Abstract
Wound-induced hair follicle neogenesis (WIHN) is a phenomenon that occurs in adult mammalian skin, where fully functional hair follicles are regenerated in the center of large full-thickness excisional wounds. Although originally discovered over 50 years ago in mice and rabbits, within the last decade it has received renewed interest, as the molecular mechanism has begun to be defined. This de novo regeneration of hair follicles largely recapitulates embryonic hair development, requiring canonical WNT signaling in the epidermis, however, important differences between the two are beginning to come to light. TLR3 mediated double stranded RNA sensing is critical for the regeneration, activating retinoic acid signaling following wounding. Inflammatory cells, including Fgf9-producing γ-δ T cells and macrophages, are also emerging as important mediators of WIHN. Additionally, while dispensable in embryonic hair follicle development, Shh signaling plays a major role in WIHN and may be able to redirect cells fated to scarring wounds into a regenerative phenotype. The cellular basis of WIHN is also becoming clearer, with increasing evidence suggesting an incredible level of cellular plasticity. Multiple stem cell populations, along with lineage switching of differentiated cells all contribute towards the regeneration present in WIHN. Further study of WIHN will uncover key steps in mammalian development and regeneration, potentially leading to new clinical treatments for hair-related disorders or fibrotic scarring.
Introduction
The ability of animals to regenerate lost structures and appendages varies dramatically across different phyla, with certain animals like urodele salamanders being able to fully regenerate lost limbs, while others, like mammals, having a severely limited capacity1. Although most mammals recover from cutaneous wounds with fibrotic scarring, a select few, including mice and rabbits, are able to bypass this, completely regenerating hair follicles, sebaceous glands, and adipocytes 2–4. This phenomenon is known as wound-induced hair neogenesis (WIHN), and is a rare example of organogenesis -- if small in scale -- in adult mammals. Although this regenerative process closely recapitulates the molecular and morphological events of embryonic hair follicle development, much about the mechanism has yet to be elucidated.
The hair follicle is a defining anatomical feature of mammals, and one that constitutes the smallest complete organ that is able to, at least partially, regenerate in humans 5. This mini-organ is comprised of epithelial and mesenchymal cells that work together to create the hair shaft. It also becomes a locus for a density of innervation and vasculature.Hair follicles follow a distinct growth cycle that consists of an active growth phase (anagen), involution (catagen), and relative quiescence (telogen) 5,6. This cycle is defined by corresponding mobilization or dormancy of a slow cycling stem cell compartment known as bulge stem cells 7,8.
WIHN offers a unique opportunity to study the molecular mechanisms that define adult mammalian regeneration. Understanding how the body is able to mobilize and coordinate distinct cell populations to initiate embryonic-like regeneration will provide insights for the future of regenerative medicine. The ability to reactivate high-fidelity tissue morphogenesis pathways in adults post injury could, at its apex, even reduce the need for costly organ transplantation therapies. Perhaps more immediately, studying the mechanisms controlling hair growth and regeneration will provide opportunities for the treatment of hair-related disorders.
WIHN model features
The phenomena of de novo hair follicle (HF) regeneration in the center of wound scars, or wound-induced hair follicle neogenesis (WIHN), was first recognized in rats, but has since be observed in rabbits, sheep, and extensively in spiny mice (Acomys cahirinus) 1,4,9–12. Interestingly, despite being first recognized in rats, recent work by Guerrero-Juarez et al. suggests that it is not a common feature of laboratory strains of rats 13. Although it has been reported in a few select cases, wounds in humans do not undergo large scale, clinically obvious, de novo hair regeneration 14–16.The molecular mechanisms of WIHN were first described by Ito and Cotsarelis et al., who developed a reproducible mouse (Mus musculus) model for studying the process 2.
In adult mice (>P21), hair follicles regenerate after large (>1cm2) full-thickness excisional wounds on the lower back of mice, with smaller wounds rarely regenerating HFs. The regenerated follicles appear in the center of the wound, or in small clusters, and are always surrounded by scar tissue. WIHN occurs 14–21 days post wounding (PWD 14–21), after reepithelization scab detachment (PWD 13–14). Unexpectedly, Rognoni et al. discovered that shortly after birth (P2), mice are able to regenerate HFs in small circular wounds (2mm biopsy punch), but this ability is lost after P10. This regeneration does not appear to be anatomically restricted, as HF regeneration also occurred in the tail 17. Regardless of age, all of the regenerated HFs have zigzag morphology, but typically share the same orientation within a cluster. The hair follicles have functioning sebaceous glands, but often lack pigmentation and never develop arrector pili muscles 2,18 (Fig. 1). Intriguingly, African spiny mice (Acomys cahirinus), which use skin autotomy to escape predation, have extremely robust WIHN. After small (<6mm) and large (1.5cm2) full thickness wounds, Acomys regenerates hair follicles that cover the entire wound, rather than simply in the center. Acomys also regenerates all three types of hair follicles (guard, awl, and zigzag), with guard and awl follicles obtaining normal pigmentation. Furthermore, unlike Mus, Acomys can regenerate smooth muscle erector pili muscles after full-thickness excisional wounding 1,12,19.
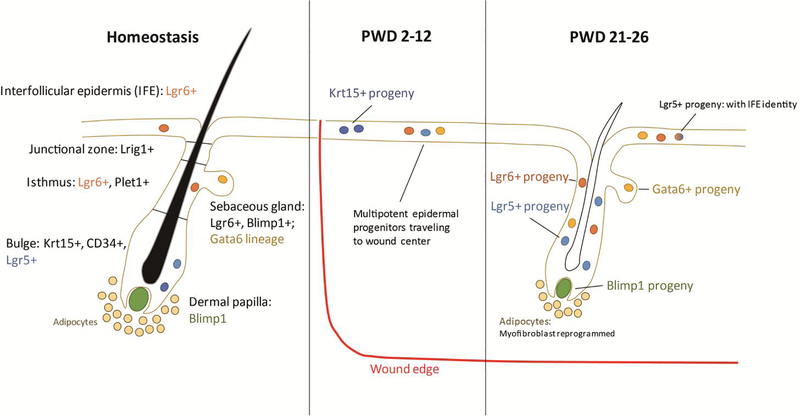
Figure 1.
Hair follicle structure and cellular origin of wound-induced hair follicle neogenesis. The hair follicle and epidermis are defined by distinct populations of stem cells that are marked by different proteins. Many of these stem cells, as well as other differentiated cell lineages, contribute to hair follicle neogenesis post wounding. Following wounding, Lgr6+ IFE/isthmus stem cells (orange) and Lgr5+ bulge stem cells (light blue) within hair follicles surrounding the wound become activated and generate progeny that participate in reepithelization and de novo hair follicle regeneration. Transcriptional plasticity in Lgr5+ stem cells in important in wound healing, allowing some Lgr5+ progeny to downregulate bulge genes while activating an IFE-like signature, allowing them to interact with the wound stroma, a property that Lgr6+ IFE/isthmus stem cells already possess during skin homeostasis (blue-orange gradient). Krt15+ bulge stem cells (dark blue) help reestablish the epidermis, however, their progeny are short-lived. Differentiated cells of the Gata6 lineage (yellow), which are normally restricted to the suprabasal layers of the sebaceous duct, are able to enter the wound site, where they undergo dedifferentiation and contribute to the stem cell compartment of the interfollicular epidermis, hair follicle, and sebaceous gland. Fibroblasts, including upper dermal fibroblasts, which can be marked by Blimp1, and myofibroblasts are important for regenerating the dermal papilla. Myofibroblasts are also able to dedifferentiate and switch lineages to regenerate cutaneous fat surrounding de novo hair follicles.
Critically, de novo HFs have repetitive hair cycles, complete with functional bulge stem cells. They begin first telogen asynchronously around PWD 35 and enter second anagen at approximately PWD 45. The number of hair follicles regenerated can vary substantially between strain, gender, and age matched mice 2,18.
Cellular basis/origin of WIHN
The regenerated HF present in WIHN exhibit all the key epithelial and mesenchymal cell types characteristic of normal body hair. Importantly, each has a new bulge with functional epithelial stem cells, a new mesenchymal dermal papilla, sebaceous gland, and adipocytes 2,3 (Fig. 1). Although the cellular origin of WIHN has not been completely elucidated, increasing evidence points to a remarkable level of lineage plasticity in those cells involved in the regeneration 2,3,8,20,21.
Unexpectedly, fate mapping studies conducted by Ito et al. definitively showed that Krt15+ bulge stem cells from HFs adjacent to the wound do not contribute to the de novo follicles 2. These Krt15+ cells do participate in wound healing by helping to reestablish the epidermis, however, their progeny are short-lived 22. This discovery was the first clue that HF regeneration may occur via other non-bulge epithelial stem cells, suggesting that they may have expanded lineage plasticity, not present in unwounded skin. Indeed, Lgr6+ interfollicular epidermis (IFE)/ isthmus stem cells were found to partially contribute to neogenic hair follicles 8. Lough et al. also found that seeding isolated Lgr6+ stem cells into wound beds could promote epithelialization, hair growth, and angiogenesis 23. Additionally, Wang et al. showed that Lgr5+ epithelial stems cells contribute to WIHN. Lineage tracing revealed that Lgr5+ cells are present in 40% of regenerated HF and that Lgr5+ cell depletion attenuates WIHN by over 50% 20. Recent work from the Kasper group examined the transcriptional plasticity of Lgr5+ hair follicle bulge and Lgr6+ expressing cells during wound healing via single-cell transcriptomics. Using pseudotemporal ordering of wound cells compared to single-cell data from telogen skin, they showed that wounding rapidly downregulates bulge genes, while activating an IFE-like signature in the progeny of Lgr5+ hair follicle stem cells.. This switch of cellular identity occurs before the cells have even migrate to the wound front, allowing the Lgr5+ progeny to interact with the wound stroma, a property that Lgr6+ IFE/isthmus stem cells already possess during skin homeostasis 24. Although this study focused on small wounds (4mm biopsy punch), which typically do not undergo WIHN, it underscores the plasticity of stem cells involved in the wound healing process and that single-cell transcriptomics will be instrumental in further defining WIHN.
The origin of the regenerated dermal papilla (DP), which forms the dermal niche that instructs hair follicle epithelial cell fate and differentiation, is also still being actively investigated 25,26. Lineage tracing experiments confirmed that CD133-positive DP from hair follicles adjacent to the wound bed do not contribute to the regenerated DP 27. Multiple lines of evidence suggest that a diverse series of skin fibroblasts are involved in wound repair and may be implicated in DP regeneration 28–32. Driskell et al. identified two distinct fibroblast lineages, the upper (papillary) dermal fibroblasts, which regulate hair growth and the formation of the arrector pili muscle, and the lower (reticular) dermal fibroblasts, which synthesize much of the fibrillar extracellular matrix. These two lineages contribute to wound repair in two consecutive waves, with the lower dermal fibroblasts proliferating first, producing collagen fibers to repair the dermis, and the upper dermal fibroblasts only migrating after re-epithelialization, to create the papillary dermis 28. The inability of mice to regenerate HFs in small wounds after P10 appears to be directly linked with these two fibroblasts types. After P10, there is a dramatic reduction in Lrig1-positive upper dermal fibroblasts and an increase in lower dermal fibroblasts 17. Furthermore, Blimp1-expressing fibroblasts promote the formation of the papillary dermis, with Blimp1-knockout mice developing fewer hair follicles than wild type after wounding 29. New evidence also suggests that myofibroblasts, a specialized type of contractile wound fibroblast, may be the source of the de novo DP and are controlled by dermal Sonic hedgehog (Shh) signaling 21. Recently, single-cell RNA-sequencing revealed a surprising diversity in fibroblasts involved in wound repair, suggesting that many of the previous classifications may be overly simplistic32. Although fibroblasts can be broadly classified into two groups based on their transcription factor signatures and PDGF receptor expression patterns (Pdgfrahigh and Pdgfralow), there is substantial heterogeneity in the populations, resulting in 12 distinct subclusters. Surprisingly, fibroblasts across all of these clusters express contractile myofibroblast markers Actin alpha 2 (Acta2) and Transgelin (Tagln), suggesting that myofibroblasts are not a homogenous population. Indeed, pseudotime and RNA velocity analyses of these 12 fibroblast populations suggest there are three distinct fibroblast-to-myofibroblast differentiation trajectories 32. These data, coupled with additional lineage tracking experiments, will be instrumental in further defining the contribution of fibroblasts to the papillary dermis and DP.
The sebaceous gland and the adipocytes that normally surround a hair follicle are also regenerated in WIHN. Remarkably, this process involves the dedifferentiation of various cell types, a level of plasticity rarely seen in mammals 3,32,33. Dontati et al. discovered that in response to wounding, differentiated cells of the Gata6 lineage, which are normally restricted to the suprabasal layers of the sebaceous duct, are able to enter the wound site, where they undergo dedifferentiation and contribute to the stem cell compartment of the interfollicular epidermis, hair follicle, and sebaceous gland 33.Interestingly, myofibroblasts are able to dedifferentiate and switch lineages to regenerate cutaneous fat. This reprogramming requires neogenic hair follicles, which trigger bone morphogenetic protein (BMP) signaling and the subsequent activation of adipocyte transcription factors normally expressed during development 3.Additionally, single-cell RNA-sequencing found a fibroblast subset that is derived from bone marrow myeloid cells. These cells that undergo the myeloid-to-fibroblast conversion are also able to convert into adipocytes in healed wounds 32.
Molecular/signaling mechanisms in WIHN
Wnt/β-catenin signaling
Since its first discovery, WIHN has been shown to involve multiple signaling pathways. Chief among these is the canonical WNT signaling pathway, which is activated and thereby mimics the development of embryonic hair follicles 2,34–37. This embryogenic process initially involves the formation of the epidermal placode, which expresses Krt17 36. Alkaline phosphatase activity and Lef1 expression define the next stage, where the placode begins to penetrate into the deep dermis. Finally, Wnt signaling mediates the interaction between epidermal and mesenchymal cells, which activate SHH signaling, and thus the formation of mature hair follicles 37. Ito et al. discovered that following wounding, de novo hair follicles expressed KRT17, Lef1, alkaline phosphatase, Wnt10b, and Shh, which are analogous to embryonic follicles.Inducible deletion of the β-catenin gene Ctnnb1 in the epidermis of mice completely abolished the formation of new hair follicles. Similarly, after reepithelialization, overexpression of the WNT antagonist Dkk1 prevented neogenic hair follicles. Conversely, the overexpression of Wnt7a, a secreted WNT ligand, throughout the wound epidermis in Krt14-Wnt7a mice doubled the number of regenerated hair follicles 2. The CXXC-type zinc finger protein 5 (CXXC5) is a known negative regulator of the Wnt/β-catenin pathway that is involved in hair regrowth via its interaction with Dishevelled 38–40. Lee et al. showed that knocking out CXXC5 accelerates hair regrowth and WIHN in mice. Consistent with this, disrupting the CXXC5-Dishevelled interaction with a competitor peptide enhanced de novo hair follicle regeneration 40.
Due to their requirement for the WIHN model, the source(s) of the WNT ligands and their regulators are actively being investigated. Epidermal WNTs were found to be critical for the WIHN. Inducible ablation of the Wntless gene (G-protein-coupled receptor 177), which is essential for Wnt ligand secretion, in the wound epidermis dramatically attenuated hair follicle regeneration 41,42. Driskell et al. also found that epidermal β-catenin activation stimulates the expansion of the upper papillary dermal lineage, allowing wounds to be more permissive for hair follicle regeneration 28.
The role of dermal WNTs in WIHN are not as straight-forward, with the source and abundance either enhancing or reducing hair follicle regeneration. Intriguingly, Gay et al. found that dermal γ-δ T cells modulate WNT activation by secreting fibroblast growth factor 9 (Fgf9). Mice lacking γ-δ T cells, as well as mice with Fgf9-defiecnt γ-δ T cells, have dramatically attenuated WIHN, with a reduction of hair follicles by greater than 60% compared to WT controls. Furthermore, overexpression of Fgf9 increased de novo hair follicles by three-fold. Specifically, Fgf9 from γ-δ T cells triggers Wnt2a secretion and subsequent Wnt activation in myofibroblasts. This creates a feedback mechanism where activated fibroblasts begin to express additional Fgf9, amplifying WNT activity throughout the wound dermis during wound repair (Fig. 2). Notably, overexpression of WNT ligands in wound epidermis of the Krt14-Wnt7a mice was not able to rescue WIHN in mice deficient in γ-δ T cells, suggesting that dermal WNTs are critical for WIHN 43. Counterintuitively, when studying why older mice (>3 weeks) have a reduced capacity for WIHN, Rognoni et al. discovered that elevated dermal Wnt/β-catenin correlated with reduced hair follicle regeneration. This increase in dermal β-catenin activity was accompanied by an increase in the number fibroblasts expressing reticular markers, a cell type that is unable to induced hair follicle formation 17,28. Furthermore, β-catenin ablation in dermal fibroblasts promoted hair follicle regeneration in neonatal and adult mouse wounds. These data support a model that as mice age, dermal Wnt/β-catenin signaling increases, which preferentially recruits lower dermal fibroblasts at the expense of papillary fibroblasts, thereby decreasing their ability to efficiently form hair follicles post wounding 17. Ultimately, more work needs to be done to completely elucidate the interplay between epidermal and dermal Wnt signaling in WIHN.
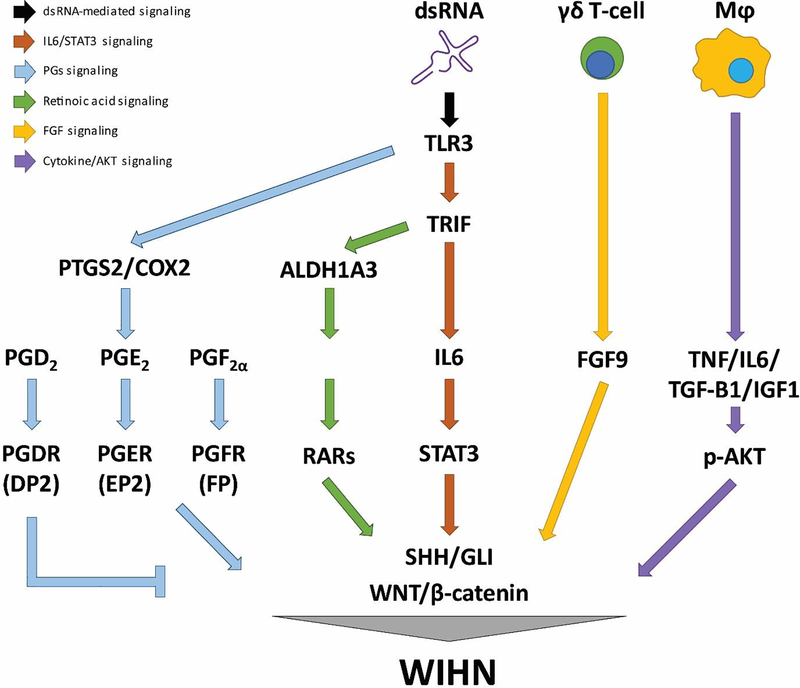
Figure 2.
Molecular mechanisms of wound-induced hair follicle neogenesis. Double strand RNA (dsRNA) sensing via Toll-like receptor 3 (TLR3) is important for WIHN, signaling through the IL6/STAT3 axis 57. TLR3-mediated dsRNA sensing also induces retinoic acid (RA) synthesis, that promotes de novo hair follicle regeneration 75. Prostaglandin (PG) signaling influences hair follicle regeneration, with PGE2 promoting and PGD2 inhibiting regeneration 58,71. Fibroblast growth factor 9 (FGF9) is secreted by γδ T-cells, which leads to Wnt2a secretion and WNT activation in myofibroblasts 43. Macrophages (Mφ) release various cytokine, TNF and TGF-β1, which induces AKT phosphorylation and β-catenin activation in Lgr5+ hair follicle stem cells 20,86,87.
The extracellular matrix (ECM) serves as an important niche for hair follicle stem cells, regulating hair growth and development 44. The ECM also plays a critical role in wound healing in the skin 45. Chen et al. discovered that collagen VI is abundantly deposited in hair follicles and dramatically upregulated upon skin wounding. Intriguingly, genetic ablation of collagen VI by knocking out Col6a1, a key regulator of collagen synthesis, delays hair cycling and growth under physiological conditions, but enhances WIHN. The addition of exogenous collagen to these Col6a−/− reduces hair follicle regeneration to its basal level. This enhancement of WIHN was found to be dependent on Wnt/β-catenin signaling, with Col6a1- knockout mice expressing significantly higher levels of β-catenin, as well as reduced expression of DKK1 46.
Hedgehog signaling
Sonic hedgehog (Shh) signaling is critical for hair follicle development and hair cycling 47–53. Ma et al. provided the first evidence the Shh pathway is important for WIHN 54. Musashi (Msi) is an evolutionarily conserved RNA binding protein that functions as an important regulator of tissue-specific stem cells 55. If Msi is knocked out in mice, WIHN is elevated over wild type controls. Conversely, if Msi is overexpressed in mice, it dramatically inhibits de novo hair follicle regeneration 54. Transcriptome profiling revealed that Msi2 represses Hedgehog (Hh) signaling activity by directly targeting Shh in the hair follicle. The importance of Shh signaling in hair follicle neogenesis was further highlighted by Lim et al., who showed that is it critical for reestablishing the dermal papilla (DP) in wounds that would typically heal with fibrotic scarring. Genetic ablation of Shh in the epidermis resulted in the loss of DP and de novo hair follicle regeneration. Conversely, overexpression of Shh in the epidermis resulted in robust hair follicle neogenesis, including in small wounds that typically do not undergo WHIN. Furthermore, epidermal Shh overexpression enhanced regeneration in large wounds, allowing hair follicles to regenerate across the entire wound, rather than only the center. They also examined the role of Shh in the dermis by constitutively activating Smoothened (Smo), an essential component of the Shh pathway, in dermal myofibroblasts. Constitutive Smo activation resulted extensive DP and hair follicle formation in small wounds. Lim et al. also explored the relationship between Shh and Wnt signaling in the dermis, due to previous reports that prolonged Wnt signaling within dermal fibroblasts correlates with injury-induced fibrosis 17,21,56. Constitutive β-catenin activation in the dermis was not able to generate DP or neogenic hair follicles in small wounds, however, forced Shh activation in Wnt-active dermal fibroblasts resulted in pronounced hair follicle regeneration. This suggests that Wnt-active dermal cells (reticular), which promote scarring, can be redirected to a regenerative phenotype if Hh signaling is activated 21. Generally, the above results demonstrate the importance of Shh in WIHN, likely in an earlier stage compared to embryonic hair development.
dsRNA/IL-6/STAT3/RA
Intriguingly, double strand RNA (dsRNA) sensing via Toll-like receptor 3 (TLR3) has also been found to be critical for WIHN 57,58. Toll-like receptors (TLRs) are highly conserved receptors that recognize distinct molecular components of invading pathogens and activate an array of inflammatory signaling pathways 59. TLRs have increasingly been found to influence aspects of wound repair. Rather than being exclusively activated by exogenous pathogens, TLRs can also be activated by endogenous ligands released after tissue damage. This results in a “sterile” inflammation process that plays a critical role in the recruitment of immune cells and the initiation of wound healing 60. TLR3, which is activated by dsRNA, has classically been studied in the context of viral infection, although there is gathering evidence that it also plays a role in wound repair 61–66.
When examining mouse strain differences in WIHN, Nelson et al. found that high regenerating mice upregulated various RNA sensing and interferon-signaling genes. Strikingly, many of these genes were also upregulated in polyriboinosinic-polyribocytidylic acid (poly(I:C)), a synthetic dsRNA mimic, treated human keratinocytes. This overlap of dsRNA sensing genes suggested a conserved role of TLR3-dsRNA sensing in early wound healing. Indeed, a single addition of poly(I:C) 3 days after wounding resulted in a robust increase in WIHN, which was abolished if RNase III, a dsRNA-specific endonuclease, was administered. Furthermore, hair follicle regeneration in TLR3 null mice was dramatically reduced compared to wild-type controls, while re-epithelialization kinetics were not affected 57. TLR3 has been shown to signal via the Interleukin-6 (IL-6)-signal transducer and activator of transcription (STAT) 3 axis in response to dsRNA 67.Consistent with this, IL-6 levels were found to be significantly elevated in high regeneration mice compared to those with lower follicle neogenesis ability. Additionally, mice treated with recombinant IL-6 exhibited enhanced WIHN, while treatment with cucurbitacin I, a selectivity IL-6/STAT3 inhibitor, inhibited WIHN. In vitro experiments in primary normal human epidermal keratinocytes (NHEK) showed that poly(I:C) treatment led to increased expression of hair follicle progenitor markers, including leucine-rich-repeat containing G proteins (LGR) 5 and 6, and keratin 15 (KRT15). Furthermore, TLR3 activation via poly(I:C) in NHEK promoted expression of key markers of hair follicle morphogenesis, including members of the Wnt/β-catenin and Shh signaling pathways 57. Collectively, these data suggest that dsRNA sensing by TLR3 is one of the earliest molecular events in hair regeneration (Fig. 2).
Unexpectedly, knocking out IL-6 in mice significantly increased WIHN when compared to wild type controls. Further investigation revealed that other IL-6-type cytokine family members, such as oncostatin M and IL-11, are elevated—along with activated STAT3-- in IL-6 null mice, compensating for its absence. Treatment of IL-6 null mice with cucurbitacin I, a selective JAK2/STAT3 pharmacological inhibitor, significantly reduced hair follicle regeneration, consistent with the fact that oncostatin M and IL-11 signal via STAT3 68. Nelson et al. also found evidence suggesting that the IL-6/STAT3 signaling pathway can modulate p63, a transcription factor vital for skin development, homeostasis, and wound healing. A single intradermal injection of pan-p63 siRNA was able to substantially reduce the number of de novo hair follicles, suggesting its role in WIHN. IL-6 was able to reduce the expression of ΔNp63, a p63 isoform that is expressed in ectoderm that has committed to stratification, while increasing the expression of TAp63, an isoform that inhibits terminal differentiation, in NHEKs 69,70. Furthermore, cucurbitacin I significantly decreased TAp63 and increased ΔNp63 protein expression in vivo. Therefore, it is speculated that the IL-6/STAT3 signaling pathway is important for regulating p63 during WIHN 69.
Interestingly, noncoding dsRNA is able to coordinate prostaglandin and Wnt signaling to promote de novo hair follicle regeneration 58. Prostaglandins (PGs) are bioactive lipids that mediate inflammation and regulate wound repair. During wound healing in the skin, PGD2 and PGE2 are reciprocally expressed, with PGD2 levels being associated with low WIHN regeneration 71. Contrastingly, PGE2 promotes regeneration in various tissue types, including the blood, colon, and liver, as well as regulate hair growth 72,73. PGE2 is also able to promote the activation of Wnt pathway in the context of regeneration and development of stem cells 74. Zhu et al. found that poly(I:C) induced strong expression of multiple Wnt ligands in NHEKs, including Wnt7b, and Prostaglandin-endoperoxide synthase 2 (PTGS2), a key upstream enzyme in the PG synthesis pathway. Poly(I:C) also increased expression of Wnt7b in mouse wounds. Consistent with the role of TLR3 in dsRNA sensing via the IL-6/STAT3 axis, mice lacking TLR3 exhibited a dramatic reduction in Wnt7b expression in the wound bed of mice. The administration of recombinant IL-6 bypasses the need for TLR3 and rescues Wnt7b levels in TLR3 null mice. Furthermore, siRNA silencing of PTGS2 abrogates the induction of WNT7B by poly(I:C). Inhibition of prostaglandin synthesis by celecoxib, a selective PTGS2 inhibitor, significantly reduced WIHN, which could be reversed with the addition of exogenous PGE2. Taken together, this suggests that noncoding dsRNA coordinates prostaglandin and Wnt signaling to promote de novo hair follicle regeneration in wounds 58 (Fig. 2).
Recently, Kim et al. discovered that dsRNA sensing via TLR3 leads to the induction of retinoic acid (RA) synthesis that promotes wound induced de novo hair follicle regeneration 75. RA plays a key role in appendage development and regeneration, such as modulating limb regrowth following amputation in salamanders 76,77. Aberrant RA signaling can also promote progressive hair loss and disrupt hair follicle morphogenesis and differentiation 78,79. Kim et al. found that poly(I:C) and RA treated NHEKs shared substantial overlap in gene transcripts and protein expression, many of which involved keratinocyte differentiation. RA levels were also markedly increased in poly(I:C) treated NHEKs, in a TLR3 dependent fashion. TLR3 null mice, which regenerate very few hair follicles after wounding, had substantially less RA in wounded and non-wounded skin. Furthermore, RA treatment was able improve WIHN in TLR3 null mice towards levels of wild type mice. Interestingly, aldehyde dehydrogenase 1 family A3 (ALDH1A3), an enzyme that converts retinal to RA, was robustly induced in NHEKs following RA or poly(I:C) treatment. Poly(I:C) also induced Aldh1a3 transcription during wound healing in wild type, but not TLR3 null mice. To explore the mechanism that RA controls regeneration, RA signaling was inhibited using BMS493, a pharmacological pan-retinoic acid receptor (RAR) antagonist. BMS493 almost completely abolished WIHN, with or without poly(I:C). WIHN was also substantially reduced in mice that were deficient in Rarα, suggesting that dsRNA-induced retinoic acid signaling following wounding is important to promote de novo hair follicle regeneration 75 (Fig. 2).
Innate immune system/macrophages
Although γ-δ T cells have a critical role in WIHN by secreting fibroblast growth factor 9 (Fgf9), the role of innate immune cells in hair follicle regeneration is largely unknown 43. After wounding, there is a robust inflammatory phase that precedes the ingress of keratinocytes. This begins with the rapid recruitment of neutrophils, which provide vital defense against microbial pathogens 80,81. Shortly afterwards, chemotactic factors produced by neutrophils and keratinocytes recruit macrophages to the wound site, where they continue the process of phagocytosis, but also promote the transition to the proliferative phase of wound healing 82–84. These distinct functions are modulated by specific macrophages subsets: M1 macrophages secrete high levels of inflammatory cytokines and have enhanced phagocytic functions, while M2 macrophages reduce proinflammatory cytokine release and promote angiogenesis and extracellular matrix deposition 83,85. While this inflammatory response is vital for combating infection, how these processes affect wound regeneration or WIHN is incompletely understood, although increasing evidence implicates macrophages in the process 20,86,87.
Wang et al. found that myeloid-macrophages (Ly6C+), but not tissues resident ones, are essential for WIHN. Depletion of macrophages via clodronate or genetic ablation via diphtheria toxin completely abolishes de novo hair follicle regeneration. Microarray analysis of Ly6C+ macrophages compared to neutrophils and tissue residence macrophages, revealed that they express markedly higher levels of various cytokines, including IL6 and TNFA. Furthermore, they found that knocking out TNF diminishes WIHN. Unexpectedly, overexpressing TNF also inhibits hair follicle regeneration, suggesting a dose-dependent mechanism for TNF induction of WIHN. Proteomic quantification of epidermal stem cells showed that TNF induces AKT phosphorylation (p-AKT) in a dose-dependent manner, and that AKT signals positively regulate β-catenin signaling, independent if Wnt ligand binding. Additionally, in vivo analysis revealed that AKT signaling was highly activated in Lgr5+ HF stem cells after wounding, and that depletion of Lgr5+ cells markedly reduced WIHN. This suggests that macrophage derived TNF regulates WIHN by activating hair follicle stem cells via AKT/ β-catenin signaling rather than Wnt mediated β-catenin activation 20 (Fig. 2).
Rahmani et al. found that WIHN is dependent on CD11b+ F4/80+ macrophages at 7–11 PWD, correlating to the onset of de novo hair follicle regeneration. Using a dual-reporter system, they showed that CX3CR1medCCR2+ macrophages dominate during the inflammatory phase of wound healing, before transitioning to CX3CR1hiCCR2+ macrophages during the remodeling phase (6–12 days post injury), which proceeds WIHN. Furthermore, WIHN is eliminated in CX3CR1 knockout mice. On a molecular level, they discovered that CX3CR1hiCCR2+ macrophages express TGF-β1 and TNF, and that TGF-β signaling is required for WIHN. They propose that TGF-β1, a known chemoattractant, is necessary for recruiting CX3CR1hiCCR2+ macrophages, which promote WIHN via TNF-induced AKT/ β-catenin signaling in Lgr5+ hair follicle stem cells 20,87. The importance of macrophages in WIHN was further confirmed by Kasuya et al., who found that there was an increase in CD11b+/CD206+ M2 macrophages during the second and third weeks after wounding and that they localized around regenerated hair follicles. Macrophage ablation between 7 and 13 days (when M2 macrophages predominate) after wounding dramatically reduced WIHN. Furthermore, they found that CD206+ M2 macrophages produced a series of growth factors important in Wnt signaling, including insulin-like growth factor 1 (Igf1) and fibroblast growth factor 2 (Fgf2), that when added exogenously increase WIHN 86,88,89. Interestingly, mechanical stretch has been implicated in hair regeneration, where it stimulates chemokine production and subsequent macrophage recruitment, which undergo M2 polarization and produce various growth factors including Igf1 to promote regeneration 90 (Fig. 2).
Unanswered questions
Although considerable progress has been made in the last decade to understand the phenomenon of WIHN, many questions still remain. Despite many advances, the cellular basis of WIHN is still nebulous. It is becoming increasingly clear that the regenerative process does not rely on a single population of cells, and instead relies on an array of stem cells and differentiated cells. Initial work suggested that bulge stem cells do not contribute to the de novo follicles; Ito et al. found that Krt15+ cells were not present in regenerated hair follicles 2. Wang et al., however, showed that Lgr5+ dermal papilla/bulge stem cells are critical for WIHN, with lineage tracing analysis revealing that Lgr5+ cells are present in 40% of regenerated HFs 20. Lgr6+ interfollicular epidermis stem cells were also found to contribute to neogenic hair follicles, suggesting lineage plasticity 8,23. Similar lineage plasticity is also important in regenerating the dermal papilla (DP), sebaceous gland, and adipocytes 3,21,28–33,63. Upper dermal fibroblasts are critical for the formation of the new papillary dermis, and the reduction of these fibroblasts, at the expense of lower dermal fibroblasts, appears to explain the early loss of HF regenerative capacity in small wounds after P10 17,28. Increasing evidence suggests that myofibroblasts are likely the source of the neogenic DP 21,28,29. Remarkably, myofibroblasts are able to dedifferentiate and switch lineages to regenerate adipocytes that surround the de novo hair follicles 3. Furthermore, Gata6+ sebaceous duct cells are also able to dedifferentiate and contribute to the stem cell compartment of the interfollicular epidermis, hair follicle, and sebaceous gland 33. All of these studies show that WIHN is controlled by a complex interplay of different cell populations, stem cells and otherwise. Deciphering this interplay is a complex task, but one that is beginning to be tackled with advanced lineage tracing techniques and single-cell sequencing techniques 21,24,32.
Much work still needs to be done to elucidate mouse strain differences, hair morphology, growth orientation, and depigmentation phenomenon. Although Nelson et al. provided convincing evidence that certain high regenerating strains of mice have upregulated dsRNA sensing abilities, detailed systematic and mechanistic studies have not been done with different mouse strains 57. It would be particularly interesting to access whether lower regeneration mouse strains have reduced levels of retinoic acid or Shh within the center of healing wounds 21,75. Although the majority of the appendageal structures of the hair follicle are regenerated in WIHN, the arrector pili muscle is not regenerated. Driskell et al. defined two fibroblast lineages in the skin, the upper and lower dermis, and showed that the upper dermis is important for regulating hair growth and the arrector pili muscle. In the context of wounding, the upper dermis is required for de novo hair follicle regeneration, but it does not appear to aid in the arrector pili regeneration 28. The role of the upper dermis in WIHN, coupled with the ability of myofibroblasts to regenerate the DP and adipocytes, show that fibroblasts are essential for WIHN 3,21,28. This underscores the relevance of the recent discovery of 12 distinct fibroblast subtypes in wound healing, and a critical area of for future study 32. The ability of African spiny mice to regenerate the arrector pili muscle, along with pigmented hair follicles, make it promising model for further comparative studies between laboratory mice (Mus musculus) 1,12,19.
Two of the most defining features of WIHN, the necessity of a large wound and the clustering of neogenic hair follicles in the center of the healed scar, are still largely a mystery. Ito et al.’s landmark paper found that full-thickness wounds less than 1cm2 rarely develop de novo hair follicles. Furthermore, any regenerated follicles are clustered in the center with a surrounding ring of scar tissue 2. Recent studies from the Garza and Ito laboratories are beginning to shed light on this matter, suggesting that hair follicle spatial orientation may be regulated by gradients of important signaling and regenerative molecules 21,75. Kim et al. found that RA, an important mediator of WIHN, is highly concentrated in the center of recently reepithelialized wounds and less abundant at the edges. This gradient is reminiscent to one that RA forms in embryonic development to control anterior/posterior axis formation 76. This suggests that other essential regulators of WIHN may also be preferentially localized in the center of wounds. Indeed, Lim et al. found that key components of the Shh pathway are localized in the center of wounds, concomitant with regenerated hair follicles. Additionally, overexpression of epidermal Shh or constitutive dermal activation of Smoothened resulted in abundant WIHN, even in small scarring wounds 21. Surprisingly, spiny mice fully regenerate hair follicles in small and large wounds, underscoring the importance of studying WIHN in Acomys in more molecular detail follicle formation; early components in the phenomenon, such as dsRNA may be particularly fruitful because they could provide clues to the initiation of WIHN 57,58,75.
Finally, the process of WIHN only occurs in a relatively narrow window of time, between PWD 14 and PWD 21, suggesting that the conditions from regeneration are temporally restricted in the lifespan of a wound 2. Increasing evidence suggests that the inflammatory phase, which precedes the ingress of the keratinocytes in the proliferative phase, is critical to hair follicle regeneration. Initially, Gay et al. showed that Fgf9-producing γ-δ T cells infiltrated into wound bed directly before reepithelialization and onset of hair follicle regeneration 43. More recently it has become clear that macrophages play a role in the process as well, with hair follicle regeneration being dramatically attenuated with their ablation. These studies have also implicated a range of inflammatory cytokines and chemokines in the regenerative process, including IL6, TNF, and TGF-β1, as well as growth factors like Igf1 and Fgf1 20,86,87. Furthermore, the addition of the dsRNA mimic poly(I:C), as early as PWD 3, dramatically enhances WIHN 57. This coupled with the fact that poly(I:C) treatment has been shown to recruit inflammatory monocytes, macrophages, and neutrophils to the wound bed, suggests that other inflammatory cells may also be implicated in WIHN 64. Fascinatingly, spiny mice have a reduced inflammatory response after wounding, with dramatically less chemokines and cytokines than Mus, as well as virtually no macrophage infiltration until late in the healing process 12,19. Altogether, these data point to a regenerative model with a complex network of interacting inflammatory cells.
Conclusion
It has been over 60 years since the discovery of WIHN, and it is still a leading model for studying the mechanisms underpinning regeneration and the reactivation of embryonic-like repair in mammals. The WIHN model is powerful due to its quantifiable and reproducible timeline. The advent of more powerful lineage tracing mechanisms, coupled with advanced single-cell techniques will not only expand our understanding of the phenomenon, but also allow us to gain insights that will further regenerative medicine. The ability of WIHN to regenerate hair follicles within fibrous scar tissue makes it a promising candidate for assisting in curing diseases such as cicatricial alopecia, as well as treating scarring injuries like burns. Recapitulating organogenesis events of embryogenesis in adults in situ would reduce the burden of costly transplantations and surgeries. As one of the smallest complete organ and appendage in the human body, studying hair follicle regeneration offers a tractable model for studying mammalian growth and development, and developing clinical therapies for hair-related diseases and regenerative medicine as a whole. Already the sum of research in WIHN shows that the old adage that inflammation is bad for regeneration is overly simplistic, and future work will likely continue to upend conventional wisdom.
Acknowledgement
The composition of this review was supported by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases, part of the National Institutes of Health, under 1F32AR074865-01 to EW and R01AR064297/AR068280 to LAG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Plikus MV, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breedis C Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer research 14, 575–579 (1954). [PubMed] [Google Scholar]
- 5.Schneider MR, Schmidt-Ullrich R & Paus R The hair follicle as a dynamic miniorgan. Current biology : CB 19, R132–142 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Stenn KS & Paus R Controls of hair follicle cycling. Physiological reviews 81, 449–494 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Cotsarelis G, Sun TT & Lavker RM Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Dann L, Glücksmann A & Tansley K The Healing of Untreated Experimental Wounds. British Journal of Experimental Pathology 22, 1–9 (1941). [Google Scholar]
- 10.Billingham RE & Russell PS Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature 177, 791–792 (1956). [DOI] [PubMed] [Google Scholar]
- 11.Brook AH, Short BF & Lyne AG Formation of new wool follicles in the adult sheep. Nature 185, 51 (1960). [DOI] [PubMed] [Google Scholar]
- 12.Jiang TX, Harn HI, Ou KL, Lei M & Chuong CM Comparative regenerative biology of spiny (Acomys cahirinus) and laboratory (Mus musculus) mouse skin. Experimental dermatology 28, 442–449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero-Juarez CF, et al. Wound Regeneration Deficit in Rats Correlates with Low Morphogenetic Potential and Distinct Transcriptome Profile of Epidermis. The Journal of investigative dermatology 138, 1409–1419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kligman AM & Strauss JS The formation of vellus hair follicles from human adult epidermis. The Journal of investigative dermatology 27, 19–23 (1956). [DOI] [PubMed] [Google Scholar]
- 15.Sun ZY, Diao JS, Guo SZ & Yin GQ A very rare complication: new hair growth around healing wounds. The Journal of international medical research 37, 583–586 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Wong TW, Hughes M & Wang SH Never too old to regenerate? Wound induced hair follicle neogenesis after secondary intention healing in a geriatric patient. Journal of tissue viability 27, 114–116 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Rognoni E, et al. Inhibition of beta-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143, 2522–2535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan C, et al. Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy. Skin research and technology : official journal of International Society for Bioengineering and the Skin 17, 387–397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brant JO, Yoon JH, Polvadore T, Barbazuk WB & Maden M Cellular events during scar-free skin regeneration in the spiny mouse, Acomys. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 24, 75–88 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Wang X, et al. Macrophages induce AKT/beta-catenin-dependent Lgr5(+) stem cell activation and hair follicle regeneration through TNF. Nature communications 8, 14091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim CH, et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nature communications 9, 4903 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature medicine 11, 1351–1354 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Lough DM, et al. Transplantation of an LGR6+ Epithelial Stem Cell-Enriched Scaffold for Repair of Full-Thickness Soft-Tissue Defects: The In Vitro Development of Polarized Hair-Bearing Skin. Plastic and reconstructive surgery 137, 495–507 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Joost S, et al. Single-Cell Transcriptomics of Traced Epidermal and Hair Follicle Stem Cells Reveals Rapid Adaptations during Wound Healing. Cell reports 25, 585–597 e587 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Morgan BA The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harbor perspectives in medicine 4, a015180 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rompolas P & Greco V Stem cell dynamics in the hair follicle niche. Seminars in cell & developmental biology 25–26, 34–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushal GS, et al. Fate of Prominin-1 Expressing Dermal Papilla Cells during Homeostasis, Wound Healing and Wnt Activation. The Journal of investigative dermatology 135, 2926–2934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telerman SB, et al. Dermal Blimp1 Acts Downstream of Epidermal TGFbeta and Wnt/beta-Catenin to Regulate Hair Follicle Formation and Growth. The Journal of investigative dermatology 137, 2270–2281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinkevich Y, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shook BA, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrero-Juarez CF, et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nature communications 10, 650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donati G, et al. Wounding induces dedifferentiation of epidermal Gata6(+) cells and acquisition of stem cell properties. Nature cell biology 19, 603–613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andl T, Reddy ST, Gaddapara T & Millar SE WNT signals are required for the initiation of hair follicle development. Developmental cell 2, 643–653 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Developmental cell 17, 49–61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGowan KM & Coulombe PA Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. The Journal of cell biology 143, 469–486 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn Y Signaling in tooth, hair, and mammary placodes. Current topics in developmental biology 111, 421–459 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Andersson T, et al. CXXC5 is a novel BMP4-regulated modulator of Wnt signaling in neural stem cells. The Journal of biological chemistry 284, 3672–3681 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Kim MS, et al. A novel Wilms tumor 1 (WT1) target gene negatively regulates the WNT signaling pathway. The Journal of biological chemistry 285, 14585–14593 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SH, et al. Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis. The Journal of investigative dermatology 137, 2260–2269 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Banziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Myung PS, Takeo M, Ito M & Atit RP Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. The Journal of investigative dermatology 133, 31–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature medicine 19, 916–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gattazzo F, Urciuolo A & Bonaldo P Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochimica et biophysica acta 1840, 2506–2519 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olczyk P, Mencner L & Komosinska-Vassev K The role of the extracellular matrix components in cutaneous wound healing. BioMed research international 2014, 747584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Cescon M & Bonaldo P Lack of Collagen VI Promotes Wound-Induced Hair Growth. The Journal of investigative dermatology 135, 2358–2367 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Carney TJ & Ingham PW Drugging Hedgehog: signaling the pathway to translation. BMC biology 11, 37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang C, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Developmental biology 205, 1–9 (1999). [DOI] [PubMed] [Google Scholar]
- 49.St-Jacques B, et al. Sonic hedgehog signaling is essential for hair development. Current biology :CB 8, 1058–1068 (1998). [DOI] [PubMed] [Google Scholar]
- 50.Woo WM, Zhen HH & Oro AE Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes & development 26, 1235–1246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouspenskaia T, Matos I, Mertz AF, Fiore VF & Fuchs E WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell 164, 156–169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato N, Leopold PL & Crystal RG Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. The Journal of clinical investigation 104, 855–864 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan BA, Orkin RW, Noramly S & Perez A Stage-specific effects of sonic hedgehog expression in the epidermis. Developmental biology 201, 1–12 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Ma X, et al. Msi2 Maintains Quiescent State of Hair Follicle Stem Cells by Directly Repressing the Hh Signaling Pathway. The Journal of investigative dermatology 137, 1015–1024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiyama-Nakagiri Y, Akiyama M, Shibata S, Okano H & Shimizu H Expression of RNA-binding protein Musashi in hair follicle development and hair cycle progression. The American journal of pathology 168, 80–92 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamburg-Shields E, DiNuoscio GJ, Mullin NK, Lafyatis R & Atit RP Sustained beta-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. The Journal of pathology 235, 686–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson AM, et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell stem cell 17, 139–151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu AS, Li A, Ratliff TS, Melsom M & Garza LA After Skin Wounding, Noncoding dsRNA Coordinates Prostaglandins and Wnts to Promote Regeneration. The Journal of investigative dermatology 137, 1562–1568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeuchi O & Akira S Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Huebener P & Schwabe RF Regulation of wound healing and organ fibrosis by toll-like receptors. Biochimica et biophysica acta 1832, 1005–1017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawai T & Akira S Toll-like receptor and RIG-I-like receptor signaling. Annals of the New York Academy of Sciences 1143, 1–20 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Cavassani KA, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. The Journal of experimental medicine 205, 2609–2621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Q, et al. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. Journal of immunology 186, 3710–3717 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Lin Q, et al. Toll-like receptor 3 ligand polyinosinic:polycytidylic acid promotes wound healing in human and murine skin. The Journal of investigative dermatology 132, 2085–2092 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Bernard JJ, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nature medicine 18, 1286–1290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borkowski AW, et al. Toll-like receptor 3 activation is required for normal skin barrier repair following UV damage. The Journal of investigative dermatology 135, 569–578 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melkamu T, Kita H & O’Grady SM TLR3 activation evokes IL-6 secretion, autocrine regulation of Stat3 signaling and TLR2 expression in human bronchial epithelial cells. Journal of cell communication and signaling 7, 109–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson AM, et al. Interleukin-6 Null Mice Paradoxically Display Increased STAT3 Activity and Wound-Induced Hair Neogenesis. The Journal of investigative dermatology 136, 1051–1053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson AM, Katseff AS, Ratliff TS & Garza LA Interleukin 6 and STAT3 regulate p63 isoform expression in keratinocytes during regeneration. Experimental dermatology 25, 155–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koster MI, Kim S, Mills AA, DeMayo FJ & Roop DR p63 is the molecular switch for initiation of an epithelial stratification program. Genes & development 18, 126–131 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson AM, et al. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. The Journal of investigative dermatology 133, 881–889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. TISSUE REGENERATION. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348, aaa2340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geng L, Hanson WR & Malkinson FD Topical or systemic 16, 16 dm prostaglandin E2 or WR-2721 (WR-1065) protects mice from alopecia after fractionated irradiation. International journal of radiation biology 61, 533–537 (1992). [DOI] [PubMed] [Google Scholar]
- 74.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim D, et al. Noncoding dsRNA induces retinoic acid synthesis to stimulate hair follicle regeneration via TLR3. Nature communications 10, 2811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duester G Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stocum DL Mechanisms of urodele limb regeneration. Regeneration 4, 159–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li M, et al. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 128, 675–688 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Okano J, et al. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. The Journal of biological chemistry 287, 39304–39315 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim MH, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. The Journal of investigative dermatology 128, 1812–1820 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo S & Dipietro LA Factors affecting wound healing. Journal of dental research 89, 219–229 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acosta JB, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. International wound journal 5, 530–539 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singer AJ & Clark RA Cutaneous wound healing. The New England journal of medicine 341, 738–746 (1999). [DOI] [PubMed] [Google Scholar]
- 84.DiPietro LA Wound healing: the role of the macrophage and other immune cells. Shock 4, 233–240 (1995). [PubMed] [Google Scholar]
- 85.Sindrilaru A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. The Journal of clinical investigation 121, 985–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasuya A, Ito T & Tokura Y M2 macrophages promote wound-induced hair neogenesis. Journal of dermatological science 91, 250–255 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Rahmani W, et al. Macrophages Promote Wound-Induced Hair Follicle Regeneration in a CX3CR1- and TGF-beta1-Dependent Manner. The Journal of investigative dermatology 138, 2111–2122 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Ding VM, et al. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. Journal of cellular physiology 225, 417–428 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Desbois-Mouthon C, et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20, 252–259 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Chu SY, et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nature communications 10, 1524 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]