Abstract
Cancer is a leading cause of death for people with HIV (PWH). The Veterans Healthcare System (VA) is the largest single institutional provider of HIV care in the United States. Cancer among Veterans with HIV is major issue and clinical research has expanded significantly during the antiretroviral therapy (ART) era providing numerous insights regarding cancer incidence, risk factors, prevention, treatment and outcomes for this unique group of patients. This work has been greatly facilitated by the availability of national VA data sources. Notably, patterns of cancer incidence have changed for Veterans with HIV during the ART era; non-AIDS defining malignancies now are the most common tumors. Despite better HIV control in the ART era, immunosuppression measured by low CD4 counts and HIV viremia have been associated with increased cancer risk. Cancer outcomes for Veterans with HIV may now be similar to uninfected Veterans, but information on outcomes and cancer treatment patterns remains limited, requiring further study to help inform prevention and treatment strategies.
1.1. Overview of HIV and Cancer
Cancer has been associated with HIV infection since the first days of the AIDS epidemic. Unexplained clusters of Kaposi Sarcoma (KS) cases among men who have sex with men in US metropolitan areas heralded the emergence of AIDS in the early 1980s.[1] In the pre-antiretroviral therapy era the most prevalent cancers among people with HIV (PWH) were those associated with severe immunosuppression: KS, non-Hodgkin lymphoma, and invasive cervical cancer, a group of cancers commonly referred to as “AIDS-defining cancers” (ADC). With the introduction of combination antiretroviral therapy (ART) in the mid-1990s and subsequent improved immune function among US persons with HIV infection the pattern of cancer diagnoses changed: ADCs became less common and non-AIDS defining cancers (NADCs) have emerged as the primary sources of cancer morbidity and mortality among PWH [2].
As AIDS-related morbidity declined, cancer has become a leading cause of death among PWH[3, 4]. However, several non-AIDS defining cancers are found more frequently among PWH than in the general population. Immunodeficiency and dysfunction induced by HIV, high prevalence of co-infections with oncogenic viruses, and elevated prevalence of traditional cancer risk factors, such as smoking and alcohol abuse/dependence all contribute risk[5]. Cancers associated with oncogenic coinfections including human papillomavirus-related tumors (anal, cervical, genital, oropharynx), Kaposi sarcoma–associated herpesvirus, Epstein–Barr virus (non-Hodgkin and Hodgkin lymphoma), and hepatitis B and C viruses (hepatocellular carcinoma) are increased in PWH compared to uninfected persons[6, 7]. Of note, lung cancer, a tumor with no identified oncogenic virus has been particularly prevalent in PWH[8, 9]. Increased risk of lung cancer among PWH has been consistently found in epidemiologic studies and has been linked to higher smoking rates among PWH as well as immune dysfunction and injury from recurrent lung infections[9].
Although improved since the pre-ART-era, population-based studies have continued to find poorer survival after a diagnosis of cancer for PWH even after accounting for potential differences in cancer stage at diagnosis and treatment[11]. These differences have been attributed to disparities in cancer treatment for PWH, impaired tolerability of cancer therapies in PWH and increased cancer aggressiveness in the setting of HIV-related immune suppression and dysfunction. Cancer treatment disparities have been well documented among PWH and structural barriers to healthcare access that affect population groups with higher rates of HIV infection may affect cancer outcomes[12]. Additionally, national surveys of oncologists suggest that a substantial proportion modify their practices when treating patients with HIV[13]. Increased treatment complications due to HIV-related factors and potential drug interactions from antiretroviral therapy have been demonstrated for some cancer types[14]. Last, mechanistic studies and detailed epidemiologic analyses suggest that HIV-related factors may directly impact cancer aggressiveness, thereby promoting worse outcomes in this population[11, 15, 16].
2.1. Role of the VA in Cancer Care for Persons with HIV in the US
The Veterans Health Administration (VA) System is the largest single institutional provider of HIV care in the United States[17] with nearly 60,000 individuals with HIV infection and an estimated prevalence of 0.48% among those in care[18]. The majority of these individuals are between 50 and 65 years of age and have other significant risk factors for cancer including smoking and viral hepatitis. It is therefore not surprising that cancer is emerging as major source of morbidity and mortality for Veterans with HIV.
2.2. Cancer Research Among US Veterans with HIV
Studies involving cancer and HIV among Veterans have been increasing during the antiretroviral era (Figure 1). Large observational studies have been greatly facilitated by VA data systems. The VA has been a pioneer in the adoption of electronic medical records (EMR), implementing a comprehensive record system more than 20 years ago. Starting in 2008 access to these data was streamlined by storage in a national relational Corporate Data Warehouse (CDW). This early incorporation of an EMR in the US VA system has also allowed for robust clinical research regarding HIV complications. These information systems have facilitated development of new methods of observational and clinical research by capitalizing on efficiencies made possible by a fully mature national EMR[19]. In addition, as the US HIV cohort ages, the VA experience with cancer in Veterans with HIV can provide an important view of what can be expected for the overall US population of persons with HIV, as the HIV population within the VA system is older than the comparable general HIV population[26].
Figure 1.
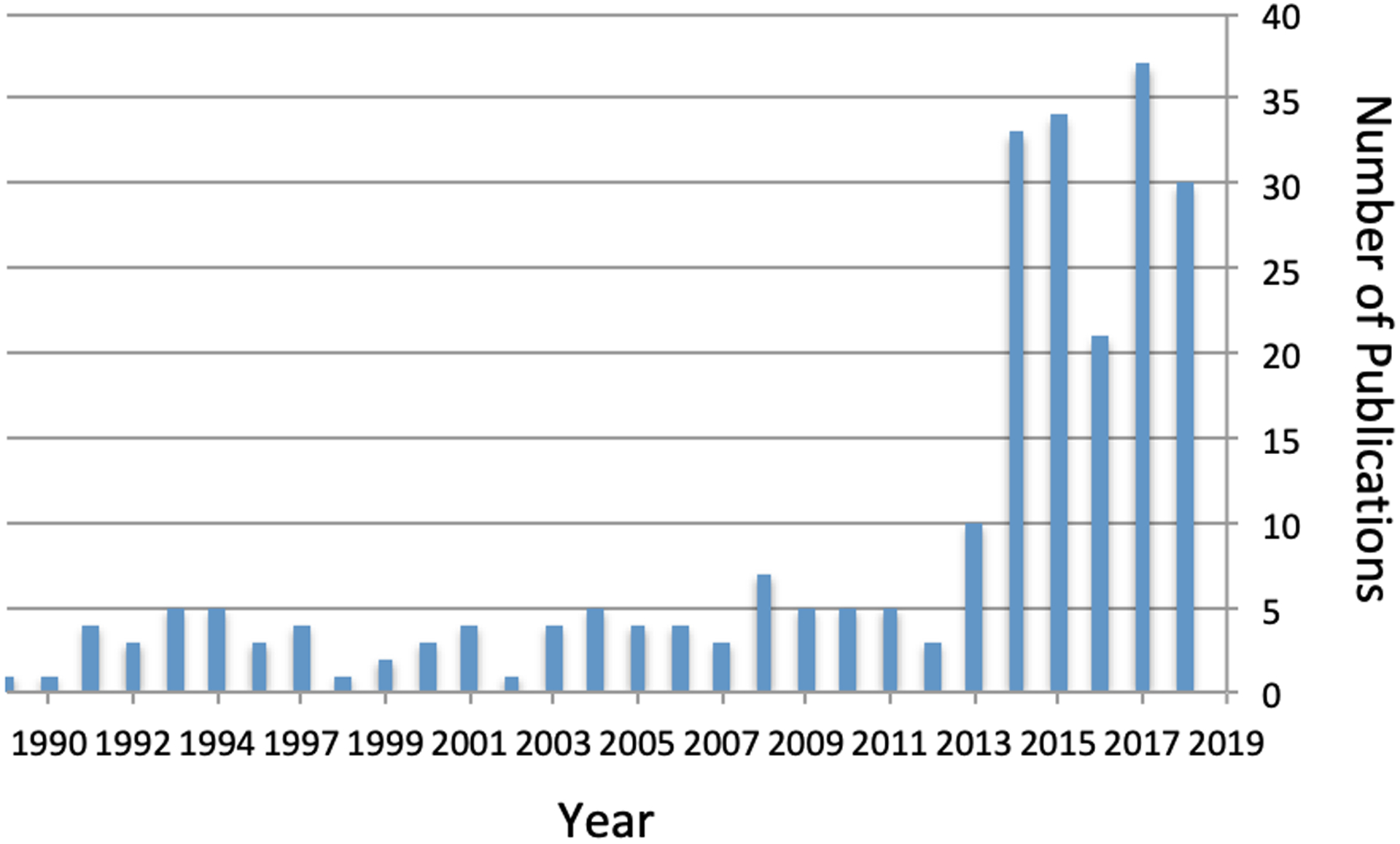
Number of Pubmed Indexed Publications Related to HIV/Cancer Among Veterans by Year
The Veterans Aging Cohort Study (VACS) has published the most research related to cancer and HIV among veterans. The single largest HIV cohort in North America, VACS is also one of the few that include demographically matched (and behaviorally similar) uninfected comparators. Beginning in September 1996, VACS has included 55,986 Veterans with HIV and 116,335 demographically matched uninfected comparators[20] and 20+ years of follow up. VACS collects data from multiple data sources inside and outside the VA and includes data from clinical notes and reports, inpatient and outpatient encounters, diagnostic and procedure codes, vital signs, pharmacy data, clinical laboratory data, and vital status. The VA Central Cancer Registry (providing detailed data on most, but not all, cancer cases diagnosed or treated at the VA since 1996[21]) has further enhanced VACS, adding data on more than 20,000 cancer cases within the cohort. These linkages with national VA cancer registry data have allowed for detailed evaluations of cancer risk, incidence and outcomes associated with both HIV infection and concomitant exposures such as behavioral factors, medications and comorbid illnesses. VACS also contributes data to the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), comprising 25 collaborating HIV epidemiologic databases, giving Veterans a substantial presence in nationally-representative HIV-related cancer research[22]. VACS also contributes data to other large collaborative studies including ART-CC and HIV-Causal.
Multiple other research groups within the VA system have also utilized both local and national data systems to evaluate cancer incidence, patterns of care and outcomes for Veterans with HIV. This combined body of work has provided greater insight into trends regarding cancer incidence among persons with HIV, information regarding HIV-related cancer risk factors, cancer prognostic factors associated with HIV infection, and unique issues related to cancer prevention and treatment in persons with HIV.
3.1. Insights on Cancer Epidemiology, Prevention and Treatment from VA HIV Research
The VA has provided unique insights into cancer epidemiology for PWH, both establishing cancer incidence in the ART-era and helping to confirm findings outside VA. A 2016 VACS analysis compared cancer incidence over four calendar periods during the ART-era (Figure 2; 1997–2000, 2001–2004, 2005–2008 and 2009–2012) and found overall declines in cancer incidence with the largest decline among AIDS defining cancers (55%) but also with significant declines in non-AIDS defining cancers (20%) as well as non-virally associated cancers (15%)[23].
Figure 2.
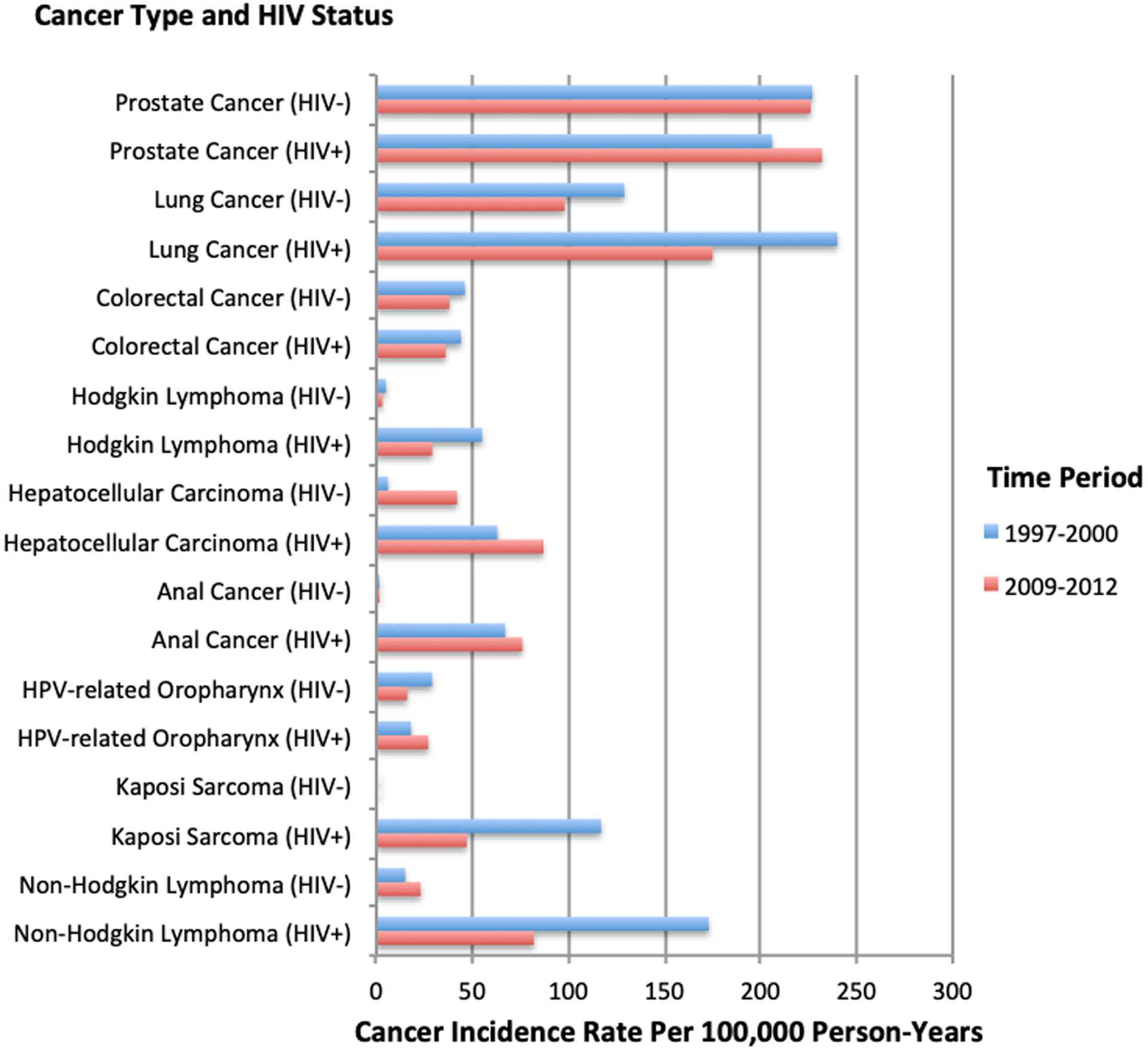
Incidence rates for specific cancers in the Veterans Aging Cohort Study by cancer type and HIV status during 1997–2000 and 2009–2012.
Decreases in cancer incidence among PWH have been attributed to improvements in immune function with antiretroviral therapy as well as population-level changes in behavioral risk factors such as smoking[24]. Despite this, cancer risk for Veterans with HIV still appears to be greater than those without HIV, even after accounting for important confounders[25].
A number of unique HIV-related factors appear to account for some of the excess cancer risk in persons with HIV. Determination of these HIV-specific risk factors for cancer has also been a focus of VA research. Unlike many other national data sources, VA data sources have detailed information on numerous important cancer-related exposures as well as HIV-related biomarker data. HIV viral suppression has been investigated as a cancer risk factor; using data from 42,441 Veterans with HIV from the VACS cohort and 4,169 incident cancers, Park et al. found that Veterans who experienced periods without viral suppression were at the highest risk of incident cancer (risk ratio [RR] 2.35; 95% confidence interval [95% CI] 2.19–2.51; compared to uninfected persons) and that even Veterans with long-term viral suppression still had higher cancer risk after adjustment than uninfected persons (RR 1.52; 95% CI: 1.44–1.61). This relationship was strongest for AIDS-defining cancers and virally associated cancers[27]. Another study using national VA data investigated the association of the incidence of three of the most common virally associated non-AIDS malignancies (anal cancer, Hodgkin lymphoma and hepatocellular carcinoma) with cumulative HIV viremia as measured by HIV RNA copy-years. That analysis found that each log increase in cumulative HIV copy-years was associated with a greater risk of anal cancer and Hodgkin lymphoma but there was no association with hepatocellular carcinoma[28].
As mentioned above, survival for persons with HIV and cancer has been worse than in cancer patients without HIV in several population-based studies. As some of these differences may relate to structural barriers to appropriate health care in demographic groups with a higher prevalence of HIV, it might be expected that those with HIV receiving care in the VA would experience less disparity in outcomes. There has been limited published data regarding outcomes for PWH and cancer in the VA system. Nevertheless, three ART-era studies have demonstrated comparable survival outcomes and little treatment disparity by HIV status for anal cancer and lung cancer[29–31].
4.1. Studies of Specific Cancers Among Veterans With HIV
As VA national data sources have matured during the antiretroviral era where NADCs have become prominent, much of the focus on HIV and cancer research within the VA system has focused on these tumor types with some continued research on AIDS-defining cancers:
4.2. AIDS-Defining Cancers: Kaposi Sarcoma and Non-Hodgkin Lymphoma
During the ART-era non-Hodgkin lymphoma (NHL) has been one of the most prevalent cancers in the general population of PWH as well as among Veterans with HIV. Although NHL risk has been markedly increased for Veterans with HIV compared to those without HIV[32], consistent with general trends among PWH, incidence rates for NHL have declined for Veterans with HIV throughout the ART-era[23]. A recent NA-ACCORD analysis (including data from VACS) evaluated immunologic risk factors for NHL in the ART-era and found that recent low CD4 count (as well as low nadir CD4, and cumulative exposure to low CD4) and prolonged HIV viremia were independent risk factors for NHL, but that risk factors differed across NHL subtypes. In addition, risk associated with cumulative HIV viremia measures were independent of CD4 count, suggesting lymphomagenesis mechanisms for PWH potentially relate to both immunosuppression and direct viral activity[33].
Kaposi Sarcoma (KS), another AIDS-defining tumor, has declined in incidence among Veterans with HIV with widespread ART use[23]. KS has received limited specific attention in large VA studies. One analysis of pre-ART era Veterans with HIV evaluated ultraviolet radiation (UVR) exposure and KS risk, and found that increasing UVR exposure was associated with greater KS risk[34]. The largest studies of KS using data from VACS have come from the NA-ACCORD collaboration.[35] In one of the largest studies of KS incidence in the ART-era, NA-ACCORD investigators estimated the effect of HIV-related immunologic biomarkers and ART-use on KS incidence and found that high cumulative HIV viremia and low recent CD4 counts were both strong KS risk factors, but that after adjusting for immunologic risk factors there was no independent effect of ART use on KS incidence. The investigators concluded that prompt control of HIV replication after HIV diagnosis was an important measure in preventing KS and that ART may not have a direct anti-KS effect. Although that analysis did not find an independent association with ART use and KS risk, a large analysis of national VA data investigated the role of different ART regimens on KS incidence, and found lower risk associated with the use of boosted protease inhibitor-based regimens[36].
4.3. Lung Cancer
Tobacco smoking and advancing age are the most potent lung cancer risk factors; High rates of smoking among Veterans with HIV have played a role in the large numbers of lung cancers and resultant morbidity and mortality in this group[37]. The independent contribution of HIV infection to lung cancer risk has been a frequent source of investigation in the ART-era. Although several early studies in non-Veteran populations found increased risk of lung cancer in persons with HIV compared to uninfected persons, these studies were often limited by small numbers of cancers or a lack of smoking data[38–41]. To address these limitations, a 2012 study using VACS data compared lung cancer incidence rates for lung cancer among 37,294 Veterans (457 incident lung cancer cases) with HIV to 75,750 uninfected Veterans, finding a 60% increase in lung cancer risk associated with HIV infection after adjusting for smoking status and other potential confounders providing strong evidence for an independent association between HIV and lung cancer risk. Interestingly, that study found no significant difference between lung cancer histologic subgroups or cancer stage at diagnosis by HIV status[42].
To clarify the potential HIV-related factors potentially responsible for increased lung cancer risk among PWH a subsequent VACS study evaluated immunologic and infectious risk factors for lung cancer using data from 21,666 Veterans with HIV. This longitudinal study found that a low CD4/CD8 ratio, suggestive of chronic immune activation, and severe, recurrent bacterial pneumonias were independent risk factors for incident lung cancer, consistent with findings in other large HIV cohorts[43–45].
Lung cancer outcomes for Veterans with HIV have been less well studied. Preliminary data has suggested that minimal treatment disparities for lung cancer by HIV status exist within the VA, differing from data in the non-VA HIV populations. Furthermore, a recent VA study on lung cancer surgery, the most common and effective therapy for early stage lung cancer, has suggested no difference in short-term mortality or major complications by HIV status[31].
Early detection of lung cancer has received increasing attention with the reporting of mortality benefits from the National Lung Screening Trial. The safety and efficacy of lung cancer screening with low dose CT has been evaluated using VA data; a multisite cohort study evaluated the risk of lung cancer screen positivity to determine if HIV infection might be associated with an excess of lung nodules potentially leading to false positive screening examinations with subsequent invasive evaluation. That analysis of CT scan findings in asymptomatic Veterans with and without HIV found that suspicious nodules were similarly prevalent in both groups, suggesting a lack of excess risk of false positive testing associated with HIV infection. However, Veterans with HIV in that study with CD4 counts less than 200 cells/mm3 did have very high proportions of suspicious nodules, potentially raising screening risks in that group[46]. As a more comprehensive evaluation of lung cancer screening with low dose CT data regarding long-term survival for Veterans with HIV who smoke as well as the findings from the aforementioned studies regarding lung cancer risk and treatment outcomes have been incorporated into an existing, well-validated lung cancer natural history model to evaluate the harms and benefits of low dose screening in persons with HIV. The output from that model demonstrated that the benefits and harms associated with low dose CT lung cancer screening for heavy smokers with HIV were similar to uninfected persons and that screening was likely to be both safe and effective in Veterans with HIV[47].
4.4. Anal Cancer
Anal cancer, a rare malignancy in the general population, has been found at higher than expected rates among PWH. Using national data VA researchers confirmed this trend among Veterans with HIV, finding an incidence rate ratio of 14.9 (95% CI: 10.1–22.1)[48]. In the same study, low CD4 counts were associated with increased risk of anal cancer, consistent with findings observed for other cancer types. A different analysis of national VA data also found that high levels of HIV viral suppression were also associated with a 45% decrease in the odds of anal cancer[49].
Outcomes for Veterans with HIV and anal cancer have been comparable to uninfected Veterans; Chiao et al evaluated outcomes for 1,112 Veterans with anal cancer (175 PWH) and found no differences in treatment patterns or survival associated with HIV status. Independent predictors of survival in that study included age, sex, advanced cancer stage and increasing comorbidity burden[29]. A contemporary VA-based study of anal cancer treatment outcomes for Veterans with HIV assessed the association of pretreatment CD4 level and post-definitive chemoradiotherapy outcomes, finding increased hematologic toxicity, risk of recurrence and lower cancer-specific survival associated with lower CD4 counts[50].
The efficacy of screening for anal cancer (most commonly with anal cytology and high resolution anoscopy with biopsy) among PWH has been inferred from cervical cancer screening and has been supported by several guidelines[51, 52]. Although a large multicenter randomized trial of treatment of high grade anal dysplastic lesions is ongoing, and is expected to provide pivotal guidance on the utility of screening, widespread implementation of anal dysplasia screening has already been undertaken[53]. Correspondingly, a single site study by a Florida VA center evaluated the results and acceptability of anal cancer screening in 162 Veterans and after finding high rates of positive screening tests concluded that these procedures could be feasible and well accepted in the context of VA HIV care[54].
4.5. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is also increased in persons with HIV compared to uninfected persons and has been frequently studied in Veterans with HIV.[55] Data regarding whether HIV is an independent risk factor for HCC in the ART-era has been conflicting; two large early ART-era studies of Veterans did not find an independent association with HIV[56, 57] while a later analysis did find that HIV was associated with increased HCC incidence after adjustment (hazard ratio 4.65; 95% CI: 2.70–8.02)[58]. The later study was the only one stratified by level of fibrosis/cirrhosis and found that HIV was a powerful risk factor among those who did not have cirrhosis. Common risk factors for HCC among Veterans with HIV include chronic hepatitis C virus infection (HCV) and alcohol use, and less commonly chronic hepatitis B virus (HBV) infection and non-alcoholic fatty liver disease[59, 60]. The prevalence of both cirrhosis and HCC has been increasing among Veterans with HIV[61]. Regarding HIV-specific HCC risk factors, large VA studies have found lower CD4 counts to be associated with HCC risk[58]. Among Veterans with HIV and HCV coinfection, key additional risk factors for HCC appear to be diabetes and severity of liver fibrosis as measured by FIB-4 index values[62]. VA research has also suggested that patients with HCV/HIV coinfection may also develop HCC sooner after HCV infection than HCV monoinfected patients[63].
HIV may adversely affect HCC outcomes; although patients with HIV who receive HCC treatment may not have worse survival[63], several multicenter studies including participating VA sites have found that HIV infection worsens survival for HCC patients who receive no cancer therapy[63, 64].
4.6. Hodgkin Lymphoma
Lymphoma has been associated with HIV infection since the beginning of the AIDS epidemic, and is still a leading tumor arising in Veterans with HIV. Hodgkin lymphoma (HL), an NADC that has been associated with Epstein-Barr virus infection, has been the focus of several analyses using national VA data. Three studies including similar cohorts of Veterans with HIV evaluated the risk of HL according to cumulative HIV exposure, as measured by HIV RNA copy-years, immunosuppression (CD4 count) and ART use; the studies found a dose-response increase in HL risk with each log increase in copy year, and also found that lower CD4 counts were also a risk factor for HL[28, 65, 66]. Long-term ART use was also protective for HL[66]. The 12-month period after ART initiation was the highest risk period for HL incidence in one study leading the authors to suggest that immune reconstitution may be related to HL risk[65].
4.7. Head and Neck Cancer
The incidence and outcomes for head and neck cancer for Veterans with HIV have not been extensively studied, likely due to a small number of cases; a 2016 analysis from the VACS cohort found only 7 HPV-related oral cavity cancer cases, 15 HPV-unrelated oral cavity cancers and 15 laryngeal cancers within their group of Veterans with HIV[23]. The authors of that study reported no change in head and neck cancer incidence trends over time, although it is likely that there was limited power to make this evaluation. Veteran data is contributed to the NA-ACCORD, which conducted a much larger study to estimate head and neck cancer risks associated with HIV; that analysis of 82,375 PWH still found a relatively small number of head and neck tumors (66 HPV-related, 182 HPV unrelated) but in comparison to general population rates the incidence associated with HIV was elevated (standardized incidence ratio [SIR] for HPV-related head and neck cancers: 3.2; 95% CI 2.5–3.4; SIR for HPV-unrelated: 3.0; 95% CI: 2.5–4.1)[67].
Risk factors for Veterans with HIV for oropharyngeal cancers have been evaluated; Chew et al. analyzed a cohort of 40,996 Veterans with HIV evaluating the association of demographics, HIV disease factors, smoking and previous HPV-related disease on oropharynx cancer incidence. They found that older age and poorer viral suppression were independently associated with oropharyngeal cancer risk but that previous HPV-related disease was not a risk factor for these tumors. Oropharynx cancer patients also had lower CD4 counts at cancer diagnosis (although decline of CD4 at cancer diagnosis has often been associated with the presence of cancer in PWH raising concerns of reverse causality)[68].
We found no large contemporary evaluations of head and neck cancer outcomes for Veterans with HIV.
4.8. Prostate Cancer
Prostate cancer is the most common cancer diagnosed in the VA system (as well as the most prevalent cancer for Veterans with HIV[27]) and will likely be the most common malignancy for all men with HIV by 2030, but otherwise has received relatively little study among Veterans with HIV[69, 70]. Prostate cancer is notable for its unique risk profile among persons with HIV; most studies have found lower incidence of prostate cancer than expected among men with HIV[48, 71, 72]. The reasons for this lower risk are unclear; it has been suggested that lower rates of prostate cancer screening may occur in men with HIV, although preliminary data from the VACS cohort evaluating prostate specific antigen testing and prostate cancer incidence among Veterans with and without HIV suggest that differential screening rates do not fully explain lower prostate cancer incidence[73].
We did not identify any large outcomes studies describing Veterans with HIV and prostate cancer, although one contemporary series from a single center described outcomes for external beam radiotherapy (EBRT) treatment of localized prostate cancer in Veterans with HIV. In that series of 15 patients the investigators found that EBRT was well tolerated, but was associated with a consistent CD4 decline[74].
5.1. Conclusions and Future Directions
Cancer is a growing source of morbidity and mortality in the aging US HIV population and is a significant issue for the VA health system, as the largest single institutional HIV care provider. National, regional and institutional VA data sources have allowed for much insight regarding cancer epidemiology and clinical management for Veterans with HIV, with significant focus on the non-AIDS defining malignancies that have become more prevalent in the antiretroviral era. More data is needed regarding the impact of cancer treatment and prevention in Veterans with HIV, given the unique clinical issues that affect this population. As several cancers have both increased incidence associated with HIV as well as HIV-specific risk factors, early detection and treatment strategies that consider these differences may be worthwhile areas of study to address the emerging cancer burden among Veterans with HIV.
Acknowledgement of Funding:
This work was funded by the National Cancer Institute (R01CA210806, K07CA180782 to KS).
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Centers for Disease C. Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30(25):305–8. [PubMed] [Google Scholar]
- 2.Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51(8):957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 5.Park LS, Hernandez-Ramirez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30(2):273–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–94. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigel K, Makinson A, Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS. 2017;12(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigel K, Pitts R, Crothers K. Lung Malignancies in HIV Infection. Semin Respir Crit Care Med. 2016;37(2):267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess Mortality among HIV-Infected Individuals with Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rositch AF, Jiang S, Coghill AE, Suneja G, Engels EA. Disparities and Determinants of Cancer Treatment in Elderly Americans Living With Human Immunodeficiency Virus/AIDS. Clin Infect Dis. 2018;67(12):1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suneja G, Boyer M, Yehia BR, Shiels MS, Engels EA, Bekelman JE, et al. Cancer Treatment in Patients With HIV Infection and Non–AIDS-Defining Cancers: A Survey of US Oncologists. J Oncol Pract. 2015;11(3):e380–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makinson A, Tenon JC, Eymard-Duvernay S, Pujol JL, Allavena C, Cuzin L, et al. Human immunodeficiency virus infection and non-small cell lung cancer: survival and toxicity of antineoplastic chemotherapy in a cohort study. J Thorac Oncol. 2011;6(6):1022–9. [DOI] [PubMed] [Google Scholar]
- 15.Okuma Y, Hishima T, Kashima J, Homma S. High PD-L1 expression indicates poor prognosis of HIV-infected patients with non-small cell lung cancer. Cancer Immunol Immunother. 2018;67(3):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grover S, Desir F, Jing Y, Bhatia RK, Trifiletti DM, Swisher-McClure S, et al. Reduced Cancer Survival Among Adults With HIV and AIDS-Defining Illnesses Despite No Difference in Cancer Stage at Diagnosis. J Acquir Immune Defic Syndr. 2018;79(4):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens DK, Sundaram V, Lazzeroni LC, Douglass LR, Sanders GD, Taylor K, et al. Prevalence of HIV infection among inpatients and outpatients in Department of Veterans Affairs health care systems: implications for screening programs for HIV. Am J Public Health. 2007;97(12):2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of Human Immunodeficiency Virus, Hepatitis C Virus, and Hepatitis B Virus Among Homeless and Nonhomeless United States Veterans. Clin Infect Dis. 2017;65(2):252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs Healthcare System: A unique laboratory for observational and interventional research. Med Care. 2006;44(8 Suppl 2):S7–12. [DOI] [PubMed] [Google Scholar]
- 20.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the national VA health information system. Medical Care. 2006;44(8):S25–S30. [DOI] [PubMed] [Google Scholar]
- 21.Park LS, Tate JP, Rodriguez-Barradas MC, Rimland D, Goetz MB, Gibert C, et al. Cancer Incidence in HIV-Infected Versus Uninfected Veterans: Comparison of Cancer Registry and ICD-9 Code Diagnoses. J AIDS Clin Res. 2014;5(7):1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park LS, Tate JP, Sigel K, Rimland D, Crothers K, Gibert C, et al. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997–2012. AIDS. 2016;30(11):1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med. 2015;163(7):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60(4):627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67(4):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park LS, Tate JP, Sigel K, Brown ST, Crothers K, Gibert C, et al. Association of Viral Suppression With Lower AIDS-Defining and Non-AIDS-Defining Cancer Incidence in HIV-Infected Veterans: A Prospective Cohort Study. Ann Intern Med. 2018;169(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr. 2014;67(2):204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiao EY, Giordano TP, Richardson P, El-Serag HB. Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26(3):474–9. [DOI] [PubMed] [Google Scholar]
- 30.Sigel K, Crothers K, Brown S, Gibert C, Goetz M, Bedimo R, et al. Treatment and Outcomes of Non-Small Cell Lung Cancer in Later ART-Era HIV Infection. Conference on Retroviruses and Opportunistic Infections; Seattle, WA: 2017. [Google Scholar]
- 31.Sigel KM, Stone K, Wisnivesky JP, Park LS, Kong CY, Silverberg MJ, et al. Short-term outcomes for lung cancer resection surgery in HIV infection. AIDS. 2019. [DOI] [PMC free article] [PubMed]
- 32.McGinnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular carcinoma and non-Hodgkin’s lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol. 2006;24(31):5005–9. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez-Ramirez RU, Qin L, Lin H, Leyden W, Neugebauer RS, Althoff KN, et al. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV. 2019. [DOI] [PMC free article] [PubMed]
- 34.Cahoon EK, Engels EA, Freedman DM, Norval M, Pfeiffer RM. Ultraviolet Radiation and Kaposi Sarcoma Incidence in a Nationwide US Cohort of HIV-Infected Men. J Natl Cancer Inst. 2017;109(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubrow R, Qin L, Lin H, Hernandez-Ramirez RU, Neugebauer RS, Leyden W, et al. Association of CD4+ T-cell Count, HIV-1 RNA Viral Load, and Antiretroviral Therapy With Kaposi Sarcoma Risk Among HIV-infected Persons in the United States and Canada. J Acquir Immune Defic Syndr. 2017;75(4):382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalkowski MA, Kramer JR, Richardson PR, Suteria I, Chiao EY. Use of boosted protease inhibitors reduces Kaposi sarcoma incidence among male veterans with HIV infection. Clin Infect Dis. 2015;60(9):1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Goetz MB, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(3 Suppl):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383–8. [DOI] [PubMed] [Google Scholar]
- 39.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. Aids. 2007;21(2):207–13. [DOI] [PubMed] [Google Scholar]
- 40.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr.55(4):510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine AM, Seaberg EC, Hessol NA, Preston-Martin S, Silver S, Cohen MH, et al. HIV as a risk factor for lung cancer in women: data from the Women’s Interagency HIV Study. J Clin Oncol. 2010;28(9):1514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26(8):1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. The lancet HIV. 2017;4(2):e67–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifford GM, Lise M, Franceschi S, Egger M, Bouchardy C, Korol D, et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer. 2012;106(3):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcus JL, Leyden WA, Chao CR, Horberg MA, Klein DB, Quesenberry CP Jr., et al. Immunodeficiency, AIDS-related pneumonia, and risk of lung cancer among HIV-infected individuals. AIDS. 2017;31(7):989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigel K, Wisnivesky J, Shahrir S, Brown ST, Justice A, Kim J, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014;28(7):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong CY, Sigel K, Criss SD, Sheehan DF, Triplette M, Silverberg MJ, et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/mul. AIDS. 2018;32(10):1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52(2):203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiao EY, Hartman CM, El-Serag HB, Giordano TP. The impact of HIV viral control on the incidence of HIV-associated anal cancer. J Acquir Immune Defic Syndr. 2013;63(5):631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryant AK, Mudgway R, Huynh-Le MP, Simpson DR, Mell LK, Gupta S, et al. Effect of CD4 Count on Treatment Toxicity and Tumor Recurrence in Human Immunodeficiency Virus-Positive Patients With Anal Cancer. Int J Radiat Oncol Biol Phys. 2018;100(2):478–85. [DOI] [PubMed] [Google Scholar]
- 51.Gaisa M, Ita-Nagy F, Sigel K, Arens Y, Hennessy MA, Rodriguez-Caprio G, et al. High Rates of Anal High-Grade Squamous Intraepithelial Lesions in HIV-Infected Women Who Do Not Meet Screening Guidelines. Clin Infect Dis. 2017;64(3):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite S, Justice A. Cancer screening in patients infected with HIV. Curr HIV/AIDS Rep. 2011;8(3):142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rider P, Hunter J, Grimm L. The Diagnostic and Therapeutic Challenge of Anal Intraepithelial Neoplasia. Curr Gastroenterol Rep. 2018;20(8):38. [DOI] [PubMed] [Google Scholar]
- 54.Rosa-Cunha I, Degennaro VA, Hartmann R, Milikowski C, Irizarry A, Heitman B, et al. Description of a pilot anal pap smear screening program among individuals attending a Veteran’s Affairs HIV clinic. AIDS Patient Care STDS. 2011;25(4):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo Re V 3rd, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56–63. [DOI] [PubMed] [Google Scholar]
- 57.Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. J Acquir Immune Defic Syndr. 2015;68(4):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164(21):2349–54. [DOI] [PubMed] [Google Scholar]
- 60.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–82 e5; quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 61.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–57. [DOI] [PubMed] [Google Scholar]
- 62.Park LS, Tate JP, Justice AC, Lo Re V 3rd, Lim JK, Brau N, et al. FIB-4 index is associated with hepatocellular carcinoma risk in HIV-infected patients. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brau N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47(4):527–37. [DOI] [PubMed] [Google Scholar]
- 64.Pinato DJ, Allara E, Chen TY, Trevisani F, Minguez B, Zoli M, et al. Influence of HIV Infection on the Natural History of Hepatocellular Carcinoma: Results From a Global Multicohort Study. J Clin Oncol. 2019;37(4):296–304. [DOI] [PubMed] [Google Scholar]
- 65.Kowalkowski MA, Mims MP, Amiran ES, Lulla P, Chiao EY. Effect of immune reconstitution on the incidence of HIV-related Hodgkin lymphoma. PLoS One. 2013;8(10):e77409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kowalkowski MA, Mims MA, Day RS, Du XL, Chan W, Chiao EY. Longer duration of combination antiretroviral therapy reduces the risk of Hodgkin lymphoma: A cohort study of HIV-infected male veterans. Cancer Epidemiol. 2014;38(4):386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beachler DC, Abraham AG, Silverberg MJ, Jing Y, Fakhry C, Gill MJ, et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol. 2014;50(12):1169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chew EY, Hartman CM, Richardson PA, Zevallos JP, Sikora AG, Kramer JR, et al. Risk factors for oropharynx cancer in a cohort of HIV-infected veterans. Oral Oncol. 2017;68:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med. 2018;168(12):866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leapman M, Park LS, Stone K, Gibert C, Goetz M, Bedimo R, et al. Prostate Cancer Screening and Incidence in Aging Veterans Infected with HIV. Conference on Retroviruses and Opportunistic Infections; Seattle, WA: 2019. [Google Scholar]
- 74.Schreiber D, Chhabra A, Rineer J, Nabhani T, Katsoulakis E, Morkos R, et al. Outcomes and tolerance of human immunodeficiency virus--positive U.S. veterans undergoing dose-escalated external beam radiotherapy for localized prostate cancer. Clin Genitourin Cancer. 2014;12(2):94–9. [DOI] [PubMed] [Google Scholar]


