Significance
Metal ions in the brain exhibit both functional (e.g., signal transduction, oxidative metabolism, and antioxidant defense) and pathological qualities (e.g., oxidative damage). Impaired metal ion homeostasis is linked to the decrease in enzymatic activities, the elevation of protein aggregation, and oxidative stress, leading to neurodegeneration. Particularly, copper coordinates to amyloid-β (Aβ) peptides, a pathological factor of Alzheimer’s disease, facilitating Aβ aggregation and inducing oxidative stress. Our work presents a mechanistic strategy to modify the coordination sphere of Cu(II) bound to Aβ using a chemical reagent by promoting copper–O2 chemistry, which can inhibit Cu(II) binding to Aβ and alter Aβ’s aggregation and toxicity. Our multidisciplinary studies demonstrate a direction for modulating copper-interacting amyloidogenic proteins.
Keywords: copper, amyloid-β, small molecule, copper–O2 chemistry, residue-specific modifications
Abstract
Neurotoxic implications of the interactions between Cu(I/II) and amyloid-β (Aβ) indicate a connection between amyloid cascade hypothesis and metal ion hypothesis with respect to the neurodegeneration associated with Alzheimer’s disease (AD). Herein, we report a mechanistic strategy for modifying the first coordination sphere of Cu(II) bound to Aβ utilizing a rationally designed peptide modifier, L1. Upon reacting with L1, a metal-binding histidine (His) residue, His14, in Cu(II)–Aβ was modified through either covalent adduct formation, oxidation, or both. Consequently, the reactivity of L1 with Cu(II)–Aβ was able to disrupt binding of Cu(II) to Aβ and result in chemically modified Aβ with altered aggregation and toxicity profiles. Our molecular-level mechanistic studies revealed that such L1-mediated modifications toward Cu(II)–Aβ could stem from the molecule’s ability to 1) interact with Cu(II)–Aβ and 2) foster copper–O2 chemistry. Collectively, our work demonstrates the development of an effective approach to modify Cu(II)–Aβ at a metal-binding amino acid residue and consequently alter Aβ’s coordination to copper, aggregation, and toxicity, supplemented with an in-depth mechanistic perspective regarding such reactivity.
Transition metal ions are critical components in the nervous systems, playing various structural and catalytic roles (1–3). In particular, copper is an indispensable element for energy metabolism, antioxidant defense, and the synthesis of neurotransmitters (4–7). Thus, the uptake and efflux of intracellular copper are tightly regulated in the brain (4, 6). Upon copper ion dyshomeostasis, vital copper-mediated functions become compromised with neurotoxicity observed in neurodegenerative disorders such as Alzheimer’s disease (AD) (4–9).
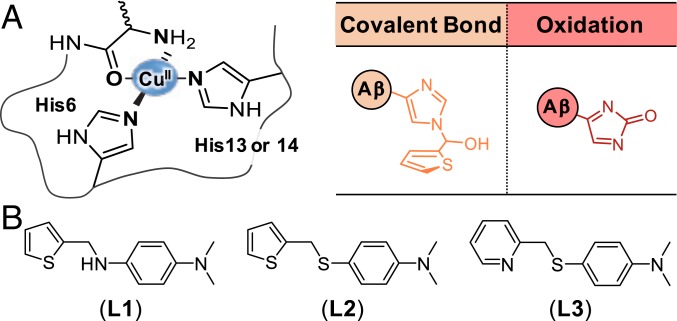
In the AD-afflicted brain, copper can be detected at concentrations as high as 400 μM in senile plaques, mainly composed of amyloid-β (Aβ) aggregates (4). The complexation between Cu(II) and Aβ and its neurotoxic implications have been previously reported (4, 8–12). The first coordination sphere of Cu(II) bound to Aβ typically consists of three nitrogen (N) donor atoms and one oxygen (O) donor atom, as depicted in Fig. 1A (11, 13). The N donor atoms consist of a combination of two histidine (His) residues (i.e., His6 and His13 or His14), an amide backbone, and the primary amine at the amino terminus (N terminus), while a carbonyl backbone between Asp1 and Ala2 serves as the O donor atom under physiological conditions (13, 14). The dissociation constant (Kd) of Cu(II) for Aβ is approximately 10−10 M (4, 11, 12, 15). Cu(II) binding to Aβ can accelerate Aβ aggregation and stabilize toxic structured Aβ oligomers (4, 16–18). In addition, redox-active Cu(I/II)–Aβ complexes can overproduce reactive oxygen species (ROS) via Fenton-like reactions, contributing to oxidative stress (4, 15, 19, 20).
Fig. 1.
Modifications at the Cu(II) coordination site and small molecules studied in this work. (A) Chemical modifications obtained from the reaction of Cu(II)–Aβ with L1. (B) Chemical structures of L1 [N1,N1-dimethyl-N4-(thiophen-2-ylmethyl)benzene-1,4-diamine], L2 [N,N-dimethyl-4-((thiophen-2-ylmethyl)thio)aniline], and L3 [N,N-dimethyl-4-((pyridin-2-ylmethyl)thio)aniline].
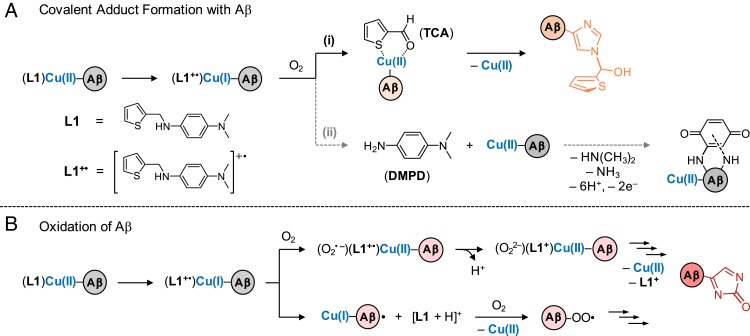
In an effort to regulate the reactivities of Cu(II)–Aβ complexes such as their aggregation and ROS generation, several metal chelators have been utilized to extract copper from Cu(II)–Aβ (21–26). Furthermore, rationally designed small molecules were reported to form a ternary complex with Cu(II)–Aβ and consequently change its properties (27–29). Upon reacting with such small molecules, Cu(II)–Aβ was indiscriminately oxidized (29), suggesting the potential significance of copper–O2 chemistry to modify Aβ (15, 19, 20). In this study, we hypothesize that specifically transforming the coordination sphere of Cu(II)–Aβ can be an effective strategy for disrupting Cu(II) binding to Aβ and chemically modifying Aβ to alter its aggregation and toxicity profiles. Herein, a mechanistic strategy is presented for specifically modifying His14, an amino acid residue involved in Cu(II)–Aβ coordination, through either covalent bond formation, oxidation, or both (Fig. 1A).
Results
Design and Preparation of a Small Molecule as a Peptide Modifier toward Cu(II)–Aβ.
Three main criteria were considered in designing our peptide modifier, L1 (Fig. 1B): 1) the accommodation of the coordination geometries for both Cu(I) and Cu(II) centers with respect to the formation of transient ternary complexes with Cu(I/II)–Aβ; 2) the redox potential of the designed molecule; and 3) the promotion of copper–O2 chemistry for Cu(I/II)–Aβ. First, to tailor the bidentate coordination to the copper center of Cu(I/II)–Aβ complexes, the sulfur (S) and N donor atoms in thiophen-2-ylmethanamine and the N,N-dimethylaniline (DMA) moiety, reported to be important for interacting with Cu(I/II) and Aβ, respectively (29, 30), were incorporated to produce L1. Under the assumption that redox chemistry of Cu(I/II)–Aβ at the copper center accounts for a major facet of a compound’s ability to modify Aβ, the chemical structure of L1 manifests S and N donor atoms anticipated to accommodate the coordination geometries of both Cu(I) and Cu(II) bound to Aβ based on the hard soft acid base (HSAB) principle. The redox potential of L1 was also considered with respect to the redox cycling between Cu(I)–Aβ and Cu(II)–Aβ. The redox property of the N,N-dimethyl-p-phenylenediamine (DMPD) moiety in L1 {E1/2: 0.11 V vs. Ag/Ag(I) in H2O (31, 32)} could be critical in the molecule’s capacity as a reducing agent for Cu(II)–Aβ {E0: approximately 0.083 V vs. Ag/Ag(I) in H2O (33)}. Lastly, the thiophene moiety acts as a weak σ-bonding ligand for copper (34), which suggests the potential for O2 binding at the metal center in Cu(I/II)–Aβ to promote copper–O2 chemistry leading to modifications of Aβ.
Based on the structure and properties of L1 as well as previous reports of copper–O2 chemistry (35–37), chemical transformation and reactivity of the compound toward Cu(II)–Aβ may be expected. For instance, N-dealkylation of L1 can be driven by Cu(I/II)–O2 chemistry to yield thiophen-2-carboxaldehyde (TCA) and DMPD (Scheme 1A) (38). Both TCA and DMPD may then undergo the conjugation with Aβ through multiple nucleophilic amino acid residues (31, 39). Moreover, oxidation of L1 to its radical cation (L1+•) can drive the reduction of Cu(II)–Aβ to Cu(I)–Aβ. The resultant Cu(I)–Aβ could then react with O2 to generate copper–O2 intermediates and subsequently modify Aβ (Scheme 1B).
Scheme 1.
Potential mechanisms for His14-specific modifications obtained upon the reaction of Cu(II)–Aβ with L1: (A) covalent adduct formation with Aβ and (B) oxidation of Aβ.
To better understand the structure-to-function relationship of L1, two additional compounds, L2 and L3, were constructed by replacing the C–N bond of L1 with a C–S bond (Fig. 1B). The DMA moiety, thought to be important for interacting with Aβ (29, 30), was maintained. The metal-interacting portions were also retained by incorporating a thiophen-2-ylmethanethioether group and a pyridin-2-ylmethanethioether group for L2 and L3, respectively. According to the HSAB theory, L2 can exhibit difficulty in accommodating the coordination geometries of both Cu(I) and Cu(II); L3 can be bound to both Cu(I) and Cu(II), similar to L1 (34). Both L2 and L3 are expected to be less oxidizable in comparison to L1 based on their structural portion, N,N-dimethyl-4-(methylsulfanyl)aniline (32). Overall, structural distinctions between L1 and L2 or L3 could allow us to investigate the significance of a molecule’s ability to directly bind to Cu(II)–Aβ and foster redox chemistry at the copper center to drive copper–O2 chemistry and, ultimately, modify Aβ.
As depicted in SI Appendix, Scheme S1, L1, L2, and L3 were prepared through reductive amination or substitution reactions. Detailed information for the synthesis and characterization of the compounds is provided in the SI Appendix, Scheme S1 and Figs. S1–S3. L1, L2, and L3 are predicted to cross the blood–brain barrier (BBB) with logBB values (logarithm of the ratio of the concentration of the compound in the brain to concentration in the blood) of 0.345, 0.702, and 0.338 and adhering to the Lipinski rule, as shown in SI Appendix, Table S1 (40, 41). In addition to the theoretical logBB values, the experimental permeability values (−logPe) of the compounds were obtained by the in vitro parallel artificial membrane-permeability assay adapted for the BBB (PAMPA–BBB). The values of −logPe of L1, L2, and L3 were 4.31 (± 0.13), 4.61 (± 0.22), and 4.35 (± 0.07), respectively, indicating the potential of the molecules to penetrate the BBB (40, 41).
Modifications of the Coordination Sphere of Cu(II)–Aβ.
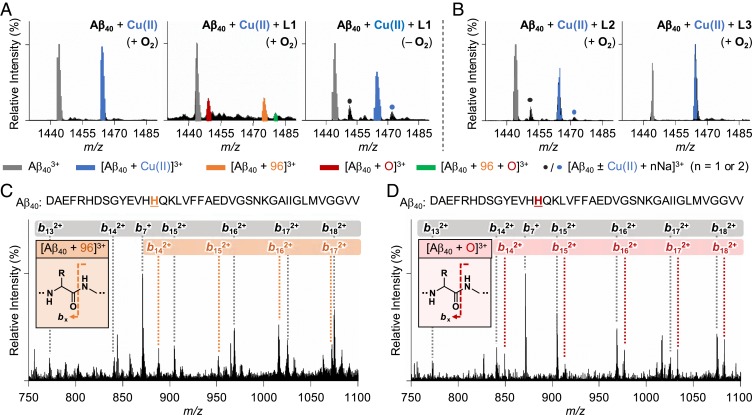
Cu(II)–Aβ complexes upon treatment with L1, L2, and L3 were analyzed by electrospray ionization–mass spectrometry (ESI–MS) and tandem MS (ESI–MS2). The sample of Aβ40 incubated with Cu(II) indicated a peak corresponding to Cu(II)-bound Aβ40 at 1,464 m/z (Fig. 2A). When L1 was introduced to Cu(II)–Aβ40, new peaks indicating modifications of Aβ were detected at 1,449, 1,475, and 1,480 m/z (vide infra for mechanistic details): corresponding to oxidation, covalent adduct formation, and dual-modification (both oxidation and covalent adduct formation), respectively. L2 and L3 did not noticeably affect Cu(II) binding to Aβ40, as presented in Fig. 2B. In the absence of Cu(II), such mass shifts from Aβ40 were not induced by the compounds (SI Appendix, Fig. S4).
Fig. 2.
Analysis of Cu(II)-treated Aβ40 upon incubation with L1 by ESI–MS and ESI–MS2. (A) ESI–MS spectra of the +3-charged Cu(II)-added Aβ40 monomer incubated with L1 in the absence and presence of O2. (B) ESI–MS spectra of the +3-charged Cu(II)-added Aβ40 monomer treated with L2 and L3 under aerobic conditions. The peaks of the covalent adduct between Aβ40 and 2-methylthiophene (1,475 m/z) and the singly oxidized Aβ40 (1,449 m/z) are highlighted in orange and red, respectively. The singly oxidized covalent adduct (1,480 m/z) is presented as a green peak. Na+ adducts of Aβ40 with or without Cu(II) are shown with blue and black dots. (C and D) ESI–MS2 spectra of the covalent adduct (1,475 m/z) and the singly oxidized peptide (1,449 m/z). The gray, orange, and red boxes indicate bx fragments corresponding to Aβ, Aβ bound to 2-methylthiophene, and singly oxidized Aβ, respectively. Conditions were as follows: [Aβ40], 100 μM; [CuCl2], 100 μM; [compound], 500 μM; incubation for 1 h; 20 mM ammonium acetate, pH 7.2; 37 °C; no agitation. The samples were diluted with H2O by 10-fold prior to injection to the mass spectrometer.
Covalent adduct formation.
Upon analyzing the Cu(II)–Aβ40 sample treated with L1, a new peak was detected at 1,475 m/z, assigned as [Aβ40 + 96]3+ (Fig. 2A, orange peak). To identify the modified amino acid residue, ESI–MS2 was performed for the selected ion at 1,475 m/z. Note that the collision-induced dissociation (CID) of the target ion results in the detection of b fragments, which could be analyzed for residue-specific peptide modifications (32, 42). Upon applying the CID to the peak at 1,475 m/z, a mass shift of 96 Da was observed from b14, suggesting that His14 was transformed by L1 (Fig. 2C).
The peak at 1,475 m/z is suspected to be a product of the reaction between His14 and TCA, an N-dealkylation product of L1 mediated by copper–O2 chemistry, as shown in Scheme 1A, (i) (35, 38). The interaction between TCA and Cu(II)–Aβ may form a transient ternary TCA–Cu(II)–Aβ complex, as shown in Scheme 1A, (i), where the aldehyde group in TCA could be subject to a nucleophilic attack by a proximal amino acid residue in Aβ (e.g., His14) (43). Thus, an increase in 96 Da from Aβ40 could be assigned to Aβ covalently bound to the 2-methylthiophene moiety. Note that 2-methylthiophene could be produced through the dehydration of thiophene-2-ylmethanol. Such dehydration of molecules is frequently observed in mass spectrometric studies (44). To further confirm the role of L1’s transformation to TCA in covalently modifying Cu(II)–Aβ, the Cu(II)–Aβ sample directly treated with TCA was analyzed by ESI–MS. A peak corresponding to [Aβ40 + 96]3+ was also detected at 1,475 m/z in the sample incubated with TCA (SI Appendix, Fig. S5). ESI–MS2 studies showed that the His14 residue in Aβ40 was modified by TCA in the presence of Cu(II), indicating that copper–O2 chemistry driving N-dealkylation of L1 is essential for generating the covalent adduct with Aβ40.
Oxidation.
Upon incubation of Cu(II)–Aβ40 with L1, a peak denoting a mass shift of 16 Da from monomeric Aβ40 was monitored at 1,449 m/z, as depicted in Fig. 2A (red peak). ESI–MS2 revealed that this mass shift took place starting from b14, indicating the modification of His14 by treatment of L1 in the presence of Cu(II) (Fig. 2D). Such His14 modification affects Cu(II) binding to Aβ, as evidenced by the decrease in the peak intensity of Cu(II)–Aβ, as shown in Fig. 2A.
The peak at 1,449 m/z could denote L1-mediated oxidation of monomeric Aβ40. Aβ oxidation may occur through a process involving the reduction of Cu(II)–Aβ to Cu(I)–Aβ coupled with the oxidation of L1 to its cationic radical, L1+•, as described in Scheme 1B {E0 [for Cu(II)–Aβ]: approximately 0.083 V (33); E1/2 (for L1): 0.098 V (SI Appendix, Fig. S6; vide infra) vs. Ag/Ag(I) in H2O, respectively, at a similar scan rate}. Cu(I)–Aβ can then react with O2 to form a transient intermediate such as Cu(II)(Aβ40)(O2•–)(L1+•) and Cu(II)(Aβ40)(O22–)(L1+) under aerobic conditions (Scheme 1B, top reaction) (10, 45, 46), finally resulting in the oxidation of Aβ (e.g., His oxidation) (47). In parallel, L1+• could abstract a hydrogen atom from Aβ to form a carbon-centered peptide radical that may further interact with O2 to produce a peroxyl radical form of Aβ followed by peptide oxidation (Scheme 1B, bottom reaction) (48). To determine whether the transformation of L1 to TCA and DMPD is a prerequisite process for the oxidation of Aβ (Scheme 1A), the samples containing Cu(II) and Aβ40 with TCA or DMPD were prepared separately and analyzed under the same conditions used to study L1. Oxidation of Aβ40 was not observed in the TCA-containing samples, while DMPD oxidized Lys16, not His14, as shown in SI Appendix, Figs. S5 and S7, the latter of which is in agreement with previously reported results (31). These data suggest that such peptide oxidation at His14 occurs through the oxidation of L1 in the presence of Cu(II)–Aβ over the compound’s N-dealkylation to TCA and DMPD.
To further confirm that the His14-specific oxidation of Aβ is mediated by L1, the peptide oxidation by ROS, broadly present in biological systems, was investigated. More specifically, Aβ40 incubated with hydrogen peroxide (H2O2) was analyzed by ESI–MS, the dot blot assay, and transmission electron microscopy (TEM) (SI Appendix, Figs. S8 and S9). Upon treatment of H2O2 to metal-free Aβ40, the oxidation of Met35 was solely observed (SI Appendix, Fig. S8), as reported previously (49). In the case of Cu(II)-treated Aβ40 with H2O2, all ESI–MS peaks from Aβ40 completely disappeared under our experimental conditions (SI Appendix, Fig. S9A). To verify the modified regions in Aβ40, the dot blot assay employing an anti-Aβ antibody, 6E10 (for N terminus) (27), and an anti-Aβ40 antibody (for carboxyl terminus [C terminus]) (50) was carried out (SI Appendix, Fig. S9 B and C). Upon incubation of Cu(II)–Aβ40 with H2O2 for 1 and 24 h, the signal intensity of 6E10 was noticeably decreased, indicating that H2O2 modified the Aβ16 region of Cu(II)–Aβ40, whereas the dot blot using the anti-Aβ40 antibody showed a similar signal intensity to that of the H2O2-free control samples, suggesting that the C terminus was not altered. In order to clarify the effects of H2O2 on the fibrillization of Cu(II)–Aβ40, the dot blot assay using an anti-fibril antibody (OC) (51) and TEM were performed (SI Appendix, Fig. S9 B and C). Upon incubation with H2O2 for 1 h, Cu(II)–Aβ40 exhibited the signal of OC at a level comparable to that of the H2O2-free control sample. At 24 h incubation of H2O2 with Cu(II)–Aβ40, the signal intensity of OC was significantly reduced, possibly due to the altered aggregation of Cu(II)–Aβ40. The presence of H2O2 varied the overall size and shape of the resultant Aβ40 aggregates, as visualized by TEM. The morphologies of H2O2- and L1-treated Aβ aggregates were notably different (for H2O2-treated aggregates, see SI Appendix, Fig. S9 B and C; vide infra for L1-incubated Aβ aggregates). These observations support that the His14-specific oxidation toward Cu(II)–Aβ mediated by L1 is distinct from the peptide oxidation by general oxidants.
Dual-modification.
The peak detected at 1,480 m/z was assigned as [Aβ40 + 96 + O]3+ (green peak in Fig. 2A). This peak implicates the dual-modification of Aβ40 (both covalent bond formation and oxidation). To verify which amino acid residue was chemically modified by L1, ESI–MS2 was performed for the singly oxidized covalent adduct at 1,480 m/z. As illustrated in SI Appendix, Fig. S10, covalent adduct formation, oxidation, and both were detected from b14.
Taken together, L1 was able to chemically modify the metal-binding residue, His14, in Cu(II)–Aβ40 through either covalent bond formation, oxidation, or both. Such modifications could disrupt the complexation between Cu(II) and Aβ and change the secondary structure of the resultant Aβ, which was supported by the results obtained by inductively coupled plasma–MS (ICP–MS) and circular dichroism (CD) spectroscopy, respectively. Upon incubation of L1 with Cu(II)–Aβ40, the concentration of free copper in the supernatant was noticeably increased in comparison to that of the L1-free sample, detected by ICP–MS (SI Appendix, Fig. S11). This observation suggests that L1-mediated peptide modifications can dissociate copper from Aβ40. Moreover, a slight change in the secondary structure of Aβ40 was monitored by CD spectroscopy, when Cu(II)-added Aβ40 was treated with L1 for 24 h (α-helix, 6.1 to 0%; β-strand, 26 to 34%) (SI Appendix, Fig. S12).
Cu(II)–Aβ-Mediated Transformations of L1.
To determine the molecular mechanisms regarding L1-mediated modifications at the coordination sphere of Cu(II)–Aβ, the transformation of L1, L2, and L3 was investigated in the presence of Cu(II) and Aβ through ultraviolet–visible spectroscopy (UV–vis), ESI–MS, and cyclic voltammetry (Fig. 3 and SI Appendix, Figs. S13–S16).
Fig. 3.
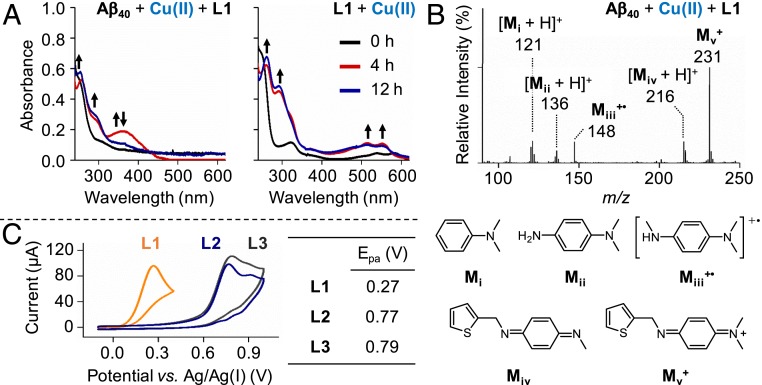
Transformations and anodic peak potentials (Epa) of L1, L2, and L3. (A) Optical changes of L1 upon addition of Cu(II) with and without Aβ40 under aerobic conditions. The new appearance of the peaks is indicated with black arrows. Conditions were as follows: [Aβ40], 25 μM; [CuCl2], 25 μM; [compound], 50 μM; room temperature; incubation for 1 h (black), 4 h (red), and 12 h (blue). (B) Structural variations of L1 in the presence of Cu(II)-treated Aβ40, monitored by ESI–MS. Conditions were as follows: [Aβ40], 100 μM; [CuCl2], 100 μM; [L1], 500 μM; incubation for 1 h; 20 mM ammonium acetate, pH 7.2; 37 °C; no agitation. The sample was diluted with H2O by 10-fold prior to injection to the mass spectrometer. (C) Cyclic voltammograms of L1, L2, and L3 in DMSO at the scan rate of 250 mV/s. The Epa values of the compounds at various scan rates are summarized in SI Appendix, Table S2. Conditions were as follows: [compound], 1 mM; 0.1 M tetra-N-butylammonium perchlorate; room temperature; three electrodes composed of the glassy carbon working electrode, platinum counter electrode, and Ag/Ag(I) reference electrode.
Cleavage.
N-dealkylation of L1 to generate TCA and DMPD in the presence of Cu(II) with or without Aβ40 was monitored by UV–vis and ESI–MS. As illustrated in Fig. 3A, when L1 was incubated with CuCl2 and Aβ40, an increase in absorbance at approximately 375 nm followed by the disappearance of the same peak after 12 h was observed. This absorption band at approximately 375 nm may correspond to the charge transfer between L1 and Cu(II)–Aβ40 or the quinone-imine moiety of L1+ (vide infra) (52). Additionally, the peaks at approximately 260 and 300 nm were slightly enhanced, indicating the presence of a mixture of TCA and DMPD. DMPD was also detected by ESI–MS (Mii in Fig. 3B) upon treatment of L1 with Cu(II)–Aβ40. Under Aβ40-free conditions, the optical bands of Cu(II)-treated L1 at 260 and 300 nm were noticeably increased, as depicted in Fig. 3A, suggesting the production of both TCA and DMPD. Fragmentation of L1 in the presence of Cu(II) without Aβ was also confirmed by ESI–MS (SI Appendix, Fig. S13A).
Unlike L1, L2 did not undergo notable transformation in the presence of Cu(II) or Cu(II)–Aβ under our experimental conditions, as shown in SI Appendix, Figs. S13B and S14A. Interaction between the compound and Cu(II) manifested a charge transfer band in the range from approximately 300 to 500 nm. In the case of L3, no significant optical changes were observed upon incubation with Cu(II) or Cu(II)–Aβ (SI Appendix, Fig. S14B), but a peak at 261 m/z was detected by ESI–MS from the sample containing Cu(II)–Aβ and the compound, implicating the oxidation of its thioether moiety (SI Appendix, Fig. S13B). N-dealkylation of L2 and L3 was not observed, indicating that these two compounds do not couple with the copper–O2 chemistry discussed above for L1.
Oxidation.
When L1 was incubated with Cu(II)–Aβ40, L1+• was not detected by either ESI–MS or UV–vis, as indicated in Fig. 3 A and B. Instead, the two-electron oxidized form, L1+ (Mv+; Fig. 3B), was monitored in the low molecular-weight region of the ESI–MS spectrum from the sample containing L1 and Cu(II)–Aβ40. In the presence of Cu(II) without Aβ40, the peak at 232 m/z corresponding to L1+• was detected by ESI–MS (SI Appendix, Fig. S13A). Moreover, as presented in Fig. 3A, the absorbance peaks at approximately 500 and 550 nm were displayed upon incubation of L1 and Cu(II), indicative of the one-electron oxidation of the compound (31, 32). These double peaks may also represent the one-electron oxidation of DMPD (i.e., DMPD+•) (31), an N-dealkylation product of L1 (Scheme 1A). Note that such optical changes were not notably observed in the absence of Cu(II) under our experimental conditions (SI Appendix, Fig. S15). In the case of L2 and L3, neither one- nor two-electron oxidation was monitored in the presence of Cu(II) with and without Aβ, as depicted in SI Appendix, Figs. S13B and S14, suggesting that these two compounds may not be able to reduce Cu(II)–Aβ to Cu(I)–Aβ, a prerequisite for inducing the oxidation of Aβ.
In addition to the spectroscopic and spectrometric studies, the redox potentials of L1, L2, and L3 were measured at various scan rates in dimethyl sulfoxide (DMSO) and H2O, as summarized in Fig. 3C and SI Appendix, Figs. S6 and S16. Due to the irreversible nature of the electrochemical waves in DMSO (SI Appendix, Fig. S16 and Table S2), the E1/2 values could not be obtained. The anodic peak potential (Epa) of L1 was 0.27 V at 250 mV/s vs. Ag/Ag(I), which is significantly lower than those of L2 (0.77 V) and L3 (0.79 V) at the same scan rate. It can be inferred from the difference in the Epa values of the abovementioned molecules that the DMPD moiety may be responsible for stabilizing L1+•, as reported previously (29, 32). Under aqueous conditions, the E1/2 value of L1 was determined as 0.10 V, which is lower than that of L3 (0.51 V) at 250 mV/s (SI Appendix, Fig. S6 and Table S3). Note that the cyclic voltammogram of L2 in H2O could not be obtained due to its limited solubility. The comparable redox potentials of Cu(II)–Aβ42 and L1 [approximately 0.083 V (33) and 0.098 V vs. Ag/Ag(I) in H2O with a similar scan rate] suggest that Cu(II)–Aβ could be reduced to Cu(I)–Aβ upon oxidation of L1. Together, N-dealkylation of L1 coupled with copper–O2 chemistry could yield TCA and DMPD, the former of which may be required for the covalent adduct formation with Aβ in the presence of Cu(II). Furthermore, the oxidation of L1 conjugated with the reduction of Cu(II)–Aβ to Cu(I)–Aβ could direct the oxidation of His14.
Specificity of L1-Induced Dual-Modification against Cu(II)–Aβ.
To confirm that our proposed mechanism for L1-induced modifications toward Cu(II)–Aβ is driven by the direct interaction between L1 and Cu(II)–Aβ and copper–O2 chemistry (Scheme 1), additional ESI–MS studies employing multiple metal ions and proteins were performed. In the absence of O2, Cu(I) and Cu(II) could not induce the modification of Aβ even with the treatment of L1, as shown in Fig. 2A and SI Appendix, Fig. S17A. The presence of Cu(I) without L1, under aerobic conditions, did not result in any notable modifications of Aβ. In the presence of O2, L1 induced the covalent adduct formation and the oxidation at His14 in the sample of Cu(I)–Aβ, as observed upon treatment of Cu(II)–Aβ with L1 (SI Appendix, Fig. S17 B–D). Based on the lack of noticeable reactivity against Aβ in the absence of either Cu(I/II) or O2, it can be inferred that L1’s capacity for altering His14 in Aβ requires the direct interaction with Cu(II)–Aβ to foster the underlying copper–O2 chemistry presented in Scheme 1.
The pertinent role of the redox chemistry presented by Cu(I/II) indicates the potential of other redox-active metal ions for driving L1-mediated Aβ modifications. This notion was directly investigated with three redox-active metal ions [i.e., Fe(II), Fe(III), Co(II)] and a redox-inactive metal ion [i.e., Zn(II)]. Interestingly, the presence of Fe(II), Fe(III), Co(II), and Zn(II) did not prompt the His14-specific modifications of Aβ that were induced by L1 in the presence of Cu(I/II). Upon introduction of Fe(II) to Aβ40, the Fe(II)–2Aβ405+ complex was detected; however, no modification of Aβ was observed with the treatment of L1 (SI Appendix, Fig. S18 A, Top). Upon incubation of Fe(III)-added Aβ40 with L1, Fe(III) binding to Aβ40 was not found, and no discernible new peaks were observed relative to the control (SI Appendix, Fig. S18 A, Bottom). The analysis of the sample containing Co(II)-treated Aβ40 with L1 led to the detection of the Co(II)–Aβ403+ complex, along with Aβ12+ at 1,424 m/z (SI Appendix, Fig. S18B), previously reported to indicate the hydrolytic cleavage of Aβ40 (53). Lastly, when redox-inactive Zn(II) was incubated with Aβ40 in the presence of L1, Zn(II) was able to bind to 2Aβ405+, but L1 did not trigger any modifications of Aβ (SI Appendix, Fig. S18C). Based on our spectrometric analyses of the Aβ samples incubated with Cu(I/II), Fe(II/III), Co(II), and Zn(II), the dual-modification of Aβ by L1 at His14 appears to exhibit a degree of specificity toward Cu(II)–Aβ.
To further confirm that such dual-modification by L1 is specific for Cu(II)–Aβ, the interactions of L1 with α-synuclein (α-Syn) and human islet amyloid polypeptide (hIAPP) (amyloidogenic) and ubiquitin (nonamyloidogenic) in the presence of Cu(II) were further analyzed by ESI–MS (SI Appendix, Fig. S19). The covalent adduct formation between L1 and the three abovementioned proteins was not observed even with Cu(II). In the case of oxidation, a mass shift by 16 Da from α-Syn or Cu(II)-bound hIAPP was indicated in the samples of Cu(II)-added proteins and L1. Note that the oxidized residue could not be determined due to limitations on resolution. No oxidation of ubiquitin was shown following treatment of both Cu(II) and L1. Overall, these results suggest that the L1-induced dual-modification is specific toward Cu(II)–Aβ.
Modulation of Cu(II)–Aβ Aggregation.
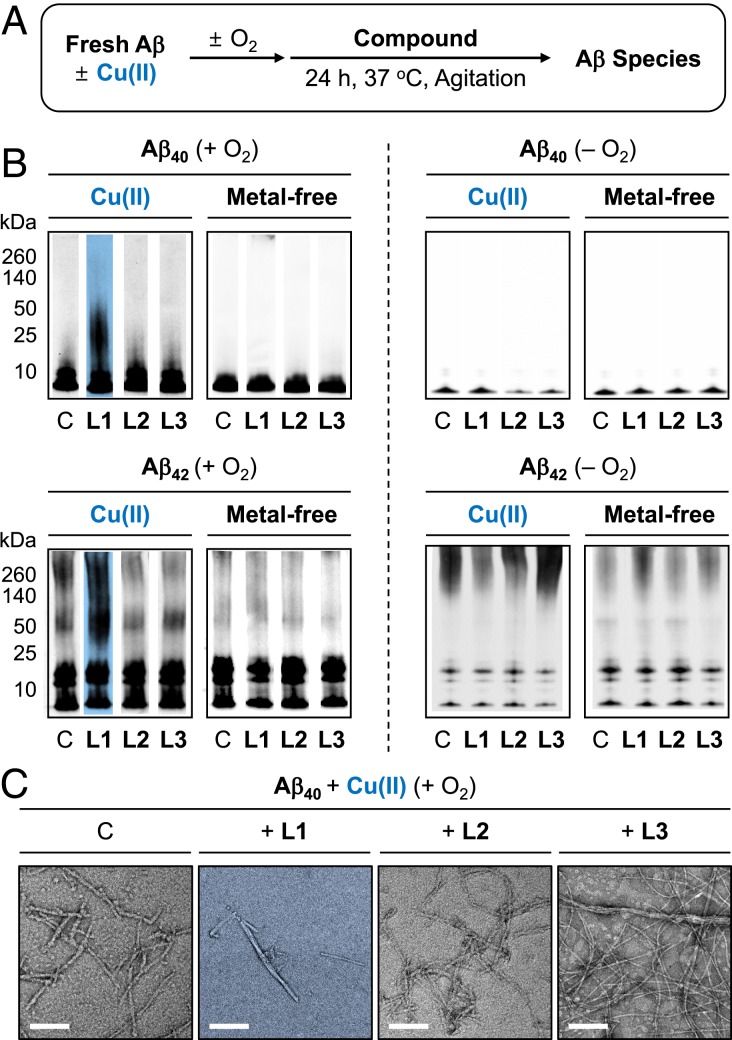
To identify whether L1-induced modifications at His14 affect the aggregation of Cu(II)–Aβ, the size distribution and morphology of the resultant Aβ species were verified by gel electrophoresis with Western blotting (gel/Western blot) and TEM, respectively. Note that the thioflavin-T assay could not be performed in this study due to the interference of the absorption of L1 with the fluorescence window for analysis. Two types of experiments were conducted employing Aβ40 and Aβ42, two major isoforms of Aβ (18): 1) inhibition experiments (Fig. 4A): freshly prepared Aβ species were treated with L1, L2, and L3 in the absence and presence of Cu(II) for 24 h; and 2) disaggregation experiments (SI Appendix, Fig. S20A): Aβ peptides were preincubated with or without Cu(II) for 24 h followed by introduction of the compounds for an additional 24 h.
Fig. 4.
Influence of L1, L2, and L3 on the aggregation of Cu(II)-bound and metal-free Aβ. (A) Scheme of the inhibition experiments. (B) Analysis of the size distribution of the resultant Aβ40 and Aβ42 species in the presence (Left) and absence (Right) of O2 by gel/Western blot using an anti-Aβ antibody (6E10). Lanes are as follows: C, Aβ with or without CuCl2; L1, C with L1; L2, C with L2; L3, C + L3. The original gel images are shown in SI Appendix, Fig. S24A. Conditions were as follows: [Aβ], 25 μM; [CuCl2], 25 μM; [compound], 50 μM; 37 °C, constant agitation. (C) Morphologies of Cu(II)-bound Aβ40 aggregates from B, monitored by TEM. (Scale bars, 200 nm.)
In the inhibition experiments, the aggregation of both Cu(II)–Aβ40 and Cu(II)–Aβ42 was altered by L1 under aerobic conditions, as illustrated in Fig. 4B. When L1 was incubated with Cu(II)-treated Aβ species, noticeable smearing bands were indicated in the gel/Western blots, compared to those obtained with compound-free Cu(II)–Aβ samples. The resultant Aβ aggregates produced in the presence of L1 were visualized by TEM to be shorter fibrils or amorphous aggregates, relative to compound-free Cu(II)–Aβ samples, as indicated in Fig. 4C and SI Appendix, Fig. S21B. Additionally, upon incubation of L1 with Cu(II)–Aβ40, the signal of an anti-oligomer antibody (A11), able to detect structured oligomers (54), was reduced, further supporting the alteration of Cu(II)-mediated Aβ aggregation by treatment of L1 (SI Appendix, Fig. S21C). In the absence of O2, L1 did not affect the aggregation of Cu(II)–Aβ (Fig. 4 B, Right). Furthermore, the aggregation of metal-free Aβ40 and Aβ42 was not modulated by L1 (Fig. 4B and SI Appendix, Fig. S22). Moreover, as expected from ESI–MS studies (vide supra), L2 and L3 were not able to vary the aggregation of both Cu(II)-added Aβ40/Aβ42 and metal-free Aβ40/Aβ42, which was confirmed by gel/Western blots and TEM (Fig. 4 and SI Appendix, Figs. S21 and S22).
The disassembly or modulation of further aggregation of preformed Cu(II)-treated and metal-free Aβ aggregates by the compounds was also investigated (SI Appendix, Figs. S20 and S23). Similar to the inhibition studies, in the disaggregation experiments, the gel/Western blots showed 1) the noticeable smearing bands in preformed Cu(II)-treated Aβ40 and Aβ42 aggregates in the presence of L1 and 2) no influence of L1 on preformed metal-free Aβ40 and Aβ42 aggregates. Short Aβ fibrils were monitored by TEM upon treatment of preformed Cu(II)–Aβ aggregates with L1, while long and large Aβ fibrils were still found under metal-free conditions. Moreover, L2 and L3 exhibited no effects on preformed Cu(II)–Aβ40/Aβ42 and metal-free Aβ40/Aβ42 aggregates according to the data obtained by gel/Western blots and TEM. Collectively, the aggregation of both Cu(II)–Aβ40 and Cu(II)–Aβ42 was noticeably impacted by L1, while L2 and L3 could not significantly affect Aβ aggregation even in the presence of Cu(II).
Regulation of the Toxicity Triggered by Cu(II)–Aβ, Free Organic Radicals, and ROS.
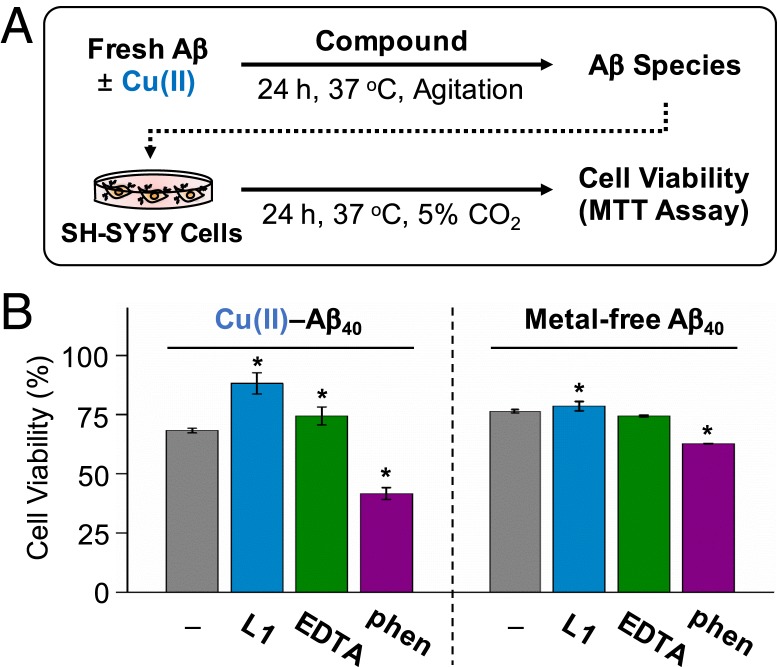
To determine the effect of L1 on the toxicity of Cu(II)–Aβ, the MTT assay employing a human neuroblastoma SH-SY5Y cell line was carried out [MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. For the cytotoxicity studies, Aβ with either Cu(II), L1, or both was preincubated for 24 h, and the resultant samples were treated to SH-SY5Y cells. As shown in Fig. 5, cell toxicity of the Aβ40 species generated with Cu(II) and L1 was reduced by approximately 15 to 20%, relative to that of compound-free Cu(II)–Aβ40. In the case of Cu(II)-treated Aβ42, cell viability was slightly increased by L1 under our experimental conditions (approximately 5%; SI Appendix, Fig. S25A). The cytotoxicity of metal-free Aβ40 and Aβ42 was lowered by less than 10% upon treatment of L1. In addition to L1, the effects of other copper chelators, i.e., 2,2′,2″,2‴-(ethane-1,2-diyldinitrilo)tetraacetic acid (EDTA) and 1,10-phenanthroline (phen), on the cytotoxicity of Cu(II)–Aβ40 and metal-free Aβ40 were also evaluated (Fig. 5 and SI Appendix, Fig. S25B). Cell survival of the Aβ40 samples incubated with the two chelators in the absence and presence of Cu(II) was equal to or lower than that of compound-free samples under our experimental conditions. These overall results support that L1-mediated modifications toward Cu(II)–Aβ could reduce the cytotoxicity induced by the metal–Aβ complex. Note that L1, L2, and L3 are relatively less toxic showing higher than 80% of cell survival up to 25 μM, as presented in SI Appendix, Fig. S26.
Fig. 5.
Regulation of the toxicity triggered by Cu(II)-treated Aβ40 and metal-free Aβ40 by L1 and other metal chelators (i.e., EDTA and phen) in living cells. (A) Scheme of the cell viability experiments. (B) Survival of the cells treated with Cu(II)-added Aβ40 and metal-free Aβ40 that were preincubated with L1, EDTA, and phen. Cell viability, determined by the MTT assay, was calculated in comparison to that with an equivalent amount of DMSO. Conditions were as folows: [Aβ40], 10 μM, [CuCl2], 10 μM, [compound], 10 μM. *P < 0.05 by Student’s t test.
In addition to modifying Aβ, the antioxidant activity of L1 may also contribute toward the molecule’s cytoprotective effects based on its redox properties (32, 55). The regulation of free organic radicals by the compounds was monitored by the Trolox equivalent antioxidant capacity (TEAC) assay. The TEAC value indicates the ability of compounds to quench free organic radicals such as ABTS+•, relative to that of Trolox, a vitamin E analog [ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); Trolox: 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid] (56). The TEAC value of L1 was 1.86 (± 0.10), while those of L2 and L3 were 0.86 (± 0.10) and 0.96 (± 0.13), respectively. The trend of the compounds’ antioxidant properties is correlated to their Epa values, indicated in Fig. 3C. These observations are consistent with the previous reports regarding a relation between the redox properties of compounds and their antioxidant capabilities (32, 55).
Moreover, the ability of L1 to 1) remove ROS and 2) inhibit the production of ROS by Fenton-like reactions of Cu(I/II)–Aβ was evaluated. The quantity of H2O2, as a ROS, was measured by the absorption of resorufin, a red chromophore produced by the reaction of Amplex Red and H2O2 in a 1:1 stoichiometry in the presence of peroxidase (57). As shown in SI Appendix, Fig. S27A, L1 was able to reduce the amount of H2O2 in a concentration-dependent manner. Furthermore, L1 could scavenge H2O2 generated from Cu(I/II) with and without Aβ40 in the presence of a reducing agent (e.g., L-ascorbate; SI Appendix, Fig. S27B). Note that the samples of Cu(II)–Aβ40 with and without L1 in the absence of L-ascorbate did not generate detectable amounts of H2O2 (SI Appendix, Fig. S27B). The notable decrease in the levels of H2O2 upon treatment of L1 may be a consequence of 1) the antioxidant capability of L1 itself based on its redox potential; 2) the antioxidant activity of DMPD, an N-dealkylation product of L1 (31); or 3) the L1-directed regulation of Fenton-like reactions of Cu(I/II) or Cu(I/II)–Aβ (58). Overall, the TEAC and H2O2 assays demonstrate the ability of L1 to scavenge free organic radicals and ROS, along with its potential to control Cu(I/II)- or Cu(I/II)–Aβ-mediated production of ROS.
Discussion
Cu(II)–Aβ complexes represent a pathological connection between Aβ and metal ions in AD (2, 4, 8–12, 15). Recent findings indicate that Cu(II)–Aβ could directly contribute toward neurodegeneration through the production of toxic oligomers and ROS (2, 4, 15, 19, 20). Based on the potential neurotoxic implications of Cu(II)–Aβ, research endeavors have led to the development of several chemical reagents [e.g., N1-(pyridin-2-ylmethyl)benzene-1,4-diamine (1), N1,N1-dimethyl-N4-(quinolin-2-ylmethyl)benzene-1,4-diamine (3), and N1,N1-dimethyl-N4-(pyridin-2-ylmethyl)benzene-1,4-diamine (L2-b)] reported to alter the aggregation of Cu(II)–Aβ by triggering the modifications of Aβ (27, 29). The abovementioned molecules are able to form a ternary complex with Cu(II)–Aβ and elicit the oxidative degradation of Aβ (27, 29), and 1 and 3 were able to oxidize Aβ (29). The exact locations of such peptide modifications against Cu(II)–Aβ with respect to the transformed amino acid residue have not been fully identified, however. In this study, an effective mechanistic approach was developed to chemically modify the coordination sphere of Cu(II)–Aβ with a rationally designed molecule, L1, by binding to the metal–peptide complex and promoting copper–O2 chemistry at the metal center. This study presents experimental evidence that the His14 residue in Cu(II)–Aβ was specifically modified via either covalent bond formation, oxidation, or both. It should be noted that the L1-induced dual-modification (both covalent bond formation and oxidation) at His14 was not observed against Aβ in the presence of 1) other metal ions [i.e., Fe(II), Fe(III), Co(II), and Zn(II)] and 2) other proteins (i.e., α-Syn and hIAPP [amyloidogenic] as well as ubiquitin [nonamyloidogenic]) even with treatment of Cu(II). Considering the neurotoxic implications of the interactions between Cu(I/II) and Aβ, such modifications at the coordination sphere of Cu(II)–Aβ could effectively alter the properties of the metal–Aβ complex. As expected, the aggregation and toxicity profiles of the resultant products from the reaction between Cu(II)–Aβ and L1 under aerobic conditions were significantly modulated. Overall, our multidisciplinary studies with emphasis on approaches, reactivities, and mechanisms demonstrate a direction for modulating Cu(II)–Aβ complexes related to the pathology of AD.
Materials and Methods
All reagents were purchased from commercial suppliers and used as received unless otherwise noted. Aβ40 and Aβ42 (Aβ42: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAII-GLMVGGVVIA) were purchased from Anaspec (Fremont, CA) or Peptide Institute, Inc. (Osaka, Japan). EDTA and phen were purchased from Sigma-Aldrich (St. Louis, MO) and Thermo Fisher Scientific (Waltham, MA), respectively. Trace metal ions were removed from the solutions used for the studies by treatment with Chelex overnight (Sigma-Aldrich). ESI–MS and ESI–MS2 analyses were performed by an Agilent 2530 mass spectrometry dual AJS-ESI (Santa Clara, CA) or a Waters Synapt G2-Si quadrupole time-of-flight ion-mobility mass spectrometer (Waters, Manchester, UK) equipped with an ESI source (Daegu Gyeongbuk Institute of Science & Technology Center for Core Research Facilities, Daegu, Republic of Korea). CD spectra were obtained by a J-815 spectropolarimeter (Korea Advanced Institute of Science and Technology Analysis Center for Research Advancement [KARA], Daejeon, Republic of Korea). UV–vis spectra were recorded on an Agilent 8453 UV–vis spectrophotometer. Cyclic voltammograms were obtained under N2 (g) on a CHI620E potentiostat (Qrins, Seoul, Republic of Korea). The concentration of copper in solution was measured by an Agilent ICP–MS 7700S (KARA). TEM images were taken by a JEOL JEM-2100 transmission electron microscope (Ulsan National Institute of Science and Technology Central Research Facilities [UCRF], Ulsan, Republic of Korea). Absorbance values for biological assays were obtained by a Molecular Devices SpectraMax M5e microplate reader (Sunnyvale, CA). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a 400-MHz Agilent NMR spectrometer (UCRF). The high-resolution mass spectra of compounds were taken by a Q Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific). The details of experimental procedures and methods are presented in SI Appendix. All data discussed in this study are included in the main text and SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (Grants NRF-2016R1A5A1009405 and NRF-2017R1A2B3002585 [to M.H.L.] and NRF-2015R1D1A1A01060188 [to J.C.]). J.H. acknowledges the Global Ph.D. Fellowship Program for support through the NRF, funded by the Ministry of Education (Grant NRF-2019H1A2A1073754).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916944117/-/DCSupplemental.
References
- 1.Que E. L., Domaille D. W., Chang C. J., Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Savelieff M. G., et al. , Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem. Rev. 119, 1221–1322 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Chang C. J., Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 11, 744–747 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Kepp K. P., Squitti R., Copper imbalance in Alzheimer’s disease: Convergence of the chemistry and the clinic. Coord. Chem. Rev. 397, 168–187 (2019). [Google Scholar]
- 5.Barnham K. J., Masters C. L., Bush A. I., Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Scheiber I. F., Mercer J. F. B., Dringen R., Metabolism and functions of copper in brain. Prog. Neurobiol. 116, 33–57 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Nam E., Han J., Suh J.-M., Yi Y., Lim M. H., Link of impaired metal ion homeostasis to mitochondrial dysfunction in neurons. Curr. Opin. Chem. Biol. 43, 8–14 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Bush A. I., Masters C. L., Tanzi R. E., Copper, β-amyloid, and Alzheimer’s disease: Tapping a sensitive connection. Proc. Natl. Acad. Sci. U.S.A. 100, 11193–11194 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faller P., Hureau C., La Penna G., Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 47, 2252–2259 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Savelieff M. G., Lee S., Liu Y., Lim M. H., Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem. Biol. 8, 856–865 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Faller P., Hureau C., Berthoumieu O., Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 52, 12193–12206 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Kepp K. P., Alzheimer’s disease: How metal ions define β-amyloid function. Coord. Chem. Rev. 351, 127–159 (2017). [Google Scholar]
- 13.Drew S. C., Barnham K. J., The heterogeneous nature of Cu2+ interactions with Alzheimer’s amyloid-β peptide. Acc. Chem. Res. 44, 1146–1155 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Suh J.-M., Kim G., Kang J., Lim M. H., Strategies employing transition metal complexes to modulate amyloid-β aggregation. Inorg. Chem. 58, 8–17 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Atrián-Blasco E., et al. , Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev. 375, 38–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeToma A. S., Salamekh S., Ramamoorthy A., Lim M. H., Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem. Soc. Rev. 41, 608–621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitkiewicz E., Kłoniecki M., Poznański J., Bal W., Dadlez M., Factors influencing compact-extended structure equilibrium in oligomers of aβ1-40 peptide–An ion mobility mass spectrometry study. J. Mol. Biol. 426, 2871–2885 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Lee S. J. C., Nam E., Lee H. J., Savelieff M. G., Lim M. H., Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 46, 310–323 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Smith D. G., Cappai R., Barnham K. J., The redox chemistry of the Alzheimer’s disease amyloid β peptide. Biochim. Biophys. Acta 1768, 1976–1990 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Rauk A., The chemistry of Alzheimer’s disease. Chem. Soc. Rev. 38, 2698–2715 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Rajasekhar K., Madhu C., Govindaraju T., Natural tripeptide-based inhibitor of multifaceted amyloid β toxicity. ACS Chem. Neurosci. 7, 1300–1310 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Chen T., et al. , Effects of cyclen and cyclam on zinc(II)- and copper(II)-induced amyloid β-peptide aggregation and neurotoxicity. Inorg. Chem. 48, 5801–5809 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Wu W. H., et al. , Sequestration of copper from β-amyloid promotes selective lysis by cyclen-hybrid cleavage agents. J. Biol. Chem. 283, 31657–31664 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Robert A., Liu Y., Nguyen M., Meunier B., Regulation of copper and iron homeostasis by metal chelators: A possible chemotherapy for Alzheimer’s disease. Acc. Chem. Res. 48, 1332–1339 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Nguyen M., Robert A., Meunier B., Metal ions in Alzheimer’s disease: A key role or not? Acc. Chem. Res. 52, 2026–2035 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Esmieu C., et al. , Copper-targeting approaches in Alzheimer’s disease: How to improve the fallouts obtained from in vitro studies. Inorg. Chem. 58, 13509–13527 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Beck M. W., et al. , A rationally designed small molecule for identifying an in vivo link between metal-amyloid-β complexes and the pathogenesis of Alzheimer’s disease. Chem. Sci. 6, 1879–1886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong S., et al. , Advanced electron paramagnetic resonance studies of a ternary complex of copper, amyloid-β, and a chemical regulator. Inorg. Chem. 57, 12665–12670 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Beck M. W., et al. , Structure-mechanism-based engineering of chemical regulators targeting distinct pathological factors in Alzheimer’s disease. Nat. Commun. 7, 13115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayne D. J., Lim S., Donnelly P. S., Metal complexes designed to bind to amyloid-β for the diagnosis and treatment of Alzheimer’s disease. Chem. Soc. Rev. 43, 6701–6715 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Derrick J. S., et al. , A redox-active, compact molecule for cross-linking amyloidogenic peptides into nontoxic, off-pathway aggregates: In vitro and in vivo efficacy and molecular mechanisms. J. Am. Chem. Soc. 137, 14785–14797 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J., et al. , Tuning structures and properties for developing novel chemical tools toward distinct pathogenic elements in Alzheimer’s disease. ACS Chem. Neurosci. 9, 800–808 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Jiang D., et al. , Redox reactions of copper complexes formed with different β-amyloid peptides and their neuropathalogical relevance. Biochemistry 46, 9270–9282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combariza M. Y., Vachet R. W., Gas-phase ion-molecule reactions of transition metal complexes: The effect of different coordination spheres on complex reactivity. J. Am. Soc. Mass Spectrom. 13, 813–825 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Elwell C. E., et al. , Copper–oxygen complexes revisited: Structures, spectroscopy, and reactivity. Chem. Rev. 117, 2059–2107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J. Y., Karlin K. D., Elaboration of copper-oxygen mediated C-H activation chemistry in consideration of future fuel and feedstock generation. Curr. Opin. Chem. Biol. 25, 184–193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itho S., “Chemical reactivity of copper active-oxygen complexes” in Copper-Oxygen Chemistry, Karlin K. D., Itho S., Rokita S., Eds. (Wiley on Reactive Intermediates in Chemistry and Biology, John Wiley & Sons, 2011) pp. 225–282. [Google Scholar]
- 38.Kim S., et al. , Amine oxidative N-dealkylation via cupric hydroperoxide Cu-OOH homolytic cleavage followed by site-specific fenton chemistry. J. Am. Chem. Soc. 137, 2867–2874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M., et al. , Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 288, 23212–23224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di L., Kerns E. H., Fan K., McConnell O. J., Carter G. T., High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 38, 223–232 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Avdeef A., et al. , PAMPA–critical factors for better predictions of absorption. J. Pharm. Sci. 96, 2893–2909 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Swaney D. L., McAlister G. C., Coon J. J., Decision tree-driven tandem mass spectrometry for shotgun proteomics. Nat. Methods 5, 959–964 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao S.-M., Du Q.-S., Meng J.-Z., Pang Z.-W., Huang R.-B., The multiple roles of histidine in protein interactions. Chem. Cent. J. 7, 44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demarque D. P., Crotti A. E. M., Vessecchi R., Lopes J. L. C., Lopes N. P., Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 33, 432–455 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Arrigoni F., et al. , Copper reduction and dioxygen activation in Cu-amyloid beta peptide complexes: Insight from molecular modelling. Metallomics 10, 1618–1630 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Hewitt N., Rauk A., Mechanism of hydrogen peroxide production by copper-bound amyloid beta peptide: A theoretical study. J. Phys. Chem. B 113, 1202–1209 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Schöneich C., Mechanisms of metal-catalyzed oxidation of histidine to 2-oxo-histidine in peptides and proteins. J. Pharm. Biomed. Anal. 21, 1093–1097 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Davies M. J., Protein oxidation and peroxidation. Biochem. J. 473, 805–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmblad M., Westlind-Danielsson A., Bergquist J., Oxidation of methionine 35 attenuates formation of amyloid β -peptide 1-40 oligomers. J. Biol. Chem. 277, 19506–19510 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Kang J., et al. , Chemical strategies to modify amyloidogenic peptides using iridium(iii) complexes: Coordination and photo-induced oxidation. Chem. Sci. 10, 6855–6862 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kayed R., et al. , Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2, 18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi J.-S., Braymer J. J., Nanga R. P., Ramamoorthy A., Lim M. H., Design of small molecules that target metal-Aβ species and regulate metal-induced Aβ aggregation and neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 107, 21990–21995 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derrick J. S., et al. , Mechanistic insights into tunable metal-mediated hydrolysis of amyloid-β peptides. J. Am. Chem. Soc. 139, 2234–2244 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Kayed R., et al. , Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Chevion S., Roberts M. A., Chevion M., The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 28, 860–870 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Lee H. J., et al. , Structural and mechanistic insights into development of chemical tools to control individual and inter-related pathological features in Alzheimer’s disease. Chemistry 23, 2706–2715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R. P., A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253, 162–168 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Atrián-Blasco E., Del Barrio M., Faller P., Hureau C., Ascorbate oxidation by Cu(amyloid-β) complexes: Determination of the intrinsic rate as a function of alterations in the peptide sequence revealing key residues for reactive oxygen species production. Anal. Chem. 90, 5909–5915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.