Abstract
Background
Up to 30% of women with vaginal symptoms are not assigned a diagnosis after standard diagnostic assessment.
Methods
We compared premenopausal women with idiopathic vaginitis (IV) or vulvodynia (VVD) to healthy controls. Microbiota were characterized using rRNA sequencing. Cytokines/chemokines (IL-10, IL-1α, IL-1β, IL-6, IL-8, IL-2, IL-18, IL-4, IL-9, and IL-13) were measured in vaginal lavage fluid using the Meso Scale Discovery platform or ELISA (IL-1ra). Immunoglobulins were measured in vaginal lavage fluid using a bead-based immunoassay (Millipore). Cases and controls were compared using Kruskal Wallis, ANOVA and linear regression or (for microbiome composition) the Bray-Curtis dissimilarity statistic.
Results
We compared 20 women with IV, 30 with VVD and 52 controls. Most (80%) had > 90% 16S rRNA gene sequences from Lactobacillus crispatus, L. jensenii, L. gasseri or L. iners. In analyses adjusted for age and hormonal contraception (HC), Gardnerella vaginalis was less prevalent and abundant in women with VVD (2/30, 7%) vs. controls (16/52, 31%) or IV (5/20, 25%) (p = 0.030). Bray-Curtis dissimilarity was not significantly different between IV and controls or VVD. Fungal sequences were only detected in 5 participants: 2 control, 1 IV, 2 VVD. In univariate analysis, cytokines were not associated with diagnosis. Median vaginal concentration of IgE (but not other immunoglobulins) was lower in women with vulvodynia (p = 0.006).
Conclusions
Minimal differences in vaginal microbiota and inflammatory markers between women with IV, VVD or controls suggest no striking association between vaginal bacteria, fungi or inflammation and diagnosis in these women.
Keywords: Vaginitis, Vulvodynia, Vaginal microbiota, Vaginal discharge
Short summary
Women with idiopathic vulvovaginal symptoms had no differences in bacteria, fungi, or vaginal cytokines/chemokines compared to healthy controls, suggesting symptoms are not due to occult infection.
Introduction
Vaginal complaints account for 5–10 million health care visits annually.(1, 2) Vaginal symptoms are associated with significant distress, including fears about cancer, feelings of shame and stigma, and sexual dysfunction.(3) In evaluating such concerns it is often difficult for patients and providers to distinguish between what is normal and what is pathologic(4) as symptoms are by definition subjective. Current diagnostic algorithms aim to identify BV, yeast vaginitis or Trichomonas vaginalis (TV) vaginitis using pH testing and light microscopy or, alternately, by using point-of-care testing.(5) Even with a thorough evaluation with molecular assays, up to 30% of symptomatic women have no infectious etiology to explain their vaginal symptoms.(6)
Alternative causes of vaginal discomfort include the vulvovaginal pain syndrome vulvodynia,(7) or desquamative inflammatory vaginitis (DIV), which is characterized by pain, copious vaginal discharge and vaginal inflammation.(8) The condition aerobic vaginitis likely falls on the same spectrum as DIV, and is characterized by vaginal inflammation and loss of vaginal lactobacilli.(9) However, all of these diagnoses describe syndromes and constellations of clinical findings, rather than a clear cause for symptoms. In the absence of a known cause, finding an effective treatment is often difficult.
To identify possible etiologic pathways or rule out other plausible explanations for bothersome symptoms of pain and vaginitis, we evaluated microbial and immunologic characteristics of women with idiopathic vaginitis and vulvodynia and compared them to healthy women.
Methods
We enrolled symptomatic, premenopausal women presenting to the Vulvovaginal Disorders Program at Massachusetts General Hospital in Boston between August 2014 and May 2017. All participants signed informed consent, completed questionnaires, and underwent standardized exam and vaginal sample collection. In addition to clinically indicated testing, each participant had a vaginal swab collected for Gram stain, a vaginal swab for molecular characterization of the microbiota and (after May 2015) cervicovaginal lavage with 5 mL of sterile saline. Samples were transported on ice and stored at −80C until thawed for analysis. Participants completed questionnaires about health history, recent vaginal product use, presence and severity of vulvovaginal symptoms (vulvar itch, burn, irritation; vaginal itch, burn, irritation, vulvar or vaginal pain, vaginal dryness or vaginal discharge), and the Female Sexual Distress Scale (FSDS). Gram-stained slides of smeared vaginal fluid underwent evaluation using the Nugent criteria.(10) In addition, the numbers of polymorphonuclear cells and epithelial cells per 100X field were counted in 5 non-contiguous fields and an average ratio calculated.
The final diagnosis for the purposes of this study was made through a combination of clinical data, study-specific Gram stain, and review of the medical record. Women who had a study Nugent score of ≥ 7, even if not clinically diagnosed with BV were considered to have BV for the purposes of the study and were not included as cases or controls. Women ultimately diagnosed with idiopathic vaginitis (IV) (moderate-severe vaginal discharge, itching, irritation and no other diagnosis) or vulvodynia (VVD) (vulvar or vaginal pain of at least 3 months duration)(7) were included as cases. All cases had either negative testing for gonorrhea, chlamydia and trichomonas upon review of medical records or had negative testing as part of their clinical evaluation, and all cases had a negative fungal culture. When the diagnosis was not clear, each case was reviewed by two investigators (AP and CM) who made the assignment together. Women presenting to the Massachusetts General Hospital Gynecology clinic for annual exam were enrolled as healthy controls, signed informed consent and completed the same procedures as cases. Healthy controls underwent testing for sexually transmitted infections as clinically indicated.
DNA was extracted from each swab using the QIAamp BiOstic Bacteremia DNA Isolation Kit (Qiagen, Hilden, Germany). To assess contamination from reaction buffers or collection swabs, sham digests from swabs without human contact were processed in parallel (negative control). No-template water controls were also included with every PCR assay to assess contamination from PCR reagents. DNA samples were tested for PCR inhibitors (11) and 16S rRNA gene copies in each sample (total bacterial load) were measured using a broad-range qPCR assay.(12) The microbiota (relative abundances) was characterized using broad-range 16S rRNA gene PCR with deep sequencing of the V3-V4 region on Illumina MiSeq platform (Illumina, San Diego, CA).(13) The DADA2 pipeline was used to infer sequence variants from raw reads for subsequent analysis. Sequences were classified using the phylogenetic placement tool pplacer and a curated reference set of vaginal bacteria.(12) A mean of 81,158 sequence reads and median of 77,731 sequence reads per sample were obtained. There was one sample (a healthy control) with less than 1000 reads which was excluded from the analysis. Sequence reads can be accessed from the NCBI Short Read Archive (in submission, accession numbers pending).
Additionally, PCR amplification and deep sequencing of the 28S and ITS regions of the fungal rRNA operon were performed to identify fungal sequences. A 28S-10 forward/28S-12 reverse primer pair was used to target a selected region of the 28S rRNA gene, while a 5.8S forward/28S-1 reverse primer pair was used to target the ITS2 region (primers in Supplemental Table 1).(14) Two types of negative controls were run for each PCR, one with PCR reagents only and one with a clean swab that was processed in the same way as study samples (extraction control). Each 30 μL reaction contained 1X Accuprime Buffer II, 0.4 μM of forward primer, 0.4 μM of reverse primer, and 0.9 U Accuprime™ High Fidelity Taq polymerase (Thermo Fisher Scientific, Waltham, MA). Cycling conditions were an initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 40 seconds. Final elongation was at 72°C for 7 minutes. Band size (~450 bp for both 28S, and ITS2 amplicons) was confirmed with gel electrophoresis. Clean-up of amplicons was performed with Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN) per Illumina’s 16S Metagenomic sequencing library preparation protocol for the Illumina MiSeq System. After clean-up, amplicons were subjected to Index PCR using Nextera®XT Index kits v2 (Set A), KAPA HiFi HotStart Ready Mix (KAPA Biosystems, Wilmington, MA) and 5 uL DNA in order to multiplex all samples for a single sequencing run (Illumina Reference). Index PCR cycling conditions were an initial denaturation at 95°C for 3 minutes, followed by 8 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Final elongation was at 72°C for 5 minutes. Indexed PCR products were cleaned using Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN), per protocol then air dried for 8 minutes and eluted in 30 μL filtered (100,000 mwco) 1X TE buffer. DNA concentration of each sample was assessed using the Quant-iT dsDNA Assay Kit, high sensitivity (Thermo Fisher Scientific, Waltham, MA), and concentration was used to determine sample volumes to pool. High concentration samples (>9 nM) were pooled together at equimolar quantities, while low concentration samples (<9 nM) were pooled together separately at equal volumes, using the entirety of available PCR product. Pools were combined to a final concentration of 7.5 nM.
Sequencing was performed on the Illumina MiSeq instrument (Illumina, San Diego, CA) with the MiSeq® Reagent Kit v3– 600 cycle to capture paired-end reads (2 × 300). PhiX Control Library v3 (Illumina, San Diego, CA) was combined with the amplicon library at 15%. The combined library was denatured with NaOH, diluted with hybridization buffer, heat denatured, and was sequenced as PE300 reads using standard Illumina primers. Raw sequence reads were demultiplexed using Illumina’s onboard software and barcodecop (available at https://github.com/nhoffman/barcodecop). Because of the length heterogeneity of the ITS region, only the forward reads were used in the analysis. Forward reads from all specimens were pooled and dereplicated using swarm version 2.2.2 (https://doi.org/10.7717/peerj.1420) with default parameters. Operational Taxonomic Units (OTUs) with a read mass < 2 were discarded. The remaining OTUs were used to search a version the NCBI nt database downloaded 3/25/2019 using NCBI BLAST+ 2.8.1 (https://www.ncbi.nlm.nih.gov/pubmed/20003500?dopt=Citation) with parameters -perc_identity 95 and -qcov_hsp_perc 90. Taxonomic lineages were derived from a version of the NCBI taxonomy downloaded March 28, 2019. Taxonomic assignment of OTUs was performed based on percent identities of BLAST alignments to sequence records in the nt database: for example, a species-level classification was assigned to an OTU based on species-level taxonomic names of all nt records aligned with a percent identity of at least 99%. For OTUs with no matches at that threshold, the process is repeated for each rank (genus, family, etc.) with correspondingly higher thresholds until an assignment is obtained. Rank-specific thresholds are provided in Supplementary Table 2. Code for taxonomic assignment is available at https://github.com/crosenth/moose version 0.4. The average number of reads for the combined controls was 1130 (ITS) and 1736 (28S) (range 2–10343), which was an order of magnitude lower than the average number for samples [132770 (ITS); 102007 (28S)]).
Vaginal cytokines (IL-10, IL-1α, IL-1β, IL-6, IL-8, IL-2, IL-18, IL-4, IL-9, and IL-13) were measured in vaginal swab eluate or vaginal lavage fluid using the Meso Scale Discovery platform (Meso Scale Discovery, Rockville, MD). IL1ra was measured using ELISA (R&D Systems, Minneapolis, MN). These analytes were selected based on what was detectable in vaginal fluid, and to represent a variety of possible immune pathways: pro-inflammatory (IL-1α, IL-1β, IL-2, IL-6, IL-18), anti-inflammatory (IL-1ra, IL-10), Th2 (IL-9, IL13), and chemokines (IL-8). Values were log transformed for analysis. Undetectable values were assigned a value of half the lower limit of normal. Vaginal immunoglobulins were measured in vaginal swab eluate or vaginal lavage fluid using Milliplex kits (Millipore Sigma, Burlington, MA) according to manufacturer instructions. Laboratory staff performing analyses were blinded to the case vs. control status of participants.
In univariate analysis, relative abundances and total quantity of 16S rRNA in cases and controls were compared using the Kruskal-Wallis test, the Skillings-Mack test controlling for batch, and linear regression adjusting for other important covariates (e.g., age, hormonal contraception). Prevalence rates were compared between groups using Fisher’s exact test. In multivariate analysis, Bray-Curtis dissimilarities and permutational ANOVA (permANOVA) were used to assess the compositional differences between controls and women with IV or VVD. Principal coordinates analysis (PCoA) was used for dimensionality reduction. We compared cytokine and immunoglobulin values in swab eluate and cervicovaginal lavage within each diagnosis group using permANOVA to evaluate how the sampling methodology impacted results, and controlled for sample type in analyses of cytokines and immunoglobulins. All tests were two sided.
Results
This analysis includes 20 women with IV, 30 with VVD and 52 controls. Participants were mostly White (87%), Asian (7%), and Black (3%), and had very similar demographic characteristics. Women with vulvodynia were more likely to have a history of receiving treatment for seasonal allergies (Table 1). None of the participants with IV met diagnostic criteria for desquamative inflammatory vaginitis (DIV). Not surprisingly, women with vulvodynia were significantly more likely to report moderate-severe vulvovaginal pain in the past month than the other two groups, but they were also very likely to report moderate-severe vulvovaginal itch, burn or irritation. Women with IV were most likely to report moderate-severe discharge. On exam, no cervical or vaginal abnormalities were noted for any of the participants. On Gram stain, an equal proportion had a ratio of WBC/epithelial cells equal to 1 or greater: control 12/50 (24%), IV 6/19 (30%), VVD 8/20 (26%).
Table 1:
Demographic and clinical data for each diagnostic group
| Control (n = 50) | Idiopathic vaginitis (n = 20) | Vulvodynia (n = 30) | P value | |
|---|---|---|---|---|
| Age | 36 ± 9 | 34 ± 9 | 31 ± 8 | 0.06 |
| Race | 0.72 | |||
| White | 45 (90%) | 19 (95%) | 24 (80%) | |
| Black | 1 (2%) | 0 | 2 (7%) | |
| Asian | 3 (6%) | 1 (5%) | 3 (10%) | |
| Other | 1 (2%) | 0 | 1 (3%) | |
| Hispanic ethnicity | 2 (4%) | 1 (5%) | 0 (0%) | 0.50 |
| Hormonal contraception | 18 (36%) | 5 (25%) | 10 (33%) | 0.68 |
| Any condom use | 9 (18%) | 5 (25%) | 3 (10%) | 0.37 |
| Asthma | 7 (14%) | 7 (35%) | 6 (20%) | 0.14 |
| Eczema | 6 (12%) | 3 (15%) | 6 (20%) | 0.63 |
| Seasonal allergies | 12 (24%) | 6 (30%) | 17 (57%) | 0.01 |
| History of STI | 8 (16%) | 2 (10%) | 5 (17%) | 0.78 |
| Sexual partner | 0.15 | |||
| Male | 47 (96%) | 20 (100%) | 28 (93%) | |
| Female | 0 | 0 | 2 (7%) | |
| Both/either | 2 (4%) | 0 | 0 | |
| Sex in past week | 20 (40%) | 8 (40%) | 12 (40%) | 1.0 |
| Sexual distress (FSDS) | 6 (13%) | 8 (42%) | 23 (77%) | < 0.001 |
| Vaginal products in the past week | ||||
| Douche | 2 (4%) | 0 (0%) | 0 (0%) | 0.23 |
| Lubricant | 1 (2%) | 4 (20%) | 6 (20%) | 0.02 |
| Antifungal cream | 2 (4%) | 1 (5%) | 3 (10%) | 0.54 |
| Antibiotic | 3 (6%) | 1 (5%) | 2 (7%) | 0.97 |
| Probiotic | 1 (2%) | 1 (5%) | 1 (3%) | 0.80 |
| Other | 0 | 1 (5%) | 4 (21%) | |
| Symptoms in past month | ||||
| Discharge | 5 (10%) | 12 (60%) | 9 (30%) | < 0.001 |
| Odor | 3 (6%) | 6 (30%) | 4 (13%) | 0.03 |
| Itch/burn/irritation | 4 (8%) | 6 (30%) | 24 (80%) | < 0.001 |
| Pain | 4 (8%) | 5 (25%) | 24 (80%) | < 0.001 |
| Dryness | 2 (4%) | 3 (15%) | 10 (33%) | 0.001 |
STI = sexually transmitted infection (i.e. gonorrhea, chlamydia, trichomonas, herpes, syphilis); FSDS = Female Sexual Distress Scale; sexual distress is defined as a score of 15 or higher out of a maximum 48
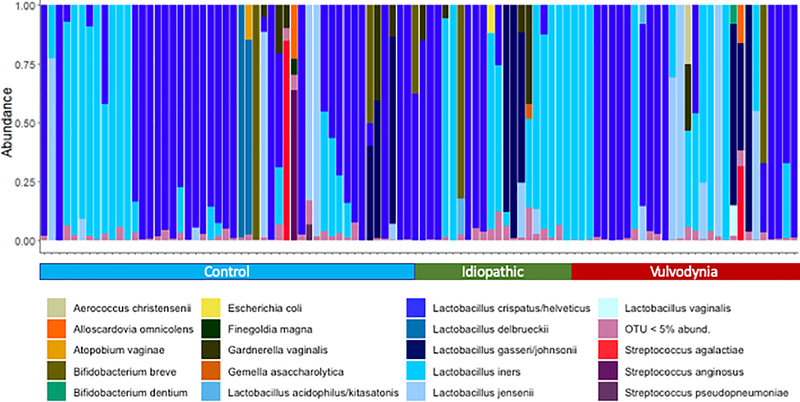
In a majority of women (80%) greater than 90% of sequences were from Lactobacillus species (Figure 1). Study group was not significantly associated with relative abundance of any individual Lactobacillus species (Figure 2). Gardnerella vaginalis was less prevalent and abundant in women with VVD (2/30, 7%) vs. control (16/52, 31%) or IV (5/20, 25%) (p= 0.03). Adjustment for age and use of hormonal contraception did not quantitatively affect results. PCoA and permANOVA analysis using Bray-Curtis dissimilarity did not show any difference in the microbiota between the three groups (Supplemental Figure 1). There was no difference in median (IQR) total 16S rRNA copies between groups: controls 1.27 × 108 (5.58 × 107, 2.51 × 108) vs. IV 6.73 × 107 (1.46 × 107, 1.6 × 108) vs. VVD 8.55 × 107 (3.25 × 107, 1.87 × 108) (p = 0.13).
Figure 1:
Relative abundance of 20 most commonly detected taxa. Participants were grouped according to diagnosis and then hierarchical clustering was performed within diagnosis group.
Figure 2:
Comparison of median relative abundance for Lactobacillus genus, L. crispatus/jensenii and L. iners between diagnosis groups. Using the Kruskall-Wallis test, no significant difference was demonstrated between groups.
By PCR, fungal sequences were detected in only 5 participants: 2 control women (4%) had Candida albicans by both assays, 1 woman with IV (5%) had C. albicans detected with both assays and also a small proportion of C. lusitaniae by 28S rRNA gene sequence analysis, and 2 women with vulvodynia (7%) had C. albicans by both assays.
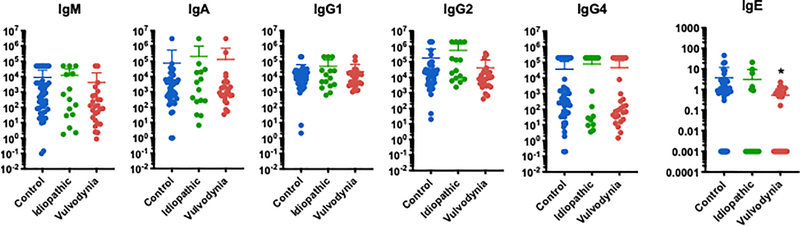
Analysis of vaginal fluid immunoglobulin levels demonstrated a lower median concentration of IgE in women with vulvodynia, but no differences in other immunoglobulin levels between the study groups (Figure 3). In univariate and multivariate analyses, no cytokines were associated with diagnosis (p values all > 0.05) (Figure 4). Stratified analysis by sample type did not reveal clustering by diagnosis in either swabs (p = 0.54; permutational ANOVA), or CVL (p = 0.91; permutational ANOVA) (Supplemental Figure 2).
Figure 3:
Comparison of median vaginal immunoglobulin concentration between diagnosis groups, demonstrating lower level of IgE in women with vulvodynia compared to healthy controls.
Figure 4:
Heatmap of log-transformed soluble immune marker values. No significant differences in values were seen between diagnosis groups.
Discussion
The primary objective of this case-control analysis of prospectively enrolled women was to evaluate whether a distinct microbial or immunologic signature was associated with our study groups. Our study population was demographically relatively homogenous to limit potential confounding, and participants were phenotypically well characterized. Cases reported significantly higher symptom burden than controls, and significantly more sexual distress, suggesting that the lack of identifiable differences was not due to misclassification of diagnoses. We included women with idiopathic vaginitis and those who met the diagnostic criteria for vulvodynia, as these two groups are sometimes difficult to separate. Many women with vulvodynia are seen by multiple providers, and may be treated for infectious vaginitis multiple times prior to receiving a diagnosis of vulvodynia.(15) Report of vulvovaginal symptoms is common in both pre- and postmenopausal women,(16) and is not predictive for a specific diagnoses – even when symptoms are considered “classic” for a particular condition.(5)
Previous studies have attempted to link the vaginal microbiota and report of vulvovaginal symptoms but have also not demonstrated clear associations. In a cohort of women with localized provoked vulvodynia, there were only limited associations between pain severity and vaginal microbiota.(17) A separate study found no differences in vaginal or vestibular microbiota in women with vulvodynia and controls.(18) In a cross-sectional analysis of post-menopausal women, no association between vaginal microbial communities and report of vulvovaginal discomfort was identified.(19) Our results, demonstrating that a majority of women had Lactobacillus-dominant vaginal microbiota regardless of whether they had a diagnosis of IV, VVD, or neither, confirm these earlier reports, and suggest that idiopathic vaginal discharge, irritation or pain are not the result of disruption of a healthy vaginal microbial community. In addition, fungal community profiling did not identify a significant number of occult fungal infections.
An alternate hypothesis to explain symptoms is that microbiota is not different between diagnostic groups, but rather that immune response to those microbes are different. A previous study demonstrated a higher rate of skin test positivity to aeroallergens in women with a history of idiopathic vulvovaginal discomfort compared to controls.(20) Another found a higher rate of patch test sensitivity to C. albicans in women with vulvodynia compared to those with atopic dermatitis or controls.(21) In our study, women with vulvodynia were more likely to report a history of seasonal allergies. However, when we measured both vaginal fluid IgE and cytokines typically considered Th2 type (IL4, IL9) we did not find any differences between our diagnosis groups. Nor did we find differences in cytokine classically considered pro-inflammatory (IL1β, IL18, IL6). Our findings are in line with work by Jayaram and colleagues who found no difference in vaginal fluid IL1β between women with and without vulvodynia.(18) In contrast to our findings, Zanotta et al compared 48 cytokines and chemokines in vaginal fluid between women with vulvar pain syndrome and healthy controls and found 6 analytes that were consistently different between groups in both pre- and postmenopausal women: IL3, IL12(p40), SDF1α, TNF-β, IL18 and IL10.(22) Of these, our analysis only included IL10 and IL18, and we did not find a difference between groups. Both studies included similar numbers of cases and controls, but ours only enrolled women with Nugent score under 7 who did not have Candida by microscopy or culture. The Zanotta study did not evaluate vaginal bacteria or Candida, which are known to be associated with differences in vaginal immune markers and may have been potential unmeasured confounders biasing their results.(23)
The lack of significant difference in superficial vaginal inflammation between women with and without vulvovaginal discomfort (in the presence of very similar microbiota), suggests that these symptoms may be related to structures below the epithelium, such as neurons, cellular inflammation or muscular spasm or dysfunction. Prior studies of vestibular tissue showed higher levels of TNFα and IL1β in tissue from women with vulvar vestibulitis compared to pain-free women undergoing rectocele repair.(24) In vitro culture of vestibular fibroblasts from women with vestibulitis demonstrated higher production of IL6, IL8 and IL1β in response to Candida albicans or α-melanocyte stimulating hormone compared to fibroblasts from pain-free control women.(25) Tissue removed at vestibulectomy for vulvodynia has more inflammation than vestibular tissue removed during non-pain related surgery.(26) However, these associations may be due to the impact of vulvodynia on the tissue rather than an indication that inflammation causes vulvodynia. In a mouse model of cystitis, the neuromodulating molecule Substance P was induced in nerve cells upon infection with E. coli, and subsequent inflammation could be blocked by inhibiting the receptor for Substance P.(27) In both human cases and mouse models of psoriasis, nerve injury can lead to clearing of psoriatic inflammation in the distribution of the nerve (reviewed in (28)).These data suggest that tissue based inflammation may be due in part to neural activation.
Over half of the women in the idiopathic vaginitis group reported moderate-severe vaginal discharge. None of these women had significant cervical ectropion on exam, which has been proposed as a potential non-infectious cause of vaginal discharge.(29) Many of these women also reported itching or irritation. Non-allergic rhinitis, which occurs more commonly in women, may be a similar phenomenon, with no clear pathogen identified, and no clear allergic trigger (reviewed in (30)). However, that disorder is often characterized by eosinophilia, and we did not find any differences in Th2 cytokines commonly associated with eosinophils (IL4, IL9, IL13).
Strengths of our study include clear definitions of study groups, prospective collection of samples for planned measurement of study variables, and concurrent measurement of microbes, soluble cytokines and chemokines, and vaginal immunoglobulin levels. Although our study may not have enough power to detect small differences between groups, it provides a comprehensive evaluation of the superficial vaginal environment in women with vulvovaginal discomfort of uncertain etiology. It is possible that symptoms may be due to an organism we did not test for, such as M. genitalium in low abundance or a viral infection. Alternatively, metabolically active but low prevalence species associated with symptoms might not be identified with 16S rRNA sequencing of the dominant bacteria. However, the lack of any significant differences in soluble immune markers in the vaginal fluid suggests that this scenario is unlikely. Our study population is very homogenous with regard to self-identified race, which facilitates comparisons between small groups but limits the generalizability of our findings. This is a cross-sectional analysis, which may not capture important daily or weekly variations in microbiota or immune responses that could influence overall pathology and symptoms.
In summary, our findings suggest that idiopathic vulvovaginal discomfort is not associated with vaginal microbial colonization or epithelial immune responses. To identify the cause of these symptoms may require more extensive sampling of the vaginal tissue and deep cellular immune responses.
Supplementary Material
Supplemental Figure 1: Principle coordinates analysis using Bray Curtis dissimilarity does not demonstrate any clustering of microbial communities by diagnosis.
Supplemental Figure 2: Stratified analysis of cytokines by sample type. Vaginal fluid cytokines were measured in either swabs or CVL, based on timing of participant enrollment (A). Stratified analysis by sample type did not reveal clustering by diagnosis in either swabs (B) (p = 0.54; permutational ANOVA), or CVL (C) (p = 0.91; permutational ANOVA)
Acknowledgments
Dr. Mitchell has received consulting fees from Lupin Pharmaceuticals and Scynexis, and receives research funding from Merck. Dr. Fredricks receives royalties from BD for unrelated work. All other authors have no conflicts of interest to disclose.
This work was funded by NIH/NIAID/1R21AI113439 (CM)
References
- 1.Lipsky MS, Waters T, Sharp LK. Impact of vaginal antifungal products on utilization of health care services: evidence from physician visits. J Am Board Fam Pract. 2000;13(3):178–82. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 Pt 2):924–35. [DOI] [PubMed] [Google Scholar]
- 3.Karasz A, Anderson M. The vaginitis monologues: women’s experiences of vaginal complaints in a primary care setting. Soc Sci Med. 2003;56(5):1013–21. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M, Karasz A, Friedland S. Are vaginal symptoms ever normal? a review of the literature. MedGenMed. 2004;6(4):49. [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291(11):1368–79. [DOI] [PubMed] [Google Scholar]
- 6.Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima Bacterial Vaginosis and Aptima Candida/Trichomonas Vaginitis Assays: results from a prospective multi-center clinical study. J Clin Microbiol. 2019. November 20;EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. Obstet Gynecol. 2016;127(4):745–51. [DOI] [PubMed] [Google Scholar]
- 8.Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol. 1994;171(5):1215–20. [DOI] [PubMed] [Google Scholar]
- 9.Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109(1):34–43. [DOI] [PubMed] [Google Scholar]
- 10.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis. 2008;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golob JL, Pergam SA, Srinivasan S, et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin Infect Dis. 2017;65(12):1984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khot PD, Ko DL, Fredricks DN. Sequencing and analysis of fungal rRNA operons for development of broad-range fungal PCR assays. Appl Environ Microbiol. 2009;75(6):1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc (1972). 2003;58(2):82–8. [PubMed] [Google Scholar]
- 16.Watson LJ, James KE, Hatoum Moeller IJ, Mitchell CM. Vulvovaginal Discomfort Is Common in Both Premenopausal and Postmenopausal Women. J Low Genit Tract Dis. 2019;23(2):164–9. [DOI] [PubMed] [Google Scholar]
- 17.Donders GGG, Bellen G, Ruban KS. Abnormal vaginal microbioma is associated with severity of localized provoked vulvodynia. Role of aerobic vaginitis and Candida in the pathogenesis of vulvodynia. Eur J Clin Microbiol Infect Dis. 2018;37(9):1679–85. [DOI] [PubMed] [Google Scholar]
- 18.Jayaram A, Witkin SS, Zhou X, et al. The bacterial microbiome in paired vaginal and vestibular samples from women with vulvar vestibulitis syndrome. Pathog Dis. 2014;72(3):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell CM, Srinivasan S, Zhan X, et al. Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause. 2017;24(10):1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozturk S, Caliskaner Z, Karaayvaz M, Dede M, Gulec M. Hypersensitivity to aeroallergens in patients with recurrent vulvovaginitis of undetermined etiology. J Obstet Gynaecol Res. 2007;33(4):496–500. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez De Knott HM, McCormick TS, Do SO, et al. Cutaneous hypersensitivity to Candida albicans in idiopathic vulvodynia. Contact Dermatitis. 2005;53(4):214–8. [DOI] [PubMed] [Google Scholar]
- 22.Zanotta N, Campisciano G, Scrimin F, et al. Cytokine profiles of women with vulvodynia: Identification of a panel of pro-inflammatory molecular targets. Eur J Obstet Gynecol Reprod Biol. 2018;226:66–70. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell C, Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol. 2014;71(6):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster DC, Hasday JD. Elevated tissue levels of interleukin-1 beta and tumor necrosis factor-alpha in vulvar vestibulitis. Obstet Gynecol. 1997;89(2):291–6. [DOI] [PubMed] [Google Scholar]
- 25.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346 e1–8. [DOI] [PubMed] [Google Scholar]
- 26.Tommola P, Butzow R, Unkila-Kallio L, Paavonen J, Meri S. Activation of vestibule-associated lymphoid tissue in localized provoked vulvodynia. Am J Obstet Gynecol. 2015;212(4):476 e1–8. [DOI] [PubMed] [Google Scholar]
- 27.Butler DSC, Ambite I, Nagy K, et al. Neuroepithelial control of mucosal inflammation in acute cystitis. Sci Rep. 2018;8(1):11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cekmez Y, Sanlikan F, Gocmen A, Vural A, Turkmen SB. Is Cryotherapy Friend or Foe for Symptomatic Cervical Ectopy? Med Princ Pract. 2016;25(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Principle coordinates analysis using Bray Curtis dissimilarity does not demonstrate any clustering of microbial communities by diagnosis.
Supplemental Figure 2: Stratified analysis of cytokines by sample type. Vaginal fluid cytokines were measured in either swabs or CVL, based on timing of participant enrollment (A). Stratified analysis by sample type did not reveal clustering by diagnosis in either swabs (B) (p = 0.54; permutational ANOVA), or CVL (C) (p = 0.91; permutational ANOVA)