Abstract
Objective
At present, little is known regarding the specific risk factors of ocular metastasis (OM) in elderly patients with lung cancer. This study aimed to find out the risk factors of ocular metastasis.
Methods
A total of 1615 elderly patients with lung cancer were recruited into this retrospective study between April 2001 and July 2016. These patients were divided into two groups, namely OM and non-ocular metastasis (NOM). Student’s t-tests, nonparametric rank sum tests, and Chi-square tests were applied to describe whether there were significant differences between the OM group and NOM group. We compared a range of serum biomarkers between the two groups of patients, including alkaline phosphatase, calcium, hemoglobin, alpha-fetoprotein, carcinoembryonic antigen, CA-125, CA-199, CA-153, CA-724, cytokeratin-19 fragment (CYFRA21-1), TPSA and neuron specific enolase (NSE). We used binary logistic regression analysis to determine the risk factors and receiver operating curve (ROC) analyses to assess the diagnostic value for OM in CRC patients.
Results
The incidence of OM in elderly patients with lung cancer was 2.0%. Binary logistic regression indicated that CA-125, CA-153, and total prostate specific antigen (TPSA) were identified as independent risk factors of OM in patients with lung cancer (P<0.001, P<0.001, and P=0.003, respectively). The sensitivity and specificity of OM diagnosis were as follows: CA-125, 81.25% and 81.57%; CA-153, 68.75% and 83.78%; and TPSA, 81.25% and 90.03%, respectively.
Conclusion
The serum concentrations of CA-125, CA-153, and TPSA have predictive value in the diagnosis of OM in elderly patients with lung cancer.
Keywords: elderly people, lung cancer, ocular metastases, risk factors
Introduction
Previous studies have shown that lung cancer is closely related to age1,2 for a variety of reasons, including the fact that elderly people have poor immune mechanisms3 and because the evolution of lung cancer is shaped via immunoediting mechanisms. Furthermore, lung cancer is associated with genetic4 and environmental factors, including microbes of the airway5 and second-hand smoke.6 The susceptibility of lung cancer in elderly people increases with prolonged exposure to such factors.
The incidence of lung cancer with ocular metastasis (OM) is more common with lung adenocarcinoma.7 In the review of 42 lung cancer cases with OM, small cell lung cancer (SCLC) accounted for 21.4% (n=9), adenocarcinoma accounted for 47.6% (n=20), other types of lung cancer also included neuroendocrine tumors (n=5), large cell carcinoma (n=2), lung squamous cell carcinoma (n=3), lung adenosquamous carcinoma (n=1), and 2 cases of non-small cell lung cancer (NSCLC).8 Furthermore, lung adenocarcinoma often metastasizes to the brain, liver, kidneys, bone, bone marrow, and adrenal glands.8 Metastasis can also occur to other parts of the body, albeit rarely, including the eyes, nose, parotid gland, and paranasal sinus.9 The eye is a rare site for the metastasis of lung cancer because of the absence of a lymphatic system.10 However, OM remains one of the greatest challenges to the quality of life (QOL) of elderly patients with lung cancer.8 Unfortunately, the process that determines whether cancer spreads from the original tumor site to reach distant organs remains poorly understood, despite the devastating consequences of metastasis on disease prognosis.11 For the occurrence of metastasis, the tumor must travel through the body, including the bloodstream. Therefore, serum biomarkers may be significant indicators of unusual activity in blood vessels.
Metastases to the ocular structures occur by hematogenous spread. Therefore, the parts of the eye with the best vascular supply are most likely to be affected.10 Ocular metastasis involves both intraocular metastasis and orbital metastasis. Most cases of intraocular metastasis involve carcinomas; the majority of this form of metastasis originate from breast cancer in females and lung cancer in males.7 Orbital metastatic disease, as the initial presentation of lung adenocarcinoma, is very rare. Consequently, our lack of knowledge regarding this process and the variety of different clinical presentations, may result in misdiagnosis and an unfavorable prognosis.12,13
However, for lung cancer patients with ocular symptoms as the initial manifestation, the diagnosis of OM and primary lung cancer is relatively difficult. Furthermore, the length of time required for the diagnosis is relatively long.7 Consequently, there is an urgent need to improve the diagnosis of OM in elderly patients with lung cancer. Computed tomography (CT) scans and the subsequent pathological analysis of tissue biopsies remain the gold standard approach for the diagnosis of cancer in numerous Chinese hospitals. However, the serological analysis of serum biomarkers is also routinely used in clinical practice. In this study, we investigated whether OM may be detected by the presence of cancer biomarkers in elderly patients with lung cancer. We also attempted to identify correlations between a variety of risk factors and OM, and establish an accurate indicator with which to distinguish OM from non-ocular metastasis (NOM) in this type of patients. Our aim was to lay the foundation for targeted local and systematic anti-cancer treatment strategies.7
Materials and Methods
Study Design
This was a retrospective study, which included a series of consecutive elderly patients (aged ≥60 years) diagnosed with lung cancer between April 2001 and July 2016. A total of 1615 elderly patients with lung cancer were recruited. These patients were divided into two groups, namely OM and NOM. The diagnosis of OM was reached through both CT and magnetic resonance imaging (MRI). The inclusion criteria for the OM group were as follows: 1) patients without primary ocular malignant cancer, such as extraocular muscle lymphoid neoplasms and orbital primary tumors; and 2) patients without ocular benign tumors, such as orbital inflammatory pseudotumors. The inclusion criteria for the NOM group were patients without organ metastases or lymph node metastases. All medical records were updated throughout the study. We also collected basic demographic and clinical data prior to the introduction of any form of treatment (i.e., surgery, chemotherapy, radiotherapy, and symptomatic treatment).
Data Collection
Relevant serological data (e.g., age, gender, histopathological types, and treatments) were acquired from the diagnostic reports of the patients. We then compared a range of serum biomarkers between the two groups of patients, including alkaline phosphatase (ALP), calcium, hemoglobin (HB), alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA-125, CA-199, CA-153, CA-724, cytokeratin-19 fragment (CYFRA21-1), TPSA and neuron specific enolase (NSE). All clinical data were collected at the time of initial diagnosis of lung cancer.
Statistical Analyses
Chi-squared tests and Student’s t-tests were used to evaluate differences in gender and age between the OM and NOM groups. Independent risk factors of OM were investigated using binary logistic regression models. Receiver operating characteristic (ROC) curves were created, and the areas under the curve (AUC) were used to assess the diagnostic value of OM in elderly patients with lung cancer and estimate the accuracy of OM diagnosis. A P < 0.05 denoted statistical significance. Statistical tests were performed using the Microsoft Office Excel 2016 (Microsoft Corp, USA), SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA), and MedCalc18.6.0 statistical software (MedCalc, Ostend, Belgium). Continuous data, represented as means ± standard deviation.
Results
Demographics and Clinical Characteristics
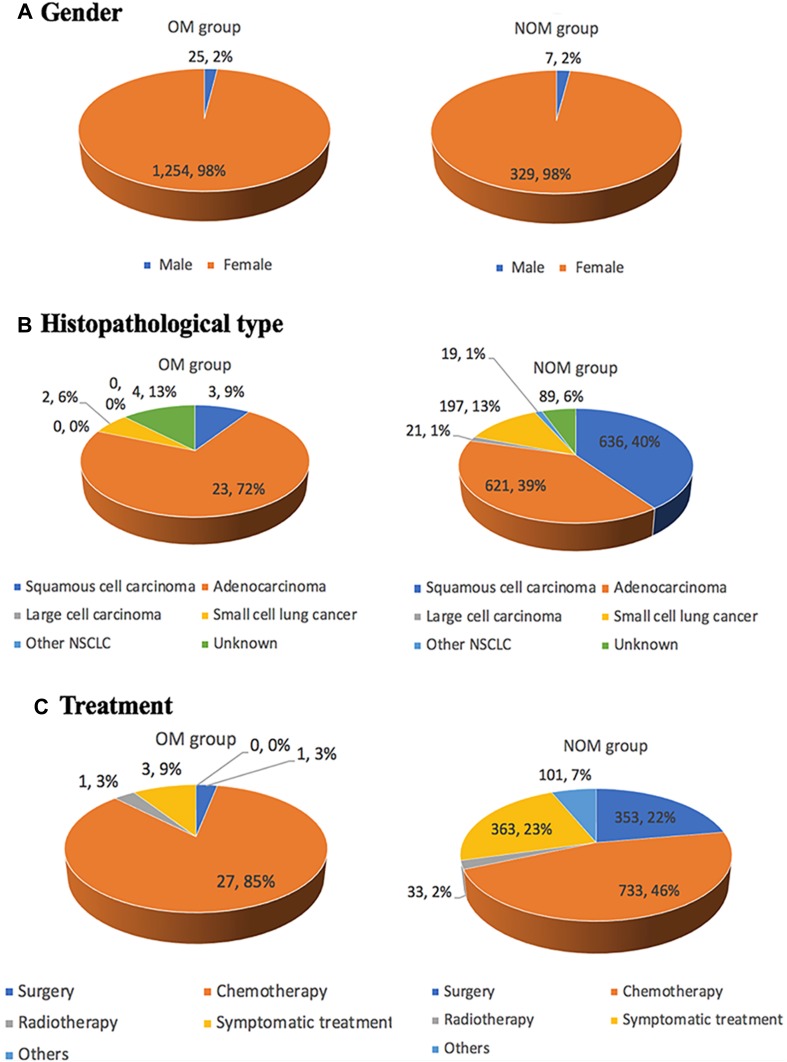
In total, 32 OM patients (i.e., 22 and 10 patients with orbital and intraocular metastasis, respectively), and 1583 NOM patients, were analyzed in this study. The mean age in the OM and NOM groups was 67.7±5.3 and 68.3±6.2 years, respectively. Chi-squared tests and Student’s t-tests did not show significant differences (P > 0.05) in terms of gender or age between the OM and NOM groups. However, there was a significant difference observed between these two groups in the histopathological type (P < 0.05). Notably, adenocarcinoma was diagnosed in a large proportion of elderly lung cancer patients with OM using histopathology. Therefore, the histological subtype of adenocarcinoma may be associated with a higher risk of developing OM in elderly patients with lung cancer. Squamous cell carcinoma was the most common type reported in the NOM group. From 2001 to 2016, an increasing number of elderly patients selected chemotherapy and symptomatic treatment. This was attributed to the efficacy of chemotherapy, and the delay in the presentation of individual symptoms following the administration of symptomatic treatment. Table 1 and Figure 1 show the detailed clinical data of all elderly patients included in this study.
Table 1.
Clinical Characteristics of Elderly Patients with Lung Cancer
| Patient Characteristics | OM Groupa (%) | NOM Group (%) | P valued |
|---|---|---|---|
| (n=32) | (n=1583) | ||
| Genderb | |||
| Male | 25(78.1) | 1254(79.22) | 0.880 |
| Female | 7(21.9) | 329(20.78) | |
| Agec | |||
| Mean | 67.7±5.3 | 68.3±6.2 | 0.980 |
| Histopathological typeb | |||
| Squamous cell carcinoma | 3(9.4) | 636(40.2) | 0.001 |
| Adenocarcinoma | 23(71.9) | 621(39.2) | |
| Large cell carcinoma | 0(0.0) | 21(1.3) | |
| Small cell lung cancer | 2(6.3) | 197(12,4) | |
| Other NSCLC | 0(0.0) | 19(1.2) | |
| Unknown | 4(12.4) | 89(5.7) | |
| Treatment | |||
| Surgery | 1(3.1) | 353(22.3) | |
| Chemotherapy | 27(84.4) | 733(46.3) | |
| Radiotherapy | 1(3.1) | 33(2.1) | |
| Symptomatic treatment | 3(9.4) | 363(22.9) | |
| Others | 0(0.0) | 101(6.4) |
Notes: aThe OM group included 10 patients with orbital metastasis and 22 patients with intraocular metastasis. bChi-squared test. cStudent’s t-test. dComparison between the OM and NOM groups. P < 0.05 denoted statistical significance.
Abbreviations: OM, ocular metastasis; NOM, non-ocular metastasis; NSCLC, non-small cell lung cancer.
Figure 1.
Clinical characteristics of elderly patients with lung cancer.
Notes: (A), Gender; (B), Histopathological type; (C), Treatment.
Clinical Data and Risk Factors of OM
Comparison of the tumor biomarker data obtained from patients in the OM and NOM groups showed that the concentrations of AFP, CEA, CA-125, CA-199, CA-153, CYFRA21-1, and TPSA in the OM group were significantly higher compared with those measured in the NOM group (P < 0.05). In contrast, the concentration of HB was lower in the OM group (P < 0.05). There were no significant differences in the serum concentrations of ALP, calcium, CA-724, and NSE between the two groups (P > 0.05). The results from this analysis are shown in Table 2. Moreover, binary logistic regression analysis showed that CA-125, CA-153, and TPSA were independent risk factors of OM (Table 3).
Table 2.
Differences in the Concentration of Various Tumor Biomarkers Between Elderly Lung Cancer Patients with and Without OM
| Tumor Biomarkers | OM Group | NOM Group | t-test | P value |
|---|---|---|---|---|
| ALP (U/L) | 108.25±10.39 | 94.73±1.89 | 1.281 | 0.209 |
| Calcium (mmol/L) | 2.19±0.03 | 2.25±0.01 | −1.787 | 0.083 |
| AFP (ng/mL) | 3.22±0.39 | 1.75±0.04 | 5.559 | <0.001 |
| CEA (ng/mL) | 416.67±118.08 | 38.66±4.62 | 10.377 | <0.001 |
| CA-125 (U/mL) | 378.63±78.31 | 64.39±4.22 | 9.930 | <0.001 |
| CA-199 (U/mL) | 160.42±53.37 | 38.95±6.65 | 2.563 | 0.010 |
| CA-153 (U/mL) | 54.99±10.63 | 19.32±0.75 | 6.483 | <0.001 |
| CA-724 (U/mL) | 9.05±3.88 | 12.80±3.47 | −0.721 | 0.484 |
| CYFRA21-1(ng/mL) | 29.28±4.16 | 9.37±0.75 | 4.714 | <0.001 |
| TPSA (ng/L) | 4.59±0.45 | 1.63±0.10 | 6.420 | <0.001 |
| NSE (μg/L) | 25.77±2.82 | 25.25±0.97 | 0.176 | 0.861 |
| HB (g/L) | 108.34±3.55 | 117.41±0.47 | −2.529 | 0.017 |
Notes: Independent sample t-test. P < 0.05 denoted statistical significance.
Abbreviations: ALP, Alkaline phosphatase; OM, ocular metastasis; NOM, non-ocular metastasis; HB, hemoglobin.
Table 3.
Risk Factors of OM in Elderly Patients with Lung Cancer
| Factors | B | Exp(B) | OR (95% CI) | P |
|---|---|---|---|---|
| AFP | 0.311 | 1.365 | 1.204–1.548 | <0.001 |
| CEA | 0.002 | 1.002 | 1.001–1.003 | <0.001 |
| CA-125 | 0.002 | 1.002 | 1.002–1.003 | <0.001 |
| CA-199 | <0.001 | 1.000 | 1.000–1.001 | <0.001 |
| CA-153 | 0.011 | 1.011 | 1.006–1.016 | <0.001 |
| CYFRA21-1 | 0.008 | 1.008 | 1.003–1.012 | 0.002 |
| TPSA | 0.043 | 1.044 | 1.015–1.074 | 0.003 |
| HB | −0.025 | 0.975 | 0.958–0.993 | 0.007 |
Notes: Binary logistic regression analysis. P < 0.05 denoted statistical significance
Abbreviations: B, coefficient of regression; OR, odds ratio; CI, confidence interval; OM, ocular metastasis.
Cut-Off Value, Sensitivity, Specificity, and AUC of CA-125, CA-153, and TPSA in the Diagnosis of OM
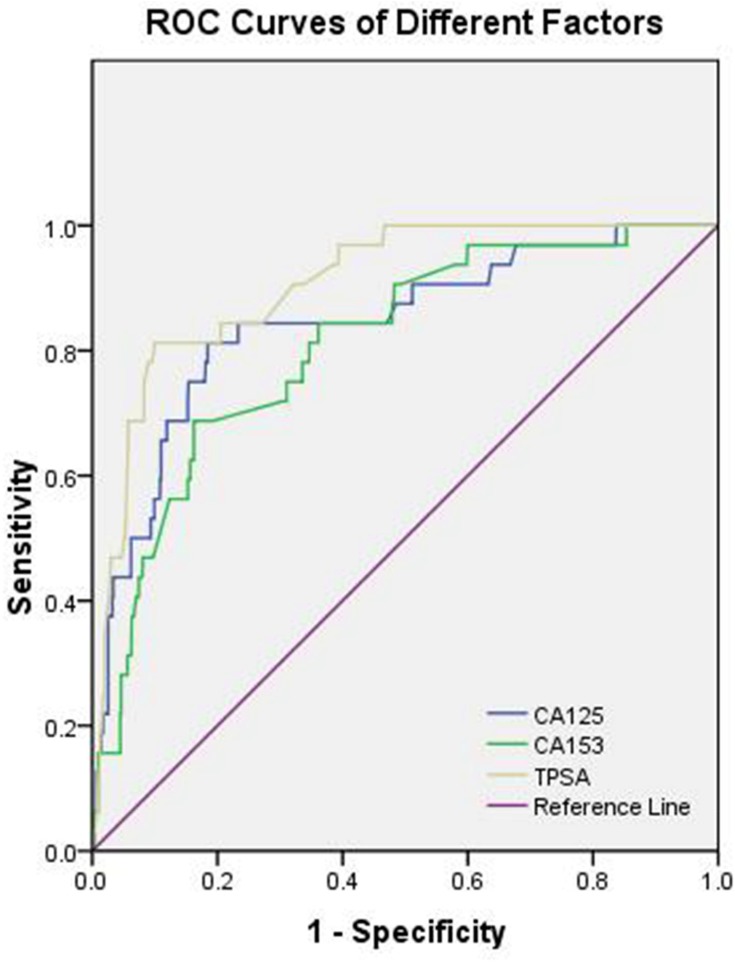
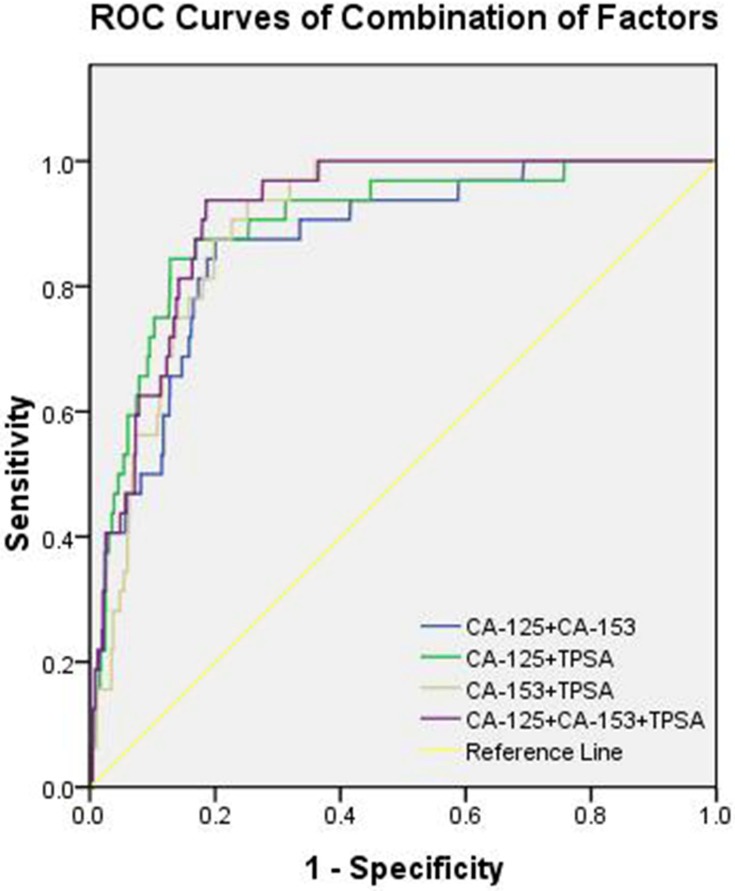
Table 4 shows that the cut-off values for CA-125, CA-153, and TPSA were 66.00 U/mL, 23.27 U/mL, and 2.53 ng/mL, respectively. Of note, the AUC of TPSA yielded the highest value. Figure 2 shows the ROC curves for CA-125, CA-153, and TPSA as single factors. We subsequently tested different combinations of these three risk factors in pairs, as well as in combination. Figure 3 shows the ROC curves of the following combinations: CA-125 + CA-153, CA-125 + TPSA, CA-153 + TPSA, and CA-125 + CA-153 + TPSA. We found that the combination of CA-125 + CA-153 + TPSA showed the highest AUC values, which reached 0.913. Of these indicators, CA-153 + TPSA, CA-125 + CA-153 + TPSA, and CA-153 + TPSA showed the highest sensitivity (93.75%), while TPSA showed the highest specificity (90.03%). All these findings were statistically significant (P < 0.05).
Table 4.
The Cut-off Value, Sensitivity, Specificity, and AUC of Single Risk Factors for the Prediction of OM in Elderly Lung Cancer Patients
| Factor | Cut-Off Value | Sensitivity (%) | Specificity (%) | AUC | P |
|---|---|---|---|---|---|
| CA-125 (U/mL) | 66.00 | 81.25 | 81.57 | 0.842 | <0.001 |
| CA-153 (U/mL) | 23.27 | 68.75 | 83.78 | 0.808 | <0.001 |
| TPSA (ng/mL) | 2.53 | 81.25 | 90.03 | 0.906 | <0.001 |
| CA-125+CA-153 | – | 87.50 | 79.92 | 0.868 | <0.001 |
| CA −125+TPSA | – | 84.37 | 87.18 | 0.898 | <0.001 |
| CA-153+TPSA | – | 93.75 | 74.81 | 0.895 | <0.001 |
| CA-125+CA-153+TPSA | – | 93.75 | 81.44 | 0.913 | <0.001 |
Notes: Sensitivity and specificity were acquired at the cut-off value. P < 0.05 denoted statistical significance.
Abbreviations: AUC, area under the curve; TPSA, total prostate specific antigen.
Figure 2.
The receiver operating characteristics (ROC) curves of risk factors for detecting OM in elderly lung cancer patients, the ROC curve of CA-125, CA-153 and TPSA.
Figure 3.
The receiver operating characteristics (ROC) of different combinations of risk factors for diagnosing OM, the ROC curves of CA-125+CA-153, CA-125+TPSA, CA-153+TPSA and CA-125+CA-153+TPSA.
Discussion
Herein, we review recent data and provide an in-depth discussion regarding the clinical features and course of OM in lung cancer. Previous studies have shown that the incidence of lung cancer is closely related to age, with the highest incidence rates reported in elderly individuals.2 Men are at a higher risk of developing lung cancer versus women due to the long-term use of tobacco. In our study, this ratio was 3.8:1. However, in recent years, an unusually large number of non-smoking women from East Asia develop lung cancer.11 The study of patients who have never smoked revealed that the causes of lung cancer are variable.11
The most common sites of metastasis in elderly patients with lung cancer are the brain, liver, kidneys, bone, bone marrow, and adrenal glands. Rarely, lung cancer metastasizes to the eye, nose, parotid gland, and paranasal sinus. Although the rate of OM is lower than those of other metastases, it remains one of the most significant diagnostic criteria for lung cancer. Symptomatic OM may be an early event of lung cancer.14 Moreover, widespread OM is the first sign of systemic metastasis from a primary neoplasm.15 However, metastasis of lung cancer to the eye is rare and thus, may be recognized late.16
In recent years, there have been considerable developments in certain medical treatments. For example, Hoffman suggested that low-dose computed tomography may be a useful prospective treatment. Moreover, a National Lung Screening Trial showed that screening through low-dose computed tomography may reduce lung cancer-related mortality in high-risk patients by 20% compared with chest radiography.17 Furthermore, the use of regenerative therapy with plasma rich in growth factors (PRGF) can stimulate the healing process, and permit the management of inflammation in the ocular surfaces.18 Nevertheless, such medical treatments rely on the accurate and timely diagnosis of disease. In the diagnosis of cancer, traditional histopathological examination, immunohistochemistry, and cytogenetic analysis are cumbersome and time-consuming. In contrast, serological examination is convenient and routinely practiced compared with CT or MRI. Some serological indicators may represent risk factors of lung cancer. Therefore, the identification of a panel of predictive risk factors may assist timely intervention, resulting in the prevention and delay of OM. Following the diagnosis of lung cancer, the treatment of choice is multimodal treatment, including surgery, radiotherapy and chemotherapy.
In Table 5, we reviewed some risk factors of metastases of lung cancer. However, very few studies have investigated the association between OM and lung cancer. In the present study, we collected serum from appropriate patients and determined the levels of ALP, calcium, HB, AFP, CEA, CA-125, CA-199, CA-153, CA-724, CYFRA21-1, TPSA, and NSE. Based on the analysis of a large population, we measured the concentrations of CA-125, CA-153, and TPSA as independent risk factors of OM in elderly individuals with lung cancer (P<0.01, P<0.01, and P=0.03). Subsequently, we determined the cut-off value, sensitivity, specificity, and AUC for each of these biomarkers. Finally, we inferred that CA-125, CA-153, and TPSA represent important risk factors of OM in elderly patients with lung cancer. Using the ROC curves of these biomarkers, it is possible to develop reliable clinical tests. Blood levels above the cut-off values for CA-125 (> 66.00 U/mL), CA-153 (> 23.37 U/mL), and TPSA (> 2.53 ng/mL) represent a critical point for the occurrence of OM in patients with lung cancer. Based on this, further detailed diagnostic techniques (e.g., CT and MRI of the eye) should be utilized. These methods have an excellent capability to detect OM and therefore, facilitate clinical management. TPSA exhibited the highest AUC and therefore, showed the greatest accuracy in distinguishing elderly lung cancer patients with OM. In the current study, we combined independent risk factors to improve accuracy. A particularly novel aspect of our research was the identification of four different combinations of the markers CA-125, CA-153, and TPSA. The combination of CA-125 + CA-153 + TPSA showed the highest accuracy for predicting OM in elderly patients with lung cancer, while CA-125 and CA-153 + TPSA showed relatively high sensitivity and specificity. Thus, the combination of CA-125, CA-153, and TPSA appears to be more accurate in the prediction of OM.
Table 5.
The Risk Factors of Metastases of Lung Cancer
| Author | Year | Histopathological Type | Metastatic Sites | Risk Factor |
|---|---|---|---|---|
| Morita et al20 | 2019 | NSCLC | Intertrabecular Vertebral | CEA |
| Zhou et al21 | 2017 | NS | Bone | CA-125, ALP |
| Liu et al22 | 2017 | Adenocarcinoma | Brain, lymph node | CYFRA21-1 |
| Wu et al23 | 2017 | NSCLC | Lymph node | MicroRNA-422a |
| Chu et al24 | 2017 | Adenocarcinoma | Lymph node | CLSTN1, CLU, NGAL |
| Chen et al25 | 2015 | NSCLC | Brain | NSE |
| Chen et al26 | 2015 | NS | Lymph node | CYFRA21-1, CEA |
| Lee et al27 | 2012 | NSCLC | Brain | CEA |
| Cabreraalarcon28 | 2011 | NS | NS | CYFRA21-1 |
| Cedrés29 | 2011 | NSCLC | Brain | CEA, CYFRA21-1, CA-125 |
| Oshiro et al30 | 2004 | Adenocarcinoma | Liver | AFP |
| Pollán et al31 | 2003 | NSCLC | NS | CA-125 |
| NiklińskiJ32 | 1992 | NSCLC | Lymph node | SCC |
Abbreviations: NS, not specific; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma antigen; CLSTN1, calsyntenin-1; CLU, clusterin; NGAL, neutrophil gelatinase-associated lipocalin.
Our study was characterized by limitations, which need to be considered. Firstly, this was a retrospective study. The exclusive enrollment of elderly patients may limit the generalization of these findings. Furthermore, we did not include transient data from the time of primary diagnosis, the survival rate of elderly patients, and the duration of follow-up. In addition, the study was conducted in a single medical center with a relatively small sample size. Consequently, our results may not represent the whole population of patients with OM. Thus, further studies involving a larger sample size and several centers are required. Such studies may improve the effectiveness of these risk factors in predicting OM among elderly patients with lung cancer. Finally, the metastatic stage of cancer is usually lethal.19 The majority of our patients were not classified by tumor type. Therefore, we were unable to identify risk factors of OM according to tumor stage.
In conclusion, based on our investigation, the incidence of OM in lung cancer patients was 1.98%. The most common histopathological subtype among patients with OM was adenocarcinoma. The serum concentrations of CA-125, CA-153, and TPSA were identified as independent risk factors of OM in elderly patients with lung cancer. Moreover, we found that the combination of CA-125, CA-153, and TPSA has great predictive value due to the high sensitivity and specificity of these factors for OM. Prospective, multicenter studies are necessary to verify the present results.
Ethics Statement
This study was approved by the medical research ethics committee of the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China. Informed written informed consent was provided by all patients included in this study. All methods were performed in accordance with the Declaration of Helsinki.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This research is supported by National Natural Science Foundation of China (Nos: 81660158, 81400372, and 81460092).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Eguchi T, Bains S, Lee MC, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35(3):281–290. doi: 10.1200/JCO.2016.69.0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara R, Mezquita L, Auclin E, Chaput N, Besse B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: does age really matter. Cancer Treat Rev. 2017;60:60–68. doi: 10.1016/j.ctrv.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal R, Cadieux EL, Salgado R, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567(7749):479–485. doi: 10.1038/s41586-019-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134(7):728–733. doi: 10.1001/jamaophthalmol.2016.0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacks T. Lung tumours swell when prodded by airway microbes. Nature. 2019;566:11. doi: 10.1038/d41586-019-00357-w [DOI] [Google Scholar]
- 6.Deweerdt S. Aetiology: crucial clues. Nature. 2014;513(7517):S12–S13. doi: 10.1038/513S12a [DOI] [PubMed] [Google Scholar]
- 7.Westerwick D, Driever F, Le Guin CHD, Schmid KW, Metz KA. Intraocular metastases. Pathologe. 2017;38(6):500–506. doi: 10.1007/s00292-017-0373-y [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Sun Y, Zhao J, et al. Ocular metastasis in lung cancer: a retrospective analysis in a single chinese hospital and literature review. Chin J Lung Cancer. 2017;20(5):326–333. doi: 10.3779/j.issn.1009-3419.2017.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ates I, Yazici O, Ates H, Ozdemir N, Zengin N. Unusual metastases of lung cancer: bulbus oculi and maxillary sinus. Exp Oncol. 2015;37(3):231–232. doi: 10.31768/2312-8852.2015.37(3):231-232 [DOI] [PubMed] [Google Scholar]
- 10.Cohen VM. Ocular metastases. Eye (Lond). 2013;27(2):137–141. doi: 10.1038/eye.2012.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbaraman N. Public health: a burning issue. Nature. 2014;513:S16–S17. doi: 10.1038/513S16a [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Qi Y, Sun X, Yu J, Meng X. Orbital metastasis as the initial presentation of lung adenocarcinoma: a case report. Onco Targets Ther. 2016;9:2743–2748. doi: 10.2147/OTT.S99583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa-Barberi G, Alba Linero C, Galván González FJ, Álvarez González E, Rey López A, Medina Rivero F. Study of aggressive carcinomas through orbital metastasis. Arch Soc Esp Oftalmol. 2018;S0365-6691(18):30336–30338. [DOI] [PubMed] [Google Scholar]
- 14.Su HT, Chen YM, Perng RP. Symptomatic ocular metastases in lung cancer. Respirology. 2008;13(2):303–305. doi: 10.1111/j.1440-1843.2007.01203.x [DOI] [PubMed] [Google Scholar]
- 15.Shields JA, Shields CL, Eagle RC, Gündüz K, Lin B. Diffuse ocular metastases as an initial sign of metastatic lung cancer. Ophthalmic Surg Lasers. 1998;29(7):598–601. [PubMed] [Google Scholar]
- 16.Schalenbourg A, Mantel I. The eye and cancer. Rev Med Suisse. 2015;11(499):2395–2398. [PubMed] [Google Scholar]
- 17.Hoffman RM, Sanchez R. Lung cancer screening. Med Clin North Am. 2017;101(4):769–785. doi: 10.1016/j.mcna.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarnieri A, Alfonso-Bartolozzi B, Ciufo G, Moreno-Montañés J, Gil-Bazo I. Plasma rich in growth factors for the treatment of rapidly progressing refractory corneal melting due to erlotinib in nonsmall cell lung cancer. Medicine (Baltimore). 2017;96(22):e7000. doi: 10.1097/MD.0000000000007000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egeblad M, de Visser KE. Sticking together helps cancer to spread. Nature. 2019;566(7745):459–460. doi: 10.1038/d41586-019-00341-4 [DOI] [PubMed] [Google Scholar]
- 20.Morita S, Suda T, Oda C, et al. The value of F-FDG PET in the diagnosis of intertrabecular vertebral metastasis in a small cell lung cancer patient with a high serum CEA level. Intern Med. 2019;58(3):415–418. doi: 10.2169/internalmedicine.1394-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Yu QF, Peng AF, et al. The risk factors of bone metastases in patients with lung cancer. Sci Rep. 2017;7(1):8970. doi: 10.1038/s41598-017-09650-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Liu D, Li L, et al. The important role of circulating CYFRA21-1 in metastasis diagnosis and prognostic value compared with carcinoembryonic antigen and neuron-specific enolase in lung cancer patients. BMC Cancer. 2017;17:96. doi: 10.1186/s12885-017-3070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu L, Hu B, Zhao B, et al. Circulating microRNA-422a is associated with lymphatic metastasis in lung cancer. Oncotarget. 2017;8(26):42173–42188. doi: 10.18632/oncotarget.15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Lai Y-H, Lee M-C, et al. Calsyntenin-1, clusterin and neutrophil gelatinase-associated lipocalin are candidate serological biomarkers for lung adenocarcinoma. Oncotarget. 2017;8(64):107964–107976. doi: 10.18632/oncotarget.22438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Peng W, Huang Y, et al. Significance of serum neuron-specific enolase before treatment in predicting brain metastases and prognosis of advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2015;37(7):508–511. [PubMed] [Google Scholar]
- 26.Chen F, Yan CE, Li J, et al. Diagnostic value of CYFRA 21-1 and CEA for predicting lymph node metastasis in operable lung cancer. Int J Clin Exp Med. 2015;8(6):9820–9824. [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol. 2012;33(4):1065–1073. doi: 10.1007/s13277-012-0344-0 [DOI] [PubMed] [Google Scholar]
- 28.Cabreraalarcon JL, Carrillovico A, Santotoribio JD, et al. CYFRA 21-1 as a tool for distant metastasis detection in lung cancer. Clin Lab. 2011;57(11–12):1011–1014. [PubMed] [Google Scholar]
- 29.Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2011;12(3):172–179. doi: 10.1016/j.cllc.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 30.Oshiro Y, Takada Y, Enomoto T, et al. A resected case of metachronous liver metastasis from lung cancer producing alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II)]. Hepatogastroenterology. 2004;51(58):1144–1147. [PubMed] [Google Scholar]
- 31.Pollan M, Varela G, Torres A, et al. Clinical value of p53, c-erbB-2, CEA and CA125 regarding relapse, metastasis and death in resectable non-small cell lung cancer. Int J Cancer. 2003;107(5):781–790. doi: 10.1002/ijc.v107:5 [DOI] [PubMed] [Google Scholar]
- 32.Nikliński J, Furman M, Laudański J, Kozłowski M. Evaluation of squamous cell carcinoma antigen (SCC-Ag) in the diagnosis and follow-up of patients with non-small cell lung carcinoma. Neoplasma. 1992;39(5):279–282. [PubMed] [Google Scholar]