Abstract
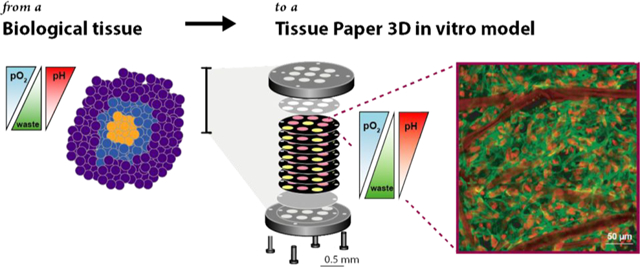
Paper-based scaffolds support the three-dimensional culture of mammalian cells in tissue-like environments. These Tissue Papers, a name that highlights the use of materials obtained from (plant) tissue to generate newly functioning (human) tissue structures, are a promising analytical tool to quantify cellular responses in physiologically relevant extracellular gradients and coculture architectures. Here, we highlight current examples of Tissue Papers, commonly used methods of analysis, and current measurement challenges.
GRAPHICAL ABSTRACT
The lateral flow immunoassay that is the workhorse of over-the-counter pregnancy tests utilizes paper strips to wick analyte-containing fluids over capture antibodies, immobilized in defined locations for an easily interpretable (often colorimetric) readout. These tests have not significantly evolved since their initial design and commercialization in 19851,2 but highlight the level of selective bioanalysis that is possible with the appropriate combination of reagents and support materials. Research focused on paper as a support material for analytical assays and clinical diagnostics has exploded since its reintroduction by Whitesides and Yager in the early 2000s as a medium for performing low-cost diagnostics.3–6 The incorporation of microfluidic-inspired architectures has resulted in a number of single-input paper devices capable of performing multistep procedures to separate and analyze biological and environmental samples, in both serial and parallel fashions.7–11
Paper can also support cell culture, allowing for prolonged incubation periods and bioanalysis of mammalian cells. The paper not only acts as a support material for the cells but also provides a three-dimensional (3D) environment that more accurately mimics the structural and chemical aspects of in vivo tissues than two-dimensional (2D) monolayers of cells on a glass or plastic substrate. This Feature highlights creative uses of paper for the assembly and analysis of 3D cultures, each demonstrating paper’s utility as a platform for investigating biochemical questions about cellular regulation, generating tissue-inspired structures, and serving as an implantable scaffold for tissue regeneration.
NEED FOR NEW 3D CULTURE FORMATS
3D cultures are prepared by depositing cells into porous scaffolds, suspending them in biomimetic hydrogels, or by forming cellular aggregates such as the spheroid depicted in Figure 1A.12–14 These setups provide tissue-relevant structural aspects, including an increased number of cell–cell contacts over monolayer structures as well as the increased production and modification of extracellular matrixes needed to regulate proliferation and promote differentiation.15–18 Bissell showed that placing mammary epithelial cells in a collagen overlay resulted in polarization and the formation of acini-like structures, neither of which are observed in 2D.19 They also showed that mammary epithelial cells suspended in collagen secreted casein, which was not detected in monolayer cultures.20
Figure 1.
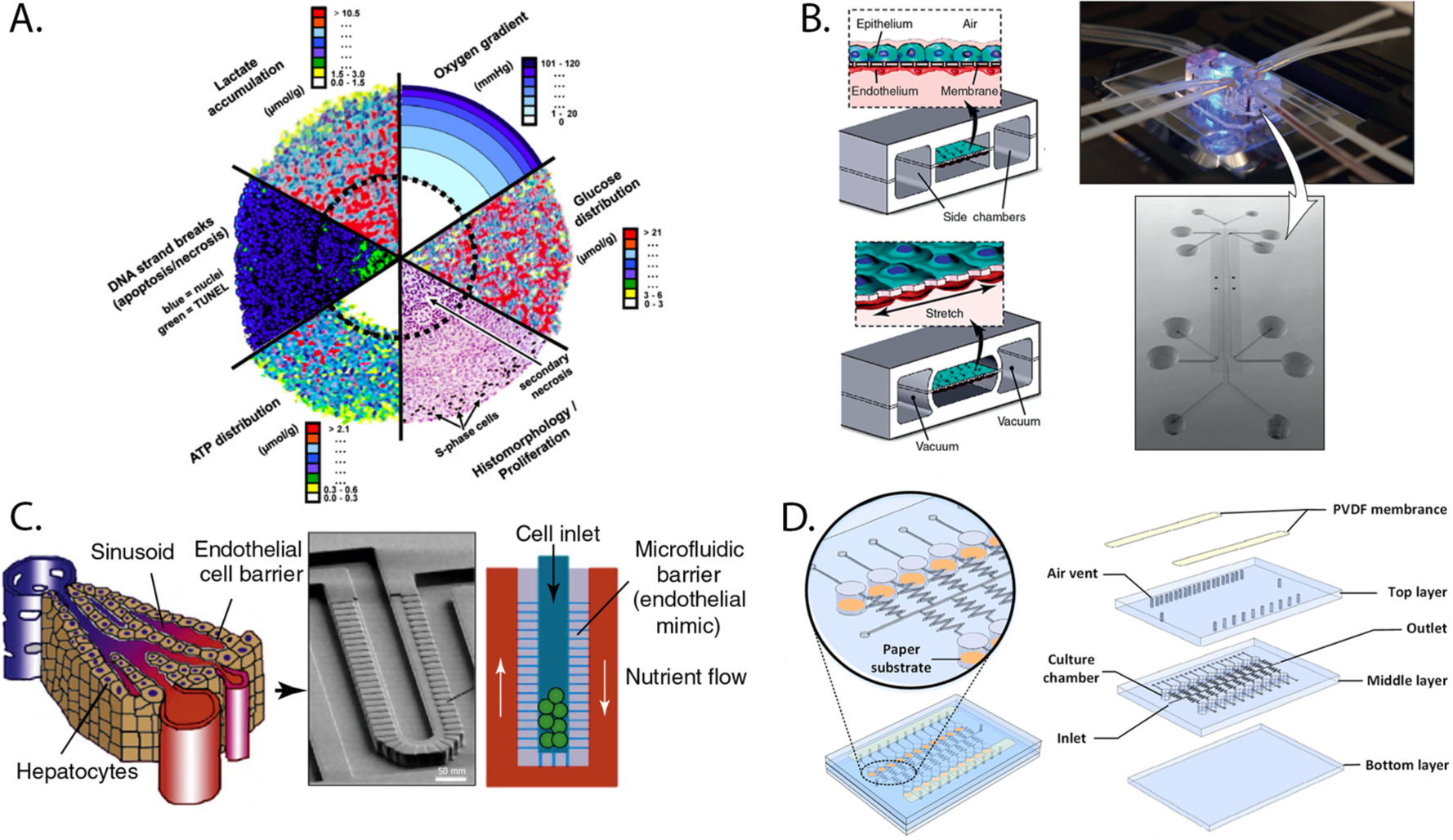
Various preparations of 3D cultures. (A) Compilation of images displaying the gradients of proliferation, viability, oxygen, and nutrients that form across spheroids. Part A was reproduced with permission from ref 12. Copyright 2010 Elsevier. (B) Schematic of a lung-on-a-chip model containing a coculture of human alveolar epithelial cells and pulmonary capillary endothelial cells on the opposite sides of a membrane (left), image of the assembled device (top right), and scanning electron micrograph of the device before assembly (bottom right). (C) A liver-on-a-chip device that uses a series of microfabricated posts to mimic the endothelial barrier in the liver sinusoid, separating hepatocytes from a surrounding flow channel. Parts B and C were adapted with permission from ref 30. Copyright 2011 Elsevier. (D) Three-layer PDMS/paper hybrid microfluidic device used for uropathogen testing. The orange areas (left) contain the cellular culture chambers in paper. Part E was reproduced with permission from ref 97. Copyright 2016 American Chemical Society.
3D culture setups also provide tissue-relevant chemical aspects, in particular, gradients of soluble factors that arise from diffusional limitations at the interface of the culture medium and the cell-containing construct.21–23 Monotonic gradients of oxygen and nutrients extend into the culture as a result of cellular consumption outpacing the rates of diffusion. Gradients of waste products and signaling molecules also form as cellular production outpaces removal. These gradients account for the phenotypic differences observed between cells cultured in 2D and 3D environments. For example, early work by Sutherland showed that spheroids were less sensitive to ionizing radiation than monolayers of the same cell type.24–26 This decreased sensitivity can be attributed to oxygen gradients spanning the spheroids, with low-oxygen regions at the center of the aggregate being less susceptible to DNA-damage than cells in the outer, well-oxygenated rim.
Despite the increased physiological relevance of 3D culture formats, cell-based assays continue to rely on monolayer cultures, due in part to the convenience of well plate formats. Well plates have standardized dimensions for easy loading of cells or exchanging of culture medium with multichannel micropipettes or automated liquid handlers. Cellular contents are commonly analyzed with plate readers via luminescence-based readouts; cellular morphology and protein localization are assessed with high-content imaging platforms. While spheroids can also be prepared and dosed in a similar high-throughput manner,27–29 spatially resolved analyses can require extensive sample preparation and histological slicing, both of which have proven difficult to automate.
Microfabricated devices accommodate 3D cultures by filling channels with cell-laden biomimetic hydrogels or by selectively placing preformed spheroids or organoid structures in the device (Figure 1B–D).21,22,30–32 Organ-on-a-chip devices contain tissue-inspired structures, incorporating multiple cell types to generate functional tools capable of systemically evaluating drug toxicity or recapitulating physiological events such as the menstrual cycle.30,33–36 Despite their modular nature, highly parallelized multiorgan devices that are akin to well plates have yet to be demonstrated. The specialized equipment and engineering expertise needed to set up, maintain, and analyze cells in microfabricated devices has also impeded their adoption by tissue culture laboratories.37,38
Paper-based cultures hold the potential to revolutionize the preparation and analysis of tissue-like structures. We refer to these cultures as Tissue Papers, an appropriate name as the setups use materials from (plant) tissue to generate functional (human) tissue structures. In Tissue Papers, cells or cell-laden hydrogels are seeded directly into paper-based scaffolds with readily available lab equipment (e.g., a micropipette). The patterning and chemical modification strategies developed for paper-based microfluidics are translatable, generating defined cell culture regions or enhancing cellular attachment to the cellulose fibers. The scaffolds, which can be stacked to form thick structures composed of multiple cell types or extracellular matrixes, are suitable for prolonged culture periods and are compatible with many standard cell-based analyses. Unlike other 3D culture platforms, cells can be readily retrieved from the scaffolds without fixation, allowing for spatially resolved characterization or further culture.
CONSTRUCTING PAPER-BASED CULTURES
In the first example of paper-based cultures, Whitesides seeded 200 μm-thick sheets of chromatography paper with cell-laden Matrigel.39 The examples highlighted throughout this Feature use a variety of paper types, resulting in cultures of varying thickness. We, for example, regularly use 40 μm-thick sheets of lens cleaning tissue paper.40–43 Regardless of its thickness, the paper is crucial to generate 3D culture environments, serving as a scaffold capable of supporting the cell-laden hydrogel slabs, which are prone to cracking or breaking upon manipulation.
In addition to supporting cell-laden hydrogel slabs, the cellulose fibers can also support the cells directly. Qin noted human-induced hepatocytes (hiHEPs) selectively formed spheroid-like aggregates in high fiber density regions of filter paper scaffolds.44 This work also showed that endothelial cells (HUVECs) readily attached to the fibers through a nonligand mediated process. Others demonstrated that the topography and chemical composition of unmodified fibers affect cell-scaffold interactions.45,46 Cho found that adipose-derived stem cell culture was compatible with a number of paper sources, but the cells only attached to the fibers of the weighing paper.46 Qin showed that cardiomyocytes were sensitive to texture and chemical composition of the paper source, readily forming monolayers of beating cells on printer and filter paper but not on nitrocellulose membranes.45 They also found that only cardiomyocytes on printer paper exhibited differentiated morphologies, suggesting an inherent benefit of certain fiber microstructures.
These observations highlight the importance of selecting the appropriate scaffold, as each paper type is more than a collection of cellulose fibers. Parameters such as fiber density, surface area, and chemical composition are likely to affect cellular behavior and, therefore, must be considered and optimized for each culture application. Systematic studies into the bulk properties of different paper sources have shown that these fiber characteristics have a profound influence on the paper’s ability to wick fluids and maintain a high wet strength.8
Defining Culture Volume with Thickness, Porosity, and Patterning.
Porosity provides an easy but misleading metric for comparing paper sources as it offers little insight into the number of cells that can be accommodated in a given volume. Laiwattanapaisal illustrated the importance of considering a scaffold’s average pore size with suspensions of mouse melanoma cells, which have an average diameter of 10 μm.47 These cells readily permeated into papers whose pore size was greater than 11 μm but formed monolayers atop scaffolds with smaller pore sizes. Choosing a paper with very large average pore sizes can also have its limitations if there are too few fibers to reinforce the gels, limiting the scaffold’s ability to retain cells.
The total number of cells a paper scaffold can accommodate is defined by its average pore size, thickness, and the length and width of its borders. These borders can be defined by cutting the scaffold to a specific size with a laser, a craft cutter, scissors, or a hole punch. Demko cut Whatman papers into shapes that fit into commercial well plates (Figure 2A).48,49 Cho cultured adipose-derived stem cells in paper scaffolds that were cut with scissors into the shape of a calvarial bone defect.46
Figure 2.
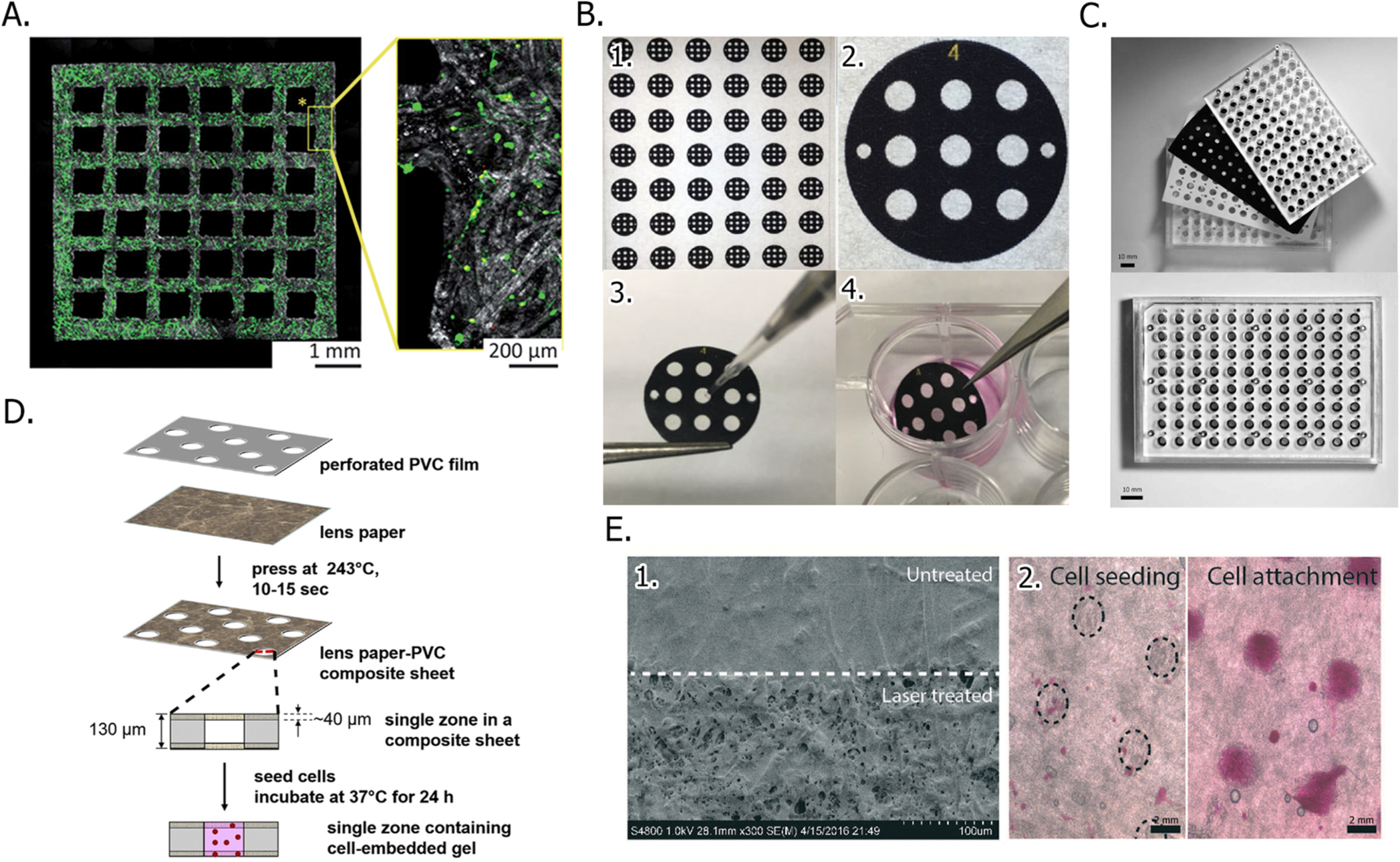
Cutting and patterning of paper scaffolds to define cell culture regions. (A) Fluorescence image of a cortical neuron-containing paper scaffold, which was prepatterned with a laser cutter. Cells were stained prior to imaging. Viable cells are green, and dead cells are red. Part A was reproduced with permission from ref 48. Copyright 2017 The Royal Society of Chemistry. (B) Patterning procedure in which (1) a sheet of Whatman 105 paper is wax patterned with a large number scaffolds. (2) Close-up of a single scaffold, which is 3.0 mm in diameter. (3) Cell-containing Matrigel suspension is seeded into the culture zones with a micropipet. (4) Cell-containing scaffolds are incubated in culture medium until needed. Part B was adapted with permission from ref 41. Copyright 2017 John Wiley and Sons. (C) Top-view of a 96-zone, paper-based screening assay format, before (top) and after (bottom) assembly. Part C was adapted with permission from ref 52. Copyright 2018 American Chemical Society. (D) Schematic of a PVC-Whatman 105 paper hybrid scaffold fabrication process. Part D was adapted with permission from ref 58. Copyright 2016 John Wiley and Sons. (E) Laser ablation-patterned parchment paper with a series of hydrophilic zones, imaged with (1) a scanning electron microscope and (2) a brightfield microscope after the hydrophilic zones were seeded with cells. Part E was adapted with permission from ref 63. Copyright 2016 The Royal Society of Chemistry.
Borders can also be defined with patterning techniques.3,50,51 Patterning generates chemically isolated zones that support individual cultures. Whitesides first published on wax-patterned paper scaffolds for cell culture in 2009.39 We adopted this wax printing method to pattern Whatman 105 lens paper. With a combination of patterning and cutting, we prepare scaffolds containing between 1 and 96 zones (Figure 2B,C).40,52–54 While wax patterning is accessible to anyone with the appropriate printer (e.g., a Xerox ColorQube printer), the wax is both temperature and solvent sensitive, requiring ethylene oxide treatment or prolonged exposure to UV light for sterilization.
Patterning techniques that overcome the limitations of wax are available but require access to photolithography or microcontact printing equipment. Kang used SU-8 photoresist to pattern scaffolds with culture zones connected to a series of channels that formed a gradient mixer,55 while He employed flash foam stamp lithography to transfer PDMS from a stamp directly onto the paper.56 Derda developed a Teflon patterning technique that supported the organic solvent-intensive synthesis of peptides as well as cell culture in a single paper scaffold.57
Hybrid scaffolds, which incorporate sheets of paper into patterned thermoplastic films, also forego the need for wax printing but require multistep procedures. Whitesides used a heated pneumatic press to generate scaffolds by sandwiching a polyvinyl chloride (PVC) sheet, cut with a steel-rule die to make a predetermined pattern of void spaces, between two sheets of Whatman 105 paper (Figure 1D).58,59 In this design, the PVC prevented chemical crosstalk between zones and the paper sheets ensured that the cell-laden gels were retained within the voids of the PVC. Xue prepared 96-well hybrid scaffolds using a similar approach, hot molding Kimwipes into a precut paraffin wax film.60
Chemical Modification of Cellulose to Improve Cellular Viability and Promote Differentiation.
Chemical modification of the scaffolds can further increase their functionality, promoting cellular attachment to the fibers and increasing its overall durability. These modifications range in complexity from coating the cellulose fibers uniformly to imparting specific functional groups in a spatially resolved manner. Demko found that coating oxygen plasma-sterilized paper scaffolds with laminin guided the formation of neurite and axonal projections along the paper fibers (Figure 2A).48 When cultured in untreated scaffolds, the neurons clumped and failed to form these extended networks. Voros found that paper fibers coated with Matrigel contained twice as many astrocytes as fibers coated with laminin or fibronectin.49
Maiti functionalized paper by attaching PAMAM dendrimers onto cellulose fibers with glutaraldehyde linkages.61 The dendrimers increased both the hydrophilicity and the roughness of the scaffolds, promoting adhesion as well as increased cellular health markers in hepatoma (HepG2) cells. Using standard peptide chemistries, Derda synthesized a library of integrin-binding peptide motifs directly into the cell culture regions of a patterned paper scaffold.57,62 Using a combinatorial synthesis approach, they were able to identify peptide sequences that could potentiate cellular differentiation or migration.
Solution-phase reactions limit the throughput of scaffold modification, requiring large volumes of reagent as well as multistep wash procedures to ensure the removal of unreacted analytes. Gas-phase chemistries eliminate the wash steps and the need to dry newly modified scaffolds before usage. Using a laser ablation technique, Ziaie patterned parchment paper with a series of hydrophilic zones (Figure 2E).63 The laser increased the roughness of the 60-μm thick scaffold by 125% and the number of surface-bound hydroxyl and carbonyl groups. Initiated chemical vapor deposition (iCVD) can incorporate multiple functional groups onto paper scaffolds by introducing gaseous reagents in a serial fashion. Cho polymerized a perfluorodecyl acrylate molecule directly onto the cellulose fibers with iCVD to generate a hydrophobic scaffold.46 The scaffolds were further reacted with a glycidyl methacrylate, which was thought to react with cell surface proteins. Hwang also used iCVD to increase the hydrophobicity of the paper scaffolds by attaching a poly(styrene-co-maleic anhydride) layer that was further reacted with poly-L-lysine.64
Increasing Culture Complexity by Imparting Defined Extracellular Gradients.
All in vivo tissues contain over-lapping gradients that extend radially from blood or lymphatic vessels. In healthy epithelial tissues, blood vessels are spaced at approximately 100-μm intervals to ensure an adequate supply of oxygen and nutrients while limiting prolonged exposure to waste products.65,66 These gradients become exaggerated in ischemic tissues or in poorly vascularized tumors, where the vessels are aberrant and leaky.
Controlling and quantifying the gradients that form across cell-containing structures is challenging from both an engineering and measurement perspective but necessary to ensure the culture maintains a physiologically relevant environment. In monolayer cultures with high densities of cells, pericellular hypoxia and nutrient depletion occur as the rate of cellular consumption outpaces the rate of oxygen diffusion. Continuous perfusion systems alleviate these stresses through a constant exchange of nutrients and waste products between the culture and flowing medium. Systematic studies of culture conditions in cell-containing microchannels found that the rate of medium exchange can result in shear stress-induced phenotypes, detachment, and even death in monolayer cultures.67,68 The incorporation of extracellular matrixes or biomimetic hydrogels reduces the susceptibility of cells to shear stress but cannot eliminate gradient formation across the cell-containing structures, due to the diffusion-limited steady-state exchange between the flowing medium and culture.
Relying on the wicking nature of paper, continuous perfusion systems have been generated with cell-containing scaffolds. In these setups (Figure 3), capillary action delivers a steady supply of fresh medium to the scaffolds. In one example, Kang patterned paper scaffolds with a network of serpentine channels, capable of generating a gradient of doxorubicin concentrations that were introduced to five cell-containing zones.55 Takeda and He independently generated self-replenishing perfusion setups.56,69 He used a computer-controlled system to supply medium to three separate scaffolds, each containing murine fibroblasts. While fibroblast viability was comparable in the static and flow-based culture setups, the rate of proliferation in the flow-based cultures was significantly higher. Ziaie continually perfused lung adenocarcinoma cells in a paper scaffold by placing it atop an open channel microfluidic device.63 When compared to static cultures, continuous perfusion promoted the expression of zonula occludens-1, a tight-junction protein associated with the epithelial barrier function found in the lung.
Figure 3.
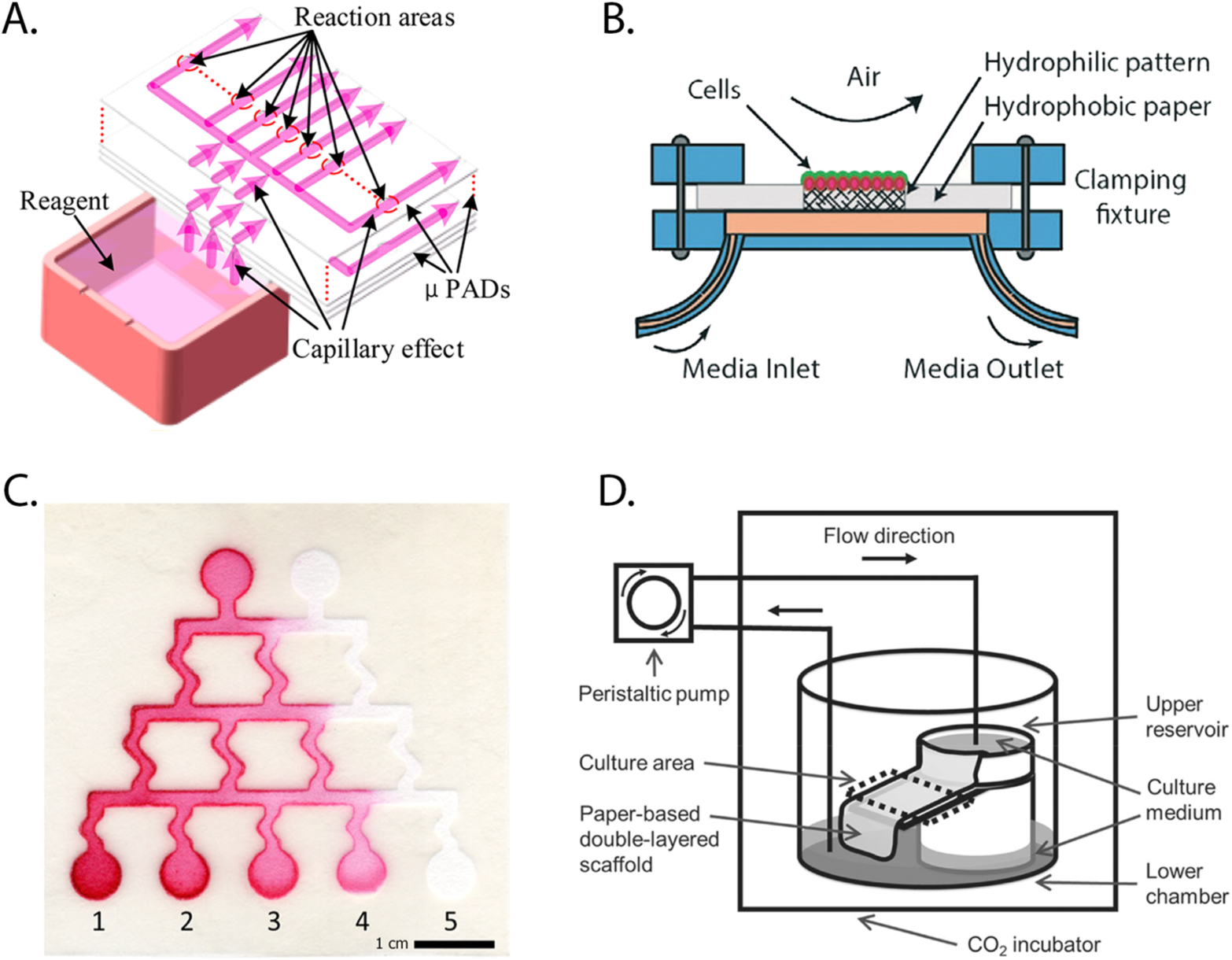
Perfusion-based setups, supplying Tissue Papers with a continuous supply of fresh culture medium. These setups rely on: (A) Capillary action of paper substrates to deliver medium of a fixed composition to cell-containing regions. Part A was reproduced with permission from ref 56. Copyright 2017 American Chemical Society. (B) Exchange with medium of a fixed composition passing under a cell-containing region in a microfabricated device. Part B was reproduced with permission from ref 63. Copyright 2016 The Royal Society of Chemistry. (C) Capillary action through serpentine channels, which mix input components to supply a gradient of concentrations to cell-containing regions. Part C was reproduced with permission from ref 55. Copyright 2016 Springer. (D) Gravity-based flow to wick medium of a fixed composition from one reservoir to the next. Part D was reproduced with permission from ref 69. Copyright 2016 IOP Publishing.
In our work, we impose monotonic gradients across the Tissue Paper structures by limiting exchange with the culture medium.41,70 To maintain gradients of oxygen and nutrients throughout the experiment, the cell-containing scaffolds are placed in custom-made holders that ensure one end is in direct contact with the culture medium while the other is blocked by a polyethylene terephthalate (PET) film. By replacing the PET films with a thin sheet of PDMS, which is highly gas permeable, we can decouple the oxygen and nutrient gradients.71,72 We also have modulated the steepness and range of these consumption-based gradients by changing the density of cells in each scaffold or changing the overall size of the cell-containing regions.
We have shown that oxygen gradients form in our culture setups with numerous assays, including Pimonidazole staining, the stabilization and transcriptional activation of hypoxia inducible factor 1 alpha (HIF1α), and oxygen-sensitive luminescent films.70,71 Biological readouts provide a semi-quantitative correlation of change in cellular response as a function of oxygen concentration. The oxygen-sensitive films are quantitative between 0–150 mmHg and are particularly sensitive in physiologically relevant oxygen concentrations. With these films, we showed that by decreasing the density of cells in a 12-scaffold colon carcinoma stack 4-fold, the range of oxygen tensions that spanned the culture after 24 h increased from 8.3–0.1 mmHg to 117–5.4 mmHg.40
DIVERSITY OF TISSUE PAPER APPLICATIONS
Tissue Papers are filling an important gap in 3D culture techniques, providing an experimentally accessible platform to (1) probe cellular responses and biochemical regulation in physiologically relevant tissue microenvironments and (2) evaluate cellular differentiation and potential implantation of induced human stem cells.
Tissue Papers to Quantify Cellular Invasion.
The development and maintenance of tissue architectures, whether healthy or malignant, rely on cellular movement. 3D cultures have proven invaluable as models to tease apart the role of extracellular cues in regulating cellular movement. We have developed three different invasion assay formats using paper-based scaffolds (Figure 4).
Figure 4.

Paper-based invasion assays. (A) Photograph of a single-zone scaffold (left), which fits directly into a commercial 96-well plate. Schematics of a paper-based version of the Transwell assay in which invasive and nonmotile cells are separated in the presence of chemokines (top) or cocultures (bottom). Part A was adapted with permission from ref 54. Copyright 2019 The Royal Society of Chemistry. (B) Schematic of an invasion stack in which cells were separated by the distance they travel in the presence of an oxygen gradient (left). Representative data set of cell distribution throughout the stack after 48 h, quantified with a qPCR assay. Part B was adapted with permission from ref 43. Copyright 2015 American Chemical Society. (C) Schematic of channel-based invasion assay in which cellular movement along in an oxygen gradient was tracked in situ with an inverted fluorescence microscope (left). Representative image of cellular distribution in the channel (top, right) and data sets summarizing movement after 48 h (bottom, right). Part C was adapted with permission from ref 53. Copyright 2016 The Royal Society of Chemistry.
Using single zone scaffolds that fit directly into the wells of a commercial 96-well plate, we separated invasive and nonmotile cells in mono- and coculture setups with a paper-based version of the Transwell assay (Figure 4A).54 Whatman 105 scaffolds containing a single zone surrounded by a wax border remained at the air–liquid interface throughout the experiment. The invasive cells were collected at the bottom of the well plate and enumerated with a widefield microscope; the noninvasive cells that remained in the paper scaffold were quantified with fluorescence imaging or other viability assays. First, we compared traditional and paper-based Transwell formats. In both setups, we observed identical trends of statistically significant increases in MDA-MB-231 cell invasion with increasing concentrations of fetal bovine serum. In subsequent coculture experiments, we showed that MCF7 cell invasion increased in the presence of reductive mammary fibroblasts. The extent of this invasion increased with decreasing distance between the two cell types, a confirmation of a concentration-dependent signaling response. Whitesides also found that including tumor-associated fibroblasts in Tissue Paper structures influenced the extent and direction of patient-derived lung carcinoma cell invasion.59
Unlike the Transwell assay, the invasion stack format can segment cell populations based on the distance invaded (Figure 4B).41–43 Each invasion stack consists of a cell-containing scaffold sandwiched between layers containing only hydrogel. After incubation, each layer is separated and the cells quantified. In the invasion stacks, we found that cells with increased metastatic potential in xenograft models had higher numbers of invasive cells than those with low metastatic potential.42,43,71 We also discovered that multiple cell lines preferentially invade into regions of higher oxygen and nutrient concentrations.41–43,70 By decoupling the oxygen and nutrients gradients in our setups, we determined that oxygen is the primary driving force behind this selective movement. Cellular migration studies in microfluidic devices show cells migrate toward regions of higher oxygen in some setups but away from that source in others.73–75 Although it is difficult to definitively say if oxygen is a chemoattractant, these conflicting results highlight that context is critical for predicting cellular responses in vivo. Further studies, which systematically compare cellular movement in imposed and consumption-generated oxygen gradients of different steepness, are needed to elucidate oxygen’s role in directing invasion.
The invasion stacks are limited to end-point analyses with fluorescence microscopy and imaging techniques, due to the highly scattering nature of the cellulose fibers. To track cellular movement in a time-resolved manner along extracellular gradients, we patterned single sheets of Whatman 105 lens paper with three 11.0 mm × 2.5 mm channels (Figure 4C).53 In these experiments, we used a center-of-mass algorithm to track the movement of cell populations engineered to constitutively express a fluorescent protein. With a limited number of images taken over a 48 h period, we confirmed our observations from the invasion stacks that oxygen was the major driver of directional movement. Increased time-resolution snapshots are needed to determine if cells are undergoing a chemotactic (directed) response to the oxygen gradient.
Tissue Papers to Model the Tumor Microenvironment.
Much like the invasion stacks described above, tissue stacks are multilayer structures of cell-containing scaffolds. Monoculture stacks have evaluated cellular responses to chemotherapies and ionizing radiation along the oxygen and nutrient gradients that form in poorly vascularized tumors.40,58,76 This setup has several advantages over the commonly used spheroid model. First, tissue stacks can be assembled a few hours after seeding whereas spheroids can require several weeks of culture to achieve structures with millimeter diameters. Second, the stacked structures can accommodate any cell type, where spheroids are limited to cells capable of forming tight cell–cell interactions. Of the 60 human cell lines used by the National Cancer Institute to evaluate potential anticancer drugs, only 26 are capable of forming spheroids in a liquid overlay setup.27 Finally, the tissue stacks have a level of experimental control unattainable in spheroids. The gradients that span the tumor stacks can be modulated by varying the number of layers, seeding density, and by manipulating access to nutrients and oxygen. This level of control enables fundamental studies of the microenvironment’s impact on cellular phenotype.
Whitesides first demonstrated tissue stacks,39 generating a 1600 μm-thick structure composed of eight, breast adenocarcinoma cell-containing layers. Using a similar tissue stack structure containing a patient-derived lung carcinoma cell line, they showed a direct relationship between resistance to ionizing radiation and distance from the nutrient source.58 These resistant cell populations correlated with hypoxia-associated phenotypes, including a decreased rate of proliferation and stabilization of HIF1α. Sutherland found similar results with spheroid models, where the poorly oxygenated center regions had the greatest chemo- and radiation-resistance.77–79
We characterized the oxygen gradients that form across tissue stacks containing colon carcinoma cells40 with culture-compatible luminescent thin films developed in our lab.71 Due to their reduced proliferation, cells in hypoxic regions were resistant to SN-38, the active metabolite of the neoplastic agent irinotecan. The Tissue Roll for the Analysis of Cellular Environment and Response (TRACER) system developed by McGuigan (Figure 5) also generates tumorlike environments.80–82 TRACER cultures are composed of a long strip of paper patterned with cell-containing regions, which align to form a stacked structure, similar to those employed by our lab, when wrapped around a spindle. Using liquid chromatography–mass spectrometry (LC–MS), they found that increasing the distance of ovarian adenocarcinoma cells from the nutrient source resulted in an increased reliance on glycolysis and higher levels of oxidized fatty acids.80 In a coculture setup of squamous carcinoma cells and cancer-associated fibroblasts, they also showed that fibroblast-associated paracrine signaling increased the resistance of cancer cells to ionizing radiation in normoxic and hypoxic regions.82
Figure 5.

TRACER culture platform developed by McGuigan. (A) Workflow of the setup in which (1) cell-laden gels are seeded into a paper strip, (2) rolled around an oxygen impermeable spindle, (3) incubated in medium, and (4) unrolled and analyzed. (B) Schematic of the assembled TRACER setup, highlighting the formation of gradients across the culture. Parts A and B were reproduced with permission from ref 80. Copyright 2016 Nature Publishing Group.
Tissue Papers to Model Cardiac Tissue.
Three stacked structures, each representing different aspects of cardiac tissue,83–86 further highlight the adaptability of Tissue Papers. With cocultures composed of neonatal rat cardiomyocyte- and cardiac fibroblast-containing Whatman 114 chromatography paper scaffolds, Whitesides modeled the response of stromal cells to cardiac ischemia.83 This work showed that fibroblast invasion increased with increasing levels of ischemic damage in cardiomyocytes. Grande-Allen modeled different regions of an adult aortic valve leaflet by stacking different numbers of Whatman 114 scaffolds containing aortic valvular interstitial cells (VICs).84 These studies confirmed the importance of hypoxia in the calcification process associated with hardening of the arteries, as smooth muscle alpha-actin expression in the VICs increased with increasing distances from the medium. In another model, Qin differentiated human pluripotent stem cells into cardiomyocytes on printer and filter paper scaffolds, which were able to maintain the differentiated and beating cells for up to 3 months.45
Tissues Papers for Implantation.
Bacterial- and plant-derived cellulosic materials do not elicit an inflammatory response when implanted in animal models.85,86 Two recent examples demonstrate the feasibility of implanting Tissue Papers to repair tracheal injuries and bone defects. In one example, Hwang showed that origami structures of poly-L-lysine modified paper scaffolds supported the prolonged culture of chondrocytes (Figure 6A).64 When transplanted properly into a three-ring defect trachea in rabbits, these chondrocyte-containing scaffolds provided a tight seal without the need for sutures and showed no tissue granulation or stenosis for up to 4 weeks after implantation. In the other example, Cho implanted tissue stacks containing alternating layers of adipose-derived stem cells and endothelial cells (HUVECs) into mice with calvarial (skull) fractures (Figure 6B).46 In these stack structures, the stem cells differentiated into cells with osteogenic markers. When implanted, they readily formed bonelike structures that were indistinguishable from the original bone in micro-CT scans.
Figure 6.

(A) Schematic of fabrication and usage of origami-style Tissue Papers developed by Hwang to repair three-ring defect tracheas in rabbit models. Scaffolds were chemically modified with an iCVD process. Prior to implantation, the scaffolds were loaded with chondrocyte-laden alginate gels. Part A was reproduced with permission from ref 64. Copyright 2015 United States National Academy of Sciences. (B) Poly(glycidyl methacrylate)-modified paper scaffolds cut into the shape of calvarial bone defects, seeded with adipose-derived stem cells and HUVECs, and implanted into the mouse models. (bottom) Micro-CT images of different paper grades, 8 weeks after implantation. Part B was adapted with permission from ref 46. Copyright 2014 Elsevier. (C) Schematic of biomineralized Tissue Papers preparation. Osteoblast-containing collagen gels were seeded into scaffolds, which were prefolded into a particular shape. Part C was reproduced with permission from ref 87. Copyright 2016 Nature Publishing Group.
Whitesides was also able to use paper as a scaffold to engineer bonelike structures in free-standing paper scaffolds (Figure 6C).87 As paper resembles the fibrous structure of collagen in bone and has a porous nature, osteoblasts received the oxygen and nutrients needed for cell growth and calcium phosphate deposition. By folding the paper into different shapes before rolling into a cylindrical structure, they were able to observe how various shapes affected hydroxyapatite deposition. These results further demonstrate the applicability of paper-based cell cultures and are promising for future use of Tissue Papers in a more clinical setting.
MEASUREMENT CHALLENGES IN 3D CULTURES
Quantitatively assessing differences in the average cellular response to a particular stress can be straightforward in both monolayer and 3D cultures. For example, cellular viability or the presence of a particular metabolite can be assessed after cells are lysed and their contents collected. Unlike monolayer cultures where cells are exposed to uniform culture conditions, bulk analyses of 3D cultures cannot account for spatial heterogeneities across the structure. Insights into these heterogeneities are needed to develop more effective treatments capable of targeting a particular cell type or phenotype; they are also important for evaluating the microenvironment’s role in cellular regulation. The diffusion-dominated environments inherent in 3D cultures give rise to a range of cellular phenotypes as well gene, protein, and metabolite profiles. In poorly vascularized tumors, spheroids, and the Tissue Paper tumor stacks, metabolically quiescent and necrotic cells are surrounded by rapidly dividing cells.88
Intercellular signaling also plays a key role in tissue homeostasis and repair, affecting the composition of the extracellular matrix and its soluble components. As demonstrated in many of the examples above, cellular behavior can change drastically when more than one cell type is included. While cocultures increase the biological relevance of tissue models, they offer the added challenge of assigning the source and sink of these spatially and temporally changing signaling gradients.
Visualizing Cells in Paper-Based Scaffolds.
Optical microscopy is the gold standard for assessing cellular morphology and the localization of organelles or proteins. High-content imaging systems automate image collection of monolayer cultures and confocal imaging systems are now available to image spheroids.89–91 We and others image cells in paper scaffolds with fluorescence imagers as well as widefield and confocal fluorescence microscopes. These measurements require the cells to be labeled with a small fluorescent dye, engineered to express a fluorescent protein constitutively, or immunostained with fluorescently labeled antibodies. A confocal laser scanning imager can acquire fluorescence images from a large number of scaffolds in parallel, at a resolution of tens to hundreds of micrometers. The integrated fluorescence intensity over zones that are millimeters in diameter can be related to cell number with the appropriate calibration curves. These low-resolution images can suffer from high background fluorescence caused by the chemical agents used to bleach paper, limiting the ability to detect a small number of cells or low-abundance fluorescently labeled molecules.
Cells in Whatman 105 scaffolds, which are 80% void volume and 40 μm thick, are easily visualized with widefield microscopy. The void volume of the 200-μm thick Whatman 1 chromatography paper is significantly less, making cellular visualization without confocal capabilities difficult. The depth of focus is dependent upon the scaffold’s fiber density, due to their highly scattering nature and the opacity in the visible region. In Tissue Paper structures containing greater than four sheets of Whatman 105, we are unable to visualize cells throughout stack in situ. Physically separating the layers prior to analysis affords high-quality images of cells in each region of the Tissue Stack but prohibits real-time imaging of changes in cellular structure or the tracking of cellular invasion. By changing the format from a stacked structure to a single sheet of Whatman 105 containing millimeter-long channels, we were able to image cellular movement and gradient formation in real-time.53,71,72 Here, images collected on a widefield microscope were stitched together, providing a single image of the 11 mm-long channel. While single sheet cultures allow for real-time imaging, they lack the attractive features of the stacked setups: a straightforward setup, separation, and analysis of individual scaffolds containing different cell types or extracellular matrixes.
Optical methods such as multiphoton fluorescence microscopy, light-sheet microscopy, and optical coherence tomography can visualize cells in spheroid models.90,92–94 These techniques are compatible with highly scattering materials and could conceivably be used to image cells in Tissue Paper stacks in situ. Another option to reduce these spurious fluorescent signals and to combat the limited depth of focus would be to generate a fibrous material that is index matched with extracellular matrixes. Demko demonstrated the feasibility of this approach, applying an index matching fluid to cell-containing paper scaffolds.48 Cellulose fiber scattering was significantly reduced but this technique required the cells to be fixed prior to analysis.
Biochemical Analyses: Targeted and Discovery Mode.
Cells can be removed from the paper scaffolds after enzymatic or nonenzymatic degradation of the extracellular matrix. They can also be lysed directly in the scaffolds, employing reagents commonly used to extract proteins, nucleic acids, or metabolites from intact tissues. Using an HPLC-UV method, He monitored the concentration of paclitaxel in continuously perfused cultures of fibroblasts, relating cellular viability to drug concentration.56 McGuigan compared the concentration of glycolysis-associated metabolites extracted from cells in different regions of an oxygen gradient in the TRACER setup.80 These extracts were separated on a HILIC column and analyzed on an Orbitrap instrument. Alternatively, Lin used a paper-spray mass spectrometry (PSMS) method to ionize and quantify parent drugs and their metabolites from scaffolds containing hepatocytes (HepG2 cells) or cocultures of hepatocytes and fibroblasts.95
These examples further highlight the difficulty of acquiring spatially and temporally resolved information simultaneously from 3D cultures. Sampling techniques such as microdialysis or capillary electrophoresis have the potential to provide both spatially (based on probe placement) and temporally resolved analyses of intercellular signaling or metabolomic changes at the single cell level, but these techniques have yet to be incorporated into paper-based cultures.
Transition from Monolayers to 3D Structures: Finding the Appropriate Method.
Despite the successful examples of quantifying cellular responses in 3D cultures, one must be aware of the challenges of applying an assay optimized for monolayer cultures without thorough characterization and validation. We and others have noted that the potency and the efficacy of small molecule modulators can change when moving from a monolayer to a 3D culture format. In one example, we found the potency of SN-38 decreased 2.3-fold compared to monolayer cultures when HCT116 cells were in the paper scaffolds.40 In another example, the efficacy of ERα agonists decreased when moving from a monolayer to a 3D environment, but the potencies remained the same.52 There are several explanations for these differences. One is the inability of the method to quantify cellular responses accurately. More likely it is because of environmentally induced cellular differences caused by factors such as reduced oxygen or nutrient levels, modulated protein expression and therefore activity, or from a decreased effective concentration due to molecular loss from nonspecific adsorption to the scaffolds or extracellular matrixes. To better understand these differences between 2D and 3D environments, better analytical characterization of the cells and ECM in each culture format is needed. Quantitative -omic assessments of cells would determine how ECM composition and stiffness influence protein expression and pathway regulation. Quantitative partitioning models would allow for future predictions of small molecule loss in these systems due to nonspecific interactions.
Another confounding variable that can lead to differences between monolayer and 3D cultures is the failure to account for extracellular gradients. Proliferation, metabolism, and cellular signaling are affected by oxygen and nutrient concentrations. Eddington recently showed that monolayers of cells placed along an oxygen gradient showed differential activation of the two proteins responsible for regulating hypoxic phenotypes: HIF1α and HIF2 α.96 We also observed these changes in Tissue Paper structures containing breast adenocarcinoma cells.70 The variable conditions cells experience in diffusion-dominated culture environments would benefit most from single-cell analyses. Although such analyses are possible with microscopy, they are limited in scope as cells must be engineered to express fluorescent proteins in a pathway-regulated manner. The number of pathways that can be evaluated simultaneously in fluorescence microscopy is also limited by spectral overlap and interferences that can arise from neighboring cells. On the other hand, flow cytometry is capable of single-cell analysis and can increase the number of proteins that could be analyzed; however, it lacks the spatial and temporal capabilities of microscopy.
CONCLUSIONS AND FUTURE DIRECTIONS
Tissue Papers are modular in construction, combining the simplicity of monolayer culture set up with the gradient control of a microfluidic device. The versatility of paper as a scaffold is evidenced by its ability to quantify microenvironment effects on cellular response as well as its ability to be implanted to promote tissue growth and healing. Each Tissue Paper structure relies on the porous nature of paper, whose fibrous structure not only provides support during prolonged incubation periods but also acts as a guide for cellular growth. The fibers reinforce the thin cell-laden gels the scaffolds contain, allowing them to be easily assembled and disassembled for analysis without loss of or damage to the sample.
There are many measurement challenges that await analytical chemists interested in 3D culture models. The challenges of providing molecular level information with high spatial and temporal resolution are not new, as we have witnessed profound advances in targeted in vivo bioanalysis. Drawing the parallels between in vivo and in vitro analytical challenges is an important reminder that the predictive value of a model is only as good as the measurements that correlate cellular responses. Nevertheless, the variety and widespread use of paper-based platforms are possible due to a common theme. They are made from readily accessible materials, allowing any lab to develop a range of devices whose final complexity and functionality far surpass the simplicity of the starting material.
ACKNOWLEDGMENTS
This work was supported by funded provided by the National Institute of General Medical Sciences through Grant Award Number R35GM128697. We thank Julie McIntosh, Melanie Sinanian, and Nathan Whitman for helpful discussions as we prepared this Feature.
Biographies
Sabrina M. Cramer is a graduate student in the research group of Professor Matthew Lockett in the Department of Chemistry at the University of North Carolina at Chapel Hill. She graduated from Ohio University in 2012 with a B.S. in Forensic Chemistry. Her research focuses on the development of a 3D placental cell model to examine the effect of potentially toxic perfluorinated molecules.
Tyler S. Larson is a graduate student in the research groups of Professor Matthew Lockett and Professor Gary Glish in the Department of Chemistry at the University of North Carolina at Chapel Hill. He graduated from The College of William and Mary in 2018 with a B.S. in Chemistry. His research interests focus on using mass spectrometry to study drug penetration and metabolism in paper-based tumor models.
Matthew R. Lockett is an assistant professor in the Department of Chemistry at the University of North Carolina at Chapel Hill. He earned his B.S. in Chemistry from the University of Pittsburgh in 2004 and his Ph.D. in Chemistry in 2009 from the University of Wisconsin at Madison under the guidance of Lloyd M. Smith. Prior to joining the faculty at UNC, Matthew was a postdoctoral fellow in the laboratory of George M. Whitesides in the Department of Chemistry and Chemical Biology at Harvard University. Current research in the Lockett group focuses on the development of Tissue Paper structures to quantify cellular invasion, identify drug resistance mechanisms, and metabolic regulation in physiologically relevant oxygen gradients. The lab is also developing Tissue Papers to assess potential environmental toxins and their effects on breast cancer development and alteration of the placental barrier function.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Jones G; Kraft A Bus. Hist 2004, 46 (1), 100–122. [Google Scholar]
- (2).Gnoth C; Johnson S Geburtshilfe Frauenheilkd. 2014, 74 (7), 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Martinez AW; Phillips ST; Butte MJ; Whitesides GM Angew. Chem, Int. Ed 2007, 46 (8), 1318–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Martinez AW; Phillips ST; Carrilho E; Thomas SW; Sindi H; Whitesides GM Anal. Chem 2008, 80 (10), 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Nash MA; Hoffman JM; Stevens DY; Hoffman AS; Stayton PS; Yager P Lab Chip 2010, 10 (17), 2279–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Fu E; Liang T; Houghtaling J; Ramachandran S; Ramsey SA; Lutz B; Yager P Anal. Chem 2011, 83 (20), 7941–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Salentijn GIJ; Grajewski M; Verpoorte E Anal. Chem 2018, 90 (23), 13815–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fernandes SC; Walz JA; Wilson DJ; Brooks JC; Mace CR Anal. Chem 2017, 89 (11), 5654–5664. [DOI] [PubMed] [Google Scholar]
- (9).Gong MM; Sinton D Chem. Rev 2017, 117 (12), 8447–8480. [DOI] [PubMed] [Google Scholar]
- (10).Cate DM; Adkins JA; Mettakoonpitak J; Henry CS Anal. Chem 2015, 87 (1), 19–41. [DOI] [PubMed] [Google Scholar]
- (11).Yamada K; Shibata H; Suzuki K; Citterio D Lab Chip 2017, 17 (7), 1206–1249. [DOI] [PubMed] [Google Scholar]
- (12).Hirschhaeuser F; Menne H; Dittfeld C; West J; Mueller-Klieser W; Kunz-Schughart LA J. Biotechnol 2010, 148 (1), 3–15. [DOI] [PubMed] [Google Scholar]
- (13).Kenney RM; Lloyd CC; Whitman NA; Lockett MR Chem. Commun 2017, 53 (53), 7194–7210. [DOI] [PubMed] [Google Scholar]
- (14).Fennema E; Rivron N; Rouwkema J; van Blitterswijk C; de Boer J Trends Biotechnol. 2013, 31 (2), 108–115. [DOI] [PubMed] [Google Scholar]
- (15).Yamada KM; Cukierman E Cell 2007, 130 (4), 601–610. [DOI] [PubMed] [Google Scholar]
- (16).Rianna C; Kumar P; Radmacher M Semin. Cell Dev. Biol 2018, 73, 107–114. [DOI] [PubMed] [Google Scholar]
- (17).Tam RY; Smith LJ; Shoichet MS Acc. Chem. Res 2017, 50 (4), 703–713. [DOI] [PubMed] [Google Scholar]
- (18).Vogel V Annu. Rev. Physiol 2018, 80, 353–387. [DOI] [PubMed] [Google Scholar]
- (19).Hall HG; Farson DA; Bissell MJ Proc. Natl. Acad. Sci. U. S. A 1982, 79 (15), 4672–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lee EYH; Parry G; Bissell MJ J. Cell Biol 1984, 98 (1), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Keenan TM; Folch A Lab Chip 2008, 8 (1), 34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).van Duinen V; Trietsch SJ; Joore J; Vulto P; Hankemeier T Curr. Opin. Biotechnol 2015, 35 (1), 118–126. [DOI] [PubMed] [Google Scholar]
- (23).Rothbauer M; Zirath H; Ertl P Lab Chip 2018, 18 (2), 249–270. [DOI] [PubMed] [Google Scholar]
- (24).Durand RE; Sutherland RM Cancer Res. 1973, 33 (2), 213–219. [PubMed] [Google Scholar]
- (25).Sutherland RL; Inch WR; McCredie JA; Kruuv J Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med 1970, 18 (5), 491–495. [DOI] [PubMed] [Google Scholar]
- (26).Sutherland RM Science 1988, 240 (4849), 177–184. [DOI] [PubMed] [Google Scholar]
- (27).Friedrich J; Seidel C; Ebner R; Kunz-Schughart LA Nat. Protoc 2009, 4 (3), 309–324. [DOI] [PubMed] [Google Scholar]
- (28).Hsiao AY; Tung YC; Qu XG; Patel LR; Pienta KJ; Takayama S Biotechnol. Bioeng 2012, 109 (5), 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Tung YC; Hsiao AY; Allen SG; Torisawa YS; Ho M; Takayama S Analyst 2011, 136 (3), 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Huh D; Hamilton GA; Ingber DE Trends Cell Biol. 2011, 21 (12), 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Jiang WQ; Li MQ; Chen ZZ; Leong KW Lab Chip 2016, 16 (23), 4482–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lee GH; Lee JS; Wang X; Hoon Lee S Adv. Healthcare Mater 2016, 5 (1), 56–74. [DOI] [PubMed] [Google Scholar]
- (33).Benam KH; Dauth S; Hassell B; Herland A.; Jain A; Jang KJ; Karalis K; Kim HJ; MacQueen L; Mahmoodian R; Musah S; Torisawa YS; van der Meer AD; Villenave R; Yadid M; Parker KK; Ingber DE Annu. Rev. Pathol.: Mech. Dis 2015, 10 (1), 195–162. [DOI] [PubMed] [Google Scholar]
- (34).Zhang B; Korolj A; Lai BFL; Radisic M Nat. Rev. Mater 2018, 3 (8), 257–278. [Google Scholar]
- (35).Xiao S; Coppeta JR; Rogers HB; Isenberg BC; Zhu J; Olalekan SA; McKinnon KE; Dokic D; Rashedi AS; Haisenleder DJ; Malpani SS; Arnold-Murray CA; Chen KW; Jiang MY; Bai L; Nguyen CT; Zhang JY; Laronda MM; Hope TJ; Maniar KP; Pavone ME; Avram MJ; Sefton EC; Getsios S; Burdette JE; Kim JJ; Borenstein JT; Woodruff TK Nat. Commun 2017, 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Zhang YS; Aleman J; Shin SR; Kilic T; Kim D; Mousavi Shaegh SA; Massa S; Riahi R; Chae S; Hu N; Avci H; Zhang W; Silvestri A; Sanati Nezhad A; Manbohi A; De Ferrari F; Polini A; Calzone G; Shaikh N; Alerasool P; Budina E; Kang J; Bhise N; Ribas J; Pourmand A; Skardal A; Shupe T; Bishop CE; Dokmeci MR; Atala A; Khademhosseini A Proc. Natl. Acad. Sci. U. S. A 2017, 114 (12), E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sackmann EK; Fulton AL; Beebe DJ Nature 2014, 507 (7491), 181–189. [DOI] [PubMed] [Google Scholar]
- (38).Caicedo HH; Brady ST Trends Biotechnol. 2016, 34 (1), 1–3. [DOI] [PubMed] [Google Scholar]
- (39).Derda R; Laromaine A; Mammoto A; Tang SK; Mammoto T; Ingber DE; Whitesides GM Proc. Natl. Acad. Sci. U. S. A 2009, 106 (44), 18457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Boyce MW; LaBonia GJ; Hummon AB; Lockett MR Analyst 2017, 142 (15), 2819–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lloyd CC; Boyce MW; Lockett MR Curr. Prot. Chem. Biol 2017, 9 (2), 75–95. [DOI] [PubMed] [Google Scholar]
- (42).Mosadegh B; Lockett MR; Minn KT; Simon KA; Gilbert K; Hillier S; Newsome D; Li H; Hall AB; Boucher DM; Eustace BK; Whitesides GM Biomaterials 2015, 52 (1), 262–71. [DOI] [PubMed] [Google Scholar]
- (43).Truong AS; Lochbaum CA; Boyce MW; Lockett MR Anal. Chem 2015, 87 (22), 11263–70. [DOI] [PubMed] [Google Scholar]
- (44).Wang Y; Su W; Wang L; Jiang L; Liu Y; Hui L; Qin J Toxicol. Res 2018, 7 (1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Wang L; Xu C; Zhu YJ; Yu Y; Sun N; Zhang XQ; Feng K; Qin HJ Lab Chip 2015, 15 (22), 4283–4290. [DOI] [PubMed] [Google Scholar]
- (46).Park HJ; Yu SJ; Yang K; Jin Y; Cho AN; Kim J; Lee B; Yang HS; Im SG; Cho SW Biomaterials 2014, 35 (37), 9811–9823. [DOI] [PubMed] [Google Scholar]
- (47).Pupinyo N; Chatatikun M; Chiabchalard A; Laiwattanapaisal W Analyst 2019, 144 (1), 290–298. [DOI] [PubMed] [Google Scholar]
- (48).Dermutz H; Thompson-Steckel G; Forro C; de Lange V; Dorwling-Carter L; Voros J; Demko L RSC Adv. 2017, 7 (62), 39359–39371. [Google Scholar]
- (49).Aebersold MJ; Thompson-Steckel G; Joutang A; Schneider M; Burchert C; Forro C; Weydert S; Han H; Voros J Front. Neurosci 2018, 12, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Coltro WKT; de Jesus DP; da Silva JAF; do Lago CL; Carrilho E Electrophoresis 2010, 31 (15), 2487–2498. [DOI] [PubMed] [Google Scholar]
- (51).Liana DD; Raguse B; Gooding JJ; Chow E Sensors 2012, 12 (9), 11505–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Whitman NA; Lin ZW; DiProspero TJ; McIntosh JC; Lockett M R Anal. Chem 2018, 90 (20), 11981–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kenney RM; Boyce MW; Truong AS; Bagnell CR; Lockett MR Analyst 2016, 141 (2), 661–8. [DOI] [PubMed] [Google Scholar]
- (54).Kenney RM; Loeser A; Whitman NA; Lockett MR Analyst 2019, 144 (1), 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hong B; Xue P; Wu Y; Bao J; Chuah YJ; Kang Y Biomed. Microdevices 2016, 18 (1), 21. [DOI] [PubMed] [Google Scholar]
- (56).Wu Y; Gao Q; Nie J; Fu JZ; He Y ACS Biomater. Sci. Eng 2017, 3 (4), 601–607. [DOI] [PubMed] [Google Scholar]
- (57).Deiss F; Matochko WL; Govindasamy N; Lin EY; Derda R Angew. Chem., Int. Ed 2014, 53 (25), 6374–6377. [DOI] [PubMed] [Google Scholar]
- (58).Simon KA; Mosadegh B; Minn KT; Lockett MR; Mohammady MR; Boucher DM; Hall AB; Hillier SM; Udagawa T; Eustace BK; Whitesides GM Biomaterials 2016, 95 (1), 47–59. [DOI] [PubMed] [Google Scholar]
- (59).Camci-Unal G; Newsome D; Eustace BK; Whitesides GM Adv. Healthcare Mater 2016, 5 (6), 641–647. [DOI] [PubMed] [Google Scholar]
- (60).Zhang L; Sun LH; Hou MM; Xu ZG; Kang YJ; Xue P Biomed. Microdevices 2018, 20 (3), 68. [DOI] [PubMed] [Google Scholar]
- (61).Agarwal T; Rustagi A; Das J; Maiti TK Colloids Surf., B 2018, 172, 346–354. [DOI] [PubMed] [Google Scholar]
- (62).Deiss F; Yang Y; Derda R Parallel synthesis of peptides on Teflon-patterned paper arrays (SyntArrays) In Microarray Technology; Li P, Sedighi A, Wang L, et al. ; Humana Press: New York, NY, 2016; Vol. 1368, pp 249–271. [DOI] [PubMed] [Google Scholar]
- (63).Rahimi R; Htwe SS; Ochoa M; Donaldson A; Zieger M; Sood R; Tamayol A; Khademhosseini A; Ghaemmaghami AM; Ziaie B Lab Chip 2016, 16 (22), 4319–4325. [DOI] [PubMed] [Google Scholar]
- (64).Kim S-H; Lee HR; Yu SJ; Han M-E; Lee DY; Kim SY; Ahn H-J; Han M-J; Lee T-I; Kim T-S; Kwon SK; Im SG; Hwang NS Proc. Natl. Acad. Sci U. S. A 2015, 112 (50), 15426–15431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Jain RK; Au P; Tam J; Duda DG; Fukumura D Nat. Biotechnol 2005, 23 (7), 821–823. [DOI] [PubMed] [Google Scholar]
- (66).Rouwkema J; Rivron NC; van Blitterswijk CA Trends Biotechnol. 2008, 26 (8), 434–441. [DOI] [PubMed] [Google Scholar]
- (67).Lu H; Koo LY; Wang WM; Lauffenburger DA; Griffith LG; Jensen KF Anal. Chem 2004, 76 (18), 5257–5264. [DOI] [PubMed] [Google Scholar]
- (68).Kim L; Toh YC; Voldman J; Yu H Lab Chip 2007, 7 (6), 681–694. [DOI] [PubMed] [Google Scholar]
- (69).Ozaki A; Arisaka Y; Takeda N Biofabrication 2016, 8 (3), 035010. [DOI] [PubMed] [Google Scholar]
- (70).Truong AS; Lockett M R Analyst 2016, 141 (12), 3874–3882. [DOI] [PubMed] [Google Scholar]
- (71).Boyce MW; Kenney RM; Truong AS; Lockett MR Anal. Bioanal. Chem 2016, 408 (11), 2985–92. [DOI] [PubMed] [Google Scholar]
- (72).Kenney RM; Boyce MW; Whitman NA; Kromhout BP; Lockett MR Anal. Chem 2018, 90 (3), 2376–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Chang CW; Cheng YJ; Tu M; Chen YH; Peng CC; Liao WH; Tung YC Lab Chip 2014, 14 (19), 3762–3772. [DOI] [PubMed] [Google Scholar]
- (74).Wang LX; Zhou YF; Fu JJ; Lu Z; Yu L Micromachines 2018, 9 (12), 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Sleeboom JF; den Toonder JMJ; Sahlgren CM Int. J. Mol. Sci 2018, 19 (10), 3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Deiss F; Mazzeo A; Hong E; Ingber DE; Derda R; Whitesides GM Anal. Chem 2013, 85 (17), 8085–8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Franko AJ; Sutherland RM Radiat. Res 1979, 79 (3), 439–453. [PubMed] [Google Scholar]
- (78).Franko AJ; Sutherland RM Radiat. Res 1979, 79 (3), 454–467. [PubMed] [Google Scholar]
- (79).West CML; Sutherland RM Radiat. Res 1987, 112 (1), 105–115. [PubMed] [Google Scholar]
- (80).Rodenhizer D; Gaude E; Cojocari D; Mahadevan R; Frezza C; Wouters BG; McGuigan AP Nat. Mater 2016, 15 (2), 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Rodenhizer D; Cojocari D; Wouters BG; McGuigan AP Biofabrication 2016, 8 (4), 045008. [DOI] [PubMed] [Google Scholar]
- (82).Young MK; Rodenhizer D; Dean T; D’Arcangelo E; Xu B; Ailles L; McGuigan AP Biomaterials 2018, 164 (1), 54–69. [DOI] [PubMed] [Google Scholar]
- (83).Mosadegh B; Dabiri BE; Lockett MR; Derda R; Campbell P; Parker KK; Whitesides GM Adv. Healthcare Mater 2014, 3 (7), 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Sapp MC; Fares HJ; Estrada AC; Grande-Allen KJ Acta Biomater. 2015, 13 (1), 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Modulevsky DJ; Cuerrier CM; Pelling AE PLoS One 2016, 11 (6), e0157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Helenius G; Backdahl H; Bodin A; Nannmark U; Gatenholm P; Risberg BJ Biomed. Mater. Res. Part A 2006, 76A (2), 431–438. [DOI] [PubMed] [Google Scholar]
- (87).Camci-Unal G; Laromaine A; Hong E; Derda R; Whitesides GM Sci. Rep 2016, 6, 27693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Liu X; Weaver EM; Hummon AB Anal. Chem 2013, 85 (13), 6295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Carragher N; Piccinini F; Tesei A; Trask OJ; Bickle M; Horvath P Nat. Rev. Drug Discovery 2018, 17 (8), 606. [DOI] [PubMed] [Google Scholar]
- (90).Rios AC; Clevers H Nat. Methods 2018, 15 (1), 24–26. [DOI] [PubMed] [Google Scholar]
- (91).Cella Zanacchi F; Lavagnino Z; Perrone Donnorso M; Del Bue A; Furia L; Faretta M; Diaspro A Nat. Methods 2011, 8 (12), 1047–1049. [DOI] [PubMed] [Google Scholar]
- (92).Konig K; Uchugonova A; Gorjup E Microsc. Res. Tech 2011, 74 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- (93).Pampaloni F; Ansari N; Stelzer EHK Cell Tissue Res. 2013, 352 (1), 161–177. [DOI] [PubMed] [Google Scholar]
- (94).Pampaloni F; Reynaud EG; Stelzer EHK Nat. Rev. Mol. Cell Biol 2007, 8 (10), 839–845. [DOI] [PubMed] [Google Scholar]
- (95).Chen QS; He ZY; Liu W; Lin XX; Wu J; Li HF; Lin JM Adv. Healthcare Mater 2015, 4 (15), 2291–2296. [DOI] [PubMed] [Google Scholar]
- (96).Rexius-Hall ML; Rehman J; Eddington DT Integ. Biol 2017, 9 (9), 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Xu B; Du Y; Lin J; Qi M; Shu B; Wen X; Liang G; Chen B; Liu D Anal. Chem 2016, 88 (23), 11593–11600. [DOI] [PubMed] [Google Scholar]


