 | Parasitism qx1 | Infection qx2 | Other causes qx3 | ||||
| Exposed to Coptera haywardi + Beauveria bassiana | |||||||||||||||||
| Larvae (8–9 d) | 500 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pupae | 500 | 435 | 343 | 0 | 92 | 0.87 | 0.69 | 0.00 | 0.18 | 1.00 | 0.87 | 0.69 | 0.00 | 0.18 | 0.69 | 0.00 | 0.18 |
| Adult (1–7 d) | 65 | 1 | 0 | 0 | 1 | 0.02 | 0.00 | 0.00 | 0.02 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 |
| Adult (8–15 d) | 64 | 3 | 0 | 3 | 0 | 0.05 | 0.00 | 0.05 | 0.00 | 0.13 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.05 | 0.00 |
| 0.88 | 0.69 | 0.01 | 0.18 | 0.69 | 0.05 | 0.20 | |||||||||||
| Exposed to Coptera haywardi | |||||||||||||||||
| Larvae (8–9 d) | 500 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pupae | 500 | 445 | 341 | 0 | 104 | 0.89 | 0.68 | 0.00 | 0.21 | 1.00 | 0.89 | 0.68 | 0.00 | 0.21 | 0.68 | 0.00 | 0.21 |
| Adult (1–7 d) | 55 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Adult (8–15 d) | 55 | 2 | 0 | 0 | 2 | 0.04 | 0.00 | 0.00 | 0.04 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| 0.89 | 0.68 | 0.00 | 0.21 | 0.68 | 0.00 | 0.24 | |||||||||||
| Exposed to Beauveria bassiana | |||||||||||||||||
| Larvae (8–9 d) | 500 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pupae | 500 | 20 | 0 | 0 | 20 | 0.04 | 0.00 | 0.00 | 0.04 | 1.00 | 0.04 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.04 |
| Adult (1–7 d) | 480 | 50 | 0 | 14 | 36 | 0.10 | 0.00 | 0.03 | 0.08 | 0.96 | 0.10 | 0.00 | 0.03 | 0.07 | 0.00 | 0.03 | 0.07 |
| Adult (8–15 d) | 430 | 53 | 0 | 15 | 38 | 0.12 | 0.00 | 0.03 | 0.09 | 0.86 | 0.11 | 0.00 | 0.03 | 0.08 | 0.00 | 0.03 | 0.09 |
| 0.25 | 0.00 | 0.06 | 0.19 | 0.00 | 0.06 | 0.20 | |||||||||||
Mortality factors were parasitism by Coptera haywardi and infection by Beauveria bassiana. Larvae were exposed separated and sequential to each cause of death.
Results
Selection of the Method of B. bassiana Application to Adults of A. obliqua
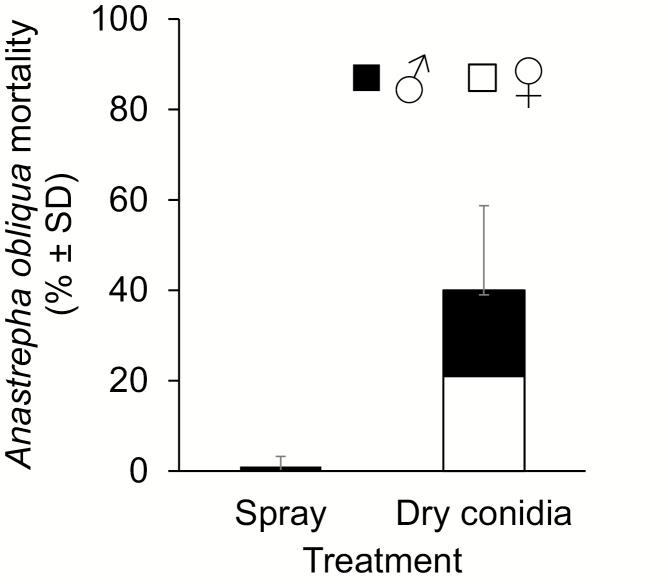
Under laboratory conditions, A. obliqua mean emergence was 98%, with a sex ratio of 1♀:1♂. The mortality caused by the application of dry conidia was 40%, compared to only 1% mortality with the spraying method. However, given the absence of data variation in this treatment, statistical analysis was not possible. Application of the fungus by dry conidia caused deaths in adults from 3 to 10 d old, with no statistical was found between the females (52.5%) and males (47.5%) (t = 0.221; df = 6; P = 0.832) (Fig. 1).
Fig. 1.
Mortality of Anastrepha obliqua adults (% ± SD) produced by Beauveria bassiana applied by spray or as dry conidia to the soil (n = 20).
Interaction of B. bassiana and C. haywardi
Emergence of A. obliqua adults from larvae placed in trays with soil occurred from 17 to 21 d. Emergence of flies decreased from 92.2% to 6.6% when the pupae were exposed to C. haywardi only (χ 2 = 185.52; df = 3; P = 2.2 e−16) (Table 1). The percentage of adult mortality caused by the fungus and other causes was calculated from the number of emerged flies. When the parasitoid and the fungus were both tested, adult mortality induced by B. bassiana was 7.7%, which is lower than the 21.25% mortality caused with application of B. bassiana alone, although this difference was not significant (χ 2 = 1.501; df = 1; P = 0.220). Mortality by other causes was greater in the treatments with no release of C. haywardi (χ 2 = 214.93; df = 3; P = 2.2 e−16).
Table 1.
Anastrepha obliqua emergence and adult mortality (% ± SD) when exposed to Coptera haywardi and Beauveria bassiana separately and in the control (n = 500 larvae / treatment)
| Treatments | A. obliqua emergency a | Adults mortality A. obliqua | ||||
|---|---|---|---|---|---|---|
| By B. bassianab | Others causesc | |||||
| n | % | n | % | n | % | |
| Coptera haywardi + Beauveria bassiana | 39 | 7.80 ± 7.59a | 3 | 7.69 ± 43.46a | 1 | 2.57 ± 2.35a |
| Coptera haywardi | 33 | 6.60 ± 7.66a | 2 | 6.06 ± 4.38a | ||
| Beauveria bassiana | 480 | 96.0 ± 8.94b | 102 | 21.25 ± 5.73a | 76 | 15.83 ± 5.36b |
| Control | 461 | 92.2 ± 16.88b | 96 | 20.82 ± 8.63b | ||
Mortality percentage calculated from the number flies emerged. Different letters show significant differences with a level of 0.05.
Statistical analysis performed using the following models:
aGeneralized Linear Model with binomial response with overdispersion correction, and with comparison of means by means of linear ANOVA hypothesis, Tukey test.
bbGeneralized Linear Model with quasibinomial response.
cGeneralized Linear Model with Poisson response with comparison of means with linear hypothesis.
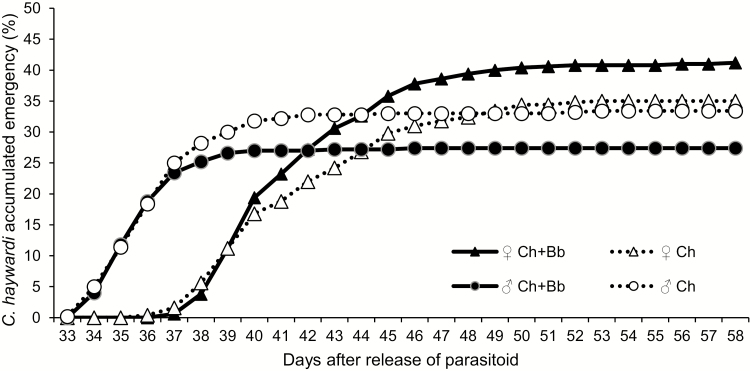
The percentage of parasitism by C. haywardi was unaffected by the application of B. bassiana, at 68.6 ± 3.2%, compared to 68.4 ± 2.8% when only parasitoids were released (t = −0.093; df = 8; P = 0.928). However, a greater proportion of females (1.5:1 female: male ratio) was observed when the fungus was applied alone than when only parasitoids were released (1:1) (χ 2 = 5.299; df = 1; P = 0.021) (Fig. 2).
Fig. 2.
Coptera haywardi cumulative emergence (%) from Anastrepha obliqua pupae when released alone (Ch) and when released jointly the application of Beauveria bassiana (Ch+Bb), by sex.
The mean time for C. haywardi male offspring to emerge from fungus treated pupae was 36.1 ± 1.8 d after the release of the parasitoid (DARP), which was similar when only the parasitoid was released (36.7 ± 2.6 DARP) (t = −2.475; df = 292; P = 0.014) (n = 304). The mean time of emergence for C. haywardi female offspring was greater when the fungus was applied (41.85 ± 3.38 DARP) but did not differ statistically when only the parasitoid was released (41.87 ± 3.63 DARP) (t = 0.135; df = 359; P = 0.89).
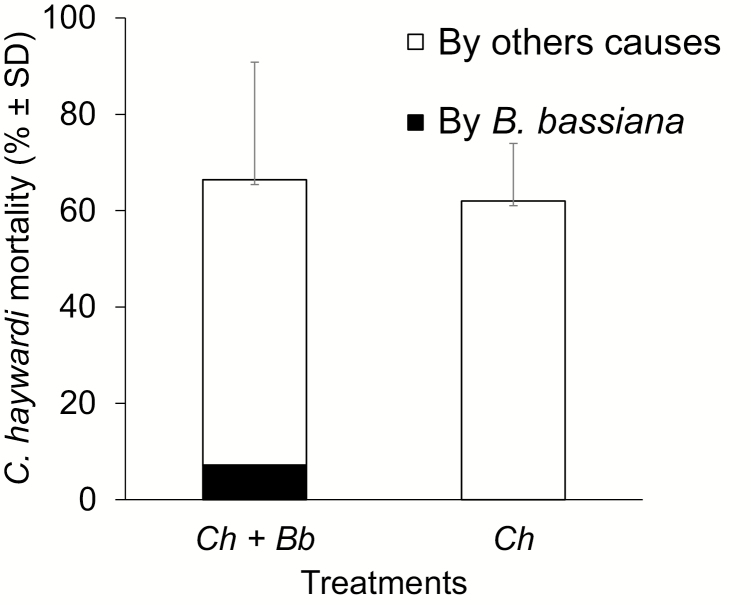
When they acted alone, released parasitoids showed 62% mortality due to other causes during the test period. When released together with application of B. bassiana, the mortality of C. haywardi was 66.4%, which was not significantly different (χ 2 = 0.0533; df = 1; P = 0.817). Of this 66.4%, only 7.2% mortality was attributable to the B. bassiana infection, the rest was due to other causes (Fig. 3).
Fig. 3.
Coptera haywardi (Ch) mortality (% ± SD), when released alone and with application of Beauveria bassiana (Bb), on Anastrepha obliqua pupae.
The proportion of pupae of A. obliqua that did not emerge was greater when only the parasitoids were released (20.8%) than when they were applied together with the fungus (18.4%) (Table 2). In the treatment with fungus application only, this proportion was 4.0%, while in control, it was 4.2% (Table 2). Different dry mass contents were observed in the pupae of the flies that did not emerge, as well as other deformities (pupae of unusual size or of gelatinous content) and what was considered to be a possible parasitism due to the fact that there was formation of the parasitoid pupa, although no development to the adult stage took place (Fig. 4).
Table 2.
Unviable pupae of Anastrepha obliqua with dry masses and other deformations (% ± SD) when exposed to Beauveria bassiana and Coptera haywardi separately, in combinations and in the control (n = 500 larvae/treatment)
| Treatments | Emergency C. haywardia (% ± SD) | Inviable pupae (%) | ||
|---|---|---|---|---|
| Dry massb | Other deformationsc | Possible parasitismd | ||
| Coptera haywardi + Beauveria bassiana (Ch+Bb) | 68.6 ± 3.21a | 12.4 ± 10.31a | 2.00 ± 1.00a | 4.00 ± 4.24a |
| Coptera haywardi (Ch) | 68.2 ± 2.85a | 17.0 ± 7.96a | 0.40 ± 0.54a | 3.4 ± 4.39a |
| Beauveria bassiana (Bb) | 0.20 ± 0.44b | 3.80 ± 8.49a | 0 | |
| Control | 1.60 ± 3.56b | 2.60 ± 5.81a | 0 | |
Different letters indicate significant differences with a level of 0.05. Analysis performed by at test. bGeneralized Linear Model (GLM) with quisipoisson response, with mean comparison by means of linear ANOVA hypothesis, Tukey test.
cGLM with negative binomial response.
dGLM with quisipoisson response.
Fig. 4.
Pupae of Anastrepha obliqua possibly parasitized by Coptera haywardi but without adult development.
The presence of pupae with dry mass content differed significantly among the treatments where the parasitoid was released alone and in conjunction with the fungus, compared to that in which only the fungus was applied, or the control treatment (χ 2 = 47.684; df = 3; P = 2.486 e−10) (Table 2). In contrast, no significant differences were found among treatments in terms of the quantity of deformed fly pupae (χ 2 = 2.6519; df = 3; P = 0.448).
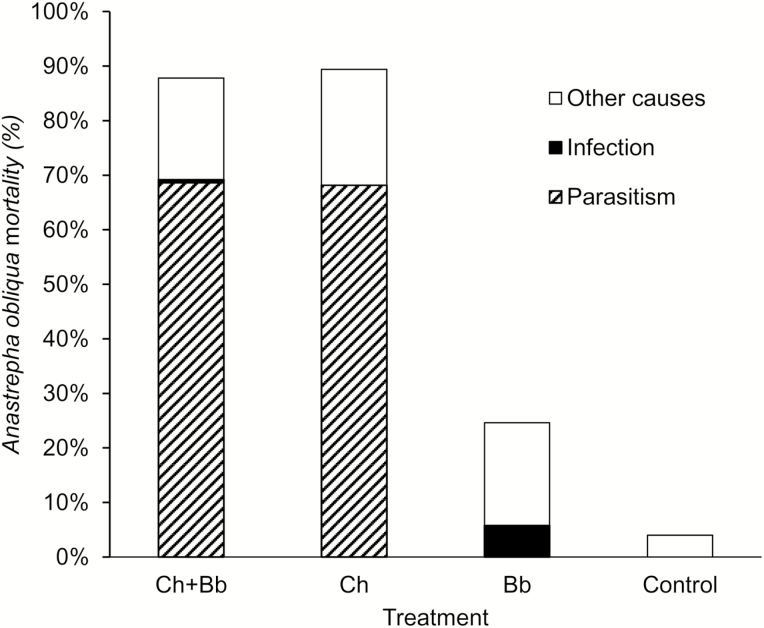
According to the multiple decrement data (Table 3), release of the parasitoid caused the greatest mortality fraction, with values of 68 and 69% when used alone and in combination with B. bassiana, respectively. The lowest proportion of mortality was caused by B. bassiana on the adult stage, and this value was lower when used with the parasitoid (1%) than when applied alone (6%). Death due to other causes was found at 18, 21, and 19%, when applying the fungus and parasitoid together, the parasitoid alone and the fungus alone, respectively. The total induced mortality was very similar between when the two natural enemies were used in combination and when the parasitoid was released alone.
When all of the causes of death were included, the parameters indicated that the population reduction of flies was similar when the parasitoid was released alone (89%) and when the parasitoid and fungus were applied together (88%), which caused a greater pupal suppression in both cases, compared to the control (Table 1). Application of B. bassiana generated lower induced mortality (25%), mainly in the adult stage of the fly. The natural A. obliqua mortality recorded in control was 4% (Fig. 5).
Fig. 5.
Anastrepha obliqua percentage of mortality by Coptera haywardi (Ch) parasitism, Beauveria bassiana (Bb) infection, and other causes, when they acted alone or together.
Discussion
Our results suggest that C. haywardi and B. bassiana could be used simultaneously for the control of A. obliqua, since no antagonistic interaction was observed between them, although there was also no evidence of an additive effect when the fungus was applied to the soil. Analysis of the multiple decrement life table of A. obliqua showed that C. haywardi was the organism that caused the greatest mortality, followed by other causes (unviable pupae and natural mortality in adults), and finally by infection caused by B. bassiana, in which no significant decrease in the number of A. obliqua adults was observed. The data also show no antagonistic interaction, since no interference was observed between the two control agents, as B. bassiana alone caused a mortality of 7.20 ± 4.1% in C. haywardi.
A low percentage of fungal infection in parasitoids in semi-natural conditions is an advantage because it more closely resembles reality compared to what happens under controlled conditions in the laboratory. This low infection makes it possible to adjust the application times for the use of the parasitoid and the fungus, so that the parasitoid infection in the field is minimal or nil, as reported with the use of B. bassiana together with the parasitoid Diadegma semiclausum (Hellen) (Hymenoptera: Ichneumonidae) in Plutella xylostella (L.) (Lepidoptera: Yponomeutidae) (Madurappulige 2005).
The high percentages of parasitism presented by C. haywardi are indicators of its potential use for suppression of the population of A. obliqua, regardless of the B. bassiana application method utilized. Some reports indicate that certain parasitoids, such as Ascogaster quadridentatus Wesm. (Hymenoptera: Braconidae) and Aphelinus asychis (Hymenoptera: Aphelinidae), can produce a fungistatic substance in the hemolymph of the host that impedes the successful development of pathogenic fungi, thus favoring the development and emergence of adults (El-Sufty and Führer 1985, Mesquita and Lacey 2001), although this is influenced by the time interval between the parasitism and the presence of the fungus in the insect (Powell et al. 1986). It is probable that this inhibitor effect was present in our study, preventing the development of B. bassiana. However, further research is required in order to determine whether C. haywardi secretes this type of substance.
When C. haywardi was released, the reduction in the emergence of flies was higher, presenting a high proportion of unviable pupae that were possibly parasitized but had suffered the effects of the defense reactions of A. obliqua, a fly species typified by a high capacity for antagonistic reaction to parasitism (Silva et al. 2002, Carton et al. 2008). These reactions could be physiological (Carton and Nappi 1997), biochemical (Kohler et al. 2007), genetic or ecological (Carton and Nappi 2001, Carton et al. 2005, Dubuffet et al. 2007) in type. However, the mortality is important in terms of control since it acts to diminish the pest population.
When B. bassiana was applied using the dry conidia method, A. obliqua mortality was much lower than that caused by the parasitoid. There are reports indicating that this fungus generates a high mortality in A. ludens when applied as dry conidia under controlled conditions (Wilson et al. 2017). In our case, the percentage of mortality caused by B. bassiana in A. obliqua was similar to that obtained under natural conditions (~25%) with the use of disseminator devices of B. bassiana conidia (Campos 2017), confirming the susceptibility of A. obliqua to this entomopathogenic fungi (Hernández Díaz-Ordaz et al. 2010, Osorio-Fajardo and Canal 2011).
One cause of an antagonistic interaction between an entomopathogenic fungus and a parasitoid is the degree of virulence presented by the fungus (Feng et al. 1994, Danfa and Van der Valk 1999, Roy and Pell 2000), given that B. bassiana is a generalist fungus that can affect nontarget arthropods (Bruce et al. 1997). However, the mortality presented in this study under semi-protected conditions was much lower than that observed under laboratory conditions (Martínez-Barrera et al. 2019), which could be due in part to the fact that environmental factors, such as temperature, humidity, and solar radiation could act to reduce the survival and virulence of B. bassiana (Shipp et al. 2003, Labbé et al. 2009, Castrillo et al. 2010).
Nevertheless, the interaction that takes place between the parasitoid and the fungus could also depend on the time elapsed between the applications of the two organisms. Different cases have been reported in the control of aphids (Hemiptera: Aphididae) in which a reduced impact has been observed when the parasitoid is released first and the entomopathogenic fungus applied afterward (Rashki et al. 2009). It has also been documented that, with short time intervals between applications, the parasitoids cannot complete their development, evidencing an antagonistic interaction that diminishes when the time interval between application of the fungus and release of the parasitoid is longer (Powell et al. 1986, Askary and Brodeur 1999, Emami et al. 2013, Martins et al. 2014). In this study, it was observed that an early (7–10 d) release of the parasitoids before application of the fungus could represent a suitable time interval in which to avoid an antagonistic interaction.
Previous studies indicate that B. bassiana does not affect the development, survival or fecundity of C. haywardi (Martínez-Barrera et al. 2019), which could allow an additive interaction. However, the results revealed a neutral interaction. This type of interaction is presented when the mortality of the pest population does not increase following an increase in the number of natural enemies, for which reason biological control agents can sometimes be equally effective alone and/or functionally redundant (Straub et al. 2008). In this case, it was found that the two natural enemies did not produce a functional redundancy, since the mortality caused by B. bassiana in adults of A. obliqua was lower than that caused by C. haywardi in the pupae. This neutral interaction can be favorable in terms of proposing the use of these natural enemies in an independent manner. For example, C. haywardi could be used as a pupa biocontrol agent, whereas B. bassiana could be used for adult control through conidia disseminating devices.
Peterson et al. (2009) reported a compilation of 73 life tables of 28 insect species belonging to 5 orders, including the Diptera. In one of their findings, the mortality caused by other factors was greater than that caused by natural enemies, with the parasitoids and predators contributing the least to the accumulated mortality, compared to the effect of entomopathogenic microorganisms. However, it should be noted that these life tables were obtained under different circumstances, in which some studies included the release of parasitoids. In the case of the fruit fly Rhagoletis pomonella (Walsh) (Diptera: Tephritidae), the life table revealed that mortality due to other causes was also greater than that caused by natural enemies. However, in this case, the parasitoids caused a greater decline in the pest population than the predators and the pathogens (Cameron and Morrison 1977).
We conclude that the combination of the parasitoid C. haywardi and fungi B. bassiana for the control of A. obliqua did not produce a synergistic, additive, or antagonistic interaction. There was no interference in the control by each of these natural enemies, which suggests that both control agents could be used simultaneously. However, it is necessary to search for other more effective application methods of B. bassiana in order to develop strategies to strengthen its effect under field conditions.
Acknowledgments
We thank José Ernesto Sánchez Vázquez for allowing us to work in his facilities, the Moscafrut Program SADER-SENASICA for the use of their facilities and for providing the biological material (C. haywardi and A. obliqua), as well as materials, equipment and technical and administrative support. We also thank the Laboratory of Beneficial Organisms, Fungi, Insects, and Nematodes for providing of the Bb-Et strain of B. bassiana, and the National Council for Science and Technology (CONACYT) for the postgraduate scholarship awarded to Olga Yaneth Martinez Barrera, and to two anonymous reviewers’ for all comments and suggestions done to improve the quality of on our article.
References Cited
- Aluja M. 1996. Future trends in fruit fly management, pp. 309–320. InMcPheron B. A. and Steck G. J. (eds.), Fruit fly pests. A world assessment of their biology and management. St. Lucie Press, Delray Beach, FL. [Google Scholar]
- Artiaga-López T., Hernández E., Domínguez-Gordillo J., Moreno D. S., and Orozco-Dávila D... 2004. Mass-production of Anastrepha obliqua at the Moscafrut fruit fly facility, México, pp. 389–392. InProceedings of the 6th International Fruit fly Symposium, 6–10 May 2002 Stellenbosch, South Africa. [Google Scholar]
- Askary H., and Brodeur J... 1999. Susceptibility of larval stages of the Aphid parasitoid Aphidius nigripes to the entomopathogenic fungus Verticillium lecanii. J. Invertebr. Pathol. 73: 129–132. [DOI] [PubMed] [Google Scholar]
- Baverstock J., Clark S. J., Alderson P. G., and Pell J. K... 2009. Intraguild interactions between the entomopathogenic fungus Pandora neoaphidis and an aphid predator and parasitoid at the population scale. J. Invertebr. Pathol. 102: 167–172. [DOI] [PubMed] [Google Scholar]
- Bianchi F. J. J. A., Booij C. J. H., and Tscharntke T... 2006. Sustainable pest regulation in agricultural landscapes: a review on landscape composition biodiversity and natural pest control. Proc. R. Soc. B. 273: 1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur J., and Rosenheim J. A... 2000. Intraguild interactions in aphid parasitoids. Entom. Exp. Appl. 97: 93–108. [Google Scholar]
- Bruce L. P., Skinner M., Gouli V., and Brownbridge M... 1997. Impact of soil applications of Beauveria bassiana and Mariannaea sp. on nontarget forest arthropods. Biol. Control 8: 203–206. [Google Scholar]
- Cameron P. J., and Morrison F. O... 1977. Analysis of mortality in the apple maggot, Rhagoletis pomonella (Diptera: Tephritidae) in Quebec. Canadian Entomol. 109: 769–788. [Google Scholar]
- Campos S. E. 2017. Infección de adultos de Anastrepha ludens y A. obliqua con diseminadores de conidios de Beauveria bassiana en campo. M.Sc. Thesis. El Colegio de la Frontera Sur. ECOSUR, Tapachula, Chiapas, México. [Google Scholar]
- Campos-Almengor O. G. 2008. Evaluación de dos aislamientos nativos de Beauveria bassiana (Bals.) Vuillemin, para el control de la broca del fruto del cafeto, Hypothenemus hampei. El Cafetal. 16: 10–11. [Google Scholar]
- Cancino J., and Montoya P... 2008. Advances and perspectives in the mass rearing of fruit fly parasitoids in Mexico, pp. 133–142. InSugayama R., Zucchi R., Ovruski S., and Sivinski J. (eds.), Fruit Flies of Economic Importance: From Basic to Applied Knowledge Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, 10–15 September 2006, Salvador, Brazil. Press Color, Bahia, Brazil. [Google Scholar]
- Cancino J., Liedo P., Ruiz L., López G., Montoya P., Barrera J. F., Sivinski J., and Aluja M... 2012. Discrimination by Coptera haywardi (Hymenoptera: Diapriidae) of host previously attacked by conspecifics of by the larval parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biocontrol Sci. Technol. 22: 899–914. [Google Scholar]
- Carey J. R. 1993. Applied demography for biologists. Oxford University Press, New York, NY: 206 pp. [Google Scholar]
- Carton Y., and Nappi A. J... 1997. Drosophila cellular immunity against parasitoids. Parasitol. Today 13: 218–227. [DOI] [PubMed] [Google Scholar]
- Carton Y., and Nappi A. J... 2001. Immunogenetic aspects of the cellular immune response of Drosophilia against parasitoids. Immunogenetics. 52: 157–164. [DOI] [PubMed] [Google Scholar]
- Carton Y., Nappi A. J., and Poirie M... 2005. Genetics of anti-parasite resistance in invertebrates. Dev. Comp. Immunol. 29: 9–32. [DOI] [PubMed] [Google Scholar]
- Carton Y., Poirié M., and Nappi A... 2008. Insect immune resistance to parasitoids. Insect Sci. 15: 67–87. [Google Scholar]
- Castañeda M. del R., Selivon D., Hernández-Ortiz V., Soto A., and Canal N. A... 2015. Morphometric divergence in populations of Anastrepha obliqua (Diptera: Tephritidae) from Colombia and some Neotropical locations. In De Meyer, M., Clarke, A. R., Vera, M. T. and J. Hendrichs. (eds.), Resolution of Cryptic Species Complexes of Tephritid Pests to Enhance SIT Application and Facilitate International Trade. Zookeys. 540: 61–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo L. A., Griggs M. H., Liu H., Bauer L. S., and Vandenberg J. D... 2010. Assessing deposition and persistence of Beauveria bassiana GHA (Ascomycota: Hypocreales) applied for control of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), in a commercial tree nursery. Biol. Control 54: 61–67. [Google Scholar]
- Danfa A., and Van der Valk H. C. H. G... 1999. Laboratory testing of Metarhizium spp. and Beauveria bassiana on Sahelian non-target arthropods. Biocontrol Sci. Technol. 9: 187–198. [Google Scholar]
- De l. R., Lopez F. L., and Liedo P... 2002. Beauveria bassiana as a pathogen of the Mexican fruit fly (Diptera: Tephritidae) under laboratory conditions. J. Econ. Entomol. 95: 36–43. [DOI] [PubMed] [Google Scholar]
- Dias N. P., Zotti M. J., Montoya P., Carvalho I. R., and Nava D. E... 2018. Fruit fly management research: a systematic review of monitoring and control tactics in the world. Crop Prot. 112: 187–200. [Google Scholar]
- Dimbi S., Maniania N. K., Lux S. A., Ekesi S., and Mueke J. K... 2003. Pathogenicity of Metarhizium anisopliae (Metsch.) Sorokin and Beauveria bassiana (Balsamo) Vuillemin, to three adult fruit fly species: Ceratitis capitata (Weidemann), C. rosa var. fasciventris Karsch and C. cosyra (Walker) (Diptera:Tephritidae). Mycopathologia 156: 375–382. [DOI] [PubMed] [Google Scholar]
- Dubuffet A., Dupas S., Frey F., Drezen J. M., Poirié M., and Carton Y... 2007. Genetic interactions between the parasitoid wasp Leptopilina boulardi and its Drosophila hosts. Heredity (Edinb). 98: 21–27. [DOI] [PubMed] [Google Scholar]
- Ekesi S., Maniania N. K., and Lux S. A... 2002. Mortality in three African tephritid fruit fly puparia and adults caused by entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci. Technol. 12: 7–17. [Google Scholar]
- El-Sufty R., and Führer E... 1985. Interrelationships between Cydia pomonella L. (Lep., Tortricidae), Ascogaster quadridentatus Wesm. (Hym., Braconidae) and the fungus Beauveria bassiana (Bals.) Vuill. Z. Angew. Entomol. 99: 504–511. [Google Scholar]
- Emami F., Alichi M., and Minaei K... 2013. Interaction between the entomopathogenic fungus, Beauveria bassiana (Ascomycota:Hypocreales) and the parasitoid wasp, Aphidius colemani Viereck (Hymenoptera: Braconidae). J. Entomol. Acarol. Research. 45: 14–17. [Google Scholar]
- Feng M. G., Poprawski T. J., and Khachatourians G. G... 1994. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: Current status. Biocontrol Sci. Technol. 4: 3–34. [Google Scholar]
- Ferguson K. I., and Stiling P... 1996. Non-additive effects of multiple natural enemies on aphid populations. Oecologia. 108: 375–379. [DOI] [PubMed] [Google Scholar]
- Guillén L., Aluja M., Equihua M., and Sivinski J... 2002. Performance of two fruit fly (Diptera: Tephritidae) pupal parasitoids (Coptera haywardi [Hymenoptera: Diapriidae] and Pachycrepoideus vindemiae [Hymenoptera: Pteromalidae]) under different environmental soil conditions. Biol. Control 23: 219–227. [Google Scholar]
- Hernández-Ortiz V. 1992. El género Anastrepha Schiner en México (Diptera: Tephritidae). Taxonomía, distribución y sus plantas huéspedes. Instituto de Ecología y Sociedad Mexicana de Entomología. Publicación No. 33. Xalapa, Ver. México, 162 pp. [Google Scholar]
- Hernández Díaz-Ordaz N., Pérez N., and Toledo J... 2010. Patogenicidad de tres cepas de hongos entomopatógenos a adultos de Anastrepha obliqua (Macquart) (Diptera: Tephritidae) en condiciones de laboratorio. Acta Zool. Mexicana. 26: 481–494. [Google Scholar]
- Kohler L. J., Carton Y., Mastore M., and Nappi A. J... 2007. Parasite suppression of the oxidations of eumelanin precursors in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 66: 64–75. [DOI] [PubMed] [Google Scholar]
- Konstantopoulou M., and Mazomenos B... 2005. Evaluation of Beauveria bassiana and B. brongniartii strains and four wild-type fungal species against adults of Bactrocera oleae and Ceratitis capitata. Biocontrol. 50: 293–305. [Google Scholar]
- Kreutz J., Vaupel O., and Zimmermann G... 2004. Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, lps typographus L., in the laboratory under various conditions. J. App. Entomol. 128: 384–389. [Google Scholar]
- Labbé R. M., Gillespie D. R., Cloutier C., and Brodeur J... 2009. Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Sci. Technol. 19: 429–446. [Google Scholar]
- López P., Cancino J., and Montoya P... 2020. Natural parasitism and parasitoid releases to control Anastrepha obliqua (Diptera: Tephritidae) infesting Spondias spp. (Anacardaceae) in Chiapas, Mexico, pp. 267–280. InPérez Staples D., Díaz-Fleischer F., Montoya P., and Vera T. (eds.), Area-wide management of fruit fly pests. CRC Press, Boca Raton, FL. [Google Scholar]
- López-Arriaga F., Montoya P., Cancino J., Toledo J., and Liedo P... 2014. Female pupae of the genetic sexing strain “Tap-7” of Anastrepha ludens as hosts of Coptera haywardi. BioControl. 59: 149–157. [Google Scholar]
- Madurappulige D. 2005. Effect of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) on Diadegma semiclausum (Hellen Hymenoptera: Ichneumonidae), a parasitoid of Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Ph. D. Thesis. Lincoln University, Lincoln, New Zealand. [Google Scholar]
- Martínez-Barrera O. Y., Toledo J., Liedo P., Gómez J., Valle-Mora J., Cancino J., and Montoya P... 2019. Does Beauveria bassiana (Hypocreales: Cordycipitaceae) affect the survival and fecundity of the parasitoid Coptera haywardi (Hymenoptera: Diapriidae)? Environ. Entomol. 48: 156–162. [DOI] [PubMed] [Google Scholar]
- Martins I. C. F., Silva R. J., Alencar J. R., Silva K. P., Cividanes F. J., Duarte R. T., Agostini L. T., and Polanczyk R. A... 2014. Interactions between the entomopathogenic fungi Beauveria bassiana (Ascomycota: Hypocreales) and the aphid paraistoid Diaeretiella rapae (Hymenoptera: Braconidae) on Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 107: 933–938. [DOI] [PubMed] [Google Scholar]
- Meadow R., Vandenberg J. D., and Shelton A. M... 2000. Exchange of inoculum of Beauveria bassiana (Bals.) Vuill. (Hyphomycetes) between adult flies of the cabbage maggot Delia radicum L. (Diptera: Anthomyiidae). Biocontrol Sci. Technol. 10: 479–485. [Google Scholar]
- Mesquita A. L. M., and Lacey L. A... 2001. Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol. Control 22: 51–59. [Google Scholar]
- Montoya P., and Toledo J... 2010. Estrategias de control biológico, pp. 169–182. InMontoya P., Toledo J., and Hernández E. (eds.), Moscas de la fruta: Fundamentos y Procedimientos para su Manejo. S y G editores; México, D.F. [Google Scholar]
- Orozco-Dávila D., Quintero L., Hernández E., Solís E., Artiaga T., Hernández R., Ortega C., and Montoya P... 2017. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico - a review. Entomol. Exp. Appl. 164: 176–187. [Google Scholar]
- Ortu S., Cocco A., and Dau R... 2009. Evaluation of the entomopathogenic fungus Beauveria bassiana Strain ATCC 74040 for the management of Ceratitis capitata. Bull. Insectology. 62: 245–252. [Google Scholar]
- Osorio-Fajardo A., and Canal N. A... 2011. Selección de cepas de hongos entomopatógenos para el manejo de Anastrepha obliqua (Macquart, 1835) (Diptera: Tephritidae) en Colombia. Rev. Fac. Nal. Agr. Medellín. 64: 6129–6139. [Google Scholar]
- Ovruski S., Aluja M., Sivinski J., and Wharton R... 2000. Hymenopteran parasitoids on fruit infesting Tephritidae (Diptera) in Latin America and the Southern United States: diversity, distribution, taxonomic status and their use in fruit fly biological control. Int. Pest Manag. Reviews. 5: 81–107. [Google Scholar]
- Pedersen B. S., and Mills N. J... 2004. Single vs. multiple introduction in biological control: the roles of parasitoid efficiency, antagonism and niche overlap. J. Appl. Ecol. 41: 973–984. [Google Scholar]
- Peterson R. K., Davis R. S., Higley L. G., and Fernandes O. A... 2009. Mortality risk in insects. Environ. Entomol. 38: 2–10. [DOI] [PubMed] [Google Scholar]
- Powell W., Wilding N., Brobyn P. J., and Clark S. J... 1986. Interference between parasitoid (Hym: Aphidiidae) and fungi (Entomophtorales) attacking cereal aphids. Entomophaga. 31: 293–302. [Google Scholar]
- R Core Team 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Rashki M., Kharazi-Pakdel A., Allahyari H., and van Alphen J. J. M... 2009. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biol. Control 50: 324–328. [Google Scholar]
- Roy H., and Pell J... 2000. Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci. Technol. 10: 737–752. [Google Scholar]
- Shipp J. I., Zhang Y. Q., Hunt D. W. A., and Ferguson G... 2003. Influence of humidity and green house microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pest. Environ. Entomol. 32: 1154–1163. [Google Scholar]
- Silva J. E. B., Bolelli I. C., and Simões Z. L. P... 2002. Hemocyte types and total and differential counts in unparasitized and parasitized Anastrepha obliqua (Diptera: Tephritidae) larvae. Braz. J. Biol. 62: 689–699. [DOI] [PubMed] [Google Scholar]
- Sivinski J., Aluja M., and Lopez M... 1997. Spatial and temporal distribution of parasitoids of Mexican Anastrepha species (Diptera: Tephritidae) within the canopies of fruit trees. Ann. Entomol. Soc. Am. 90: 604–618. [Google Scholar]
- Sivinski J., Vuline K., Meneses E., and Aluja M... 1998. The bionomics of Coptera haywardi (Oglobin) (Hymenoptera: Diapriidae) and the other pupal parasitoids of tephritid fruit flies (Diptera). Biol. Control. 11: 193–202. [Google Scholar]
- Sookar P., Bhagwant S., and Ouna E. A... 2008. Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae). J. Appl. Entomol. 132: 778–788. [Google Scholar]
- Straub C. S., Finke D. L., and Snyder W. E... 2008. Are the conservation of natural enemy biodiversity of natural enemy biodiversity and biological control compatible goals?. Biol. Control 45: 225–237. [Google Scholar]
- Trevors J. T. 1996. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 26: 53–59. [Google Scholar]
- Vargas R. I., Jang E. B., and Klungness L. M... 2003. Area-wide pest management of fruit flies in Hawaiian fruits and vegetables, pp. 37–46. InInamine K. (ed.), Recent trends on sterile insect technique and area-wide integrated pest management. Research Institute for Subtropics, Okinawa, Japan. [Google Scholar]
- Wharton R. A., and Gilstrap F. E... 1983. Key to and status of Opiine Braconid (Hymenoptera) parasitoids used in biological control of Ceratitis and Dacus s.l. (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 76: 721–742. [Google Scholar]
- Wilson W. M., Ibarra J. E., Oropeza A., Hernández M. A., Toledo-Hernández R. A., and Toledo J... 2017. Infection of Anastrepha ludens (Diptera: Tephritidae) adults during emergence from soil treated with Beauveria bassiana under various texture, humidity, and temperature conditions. Fla. Entomol. 100: 503–508. [Google Scholar]
- Zhu H., and Jun J... 2012. Target-oriented dissemination of Beauveria bassiana by the predators, Harmonia axyridis (Coleoptera: Coccinellidae) and Chrysoperla carnea (Neuroptera: Chrysopidae) for biocontrol of Myzus persicae. Biocontrol Sci. Technol. 22: 393–406. [Google Scholar]