Abstract
The present study was designed to investigate the chemoprotective effect of green tea extract (GTE), rosmarinic acid (RA) and rosemary extract (RE) against diethylnitrosamine (DEN) initiated and ferric nitrilotriacetate (Fe-NTA) promoted nephrotoxicity in rats. Forty male rats were categorized into five: Group I included healthy rats, group II received DEN+Fe-NTA, group III received 200 mg/kg b.wt. of RE+DEN+Fe-NTA, group IV received 1 g/kg b.wt. of GTE+DEN+Fe-NTA and group V received 50 mg/kg b.wt. of RA+DEN+Fe-NTA. RE, GTE, RA were given orally for 14 days before single intraperitoneal administration of DEN (160 mg/kg) till the end of the experiment. Eighteen days after DEN, a single intraperitoneal dose of Fe-NTA (5 mg Fe/kg) was administrated to rats to promote nephrotoxicity. The biochemical parameters were analyzed in serum at time intervals while the malondialdehyde (MDA) and tumor necrosis factor-alpha (TNF-α) were assessed in both serum and renal tissues. Kidney from each group was histopathologically examined at time intervals. The administration of Fe-NTA after DEN dose to albino rats resulted in acute nephrotoxicity which was characterized by a highly significant elevation of serum urea, creatinine, uric acid (p=0.000), serum and renal MDA and TNF-α (p=0.000) with vacuolation of epithelial lining renal tubules. The administration of RE, GTE and RA prior to DEN+Fe-NTA treatment significantly ameliorated the observed increased levels of the above mentioned parameters. GTE, RA & RE exerted a protective effect against renal toxicity with GTE showing a more pronounced effect on renal function parameters while RA showed the best antioxidant impact.
Keywords: vipoxin, phospholipase A2, acidic component, acute toxicity
ABBREVIATIONS
- DEN
diethylnitrosamine
- Fe-NTA
ferric nitrilotriacetate
- RE
rosemary extract
- GTE
green tea extract
- RA
rosmarinic acid
- ROS
reactive oxygen species
- HO-1
hemeoxygenase-1
- Nrf2
nuclear factor erythroid 2
- Keap-1
kelch like ECH-associated protein 1
- ARE
antioxidant response element
- HSP90
heat shock protein 90
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase-2 enzyme
- CYP2E1
cytochrome P450 2E1
- MDA
malondialdehyde
- TNF-α
tumor necrosis factor-alpha
- GA
gallic acid
- GC
(–)-gallocatechin
- EC
(–)-epicatechin
- EGC
(–)-epigallocatechin
- ECG
(–)-epicatechin gallate
- EGCG
(–)-epigallocatechin gallate
- CA
p-coumaroylquinic
- GCG
(–)-gallocatechin-3-gallate
- GTP
green tea polyphenols
- NTA
nitrilotriacetic acid disodium salt
- LPO
lipid peroxidation
- SGLTI
sodium-dependent glucose transporter I
- NF-κB
nuclear factor kappa B.
Introduction
Renal toxicity is one of the most common problems that occur to the body upon exposure to a drug or a toxin. Free radicals are considered a potent factor for mediating oxidative damage and tissue impairments (Sundaram et al., 2015). About 20% of nephrotoxicity is induced by medications, but for the elderly the nephrotoxicity incidence increases up to 66% because of the longer average life span. Chemotherapy and anticancer agents are used cautiously due to their nephrotoxic adverse effects. Nephrotoxicity takes place when detoxification and excretion functions of the kidney are disturbed due to kidney malfunction either by exogenous or endogenous toxins (Kim & Moon, 2012).
Diethylnitrosamine (DEN) is one of the well known hepato-carcinogenic nitrosamine in the environment that has many industrial and chemical applications. DEN is used as a solvent in the fiber industry, as an additive for lubricants and as a softener for co-polymers. It is also used in the synthesis of 1,1- diethylhydrazine and in condensers to elevate the dielectric constant (Koul et al. 2007). DEN can be produced in the human body as metabolic products of many drugs and found in processed meats, tobacco smoke, cheese, soybean, and many types of foods (Ahmed et al., 2015).
Ferric nitrilotriacetate (Fe-NTA) is another potent hepato- and nephrotoxic agent. It has been used both in developing and developed countries as an alternative of polyphosphates in detergents for hospital and household use (Sundaram et al., 2015). Nitrilotriacetic acid is also widely used in the treatment of boiler water to prevent mineral accumulation, in photography, paper, textile manufacture, cellulose production, cleaning operations and metal plating (Khan et al., 2004). It induces subacute and acute necrosis of renal proximal tubules. It can also promote hepatic tumors effectively. It decreases the antioxidant content and induces oxidative stress (Kaur et al., 2010). Fe-NTA is obtained when nitrilotriacetic acid reacts with iron at neutral pH forming a chelate complex which is soluble in water (Sundaram et al., 2015). A single dose of Fe-NTA from 5 to 15 mg iron per kg body weight can induce acute nephrotoxicity, while repeated doses of Fe-NTA for 10–12 days of either 1 or 2 mg iron per kg body weight can induce sub-acute nephrotoxicity (Gupta et al., 2007).
Reactive oxygen species (ROS) are one of the main causes leading to the progression of pathophysiological changes of kidney diseases. ROS have many physiological roles in cells; however, they can exert also deleterious effects such as lipid peroxidation, cellular protein oxidation and DNA damage. These effects may lead to altered integrity of plasma membrane and mitochondrial membrane. Also, the oxidative damage can result in impaired function of the protein and suppression of both cell repair and proliferation. In addition, the free iron has a very high catalytic activity, which leads to the generation of potent reactive free radicals that finally cause oxidative injury and tissue damage (Gupta et al., 2009).
Herbal plants are becoming increasingly popular in modern society as natural remedies. It may be due to the antioxidant activity of the main constituents of many herbs that have the ability to inhibit the formation of ROS or to scavenge the formed ones. In addition, herb constituents can inhibit the production of ROS by the upregulation of vitagenes that protect the cells from various electrophiles and oxidants. Examples of such cytoprotective proteins are ferritin, glutathione reductase, hemeoxygenase-1 (HO-1) and repair enzymes such as 26S proteasome (Calabrese et al., 2010).
Rosemary (Rosmarinus officinalis) is a well known spice and medicinal herb, belonging to the Lamiaceae family. Rosemary plant contains 2 active principle constituents; flavonoids, which include 6-methoxygenkwanin, diosmetin, apigenin, diosmin, hispidulin, genkwanin, sinensetin, luteolin, and di- and triterpenoids, which include carnosic acid, oleanolic acid, rosmariquinone, ursolic acid and picrosalvin (carnosol). At least, 3% of the main constituents of rosemary are represented by phenolic acids such as rosmarinic, chlorogenic and caffeic acids (Begum et al., 2013). The predominant active compounds, carnosic acid and carnosol, and many other active polyphenolic compounds in rosemary, have the ability of both scavenging free radicals directly and increasing endogenous cellular antioxidant defenses indirectly via activation of the redox-sensitive system Nrf2/Keap-1/ARE transcriptional pathway, as well as potentially via multiple other mechanisms (Satoh et al., 2013).
Rosemary was scientifically proved to possess antioxidant activity, anti-inflammatory activity, anti-nephrotoxic activity, anti-hepatotoxic activity, antimicrobial activity and anti-tumor activity (Begum et al., 2013).
Tea (Camellia sinensis) is one of the most widely consumed beverages in the world (Feng, 2013). It is an evergreen tree (Ahmed & Stepp, 2013), belongs to the Theaceae family and from its leaves green tea is produced. Green tea (GT) includes more than 3000 phytochemical compounds and about one-third of these constituents are assorted as polyphenols (Shivashankara et al., 2013). The most abundant and common polyphenolic compounds are gallic acid (GA), (–)-epicatechin (EC),(–)-gallocatechin (GC), (–)-epicatechingallate (ECG), (–)-epigallocatechin (EGC), (–)-gallocatechin-3-gallate (GCG), p-coumaroylquinic acid (CA) and (–)-epigallocatechin gallate (EGCG) which represents the highest concentrations of polyphenols compared to others (Forester & Lambert, 2014). Catechins of GTE possess antioxidant, anti-angiogenic and anti-proliferative activities that play a marked role in the treatment of various disorders such as stomach disorders, type 2 diabetes, headaches, atherosclerosis, hypotension and myocardial infarction. GTE also can reduce the risk of ovarian cancer, gastrointestinal cancer, breast cancer and lung cancer (Shivashankara et al., 2013).
Green tea polyphenols can exert their antioxidant action by scavenging free radicals, suppressing inducible nitric oxide synthase activity (iNOS), down-regulating cyclooxygenase-2 enzyme (COX-2), upregulating the transcription of many cytoprotective (phase 2) genes and various detoxification enzymes thereby reducing ROS production and protecting cellular DNA from damage (Calabrese et al., 2010). Smarinic acid (RA) is a caffeic acid ester with 3,4- dihydroxyphenyl lactic acid. It is a polyphenolic compound that is potentially used at the pharmaceutical/nutritional interface. RA is commonly found in several plants which belong to the Lamiaceae family, such as Rosmarinus officinalis, Perilla frutescens, Salvia officinalis and many other medicinal herbs (Ramalho et al., 2014). These plant species, based on dry weight, contain more than 3% RA which has four phenolic hydrogens enabling it to control the oxidation of free radicals. RA improves its radical stability by intermolecular hydrogen bond formation between the hydrogen of its phenoxyl radical and that of its hydroxyl radical (Bhatt et al., 2013).
RA exhibits a number of important biological activities, including antioxidant, anti-inflammatory, antimicrobial, anticancer and anti-neurodegenerative activities (Ramalho et al., 2014). RA treatment might enhance the detoxification mechanisms by reducing the phase I metabolizing enzymes responsible for the conversion of chemicals into carcinogens such as cytochrome P450 2E1 and increasing the activity phase II endogenous antioxidant enzymes such as glutathione peroxidase and catalase (Ahmed, 2016). In addition, RA enhances DNA repair enzymes and induces endogenous cellular defense mechanism by activation of Nrf2 /HO-1 and caspase-3 pathways (Furtado et al., 2015).
This study was designed to evaluate the possible nephroprotective effects of rosemary, green tea and rosmarinic acid on diethylnitrosamine initiated and ferric nitrilotriacetate promoted nephrotoxicity and increased oxidative stress in Wistar albino rats. This can be achieved through assessing the role of their extracts on kidney function parameters, lipid peroxidation status, proinflammatory marker levels and histopathological changes in the nephrotoxic rat model.
Materials and methods
Materials
Diethylnitrosamine, nitrilotriacetic acid disodium salt (NTA) and iron (III) nitrate nonahydrate were purchased from Sigma-Aldrich (St. Louis, MO, USA).Sodium bicarbonate and sodium hydroxide pellets were purchased from Loba Chemie, Mumbai, India. Rosmarinic acid 96% (Cat. No.536954) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rosemary was obtained from Herbarium of Cairo University, while green tea was obtained from local market in Cairo. Chemicals were used for the determination of lipid peroxide product (MDA); the thiobarbituric acid and the trichloroacetic acid were obtained from Merck (Germany) and BDH (England) Companies, respectively. Kits used for determination of urea, creatinine and uric acid in blood samples were purchased from Erba Diagnostics Mannheim GmbH (Friedrichsring 4, Mannheim, Germany). Kits used for determination of serum albumin and glucose were purchased from Bio-diagnostic Company, Cairo, Egypt. Kits for estimation of Rat Tumor Necrosis Factor Alpha (TNF-α) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Methods
Preparation of rosemary water extract (RE)
Rosemary extract was prepared as described previously by Al-Attar & Shawush (2014) with some modifications. Fifty grams of rosemary powdered leaves were added into 2 L of hot water in a flask for 6 hours. Then, the solution was slowly boiled for 1 hour and cooled at room temperature. It was gently subjected to an electric mixer for 10 minutes and filtered. The extract was given fresh to the animals.
Preparation of green tea water extract (GTE)
GTE was prepared according to El-Beih et al. (2015) with some modifications by dissolving amount equivalent to 0.001 kg of tea/ kg body weight in glassware containing 5 ml boiling distilled water (equivalent to 5 cups for a 60-kg adult human), then covered and let stand for 10 minutes at room temperature. Finally, the extract was filtered and given fresh to the animals.
Preparation of Ferric Nitrilotriacetate (Fe-NTA)
Fe-NTA solution was freshly prepared immediately before use as described by Iqbal and Athar (1998). Ferric nitrate (0.16 mmol/5.0 ml) solution was mixed with a fourfold molar excess of disodium salt of nitrilotriacetic acid (0.64 mmol/5.0 ml) and the pH was adjusted to 7.4 with sodium bicarbonate. The concentration of iron in this solution was 9 mg Fe/10 ml.
HPLC method for determination of phenolic compounds
Phenolic compounds in the water extract of rosemary and green tea were determined according to Goupy et al. (1999). A high performance liquid chromatography system (HPLC) equipped with a variable wave length detector (Agilant, Germany) 1100 was used. The HPLC was equipped with auto sampler, quaternary pump degasser and column compartment. Analyses were performed on a C18 reverse phase (BDS 5 μm, Labio, Czech Republic) packed stainless-steel column (4×250mm, i.d.). Peaks were identified by congruent retention times and UV spectra and compared with those of the standards.
Experimental animals
Forty male adult Wistar albino rats of Sprague Dawley strain with a body weight ranging from 120–150 g were obtained from the Central Animal House of National Research Centre, Cairo, Egypt. Rats were housed in stainless steel cages in a room under controlled conditions of temperature and humidity. All rats were kept for adaptation to the environmental conditions for 2 weeks. Standard laboratory diet and water were allowed ad-libitum to animals during the experimental period. Animal experiments were carried out in compliance with the Guidelines for Institutional Animal Care and Use Committee of Cairo University, Egypt, and the study protocol was approved with the no: “CU-I-S-58-17”.
Experimental design
Out of 40, 24 rats were given protective doses for 14 consecutive days of 200 mg/kg rosemary extract, 1 g/kg green tea extract and 50 mg/kg rosmarinic acid (Alnahdi, 2012; El-Beih et al., 2015; Tavafi & Ahmadvand, 2011). The induction of toxicity was initiated with a single intraperitoneal injection of diethyl nitrosamine (DEN) at a dose level of 160 mg/kg body weight (Becker et al., 1985). After eighteen days of the initiation step, the animals were given a single intraperitoneal injection of Fe-NTA at a dose level of 5 mg Fe/kg body weight (Okada et al., 2001). The animals continued to receive the extracts of medicinal plants and rosmarinic acid daily till the end of the experiment. The animals were divided into 5 groups, each of eight animals, as follows:
Group I (control negative): received only standard diet and water.
Group II (control positive): received a single i.p. injection of DEN in saline followed by a single i.p. injection of Fe NTA on the 18th day of DEN.
Group III: pretreated rats orally with rosemary extract at a dose 200 mg/kg b.wt. for 14 days followed by i.p. injection of DEN in saline and i.p. injection of Fe NTA on the 18th day of DEN.
Group IV: pretreated rats orally with green tea extract at a dose 1g/kg b.wt. for 14 days followed by i.p. injection of DEN in saline and i.p. injection of Fe NTA on the 18th day of DEN.
Group V: pretreated rats orally with pure rosmarinic acid in distilled water at a dose of 50 mg/kg b.wt. for 14 days followed by i.p. injection of DEN in saline and i.p. injection of Fe NTA on 18th day of DEN.
Changes in body weight were followed up twice a week throughout the experimental period. Blood samples were collected after 2 weeks of DEN injection, after 2 hours, 24 hours and after 48 hours of Fe-NTA injection to evaluate serum kidney function parameters. After 48 hours of Fe-NTA injection, all animals were sacrificed, blood was collected and serum was separated for further investigations of biochemical parameters. The kidneys of each rat were separated, washed with saline and weighed. Then one kidney of each animal was kept in 10% neutral buffered formalin for histopathological examination and the other one was stored in –20 °C for further estimation of malondialdehyde and tumor necrosis factor alpha.
Preparation of kidney homogenates for malondialdehyde and TNF-α assesment
A portion with known weight of the kidney of each animal was homogenized by homogenizer stirrer Wise Stir (HS-30E) in 5–10 mL cold buffer (50 mM potassium phosphate, pH 7.5) per gram tissue. Then, the homogenates were centrifuged at 4000 rpm for 15 minutes. Finally, the supernatant of tissue homogenates was separated and stored at –80 °C for malondialdehyde determination.
The other part of the kidney of each animal was rinsed in ice-cold phosphate buffer saline (PBS) (0.01mol/L, pH 7.0–7.2) to remove excess blood thoroughly and weighed before homogenization. The tissues were minced to 1–2 mm pieces and homogenized by homogenizer stirrer Wise Stir (HS-30E) in 5–10 mL of PBS with a glass homogenizer on ice. Then the homogenates were centrifuged for 5 minutes at 5000×g. The supernatants of tissue homogenates were separated and stored at –80 °C for estimation of TNF-α.
Biochemical analysis
Serum glucose was determined according to (Trinder, 1969). Serum urea was assessed according to (Talke & Schubert, 1965). Serum creatinine was determined by Modified Jaffe’s reaction as described by (Bowers, 1980). Uric acid was estimated by Trinder’s enzymatic reaction as described by (Trinder & Barham, 1972). Albumin was determined according to Doumas et al. (1971).
Estimation of lipid peroxidation level
The rate of production of TBARS (thiobarbituric acid reactive species) was measured in serum and renal tissues according to Draper & Hadley (1990). 2.5 ml of trichloroacetic acid (10% w/v) was added to 0.5 ml of serum and renal tissue homogenates in a boiling water bath for 15 minutes, cooled rapidly under tap water and finally centrifuged for 10 minutes at 3000 rpm. 2 ml of separated supernatant of serum and tissue homogenate samples was added to 1 ml of thiobarbituric acid (0.67% w/v) in a test tube and placed in a boiling water bath for 15 minutes then cooled again rapidly under tap water. The absorbance was measured at 532 nm against air. The results were calculated using a molar extinction coefficient of 1.53×10⁵ M–1.cm–1.
Estimation of tumor necrosis factor-alpha
Tumor necrosis factor alpha (TNF-α) was assessed in serum and kidney tissue homogenates using a standard ELISA method. The assessment was done by sandwich ELISA technique according to the manufacturer’s instructions (R&D Systems).
Histopathological examination
The kidneys were cleared in xylol, embedded in paraffin wax and longitudinally sectioned with a microtome into sections with 4–6 micron thickness. These sections were stained by hematoxylin and eosin and finally they were observed under an Olympus microscope with a camera.
Statistical analysis
Differences between groups were analyzed using one way analysis of variance (ANOVA), followed by Duncan multiple comparisons test using SPSS package version “20” for Windows. Values are represented as mean ± S.E. and p<0.05 was considered statistically significant, p<0.01 was considered highly significant and p<0.001 was considered very highly significant.
Results
Phenolic acid content of water extract of rosemary and green tea as determined by HPLC
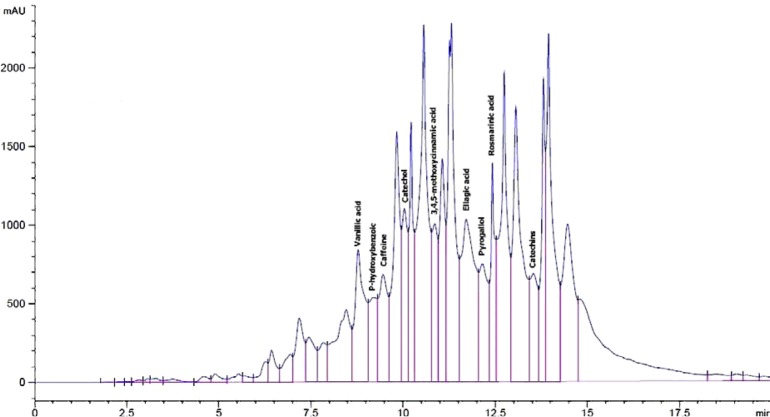
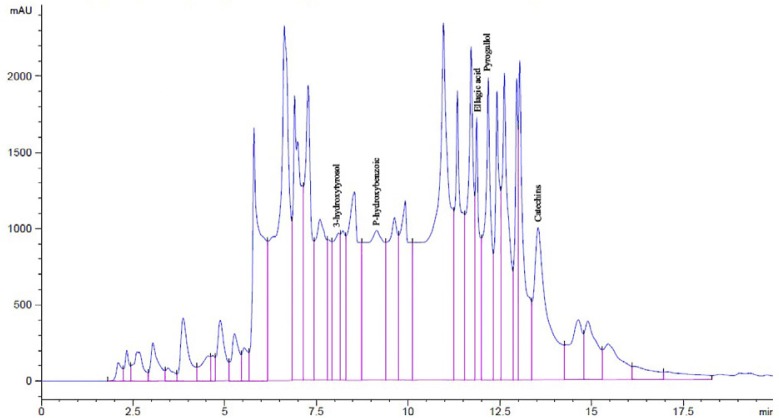
Analysis by HPLC to water extract of rosemary and green tea revealed the presence of different phenolic compounds. Using 19 standard phenolic compounds, eighteen and seventeen different phenols were detected in RE and GTE, respectively (Figures 1 & 2). A high amount of rosmarinic acid, catechol, ellagic acid, 3,4,5-methoxycinnamic acid, vanillic acid, pyrogallol, catechins, p-hydroxybenzoic acid, caffein, ferulic acid and protocatechic acid were found in the water extract of rosemary. In addition, a high amount of pyrogallol, ellagic acid, catechins, vanillic acid, p-hydroxybenzoic acid, catechol, protocatechic acid and 3-hydroxytyrosol were detected in the water extract of green tea (Table 1).
Figure 1.
HPLC profile of phenolic compounds of water extract of rosemary as standards at 280 nm. (1) Rosmarinic acid, (2) Catechol, (3) Ellagic acid, (4) 3,4,5-methoxycinnamic acid, (5) Vanillic acid, (6) Pyrogallol, (7) Catechins, (8) Caffeine, and (9) P-hydroxybenzoic.
Figure 2.
HPLC profile of phenolic compounds of water extract of green tea as standards at 280 nm. (1) Pyrogallol, (2) Ellagic acid, (3) Catechins, (4) P-hydroxybenzoic acid, and (5) 3-hydroxytyrosol.
Table 1.
Differential pattern of different phenolic compounds identified in water extracts of rosemary and green tea as detected by HPLC
| Phenolic compounds | Rosemary extract (ppm) | Green tea extract (ppm) |
|---|---|---|
| Gallic acid | 86.07 | 57.43 |
| Pyrogallol | 955.40 | 7745.85 |
| 3-hydroxytyrosol | 142.29 | 480.98 |
| Protocatechuic acid | 203.69 | 171.95 |
| Catechins | 731.80 | 1142.82 |
| Catechol | 1846.47 | 400.45 |
| P-hydroxybenzoic | 540.57 | 549.48 |
| Caffeine | 692.73 | 65.80 |
| Vanillic acid | 1269.73 | 321.09 |
| P-coumaric acid | 125.96 | 47.42 |
| Ferulic acid | 223.04 | 29.35 |
| Iso-ferulic acid | 99.48 | 16.66 |
| Alpha-coumaric | 34.31 | 5.85 |
| Ellagic acid | 1729.22 | 1583.35 |
| Coumarin | 105.49 | 21.13 |
| 3,4,5-methoxycinnamic acid | 1663.55 | 30.86 |
| Cinnamic acid | 28.29 | 4.44 |
| Rosmarinic acid | 2664.52 | – |
Effect of RE, GTE & RA on the change of body weight and kidney weight percent in DEN initiated and Fe-NTA treated rats
As compared to control negative group, the control positive group treated with DEN+Fe-NTA exhibited a marked decrease of body weight (p=0.026) and an insignificant increase of kidney weight percent (p>0.05) (Table 2). RE and GTE treated groups showed no change in body weight as compared to control negative group and that is a good response (p>0.05). Kidney weight percent of DEN+Fe-NTA group showed an insignificant change when compared to RE, RA and control negative group (p>0.05). It is important to mention that RE+DEN+Fe-NTA treated group showed a highly significant increase in kidney weight percent as compared to GT and RA pretreated rats (p=0.002 & 0.002, respectively) (Table 2).
Table 2.
Body weight change and kidney weight % after 48 hours of Fe-NTA injection, of control and treated groups.
| Group | B. wt. change (g) | Kidney Wt. % |
|---|---|---|
| Control | 17.14±2.38a | 0.84±0.03ab |
| DEN+Fe-NTA | 4.50±4.08bc | 0.88±0.03ab |
| RE+DEN+Fe-NTA | 14.13±0.05ab | 0.95±0.06a |
| GTE+DEN+Fe-NTA | 10.38±5.77ab | 0.77±0.02b |
| RA+ DEN+Fe-NTA | -2.00±3.77c | 0.78±0.03b |
*RM = rosemary, GTE = green tea extract, RA = rosmarinic acid, DEN= diethylnitrosamine, Fe.NTA = ferric nitrilotriacetate, b. wt. change = body weight change, kidney wt. % = kidney weight percentage.
*Data are represented as Mean±SE. Values that share the same letter at the same column are not significant. Values that share different letters at the same column are significant.
Effect of RE, GTE & RA on biochemical parameters of renal functions at time intervals
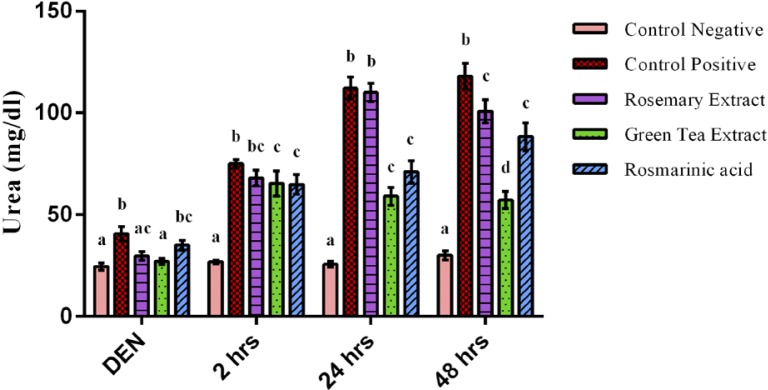
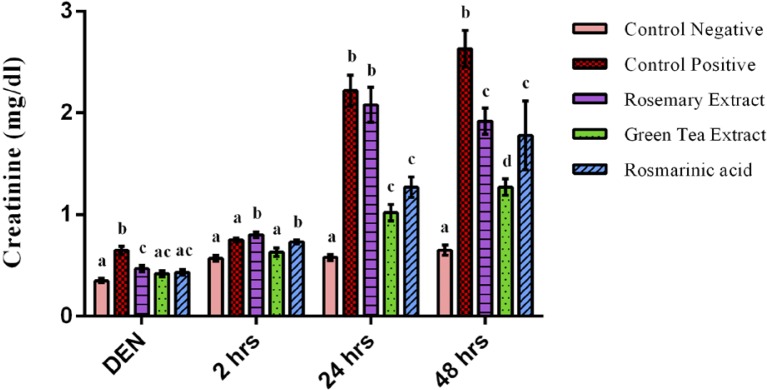
Serum levels of urea, creatinine and uric acid are clinical markers for renal function. As shown in Figures 3 & 4, after 2 weeks of DEN administration, a highly significant increase of urea and creatinine concentration of DEN treated group was observed when compared to negative control group (p<0.001). However, oral administration of RE & GTE prevented increase of urea and creatinine levels, while RA prevented only the increase of creatinine level.
Figure 3.
Urea concentration after 2 weeks of diethylnitrosamine (DEN) injection, after 2 hours, 24 hours & 48 hours of ferric nitrilotriacetate injection. Data are represented as Mean±SE. Bars that share the same letters within one series are not significant. Bars that share different letters within one series are significant.
Figure 4.
Creatinine concentration after 2 weeks of diethylnitrosamine (DEN) injection, after 2 hours, 24 hours & 48 hours of ferric nitrilotriacetate injection. Data are represented as Mean±SE. Bars that share the same letters within one series are not significant. Bars that share different letters within one series are significant.
At time intervals of Fe-NTA injection, the biochemical studies revealed a very highly significant increase of serum urea and creatinine concentration of DEN+Fe-NTA group when compared to control negative group (p=0.000). However, the administration of protective doses of GTE and RA alleviated the increase of urea and creatinine concentration highly significantly when compared to DEN+Fe-NTA treated group at time intervals. The effect of RE took more time to exert its action and that was noticed after 2 days of Fe-NTA exposure when a highly significant decrease of creatinine (p=0.000) and only a significant decrease of urea concentration (p=0.032) were observed as compared to DEN+Fe-NTA group (Figures 3 & 4).
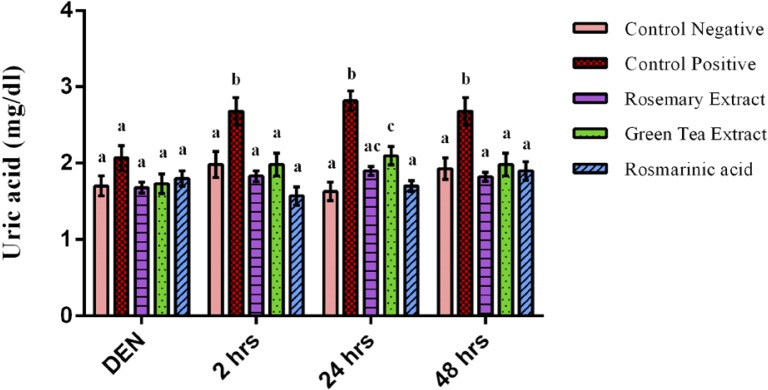
Uric acid concentration showed an insignificant difference between all groups after 2 weeks of DEN injection (Figure 5). While after Fe-NTA injection, uric acid level of DEN+Fe-NTA group showed a highly significant elevation at time intervals as compared to normal control group (p=0.000). However, the increase of uric acid levels was prevented by RA, RE and GTE administration showing a very high significant decrease at time intervals as compared to DEN+Fe-NTA group (Figure 5).
Figure 5.
Uric acid concentration after 2 weeks of diethylnitrosamine (DEN) injection, after 2 hours, 24 hours & 48 hours of ferric nitrilotriacetate injection. Data are represented as Mean±SE. Bars that share the same letters within one series are not significant. Bars that share different letters within one series are significant.
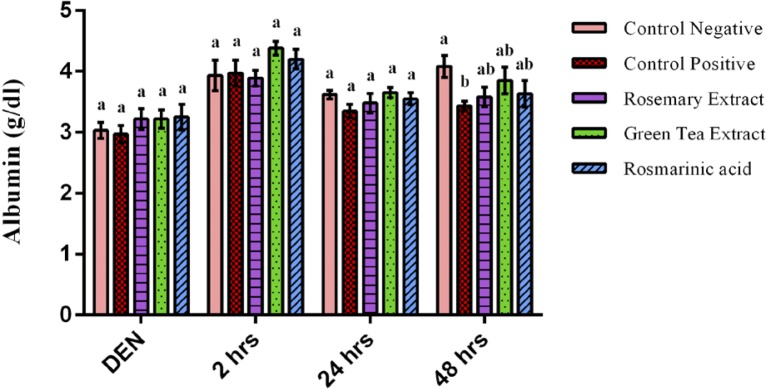
Albumin concentration showed an insignificant difference between all groups after 2 weeks of DEN, 2 hours & 24 hours of Fe-NTA injection (p>0.05). However, after 48 hours, albumin concentration of control positive group showed a significant decrease as compared to control negative group (p=0.016). With respect to groups that were pretreated with RE, GTE and RA, they exhibited an insignificant difference in their albumin levels as compared to control negative after 48 hours of Fe-NTA injection (p>0.05) (Figure 6).
Figure 6.
Albumin concentration after 2 weeks of diethylnitrosamine (DEN) injection, after 2 hours, 24 hours & 48 hours of ferric nitrilotriacetate injection. Data are represented as Mean±SE. Bars that share the same letters within one series are not significant. Bars that share different letters within one series are significant.
Effect of RE, GTE & RA on blood glucose concentration at time intervals
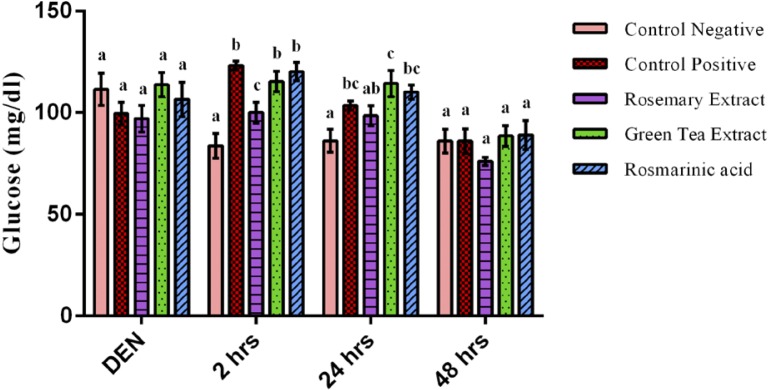
Glucose concentration showed an insignificant difference between all groups after DEN injection (p>0.05). However, Fe-NTA showed a hyperglycemic effect after 2 hours of its injection represented by a highly significant increase of serum glucose level of DEN+Fe-NTA treated group when compared to the control negative group with an increase of 47% (p=0.003).
After 24 hours, there was a slight significant elevation in the level of serum glucose of DEN+Fe-NTA group when compared to control negative group with an increase of 20% (p=0.018) (Figure 7). After 2 days of Fe-NTA injection, glucose concentration of the control positive group returned to the normal level showing an insignificant difference when compared to the control negative group and all treated groups (p>0.05). RE showed a hypoglycemic effect at time intervals reporting a highly significant decline in glucose concentration after 2 hours of Fe-NTA dose when compared to DEN+Fe-NTA treated group with a decrease of 19% (p=0.020). Both the GTE and RA treated groups showed no significant difference in their glucose concentration at time intervals when compared to control positive group (p>0.05).
Figure 7.
Glucose concentration after 2 weeks of diethylnitrosamine (DEN) injection, after 2 hours, 24 hours & 48 hours of ferric nitrilotriacetate injection. Data are represented as Mean±SE. Bars that share the same letters within one series are not significant. Bars that share different letters within one series are significant.
Effect of RE, GTE & RA on production of serum and renal malondialdehyde in DEN initiated and Fe-NTA treated rats
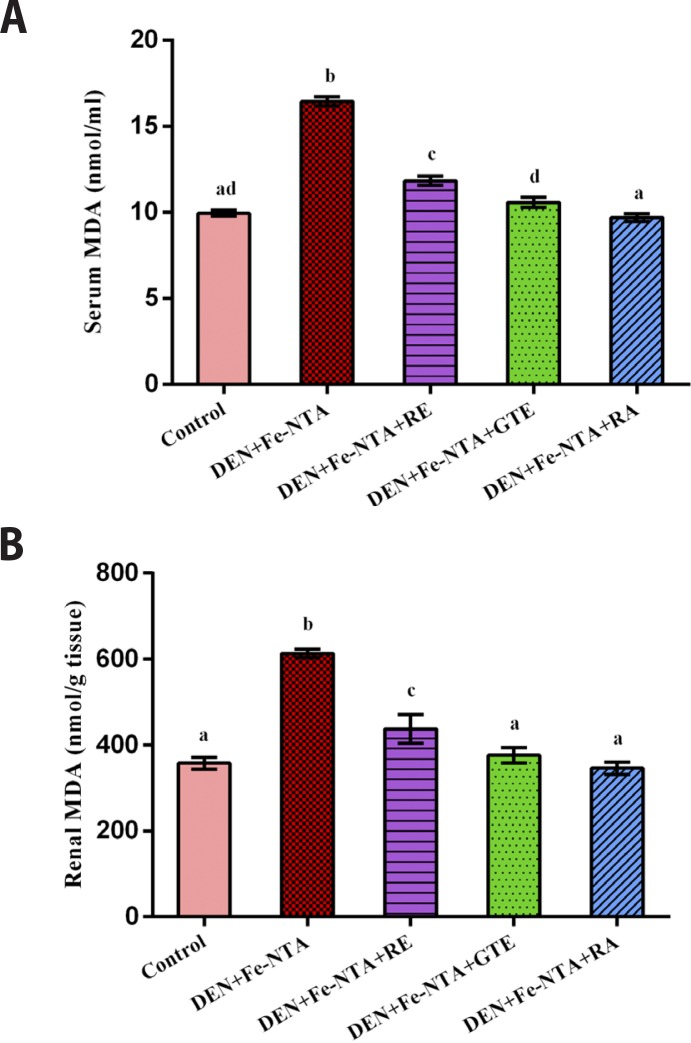
After 48 hrs of Fe-NTA injection, a very high significant increase was observed in serum and renal malondialdehyde levels (MDA) of the group that was administrated DEN+Fe-NTA (p<0.001, p<0.001) with a percentage increase of 65 % and 72 %, respectively, as compared to the control negative group. However, the administration of protective doses of RE, GTE and RA at the mentioned doses significantly prevented increase of serum and renal MDA levels (p<0.001, p<0.001 & p<0.001) for serum and renal MDA. They reduced the serum MDA level by 28%, 36% & 41% and decreased the renal MDA level by 29%, 39% & 44%, respectively (Figures 8A & 8B).
Figure 8.
Malondialdehyde (MDA) concentration after 48 hours of ferric nitrilotriacetate injection (A) in serum samples, (B) in renal tissue homogenates. Data are represented as Mean±SE.
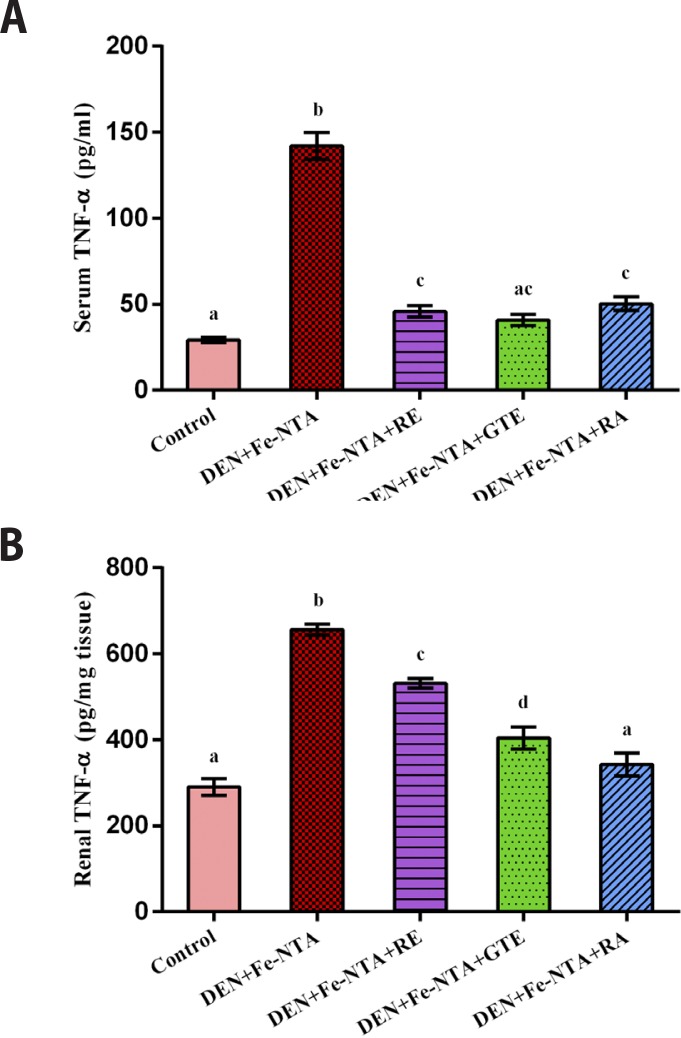
Effect of RE, GTE & RA on production of serum and renal TNF- α in DEN initiated and Fe-NTA treated rats
The exposure of rats to an acute dose of Fe-NTA increased serum and renal TNF-α levels highly significantly as compared to the control negative one (p<0.001, p<0.001) with percentage increase of 386.7 % and 126.2 %. With respect to groups that were pretreated by protective doses of RE, GTE and RA, it was obvious that these natural extracts alleviated the increasing level of serum and renal TNF-α highly significantly as compared to control positive group (p<0.001) with a percentage decrease of 68%, 71% and 65%, respectively for serum TNF-α level and 19%, 38% and 48%, respectively for renal TNF-α level (Figures 9A & 9B).
Figure 9.
Tumor necrosis factor alpha (TNF-α) concentration after 48 hours of ferric nitrilotriacetate injection (A) in serum samples, (B) in renal tissue homogenates. Data are represented as Mean±SE.
Effect of RE, GTE & RA on histopathological examination of kidney in acute nephrotoxicity rat model
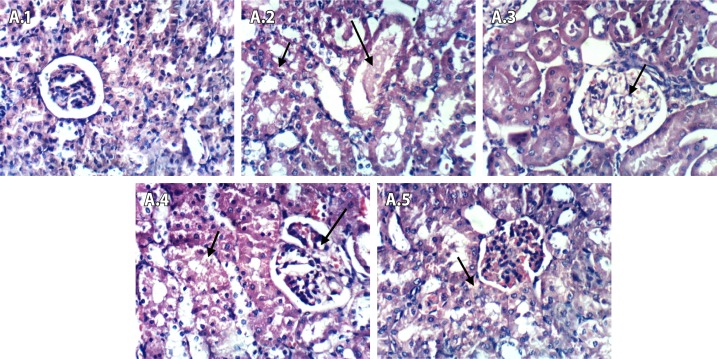
After 2 hours of Fe-NTA injection (Figure 10 (A.1–A.5)), the control negative group showed no histopathological changes in renal parenchyma (A.1). Renal tissues of control positive group (DEN+Fe-NTA) showed vacuolation of the epithelial lining renal tubules and eosinophilic proteinaceous material in the lumen of renal tubules (A.2). Renal tissues of rosemary treated group (DEN+Fe-NTA+RE) showed vacuolation of endothelial lining glomerular tuft (A.3). In case of green tea treated group (DEN+Fe-NTA+GTE), renal tissues showed vacuolation of epithelial lining of renal tubules and endothelial lining glomerular tuft (A.4). Renal tissues of rosmarinic acid treated group (DEN+Fe-NTA+RA) showed vacuolation of epithelial lining of some renal tubules (A.5).
Figure 10.
Light photomicrographs of kidney sections in rats after 2 hours of Fe-NTA injection (Series A; 1–5). (A.1) Control negative showed no pathological changes in renal parenchyma, (A.2) DEN+Fe-NTA group showed vacuolation of epithelial lining renal tubules, (A.3) DEN+Fe-NTA+RE group showed vacuolation of endothelial lining glomerular tuft, (A.4) DEN+Fe-NTA+GTE group showed vacuolation of epithelial lining renal tubules and endothelial lining glomerular tuft and (A.5) DEN+Fe-NTA+RA group showed vacuolation of epithelial lining of some renal tubules.
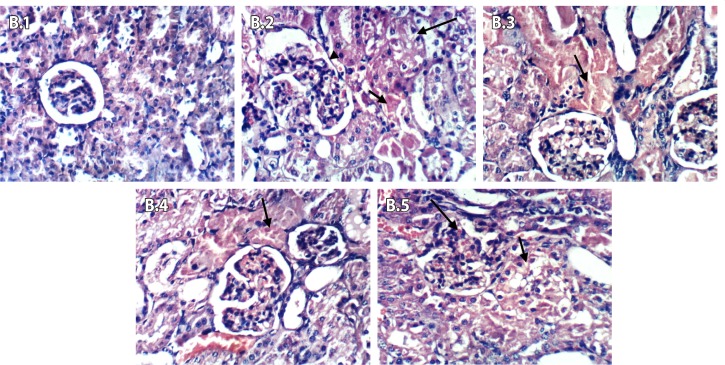
After 24 hours of Fe-NTA injection (Figures 11 (B.1–B.5)), the control negative group showed no histopathological changes in renal parenchyma (B.1). Kidneys of control positive group (DEN+Fe-NTA) showed coagulative necrosis of epithelial lining of renal tubules, vacuolation of renal tubular epithelium, hypertrophy and hypercellularity of glomerular tuft (B.2). Kidneys of the group that received rosemary extract (DEN+Fe-NTA+RE) showed focal coagulative necrosis of epithelial lining of renal tubule (B.3). As shown in B.4, green tea treated group (DEN+Fe-NTA+GTE) showed focal coagulative necrosis of epithelial lining renal tubules. Kidneys of rosmarinic acid group (DEN+Fe-NTA+RA) showed vacuolation of epithelial lining of some renal tubules and slight congestion of glomerular tuft (B.5).
Figure 11.
Light photomicrographs of kidney sections in rats after 24 hours of Fe-NTA injection (Series B; 1–5). (B.1) Control negative showed no pathological changes in renal parenchyma, (B.2) DEN+Fe-NTA group showed necrosis of epithelial lining renal tubules, (B.3) DEN+Fe-NTA+RE group showed focal coagulative necrosis of epithelial lining renal tubule, (B.4) DEN+Fe-NTA+GTE group showed focal coagulative necrosis of epithelial lining renal tubules, and (B.5) DEN+Fe-NTA+RA group showed vacuolation of epithelial lining öf some renal tubules.
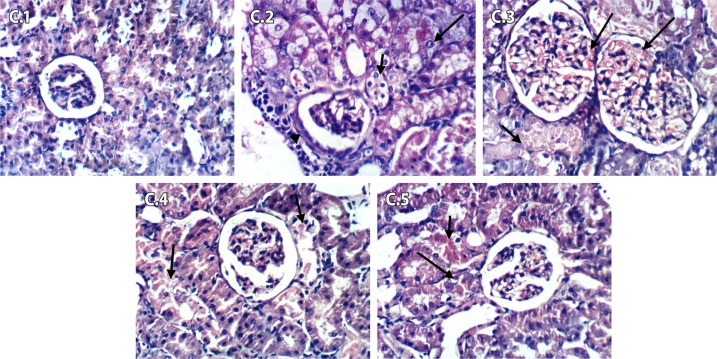
Figures 12 (C.1–C.5) shows the histopathological changes after 48 hours of Fe-NTA injection. Figure 12 (C.1) of the control negative group shows no histopathological changes in renal parenchyma. As shown in C.2, kidneys of control positive group (DEN+Fe-NTA) showed vacuolation of epithelial lining of renal tubules, karyomegaly of the nuclei of renal epithelium and periglomerular inflammatory cell infiltration. Kidneys of rosemary treated group (DEN+Fe-NTA+RE) showed focal coagulative necrosis of epithelial lining of renal tubules, hypertrophy and congestion of glomerular tufts (C.3). Kidneys of the group treated with green tea (DEN+Fe-NTA+GTE) showed necrobiosis of epithelial lining of some renal tubules (C.4). Kidneys of the group treated with a pure extract of rosmarinic acid (DEN+Fe-NTA+RA) showed focal necrosis and apoptosis of epithelial lining of some renal tubules (C.5).
Figure 12.
Light photomicrographs of kidney sections in rats after 48 hours of Fe-NTA injection (Series C; 1–5). (C.1) Control negative showed no pathological changes in renal parenchyma, (C.2) DEN+Fe-NTA group showed karyomegally of the nuclei of renal epithelium and periglomerular inflammatory cells infiltration, (C.3) DEN+Fe-NTA+RE group showed hypertrophy and congestion of glomerular tufts, (C.4) DEN+Fe-NTA+GTE group showed necrobiosis of epithelial lining of some renal tubules and (C.5) DEN+Fe-NTA+RA group showed focal necrosis and apoptosis.
Discussion
Nephrotoxicity is one of the most common issues that occurs upon exposure to toxins or medications (Sundaram et al. 2015). Oxidative stress plays a fundamental role in the pathogenesis of nephrotoxicity and tumorigenesis caused by DEN and Fe-NTA (Rashid et al., 2013). Fe-NTA is absorbed into portal vein through mesothelium upon intraperitoneal administration, and then passes into blood circulation through the liver. Because Fe-NTA has a low molecular weight, it can be easily filtered through the glomeruli into renal proximal tubules where Fe3+-NTA is reduced to Fe2+-NTA by two main reducing compounds, cysteine or cysteine glycine. This results in the generation of superoxide anion radicals (O2•–) which induce the iron to catalyze Haber-Weiss reaction forming hydroxyl radical (OH•) that leads to the progression of lipid peroxidation, thereby to renal tissue damage (Rehman & Sultana 2011). Many herbal plants have been highlighted recently for their potent antioxidant activities among which are green tea and rosemary. They possess also anti-inflammatory activities (Begum et al., 2013; Anand et al., 2015).
In the current study, HPLC for water extract of rosemary and green tea revealed the presence of many antioxidant phenolic compounds such as catechins, catechol, vanillic acid, caffeine, ellagic acid, coumarin, pyrogallol, 3-hydroxytyrosol, protocatechuic acid, 3,4,5-methoxy cinnamic acid and rosmarinic acid detected in rosemary. These bioactive constituents have a strong antioxidant impact in inhibiting the production of free radicals, thereby preventing cells from the expected damage. These results are in agreement with other authors (Begum et al., 2013; Anand et al., 2015).
The polyphenols, including catechins and epicatechins, from green tea, carnosic acid, carnosol, caffeic acid and rosmarinic acid from rosemary and many polyphenolic compounds are oxidized into electrophilic hydroquinones or quinines during their reaction with free radicals. These electrophilic compounds have the ability to activate Nrf2 transcription factor through a modification of a specific cysteine in Keap1; the inhibitor of Nrf2. Such modification converts Keap1 to its inactive form thereby activating Nrf2 transcriptional pathway. After that, Nrf2 binds to antioxidant response element (ARE) resulting in upregulation of the transcription of genes for phase II detoxification enzymes (cytoprotective genes) such as glutathione reductase, thioredoxin reductase, repair enzymes and proteasome (Forman et al., 2014).
In the present study, the rats exposed to acute doses of DEN and Fe-NTA had an obvious decline in their body weight, suggesting certain metabolic disorders (Olvera et al., 2012). In addition, the observed increase of the relative kidney weight of DEN+ Fe-NTA treated rats may be due to the inflammatory action of Fe-NTA which induces the kidney to swell. The histopathological examination was in agreement with these findings, since the renal tissues of DEN+ Fe-NTA treated group showed a vacuolation of epithelial lining renal tubules which developed into necrosis of epithelial lining renal tubules, karyomegaly of the nuclei of renal epithelium and periglomerular inflammatory cells infiltration.
However, the daily administration of RE prevented any adverse effect on body weight and that may be due to its role in controlling wasting of muscles by increasing glucose metabolism (El-Boshy et al., 2015). The supplementation of GTE could also restore the body weight and that may be attributed to the positive anabolic effect of GTE on decreasing the degeneration of adipocytes and muscle tissues through glucose metabolism improvement (Ramadan et al., 2009). On the other hand, pretreatment of GTE and RA exhibited a protective effect on renal tissues, since the deterioration of renal histology was less than that observed in DEN+ Fe-NTA treated group and that was in agreement with the obtained biochemical analysis. These findings were in accordance with Eybl et al. (2008).
Urea, creatinine and uric acid are important clinical parameters for renal injury and their significant elevation upon DEN and Fe-NTA treatment indicates an acute reduction in the glomerular filtration rate which resulted in morphological alterations of renal tissues such as tubular brush border loss that makes them impermeable to creatinine and urea thereby causing their elevation in the blood (Abd El Kader et al., 2012; Gupta et al., 2009). These results seem to be in accordance with (Rashid et al. 2013; Sharmila et al., 2016). Green tea polyphenols such as EC, ECG, EGC and EGCG are able to chelate metal ions specially iron and copper, which subsequently reduce iron deposition, inhibit hydroxyl radical generation, suppress lipid hydroperoxide degradation and cause reactive aldehyde formation, ameliorating renal functions (Weinreb et al., 2009). These results were documented previously by (Rehman et al., 2013). The oral administration of RA causes improvement in glomerular function and renal injuries and that may be due to the action of RA on the intracellular mechanisms responsible for DNA repair, instead of exerting a direct ROS-scavenging effect. On the other hand, RA enhanced an endogenous cellular antioxidant defense mechanism by activating Nrf2/HO-1 pathway resulting in upregulation of expression of many protective genes. These findings were reported previously by (Tavafi et al., 2011; Forman et al., 2014). Rosmarinic acid, carotenoids, diterpenoids and alpha-tocopherol have been documented as principal antioxidant constituents of rosemary aqueous extract which enables it to inhibit lipid peroxidation and free radical generation and improves the renal functions (Abd El-Ghany et al., 2012).
The elevation of uric acid level in DEN+Fe-NTA treated group is an indicator of its increase in joints and various tissues. Such elevation gradually leads to permanent damage of tissue and acute inflammation (Mohamed & Al-Okbi, 2008; Sundaram et al., 2015). Caffeic acid, one of phenolic phytochemical constituents of RE that have anti-gout activity, has been reported to be an inhibitor of xanthine oxidase which catalyzes the oxidation of hypoxanthine to xanthine and finally to uric acid. In addition, RA, which is the ester of caffeic acid, has the ability to inhibit xanthine oxidase and the reabsorption of renal urate, thereby reducing uric acid level (Mohamed & Al-Okbi, 2008).
The cause of the decrease of albumin concentration in the DEN+Fe-NTA treated group may be due to reabsorption impairment of albumin in the proximal tubules resulting in renal injuries which cause the remarkable release of serum protein into urine or also to the incident liver injuries and hepatocellular damage that lead to a decrease of albumin biosynthesis in the liver (Khouja & Al Nahari, 2015). The antioxidant constituents of RE such as carnosic acid, carnosol, rosmarinic acid, ursolic acid and caffeic acid play an important role in preventing lipid peroxidation, necrosis and the damage of hepatocytes (Al-Attar & Shawush, 2014). GT increases the intravascular movement of albumin, suppresses protein nitration, decreases the hepatocellular damage and improves hepatocyte functions, thereby improving the albumin concentration (Butterfield et al., 2014).
The hyperglycemic effect of Fe-NTA may be explained by its role in the induction of ROS and the overloading of iron (II) which could impair pancreatic insulin secretion, participate in the impairment of insulin signaling and interfere with insulin action and glucose uptake in adipocytes leading to an increase of glucose concentration (Al-Yousuf, 2015). The hypoglycemic effect of RE may be attributed to its role in increasing the production of insulin by regenerating the pancreatic β-cells or inhibiting the absorption of glucose in the intestine by suppressing α-glucosidase enzyme or intestinal amylase enzyme (Al-Attar & Shawush, 2014; Alnahdi, 2012).
The toxic effect of DEN and Fe-NTA compounds consists in elevation of the redox active iron levels inducing ROS formation able to attack the biomolecules of the cells causing membrane deterioration, DNA damage and mutation reported by the formation of 8-hydroxy guanosine in DNA molecules (Kaur et al., 2010). These findings correlate with (Gupta et al. 2009). However, the administration of protective doses of RE diminished the level of serum and renal MDA and this may be attributed to its antioxidant property that maintains the lipid structure of cell membrane, neutralizes the reactive free radicals protecting various biomolecules, such as proteins and DNA in such biological systems (Abd El Kader et al., 2012). Most of the antioxidant property of RE is contributed to carnosic acid and carnosol compounds which play an important role in attenuating the MDA levels in serum and renal tissues (Al-Attar & Shawush, 2014).
Green tea and rosmarinic acid extracts showed the best antioxidant activity against iron-induced oxidative stress, restoring the normal value of serum and renal MDA to that of the control negative group, considered as a good improvement. The polyphenolic constituents of GTE and polyphenolic structure of RA play a main role in redox homeostasis control in the cell by upregulating the redox-sensitive system Nrf2/Keap-1/ARE pathway leading to activation of many cytoprotective genes, activating HSF-1/Hsp90 pathways that might be involved in upregulation of protective stress response genes and inducing many other protective and detoxification mechanisms. In addition, the main GTE constituents, EGCG and ECG, have powerful antioxidant activity against DNA damage, LPO of phospholipid bilayers of cells and tumorigenesis (Weinreb et al., 2009). The formed intramolecular hydrogen bond after the abstraction of hydrogen in the chemical structure of RA increases its antioxidant activity (Bhatt et al., 2013). These investigations are in accordance with findings of other authors (Tavafi & Ahmadvand, 2011; Satoh et al., 2013).
Proinflammatory cytokine marker, tumor necrosis factor alpha (TNF-α), plays a vital role in several diseases such as cancer owing to its mutagenicity and proliferative ability. Also, it has the ability to stimulate the production of other inflammatory mediators such as ROS through oxidative stress-responsive genes which promotes and develops inflammation (Rashid et al., 2013). Previous reports stated that Fe-NTA induced the production of TNF-α which regulates the transcription of NF-κB directly (Rehman et al., 2013). It has been demonstrated that ROS act as second messengers; they are able to activate NF-κB through TNF-α and other proinflammatory cytokines (Mannaa et al., 2013). These cytokines have an essential role in vascular permeability, inflammation and cell proliferation (Rehman et al., 2013). These findings are in agreement with (Rehman et al., 2013) who reported a marked elevation in serum and renal TNF- α in DEN+Fe-NTA treated rats. RE, GTE and RA treated groups were observed to have an approximate effect on reducing serum TNF-α level near to normal range. The protective anti-inflammatory effect of rosemary, green tea and rosmarinic acid may be attributed to the redox property of polyphenolic constituents of their extracts and polyphenolic structure of RA which can modulate redox cellular homeostasis by activating the redox system Nrf2/Keap-1/ARE leading to overexpression of phase II detoxification enzymes such as glutathione reductase, glutathione peroxidases, thioredoxin reductase and thioredoxin peroxidase (Forman et al., 2014). The redox property of polyphenols can also modulate redox homeostasis in the cell by inhibiting the activity of COX-2, interfering with Nf-kB binding to DNA and suppressing the phosphorylation of kinases (Calabrese et al., 2010).
The anti-inflammatory effect of RE may be also attributed to its free radical quenching property due to its rich contents of polyphenolic constituents which inhibit the release of TNF-α (Bogdanski et al., 2012). In addition, the anti-inflammatory effect of GTE may be attributed to the ability of its polyphenolic compounds to scavenge free radicals directly through hydrogen and electron donation, suppressing the propagation of LPO. Rosmarinic acid could restore the level of serum and renal TNF-α to the range of control negative group and that may be due to the anti-inflammatory impact of RA that may have the potentiality to reduce inflammation of injured tissue in many organs like liver, kidney and lung by down-regulating of TNF-α and inhibiting the NF-kB pathway. Our findings are compatible with those of (Rocha et al., 2014).
In conclusion, the present investigations proved that green tea, rosmarinic acid and rosemary extracts are promising as nephroprotective agents against DEN and Fe-NTA induced nephrotoxicity in Wistar rats. Green tea can restore urea, creatinine and uric acid near to normal range, while rosmarinic acid showed the best antioxidant and anti-inflammatory effect in this rat toxicity model and so did rosemary but with lower extent. It can thus be stated that rosemary, green tea and rosmarinic acid rich substances are highly recommended specially for those who are more exposed to drug intoxication, environmental toxins and pollutants, such as workers in laboratories, chemical industries and factories, to obtain protection from renal toxicity. Since each item used has its own mechanisms of action resulting in improving some of the tested parameters, a combination of these plant extracts in one supplement may provie a more potent effect for detoxification of the kidney.
Acknowledgements
The authors sincerely thank Prof. Dr. Kawkab A. Ahmed, Professor of pathology, Faculty of Veterinary Medicine, Cairo University for her interest and great work on histopathological examination. The authors also thank Dr. Muhammed Taha for providing scientific information and necessary facilities for this research project.
REFERENCES
- Abd El Kader MA, El-Sammad NM, Taha H. The protective role of rosemary (rosmarinus officinalis) in lead acetate induced toxicity in rats. J Appl Sci Res. 2012;8(6):3071–3082. [Google Scholar]
- Abd El-Ghany MA, Motawee MM, El-Kewawy Biological effects of yoghurt with rosemary on injured liver rats. Aust J Basic Appl Sci. 2012;6(3):525–532. [Google Scholar]
- Ahmed MM. Rosmarinic acid attenuates the hepatotoxicity induced by ethanol in rats. American Journal of Biochemistry. 2016;6(3):82–90. [Google Scholar]
- Ahmed RR, Mahmoud AM, Ashour MB, et al. Hesperidin protects against diethylnitrosamine-induced nephrotoxicity through modulation of oxidative stress and inflammation. Natl J Physiol Pharm Pharmacol. 2015;5(5):391–397. [Google Scholar]
- Ahmed S, Stepp JR. Green tea: The plants, processing, manufacturing and production. In: Preedy VR, editor. Tea in health and disease prevention. Vol. 2. USA: Academic Press; 2013. pp. 19–31. [Google Scholar]
- Al-Attar AM, Shawush NA. Physiological investigations on the effect of olive and rosemary leaves extracts in male rats exposed to thioacetamide. Saudi J of Biol Sci. 2014;21(5):473–480. doi: 10.1016/j.sjbs.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnahdi HS. Effect of rosmarinus officinalis extract on some cardiac enzymes of streptozotocin-induced diabetic rats. J Health Sci. 2012;2(4):33–37. [Google Scholar]
- Al-Yousuf HHH. Chemopreventive effect of daidzein on renal toxicity by targeting oxidative stress and inflammation. Int J Adv Res. 2015;3(2):506–521. [Google Scholar]
- Anand J, Upadhyaya B, Rawat P, et al. Biochemical characterization and pharmacognostic evaluation of purified catechins in green tea (Camellia sinensis) cultivars of India. 3 Biotech. 2015;5(3):1–10. doi: 10.1007/s13205-014-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RA, Lu SS, Bresil H, et al. DNA ethylation in target and non-target organs of hamsters and rats treated with diethylnitrosamine. Cancer Lett. 1985;26(1):17–24. doi: 10.1016/0304-3835(85)90168-5. [DOI] [PubMed] [Google Scholar]
- Begum A, Sandhya S, Ali SS, et al. An in-depth review on the medicinal flora rosmarinus officinalis (Lamiaceae) Acta Sci Pol Technol Aliment. 2013;12(1):61–73. [PubMed] [Google Scholar]
- Bhatt R, Misha N, Bansal PK. Phytochemical, pharmacological and pharmacokinetics effects of rosmarinic acid. J Pharm Sci Innov. 2013;2(2):28–34. [Google Scholar]
- Bogdanski P, Suliburska J, Szulinska M, et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32(6):421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Bowers LD. Kinetic serum creatinine assays I, the role of various factors in determining specificity. Clin Chem. 1980;26:551–554. [PubMed] [Google Scholar]
- Butterfield A, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochimica et Biophysica Acta. 2014;1842(9):1693–1706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkov-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Comprehensive Invited Review. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as an index of lipid peroxidation. Methods Enzymol. 1990;186(B):421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- El-Beih NM, Ramadan G, Talaat RM, et al. Alleviative effects of green and black tea aqueous extracts on cellular oxidative stress and anemia in rat adjuvant-induced arthritis. Indian J Trad Know. 2015;14(3):335–343. [Google Scholar]
- El-Boshy MS, Header ElSawy NA, et al. Studies on the constituents of rosmarinus EA officinalis and their synergistic effect in experimental diabetic rats. J Invest Biochem. 2015;4(1):36–43. [Google Scholar]
- Eybl V, Kotyzova D, Cerna P, et al. Effect of melatonin, curcumin, quercetin, and resveratrol on acute ferric nitrilotriacetate (Fe-NTA)-induced renal oxidative damage in rats. Hum Exp Toxicol. 2008;27(4):347–353. doi: 10.1177/0960327108094508. [DOI] [PubMed] [Google Scholar]
- Feng WY. Metabolic fate of green tea catechins in humans. In: Preedy VR, editor. Tea in health and disease prevention. Vol. 81. USA: Academic Press; 2013. pp. 953–969. [Google Scholar]
- Forester SC, Lambert JD. Cancer preventive effects of green tea polyphenols. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in human health and disease. USA: Academia Press; 2014. pp. 1309–1322. [Google Scholar]
- Forman HJ, Davies KJA, Ursini F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis Versus Free Radical Scavenging in vivo. Free Radical Biology and Medicine. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado RA, Oliveira BR, Silva LR, et al. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. European Journal of Cancer Prevention. 2015;2(2):106–112. doi: 10.1097/CEJ.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Goupy P, Hugues M, Boivin P. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extract and of isolated phenolic compunds. J. Sci. Food Agric. 1999;79:1625–1634. [Google Scholar]
- Gupta A, Chander V, Sharma S, et al. Sodium nitroprusside and L-arginine attenuates ferric nitrilotriacetate-induced oxidative renal injury in rats. Toxicology. 2007;232(3):183–191. doi: 10.1016/j.tox.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sharma S, Kaur I, et al. Renoprotective effects of sesamol in ferric nitrilotriacetate-induced oxidative renal injury in rats. Basic Clin Pharmacol Toxicol. 2009;104(4):316–321. doi: 10.1111/j.1742-7843.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Athar M. Attenuation of iron-nitrilotriacetate (Fe-NTA)-mediated renal oxidative stress, toxicity and hyperproliferative response by the prophylactic treatment of rats with garlic oil. Food Chem Toxicol. 1998;36(6):485–495. doi: 10.1016/s0278-6915(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2010;28(5):703–713. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma S, Sultana S. Amelioration of ferric nitrilotriacetate (Fe-NTA) induced renal oxidative stress and tumor promotion response by coumarin (1,2-benzopyrone) in Wistar rats. Cancer Lett. 2004;210(1):17–26. doi: 10.1016/j.canlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Khouja H, AlNahari H. Effects of fresh, unprocessed green tea camellia sinensis extract on liver function, lipid profile (cholesterol and triglycerides), thyroid stimulating hormone (TSH) and cortisol in normal healthy subjects. J Life Sci Res. 2015;2(1):18–24. [Google Scholar]
- Kim SY, Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol Ther. 2012;20(3):268–272. doi: 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A, Binepal G, Gangar SC. Impediment of diethylnitrosamine induced hepatotoxicity in male balb/c mice by pretreatment with aqueous azadirachtaindica leaf extract. Indian J Exp Biol. 2007;45:359–366. [PubMed] [Google Scholar]
- Mannaa FA, Abdalla MS, Abdel-Wahhab KG, et al. Effect of some nutraceutical agents on aluminum-induced functional neurotoxicity in senile rats: I. Effect of rosemary aqueous extract and docosahexaenoic acid. J Appl Sci Res. 2013;9(3):2322–2334. [Google Scholar]
- Mohamed DA, Al-Okbi SY. Evaluation of anti-gout activity of some plant food extracts. Pol J Food Nutr sci. 2008;58(3):389–395. [Google Scholar]
- Okada K, Wangpoengtraku C, Tanaka T, et al. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131(8):2090–2095. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- Olvera CYV, Gonzalez DJS, Solano JD, et al. Characterization of N-diethylnitrosamine-initiated and ferric nitrilotriacetate-promoted renal cell carcinoma experimental model and effect of a tamarind seed extract against acute nephrotoxicity and carcinogenesis. Mol Cell Biochem. 2012;369(1–2):105–117. doi: 10.1007/s11010-012-1373-0. [DOI] [PubMed] [Google Scholar]
- Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br J Nutr. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- Ramalho LNZ, Pasta AAC, Terra VA, et al. Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats. Food Chem Toxicol. 2014;74:270–278. doi: 10.1016/j.fct.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Rashid S, Ali N, Nafees S, et al. Amelioration of renal carcinogenesis by bee propolis: A chemo preventive Approach. Toxicol Intl. 2013;20(3):227–235. doi: 10.4103/0971-6580.121676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H, Krishnasamy Y, Haque K, et al. Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporine a treatment in rats. PLoS One. 2013;8(6):1–12. doi: 10.1371/journal.pone.0065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman MU, Sultana Attenuation of oxidative stress, inflammation and early markers of tumor promotion by caffeic acid in Fe-NTA exposed kidneys of wistar rats. Mol Cell Biochem. 2011;357(1-2):115–124. doi: 10.1007/s11010-011-0881-7. [DOI] [PubMed] [Google Scholar]
- Rocha J, Eduardo-Figueira M, Barateiro A. Anti-inflammatory Effect of Rosmarinic Acid and an Extract of Rosmarinus officinalis in Rat Models of Local and Systemic Inflammation. Basic Clin Pharmacol Toxicol. 2014;116(5):398–413. doi: 10.1111/bcpt.12335. [DOI] [PubMed] [Google Scholar]
- Satoh T, McKercher SR, Lipton SA. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radical Biology and Medicine. 2013;65:645–657. doi: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmila R, Sindhu G, Arockianathan PM. Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J Basic Clin Physiol Pharmacol. 2016;27(5):1–10. doi: 10.1515/jbcpp-2015-0085. [DOI] [PubMed] [Google Scholar]
- Shivashankara AR, Kumar A, Ravi R, et al. Hepatoprotective effects of green tea and its polyphenols, preclinical observations. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in human health and disease. 55. Vol. 1. USA: Academic Press; 2014. pp. 715–721. [Google Scholar]
- Sundaram S, Kanniappan GV, Kannappan P. Enzymatic and non enzymatic antioxidantactivity of tabernaemontana divaricate R.Br. against DEN and Fe-NTA induced renal damage in wistar albino rats. J Appl Pharm Sci. 2015;5(05):033–037. [Google Scholar]
- Talke HN, Schubert GE. Enzymatic determination of urea in blood and serum in the optical test according to Warburg. Klin Wschr. 1965;43(3):174–175. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- Tavafi M, Ahmadvand H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 2011;43:392–397. doi: 10.1016/j.tice.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Tavafi M, Ahmadvand H, Khalatbari A, et al. Rosmarinic acid ameliorates diabetic nephropathy in uninephrectomized diabetic rats. Iran J Basic Med Sci. 2011;14(3):275–283. [Google Scholar]
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem. 1969;6(1):24–27. [Google Scholar]
- Trinder P, Barham D. An improved color reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97(1151):142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Mandel S, Youdim MBH. Neuroprotective molecular mechanisms of (2)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009;4:283–296. doi: 10.1007/s12263-009-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]