Abstract
Vibrio cholerae interacts with many organisms in the environment, including heterotrophic protists (protozoa). Several species of protozoa have been reported to release undigested bacteria in expelled food vacuoles (EFVs) when feeding on some pathogens. While the production of EFVs has been reported, their biological role as a vector for the transmission of pathogens remains unknown. Here we report that ciliated protozoa release EFVs containing V. cholerae. The EFVs are stable, the cells inside them are protected from multiple stresses, and large numbers of cells escape when incubated at 37 °C or in the presence of nutrients. We show that OmpU, a major outer membrane protein positively regulated by ToxR, has a role in the production of EFVs. Notably, cells released from EFVs have growth and colonization advantages over planktonic cells both in vitro and in vivo. Our results suggest that EFVs facilitate V. cholerae survival in the environment, enhancing their infectious potential and may contribute to the dissemination of epidemic V. cholerae strains. These results improve our understanding of the mechanisms of persistence and the modes of transmission of V. cholerae and may further apply to other opportunistic pathogens that have been shown to be released by protists in EFVs.
The aquatic bacterium Vibrio cholerae is the aetiological agent of the acute diarrheal disease cholera, which is endemic in many countries. Outbreaks are linked to inadequate access to clean water and sanitation and it is estimated that there are 1.3 to 4.0 million cases and 21,000 to 143,000 deaths annually worldwide1. Both toxigenic (producing cholera toxin (CT)) and non-toxigenic (CT-negative) V. cholerae are globally-distributed aquatic bacteria. Despite strong evidence that the primary habitat of V. cholerae is the marine environment2 (for example, estuarine and coastal waters3), there are also reports showing the persistence of V. cholerae in freshwater systems4. In the aquatic environment, V. cholerae interacts with sediments and many organisms, including protozoa, aquatic plants, phytoplankton and zooplankton, all of which may act as reservoirs. These natural reservoirs may have a critical role in survival of V. cholerae in inter-epidemic periods and may be responsible for the development of virulence5. For example, it has been shown that V. cholerae colonizes and reproduces in copepods, and copepod blooms might result in the numbers of V. cholerae required for an infective dose6. Furthermore, cholera outbreaks have been linked to ingestion of fresh fish7, shellfish, crabs and oysters8.
Protozoa take up bacterial prey into phagosomes that become acidified and filled with enzymes, resulting in digestion. However, several species of ciliates and amoebae can package and release undigested cells when feeding on certain species of bacterial pathogens. For example, the amoebae Acanthamoeba spp. and Dictyostelium discoideum and ciliates such as Tetrahymena spp., Colpodia spp. and Glaucom spp. release food vacuoles containing live bacterial cells when feeding on Salmonella enterica, Legionella pneumophila, Mycobacterium smegmatis, Listeria monocytogenes, Campylobacter jejuni, Pseudomonas aeruginosa and Helicobacter pylori9–17. Notably, bacteria inside EFVs are more resistant to acidic environments18, freeze–thaw, sonication and 24 h exposure to cooling-tower biocides19. Cells within EFVs show enhanced survival under starvation conditions and may remain viable for at least six months10. Such resistance to stress and long-term starvation may facilitate the subsequent uptake by and infection of a host11, although this has not yet been demonstrated. This represents a major gap in our understanding of epidemiology of many infectious diseases.
V. cholerae produces defences against Tetrahymena pyriformis, including the PrtV protease20, chitin-induced production of ammonia21 and the pigment pyomelanin22. However, to our knowledge, the production of V. cholerae EFVs has not previously been demonstrated. Here we report that co-incubation of V. cholerae with different ciliates results in the release of V. cholerae in EFVs to the extracellular environment. We also demonstrate that V. cholerae EFVs survive better than planktonic free-living cells under different stresses and show an increased infectious potential. Taken together, our results suggest that V. cholerae EFVs lead to increased survival of V. cholerae epidemic strains in both the natural and the host environment, contributing to the dissemination and infection of V. cholerae.
Results
The production of V. cholerae EFVs by T. pyriformis is dependent on prey cell number and enhanced by bacterial protein synthesis.
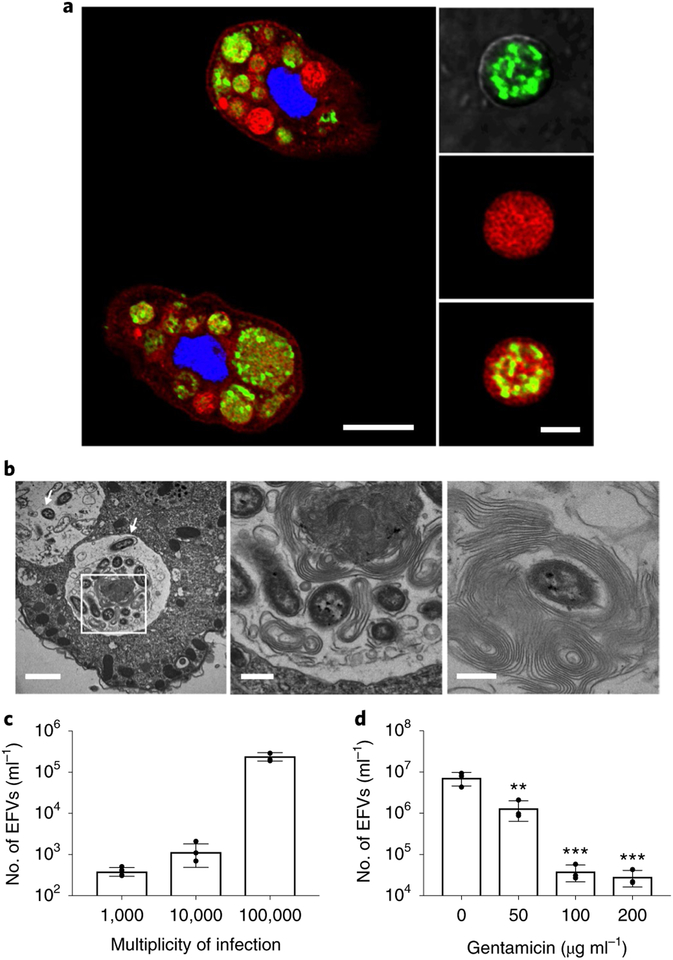
This study shows that when V cholerae and T. pyriformis are co-incubated, EFVs containing live undigested bacteria are released into the environment through the ciliate cytoproct (anus) (Fig. 1a, Supplementary Fig. 1 and Supplementary Video 1). Transmission electron microscopy (TEM) of the EFVs reveals that V. cholerae is packaged into multilamellar vacuoles, similar to previously reported EFVs containing L. pneumophila9,19 (Fig. 1b and Supplementary Fig. 2a). The production of V. cholerae EFVs increases as the bacterial concentration increases, showing that EFV production is dependent on prey cell number (Fig. 1c). EFVs are also produced when V. cholerae is co-incubated with Acanthamoeba castellanii (Supplementary Fig. 2b).
Fig. 1 |. Production of EFVs containing V. cholerae.
a, Fixed samples of green fluorescent protein (GFP)-tagged (green) V. cholerae co-incubated with T. pyriformis in 0.55× NSS. T. pyriformis was stained with 4,6-diamidino-2-phenylindole (DAPI, blue) and FM 4–64 FX (red) was used to stain the EFV membranes. Intracellular food vacuoles containing GFP-tagged V. cholerae (left; scale bar, 15 μm; Supplementary Video 2) and an expelled EFV (right; scale bar, 5 μm; Supplementary Video 3). Images are representative of three independent experiments. b, TEM of fixed samples of T. pyriformis and V. cholerae EFVs. Left, V. cholerae (white arrows) in vacuoles of T. pyriformis after overnight incubation in 0.55× NSS at room temperature. The presence of many mitochondria around the vacuole is observed. Middle, a magnified view of the bounded area in the left panel. Right, a single bacterial cell in an EFV containing multiple cells to show the presence of multiple layers of membrane surrounding V. cholerae. Scale bars: 2 μm (left), 500 nm (middle) and 500 nm (right) (see also Supplementary Fig. 2). Images are representative of three independent experiments. c, Number of EFVs after co-incubation of T. pyriformis with different numbers of V. cholerae. Data are from four independent biological replicates and are shown as the mean±s.d. d, Numbers of EFVs produced when protein synthesis is inhibited before co-incubation. Data are from three independent biological replicates and are shown as the mean±s.d. Significant differences were determined using one-way ANOVA with Dunnett’s multiple comparisons test. **P<0.01 and ***P<0.001.
To determine whether active V. cholerae is necessary for EFV production, bacteria were pre-treated with sublethal concentrations of gentamicin to inhibit protein synthesis. Inhibition of protein synthesis resulted in a significant decrease in production of EFVs by T. pyriformis (Fig. 1d and Supplementary Fig. 3a). Furthermore, there was a significant reduction in EFV production with heat-killed V. cholerae (Supplementary Fig. 3b). These results indicate that V. cholerae produces one or more specific factors when inside the protozoan phagosome, contributing to release of EFVs.
Co-incubation of different V. cholerae strains with several ciliated protozoa also results in the production of EFVs.
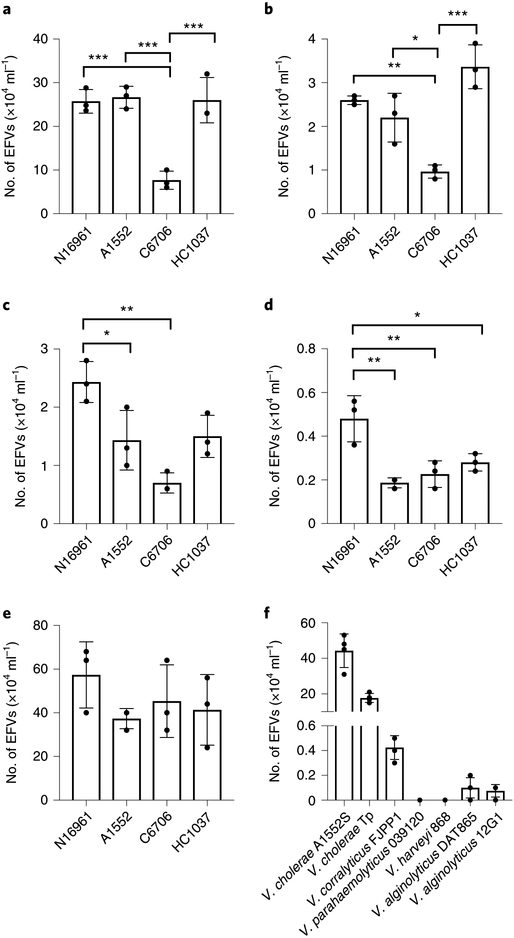
To further determine whether the production of EFVs is a general response, different V. cholerae and wild-type ciliate strains were tested for EFV production. Results showed that the co-incubation of V. cholerae O1 N16961, A1552, C6706 and HC1037 with T. pyriformis, Tetrahymena malaccensis, Tetrahymena sp., Uronema marinum and Tetrahymena thermophila led to the release of V. cholerae EFVs to the extracellular space (Fig. 2a–e). Higher numbers of EFVs were observed in the co-incubations with T. thermophila at 30 °C (Fig. 2e), suggesting that increased temperature might enhance EFV production. These results show that predation of V. cholerae by ciliated protozoa at both room temperature and 30 °C results in the production of EFVs. We also tested different Vibrio spp. (Fig. 2f), demonstrating that there are strain-dependent differences in EFV production.
Fig. 2 |. Production of EFVs by different Vibrio spp. and ciliate wild-type strains.
a–e, EFV production when V. cholerae O1 strains N16961, A1552, C6706 and HC1037 were co-incubated in 0.55× NSS with T. pyriformis (a), T. malaccensis (b), Tetrahymena sp. (c) and U. marinum (d) at room temperature, and with T. thermophila at 30 °C (e). f, Different Vibrio spp. incubated with T. pyriformis. Data are from three (a–e) or four (f) independent biological replicates and are shown as the mean±s.d. Significant differences were determined using one-way ANOVA with Tukey’s multiple comparisons test. *P<0.05, **P<0.01 and ***P<0.001.
The V. cholerae outer membrane protein OmpU is involved in the release of EFVs.
To identify the potential factor(s) responsible for the production of EFVs, various V. cholerae A1552 mutants with deletions in genes related to grazing resistance and biofilm formation22–24, transcriptional regulation25–28, motility29, acid resistance30, outer membrane proteins31,32, aminoacyl lipid modification33, type I34, II35–37 and VI38 secretion systems and intracellular survival and multiplication39 were tested. None of the mutants used in this study showed a growth defect in LB medium. Before each co-incubation, bacteria were adjusted to an OD600 nm of 1.00 to 1.04 (approximately 109 c.f.u. per ml) in 0.55× NSS (see Methods) and serially diluted to the desired concentration. Compared to the wild type, a significant decrease in the number of EFVs was observed when toxR or ompU mutants were used as prey (Table 1).
Table 1 |.
Numbers of EFVs produced by different V. cholerae A1552 mutants
| Classification | A1552 mutants testeda | Number of EFVs per ml (mean ± s.d.) |
|---|---|---|
| Grazing resistance or biofilm formation | ΔvpsAb | 4.15 × 105 ± 6.81 × 104 |
| ΔrpoSb | 3.93 ×105 ± 1.30 × 105 | |
| ΔhmgAb | 3.78 × 105 ± 1.34 × 105 | |
| ΔvpsRb | 2.73 × 105 ± 1.30 × 105 | |
| Transcriptional regulators | ΔhapRb | 4.50 × 105 ± 1.44 × 105 |
| ΔphoBb | 2.75 × 105 ± 1.02 × 105 | |
| ΔchiSb | 5.40 × 105 ± 9.76 × 104 | |
| ΔtoxRc | 1.28 × 104 ± 3.95 × 103 | |
| Motility | ΔflaAb | 3.20 × 105 ± 1.83 × 105 |
| Acid resistance | ΔcadCb | 3.50 × 105 ± 1.96 × 105 |
| Outer membrane proteins | ΔompU | 4.25 × 103 ± 5.00 × 102 |
| ΔompVb | 4.03 × 105 ± 1.44 × 105 | |
| Aminoacyl lipid modification | ΔalmEFGb | 2.97 × 105 ± 1.15 × 105 |
| Type I secretion system | ΔrtxAb | 4.00 × 105 ± 1.67 × 105 |
| Type II secretion system | ΔCTXΦb | 2.85 × 105 ± 1.98 × 105 |
| ΔgbpAb | 3.90 × 105 ± 1.95 × 105 | |
| ΔlapAb | 3.20 × 105 ± 1.68 × 105 | |
| Type VI secretion system | Δhcp1b | 3.85 × 105 ± 1.55 × 105 |
| Δhcp2b | 3.65 × 105 ± 6.19 × 104 | |
| Δhcp1,2b | 3.80 × 105 ± 1.76 × 105 | |
| Intracellular survival and multiplication in other bacteria | ΔankBb | 2.98 × 105 ± 2.08 × 105 |
Sample size, n = 4.
No significant reduction compared with wild type.
Significant reduction compared with wild type.
ToxR is the transcriptional regulator of ompU. Thus, to determine whether the defect in EFV production in the ΔtoxR strain is due to loss of ompU expression, or if other genes in the virulence operon regulated by ToxR are involved, ΔompU and ΔtoxR strains were complemented with ompU. In addition, as the operon that encodes ompU includes dacB (a carboxypeptidase located downstream of ompU), a dacB-deletion mutant was also constructed and tested. Deletion of dacB did not affect EFV production; however, complementation of the ompU gene in both ΔompU and ΔtoxR strains restored the number of EFVs back to wild-type levels (Fig. 3). These results indicate that OmpU, an outer membrane protein involved in resistance to antimicrobial peptides40, bile salts41 and organic acids42, which is positively regulated by the master regulator of virulence, ToxR, has an important role in the production of EFVs.
Fig. 3 |. Number of EFVs produced by different V. cholerae mutants.
Number of EFVs produced by wild-type, ΔtoxR, ΔdacB, ΔompU and the ompU-complemented ΔompU and ΔtoxR strains (ΔompU pBAD24::ompU and ΔtoxR pBAD24::ompU, respectively). Strains were grown in LB broth at 37°C, with agitation at 200 r.p.m. overnight except for the ompU-complemented mutants, which were supplemented with 100 μgml−1 carbenicillin and with (for ompU expression) or without (control) 0.2% arabinose. Data are from three independent biological replicates and are shown as the mean±s.d. Significant differences were determined using one-way ANOVA with Dunnett’s multiple comparisons test. ****P< 0.0001.
EFVs protect cells from stress.
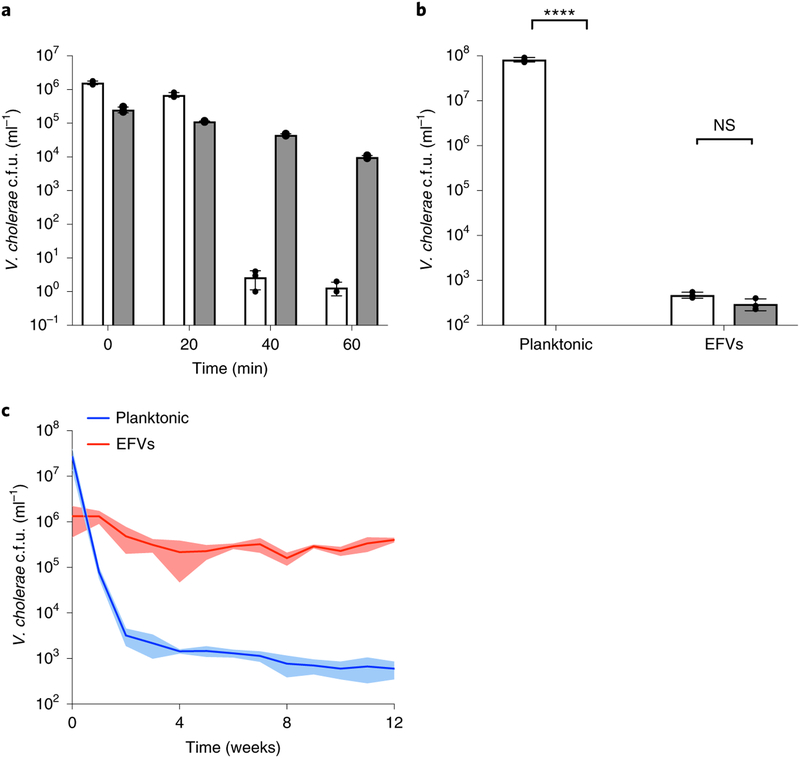
Bacterial cells inside the EFVs are potentially protected from various environmental and host stresses, such as acid stress, antimicrobials and starvation. To test this, we purified V. cholerae EFVs by filtration and washed and exposed them to pH stress (pH 3.4, the pH of the human stomach43) alongside planktonic V. cholerae cells as controls. The viability of the cells in the EFVs was only slightly affected by the treatment (less than 1-log reduction), whereas planktonic V. cholerae were completely killed after 40 min of incubation (Fig. 4a). Thus, EFVs can protect V. cholerae from low pH conditions that would be encountered on entering a human host gut. Another common stress encountered by bacteria is exposure to biocides. Thus, the experiment was repeated using gentamicin at a bactericidal concentration (300 μg ml−1) at room temperature. Again, whereas planktonic V. cholerae cells were completely eradicated, the cells in EFVs showed no loss of viability (Fig. 4b). These results therefore show that EFVs act as a protective barrier against different V. cholerae stressors.
Fig. 4 |. Survival of V. cholerae cells in EFVs under stress and starvation conditions.
a, Planktonic cells (open bars) and V. cholerae EFVs (closed bars) were incubated for 60 min in 0.55× NSS adjusted to pH 3.4. The number of colony-forming units was determined every 20 min by treating the samples with 1% Triton X-100 (Supplementary Fig. 4a–c), serial dilution and plating on LB agar plates. Data are from three independent biological replicates and are shown as the mean±s.d. b, V. cholerae EFVs and planktonic cells were treated (closed bars) or not (open bars) with gentamicin (300 μg ml−1) for 1 h at room temperature and the number of colonies was determined. Data are from three independent biological replicates and are shown as the mean±s.d. (P> 0.999). Significant differences were determined using two-way ANOVA with Sidak’s multiple comparisons test. NS, not significant; ****P< 0.0001. c, Number of cells in EFVs (red) and number of planktonic cells (blue) after incubation in 0.55× NSS for 12 weeks. Data are from three independent biological replicates and are shown as the mean ± 95% confidence intervals (shaded area).
Starvation is a common environmental stress for bacteria in aquatic environments44. Many marine bacteria can survive long periods under starvation conditions, whereas others decline in number over time. To determine whether cells in the EFVs can survive long-term starvation, EFVs were collected, resuspended in artificial seawater (0.55× NSS) and stored at room temperature. Viability was assessed and compared with that of planktonic V. cholerae maintained under the same conditions. After one week, there was an approximate 2.5-log decrease in the viability of the planktonic cells (Fig. 4c). By contrast, cells in EFVs maintained viability for at least three months (less than 0.5-log reduction). This result confirms that EFVs confer a fitness advantage to V. cholerae and increase their viability in seawater, thus contributing to their persistence in the environment.
The escape of V. cholerae from EFVs is mediated by temperature and the presence of nutrients.
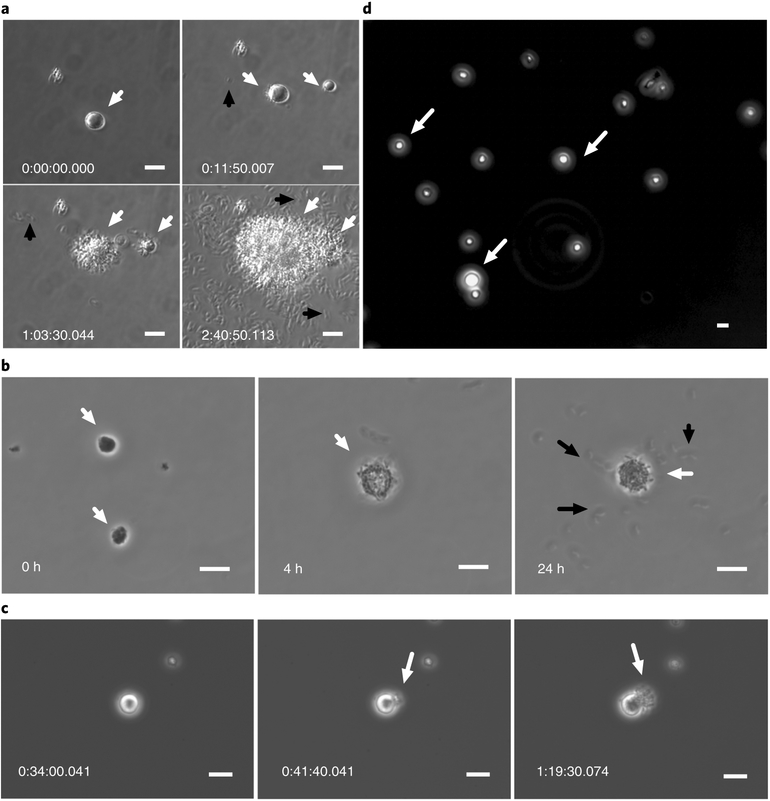
For EFVs to be an ecologically relevant mechanism of protection and transmission for pathogens in the environment, the cells inside EFVs must be able to escape and propagate. EFVs that were incubated in LB broth at 37 °C escaped quickly (in 15–30 min) and began dividing (Fig. 5a and Supplementary Videos 4 and 5). At 37 °C in 0.55× NSS without carbon or nutrient sources, motility of cells in EFVs increased and they escaped the EFVs within 4 h, but at a slower rate than in LB medium (Fig. 5b). This experiment was repeated with EFVs that had been stored in 0.55× NSS at room temperature (about 22 °C) for two months. Cell escape and propagation from EFVs in LB broth was observed within 3 h of incubation (Fig. 5c), but no EFV escape was observed during the preceding 2 months (Fig. 5d). Thus, the escape of V. cholerae from EFVs is triggered by increased temperature and the presence of nutrients.
Fig. 5 |. Escape of V. cholerae from EFVs under different nutrient and temperature conditions.
a, Escape of V. cholerae from EFVs. V. cholerae EFVs were incubated in LB broth at 37°C and were observed using time-lapse imaging for 3 h. Numbers show image time stamps (h:min:s.ms). Top left, a single V. cholerae EFV (white arrow) suspended in LB broth at the beginning of the incubation. Top right: rupture of the EFV membrane after approximately10 min (indicated by white arrows) with the subsequent release of V. cholerae cells (indicated by a black arrow). Bottom left, V. cholerae cells actively dividing and escaping from EFVs (indicated by white arrows) with more extracellular bacteria present (indicated by a black arrow). Bottom right, dense growth from EFVs (indicated by white arrows) and many extracellular V. cholerae cells, showing active division (indicated by a black arrow). b, Incubation of EFVs at 37°C without carbon or nutrient source (suspended in 0.55× NSS). Left, white arrows show two EFVs at time 0. Middle, a single EFV (indicated by a white arrow) and many V. cholerae cells after 4 h of incubation. Right, a single EFV (indicated by a white arrow) and extracellular bacteria (indicated by black arrows) are observed after 24 h of incubation. c, EFVs incubated in LB broth at room temperature. The video started recording after 2 h of incubation. Left, a single V. cholerae EFV. Middle, rupture of the EFV membrane (indicated by a white arrow). Right, V. cholerae cells showing active growth from the EFV (indicated by a white arrow). d, EFVs (indicated by white arrows) suspended in 0.55× NSS at room temperature for two months. Intact EFVs are observed without extracellular bacteria. Scale bars in a–d, 10 μm. Images are representative of three independent experiments.
Cells in EFVs have a fitness advantage in vitro.
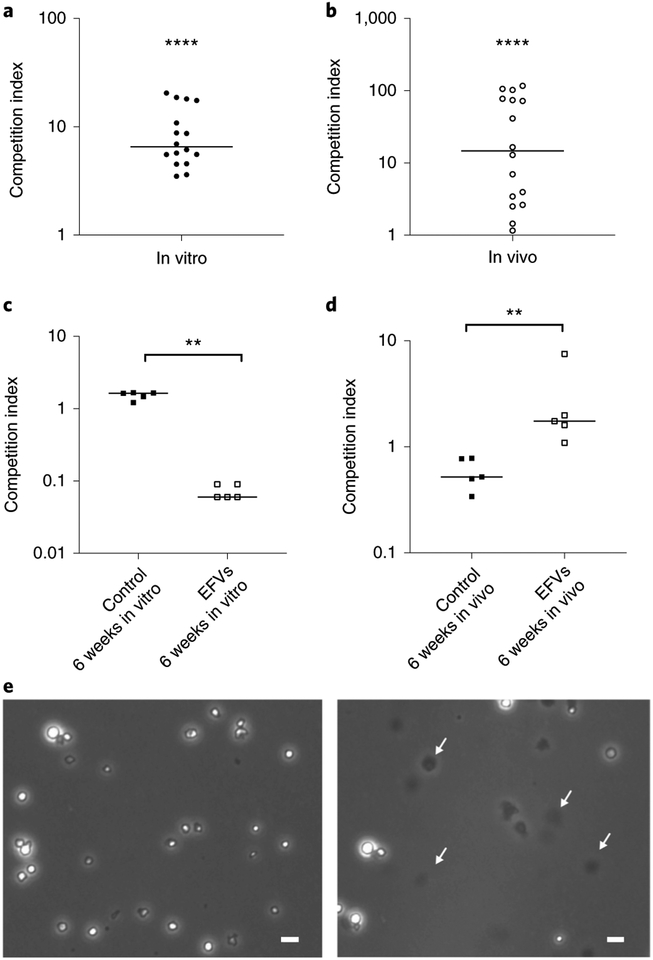
We next tested the fitness of V. cholerae cells contained in EFVs and of planktonic cells for growth in nutrient medium (LB). The V. cholerae A1552 wild-type strain was used to produce 24 h-old EFVs and competed against a ΔlacZ isogenic strain that had been grown in vitro and acclimatized in 0.55× NSS before inoculation. The in vitro competition was performed by inoculating 50 μl of a 0.55× NSS suspension containing purified EFVs (approximately 6 × 104 EFVs ml−1) and ΔlacZ isogenic strain planktonic cells (approximately 6 × 105 cells per ml; to differentiate planktonic cells from cells originating from EFVs by growth in the presence of X-gal for blue–white screening) in LB broth and incubating at 37 °C overnight with agitation. The competition index was calculated as the ratio of colony-forming units (c.f.u.) of EFVs to the c.f.u. of ΔlacZ wild type corrected by the number of viable V. cholerae cells in EFVs (Supplementary Fig. 5a–c and Supplementary Files 1 and 2). The in vitro competition index of V. cholerae in EFVs over planktonic V. cholerae (Fig. 6a, median value 6.5) suggests that the EFVs confer a growth advantage for V. cholerae when nutrients are encountered.
Fig. 6 |. Competition index for in vitro and in vivo assays of V. cholerae EFVs versus planktonic V. cholerae and working model.
a,b, Competition index of in vitro (a) and in vivo (b) assays calculated by the output ratio after incubation (in vitro: overnight, 37°C; in vivo: 24 h, 24°C) corrected by the input ratio. Number of colony-forming units was assessed by plating on LB10 agar plates supplemented with 100 μg ml−1 rifampicin to inhibit other intestinal bacteria and 80 μg ml−1 X-gal. Data in a and b are from 16 independent biological replicates and are shown as the median. The competition index of V. cholerae EFVs compared to planktonic cells in in vitro and in vivo assays is significantly higher than a hypothetical median of 1.0 (two-tailed, non-parametric, Wilcoxon signed-rank test; ****P< 0.0001). c,d, Competition index of in vitro (c) and in vivo (d) assays performed with either six-week-old EFVs (incubated in 0.55× NSS at room temperature) or six-week-old planktonic cells (control, incubated in 0.55× NSS at room temperature) compared with ΔlacZ wild type, calculated by the output ratio after incubation (in vitro: overnight, 37°C; in vivo: 24 h, 24°C) corrected by the input ratio. Colony-forming units were assessed by plating on LB10 agar plates supplemented with 100 μg ml−1 rifampicin to inhibit other intestinal bacteria and 80 μg ml−1 X-Gal. Data in c and d are from five independent biological replicates and are shown as the median. The competition index of the six-week-old V. cholerae EFVs compared with the six-week-old planktonic cells (control) was significantly different in both in vitro and in vivo conditions (two-tailed, non-parametric Mann–Whitney test; **P<0.01). e, V. cholerae EFVs incubated at 37°C for 4h in 0.55× NSS at pH 3.4 without (left) or with (right) 0.4% deoxycholic acid treatment. Images show intact V. cholerae EFVs in the untreated condition (left), with arrows showing digested EFVs after deoxycholic acid treatment. Scale bars, 10 μm. Images are representative of three independent experiments.
Purified V. cholerae EFVs are primed for infection in vivo.
Since EFVs are produced in large numbers under intense predation, and cells in the EFVs are protected against a range of stresses and can maintain long-term viability under environmental conditions, it follows that these EFVs may be infective when consumed by a host. To assess the infectivity of V. cholerae EFVs, an infant-mouse model of colonization was employed. For this, 50 μl of the same inoculum used for in vitro competition was used to infect the mice (Methods). After 24 h of infection, the competition index was calculated from cells obtained from the small intestine of each animal. Despite considerable variability in the results, V. cholerae EFVs outcompeted the in vitro grown bacteria in vivo, with median competition index significantly higher than 1.0 (P < 0.0001, Wilcoxon signed-rank test). The in vivo competition index (Fig. 6b, median value 14.7) demonstrates that V. cholerae in EFVs have a significant colonization advantage compared with planktonic cells.
V. cholerae EFVs maintain in vivo hyperinfectivity for six weeks.
The incubation of V. cholerae within EFVs in the environment might result in long periods before they are ingested by a host. To test whether aged EFVs maintain the hyperinfective phenotype, purified EFVs were incubated in 0.55× NSS for six weeks at room temperature and used for in vitro and in vivo competition assays as described above. Contrary to the earlier results, the six-week-old EFVs showed an in vitro growth disadvantage (median value 0.07) compared to the control (planktonic six-week-old V. cholerae, median value 1.43) (Fig. 6c). However, many aggregates were detected after overnight growth in LB broth, suggesting that V. cholerae from EFVs grew as aggregated bacteria, which could have affected the c.f.u. calculation for the escaped V. cholerae. Nevertheless, the six-week-old EFV V. cholerae cells still showed a colonization advantage (median value 1.74) over the control cells (median value 0.56) (Fig. 6d), confirming that long-term incubation did not affect the hyperinfective capability of V. cholerae from EFVs.
V. cholerae EFVs are not degraded at 37 °C and low pH but are digested in the presence of deoxycholic acid.
To assess whether the EFVs might be degraded, either in the stomach or the small intestine, EFVs were incubated in two conditions. First, purified EFVs were resuspended in 0.55× NSS at pH 3.4 and incubated at 37 °C for 4 h. Imaging showed that there was no escape of V. cholerae from EFVs (Fig. 6e, left). However, exposure of the EFVs to 0.4% deoxycholic acid resulted in immediate digestion of EFVs (Fig. 6e, right). Together, these results suggest that V. cholerae would remain inside EFVs when transiting through the stomach, but would be released at the site of colonization (in the small intestine) in the presence of bile.
Discussion
Our results suggest that when numbers of V. cholerae are high in the environment, such as during disease outbreaks, there would be intense predation pressure and some of these protist predators release EFVs into the water column. Although the production of EFVs has been shown for other pathogens, it has not been demonstrated whether this process is mediated by the protist or the bacteria. Here we show that OmpU has a key role in the production of EFVs containing V. cholerae, demonstrating that bacterial factors positively contribute to this process. After ingestion by T. pyriformis, V. cholerae in phagosomes encounter an adverse environment characterized by the presence of low pH and cationic antimicrobial peptides45,46. As previously shown in V. cholerae, OmpU enables resistance to such environments. For example, reports have shown that OmpU protects V. cholerae from antimicrobial peptides40,47,48, low pH42 and bile49. In addition, it has been shown that OmpU is involved in intestinal colonization by V. cholerae31 and is essential for invasion and infection of oysters by other Vibrio species47,50. In sum, the egestion of V. cholerae from EFVs is promoted by an outer membrane protein that is essential for the pathogenesis of this bacterium.
The function of OmpU in protecting V. cholerae cells from low pH and antimicrobial peptides indicates that once inside the phagosome, OmpU probably acts to resist digestion of the bacterial cells. This results in a large number of undigested cells in the vacuole. The undigested cells that remain in the phagosome may trigger the expulsion of vacuoles containing bacteria from T. pyriformis, as previously demonstrated51.
Since EFVs confer a survival advantage to V. cholerae under stressful conditions, the cells in the EFVs are protected from various environmental stresses and pH stress that would be encountered following ingestion. The EFVs would enhance survival of cells passing through the stomach, and as the EFVs contain numerous cells, would increase numbers of V. cholerae that reach the small intestine (Fig. 4). Our mouse colonization data shows that V. cholerae in EFVs can outcompete planktonic cells, suggesting that EFVs might protect cells and may enhance efficient infection, possibly through improved survival on exposure to gastric acid and increased resistance to host antimicrobial defences through active expression of ompU. Furthermore, as stated above, OmpU is critical for intestinal colonization31, suggesting that the expression of OmpU in EFVs might be responsible for the in vivo colonization advantage. We suggest that the findings reported here establish a novel understanding of the mechanisms of persistence and the modes of transmission of V. cholerae and may further apply to other opportunistic pathogens that have been shown to be released by protists via EFVs. Hence, protozoan EFVs may constitute a mechanism for transmission and infection more broadly, as has been previously speculated4,19.
Methods
Strains and growth conditions.
Organisms used in this study are listed in Supplementary Table 1. Bacterial strains were routinely grown in LB and on LB agar plates. V. cholerae mutants were constructed by splicing using overlap extension PCR52 and natural transformation53. Complementation was done using the expression vector pBAD24. Bacteria carrying the vector were grown in LB broth at 37 °C containing 100 μg ml−1 ampicillin and 0.2% arabinose for gene expression. Environmental isolates of Vibrio spp. were routinely grown in LB broth and LB agar plates supplemented with 2% NaCl and incubated at 28 °C.
Tetrahymena spp. were routinely passaged in 15 ml growth medium containing peptone–yeast–glucose (20 gl−1 proteose peptone and 1 gl−1 yeast extract) supplemented with 1 l of 0.1× M9 minimal medium (6 gl−1 NaH2PO4, 3 gl−1 K2PO4, 0.5 gl−1 NaCl and 1 gl−1 NH4Cl) and 0.1 M sterile-filtered glucose in 25 cm2 tissue culture flasks with ventilated caps (Sarstedt) and incubated statically at room temperature. U. marinum was routinely grown in 0.55× NSS medium (8.8 gl−1 NaCl, 0.735 gl−1 Na2SO4, 0.04 gl−1 NaHCO3, 0.125 gl−1 KCl, 0.02 gl−1 KBr, 0.935 gl−1 MgCl2.6H2O, 0.205 gl−1 CaCl2.2H2O, 0.004 gl−1 SrCl2.6H2O and 0.004 g l−1 H3BO3) supplemented with 1% heat-killed P. aeruginosa PAO1 in a 25 cm2 tissue culture flask, and further incubated at room temperature statically for 2 d before enumeration and use.
Before experiments, 500 μl of Tetrahymena spp. were passaged in 20 ml of 0.55× NSS medium supplemented with 1% heat-killed P. aeruginosa in a 25 cm2 tissue culture flask, and further incubated at room temperature statically for 2 d before enumeration and use. This process is necessary to remove the nutrient medium and to acclimatize the ciliate to phagotrophic feeding.
To prepare heat-killed bacteria, P. aeruginosa or V. cholerae were grown overnight in LB at 37 °C with shaking at 200 r.p.m. and adjusted to (OD600 nm = 1.0; 109cells ml−1) in 0.55× NSS. The cultures were then transferred to a water bath at 65 °C for 2 h, and then tested for viability by plating on LB agar plates at 37 °C for 2 d. Heat-killed bacteria stocks were stored at −20 °C.
Production of EFVs containing V. cholerae.
To produce EFVs, V. cholerae A1552 was co-incubated with T. pyriformis in 0.55× NSS. In brief, T. pyriformis were enumerated by microscopy and adjusted to 103 cells ml−1 and added to co-cultures of V. cholerae A1552 adjusted to 108 cells ml−1 in 0.55× NSS using a spectrophotometer (OD600 nm). After overnight incubation at room temperature, samples were analysed using an inverted epifluorescence microscope (Nikon Eclipse Ti inverted microscope) to detect the presence of EFVs in the supernatant. To purify V. cholerae EFVs, supernatants were filtered (by gravity) several times through 8 μm filters (Millipore) and the filters containing EFVs were suspended in 1 ml 0.55× NSS. The EFVs were incubated for 1 h with 300 μg ml−1 gentamicin at room temperature to kill any remaining extracellular bacteria. After gentamicin treatment, V. cholerae-EFV pellets were collected by centrifugation (3,220g for 20 min), washed three times in 0.55× NSS and suspended in 1 ml of 0.55× NSS. Finally, the number of V. cholerae EFVs was determined by microscopy after 48 h of co-incubation (the time needed for the eradication of all extracellular bacteria).
Enumeration of live/dead V. cholerae in EFVs.
To establish the number of viable V. cholerae in EFVs, a genomic staining assay was conducted. In brief, EFVs were produced and collected as above and suspended in 1 ml of 0.55× NSS. The EFVs were stained with LIVE/DEAD BacLight Bacterial Viability Kit for microscopy (Invitrogen) following the manufacturer’s instructions. After staining, the sample was centrifuged (7,607g for 5 min) to remove the staining solution and resuspended in 1 ml of 0.55× NSS. Eight microlitres of sample were placed on a glass slide, covered with a coverslip (1.5 mm thickness) and sealed with nail polish. Stained EFVs were immediately analysed by confocal microscopy (Nikon A1 confocal laser scanning microscope) to assess the number of live (green) and dead (red) bacterial cells.
Survival of V. cholerae EFVs under stress conditions.
To assess the effect of stress conditions on the viability of V. cholerae in EFVs, two treatments were performed independently. For the acid-tolerance experiments, V. cholerae EFVs were obtained as described above and suspended in either 0.55× NSS or NSS adjusted to pH 3.4 (with 1 N HCl). Incubation of the V. cholerae EFVs was carried out in triplicate for 60 min in a 96-well plate at room temperature with agitation (60 r.p.m.). The number of viable bacteria was determined at different time points by adding 1% Triton-X100 (Sigma) to each well at 0, 20, 40 and 60 min (to release the V. cholerae cells from the EFVs, Supplementary Fig. 4a–c) and plating serial dilutions on LB plates. For the gentamicin assay, V. cholerae EFVs were exposed to 300 μg ml−1 gentamicin in 0.55× NSS at room temperature with agitation at 60 r.p.m. in a 96-well plate. After 1 h incubation, 1% Triton-X100 (Sigma) was added to each well and serial dilutions were plated on LB. As a control, planktonic V. cholerae adjusted to ~ 106 cells ml−1 in 0.55× NSS was used for each of the three conditions.
Escape of V. cholerae from EFVs.
To obtain images and videos of V. cholerae cells escaping from EFVs, the EFVs were collected as described above, suspended in LB broth or 0.55× NSS and 1 ml of the suspension was added to a 24-well glass-bottom microtitre plate. Plates were incubated at 37 °C or room temperature under a confocal microscope (Nikon A1 confocal laser scanning microscope) and videos or still images were taken.
Incubation of EFVs at low pH and in the presence of deoxycholic acid.
Purified V. cholerae EFVs were incubated at 37 °C for 4 h in 0.55× NSS at pH 3.4. To test the effect of deoxycholic acid (a component of bile) on the EFVs, treatments with 0.4% deoxycholic acid were performed at 37 °C after 4 h of incubation in 0.55× NSS at pH 3.4.
Infant mouse colonization experiments.
Five-day-old litters of CD1 mice were inoculated orogastrically as described54 with 50 μl of inoculum containing ~106 rifampicin-resistant V. cholerae A1552 in EFVs (24 h old) and ~ 106 c.f.u. of an isogenic competing strain, V. cholerae A1552 ΔlacZ, which was prepared by culturing in vitro to stationary phase in LB broth at 37 °C with aeration. In parallel, 2 μl of inoculum was diluted into 2 ml of LB broth in culture tubes and competed in vitro for 18 h with aeration at 37 °C. After 24 h, mice were euthanized, and the small intestine was removed and homogenized in 1 ml of LB broth supplemented with 20% glycerol.
For the experiments with six-week-old EFVs, in vitro growth and in vivo infections were performed as described above. As a control, planktonic V. cholerae in 0.55× NSS that was starved for six weeks at room temperature was used. The ratios of wild-type to ΔlacZ V. cholerae at the input (inoculum) and outputs were determined by plating serial dilutions on LB agar supplemented with 100 μg ml−1 rifampicin and 80 μg ml−1 X-Gal. The competition index was calculated as the output ratio divided by the input ratio corrected by the number of V. cholerae in EFVs.
All animal procedures were conducted in accordance with the rules of the Department of Laboratory Animal Medicine at Tufts University School of Medicine. Five-day-old CD-1 infant mice (both male and female) were used for the infection experiments to obtain an accurate median for statistical analyses. For all experiments, mice were randomly allocated to each treatment group before inoculation of samples. All mice were obtained from Charles River Laboratories.
Transmission electron microscopy.
Cell cultures were fixed for 24 h at 4 °C by immersion in a fixative solution containing 3% glutaraldehyde in PBS buffer (0.1 M phosphate, pH 7.5) and then stored in PBS buffer at 4 °C until further processing. Samples were subsequently post-fixed for 1 h in a solution containing 1% osmium tetroxide in PBS (1×, final pH 7.5), washed with MilliQ water and dehydrated in an increasing gradient of ethanol before infiltration and embedding in Spurr resin (ProsciTech). Resin blocks were then cut into 90 nm sections using an Ultracut UC6 microtome (Leica Microsystems). Selected sections containing cells and EFVs were stained on finder grids (Electron Microscopy Sciences) with uranyl acetate and lead citrate. Stained sections on finder grids were viewed at 200 kV accelerating voltage using a FEI Tecnai G2 20 transmission electron microscope at the Mark Wainwright Analytical Centre: Electron Microscope Unit (University of New South Wales).
Data analysis.
Statistical analysis was performed using GraphPad Prism v.7.01 for Windows (www.graphpad.com). Data that did not follow a Gaussian distribution was determined by analysing the frequency distribution graphs and was transformed using natural logs. Two-tailed student’s t-tests were used to compare means between experimental samples and controls. For experiments including multiple samples, one-way or two-way analysis of variance (ANOVA) was used for the analysis and Dunnett’s multiple comparison test provided the post hoc comparisons of means. For the mouse colonization experiments, the data were analysed using a non-parametric test for medians that follow Gaussian distribution (Wilcoxon signed-rank test or Mann–Whitney test) for non-normally distributed data.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
The authors thank S. A. Rice, S. Longford and B. Morgan for critical evaluation of the manuscript, I. T. C. Hin for graphic illustrations, K. Li for assistance with TEM imaging and L. Cole for advice on confocal microscopy. This work was supported by Australian Research Council Discovery Project DP170100453, the United States NIH (AI055058), the Pew Latin American Fellows Program in the Biomedical Sciences from PEW Charitable trusts, the CONICYT Becas Chile doctoral (72140329) and postdoctoral fellowships, the ithree Institute and The Microbial Imaging Facility, Faculty of Science, University of Technology Sydney. This project was also partly funded by the Australian Centre for Genomic Epidemiological Microbiology (AusGEM), a collaborative partnership between the New South Wales Department of Primary Industries and the University of Technology Sydney and by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Programme to the Singapore Centre for Environmental Life Sciences Engineering, Nanyang Technological University.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information is available for this paper at https://doi.org/10.1038/s41564-019-0563-x.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ali M, Nelson AR, Lopez AL & Sack DA Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis 9, e0003832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colwell R & Huq A Marine ecosystems and cholera. Hydrobiologia 460, 141–145 (2001). [Google Scholar]
- 3.Martinelli Filho JE, Lopes RM, Rivera ING & Colwell RR Vibrio cholerae O1 detection in estuarine and coastal zooplankton. J. Plankton Res 33, 51–62 (2010). [Google Scholar]
- 4.Nair GB et al. Ecology of Vibrio cholerae in the freshwater environs of Calcutta, India. Microb. Ecol 15, 203–215 (1988). [DOI] [PubMed] [Google Scholar]
- 5.Vezzulli L, Pruzzo C, Huq A & Colwell RR Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ. Microbiol. Rep 2, 27–33 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Colwell RR et al. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl Acad. Sci. USA 100, 1051–1055 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta CJ et al. Cholera outbreak in southern Tanzania: risk factors and patterns of transmission. Emerg. Infect. Dis 7, 583–587 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabbani GH & Greenough WB III. Food as a vehicle of transmission of cholera. J. Diarrhoeal Dis. Res 17, 1–9 (1999). [PubMed] [Google Scholar]
- 9.Berk SG et al. Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a Dot/Icm-mediated survival mechanism. J. Appl. Environ. Microbiol 74, 2187–2199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouyer S, Imbert C, Rodier M-H & Héchard Y Long-term survival of Legionella pneumophila associated with Acanthamoeba castellanii vesicles. Environ. Microbiol 9, 1341–1344 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Denoncourt AM, Paquet VE & Charette SJ Potential role of bacteria packaging by protozoa in the persistence and transmission of pathogenic bacteria. Front. Microbiol 5, 240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourabathini P, Brandl MT, Redding KS, Gunderson JH & Berk SG Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl. Environ. Microbiol 74, 2518–2525 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koubar M, Rodier M-H, Garduño RA & Frère J Passage through Tetrahymena tropicalis enhances the resistance to stress and the infectivity of Legionella pneumophila. FEMS Microbiol. Lett 325, 10–15 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Marciano-Cabral F & Cabral G Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev 16, 273–307 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paquet VE & Charette SJ Amoeba-resisting bacteria found in multilamellar bodies secreted by Dictyostelium discoideum: social amoebae can also package bacteria. FEMS Microbiol. Ecol 92, fiw025 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Raghu Nadhanan R & Thomas CJ Colpoda secrete viable Listeria monocytogenes within faecal pellets. Environ. Microbiol 16, 396–404 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Trigui H, Paquet VE, Charette SJ & Faucher SP Packaging of Campylobacter jejuni into multilamellar bodies by the ciliate Tetrahymena pyriformis. J. Appl. Environ. Microbiol 82, 2783–2790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehfuss MYM, Parker CT & Brandl MT Salmonella transcriptional signature in Tetrahymena phagosomes and role of acid tolerance in passage through the protist. ISME J 5, 262–273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berk SG, Ting RS, Turner GW & Ashburn RJ Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol 64, 279–286 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaitkevicius K et al. A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc. Natl Acad. Sci. USA 103, 9280–9285 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun S, Tay QXM, Kjellberg S, Rice SA & McDougald D Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms. ISME J 9, 1812–1820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorian P et al. Pyomelanin produced by Vibrio cholerae confers resistance to predation by Acanthamoeba castellanii. FEMS Microbiol. Ecol 93, fix147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, Kjelleberg S & McDougald D Relative contributions of Vibrio polysaccharide and quorum sensing to the resistance of Vibrio cholerae to predation by heterotrophic protists. PLoS ONE 8, e56338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casper-Lindley C & Yildiz FH VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J. Bacteriol 186, 1574–1578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobling MG & Holmes RK Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol 26, 1023–1034 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Pratt JT, McDonough E & Camilli A PhoB regulates motility, biofilms, and cyclic di-GMP in Vibrio cholerae. J. Bacteriol 191, 6632–6642 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller VL & Mekalanos JJ Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc. Natl Acad. Sci. USA 81, 3471–3475 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chourashi R et al. Role of a sensor histidine kinase ChiS of Vibrio cholerae in pathogenesis. Int. J. Med. Microbiol 306, 657–665 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Klose KE & Mekalanos JJ Differential regulation of multiple flagellins in Vibrio cholerae. J. Bacteriol 180, 303–316 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrell DS & Camilli A Regulation of Vibrio cholerae genes required for acid tolerance by a member of the ‘ToxR-like’ family of transcriptional regulators. J. Bacteriol 182, 5342–5350 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperandio V, Girón JA, Silveira WD & Kaper JB The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect. Immun 63, 4433–4438 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohlner J, Meyer TF, Jalajakumari MB & Manning PA Nucleotide sequence of ompV, the gene for a major Vibrio cholerae outer membrane protein. Mol. Gen. Genet 205, 494–500 (1986). [DOI] [PubMed] [Google Scholar]
- 33.Hankins JV, Madsen JA, Giles DK, Brodbelt JS & Trent MS Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc. Natl Acad. Sci. USA 109, 8722–8727 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W et al. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl Acad. Sci. USA 96, 1071–1076 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toma C & Honma Y Cloning and genetic analysis of the Vibrio cholerae aminopeptidase gene. Infect. Immun 64, 4495–4500 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirn TJ, Jude BA & Taylor RK A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438, 863–866 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Waldor MK & Mekalanos JJ Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL & Wai SN Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS ONE 4, e6734 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lomma M et al. The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell Microbiol 12, 1272–1291 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Mathur J & Waldor MK The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect. Immun 72, 3577–3583 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Provenzano D, Schuhmacher DA, Barker JL & Klose KE The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun 68, 1491–1497 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merrell DS, Bailey C, Kaper JB & Camilli A The ToxR-mediated organic acid tolerance response of Vibrio cholerae requires OmpU. J. Bacteriol 183, 2746–2754 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell TL et al. Upper gastrointestinal pH in seventy-nine healthy, elderly North American men and women. Pharm. Res 10, 187–196 (1993). [DOI] [PubMed] [Google Scholar]
- 44.Östling J et al. in Starvation in Bacteria (ed. Kjelleberg S) 169–174 (Springer, 1993). [Google Scholar]
- 45.Jacobs ME et al. The Tetrahymena thermophila phagosome proteome. Eukaryot. Cell 5, 1990–2000 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinchen JM & Ravichandran KS Phagosome maturation: going through the acid test. Nat. Rev. Mol. Cell Biol 9, 781–795 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duperthuy M et al. The major outer membrane protein OmpU of Vibrio splendidus contributes to host antimicrobial peptide resistance and is required for virulence in the oyster Crassostrea gigas. Environ. Microbiol 12, 951–963 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Mathur J, Davis BM & Waldor MK Antimicrobial peptides activate the Vibrio cholerae σE regulon through an OmpU-dependent signalling pathway. Mol. Microbiol 63, 848–858 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Wibbenmeyer JA, Provenzano D, Landry CF, Klose KE & Delcour AH Vibrio cholerae OmpU and OmpT porins are differentially affected by bile. Infect. Immun 70, 121–126 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duperthuy M et al. Use of OmpU porins for attachment and invasion of Crassostrea gigas immune cells by the oyster pathogen Vibrio splendidus. Proc. Natl Acad. Sci. USA 108, 2993–2998 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurman J, Drinkall J & Parry JD Digestion of bacteria by the freshwater ciliate Tetrahymena pyriformis. Aquat. Micro. Ecol 60, 163–174 (2010). [Google Scholar]
- 52.Horton RM, Hunt HD, Ho SN, Pullen JK & Pease LR Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77, 61–68 (1989). [DOI] [PubMed] [Google Scholar]
- 53.Dalia AB, McDonough E & Camilli A Multiplex genome editing by natural transformation. Proc. Natl Acad. Sci. USA 111, 8937–8942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tischler AD & Camilli A Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun 73, 5873–5882 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.