Significance
Corals are disappearing worldwide due to both local and global stressors. Yet, our understanding of the interaction among these two types of stressors is limited, hindering efforts to conserve coral reefs. With a large dataset at a seascape scale across Moorea, French Polynesia, we showed that coral bleaching was more severe as both heat stress and nitrogen pollution increased. We also found that corals bleached more severely at comparatively low heat stress if they lived where nitrogen pollution was high. Thus, given that nitrogen pollution worsens the severity of coral bleaching, even during mild heat stress events, there is a critical need to address both local and global threats to coral reefs.
Keywords: coral reef, climate change, nutrient availability, Acropora, Pocillopora
Abstract
Climate change is increasing the frequency and magnitude of temperature anomalies that cause coral bleaching, leading to widespread mortality of stony corals that can fundamentally alter reef structure and function. However, bleaching often is spatially variable for a given heat stress event, and drivers of this heterogeneity are not well resolved. While small-scale experiments have shown that excess nitrogen can increase the susceptibility of a coral colony to bleaching, we lack evidence that heterogeneity in nitrogen pollution can shape spatial patterns of coral bleaching across a seascape. Using island-wide surveys of coral bleaching and nitrogen availability within a Bayesian hierarchical modeling framework, we tested the hypothesis that excess nitrogen interacts with temperature anomalies to alter coral bleaching for the two dominant genera of branching corals in Moorea, French Polynesia. For both coral genera, Pocillopora and Acropora, heat stress primarily drove bleaching prevalence (i.e., the proportion of colonies on a reef that bleached). In contrast, the severity of bleaching (i.e., the proportion of an individual colony that bleached) was positively associated with both heat stress and nitrogen availability for both genera. Importantly, nitrogen interacted with heat stress to increase bleaching severity up to twofold when nitrogen was high and heat stress was relatively low. Our finding that excess nitrogen can trigger severe bleaching even under relatively low heat stress implies that mitigating nutrient pollution may enhance the resilience of coral communities in the face of mounting stresses from global climate change.
Climate change is driving an increase in marine heat waves that are killing foundation species worldwide (1). On coral reefs, thermal anomalies associated with rising water temperatures cause coral bleaching, the breakdown of the mutualism between corals and their endosymbiotic algae, Symbiodiniaceae (2–4). Large heat stress events and the resulting coral bleaching can cause widespread coral mortality and alter coral community composition (5–7), as well as affect the diversity and abundance of associated fishes (8, 9). Repeated bleaching events threaten the persistence of coral reefs and the ecosystem goods and services they provide (10). As the frequency and intensity of coral bleaching events increase (6, 11), it is crucial to better understand what factors, in addition to temperature, influence the propensity of a coral colony to bleach (12, 13). One such putative stressor to the mutualism between corals and their endosymbiotic algae is excess nitrogen.
Nutrient pollution has increased to such an extent over the last century that anthropogenically derived nutrients now dwarf natural nutrient sources (14, 15). These nutrients are dramatically altering coastal marine systems, particularly oligotrophic systems such as coral reefs. Excess anthropogenic nutrients, in the form of both nitrogen (N) and phosphorous (P), can negatively impact coral reproduction, growth, and survivorship (16, 17); intensify coral diseases (18, 19); and exacerbate the adverse effects of ocean acidification on reef calcification rates (20). With respect to coral bleaching, small-scale in situ enrichment experiments have revealed that anthropogenic forms of N can increase the probability that a coral colony will bleach and subsequently die (21). Current hypotheses suggest that increased N availability makes corals more susceptible to bleaching via a variety of physiological mechanisms (16, 22), which is supported by both laboratory and field experiments (19, 21, 23–25).
It is unclear how findings from small-scale experiments examining the effect of excess N on the mutualism between coral and Symbiodiniaceae translate into real-world conditions over broad spatial scales. Results to date have been mixed. Previous studies from the Caribbean and Great Barrier Reef revealed positive correlations between water-column nutrient concentrations and coral bleaching across large reef tracts (26, 27), while others showed negative correlations between water quality and coral bleaching (28). However, data on N and bleaching in these studies are often collected at different spatial and/or temporal resolutions, across gradients of other abiotic factors, and across coral communities that vary in assemblage structure and species diversity, making it difficult to interpret unambiguously the relationships between N availability and coral bleaching.
To better understand the influence of excess N on coral bleaching in situ at a broad spatial scale, we quantified spatial patterns of N for the entire ∼50-km2 lagoon reef system that encircles the island of Moorea, French Polynesia. This was done during a moderate heat stress event in 2016, when we also quantified bleaching metrics for >10,000 coral colonies of the two common genera of branching corals (Pocillopora, Acropora) at 167 sites distributed across the lagoon (<3 m water depth) around the island. Contemporaneous estimates of heat stress for these sites were generated from continuous records of in situ water temperature. Analyses of these data revealed marked effects of N availability on coral bleaching across the seascape, as well as evidence that N alters the relationship between heat stress and bleaching. Our results suggest that local pollution can have an important impact on climate-driven disruption of coral reef ecosystems.
Results
Trends in Temperature and N Availability.
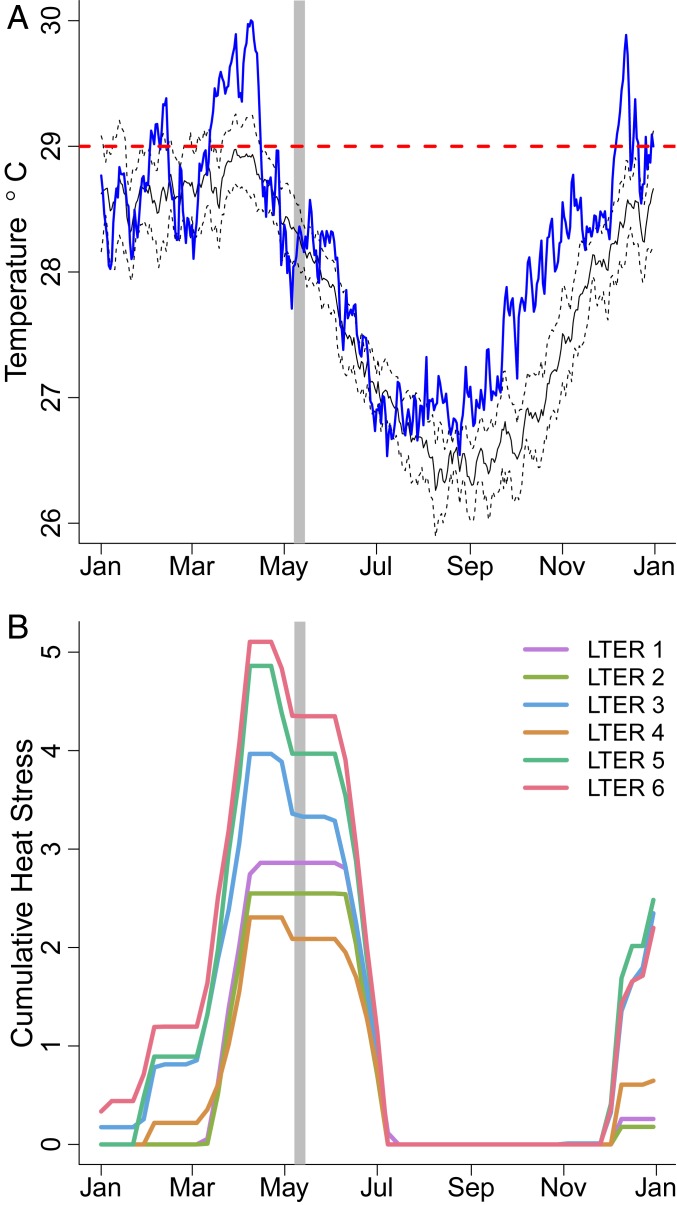
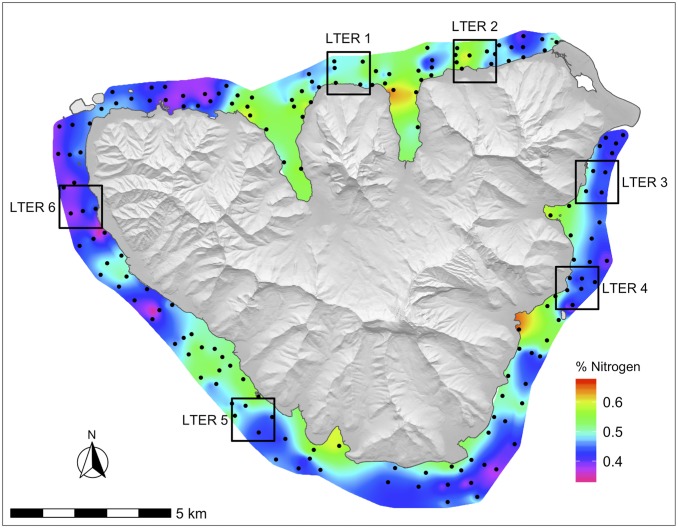
During 2016, maximum water temperature in the lagoons of Moorea peaked in early April at 30.0 °C and exceeded the maximum monthly mean (MMM) of 29 °C, a noted threshold for heat stress for corals in Moorea (29), for 70 straight days from early February through mid-April (Fig. 1A). Patterns of heat stress, defined as a 12-wk running sum of all mean weekly temperatures exceeding the MMM, were variable around the island, with heat stress occurring from as early as January to as late as mid-March, depending on the site (Fig. 1B). Heat stress was highest on the west side of Moorea (Long Term Ecological Research [LTER] sites 5 and 6, Fig. 2) with peaks of ∼5 °C weeks of cumulative heat stress. N availability, measured using the nitrogen concentration (%N) in the tissue of a ubiquitous alga Turbinaria ornata, was generally higher closer to shore, but this pattern varied spatially, with hotspots occurring near large bays and steep watersheds (Fig. 2). In general, the north shore had the highest N availability on average, except for the northwest portion of the island that had consistently low nitrogen levels. Nitrogen availability (%N) was positively correlated with δN15 ( P < 0.01), which is often used as an indicator of anthropogenic nutrient sources (SI Appendix, Fig. S1). We also explored potential covariation between heat stress and N availability and found no apparent pattern (SI Appendix, Figs. S2 and S3).
Fig. 1.
(A and B) Island-wide (A) and by site (B) temperature patterns from in situ temperature loggers at fringing reefs. (A) In 2016 (blue line) temperatures exceed the maximum monthly mean of 29 °C (red dashed line) during the Austral summer, and it was much warmer than the average long-term seasonal pattern (black line with 95% confidence intervals as dashed black lines). Bleaching surveys took place from 8 to 14 May 2016 (gray vertical bar). (B) Cumulative heat stress, measured as a 12-wk running sum for all temperatures exceeding the maximum monthly mean, peaked in April 2016 and was variable around the island as measured at LTER sites in Fig. 2. Temperature patterns from back reef sites were similar to those from fringing reefs.
Fig. 2.
Location of bleaching and nitrogen surveys (black circles, n = 167 sites) around the island of Moorea, French Polynesia. In situ temperature recorders were located at LTER sites (large boxes). Nitrogen is represented as a continuous surface where warmer colors represent greater percentage of nitrogen in the tissue of the brown alga T. ornata and cooler colors represent lower percentage of nitrogen in algal tissue. Land is displayed as a digital elevation model with a hillshade to show ridgelines and valleys.
Spatial Patterns of Coral Bleaching.
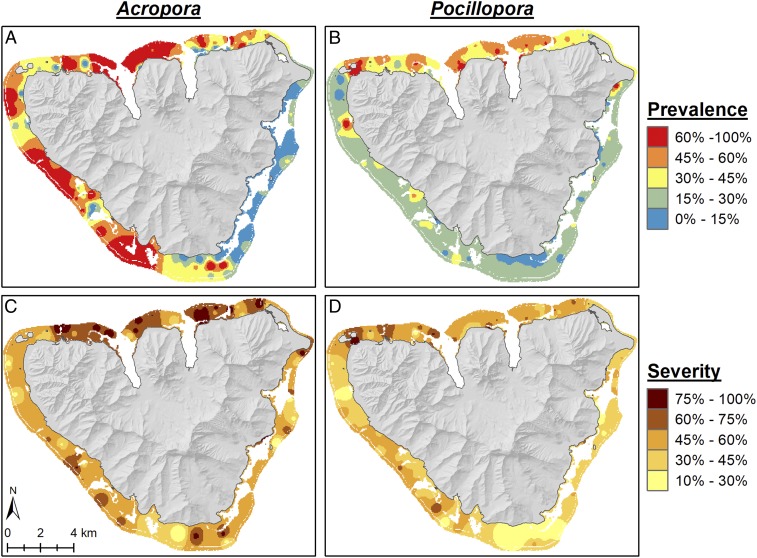
Across 167 sites around Moorea (Fig. 2), we observed Pocillopora and Acropora at 149 sites. We surveyed a total of 1,500 Acropora colonies and 8,730 Pocillopora colonies, ranging from 1 to 247 colonies per site. Overall, 37% of Acropora and 28% of Pocillopora colonies exhibited bleaching. Spatial patterns of bleaching prevalence (the probability of bleaching across colonies) and severity (the amount of bleaching per colony) were variable across genera (Fig. 3). For Acropora, bleaching prevalence was high along both the north and west shores, where up to 100% of colonies at each site were bleached (Fig. 3A). Bleaching in Pocillopora was most prevalent across the north shore (Fig. 3B), with bleaching roughly twice as prevalent on the north shore as elsewhere (47% of colonies exhibiting bleaching on the north shore vs. ∼22% of colonies elsewhere). When bleaching was observed for Acropora, severity was generally high, with the median percent of colony bleached being 55% regardless of location (Fig. 3C). Severity of bleaching in Pocillopora tended to mirror patterns in prevalence, with a median of 55% of a colony bleached along the north shore, compared to 37 to 41% elsewhere (Fig. 3D).
Fig. 3.
(A–D) Distribution of coral bleaching prevalence (A and B) and severity (C and D) for Acropora (A and C) and Pocillopora (B and D). Continuous surfaces were created using inverse distance weighting; the extent of the surface represents reef area within the lagoons, while white areas represent nonreef area (e.g., deep lagoons, sand) within lagoons or offshore reefs beyond the reef crest. Note that color ramps are different for prevalence and severity.
Predictors of Coral Bleaching.
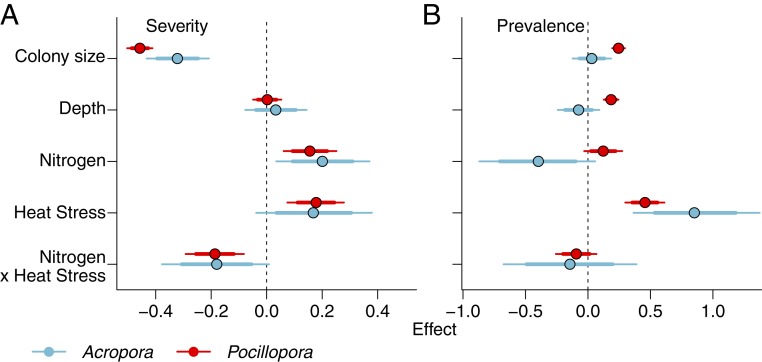
We found generally consistent results across genera and bleaching metrics for colony- and site-level predictors (Fig. 4). Coral colony size was positively related to bleaching prevalence in Pocillopora, but not Acropora, and negatively related to severity in both Pocillopora and Acropora, with larger colonies tending to bleach less severely. There was little evidence for an effect of depth, except for a small positive relationship between depth and bleaching prevalence in Pocillopora (Fig. 4B). Thus, colonies in deeper water bleached slightly more, although the depth range of our observations was small (≤3 m). In general, bleaching prevalence and severity were higher for corals at back reef sites compared to fringing reef sites (SI Appendix, Table S1).
Fig. 4.
(A and B) Relationship between predictors and coral bleaching severity (A) and prevalence (B) for Acropora (blue) and Pocillopora (red). Betas are coefficients, where colony size and depth are related to bleaching at the colony scale and nitrogen and heat stress are related to bleaching at the site scale (sites are represented by circles in Fig. 2). Thin lines are 95% credible intervals, and thick lines are 80% credible intervals. We consider there to be evidence for each effect when the 80% credible interval does not cross zero (dashed line).
Nitrogen and cumulative heat stress were each positively related with bleaching severity for both Pocillopora and Acropora, as well as with bleaching prevalence in Pocillopora (Fig. 4). Bleaching prevalence in Acropora also increased with increasing heat stress, and there was some model support that N decreased the propensity of Acropora to bleach (Fig. 4A). Thus, for both genera, N and heat stress independently affected the proportion of corals on a reef that showed any degree of bleaching (prevalence, Fig. 4B). In contrast, N and heat stress interacted to influence how much an affected coral colony bleached (severity, Figs. 4A and 5 and SI Appendix, Fig. S4). While Acropora showed higher levels of bleaching severity than did Pocillopora (Fig. 5), both genera displayed the same pattern of change in the relationship between N and bleaching severity as a function of heat stress (Fig. 5).
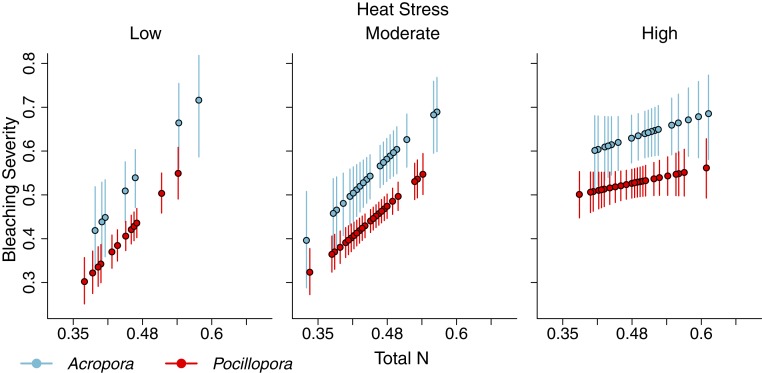
Fig. 5.
Visualization of the negative interaction between nitrogen and heat stress on bleaching severity for Acropora (blue) and Pocillopora (red) in the back reef. Plotted are the mean values (circles) and 95% credible intervals (lines) predicted from the models for bleaching severity across the range of nitrogen values when heat stress was held to a low (1.84 °C weeks), a moderate (2.14 °C weeks), and a high value (2.81 °C weeks).
The interaction between N and cumulative heat stress in shaping the severity of bleaching was negative (Figs. 4 and 5). Increasing N generally led to increasing bleaching severity, but the level of heat stress mediated the sensitivity of corals to variation in nitrogen (i.e., slope of the relationship) (Fig. 5). Sensitivity to N was greatest when heat stress was low, and it declined as heat stress increased (Fig. 5). Both coral genera were relatively unaffected by variation in N at the highest observed heat stress, a situation where the majority of an affected colony bleached regardless of N enrichment (Fig. 5). Notably, for each coral genus, the severity of bleaching that occurred under low heat stress at high N was similar to the most severe bleaching that occurred at the highest level of heat stress (Fig. 5).
Discussion
We show that nitrogen enrichment can interact with heat stress to influence coral bleaching across the seascape. Based on a dataset of >10,000 corals that were sampled around the island of Moorea during a moderate heat stress event, we found that both heat stress and N enrichment impacted spatial heterogeneity in bleaching prevalence and severity. We found a significant interaction between heat stress and N enrichment on the severity of coral bleaching. In areas with relatively low heat stress, high levels of N increased the severity of bleaching up to twofold. In contrast, at the highest levels of heat stress, coral bleaching was severe regardless of the level of N. Importantly, high N pollution at low heat stress resulted in corals bleaching as severely as corals at high heat stress. Heat-induced coral bleaching is a major driver of coral mortality on reefs worldwide. Yet, how local factors impact coral responses to heat stress is poorly understood at seascape scales. The results of this study provide critical evidence that locally generated nitrogen pollution can exacerbate the extent of climate-driven coral bleaching events.
Interaction between Nitrogen and Heat Stress.
Excess nitrogen may facilitate coral bleaching by compromising the relationship between the coral host and the Symbiodiniaceae symbionts via several nonexclusive mechanisms. For example, increased N availability may lead to an imbalance between N and P that damages coral photosystems (22); may decrease the amount of carbon that symbionts return to corals, leading to starvation (30); and may increase the production of N-based free radicals (31). Nitrogen pollution can lead to greater susceptibility of corals to heat stress by increasing algal growth rates, leading to increased competition between algae and corals (32). Our results reinforce patterns from previous small-scale experiments to show that increased N availability can promote coral bleaching at the reef-wide scale. Further, our work is consistent with laboratory (24) and field experiments (19, 21, 25), as well as large-scale surveys (26, 27) and time series (33) showing that increased N availability facilitates coral bleaching.
Our results are consistent with the hypothesis that the level of heat stress may mediate the impacts of excess N on coral bleaching, with high heat stress overwhelming any effect of excess N. We found significant heterogeneity in heat stress across the seascape that interacted negatively with N availability to influence the severity of coral bleaching. In areas with low heat stress, N increased the severity of bleaching in the two dominant genera of branching corals in Moorea (Pocillopora, Acropora), approximately doubling the mean proportion of a colony that bleached (Fig. 5). The influence of N on bleaching severity diminished with increasing heat stress such that at the greatest observed level of heat stress, the mean proportion of a coral colony that bleached was high across all levels of N.
We also found that nitrogen influenced the proportion of corals on a reef that showed any level of bleaching (prevalence), but the patterns were less certain and more nuanced. In our surveys, the proportion of a coral that bleached ranged from very minor (e.g., a few branch tips) to the entire colony. For both coral genera, we found no compelling evidence that N and heat stress interacted to influence bleaching prevalence. Thus, the effects of these factors were additive, at least over the range of heat stress that occurred in the moderate thermal event of 2016. Interestingly, the effect of N on bleaching prevalence appeared to differ qualitatively between the two coral genera. For Pocillopora, N increased both the probability a colony would suffer at least some bleaching and the severity of that bleaching. For Acropora, our evidence suggests that N tended to reduce the likelihood a coral would bleach, but increased the severity for any colony that did bleach. Because mortality of a bleached coral is directly related to the proportion of its endosymbiont (Symbiodiniaceae) population that is lost (34), the ecologically most important effect of N enrichment in our study was the increase in bleaching severity under low heat stress. Our finding that elevated N can cause severe bleaching under low heat stress implies that N pollution may induce mass mortality of corals even when typical thermal tolerances have not been crossed.
The negative interaction between N and heat stress on bleaching severity that we observed also helps explain and reconcile previously reported findings. Our result—that excess N lowered the threshold for severe coral bleaching at low but not high levels of heat stress—is consistent with other observational studies and mechanistic experiments. On the Great Barrier Reef, concentrations of inorganic N in the water column were positively correlated with coral bleaching prevalence in years with moderate heat stress (27), but were uncorrelated with bleaching during the strong thermal event associated with 2015/2016 El Niño when bleaching and mortality were extreme (35). Similarly, in a field experiment in Moorea, excess N increased bleaching prevalence in both Pocillopora and Acropora, but the effect of N on bleaching was stronger at lower levels of heat stress (21).
Seascape-Scale Effects across Habitats and Taxa.
Coral bleaching stems from multiple mechanisms operating at a variety of spatial and temporal scales (36, 37), where finer-scale processes are constrained by processes at higher levels [i.e., hierarchy theory (38)]. For example, within a coral colony, bleaching can be variable, and likewise two colonies side by side can exhibit vastly different bleaching responses (39). Given the role of different drivers at multiple scales, uncovering emergent effects across the seascape can be difficult. Despite this challenge, our study revealed strong relationships between N and bleaching (Fig. 4), helping to explain spatial patchiness in bleaching across the seascape at an island scale. To uncover these effects, we 1) explicitly accounted for scale using a hierarchical model that considered variation across colonies within sites and variation across sites within habitats and coastlines; 2) observed bleaching for two genera, allowing us to infer patterns across taxa that are somewhat different in their susceptibility to excess N and heat stress; and 3) collected data on drivers of bleaching at scales meaningful to the hypothesized effect on bleaching. Future studies can benefit by considering explicitly how multiple drivers are operating at multiple scales to explain patchiness in coral bleaching at a seascape scale.
Fringing reefs in Moorea, which often experience increased runoff and sedimentation that increase turbidity, had lower levels of bleaching prevalence and severity than did back reefs (SI Appendix, Table S1). Several mechanisms may account for this finding. Turbidity, which is often higher on fringing reefs, can potentially moderate effects of heat stress on coral bleaching by reducing light and lowering photooxidative stress (34). The long-term decline in coral cover on many of the fringing reefs in Moorea (40) may have also contributed to lower bleaching on fringing reefs because corals that persist in inherently stressful habitats may be more robust to heat stress due to selection and acclimatization. For example, corals surviving on the fringing reefs may be dominated by thermotolerant endosymbiont communities (41, 42), microbiomes that are resistant to the effects of nutrient pollution (25, 32), and/or exhibit adaptive traits such as greater energy reserves (43) or shifting endosymbiont communities (44).
The effects of N and heat stress on bleaching differed somewhat between the taxa we studied, with Acropora being more sensitive to severe bleaching than Pocillopora. Taxonomic variability of coral bleaching is well known (5), and our results are consistent with patterns previously observed on Moorea and elsewhere, with Acropora often being the most susceptible genus, followed by Pocillopora (29, 45). More recent experimental work revealed that excess N consistently increased bleaching prevalence in Pocillopora while bleaching in Acropora was more tightly linked to heat stress (21). Our results are consistent with these observations but also indicate that both taxa responded similarly to spatial heterogeneity in N availability by experiencing more severe bleaching under N-enriched conditions (Fig. 5). Thus, N pollution increases the severity of coral bleaching of multiple taxa with different underlying sensitivities to thermal stress.
Anthropogenic Influences on Coral Bleaching.
Different sources of nitrogen (e.g., nitrate, ammonium, or urea) often impact coral physiology differently. For example, anthropogenically derived N (nitrate) is more likely to impair coral physiology and growth than is naturally derived N (urea or ammonium) (17, 30). Further, in a recent field experiment, nitrate increased bleaching prevalence and duration in Pocillopora corals while urea did not affect bleaching (21). Our data suggest that the impact of N availability across the seascape was likely due to an increase in anthropogenically derived N. We found that the total N in algal tissue was positively correlated with δN15 (SI Appendix, Fig. S1). Higher levels of δN15 in algae are often indicative of N derived from human sources, suggesting that sites with higher N and δN15 had higher input from anthropogenic N sources (46, 47). We could not distinguish between the independent effects of total N and δN15 on bleaching because they were highly correlated among our sites. Yet, our data suggest that increases in anthropogenic N drove increases in the prevalence and severity of coral bleaching.
The observation that excess N can exacerbate coral bleaching is of great importance for the immediate future of coral reefs. Continued changes in land use and altered precipitation regimes from climate change will likely result in more runoff and anthropogenic nutrients from terrestrial systems reaching coastal marine systems (48). Further, climate change is expected to increase the frequency and severity of temperature anomalies, leading to more severe and widespread coral bleaching (6, 11). The combination of excess anthropogenic N with an increase in the duration of both low and high levels of heat stress suggests that corals will bleach more frequently and for longer periods in the near future. By lowering the temperature threshold for severe coral bleaching, excess N has the potential to decrease the return time between bleaching events, leaving corals with less time to recover following even relatively mild heat stress events. Together, our results suggest that although the ultimate survival of coral reefs is dependent on the immediate reduction of carbon emissions that cause global warming, management of land use and near-shore water quality are critically important for the resistance and resilience of corals to near-term increases in heat stress.
Methods
Study Site.
Moorea, French Polynesia is a volcanic high island with an ∼60-km perimeter in the central South Pacific ∼20 km west of Tahiti (17°30′S, 149°50′W). A barrier reef surrounds the island and forms lagoons ranging from 0.8 to 1.3 km in width. Shoreward of the reef crest is a distinct back reef habitat that is less than ∼3 m deep and is composed of a mosaic of coral patch reefs, coral rubble, and sand. Fringing reefs ring the lagoon at the edge of the island. Our study was conducted on the back reef and fringing reefs from January to May 2016 during a sea surface temperature anomaly when water temperatures were elevated to levels predicted to cause coral bleaching (Fig. 1).
Coral Bleaching Surveys.
We surveyed 167 sites around Moorea and recorded bleaching on colonies of Pocillopora and Acropora, which were present at 149 of the sites. Sites were a minimum of 0.5 km apart, and at each site two snorkelers conducted 10-min swims in opposite directions recording all observed colonies of Pocillopora and Acropora. Sites were distributed around the entire island and were categorized by habitat (fringing reef and back reef) and by the dominant cardinal direction of the coastline (north, east, west) (Fig. 2).
We surveyed the two most common and widespread genera of branching corals in the system, Acropora and Pocillopora. The most common Acropora species included Acropora hyacinthus, Acropora retusa, and Acropora lutkeni, while the most common Pocillopora species were Pocillopora verrucosa, Pocillopora meandrina, and Pocillopora eydouxi. Pocillopora damicornis and Acropora pulchra were present at some sites but were not included in the surveys since they were largely restricted to the fringing reef sites.
For each colony, we estimated the water depth (from 0 to 3 m deep) and its size class (size classes were 1 to 10 cm, 11 to 20 cm, 21 to 30 cm, 31 to 40 cm, and >40 cm diameter). We then recorded whether bleaching was present (prevalence) and, if so, estimated the percentage of the colony surface area that was bleached (severity). Because corals undergo natural, seasonal variation in Symbiodiniaceae density that can affect their coloration (49), we defined bleached tissue only as tissue that had lost all pigmentation.
Seawater Temperature and Nitrogen Availability.
In situ seawater temperature was recorded with loggers (64M SeaBird Water Temperature Recorder; Sea-Bird Electronics, Inc.) at 1 to 2 m depth at four back reef and six fringing reef sites distributed around the island (Fig. 1) (50). Temperature was attributed to each sampling site location based on its proximity to one of the temperature loggers within each habitat (Fig. 2). From these temperature records we calculated cumulative heat stress (in °C weeks) as a 12-wk running sum for all temperatures exceeding the MMM temperatures from February to April of 29 °C, based on an earlier study (29) that showed this metric was a good predictor of bleaching in Moorea.
To assess nitrogen availability at each site, we measured the N content (%N) in the long-lived brown macroalga Turbinaria ornata at each site. The nutrient content of macroalgae is often used as a proxy for ambient nutrient conditions as these macroalgae integrate nutrients over a relatively long time frame (i.e., weeks to months) (51). Both field surveys and experiments show that algae in consistently enriched environments typically have higher tissue nutrients (19, 52). During January and May 2016, we sampled 10 individual thalli of T. ornata at each site around the island for analysis of N content while we also surveyed coral bleaching (there was minimal bleaching in January 2016). Samples were immediately placed on ice and transported to the laboratory. One blade from each thallus was sampled at 5 cm below the apical tip of the thallus. These blades were scrubbed of epiphytes and briefly rinsed with fresh water before being dried at 60 °C to a constant weight and ground to powder. Total N content was determined via elemental analysis using a CHN Carlo-Erba elemental analyzer (NA1500) CN Analyzer at the University of Georgia, Center for Applied Isotope Studies. We also measured δN15 in the tissues of T. ornata at the Marine Science Institute Analytical Laboratory at the University of California, Santa Barbara, using standard elemental analyzer isotope ratio mass spectrometer (EA-IRMS) procedures. We averaged values from January and May for analyses to represent nutrient conditions throughout the Austral summer.
Bayesian Hierarchical Model.
We modeled bleaching prevalence and severity with Bayesian hierarchical models where prevalence was modeled as and severity was modeled as Hierarchical effects of site, habitat, and coastline were included for the mean of both components; predictors were included at the scale at which they were measured; and coefficients for each predictor were estimated separately for prevalence (p) and severity (s) at each level,
| [1] |
| [2] |
| [3] |
| [4] |
| [5] |
| [6] |
| [7] |
| [8] |
where j represents colonies, k represents sites, h represents habitat, and c represents coastlines. Predictors were transformed to a zero mean and unit variance to improve convergence and allow for comparisons across effects, and interactions were calculated with transformed predictors to alleviate potential collinearity between the interaction and the main effects. Priors for all regression coefficients and for were priors for variances were and the prior for r was
Models were fitted with JAGS using the rjags package in R (53). Each model was run with 10,000 iterations and three chains. Model convergence was assessed with Gelman–Rubin statistics (54), and model fits were assessed with posterior predictive checks and Bayesian R2 (SI Appendix, Tables S2 and S3).
We considered evidence for a predictor to be influential in explaining the response variable when the 80% credible interval did not cross zero.
Data and Code Availability.
All analyses were performed in the R language for statistical computing version 3.4 (55). Temperature data can be accessed at ref. 50, nutrient and bleaching data are available at ref. 56, and all R scripts used to perform analyses and prepare figures can be accessed at ref. 57.
Supplementary Material
Acknowledgments
This research was funded by National Science Foundation Grants OCE-1619697, OCE-1547952, OCE-1236905, OCE-1829393, and OCE-1637396 for the Moorea Coral Reef LTER, BCS-1714704, and a grant from the Zegar Family Foundation. We thank A. Brooks, A. Duran, K. Seydel, J. Verstaen, and R. Welsh for field and laboratory assistance. We acknowledge support from the University of California, Santa Barbara Center for Scientific Computing from the California NanoSystems Institute, Materials Research Laboratory: an NSF Materials Research Science and Engineering Centers (DMR-1720256) and NSF CNS-1725797. The digital terrain model used in Figs. 2 and 3 was provided courtesy of M. Troyer and was funded through ETH Zürich and the Hacettepe University Scientific Research Projects Coordination Unit. Research was completed under permits issued by the Territorial Government of French Polynesia (Délégation à la Recherche) and the Haut-Commissariat de la République en Polynésie Francaise (DTRT) (Protocole d’Accueil 2015–2017), and we thank the Délégation à la Recherche and DTRT for their continued support. We thank J. Lecky for lending us his superb cartographic skills, L. Ezzat for insightful discussions about the role of nutrients for corals, and N. Lemoine for advice on statistical analyses.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data for this article are available at the Environmental Data Initiative, https://doi.org/10.6073/pasta/57108aaeede00e77cac110bc5366a92b. R scripts used to perform analyses and prepare figures are available at Zenodo, http://doi.org/10.5281/zenodo.3575174.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915395117/-/DCSupplemental.
References
- 1.Smale D. A., et al. , Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 9, 306–312 (2019). [Google Scholar]
- 2.Brown B. E., Coral bleaching: Causes and consequences. Coral Reefs 16, S129–S138 (1997). [Google Scholar]
- 3.Lesser M. P., “Coral bleaching: Causes and mechanisms” in Coral Reefs: An Ecosystem in Transition, Dubinsky Z., Stambler N., Eds. (Springer, 2011), pp. 405–419. [Google Scholar]
- 4.LaJeunesse T. C., et al. , Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Loya Y., et al. , Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001). [Google Scholar]
- 6.Hughes T. P., et al. , Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Grottoli A. G., et al. , The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Change Biol. 20, 3823–3833 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Stuart-Smith R. D., Brown C. J., Ceccarelli D. M., Edgar G. J., Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560, 92–96 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Holbrook S. J., Schmitt R. J., Brooks A. J., Resistance and resilience of a coral reef fish community to changes in coral cover. Mar. Ecol. Prog. Ser. 371, 263–271 (2008). [Google Scholar]
- 10.Hughes T. P., et al. , Coral reefs in the Anthropocene. Nature 546, 82–90 (2017). [DOI] [PubMed] [Google Scholar]
- 11.van Hooidonk R., et al. , Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 6, 39666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cacciapaglia C., van Woesik R., Reef-coral refugia in a rapidly changing ocean. Glob. Change Biol. 21, 2272–2282 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Sully S., Burkepile D. E., Donovan M. K., Hodgson G., van Woesik R., A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett E. M., Carpenter S. R., Caraco N. F., Human impact on erodable phosphorus and eutrophication: A global perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. Bioscience 51, 227–234 (2001). [Google Scholar]
- 15.Vitousek P. M., et al. , Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 7, 737–750 (1997). [Google Scholar]
- 16.D’Angelo C., Wiedenmann J., Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 7, 82–93 (2014). [Google Scholar]
- 17.Shantz A. A., Burkepile D. E., Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology 95, 1995–2005 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Bruno J. F., Petes L. E., Harvell D. C., Hettinger A., Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061 (2003). [Google Scholar]
- 19.Vega Thurber R. L., et al. , Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Change Biol. 20, 544–554 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Silbiger N. J., et al. , Nutrient pollution disrupts key ecosystem functions on coral reefs. Proc. R. Soc. B 285, 20172718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkepile D. E., et al. , Nitrogen identity drives differential impacts of nutrients on coral bleaching and mortality. Ecosystems, 10.1007/s10021-019-00433-2 (2019). [DOI] [Google Scholar]
- 22.Morris L. A., Voolstra C. R., Quigley K. M., Bourne D. G., Bay L. K., Nutrient availability and metabolism affect the stability of coral–Symbiodiniaceae symbioses. Trends Microbiol. 27, 678–689 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Ferrier-Pagès C., Gattuso J.-P., Dallot S., Jaubert J., Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19, 103–113 (2000). [Google Scholar]
- 24.Wiedenmann J., et al. , Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164 (2013). [Google Scholar]
- 25.Wang L., et al. , Corals and their microbiomes are differentially affected by exposure to elevated nutrients and a natural thermal anomaly. Front. Mar. Sci. 5, 101 (2018). [Google Scholar]
- 26.Wagner D. E., Kramer P., Van Woesik R., Species composition, habitat, and water quality influence coral bleaching in southern Florida. Mar. Ecol. Prog. Ser. 408, 65–78 (2010). [Google Scholar]
- 27.Wooldridge S. A., Done T. J., Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 19, 1492–1499 (2009). [DOI] [PubMed] [Google Scholar]
- 28.MacNeil M. A., et al. , Water quality mediates resilience on the Great Barrier Reef. Nat. Ecol. Evol. 3, 620–627 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Pratchett M. S., McCowan D., Maynard J. A., Heron S. F., Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS One 8, e70443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezzat L., Maguer J.-F., Grover R., Ferrier-Pagès C., New insights into carbon acquisition and exchanges within the coral–dinoflagellate symbiosis under NH4+ and NO3− supply. Proc. R. Soc. B 282, 20150610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins T. D., Davy S. K., Nitric oxide production and tolerance differ among Symbiodinium types exposed to heat stress. Plant Cell Physiol. 53, 1889–1898 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Zaneveld J. R., et al. , Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 7, 11833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapointe B. E., Brewton R. A., Herren L. W., Porter J. W., Hu C., Nitrogen enrichment, altered stoichiometry, and coral reef decline at Looe Key, Florida Keys, USA: A 3-decade study. Mar. Biol. 166, 108 (2019). [Google Scholar]
- 34.Anthony K. R. N., Connolly S. R., Hoegh-Guldberg O., Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol. Oceanogr. 52, 716–726 (2007). [Google Scholar]
- 35.Hughes T. P., et al. , Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Ezzat L., et al. , Nutrient starvation impairs the trophic plasticity of reef‐building corals under ocean warming. Funct. Ecol. 33, 643–653 (2019). [Google Scholar]
- 37.Roche R. C., Williams G. J., Turner J. R., Towards developing a mechanistic understanding of coral reef resilience to thermal stress across multiple scales. Curr. Clim. Change Rep. 4, 51–64 (2018). [Google Scholar]
- 38.Wiens J. A., Spatial scaling in ecology. Funct. Ecol. 3, 385–397 (1989). [Google Scholar]
- 39.Innis T., Cunning R., Ritson-Williams R., Wall C. B., Gates R. D., Coral color and depth drive symbiosis ecology of Montipora capitata in Kāne‘ohe Bay, O’ahu, Hawai’i. Coral Reefs 37, 423–430 (2018). [Google Scholar]
- 40.Edmunds P.; Moorea Coral Reef LTER, MCR LTER : Coral Reef: Long-term Population and Community Dynamics: Corals, ongoing since 2005. knb-lter-mcr.4.37, 10.6073/pasta/721fdcde85b630d1b4548b677d9b1d86 (2019). [DOI]
- 41.Cunning R., Ritson-Williams R., Gates R. D., Patterns of bleaching and recovery of Montipora capitata in Kāne‘ohe Bay, Hawai’i, USA. Mar. Ecol. Prog. Ser. 551, 131–139 (2016). [Google Scholar]
- 42.LaJeunesse T. C., et al. , Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc. R. Soc. B 277, 2925–2934 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wall C. B., Ritson-Williams R., Popp B. N., Gates R. D., Spatial variation in the biochemical and isotopic composition of corals during bleaching and recovery. Limnol. Oceanogr. 64, 2011–2028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverstein R. N., Cunning R., Baker A. C., Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Change Biol. 21, 236–249 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Penin L., Adjeroud M., Schrimm M., Lenihan H. S., High spatial variability in coral bleaching around Moorea (French Polynesia): Patterns across locations and water depths. C. R. Biol. 330, 171–181 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Dailer M. L., Knox R. S., Smith J. E., Napier M., Smith C. M., Using δ 15N values in algal tissue to map locations and potential sources of anthropogenic nutrient inputs on the island of Maui, Hawai’i, USA. Mar. Pollut. Bull. 60, 655–671 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Kendall C., Elliott E. M., Wankel S. D., Tracing anthropogenic inputs of nitrogen to ecosystems. Stable Isot. Ecol. Environ. Sci. 2, 375–449 (2007). [Google Scholar]
- 48.Sinha E., Michalak A. M., Balaji V., Eutrophication will increase during the 21st century as a result of precipitation changes. Science 357, 405–408 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Fitt W. K., McFarland F. K., Warner M. E., Chilcoat G. C., Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685 (2000). [Google Scholar]
- 50.Leichter J., Seydel K., Gotschalk C., Data from “Moorea Coral Reef LTER: Benthic Water Temperature, ongoing since 2005.” Environmental Data Initiative. 10.6073/pasta/1a5760c3146c574c98db854ad6d3addc. Accessed 3 December 2018. [DOI]
- 51.Atkinson M. J., Smith S. V., C:N:P ratios of benthic marine plants. Limnol. Oceanogr. 28, 568–574 (1983). [Google Scholar]
- 52.Burkepile D. E., Hay M. E., Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar. Ecol. Prog. Ser. 389, 71–84 (2009). [Google Scholar]
- 53.Plummer M., rjags: Bayesian Graphical Models using MCMC (R Package Version 4-6, 2016). https://cran.r-project.org/web/packages/rjags/index.html.
- 54.Gelman A., Rubin D. B., Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992). [Google Scholar]
- 55.R Development Core Team , R: A Language and Environment for Statistical Computing (Version 3.4, R Foundation for Statistical Computing, Vienna, Austria, 2016).
- 56.Burkepile D., Adam T., MCR LTER: Coral Reef: Coral bleaching with nitrogen and heat stress: 2016 data in support of Donovan et al. submitted to PNAS. Environmental Data Initiative. 10.6073/pasta/57108aaeede00e77cac110bc5366a92b. Deposited 25 October 2019. [DOI]
- 57.Donovan M. K., et al. , Code for “Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape.” Zenodo. 10.5281/zenodo.3575174. Deposited 13 December 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyses were performed in the R language for statistical computing version 3.4 (55). Temperature data can be accessed at ref. 50, nutrient and bleaching data are available at ref. 56, and all R scripts used to perform analyses and prepare figures can be accessed at ref. 57.