Abstract
Objectives:
Symptoms are inconsistently associated with esophageal motor findings on high resolution manometry (HRM). We aimed to evaluate predictors of dysphagia severity, including esophageal hypervigilance and visceral anxiety, among patients evaluated with HRM.
Methods:
Adult patients undergoing HRM at four academic medical centers (USA and France) were prospectively evaluated. HRM was completed and analyzed per the Chicago Classification v3.0. Validated symptom scores, including the Brief Esophageal Dysphagia Questionnaire (BEDQ) and Esophageal Hypervigilance and Anxiety Scale (EHAS) were completed at the time of HRM.
Results:
236 patients, ages 18-85 (mean 53) years, 65% female, were included. 59 (25%) patients had a major motor disorder on HRM: 19 achalasia, 24 esophagogastric junction outflow obstruction, 12 absent contractility, 4 jackhammer. 177 (75%) patients did not have a major motor disorder: 71 ineffective esophageal motility, 106 normal motility. Having a major motor disorder was a significant predictor of dysphagia severity (R2adj = 0.049, P<.001), but EHAS score carried a predictive relationship of BEDQ that was two-fold higher than having a major motor disorder: R2adj = 0.118 (P<.001). This finding remained when evaluated by major motor disorder group. HRM metrics were non-significant.
Conclusions:
In a prospective, international multicenter study, we found that esophageal hypervigilance and visceral anxiety were the strongest predictors of dysphagia severity among patients evaluated with HRM. Thus, an assessment of esophageal hypervigilance and visceral anxiety is important to incorporate when evaluating symptom severity in clinical practice and research studies.
Keywords: dysphagia, patient reported outcomes, functional, motility, hypersensitivity
Introduction:
Esophageal manometry is the primary method to evaluate esophageal motility and is therefore utilized in the clinical evaluation of patients with esophageal symptoms, particularly non-obstructive dysphagia and nonerosive gastro-esophageal reflux disease (GERD).1 High-resolution manometry (HRM) with esophageal pressure topography allows detailed characterization of esophageal contractile activity and sphincter function and also provides objective metrics of esophageal function that can be applied to a standardized, hierarchical classification scheme of esophageal motility diagnoses: the Chicago Classification.1, 2 However, the association of esophageal symptoms with HRM parameters and esophageal motility diagnoses remains inconsistent.3-5 This carries potential challenges in clinical management as therapeutic strategies in esophageal motility disorders often target manometric abnormalities.
The detection of consistent physiologic esophageal abnormalities that are associated with esophageal symptoms, however, is potentially confounded by psychological and cognitive factors that likely contribute to and modulate the symptom generation process.6 Anxiety, for example, was associated with increased symptom severity among patients with GERD in several previous studies.7, 8 Anxiety was also associated with esophageal bolus perception among otherwise asymptomatic, healthy volunteers evaluated with manometry.9 Focused attention on esophageal sensations, heightened anxiety, expectations of pain, and catastrophizing about the consequences of symptoms all contribute to esophageal hypervigilance and associated hypersensitivity. We hypothesize that these features of esophageal hypervigilance and visceral anxiety are important contributors to the presence and severity of esophageal symptoms and may account for some of the disconnect between patients’ symptom reports and objective measures of esophageal function.
We therefore aimed to evaluate predictive factors of dysphagia severity, including esophageal hypervigilance and visceral anxiety, using validated patient-reported outcomes (PROs) among an international, multi-centered cohort of patients evaluated with HRM.
Methods
Subjects
Consecutive patients aged 18-85 years that completed HRM at four academic medical centers (Northwestern Medicine, Chicago, IL, USA; Washington University, St. Louis, MO, USA; Hospices Civils de Lyon, Lyon, France; and Mayo Clinic – Arizona, Scottsdale, AZ, USA) were prospectively evaluated over a two month period in 2017. Patients were included if they completed three patient-reported outcome (PRO) measures at the time of HRM. Other clinical information including recent endoscopy results and use of chronic opioids was collected when available. As the Chicago Classification is intended for evaluation of primary motility disorder, patients were excluded for previous foregut surgery (n=69), mechanical obstruction on endoscopy (n=10), Los Angeles class C or D reflux esophagitis (n=3), hiatal hernia > 3cm (n=27), technically-limited HRM (n=16).1 Patients were also excluded for incomplete PROs (i.e. any missing item among the three PROs; n=84). The study protocol was approved by the Northwestern University and Mayo Clinic Institutional Review Board; a minimal risk exemption was granted, and thus patient informed consent was not obtained. Sharing of deidentified demographic, questionnaire and HRM data with Northwestern University as part of this protocol was approved by the Human Research Protection Office at Washington University in St. Louis. According to French Law, this analysis of data obtained during clinical evaluation of patients did not require ethical review board. Patients were informed that their clinical data could be used for clinical research, after anonymization. They had the possibility to sign a document indicating their refusal to participate, in which case their files were not used for the study.
Patient reported outcomes
Patients completed three validated PROs at the time of HRM: the Brief Esophageal Dysphagia Questionnaire (BEDQ), the Esophageal Hypervigilance and Anxiety Scale (EHAS), and GastroEsophageal Reflux Disease Questionnaire (GERDQ).10-12 For the French site, the three PROs were translated to French by two native French speakers (authors SR and FM), who reached consensus agreement on translation. The PROs were subsequently validated as reliable and valid measures among a larger French cohort.13, 14
The BEDQ is a self-report measure that consists of eight 6-point Likert scale questions (scored 0-5) assessing the frequency and severity of dysphagia and odynophagia over the preceding 14-days.10 It also includes two open-ended questions regarding frequency of food impactions and related emergency room visits. The 8 Likert-scaled items are summed to yield scores ranging from 0 (asymptomatic) – 40, with greater scores indicating greater dysphagia severity. The two open-ended questions were dichotomized as Yes (at least 1 food impaction or ED visit) or No.
The EHAS is a self-report measure that consists of 15, 5-point Likert scale questions (scored 0-4) that assesses esophageal hypervigilance and esophageal symptom-specific anxiety over the preceding one month.11 The items are summed to yield total EHAS scores ranging from 0 – 60, with greater scores indicating greater esophageal hypervigilance and esophageal symptom-specific anxiety. The EHAS score was 0 in 19/20 previously-described healthy volunteers without esophageal symptoms; the other subject reported an EHAS score of 24.15
The GERDQ is a 6-item self-report measure used to support GERD diagnosis.12 The items assess the frequency of symptoms and medication use over the preceding 7 days. The GERDQ is score in generated by summing four graded Likert scale items of four positive predictors (scored 0-3) and two reverse Likert scale items of negative predictors (scored 3-0). GERDQ scores > 8 are considered positive for a GERD diagnosis.
HRIM protocol and analysis
After a minimum 6-hour fast, HRM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. After a 2-minute baseline recording, the HRM protocol was performed with ten, 5-ml liquid swallows in a supine position.
Manometry studies were analyzed using ManoView version 3.0 analysis software (Medtronic) to measure basal EGJ pressure (end-expiration), the integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency according to the Chicago Classification.1 A median IRP of > 15 mmHg was applied as the upper-limit of normal.1 Failed swallows were assigned a DCI value of 0 mmHg•cm•s. Chicago classification diagnoses of achalasia (types I, II, III), EGJ outflow obstruction, absent contractility, distal esophageal spasm, and jackhammer esophagus were considered as major motor disorders. Ineffective esophageal motility (IEM), fragmented peristalsis, and normal motility were considered not major motor disorders.
Statistical analysis
Data were consolidated from the study sites and entered into SPSS v25 for analyses (Chicago, IL). Initial assessments of Skewness and Kurtosis of +/− 2.0 to verify normal distribution were conducted to determine the need for non-parametric tests. Differences between categorical variables were compared using chi-square test with Fisher’s Exact evaluated for small sample sets, as appropriate. Correlation of continuous variables was evaluated with Pearson’s correlation or Spearman’s Rho, depending on data distribution. Groups were compared using analysis of variance (ANOVA) and independent samples t-Test or Kruskal-Wallis and Mann Whitney U tests, depending on data distribution. For illustrative purposes, a median-split to categorize patients into high EHAS (EHAS ≥ 33) or low EHAS (<33) scores was performed with additional comparisons using ANOVA.
A priori analysis strategy included multivariable linear regression for the entire study sample. Variables with significant associations to BEDQ score (Presence of major motor disorder, basal EGJ-Pressure, and EHAS Score) were selected as potential predictor variables and entered into the hierarchical regression analysis; IRP was not utilized in the regression models due to correlation with basal EGJ-pressure (rho = 0.623) and the influence of IRP on classification of major motor disorder. Due to the difference in BEDQ score by presence of major motor disorder, additional multivariable linear regressions were performed for each group. R-squared adjusted was converted to percentage variance (R2adj x 100). Standardized beta weights (β) are reported in standardized units of standard deviation (SD).
Statistical significance was set to P<.05 for correlations and regression models. Bonferroni correction (P<0.01) was applied to account for multiple comparisons and potential Type 1 error for comparative tests. For regression analyses, multicollinearity was evaluated using standard cutoff scores for VIF (< 5.0) and tolerance (> 0.2) with no violations indicated.
Results
Subjects
236 patients, mean (standard deviation, SD) age 53 (15) years, 65% female, were included. Primary indication for HRM was dysphagia in 38%, chest pain in 10%, reflux symptoms in 41%, and other (e.g. epigastric pain; nausea; pre-bariatric surgery evaluation) in 11%. Endoscopic findings included small (<3-cm) hiatal hernia in 53 patients (22%), mild reflux esophagitis in 16 patients (7%; Los-Angeles Classification A in 8, B in 8), candidiasis in 4 patients, and non-dysplastic Barrett’s esophagus in 3 patients; 49 (21%) of patients did not have available endoscopy results. Of the 218 patients with available opioid use status, 27 patients (12%) were on opioids at the time of their HRIM. Patient characteristics by center are reported in Table 1.
Table 1. Patient characteristics by center.
| Center 1 | Center 2 | Center 3 | Center 4 | |
|---|---|---|---|---|
| n (%) | 111 | 58 | 34 | 31 |
| Age, years, mean (SD) | 52 (15) | 56 (13) | 53 (15) | 50 (16) |
| % Female* | 71 | 74 | 44 | 52 |
| BMI, kg2/m, median (IQR)* | [n = 102] | [n = 58] | [n = 34] | [n = 30] |
| 31 (25 – 38) | 28 (24 – 30) | 23 (20 – 27) | 26 (23 – 32) | |
| Endoscopy | [n = 80] | [n = 47] | [n = 32] | [n = 23] |
| Hiatal Hernia, n (%) | 27 (34) | 10 (21) | 11 (34) | 5 (22) |
| Reflux esophagitis, LA-A; LA-B, n (%) | 3 (3); 3 (3) | 2 (3); 3 (5) | 2 (6); 1 (3) | 1 (3); 1 (3) |
| Indication for HRM, n (%)* | ||||
| Dysphagia | 42 (38) | 18 (31) | 7 (20) | 20 (64) |
| Chest pain | 14 (13) | 6 (10) | 3 (9) | 0 |
| Reflux | 46 (41) | 33 (57) | 11 (32) | 8 (26) |
| Other | 9 (8) | 1 (2) | 13 (38) | 3 (10) |
| Chronic opioid use, n/n available (%) | 19/75 (20) | 3/58 (5) | 5/34 (15) | 0/30 (0) |
| BEDQ score, mean (SD) | 8.0 (8.7) | 7.8 (7.6) | 8.8 (9.7) | 9.3 (9.8) |
| BEDQ ≥1 food-impaction, n (%)* | 32 (29) | 10 (17) | 1 (3) | 4 (13) |
| BEDQ ≥1 emergency room visit, n (%)* | 26 (23) | 9 (16) | 1 (3) | 2 (7) |
| GERDQ score, mean (SD) | 9.0 (2.9) | 8.3 (2.6) | 9.0 (2.5) | 8.4 (2.5) |
| EHAS score, mean (SD) | 32.4 (16.2) | 30.2 (15.4) | 30.1 (13.5) | 28.3 (15.3) |
| Esophageal motility classification, n (%) | ||||
| Achalasia | 10 (9) | 1 (2) | 2 (6) | 5 (16) |
| EGJ Outflow Obstruction | 8 (7) | 5 (9) | 5 (15) | 6 (19) |
| Absent contractility | 7 (6) | 2 (3) | 1 (3) | 2 (7) |
| Jackhammer | 1 (1) | 1 (2) | 1 (3) | 1 (3) |
| Ineffective Esophageal Motility | 35 (32) | 19 (33) | 13 (38) | 3 (10) |
| Normal motility | 50 (45) | 30 (52) | 12 (35) | 14 (45) |
P<0.05 among all centers. BMI-body mass index. LA – Los Angeles. BMI-body mass index. BEDQ – brief esophageal dysphagia questionnaire. GERDQ – gastroesophageal reflux disease questionnaire. EHAS – esophageal hypervigilance and anxiety scale.
The mean (SD) BEDQ score was 7.1 (7.8), EHAS score was 31.4 (15.7), and GERDQ score was 8.6 (2.8); 121 patients (51%) had a GERDQ score > 8. None of the three PROs had a significant correlation with age (r = −0.040, −0.103, and −0.047 for BEDQ, EHAS, and GERDQ, respectively; P-values > 0.113) nor differed by gender (P-values > 0.549). None of the PRO scores differed by opioid use status, mean (SD) for on versus off opioids, respectively: BEDQ: 11 (10) versus 8 (8), P = 0.117; EHAS: 34 (15) versus 30 (16), P = 0.447; GERDQ: 8 (2) versus 9 (3), P = 0.073.
On the BEDQ open-ended questions assessing events over the preceding 12-months, 189 (80%) patients reported zero food impactions, 15 (6%) reported 1-2 food impactions, 15 (6%) reported 3-9 food impactions, and 17 (7%) reported ≥ 10 food impactions. The number of food-impaction related emergency room visits was reported as zero in 223 (95%), 1-2 in 11 (5%), and >2 in 2 patients (<1%).
High-resolution manometry/Chicago Classification
Among the entire cohort, median (IQRs) for median IRP was 7 (3 – 12) mmHg, median DCI was 1052 (200 – 2310) mmHg•cm•s, and basal EGJ pressure was 14 (6 – 25) mmHg. A median IRP > 15 mmHg was observed in 16% of patients. EGJ morphology was classified as type I in 177 (75%) patients and type II in 59 (25%).
Esophageal motility diagnoses based on the Chicago Classification included 19 achalasia (8%; 3 type I; 12 type II; 4 type III), 24 (10%) EGJ outflow obstruction, 12 (5%) absent contractility, 4 (2%) jackhammer, 70 (30%) IEM, 1 fragmented peristalsis (this patient included among the IEM cohort for subsequent analysis), and 106 (45%) normal motility. There were no patients with a diagnosis of distal esophageal spasm. Thus, 59 (25%) patients had a major motor disorder on HRM, while the remaining 177 (75%) did not have a major motor disorder.
Associations of Manometry, Symptom-Specific Hypervigilance and Anxiety, and Dysphagia Severity
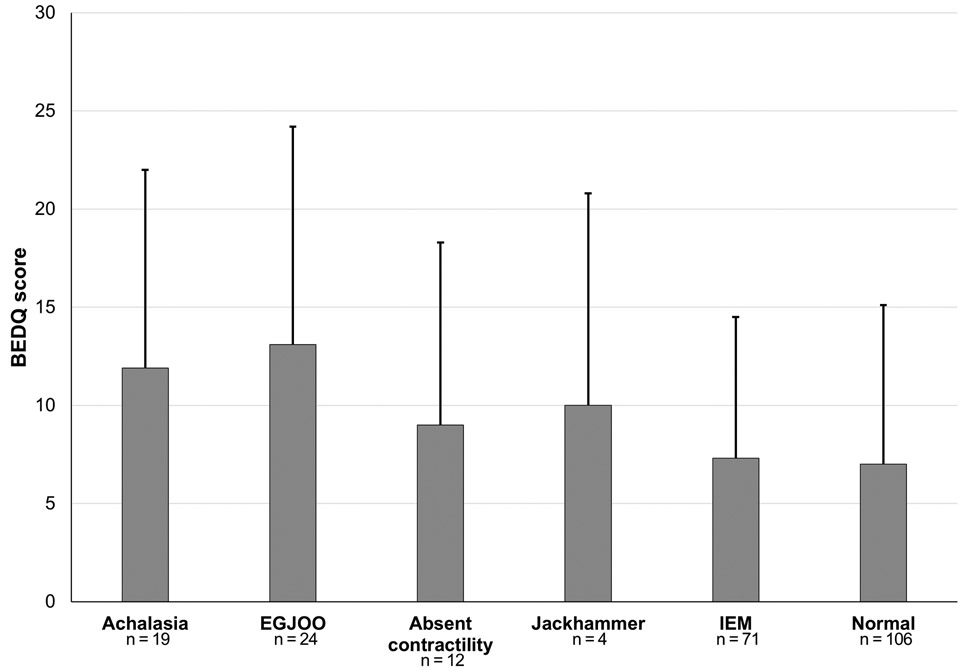
Dysphagia severity did not differ between Chicago Classification diagnoses (P = 0.132; Figure 1. However, patients with a major esophageal motor disorder had greater dysphagia severity, mean (SD) BEDQ score 12 (10), than patients without a major motor disorder, BEDQ score 7 (8); P = 0.002. EHAS and GERDQ scores did not differ among Chicago Classification diagnoses (Supplementary Figure 1; P-values 0.820 and 0.743. respectively) or major motor disorder versus without major motor disorder, mean (SD) EHAS 30 (15) versus 31 (16), P = 0.624; mean (SD) GERDQ: 9 (2) versus 9 (3), P = 0.221.
Figure 1. Dysphagia Severity by Esophageal Motility Classification.
Brief Esophageal Dysphagia Questionnaire (BEDQ) score displayed as mean (standard deviation) by Chicago Classification diagnosis. Achalasia included all subtypes: 3 type I, 12 type II, and 4 type III. EGJOO – esophagogastric junction outflow obstruction. IEM – ineffective esophageal motility. Figure used with permission from the Esophageal Center at Northwestern.
Dysphagia severity (BEDQ score) was weakly, but positively correlated with median IRP (rho = 0.174; P = 0.008) and basal EGJ pressure (rho = 0.171; P = 0.009), but poorly correlated with median DCI (rho = −0.030; P = 0.652). Dysphagia severity also differed between patients with median IRP > 15mmHg, mean (SD) BEDQ score 13 (11) and patients with median IRP ≤ 15 mmHg, BEDQ score 7 (8); P = 0.003. Dysphagia severity was positively correlated with EHAS score (r = 0.347; P <0.001). Correlation of BEDQ and EHAS scores was slightly stronger among patients with major motor disorders (r = 0.470) than patients without major motor disorders (rho = 0.327); P-values <0.001 (Supplementary Figure 2). Among the entire cohort, BEDQ and GERDQ scores did not correlate (r = 0.041; P = 0.530), though GERDQ score positively correlated with EHAS score (r = 0.255; P <0.001).
The proportion of occurrence of one or more reported food-impactions, or one of more related emergency room visits, did not differ by presence versus absence of a major motor disorders (P = 0.999 and 0.999, respectively), nor among motility classifications (P = 0.773 and 0.474, respectively); Table 2. BEDQ score, GERDQ score, and EHAS score were all greater among patients reporting one or more food-impactions or related emergency room visit, than among those reporting zero (Table 2). Two patients reported >2 food impaction associated emergency room visits: one had type I achalasia, reported 4 food impactions and 4 associated emergency room visiting in the preceding year, and had an EHAS score of 26. The other had an endoscopy with a small hiatal hernia, normal motility on HRM, reported 155 perceived food impactions with 12 associated emergency room visits in the preceding year, and had an EHAS score of 58.
Table 2. Food-impactions and related emergency room visits by esophageal motility classification.
Number of reported occurrences reflect the preceding 12 months in the open-ended responses of the Brief Esophageal Dysphagia Questionnaire (BEDQ).
| Food impaction | Emergency room visits | |||
|---|---|---|---|---|
| Reported occurrences | 0 | ≥1 | 0 | ≥1 |
| n (%) | n (%) | n (%) | n (%) | |
| Total | 189 (80) | 47 (20) | 198 (84) | 38 (16) |
| Major motor disorder | 47 (80) | 12 (20) | 50 (85) | 9 (15) |
| Without major motor disorder | 142 (80) | 35 (20) | 148 (84) | 29 (16) |
| Achalasia | 15 (79) | 41 (21) | 17 (89) | 22 (11) |
| EGJ outflow obstruction | 20 (83) | 4 (17) | 21 (88) | 3 (12) |
| Absent contractility | 8 (67) | 4 (33) | 8 (67) | 4 (33) |
| Jackhammer | 4 (100) | 0 (0) | 4 (100) | 0 (0) |
| IEM | 57 (80) | 14 (20) | 61 (86) | 10 (14) |
| Normal motility | 85 (80) | 21 (20) | 87 (82) | 19 (18) |
| Small hiatal hernia on endoscopy | 33/146 (23) | 21/38 (55)* | 39/152 (26) | 15/32 (47)* |
| BEDQ-emergency room visits | 1 (<1%) | 37 (79%) | -- | -- |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Basal EGJ pressure, mmHg | 14 (6 - 24) | 17 (9 – 30) | 14 (6 – 24) | 16 (8 – 28) |
| Median IRP, mmHg | 7 (3 – 13) | 8 (3 – 11) | 7 (3 – 13) | 7 (3 – 11) |
| Median DCI, mmHg•cm•s | 1299 (214 –2676) | 658 (85 – 1646)* | 1279 (217 – 2655) | 698 (65 – 1651) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| BEDQ score | 7.4 (8.2) | 11.5 (9.8)* | 7.4 (8.2) | 12.8 (9.6)* |
| GERDQ score | 8.6 (2.7) | 9.5 (2.5)* | 8.6 (2.7) | 9.6 (2.5)* |
| EHAS score | 29.9 (15.7) | 36 (13.9)* | 30.0 (15.5) | 37.1 (14.2)* |
P < 0.05 compared with zero food impactions or emergency room visits, respectively.
3 type I, 1 type II achalasia.
Both type I achalasia. IEM – ineffective esophageal motility. IRP – integrated relaxation pressure. DCI – distal contractile integral. EHAS – Esophageal Hypervigilance and Anxiety Scale.
For the entire cohort, having a major motor disorder was a significant predictor of dysphagia severity (R2adj = 0.049; 5% of variance, P<.001). However, total EHAS demonstrated a more than two-fold predictive relationship of BEDQ (R2adj = 0.118; 12% of variance, P<.001), Table 3A. Multivariable hierarchical linear regression also found only EHAS score to be a significant predictor of dysphagia symptom severity for both major motor disorder classifications (Table 3B). For patients with a major motor disorder, esophageal hypervigilance and anxiety accounted for 21.8% of the variance in BEDQ score and for those with no major motor disorder, EHAS accounted for 10% of the variance in BEDQ. Application of the β co-efficient provided estimates that among patients with major-motor disorders, an increase in EHAS score of 2 would be associated with an increase in BEDQ score of 1, while among patients without a major motor disorder, an increase in EHAS score of 3 would be associated with an increase in BEDQ score of 1.
Table 3. Multivariable Regression Analysis of Predictors of Dysphagia Severity.
Variables with significant associations to Brief Esophageal Dysphagia Questionnaire (BEDQ) score were included in a hierarchical linear regression analysis entailing the entire study sample (A) and by presence of a major esophageal motor disorder (B). EHAS – Esophageal Hypervigilance and Anxiety Scale. EGJP – esophagogastric junction pressure. CI-confidence interval.
| A. | |||||
|---|---|---|---|---|---|
| R2 (%) | R2adj (%) |
Unstandardized B (95% CI) |
β-coefficient (standardized) |
P | |
| Model 1 | 5.3 | 4.9 | <0.001 | ||
| Major Motor Disorder (Yes) | 4.6 (2.1 – 7.1) | 0.229 | <0.001 | ||
| Model 2 | 6.7 | 5.9 | 0.059 | ||
| Major Motor Disorder (Yes) | 3.8 (1.2 – 6.5) | 0.192 | 0.004 | ||
| Basal EGJP | 0.07 (0 – 0.14) | 0.126 | 0.059 | ||
| Model 3 | 18.8 | 17.7 | <0.001 | ||
| Major Motor Disorder (Yes) | 4.23 (1.8 – 6.7) | 0.212 | 0.001 | ||
| Basal EGJP | 0.06 (−0.01 – 0.13) | 0.101 | 0.106 | ||
| EHAS Score | 0.20 (0.13 – 0.26) | 0.348 | <0.001 | ||
| B. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Major Motor Disorder (n = 59) |
No Major Motor Disorder (n = 177) |
|||||||||
| R2 (%) |
R2adj (%) |
Unstandardized B (95% CI) |
β (standardized) |
P | R2 (%) |
R2adj (%) |
Unstandardized B (95% CI) |
β (standardized) |
P | |
| Model 1 | 1.1 | −1 | 0.423 | 1.8 | 1.3 | 0.072 | ||||
| Basal EGJP | 0.06 (−0.08–0.19) | 0.11 | 0.423 | 0.08 (−0.01- 0.17) | 0.14 | 0.072 | ||||
| Model 2 | 22.9 | 20.1 | <0.001 | 11.8 | 10.8 | <0.001 | ||||
| Basal EGJP | 0.05 (−0.08–0.17) | 0.02 | 0.446 | 0.07 (−0.02 - 0.15) | 0.11 | 0.133 | ||||
| EHAS Score | 0.32 (0.16–0.48) | 0.47 | <0.001 | 0.16 (0.09 – 0.23) | 0.32 | <0.001 | ||||
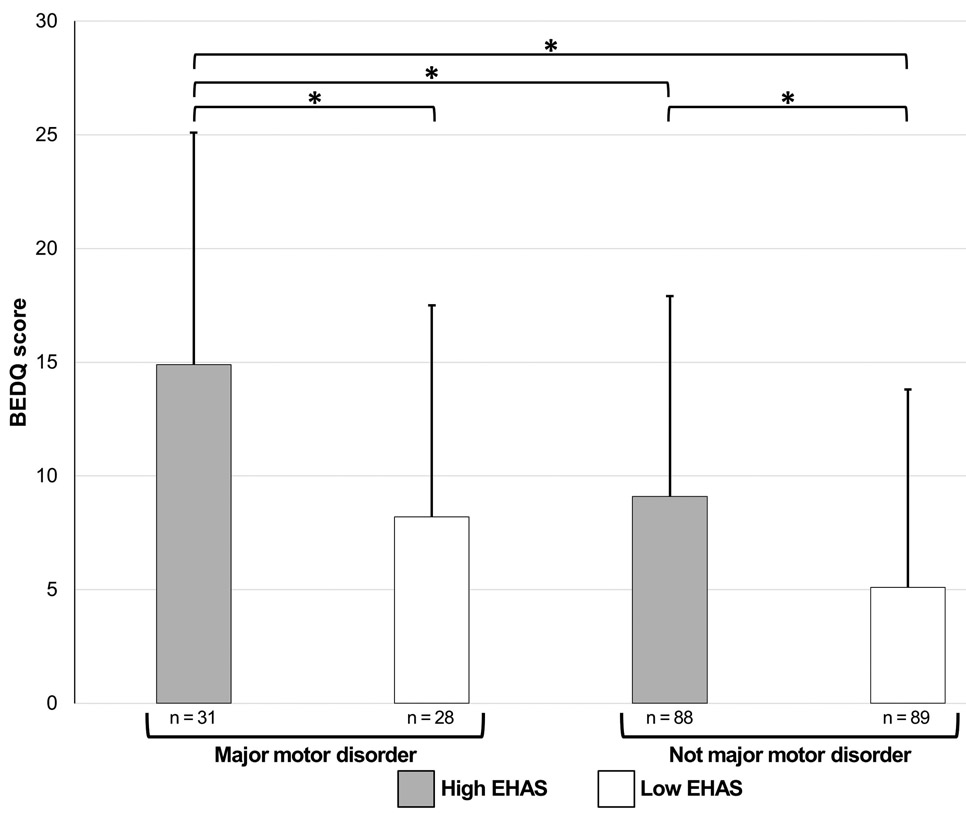
The relationship of esophageal motility diagnosis with EHAS score was illustrated by application of a median-split to categorize patients into high EHAS or low EHAS score (Figure 2): the median EHAS score among the entire cohort was 33. The group with a major motor disorder and high EHAS score had the greatest dysphagia severity. Among patients without major motor disorders, those with high EHAS scores had greater dysphagia severity than those with low EHAS scores; P = 0.001. Patients with major motor disorder and low EHAS scores had similar dysphagia severity as patients without major motor disorders (both EHAS groups).
Figure 2. Relationship of esophageal motility diagnosis and esophageal hypervigilance and visceral anxiety on dysphagia severity.
High (≥33) and low (<33) Esophageal Hypervigilance and Anxiety Scale (EHAS) scores were assigned by performing a median-split. The Chicago Classification diagnoses among the major motor disorder/high EHAS group included 8 (26%) achalasia (2 type I, 4 type II, 2 type III), 13 (42%) EGJ outflow obstruction, 9 (29%) absent contractility, and 1 (3%) jackhammer. The major motor disorder/low EHAS group included 11 (39%) achalasia (1 type I, 8 type II, 2 type III), 11 (39%) EGJ outflow obstruction, 3 (11%) absent contractility, and 3 (11%) jackhammer. The without major motor disorder/high EHAS group included 35 (40%) ineffective esophageal motility (IEM) and 53 (60%) normal motility. The without major motor disorder/low EHAS group included 36 (40%) IEM and 53 (60%) normal motility. *P=value < 0.01 to account for the multiple statistical tests. BEDQ – Brief Esophageal Dysphagia Questionnaire. Figure used with permission from the Esophageal Center at Northwestern.
Discussion
In a prospective, international, multicenter study that assessed validated PROs among patients being evaluated for potential primary esophageal motor disorders with HRM, we found that esophageal hypervigilance and visceral anxiety were major predictors of dysphagia severity. Our findings of increased dysphagia severity among patients with major esophageal motor disorders also lend validation to the Chicago Classification of esophageal motility, though the contribution of esophageal hypervigilance and visceral anxiety to symptom severity highlights the importance of recognizing and assessing these factors as part of the clinical evaluation.1, 2 Further, the association of hypervigilance and anxiety to perceived food impactions and associated emergency room visits, that was independent of HRM classification, demonstrates the impact of psychologic and cognitive factors on health care utilization. In summary, these findings demonstrate the importance of including a measure of esophageal hypervigilance and visceral anxiety to control for potential confounding in research studies in which symptom scores are utilized to classify patient outcomes.
Psychologic and cognitive factors, such as hypervigilance and anxiety, are recognized as mediators of gastrointestinal symptoms, often in the context of functional bowel disorders.16 The relationship of increased levels of generalized anxiety and other psychosocial factors with increased reflux symptom severity was described in several prior studies of patients evaluated for GERD.7, 8, 17-19 This relationship was observed in both patients with GERD (with objective pathologic esophageal acid exposure), as well an in patients categorized as functional esophageal disorders: reflux hypersensitivity and functional heartburn.7, 17, 19, with the psychosocial factors posed as major contributory factors to symptom perception in the latter groups.
There is limited previous evaluation of the relationship of psychologic and cognitive factors among esophageal motility parameters. In a multicenter study that assessed swallow perception during HRM with and without impedance among 115 healthy volunteers, anxiety carried an association with bolus perception, which was rarely observed, but otherwise carried no correlation with HRM or impedance-manometry parameters.9 In a previous study of 211 patients evaluated with HRM and validated symptom scores, increased dysphagia severity (assessed with the Mayo Dysphagia Questionnaire) was similarly reported in major esophageal motor disorders based on the Chicago Classification.20 Visceral anxiety scores (assessed with the Visceral Sensitivity Index), however, did not differ among esophageal motility classifications and the relationship between visceral anxiety and dysphagia severity was not reported. The present study, however, utilized a recently developed and validated PRO that assesses esophageal-specific hypervigilance and anxiety: the EHAS.11 In applying this brief patient-reporting tool to an international patient cohort, we demonstrated the relationship between dysphagia severity, esophageal motility, and esophageal hypervigilance and anxiety.
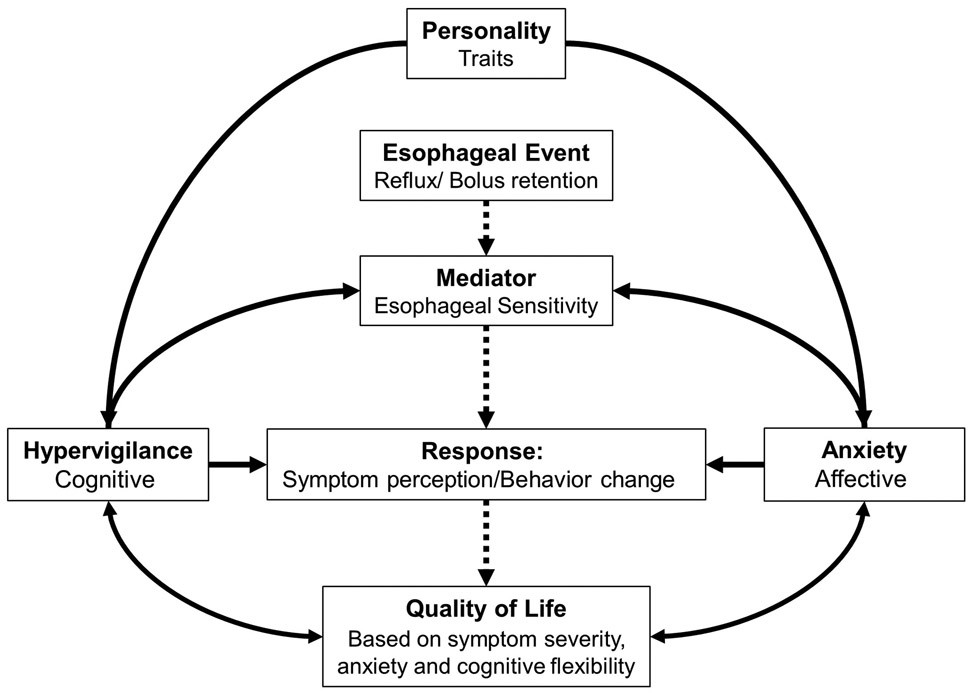
These results emphasize the concept that hypervigilance and anxiety play an important role in the esophageal patient experience, not just among patients with a functional esophageal syndrome, but also among patients with major esophageal motor disorder.1, 16 These factors may represent intrinsic primary psychological abnormalities or they may be a byproduct of a bi-directional modulatory interplay (Figure 3). Among some patients, abnormal esophageal motility (e.g. esophageal outflow obstruction) may induce esophageal hypervigilance and anxiety related to eating, thus amplifying the severity of associated dysphagia. This is likely represented by our finding of stronger BEDQ:EHAS correlation among patients with major motor disorders and with the greatest dysphagia severity in patients with major motor disorders and high levels of hypervigilance and anxiety. Other underlying features, potentially such as developed coping strategies (either cognitive or dietary), may be responsible for the lower dysphagia severity among the patients with major motor disorders and low EHAS scores. With regard to perceived food-impaction and associated emergency room visits, we observed that rates were similar across HRM-esophageal motility classifications, while the EHAS score was greater among patients reporting food impaction and seeking emergency department care, thus important regarding health care utilization. Ultimately, management strategies that target the motility abnormalities among patients with major motor disorders (regardless of hypervigilance and anxiety levels) may lead to improvement in both dysphagia severity and hypervigilance and anxiety. However, in patients with elevated levels of hypervigilance and anxiety, pursuit of multimodal management strategies that include addressing psychologic and cognitive factors (such as via cognitive-behavioral therapy), should be considered: Implementation of cognitive-behavioral therapy may be particularly worthwhile to pursue if motility-targeted therapies do not achieve symptom improvement. However, cognitive behavioral therapy may also be reasonable as an initial management strategy in some interested and motivated patients. Future longitudinal studies to evaluate predictive factors and outcomes associated with this approach are needed.
Figure 3. Potential mediating and moderating relationships of factors involved in esophageal symptom perception.
Interactions between various factors contribute to the perception of esophageal symptom presence and severity. Esophageal physiologic factors result in esophageal stimulation through reflux, obstruction, or retention. The perception of these events is moderated by cognitive-affective factors such as hypervigilance and anxiety. Dashed lines indicate the normal, unidirectional flow. Figure used with permission from the Esophageal Center at Northwestern.
Strengths of this study include utilization of a multicenter, international cohort of esophageal patients, which provides a broad and regionally-generalizable cohort. However, all four centers included are academic motility referral centers, thus these results may not translate as well to a community practice setting. Further, the utilization of the brief, validated PROs in this study provided a robust and valid characterization of patients’ symptom presence and severity; the brevity and simplicity of these patient-reporting tools does allow for generalizable use in any practice setting. We also acknowledge that BEDQ is a global symptom score, and thus differs with swallow-by-swallow bolus perception, as assessed in other studies.5, 21 However, the clinical impact is likely better reflected by the BEDQ, as it reports patient’s day-to-day experience with impact on quality of life, than a perception score obtained during a manometry test. Additionally, while the relatively large sample size is viewed as a strength and facilitated multivariable analysis, the rarity of major esophageal motility disorders (even at academic referral centers) limited evaluation of these specific disorders, such as among achalasia subtypes or distal esophageal spasm. Thus, further study to characterize the role of esophageal hypervigilance and anxiety in symptom generation among these disorders remain needed. Finally, we also acknowledge that other factors were unaccounted for in this study, both personal (e.g. personality traits) or physiologic, such as esophageal pressure-bolus flow associations that may be appreciated with impedance manometry, may also be related to symptoms severity and future study to assess the impact of these remains necessary as well.
In conclusion, we performed a prospective, international multicenter study of validated PROs in patients evaluated with HRM and demonstrated that esophageal-specific hypervigilance and anxiety were the strongest predictive factors associated with dysphagia severity among assessed clinical and manometric parameters. These findings further support the relationship between esophageal symptoms and psychologic and cognitive factors and is the first study to do so in regards to esophageal motility among a patient cohort evaluated with HRM. Esophageal hypervigilance and visceral anxiety likely account for some of the disconnect observed between symptoms and manometric findings, and thus identification of these often unaddressed features may facilitate application of appropriate management strategies in clinical practice (which may include cognitive and behavioral therapies). Further, these psychologic and cognitive factors likely carry a confounding effect in research studies that utilize PROs. Future study remains needed to assess the effects of targeted treatment on longitudinal patient outcomes, however psychologic and cognitive factors appears to be important factors related to symptom severity that should be addressed in both clinical practice and clinical research studies.
Supplementary Material
Supplementary Figure 1. Gastroesophageal Reflux Disease Questionnaire (GERDQ: A) and Esophageal Hypervigilance and Anxiety Scale (EHAS: B) Scores by Esophageal Motility Classification. EGJOO – esophagogastric junction outflow obstruction. IEM – ineffective esophageal motility. Figure used with permission from the Esophageal Center at Northwestern.
Supplementary Figure 2: Correlation of dysphagia severity and hypervigilance/anxiety scores. Darker markers indicate overlapping data points. BEDQ - Brief Esophageal Dysphagia Questionnaire. EHAS - Esophageal Hypervigilance and Anxiety Scale. Figure used with permission from the Esophageal Center at Northwestern.
STUDY HIGHLIGHTS:
1). WHAT IS KNOWN
Although high-resolution esophageal manometry (HRM) provides the primary method to evaluate esophageal motility, the relationships of symptom severity with HRM parameters and HRM classifications are inconsistent.
Anxiety is associated with symptom severity in gastroesophageal reflux patients and along with hypervigilance may play a role in dysphagia generation and severity.
2). WHAT IS NEW HERE
Esophageal hypervigilance and visceral anxiety carried a greater association with dysphagia severity than HRM parameters or HRM diagnoses among patients being evaluated for primary esophageal motility disorders.
The correlation of hypervigilance and visceral anxiety with dysphagia severity was even stronger among patients with major esophageal motor disorders than those without.
Assessment of esophageal hypervigilance and visceral anxiety may help address an important confounder in studies that utilize a subjective outcome and facilitate multidisciplinary management approaches in clinical practice.
Acknowledgments
Grant support: This work was supported by R01 DK079902 (JEP) and P01 DK117824 (JEP, THT) from the Public Health service.
Footnotes
Conflicts of interest:
Dustin A. Carlson: Medtronic (Speaking. Consulting)
C. Prakash Gyawali: Medtronic, Diversatek (Speaking, Consulting); Torax, Ironwood, Quintiles (consulting)
Sabine Roman: Medtronic (Consulting), Diversatek Healthcare (Consulting, Research support), Crospon (Research support), Biocodex (Travel grant)
Marcelo Vela: Medtronic (consulting), Diversatek (research support).
Tiffany Taft: Abbvie (Speaking), Janssen (Speaking)
Francois Mion: Laborie (speaking), Medtronic (speaking)
Laurie Keefer: Pfizer (consultant; unrestricted research funding); Abbvie (unrestricted research funding), metaME connect (scientific advisor); Rome Foundation Board Member
Peter J. Kahrilas: Ironwood (Consulting)
John E. Pandolfino: Crospon, Inc (stock options), Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking. Consulting), Torax (Speaking, Consulting), Ironwood (Consulting), Impleo (Grant).
Frederick TJ Lin, Michael Crowell, Karthik Ravi, Joseph R. Triggs, Farhan Quader, Jacqueline Prescott, Dario Biasutto: none
References
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0 Neurogastroenterol Motil 2015;27(2):160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103(1):27–37. [DOI] [PubMed] [Google Scholar]
- 3.Xiao Y, Kahrilas PJ, Nicodeme F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol 2014;109(4):521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohof WO, Myers JC, Estremera FA, et al. Inter- and intra-rater reproducibility of automated and integrated pressure-flow analysis of esophageal pressure-impedance recordings. Neurogastroenterol Motil 2014;26(2):168–75. [DOI] [PubMed] [Google Scholar]
- 5.Singendonk MJ, Lin Z, Scheerens C, et al. High-resolution impedance manometry parameters in the evaluation of esophageal function of non-obstructive dysphagia patients. Neurogastroenterol Motil 2018:e13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clouse RE, Lustman PJ. Psychiatric illness and contraction abnormalities of the esophagus. N Engl J Med 1983;309(22):1337–42. [DOI] [PubMed] [Google Scholar]
- 7.Kessing BF, Bredenoord AJ, Saleh CM, et al. Effects of anxiety and depression in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2015;13(6):1089–95 e1. [DOI] [PubMed] [Google Scholar]
- 8.Jansson C, Nordenstedt H, Wallander MA, et al. Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population-based study. Aliment Pharmacol Ther 2007;26(5):683–91. [DOI] [PubMed] [Google Scholar]
- 9.Cisternas D, Scheerens C, Omari T, et al. Anxiety can significantly explain bolus perception in the context of hypotensive esophageal motility: Results of a large multicenter study in asymptomatic individuals. Neurogastroenterol Motil 2017;29(9). [DOI] [PubMed] [Google Scholar]
- 10.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil 2016;28(12):1854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taft TH, Triggs JR, Carlson DA, et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther 2018;47(9):1270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013;37(5):564–72. [DOI] [PubMed] [Google Scholar]
- 13.Roman S, Taft TH, Decam E, et al. Validation en langue française d’un questionnaire d’hypersensibilité oesophagienne chez des patients adressés pour manométrie oesophagienne. Abstract; Journées Francophones d’Hépataogastroentérologie et d’Oncologie Digestive, Paris, 21–24 mars 2019 2019. [Google Scholar]
- 14.Roman S, Taft TH, Hastier A, et al. Validation en langue française d’un questionnaire court de dysphagie chez des patients adressés pour manométrie oesophagienne Abstract: Francophones d’Hépataogastroentérologie et d’Oncologie Digestive, Paris, 21–24 mars 2019 2019. [Google Scholar]
- 15.Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol 2019;17(4):674–81 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 17.Johnston BT, Lewis SA, Collins JS, et al. Acid perception in gastro-oesophageal reflux disease is dependent on psychosocial factors. Scand J Gastroenterol 1995;30(1):1–5. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein JH, Nojkov B, Korsnes S, et al. Oesophageal hypersensitivity is associated with features of psychiatric disorders and the irritable bowel syndrome. Aliment Pharmacol Ther 2007;26(3):443–52. [DOI] [PubMed] [Google Scholar]
- 19.Yadlapati R, Tye M, Keefer L, et al. Psychosocial Distress and Quality of Life Impairment Are Associated With Symptom Severity in PPI Non-Responders With Normal Impedance-pH Profiles. Am J Gastroenterol 2018;113(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy CA, Patel A, Gyawali CP. Impact of symptom burden and health-related quality of life (HRQOL) on esophageal motor diagnoses. Neurogastroenterol Motil 2017;29(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omari TI, Wauters L, Rommel N, et al. Oesophageal pressure-flow metrics in relation to bolus volume, bolus consistency, and bolus perception. United European Gastroenterol J 2013;1(4):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Gastroesophageal Reflux Disease Questionnaire (GERDQ: A) and Esophageal Hypervigilance and Anxiety Scale (EHAS: B) Scores by Esophageal Motility Classification. EGJOO – esophagogastric junction outflow obstruction. IEM – ineffective esophageal motility. Figure used with permission from the Esophageal Center at Northwestern.
Supplementary Figure 2: Correlation of dysphagia severity and hypervigilance/anxiety scores. Darker markers indicate overlapping data points. BEDQ - Brief Esophageal Dysphagia Questionnaire. EHAS - Esophageal Hypervigilance and Anxiety Scale. Figure used with permission from the Esophageal Center at Northwestern.