Abstract
Convergent extension is a fundamental morphogenetic process that underlies not only the generation of the elongated vertebrate body plan from the initially radially symmetrical embryo, but also the specific shape changes characteristic of many individual tissues. These tissue shape changes are the result of specific cell behaviors, coordinated in time and space, and affected by the physical properties of the tissue. While mediolateral cell intercalation is the classic cellular mechanism for producing tissue convergence and extension, other cell behaviors can also provide similar tissue-scale distortions or can modulate the effects of mediolateral cell intercalation to sculpt a specific shape. Regulation of regional tissue morphogenesis through planar polarization of the variety of underlying cell behaviors is well-recognized, but as yet is not well understood at the molecular level. Here, we review recent advances in understanding the cellular basis for convergence and extension and its regulation.
Keywords: Gastrulation, Axial elongation, Morphogenesis, Convergent extension, Planar cell polarity, Mouse
1. Introduction
Convergent extension (CE) is a kinematic description of tissue shape change in which a tissue elongates (extension) along one axis and narrows (converges) in one or both of the orthogonal axes, and it constitutes one of a continuum of tissue shape changes that occur during development. Early embryos, in general, are spherical, ovoid, or somewhat flattened collections of cells, in which regional specification of tissue identities occurs. These identities specify particular differentiated tissue types, as well as a cascade of ever more detailed changes in cell behavior, mechanical properties and force-generating mechanisms that result in tissue and embryo shape changes. We discuss here how the combination of these behaviors and properties drive CE in the mouse embryo.
2. Convergent extension
In principle, a number of cellular mechanisms can generate the tissue shape changes of CE and related morphogenetic movements. Polarized cell shape changes can coordinately narrow and lengthen a tissue (Figure 1B), as, for example during CE of the chick forebrain and C. elegans hypodermis, as well as in a region of the Drosophila leg imaginal disc during its evagination [1–4]. Polarized cell division can selectively lengthen a tissue, as seen during the formation of kidney tubules in the mouse embryo (Figure 1B) [5]. In multilayered systems, radial intercalation of cells can also generate tissue extension. Radial intercalation is movement of cells between one another along the axis normal to the plane of a multilayered tissue, thereby reducing the number of cell layers (thinning) and expanding its area (spreading or epiboly), a process first described in detail during the epiboly of the animal cap of Xenopus [6,7]. A biased form of radial intercalation occurs when cells preferentially intercalate, for example, at anterior-posterior (A/P) junctions between two cells as opposed to mediolateral junctions, resulting in extension along the A/P axis as opposed to uniform epiboly (Figure 1B). One of the most ubiquitous mechanisms, however, is polarized cell intercalation, which has been found to contribute to CE of mesenchymal tissues in frogs [8], zebrafish [9], and mouse [10] among others, as well as to CE of epithelial tissues, such as the Drosophila imaginal leg disc [11,12], the epithelial marginal zone of the frog [13], germ band elongation in Drosophila [14–16], and in the invaginating and elongating echinoderm gut [17,18], to name just a few of many examples [19–21].
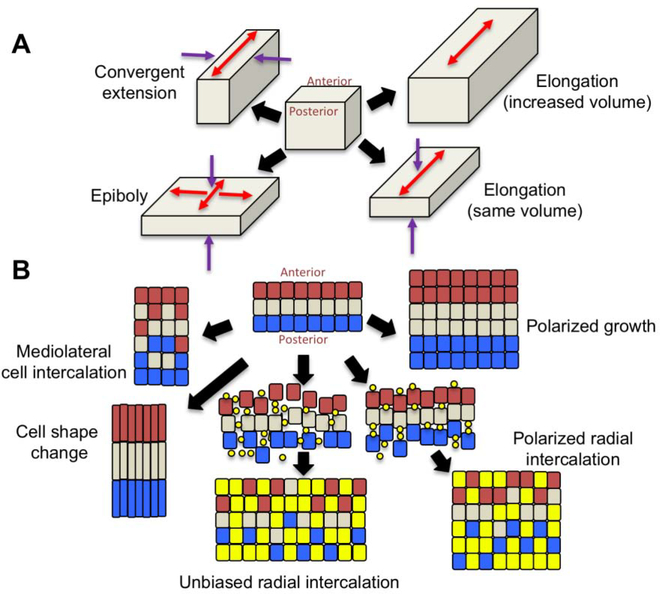
Figure 1. Tissue shape changes.
A. Ways in which a tissue can change shape. Convergent extension makes the tissue longer and narrower, epiboly makes it wider and flatter, and elongation makes the tissue longer without changing the width, and can either occur with a change in tissue volume or without. Arrows indicated the direction of tissue shape change (red = expansion; purple = narrowing or thinning). B. Cellular behaviors that can lead to the tissue shape changes shown in A. Convergent extension can be accomplished by polarized cell intercalation or by polarized cell shape change. Radial intercalation occurs when cells from an underlying layer insert themselves into the upper layer. In this example, yellow dots are cells starting to insert from underneath, and become the yellow squares as they join the overlying layer. Radial intercalation can be either unbiased, expanding the original cell layer in both anterior-posterior and mediolateral dimensions (epiboly), or polarized, expanding the tissue in only one dimension (elongation), and in both cases the tissue thins. Polarized cell division when coupled with cell growth leads to elongation with an increase in tissue volume.
2.1. General mechanisms of mesenchymal convergent extension
Mesenchymal (non-epithelial) cells provided the initial paradigm for the cellular mechanisms of CE [8,22,23]. Mosaic labeling [24] and live imaging [25] of mesodermal cells in the involuting marginal zone of Xenopus embryos demonstrated that the cells intercalate mediolaterally (perpendicular to the A/P axis of the embryo) between their neighbors to narrow (converge) the tissue in the mediolateral dimension and concomitantly lengthen (extend) it in the A/P dimension (Figure 2A) [19]. Subsequent work showed that mesodermal cell intercalation involves bipolarization and mediolateral orientation of cell protrusive activity driven internally by the actomyosin cytoskeleton, allowing cells to attach and exert traction on adjacent cells (Figure 2A) [8,26–29]. A similar process of bipolarization of cell protrusive activity giving rise to mediolateral cell intercalation has since been observed in many other systems, including the notochord of the zebrafish [30] and the presomitic mesoderm of the mouse embryo [20,31].
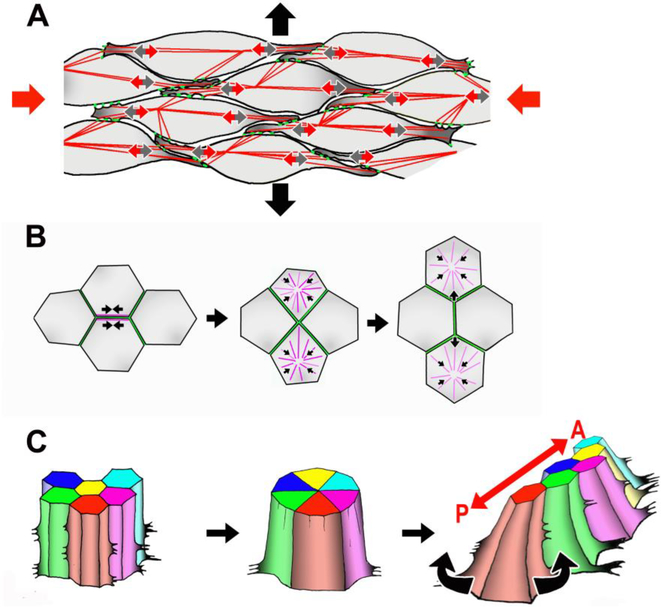
Figure 2. Cellular behaviors underlying CE.
A. Mesenchymal cells extend mediolateral lamellipodial protrusions (small grey arrows), form attachments to neighboring cells (green dots), and contraction of the actomyosin cytoskeleton (red lines) exerts traction (small red arrows) to move the cell between its neighbors. In the illustrated case, this process provides a convergence force along the mediolateral axis (large red arrows) to drive extension along the A/P axis (large black arrows). B. T1 process of epithelial cell rearrangement. Actomyosin contraction along a boundary between two cells brings four cells together at a common vertex, and apical myosin contraction elongates a new boundary between the cells not formerly in contact. C. Rosette formation involves contraction of multiple boundaries to form a common vertex between 5–10 cells, followed by the formation of new boundaries along the opposite axis to create a longer, narrower array of cells. As pictured here, the basal ends of mouse neural cells are motile, and biased protrusive activity likely drives the polarity of the rearrangement [111].
A different mechanism of mesenchymal CE has been observed in the ventrally located mesodermal cells of the zebrafish embryo, which show a directed migration from ventral to dorsal. As a group, the cells fan out such that some cells moving towards the anterior end and some towards the posterior end of the dorsal midline, which provides for simultaneous convergence and extension of the axis [32,33]. At the midline, the dorsally located, axial (notochordal) mesoderm undergoes mediolateral cell intercalation to further elongate and narrow the axis, similar to the behaviors seen in frog and mouse [30,34,35]. Interestingly, the cells of the deep lateral hindbrain and spinal cord regions of the Xenopus neural plate exhibit a similar dorsally directed motility toward the midline notoplate [36,37].
2.2. General mechanisms of epithelial convergent extension
Despite the presence of functional tight (or septate) and adherens junctions, epithelial cells rearrange by multiple mechanisms. Early studies documented epithelial cell rearrangement during evagination of the leg and wing discs of Drosophila [11,12] as well as in the epithelia of frogs [13] and killifish [38], and in the archenteron of the sea urchin [17,18]. Subsequent live imaging studies of Drosophila germ band elongation and wing development identified patterns of epithelial cell intercalation involving apical junctional rearrangement between pairs of cells (T1 process, Figure 2B [39]) or groups of 5–10 cells (rosette formation, Figure 2C) [15,16,40] (reviewed in [41]). Polarization of actomyosin contractility along cell boundaries perpendicular to the axis of extension leads to formation of a common vertex between all of the involved cells [16,42–44]. This is followed by elongation of a new boundary or boundaries along the axis of extension by active contraction of medial actomyosin in the neighboring cells [45,46]. Polarized boundary shortening thus creates narrower, longer arrays as pairs, or as groups by exchange of neighbors (Fig 2). More recently, an additional model has been proposed in which asymmetric radial force across the apex of a cell leads to movement of tricellular vertices to cause cell rearrangement [47].
Interestingly, a similar form of intercalation has also been observed in notochordal (axial mesoderm) cells during Xenopus gastrulation [48]. In this case, pulsatile actomyosin contraction in the mesoderm causes the shrinkage of anterior and posterior cell junctions similar to what is seen in the Drosophila germband [48,49] reviewed in [50]. Given the differences in continuity of intercellular junctions between mesodermal and epithelial cells, it will be very interesting to compare the underlying molecular mechanisms for this behavior in epithelial and mesenchymal cells.
A different mode of epithelial cell rearrangement is seen during CE of both the C. elegans epidermis and the ascidian notochord, where cells project basolateral protrusions to crawl between neighboring cells followed by expansion of remainder of the cell into the protrusion and formation of new junctions apically [51,52]. Thus, epithelial cell intercalation can be driven by either polarized apical junctional rearrangement or by polarized basolateral protrusive activity, or, as we discuss below (Section 3.1.2), using a combination of both apical and basolateral mechanisms.
Extension by biased radial intercalation occurs in the dorsal mesoderm of the frog and fish [25,53], and may also drive rapid CE of the neural tube in Xenopus, as radial intercalation of the multilayered neural plate results in a thinner, one-layered neural tube of smaller diameter and much greater length [54]. Radial intercalation also drives CE of the gut tube in Xenopus [55] and epiboly of the zebrafish [56], and in mouse paraxial mesoderm [10].
Finally, in addition to polarized cell intercalation, polarized epithelial cell shape change has also been implicated in CE. Cell shape changes driving CE have been observed during leg imaginal disc elongation [2], during elongation of the C. elegans embryo [3,4], and in the overall elongation of the germ band in Drosophila [57].
2.3. Molecular regulation of convergent extension: Planar Cell Polarity
The discovery that components of the starry night/frizzled (stan/fz) signaling pathway, which specifies polarity of cells across the apical plane of the wing and eye tissue in Drosophila (Fig. 3) [58,59], were also involved in CE in vertebrates [60–63] provided a way to understand how cell intercalation could be patterned. In the Drosophila wing and in the abdomen, the stan/fz pathway specifies proximal-distal polarization of cells to form a single actin-rich hair at the distal end of the apex of each cell, and coordinates this polarity between adjacent cells [58,59]. The stan/fz pathway also affects differentiation of the ommatidia of the eye in Drosophila through activation of Notch/Delta signaling [64–67].
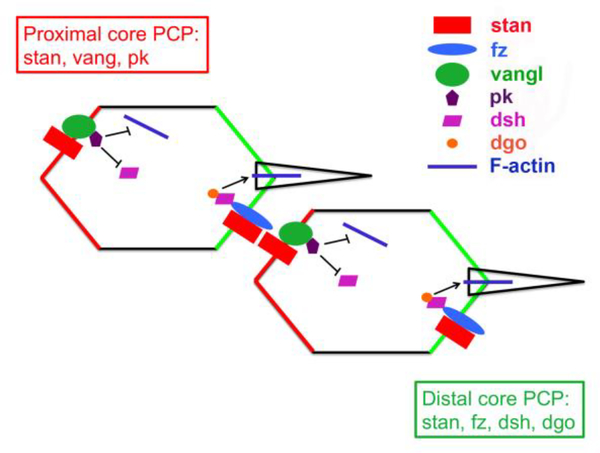
Figure 3. stan/fz planar cell polarity signaling pathway in Drosophila.
Polarized distribution of core planar polarity proteins within epithelial cells.
The core components of the stan/fz signaling pathway include the 7-pass transmembrane protein frizzled (fz), the 4-pass transmembrane protein van gogh/strabismus (vang), cytoplasmic signaling proteins (dishevelled (dsh), prickle (pk), and diego (dgo)) that act downstream of fz and vang, and starry night/flamingo (stan), an atypical cadherin [58]. A global polarity cue leads to biased localization of vang/pk and fz/dsh/dgo protein complexes to the proximal and distal borders of the cell, respectively. Stan localizes both proximally and distally within the cell, and forms homophilic adhesive intercellular bridges linking the two complexes between adjacent cells. These localizations are reinforced by antagonistic intracellular interactions, and by direct binding of vang and fz in adjacent cells [63,68].
The homologous non-canonical Wnt/PCP pathway was subsequently identified in vertebrates based on its similar function in planar polarization of cell structure and behavior (reviewed in [61]). The Wnt/PCP pathway is comprised of orthologues of Drosophila core proteins: frizzled (Fz 1–10) receptors, van gogh (Vangl1 and 2), starry night (Celsr1–3), dishevelled (Dvl1–3), prickle (Pk1, 2, Testin), and diego (Ankrd6) (reviewed in [61]). In addition, Wnt5a and Wnt11 ligands for Fz have been identified that affect planar polarity in vertebrates [69].
Initial insights came from perturbations of Wnt/PCP function in frogs [70,71] and in fish [72], which led to failure of CE of both the presumptive mesoderm and the neural plate. Wnt/PCP function in mammalian development was established when mutations of Vangl, Fz, Dvl, or Celsr genes were found to result in craniorachischisis and short body axis, implicating their function in CE [73–78]. Mammalian Wnt/PCP genes were also found to control the polarity and organization of the hair cells of the inner ear [76,79]. Here the homology of function between the stan/fz and Wnt/PCP pathways is much clearer, as the stereociliary bundles are polarized to the lateral edge of the hair cell apices, and this polarity is coordinated between all of the hair cells across the tissue (reviewed in [80,81]). Mutation of Wnt/PCP genes leads to disorganization of the polarity across the tissue [75,76,79,82] as well as reduction in the length of the cochlea [79,83] reviewed in [81]. Together, the phenotypes of craniorachischisis and inner ear polarity defects have become synonymous with PCP and CE in the mouse embryo, and have led to identification of other proteins that play a role in these morphogenetic processes in the mouse, namely protein tyrosine kinase 7 (Ptk7) and Scribble (Scrib) [79,84,85].
Human neural tube defects are generally thought to be due to complex genetic and environmental causes rather than single-gene mutations as seen in the mouse [86]. However, mutations in Wnt/PCP genes have been identified in human patients [86–90], and evidence of failure of CE has been observed in human embryos with craniorachischisis [91]. This underscores the fundamental importance of the Wnt/PCP system of planar cell polarity in promoting CE in vertebrates.
2.4. Alternative mechanisms promoting CE
While the Wnt/PCP and stan/fz signaling pathways have been intensively studied for their roles in specifying planar polarity and regulating CE, other signaling systems can induce planar polarity in epithelial tissues. For example, Drosophila germ band extension is not regulated by the stan/fz signaling system [43]. Instead, polarity at the tissue level is encoded through the AP patterning system [14,43]. Polarized cell rearrangement in this system requires the function of 3 different Toll-family receptors [92], as well as interactions between the LRR protein Tartan and the teneurin protein Ten-m to define the compartment boundaries [93]. Elongation of tubular structures in Drosophila, such as the hindgut and Malpighian tubules, requires cell rearrangement based on polarity derived from Jak-Stat signaling or EGF signaling, respectively, without involvement of stan/fz proteins [94,95].
2.5. Roles for extrinsic mechanical force during CE
CE can be either an active process, as described above, or a passive response to external forces. For example, artificial stretching of Xenopus ectodermal explants was found to induce radial and mediolateral intercalation of cells [96]. During tracheal branch elongation in Drosophila, migratory tip cells exert tension on the adjacent stalk cells, which respond by undergoing rearrangement [97]. Similarly, cell rearrangement during the late phase of sea urchin archenteron elongation is a response to axial tension exerted by the secondary mesenchyme cells [98]. Mouse notochord CE is at least in part a passive response to fluid pressure within the amniotic cavity [99], as discussed below (Section 3.1.4.).
Tissue shape changes can also result from a combination of active and passive events, as shown in both germ band extension and in wing morphogenesis in Drosophila. An A/P force generated by endoderm invagination during posterior midgut invagination (PMI) both induces cell shape changes and promotes germ band extension [45,46,100]. In the absence of PMI, cell rearrangement is not sufficient to elongate the germ band and instead causes compression of the tissue and changes to the axis of formation of new junctions [45,46]. Similarly, the Drosophila wing assumes its final shape through a constantly evolving summation of active and passive cell rearrangement and cell shape changes in response to the proximo-distally oriented force generated by wing-hinge contraction, and the countering resistance of cell adhesion to extracellular matrix molecules [101,102], reviewed in [103]. These results show that extrinsic mechanical forces can influence an active intercalation process to orient it along a particular axis and to augment its effects on tissue shape change.
3. Convergent Extension in Mammalian Development
In the mouse, CE has been most extensively studied in the early stages of development, namely gastrulation and axial elongation, as these are fundamental events that shape the overall body axis of the embryo. However, there are many other developmental events where CE also plays a role in producing the ultimate form of the tissue, and where the constraints of the particular tissue lead to the use of different cellular mechanisms to accomplish CE. We will first review the role of CE in body axis elongation, and then consider two specialized cases where CE drives morphogenesis, namely cochlear duct elongation and epididymal elongation and coiling.
3.1. Elongation of the body axis
The migratory phase of gastrulation is followed by a period of extensive morphogenetic changes in embryonic shape. Bending of the epiblast and visceral endoderm inwards toward the amniotic cavity in the anterior region creates the headfolds and the foregut pocket [104], which is followed by A/P elongation of the body axis. This is a coordinated event involving CE of the paraxial (presomitic) mesoderm, the neural ectoderm, and the axial (notochordal) mesoderm.
3.1.1. CE of Paraxial mesoderm
Mesoderm cells exiting from the distal region of the fully extended primitive streak are fated to become somitic (paraxial) mesoderm [105,106]. These cells leave the primitive streak by way of monopolar, anteriorly directed migration, but as they move anteriorly past the posterior notochord (PNC), they begin to change shape, going from relatively round to quite elongate, and orient their long axis perpendicular to the midline of the embryo [10]. Their monopolar protrusive activity becomes multipolar, and then bipolar, with protrusive activity concentrated in the medial and lateral ends.
Initially, the bipolar protrusive activity is sequential (first medial and then lateral), which leads to a pattern of cell translocation from medial to lateral and back again [10]. This type of intercalation leads to a simple exchange of place and produces inefficient CE. Eventually, though, the bipolar protrusive activity becomes simultaneous at medial and lateral ends, effectively driving mediolateral intercalation and leading to significant CE [10]. Mediolateral intercalation behavior not only promotes elongation of the presomitic mesoderm but also thickening along the dorsal-ventral axis. This is due to cells undergoing convergent thickening (CT), where they intercalate in the radial dimension of the tissue as a result of the forces generated by convergence [107]. The combination of CE and CT transforms the presomitic mesoderm into a narrower, thicker, and longer tissue that is subsequently segmented into somitic blocks.
The key elements of mesodermal CE are highlighted by comparing the normal behavior to that seen in embryos carrying a mutation in the gene encoding Protein Tyrosine Kinase 7 (Ptk7; [10,84]). In Ptk7 mutant embryos the mesodermal cells fail to convert from monopolar to bipolar protrusive activity, and as a result do not undergo CE. It is important to note that in the absence of Ptk7 mesodermal cells do not lack polarity. As they exit the primitive streak, they exhibit normal, anteriorly directed protrusive activity. What fails is the conversion of this activity to the bipolar protrusive activity required for mediolateral cell intercalation. In the Ptk7 mutant embryo, the presomitic mesoderm is very wide in the mediolateral dimension and short in the A/P dimension as well as being thin in the dorso-ventral dimension [10]. The dorso-ventral difference is more than would be expected from a simple loss of CT, and analysis of the cell behavior reveals a cryptic process of radial intercalation that normally acts to restrain CT [10].
The nature of the signal inducing reorientation of protrusive activity and cell shape is currently unknown, however there are paradigms for how it may operate intracellularly. As the cell shape change precedes the repolarization of protrusive activity, signaling through Ptk7 may lead to changes in actin and/or microtubule organization. In Xenopus, initiation of mesodermal CE requires intact microtubules [108], which act to repress RhoA and activate Rac in specific subcellular regions through sequestration of RhoGEF H1 (XLfc) [109]. Thus, changes in the microtubule array in mouse presomitic mesoderm could change the patterns of RhoA and Rac signaling, leading to cell elongation. Alternatively, the changes in cell shape and protrusive activity may depend on non-muscle myosin II activity. Ptk7 normally activates Rho kinase resulting in phosphorylation and activation of myosin light chain kinase (MLCK) and actomyosin contractility [110], and loss of Ptk7 causes disruption of myosin organization in epithelial cells [110,111]. Non-muscle myosin II has been shown to bind to, and sequester, members of the Dbl family of Rac GEF, thereby reducing the levels of active Rac and Cdc42 and increasing the levels of active RhoA [112] (reviewed in [113]). Thus, signals from Ptk7 in nascent presomitic mesoderm may act to change the pattern of myosin organization and RhoA activity along the anterior and posterior margins of the cells, thereby restricting protrusive activity to the medial and lateral ends of the cells.
3.1.2. CE of Neural Ectoderm
The neural plate elongates in concert with the paraxial mesoderm, but is an epithelial tissue, thus substantially different cellular mechanisms are important for neural CE. Similar to the patterns of cell rearrangement seen during Drosophila germ band elongation [15,16], neural plate cells undergo apical junctional rearrangement through T1 process and rosette formation [111]. Cell proliferation also plays a major role in driving cell rearrangement [111], which is not a feature of CE during germ band elongation. The basolateral ends of neural plate cells are elongated along the mediolateral axis and exhibit vigorous, mediolaterally biased protrusive activity [111], which has recently been shown to be a feature of Drosophila germ band extension as well [114]. It still remains to be determined what specific roles are played by basolateral protrusive activity and apical junctional rearrangement in the overall process of epithelial cell intercalation. However, analysis of rosette resolution during neural CE [111] and germ band extension [114] shows that both basolateral protrusive activity and apical junctional rearrangement play active roles in driving cell intercalation.
3.1.2.1. Regulation of neural cell intercalation by cell division
Similarly to mouse neural CE, proliferation plays an important role in promoting cell rearrangements during chick gastrulation [115]. In both systems, cell proliferation has been found to promote cell rearrangement in two ways. The neural plate is a pseudo-stratified epithelium, and due to their junctional adhesions to neighboring cells, cells round up and move apically to divide. As the cell begins to undergo cytokinesis, the cells attached on either side of the cleavage furrow are brought inward, and can form new interactions with one another at the end of cytokinesis, separating the newly-formed daughter cells from each other (Figure 4B, D). In addition, the daughter cells can move between two neighboring cells, causing them to separate (Figure 4A, B).
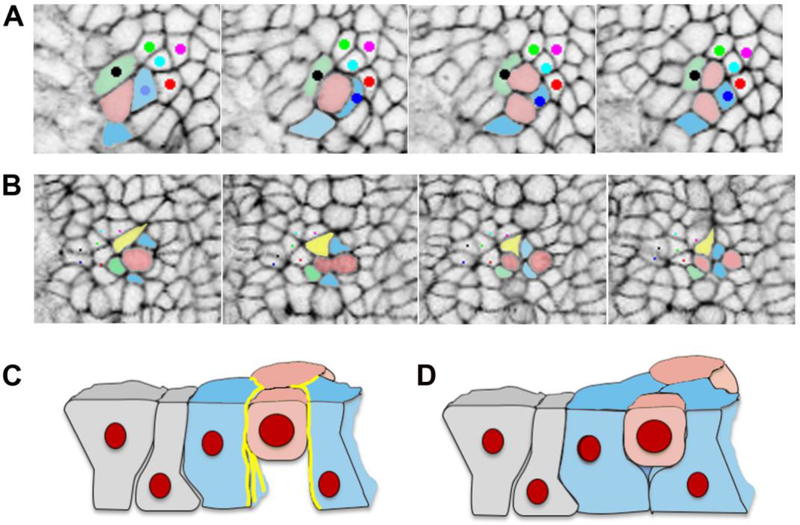
Figure 4. Cell division generating cell rearrangement in the mouse neural plate.
A. In this example, the pink cell divides, and the daughter cells stay in contact, while one pink daughter cell separates the blue and green cells. B. In this example the pink cell divides, and the two blue cells insert themselves between the two pink daughter cells, and one pink daughter cell separates the yellow and green cells. C and D. Schematics of the behavior of the basal ends of neighboring cells during cell division, based on published data for chick epiblast [115]. C. When actin and myosin localization (yellow lines) is strong and cadherin low at the lateral boundaries of cells neighboring a mitotic cell, these cells do not fill in the space basal to the mitotic cell to form new intercellular junctions, and as a result do not separate the daughter cells. D. When actin and myosin are not strongly localized at the lateral boundaries of cells neighboring a mitotic cell, these cells are able to fill in the space basal to the mitotic cell, forming new junctions and separating the daughter cells.
Cell rearrangement generated by cell division is developmentally regulated during chick gastrulation [115]. At stage X (Eyal-Giladi-Kochav staging, 0 hr of incubation), before gastrulation has begun, daughter cells do not separate at the end of cytokinesis, but remain in contact with one another (comparable mouse neural cell behavior is illustrated in Figure 4A). The result is a relatively static epithelium without much rearrangement. By stage 3 (Hamburger and Hamilton staging, 12–15 hr. of incubation), however, nearly 90% of mitoses result in rearrangement of cells, and the majority of cell rearrangements are due to separation of daughter cells during mitosis [115]. Inhibiting proliferation with aphidocoline or aminopterin reduces cell rearrangement, demonstrating that proliferation itself is a driving force for overall cell rearrangement in the chick epiblast.
The underlying molecular mechanism allowing cell division to promote cell rearrangement in the chick epiblast involves changes in E-cadherin adhesion and in the stability of the cortical acto-myosin cytoskeleton [115] (illustrated in Figure 4C, D). At stage X when cells round up for mitosis, localized actin and myosin at the lateral cortex of the neighboring cells stabilize the membrane and inhibit formation of new adhesive contacts, restricting the ability of the neighboring cells to move in between the daughter cells after cytokinesis has finished (Figure 4C). In contrast, by stage 3 the actomyosin in the cortex of the cells is more dynamic and is no longer concentrated in the basolateral regions of the cells. At this stage cells actively form new adhesions and move to fill the space opened up by the mitotic cell as it moves apically [115] (Figure 4D). Thus, dynamics of cadherins and actomyosin are critical to the morphogenetic outcome of division.
The molecular mechanisms for proliferation-generated cell rearrangement have not been studied in similar detail in the mouse neural plate, but available evidence suggests a similar process. Prospective neural cells of the mouse gastrula are quite proliferative, but undergo very little rearrangement [116]. Once epiblast cells have differentiated to neural ectoderm, however, cell division accounts for the majority of cell rearrangement in the elongating neural plate (40%), more than T1 process and rosette formation together (35%) [111]. On the basis of the paradigm provided by the chick epiblast, one would expect to see changes in actomyosin organization and dynamics as the neural ectoderm differentiates. In addition, one would predict that mutations altering cytoskeletal organization and adhesive function would affect the relative frequency of rearrangements generated by cell division.
3.1.2.2. Regulation of neural CE by PCP signaling
The Wnt/PCP signaling pathway plays a critical role in CE in the neural ectoderm. Indeed, one of the hallmarks of Wnt/PCP function is the phenotype of short and open spinal cord in embryos that lack the function of one or more of the core proteins [75–77,117,118]. Most of the effects of these mutations on neural CE have been evaluated at the tissue level by measuring axis length and width, but analysis of cellular behaviors in two mutants has provided insight into the cellular and molecular mechanisms regulated by Wnt/PCP signaling [111].
Polarity of intercalation is important, as seen in the Ptk7 mutant. In the absence of Ptk7 cells rearrange at a similar rate and with normal rates of protrusive activity, but do so randomly [111]. As a result, the neural tissue gets longer and wider by the same amount (due to proliferation and accretion), but does not undergo concerted narrowing and elongation. In addition, the normal medio-lateral elongation of neural cells at their basal ends is lost in the absence of Ptk7 [111]. Thus, Ptk7 has a similar effect on cell morphology and behavior in both neural and paraxial mesodermal cells, suggesting a fundamental role in regulating mediolateral intercalation.
In contrast, embryos homozygous for the semi-dominant loop-tail (Lp) mutation of Vangl2 show no defect in the polarity of either cell intercalation or protrusive activity. Instead, this mutation, a point mutation that leads to impaired transport of Vangl2 to the membrane [119,120], affects the frequency of apical junctional rearrangement [111]. Neural cells in these embryos rearrange only half as often as wild type, leading to a proportionate decrease in the degree of narrowing and elongation. Rosettes in these embryos often stall indefinitely once they have formed a common vertex and do not resolve. Importantly, basolateral protrusive activity in the Vangl2Lp mutant exhibits both normal polarity and normal frequency, and the pattern of rosette resolution is almost always from basal to apical, rather than equally in both directions [111]. This suggests that the function of Vangl2 is very important for apical junctional rearrangement but dispensable for the orientation of protrusive activity or the polarity of cell rearrangement, in contrast to Ptk7, which does not affect the rate of apical junctional rearrangement but is very important for its polarity. The lack of effect of Vangl2Lp on the polarity of cell behavior is unexpected given the clear effects on polarity that this mutation has in the inner ear [79], in lingual papillae [121], and in hair follicle development [122,123]. It may be that Vangl1 compensates to specify polarity in the presence of the Vangl2Lp mutation, or it may be that polarity of neural cell behavior is regulated differently than the intracellular polarity seen in the inner ear, tongue, and hair follicles.
These results demonstrate that the polarity and the frequency of cell intercalation are key elements regulating CE in the neural plate. Interestingly, the effect of the Ptk7 mutation on axial elongation is much more severe than the Vangl2Lp mutation. This may be because Ptk7 strongly affects CE in both mesoderm and neural ectoderm, while Vangl2 is not highly expressed in paraxial tissues [120] and the Vangl2Lp mutation has much less effect on the paraxial mesoderm cell behavior (W.W. Yen and A.E.S. unpublished observations).
Neither the Vangl2Lp mutant nor the Ptk7 mutant exhibit any changes in the type of rearrangement in the neural plate (i.e. T1, rosette formation, division, single cell intercalation). However, Ptk7 regulates the organization of non-muscle myosin II in the cortical cytoskeleton [110,111]. Normally myosin II is concentrated basolaterally in filaments at the anterior and posterior membranes of the cells, but in the absence of Ptk7 it appears as dynamic spots within the cytoplasm. This loss of organization may account for the loss of polarity of protrusive activity seen in the Ptk7 mutant, for the same reasons described above (Section 3.2.1) for the paraxial mesoderm. If myosin II normally acts in neural cells to promote bipolarity of protrusive activity through regulation of RhoA and Rac1 activity [112,113], loss of myosin concentrations at the anterior and posterior aspects of the cell would promote multipolar protrusive activity.
The function of Wnt/PCP signaling in the mouse neural plate has to date not been associated with polarized localization of Vangl2, Fz, or Dvl proteins within the cells [74] in contrast to the clear asymmetry shown in the frog neural plate [124]. This may be due simply to difficulty in visualizing asymmetries, but it may be that the asymmetry lies more in a polarized stability of the proteins at the membrane than in the overall amount localized [125]. How this pathway functions to polarize kinematic events such as cell rearrangement is not well understood, and is an important question for the future.
3.1.3. CE of Notochord
The notochord undergoes one of the best-studied CE events in mouse embryo development due to its accessibility during the early phases of its elongation. In the mouse the origins of the notochord are variously described, but the consensus derived from consideration of historical [126–130] and more contemporary descriptions [131–133] is that it comes from mesoderm that arises in the node at the rostral end of the primitive streak.
It extends anteriorly as a structure called the head process, initially moving along the axial midline between the epiblast and the visceral endoderm [128,130]. The cells of the head process then undergo a mesenchymal-to-epithelial transition at E7.5 –E7.75 to insert into the endodermal layer, where the now epithelial notochordal precursor cells coalesce to form the prechordal plate and the posterior notochord (PNC) [127,128,131,132]. The PNC is a teardrop shaped indentation on the distal tip of the embryo, which contains the notochordal precursor cells, and additionally functions in induction of left-right polarity through coordinated beating of the primary cilia on its cells [134]. The PNC has been variously referred to as the archenteron [127], the chordal plate [128], and the node [131,132,135], but by morphology and gene expression it is homologous to the gastrocoele roof plate of Xenopus, and to the posterior notochordal plate of the chick and rabbit [136]. Therefore, for purposes of accuracy we will refer to this structure as the PNC, and reserve the term “node” for the region immediately posterior to the PNC at the rostral end of the primitive streak, which by morphology and gene expression is homologous to Hensen’s node in the chick and rabbit [126,136].
The prechordal plate has important functions in specifying dorsal-ventral polarity and patterning of the cranial neural tube [137–139], but its cellular behaviors during cranial morphogenesis have not been well described, particularly with regard to CE. The notochordal precursors are located in the PNC [128,129,131,132,135,140]. Cells of the PNC contribute to the notochord underlying the early neural plate by addition to the caudal end, but some also migrate posteriorly, presumably to establish notochordal precursors for the later trunk and tail [132,141,142].
A number of studies have tracked notochord cells during the early somite period using fluorescent dyes or proteins [99,131–133,135,141]. Di-I labeling of the ventral layer of the PNC leads to dispersion of labeled cells throughout the notochord and prechordal plate, supporting a role for mediolateral intercalation as the mechanism of elongation of the notochord [131,135]. More recent studies have used tissue-specific nuclear-targeted EGFP to track cells, either manually [99] or by computational analysis of adaptive light sheet data [133], and both directly show notochordal cells undergoing mediolateral intercalation. The elongation of the notochord thus occurs both by accretion of cells at the caudal end and by mediolateral intercalation [99,133,143]. Interestingly, cell proliferation is relatively low in the notochord but is predominantly oriented along the rostro-caudal axis [133,143,144].
3.1.3.1. Regulation of Notochord CE by PCP Signaling
Notochord CE is clearly affected by the Wnt/PCP signaling pathway as mutations and knockouts of the core proteins Vangl2, Dvl2/3, Dvl1/2, or Ptk7 lead to a shorter, wider notochord [10,82,118,145,146]. Immunostaining of the PNC has also shown that the core proteins have polarized localization within the cells, and that PCP function in the PNC affects the subcellular localization of the primary cilium [146–149]. In embryos mutant for PCP genes Vangl2 or Dvl, or double heterozygotes for Vangl2 and cofilin (Cfl1), the primary cilia of the PNC cells are randomly localized relative to the rostro-caudal axis, while the normal pattern is for localization to the posterior pole of each cell [146–149]. The mislocalization is associated with failure of coordinated beating of these cilia, which is required for specifying the left-right axis of the embryo through asymmetric induction of Nodal expression [150].
Despite the wealth of studies demonstrating mediolateral intercalation during notochordal CE in the mouse, the cellular mechanisms of notochordal cell rearrangement have not been investigated. The notochord is an epithelial structure before it rounds up and detaches from the endodermal layer [151], and it has vigorous basolateral protrusive activity (W. Yen, M.L.K. Williams, and AES, unpublished observations) so notochordal cells may use a combination of apical junctional rearrangement and basolateral protrusive activity, similar to what is seen in the neural tube [111].
3.1.4. The role of extrinsic mechanical forces in axial elongation
There is evidence that notochordal cell rearrangement may not be an active force-producing process. Imuta et al. [99] showed that artificially reducing the fluid levels within the amniotic cavity affects notochord elongation, and results in a shortened axis overall. These observations raise interesting questions about the relative roles of active, force-producing activities and extrinsic mechanical forces in mouse axial and paraxial tissues. Fluid pressure in the amniotic cavity is clearly playing a role in facilitating notochord extension, and likely neural extension as well [99], however, it lacks any obvious directional bias that would preferentially promote A/P elongation.
While computational modeling that includes intercellular tension and elasticity supports an anisotropic effect of unbiased amniotic cavity expansion on notochord elongation [99], there may be an additional force promoting preferential elongation along the A/P axis. Head fold formation precedes the onset of axial elongation, and causes a strong invagination at the anterior end of the embryo [104]. The strain produced by the folding of both prospective neural and axial mesoderm may provide an orienting mechanical force along the A/P axis that facilitates mediolateral cell intercalation in both tissues, similar to the force provided by the PMI during Drosophila GBE [45]. Consistent with this, embryos lacking BMP2 have a shortened axis and do not undergo turning [152,153]. An interesting speculation is that the strain derived from head fold formation could also play a role in development of the ciliated cells of the PNC, similar to the effects of notochord elongation on the left-right organizer of the Xenopus embryo [154]. A more detailed examination of the process of head fold formation and its relationship to overall axial elongation is clearly called for.
While amniotic fluid pressure may play a relatively important role in facilitating cell rearrangements and tissue shape changes during the early phases of gastrulation and axial elongation, this may change as the neural tube begins to close. As shown by Brillouin microscopy, an optical technique for measuring the viscoelastic properties of tissue [155], the longitudinal modulus of the neural tube changes from E8.5 to E9.5, with the tissue becoming significantly stiffer as the neural folds close [156]. At this point the relative importance of force-producing cell intercalation behaviors in the neural tube may be increased, as well as interactions between the neural tube and the surface ectoderm to reduce mechanical stress at the point of closure [157,158]. A more thorough analysis of the material properties of embryonic tissues during this transition may provide some new insights.
3.1.5. Coordination of CE in axial and paraxial tissues
A compelling question that arises is how the morphogenesis of the three major tissues can be coordinated to produce efficient elongation. Given the general effect of reducing amniotic fluid pressure on overall axis elongation, it is possible that extrinsic mechanical forces act simultaneously on the axial and paraxial tissues to promote CE [99]. Signaling communication clearly plays a role as well, as shown by analysis of the Fgf3 mutant [159]. Loss of FGF3 leads to a shortened, kinked or curly tail, resulting from premature axis termination in the tailbud [159,160]. FGF3 is produced by the presomitic mesoderm but has its effect on the neural epithelium, where it negatively regulates BMP4 production. Neuroepithelial BMP4, in turn, promotes neural tube closure and negatively regulates the presomitic mesoderm [159]. While the major effect of these signaling pathways is seen during secondary neurulation in the tailbud, it is quite possible that they play a similar role in primary neurulation, or that there are analogous effects of other signaling pathways in the earlier stages of axial elongation.
3.2. CE during cochlear duct formation
The cochlear duct is a developing tissue that exhibits PCP-dependent CE during its morphogenesis. In addition, the highly structured organization of the cells of the organ of Corti provides a mammalian model for the cell autonomous function of the PCP pathway [81]. At the cellular level the mechanisms of cochlear duct CE provide a unique paradigm for how a variety of cell behaviors can combine to generate tissue level changes.
3.2.1. Outgrowth of the cochlear duct
The inner ear arises from the otocyst, a hollow epithelial structure formed from the otic placode, which invaginates from the surface of the embryo [161] (reviewed in [162]). Spatial specification of the future inner ear structures occurs by E10.5, and the cochlear duct is first seen as a ventral tubular evagination on E10.75, which elongates and then forms an abrupt anterior bend [161]. The anterior portion elongates and begins to coil, such that by E17.5 it has lengthened several-fold and has formed 1.75 coiled turns [161]. The duct consists of a sensory epithelium, the organ of Corti (OC), which extends the length of the duct, from base to apex, and non-sensory cells that make up the rest of the duct. The OC contains 4 rows of sensory hair cells, each of which has a polarized wedge-shaped array of microvilli on its apical surface, and is surrounded by support cells. At the cellular level the hair cells exhibit polarized organization of the microvilli into a staircase-like array within the stereociliary bundle, and the bundle has a polarized position at the abneural edge of the apex of the cell. Across the OC all of the hair cells display a coordinated polarity such that all the stereociliary bundles are oriented perpendicularly to the longitudinal axis of the duct (reviewed in [81,162]).
3.2.2. Mechanisms of CE
While the non-sensory cells of the cochlear duct proliferate throughout its elongation, the progenitor cells of the OC (PrC) become post-mitotic between E13 and E14 [163,164]. All of the subsequent elongation of this portion of the cochlear duct must thus occur through rearrangement. Analysis of cell packing, density, and organization, as well as live imaging of sparsely labeled explants provide a very detailed picture of the cellular mechanisms underlying cochlear duct CE [83,165–168]. The newly post-mitotic PrC are organized in a hexagonal array in a pseudo-stratified epithelium and the OC primordium is short and relatively thick. Differentiation of the PrC begins at the base of the duct and progresses to the apex over the next 4 days, concomitant with elongation of the duct. The progress of OC CE is exclusively from base to apex [83], which suggests that either there is an overall base-to-apex polarization of cell intercalation, or that the differentiation of the cells at the base provides a blocking activity that orients elongation towards the apex. Between E14 and E15 the OC primordium thins and extends due to radial intercalation and limited mediolateral intercalation, as well as directional migration of the PrC along the basement membrane toward the apex of the duct [83,168]. Interestingly, the OC epithelium also decreases in width during this process [169], implying that the radial intercalation is biased to promote extension along the longitudinal axis of the duct. Such biased radial intercalation promoting extension is also seen during CE of the zebrafish paraxial mesoderm [53] and during Xenopus gastrulation [170,171] and neurulation.
Cell rearrangement accounts for about 77% of OC CE before E15, but intercalation ceases at that point and changes in cell size and organization become much more important. In fact the greatest amount of elongation actually occurs between E15 and E17 [168], and is almost exclusively due to increasing cell size and collective cell migration as the PrC differentiate to form the hair cells and supporting cells [167,168].
3.2.3. Planar polarity regulates cochlear duct CE
The inner ear provides a comprehensive readout of the functions of mammalian PCP. As described above (3.2.1, 3.2.2), the formation of the cochlear duct displays CE as it elongates, and strongly organized polarity of the stereociliary bundles both within and between hair cells. The importance of PCP signaling to OC morphogenesis was recognized initially in mouse mutants of Vangl2, Celsr, and Scrib [76,79,85], followed by studies showing similar effects of mutation of Dvl1 and Dvl2 [82], Frz3/6 [75], and Ptk7 [84]. Embryos carrying mutations in these PCP genes exhibit changes in the tissue-level organization of the hair cells as well as decreased elongation and coiling of the cochlear duct [76,77,79,82–84].
The effects on CE of the cochlear duct have been best described for the Vangl2Lp and the double Dvl1/2 mutants, where the OC is wider and shorter by E18.5, and extra rows of hair cells have formed in the apex [83]. Interestingly, thinning of the OC is normal [83]. It is likely that mediolateral cell intercalation is affected in these PCP mutants, but given that radial intercalation appears to be the dominant behavior driving OC CE [168], it may be that they also affect the polarity of radial cell intercalation.
3.3. CE during epididymal morphogenesis
There are several mechanisms by which tubular organs can elongate, including oriented cell division, cell shape change, cell rearrangement, or cell recruitment [172,173]. The epididymis is a particularly compelling example of tubular morphogenesis, involving both elongation and coiling, and recent work demonstrates that cell rearrangement is a major mechanism promoting CE of this tissue.
3.3.1. Epididymal morphogenesis
The epididymis arises from the Wolffian duct on E14.5 of development in the mouse, as a straight, unbranched epithelial tube, surrounded by a connective tissue capsule derived from the mesonephric mesenchyme consisting of extracellular matrix and embedded fibroblasts (Figure 5) [174,175]. As the epithelium of the tube proliferates and elongates, the mechanical constraint of the surrounding capsule causes it to bend and then to buckle [175]. This continues through early postnatal development, until approximately one meter of unbranched epididymal duct is compacted into a one centimeter-long organ [173]. By E18.5, the coils begin to be separated into regional groups by connective tissue septa, and these regions are differentiated in function by the action of Hox proteins [173,176]. This astonishing degree of elongation, coiling, and differentiation is necessary for proper sperm maturation, as mutations that affect the ultimate length of the epididymis can lead to infertility [173,176].
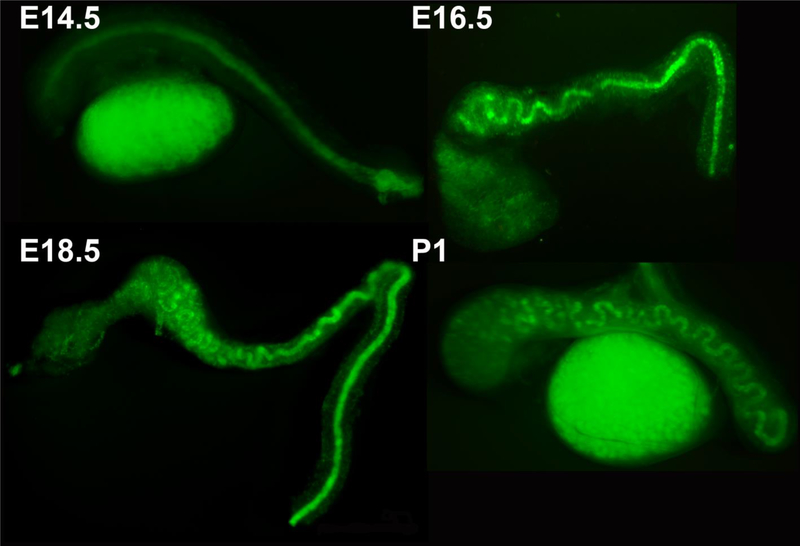
Figure 5. Initiation of coiling in the Wolffian duct.
Wolffian ducts collected from hoxb7 GFP transgenic mice at embryonic day (E)14.5, E16.5, E18.5, and postnatal (P) day 1 (P1). GFP is expressed in the epithelial cells of the ducts, allowing the process of duct coiling to be appreciated. Coiling proceeds from proximal to distal, and is initially two-dimensional at E16.5, and three-dimensional by E18.5. Republished with permission of John Wiley and Sons, Inc., from Hinton et al [174]; permission conveyed through Copyright Clearance Center, Inc.
3.3.2. Mechanisms of CE
Elongation of the epididymis begins with generalized proliferation of the epithelial cells. Analysis of the relatively straight E15.5 duct shows that cell division occurs throughout the circumference of the tube, and that the polarity of the division plane is random [175,177]. These observations rule out a role for oriented division in selectively promoting elongation, as well as a role for “hot spots” of division in driving coiling, and suggests that proliferation is mostly responsible for the overall increase in size of the duct throughout development. In addition, while there are modest overall changes in cell size over the period of elongation, there is no evidence of significant changes in cell shape that might promote elongation [177,178]. During the period from E15.5 to birth, the epididymal duct changes very little in diameter, strongly suggesting that cell rearrangements are important in the elongation process [178]. To date, difficulty in visualizing live cell behavior in the culture chamber required for epididymal explant culture has precluded a detailed direct analysis of the patterns of cell rearrangement. However, a detailed analysis of cell organization along the apical-basal axis provides evidence consistent with epithelial cell rearrangements like those seen in the neural tube, namely rosette formation, T1 process, and single-cell intercalation. Interestingly, the apical junctional boundaries change from apical to basal, consistent with a scutoid configuration accommodating the 3-dimensional structure of the tube [179,180].
Between E14.5 and E16.5, the combination of proliferation and CE drive extensive elongation of the duct, which becomes increasingly constrained by the connective tissue capsule in which it resides [175]. This leads to compressive forces on the tissue, which first cause the formation of S-shaped curves along the elongating axis [175–177], and eventually torsional buckling [175]. This coiling process begins at the rostral end and progresses to the caudal end by E18.5.
3.3.3. Regulation of epididymal CE
So far, two genes have been shown to affect the overall elongation and coiling of the duct, inhibin beta A (inhba; [181]) and Ptk7 [177]. inhba is highly expressed in the mesenchyme surrounding the Wolffian duct, and acts to promote growth and proliferation in the duct. Loss of inhba expression abolishes duct elongation, due to loss of proliferation. On the other hand, Ptk7 is expressed in both the epithelium and the mesenchyme, and affects both compartments. Loss of Ptk7 expression does not affect proliferation, but it does cause loss of polarity of cell rearrangement in the epithelium. As a result, the diameter of the duct is increased, and the number of cells per cross-sectional area is greater, and the elongation of the duct is significantly decreased. The lack of elongation in each of these mutants thus generates less compressive force within the capsule, leading to the loss of coiling. In both cases this affects the maturation of the sperm, which show little if any motility.
These results confirm the key roles that proliferation and cell rearrangement play in the tubular morphogenesis of the epididymis. It is only with adequate proliferation that sufficient numbers of cells are available for cell rearrangement to generate elongation, and only with rearrangements that are polarized along the length of the tube that a sufficiently long tube can be generated. The mechanical constraint of the surrounding connective tissue capsule produces the appropriate bending and buckling of the elongating duct required for packing it into a compact organ.
4. Conclusions
There are many, diverse roles for CE as the embryo undergoes morphogenesis. It is first exhibited during axis elongation, where CE is the major driver of tissue shape change, but numerous later events in organogenesis also employ this mechanism for achieving their final configuration. The best characterized cellular mechanism for CE is polarized cell intercalation, however, the variety of behaviors that lead to CE in other tissues demonstrates that the cellular mechanism cannot be assumed but must be determined by careful analysis at the cellular level. Our understanding of how CE is regulated during development is still in the early stages. While the Wnt/PCP pathway is clearly very important for providing positional information, we do not yet understand how it operates to polarize and promote the active cell behaviors involved. This is likely to entail understanding at the molecular level, including what is required for cells to make protrusions, exert traction, and regulate adhesion, but also how these are coordinated between cells and tissues. In addition, the intersection between active force-producing cell behavior and extrinsic forces in driving CE of the tissue is clearly an important area for future investigation. Evaluation of tissue biomechanics, how these can be affected by the cell behaviors of CE, and how they feed back on the process of intercalation will be critical to a full understanding of this important morphogenetic mechanism.
5. Acknowledgements
We thank current and former lab members and many collaborators, for their invaluable contributions to the research described and for helpful discussions. Our work on mouse axial morphogenesis was supported by grants from the National Science Foundation to AS (IOS 1051294), and from the National Institutes of Health to AS (1R01HD084181).
Abbreviations
- A/P
anterior-posterior
- CE
convergent extension
- CT
convergent thickening
- OC
Organ of Corti
- PCP
planar cell polarity
- PMI
posterior midgut invagination
- PNC
posterior notochord
- PrC
progenitor cells
- Wnt/PCP
Wingless-related integration site/ planar cell polarity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nishimura T, Honda H, Takeichi M, Planar cell polarity links axes of spatial dynamics in neural-tube closure, Cell. 149 (2012) 1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- [2].Condic ML, Fristrom D, Fristrom JW, Apical cell shape changes during Drosophila imaginal leg disc elongation: A novel morphogenetic mechanism, Development. 111 (1991) 23–33. [DOI] [PubMed] [Google Scholar]
- [3].Priess JR, Hirsh DI, Caenorhabditis elegans morphogenesis: The role of the cytoskeleton in elongation of the embryo, Dev. Biol 117 (1986) 156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- [4].Simske JS, Hardin J, Getting into shape: Epidermal morphogenesis in Caenorhabditis elegans embryos, BioEssays. 23 (2001) 12–23. doi:. [DOI] [PubMed] [Google Scholar]
- [5].Fischer E, Legue E, Doyen A, Nato F, Nicolas J-F, Torres V, Yaniv M, Pontoglio M, Defective planar cell polarity in polycystic kidney disease, Nat Genet. 38 (2006) 21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- [6].Keller RE, The cellular basis of epiboly: an SEM study of deep-cell rearrangement during gastrulation in Xenopus laevis, J Embryol Exp Morphol. 60 (1980) 201–234. [PubMed] [Google Scholar]
- [7].Keller RE, An experimental analysis of the role of bottle cells and the deep marginal zone in gastrulation of Xenopus laevis, J Exp Zool. 216 (1981) 81–101. [DOI] [PubMed] [Google Scholar]
- [8].Shih J, Keller R, Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis, Development. 116 (1992) 915–930. [DOI] [PubMed] [Google Scholar]
- [9].Glickman NS, Kimmel CB, Jones MA, Adams RJ, Shaping the zebrafish notochord, Development. 130 (2003) 873–887. [DOI] [PubMed] [Google Scholar]
- [10].Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A, PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation, Development. 136 (2009) 2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fristrom D, The mechanism of evagination of imaginal discs of Drosophila melanogaster,. III. Evidence for cell rearrangement, Dev. Biol 54 (1976) 163–171. doi: 10.1016/0012-1606(76)90296-7. [DOI] [PubMed] [Google Scholar]
- [12].Taylor J, Adler PN, Cell rearrangement and cell division during the tissue level morphogenesis of evaginating Drosophila imaginal discs, Dev. Biol 313 (2008) 739–751. doi: 10.1016/j.ydbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keller RE, Time lapse cinemicrographic analysis of superficial cell behavior during and prior to gastrulation in Xenopus laevis, J. Morphol 157 (1978) 223–247. doi: 10.1002/jmor.1051570209. [DOI] [PubMed] [Google Scholar]
- [14].Irvine KD, Wieschaus E, Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes., Development. 120 (1994) 827–41. [DOI] [PubMed] [Google Scholar]
- [15].Bertet C, Sulak L, Lecuit T, Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation, Nature. 429 (2004) 667–671. [DOI] [PubMed] [Google Scholar]
- [16].Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA, Multicellular rosette formation links planar cell polarity to tissue morphogenesis, Dev Cell. 11 (2006) 459–470. [DOI] [PubMed] [Google Scholar]
- [17].Ettensohn CA, Gastrulation in the sea urchin embryo is accompanied by the rearrangement of invaginating epithelial cells, Dev. Biol 112 (1985) 383–390. doi: 10.1016/0012-1606(85)90410-5. [DOI] [PubMed] [Google Scholar]
- [18].Hardin JD, Cheng LY, The mechanisms and mechanics of archenteron elongation during sea urchin gastrulation, Dev. Biol 115 (1986) 490–501. doi: 10.1016/0012-1606(86)90269-1. [DOI] [Google Scholar]
- [19].Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P, Mechanisms of convergence and extension by cell intercalation, Philos Trans R Soc L. B Biol Sci 355 (2000) 897–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keller R, Shaping the vertebrate body plan by polarized embryonic cell movements, Science (80-. ). 298 (2002) 1950–1954. [DOI] [PubMed] [Google Scholar]
- [21].Keller R, Mechanisms of elongation in embryogenesis, Development. 133 (2006) 2291–2302. [DOI] [PubMed] [Google Scholar]
- [22].Keller RE, The cellular basis of amphibian gastrulation, Dev Biol (N Y 1985). 2 (1986) 241–327. [DOI] [PubMed] [Google Scholar]
- [23].Wilson PA, Oster G, Keller R, Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants, Development. 105 (1989) 155–166. [DOI] [PubMed] [Google Scholar]
- [24].Keller R, Tibbetts P, Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis, Dev Biol. 131 (1989) 539–549. [DOI] [PubMed] [Google Scholar]
- [25].Wilson P, Keller R, Cell rearrangement during gastrulation of Xenopus: direct observation of cultured explants, Development. 112 (1991) 289–300. [DOI] [PubMed] [Google Scholar]
- [26].Shih J, Keller R, Cell motility driving mediolateral intercalation in explants of Xenopus laevis, Development. 116 (1992) 901–914. [DOI] [PubMed] [Google Scholar]
- [27].Pfister K, Shook DR, Chang C, Keller R, Skoglund P, Molecular model for force production and transmission during vertebrate gastrulation, Development. 143 (2016) 715–727. doi: 10.1242/dev.128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rolo A, Skoglund P, Keller R, Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB, Dev. Biol 327 (2009) 327–338. doi: 10.1016/j.ydbio.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R, Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network, Development. 135 (2008) 2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Myers DC, Sepich DS, Solnica-Krezel L, Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity?, Trends Genet. 18 (2002) 447–455. [DOI] [PubMed] [Google Scholar]
- [31].Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A, PTK7 is essential for polarized cell cell motility and convergent extension during mouse gastrulation, Development. (in press) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L, Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements, Nat Cell Biol. 4 (2002) 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sepich DS, Calmelet C, Kiskowski M, Solnica-Krezel L, Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation, Dev Dyn. 234 (2005) 279–292. [DOI] [PubMed] [Google Scholar]
- [34].Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L, Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension, Genesis. 27 (2000) 159–173. [DOI] [PubMed] [Google Scholar]
- [35].Myers DC, Sepich DS, Solnica-Krezel L, Bmp activity gradient regulates convergent extension during zebrafish gastrulation, Dev Biol. 243 (2002) 81–98. [DOI] [PubMed] [Google Scholar]
- [36].Elul T, Keller R, Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus, Dev Biol. 224 (2000) 3–19. [DOI] [PubMed] [Google Scholar]
- [37].Ezin AM, Skoglund P, Keller R, The midline (notochord and notoplate) patterns the cell motility underlying convergence and extension of the Xenopus neural plate, Dev Biol. 256 (2003) 100–114. [DOI] [PubMed] [Google Scholar]
- [38].Keller RE, Trinkaus JP, Rearrangement of enveloping layer cells without disruption of the epithelial permeability barrier as a factor in Fundulus epiboly, Dev Biol. 120 (1987) 12–24. [DOI] [PubMed] [Google Scholar]
- [39].Weairet D, Rivier N, Soap, cells and statistics-random patterns in two dimensions, Contemp. Phys 25 (1984) 59. doi: 10.1080/00107518408210979. [DOI] [Google Scholar]
- [40].Classen AK, Anderson KI, Marois E, Eaton S, Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway, Dev. Cell 9 (2005) 805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [41].Zallen JA, Blankenship JT, Multicellular dynamics during epithelial elongation, Semin Cell Dev Biol. 19 (2008) 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fernandez-Gonzalez R, de S, Simoes M, Röper JC, Eaton S, Zallen JA, Myosin II Dynamics Are Regulated by Tension in Intercalating Cells, Dev. Cell 17 (2009) 736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zallen JA, Wieschaus E, Patterned gene expression directs bipolar planar polarity in Drosophila, Dev Cell. 6 (2004) 343–355. [DOI] [PubMed] [Google Scholar]
- [44].Rauzi M, Verant P, Lecuit T, Lenne PF, Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis, Nat. Cell Biol 10 (2008) 1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- [45].Collinet C, Rauzi M, Lenne PF, Lecuit T, Local and tissue-scale forces drive oriented junction growth during tissue extension, Nat. Cell Biol 17 (2015) 1247–1258. doi: 10.1038/ncb3226. [DOI] [PubMed] [Google Scholar]
- [46].Yu JC, Fernandez-Gonzalez R, Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila, Elife. 5 (2016). doi: 10.7554/eLife.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vanderleest TE, Smits CM, Xie Y, Jewett CE, Blankenship JT, Loerke D, Vertex sliding drives intercalation by radial coupling of adhesion and actomyosin networks during drosophila germband extension, Elife. 7 (2018). doi: 10.7554/eLife.34586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shindo A, Wallingford JB, PCP and septins compartmentalize cortical actomyosin to direct collective cell movement, Science (80-. ). 343 (2014) 649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shindo A, Inoue Y, Kinoshita M, Wallingford JB, PCP-dependent transcellular regulation of actomyosin oscillation facilitates convergent extension of vertebrate tissue, Dev. Biol (2019). doi: 10.1016/j.ydbio.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huebner RJ, Wallingford JB, Coming to Consensus: A Unifying Model Emerges for Convergent Extension, Dev. Cell (2018). doi: 10.1016/j.devcel.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Williams-Masson EM, Heid PJ, Lavin CA, Hardin J, The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis, Dev Biol. 204 (1998) 263–276. [DOI] [PubMed] [Google Scholar]
- [52].Munro E, Odell G, Cell rearrangements during ascidian notochord formation, Development. 129 (2001) 1–12. [DOI] [PubMed] [Google Scholar]
- [53].Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L, Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation, J Cell Biol. 180 (2008) 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Davidson LA, Keller RE, Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension, Development. 126 (1999) 4547–4556. [DOI] [PubMed] [Google Scholar]
- [55].Dush MK, Nascone-Yoder NM, Vangl2 coordinates cell rearrangements during gut elongation, Dev. Dyn 248 (2019) 569–582. doi: 10.1002/dvdy.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Warga RM, Kimmel CB, Cell movements during epiboly and gastrulation in zebrafish., Development. 108 (1990) 569–80. [DOI] [PubMed] [Google Scholar]
- [57].Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B, Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension, Nat. Cell Biol 11 (2009) 859–864. doi: 10.1038/ncb1894. [DOI] [PubMed] [Google Scholar]
- [58].Adler PN, The frizzled/stan Pathway and Planar Cell Polarity in the Drosophila Wing, in: 2012: pp. 1–31. doi: 10.1016/b978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Strutt D, The planar polarity pathway, Curr. Biol 18 (2008) R898–R902. doi: 10.1016/j.cub.2008.07.055. [DOI] [PubMed] [Google Scholar]
- [60].Tada M, Concha ML, Heisenberg CP, Non-canonical Wnt signalling and regulation of gastrulation movements, Semin. Cell Dev. Biol 13 (2002) 251–260. doi: 10.1016/S1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- [61].Butler MT, Wallingford JB, Planar cell polarity in development and disease, Nat. Rev. Mol. Cell Biol 18 (2017) 375–388. doi: 10.1038/nrm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Davey CF, Moens CB, Planar cell polarity in moving cells: think globally, act locally, Development. 144 (2017) 187–200. doi: 10.1242/dev.122804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Goodrich LV, Strutt D, Principles of planar polarity in animal development, Development. 138 (2011) 1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fanto M, Mlodzik M, Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye, Nature. 397 (1999) 523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- [65].Das G, Reynolds-Kenneally J, Mlodzik M, The atypical cadherin flamingo links frizzled and notch signaling in planar polarity establishment in the Drosophila eye, Dev. Cell 2 (2002) 655–666. doi: 10.1016/S1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- [66].Katanaev VL, Egger-Adam D, Tomlinson A, Antagonistic PCP Signaling Pathways in the developing Drosophila eye, Sci. Rep 8 (2018). doi: 10.1038/s41598-018-24053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jenny A, Planar cell polarity signaling in the drosophila eye, in: Curr. Top. Dev. Biol, 2010: pp. 189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Strutt H, Strutt D, Asymmetric localisation of planar polarity proteins: Mechanisms and consequences, Semin. Cell Dev. Biol 20 (2009) 957–963. doi: 10.1016/J.SEMCDB.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [69].Gao B, Wnt Regulation of Planar Cell Polarity (PCP), in: Curr. Top. Dev. Biol, 2012: pp. 263–295. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- [70].Tada M, Smith JC, Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway, Development. 127 (2000) 2227–2238. [DOI] [PubMed] [Google Scholar]
- [71].Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM, Dishevelled controls cell polarity during Xenopus gastrulation, Nature. 405 (2000) 81–85. [DOI] [PubMed] [Google Scholar]
- [72].Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW, Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation, Nature. 405 (2000) 76–81. [DOI] [PubMed] [Google Scholar]
- [73].Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A, Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure., Development. 129 (2002) 5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- [74].Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A, Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation, Development. 133 (2006) 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang Y, The Role of Frizzled3 and Frizzled6 in Neural Tube Closure and in the Planar Polarity of Inner-Ear Sensory Hair Cells, J. Neurosci 26 (2006) 2147–2156. doi: 10.1523/jneurosci.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN, Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse, Curr Biol. 13 (2003) 1129–1133. [DOI] [PubMed] [Google Scholar]
- [77].Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P, Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification, Hum Mol Genet. 10 (2001) 2593–2601. [DOI] [PubMed] [Google Scholar]
- [78].Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P, Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail, Nat Genet. 28 (2001) 251–255. [DOI] [PubMed] [Google Scholar]
- [79].Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW, Identification of Vangl2 and Scrb1 as planar polarity genes in mammals, Nature. 423 (2003) 173–177. [DOI] [PubMed] [Google Scholar]
- [80].Lu X, Sipe CW, Developmental regulation of planar cell polarity and hair-bundle morphogenesis in auditory hair cells: Lessons from human and mouse genetics, Wiley Interdiscip. Rev. Dev. Biol 5 (2016) 85–101. doi: 10.1002/wdev.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tarchini B, Lu X, New insights into regulation and function of planar polarity in the inner ear, Neurosci. Lett 709 (2019) 134373. doi: 10.1016/j.neulet.2019.134373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang J, Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation, Development. 133 (2006) 1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P, Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway, Nat. Genet 37 (2005) 980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M, PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates, Nature. 430 (2004) 93–98. [DOI] [PubMed] [Google Scholar]
- [85].Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ, Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation, Genomics. 78 (2001) 55–63. [DOI] [PubMed] [Google Scholar]
- [86].Juriloff D, Harris M, Insights into the Etiology of Mammalian Neural Tube Closure Defects from Developmental, Genetic and Evolutionary Studies, J. Dev. Biol 6 (2018) 22. doi: 10.3390/jdb6030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].De Marco P, Merello E, Rossi A, Piatelli G, Cama A, Kibar Z, Capra V, FZD6 is a novel gene for human neural tube defects, Hum. Mutat 33 (2012) 384–390. doi: 10.1002/humu.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Allache R, De Marco P, Merello E, Capra V, Kibar Z, Role of the planar cell polarity gene CELSR1 in neural tube defects and caudal agenesis, Birth Defects Res. Part A - Clin. Mol. Teratol 94 (2012) 176–181. doi: 10.1002/bdra.23002. [DOI] [PubMed] [Google Scholar]
- [89].Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P, Mutations in VANGL1 associated with neural-tube defects, N Engl J Med. 356 (2007) 1432–1437. [DOI] [PubMed] [Google Scholar]
- [90].De Marco P, Merello E, Consales A, Piatelli G, Cama A, Kibar Z, Capra V, Genetic Analysis of Disheveled 2 and Disheveled 3 in Human Neural Tube Defects, J. Mol. Neurosci 49 (2013) 582–588. doi: 10.1007/s12031-012-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kirillova I, Novikova I, Augé J, Audollent S, Esnault D, Encha-Razavi F, Lazjuk G, Attié-Bitach T, Vekemans M, Expression of the sonic hedgehog gene in human embryos with neural tube defects., Teratology. 61 (2000) 347–54. doi:. [DOI] [PubMed] [Google Scholar]
- [92].Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA, A positional Toll receptor code directs convergent extension in Drosophila, Nature. 515 (2014) 523–527. doi: 10.1038/nature13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Paré AC, Naik P, Shi J, Mirman Z, Palmquist KH, Zallen JA, An LRR Receptor-Teneurin System Directs Planar Polarity at Compartment Boundaries, Dev. Cell (2019). doi: 10.1016/j.devcel.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Johansen KA, Iwaki DD, Lengyel JA, Localized JAK/STAT signaling is required for oriented cell rearrangement in a tubular epithelium, Development. 130 (2003) 135–145. doi: 10.1242/dev.00202. [DOI] [PubMed] [Google Scholar]
- [95].Saxena A, Denholm B, Bunt S, Bischoff M, VijayRaghavan K, Skaer H, Epidermal Growth Factor Signalling Controls Myosin II Planar Polarity to Orchestrate Convergent Extension Movements during Drosophila Tubulogenesis, PLoS Biol. 12 (2014) e1002013. doi: 10.1371/journal.pbio.1002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].V Belousov L, Luchinskaia NN, Zaraĭskiĭ AG, [Tensotaxis--a collective movement of embryonic cells up along the gradients of mechanical tensions]., Ontogenez. 30 (n.d.) 220–8. [PubMed] [Google Scholar]
- [97].Caussinus E, Colombelli J, Affolter M, Tip-Cell Migration Controls Stalk-Cell Intercalation during Drosophila Tracheal Tube Elongation, Curr. Biol 18 (2008) 1727–1734. doi: 10.1016/j.cub.2008.10.062. [DOI] [PubMed] [Google Scholar]
- [98].Hardin J, Weliky M, Cell rearrangement induced by filopodial tension accounts for the late phase of convergent extension in the sea urchin archenteron, Mol. Biol. Cell 30 (2019) 1911–1919. doi: 10.1091/mbc.E19-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Imuta Y, Koyama H, Shi D, Eiraku M, Fujimori T, Sasaki H, Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos, Mech. Dev 132 (2014) 44–58. doi: 10.1016/j.mod.2014.01.004. [DOI] [PubMed] [Google Scholar]
- [100].Lye CM, Blanchard GB, Naylor HW, Muresan L, Huisken J, Adams RJ, Sanson B, Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila, PLoS Biol. 13 (2015) e1002292. doi: 10.1371/journal.pbio.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Etournay R, Popović M, Merkel M, Nandi A, Blasse C, Aigouy B, Brandl H, Myers G, Salbreux G, Jülicher F, Eaton S, Interplay of cell dynamics and epithelial tension during morphogenesis of the Drosophila pupal wing, Elife. 4 (2015) e07090. doi: 10.7554/eLife.07090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper JC, Jülicher F, Eaton S, Cell Flow Reorients the Axis of Planar Polarity in the Wing Epithelium of Drosophila, Cell. 142 (2010) 773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- [103].Jülicher F, Eaton S, Emergence of tissue shape changes from collective cell behaviours, Semin. Cell Dev. Biol 67 (2017) 103–112. doi: 10.1016/j.semcdb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- [104].Gavrilov S, Lacy E, Genetic dissection of ventral folding morphogenesis in mouse: embryonic visceral endoderm-supplied BMP2 positions head and heart., Curr. Opin. Genet. Dev 23 (2013) 461–9. doi: 10.1016/j.gde.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lawson KA, Pedersen RA, Clonal Analysis of Cell Fate During Gastrulation and Early Neurulation in the Mouse, in: 2007. doi: 10.1002/9780470514221.ch2. [DOI] [PubMed] [Google Scholar]
- [106].Smith JL, Gesteland KM, Schoenwolf GC, Prospective fate map of the mouse primitive streak at 7.5 days of gestation, Dev. Dyn (1994). doi: 10.1002/aja.1002010310. [DOI] [PubMed] [Google Scholar]
- [107].Shook DR, Kasprowicz EM, Davidson LA, Keller R, Large, long range tensile forces drive convergence during Xenopus blastopore closure and body axis elongation, Elife. 7 (2018) e26944. doi: 10.7554/eLife.26944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lane MC, Keller R, Microtubule disruption reveals that Spemann’s organizer is subdivided into two domains by the vegetal alignment zone, Development. 124 (1997) 895–906. [DOI] [PubMed] [Google Scholar]
- [109].Kwan KM, Kirschner MW, A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis, Development. 132 (2005) 4599–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Andreeva A, Lee J, Lohia M, Wu X, Macara IG, Lu X, PTK7-Src Signaling at Epithelial Cell Contacts Mediates Spatial Organization of Actomyosin and Planar Cell Polarity, Dev. Cell (2014). doi: 10.1016/j.devcel.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Williams M, Yen W, Lu X, Sutherland A, Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate, Dev Cell. 29 (2014) 34–46. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG, Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: A possible link to Rho family GTPases, J. Cell Biol 190 (2010) 663–674. doi: 10.1083/jcb.201003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wu SK, Priya R, Spatio-Temporal Regulation of RhoGTPases Signaling by Myosin II, Front. Cell Dev. Biol 7 (2019) 90. doi: 10.3389/fcell.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sun Z, Amourda C, Shagirov M, Hara Y, Saunders TE, Toyama Y, Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension, Nat. Cell Biol 19 (2017) 375–383. doi: 10.1038/ncb3497. [DOI] [PubMed] [Google Scholar]
- [115].Firmino J, Rocancourt D, Saadaoui M, Moreau C, Gros J, Cell Division Drives Epithelial Cell Rearrangements during Gastrulation in Chick, Dev. Cell 36 (2016) 249–261. doi: 10.1016/j.devcel.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A, Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population, Dev. Dyn 241 (2012) 270–283. doi: 10.1002/dvdy.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Copp AJ, Greene ND, Murdoch JN, Dishevelled: linking convergent extension with neural tube closure, Trends Neurosci. 26 (2003) 453–455. [DOI] [PubMed] [Google Scholar]
- [118].Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ, Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure, Development. 134 (2007) 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD, Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure, Nat. Cell Biol 12 (2010) 41–46. doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Torban E, Wang HJ, Patenaude AM, Riccomagno M, Daniels E, Epstein D, Gros P, Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation, Gene Expr Patterns. 7 (2007) 346–354. [DOI] [PubMed] [Google Scholar]
- [121].Wang Y, Williams J, Rattner A, Wu S, Bassuk AG, Goffinet AM, Nathans J, Patterning of papillae on the mouse tongue: A system for the quantitative assessment of planar cell polarity signaling, Dev. Biol 419 (2016) 298–310. doi: 10.1016/j.ydbio.2016.09.004. [DOI] [PubMed] [Google Scholar]
- [122].Devenport D, Fuchs E, Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles, Nat. Cell Biol 10 (2008) 1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chang H, Smallwood PM, Williams J, Nathans J, The spatio-temporal domains of Frizzled6 action in planar polarity control of hair follicle orientation, Dev. Biol 409 (2016) 181–193. doi: 10.1016/j.ydbio.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ossipova O, Kim K, Sokol SY, Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling, Biol. Open (2015). doi: 10.1242/bio.201511676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chien YH, Keller R, Kintner C, Shook DR, Mechanical strain determines the axis of planar polarity in ciliated epithelia, Curr. Biol 25 (2015) 2774–2784. doi: 10.1016/j.cub.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jolly J, Féréster-Tadié M, Récherches sur l’oeuf du rat et de la souris, Arch. Anat. Microsc. Morphol Exp 32 (1936) 323–390. [Google Scholar]
- [127].Theiler K, The House Mouse: Atlas of Embryonic Development., Springer Verlag, New York, 1989. [Google Scholar]
- [128].Jurand A, Some aspects of the development of the notochord in mouse embryos., J. Embryol. Exp. Morphol 32 (1974) 1–33. [PubMed] [Google Scholar]
- [129].Poelmann RE, The head-process and the formation of the definitive endoderm in the mouse embryo, Anat. Embryol. (Berl). (1981). doi: 10.1007/BF00318093. [DOI] [PubMed] [Google Scholar]
- [130].Tam PPL, Meier S, The establishment of a somitomeric pattern in the mesoderm of the gastrulating mouse embryo, Am. J. Anat 164 (1982) 209–225. [DOI] [PubMed] [Google Scholar]
- [131].Sulik K, Dehart DB, Inagaki T, Carson JL, Vrablic T, Gesteland K, Schoenwolf GC, Morphogenesis of the murine node and notochordal plate, Dev. Dyn 201 (1994) 260–278. doi: 10.1002/aja.1002010309. [DOI] [PubMed] [Google Scholar]
- [132].Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J, Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms, Dev Cell. 13 (2007) 884–896. [DOI] [PubMed] [Google Scholar]
- [133].McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ, In Toto Imaging and Reconstruction of Post-Implantation Mouse Development at the Single-Cell Level, Cell. 175 (2018) 859–876.e33. doi: 10.1016/j.cell.2018.09.031. [DOI] [PubMed] [Google Scholar]
- [134].Shinohara K, Hamada H, Cilia in left–right symmetry breaking, Cold Spring Harb. Perspect. Biol 9 (2017) a028282. doi: 10.1101/cshperspect.a028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Beddington RSP, Induction of a second neural axis by the mouse node, Development. 120 (1994) 613–620. [DOI] [PubMed] [Google Scholar]
- [136].Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, Van Straaten HWM, Viebahn C, Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo, Differentiation. 75 (2007) 133–146. doi: 10.1111/j.1432-0436.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- [137].Camus A, Davidson BP, Billiards S, Khoo P-L, Rivera-Pérez JA, Wakamiya M, Behringer RR, Tam PPL, The morphogenetic role of midline mesendoderm and ectoderm in the development of the forebrain and the midbrain of the mouse embryo., Development. 127 (2000) 1799–813. [DOI] [PubMed] [Google Scholar]
- [138].Rubenstein JLR, Shimamura K, Martinez S, Puelles L, REGIONALIZATION OF THE PROSENCEPHALIC NEURAL PLATE, Annu. Rev. Neurosci 21 (2002) 445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- [139].Bouwmeester T, Leyns L, Vertebrate head induction by anterior primitive endoderm, BioEssays. 19 (1997) 855–863. doi: 10.1002/bies.950191005. [DOI] [PubMed] [Google Scholar]
- [140].Sausedo RA, Schoenwolf GC, Quantitative analyses of cell behaviors underlying notochord formation and extension in mouse embryos, Anat Rec. 239 (1994) 103–112. [DOI] [PubMed] [Google Scholar]
- [141].Ukita K, Hirahara S, Oshima N, Imuta Y, Yoshimoto A, Jang C-W, Oginuma M, Saga Y, Behringer RR, Kondoh H, Sasaki H, Wnt signaling maintains the notochord fate for progenitor cells and supports the posterior extension of the notochord, Mech. Dev 126 (2009) 791–803. doi: 10.1016/J.MOD.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Imuta Y, Kiyonari H, Jang CW, Behringer RR, Sasaki H, Generation of knock-in mice that express nuclear enhanced green fluorescent protein and tamoxifen-inducible Cre recombinase in the notochord from Foxa2 and T loci, Genesis. 51 (2013) 210–218. doi: 10.1002/dvg.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sausedo RA, Schoenwolf GC, Quantitative analyses of cell behaviors underlying notochord formation and extension in mouse embryos, Anat. Rec 239 (1994) 103–112. doi: 10.1002/ar.1092390112. [DOI] [PubMed] [Google Scholar]
- [144].Bellomo D, Lander A, Harragan L, Brown NA, Cell proliferation in mammalian gastrulation: The ventral node and notochord are relatively quiescent, Dev. Dyn 205 (1996) 471–485. doi:. [DOI] [PubMed] [Google Scholar]
- [145].Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P, Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification, 2001. [DOI] [PubMed] [Google Scholar]
- [146].Mahaffey JP, Grego-Bessa J, Liem KF, Anderson KV, Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo, J. Cell Sci 140 (2013) 1262–1271. doi: 10.1242/jcs.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H, Planar polarization of node cells determines the rotational axis of node cilia, Nat. Cell Biol 12 (2010) 170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- [148].Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y, Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning, Nature. 466 (2010) 378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD, Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis, PLoS One. 5 (2010) e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N, Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein, Cell. 95 (1998) 829–837. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- [151].Balmer S, Nowotschin S, Hadjantonakis A-K, Notochord Morphogenesis in Mice: Current Understanding and Open Questions, Dev. Dyn 245 (2016) 547–55. doi: 10.1002/dvdy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Madabhushi M, Lacy E, Anterior visceral endoderm directs ventral morphogenesis and placement of head and heart via BMP2 expression, Dev. Cell 21 (2011) 907–919. doi: 10.1016/j.devcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zhang H, Bradley A, Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development., Development. 122 (1996) 2977–86. [DOI] [PubMed] [Google Scholar]
- [154].Chien YH, Srinivasan S, Keller R, Kintner C, Mechanical Strain Determines Cilia Length, Motility, and Planar Position in the Left-Right Organizer, Dev. Cell 45 (2018) 316–330.e4. doi: 10.1016/j.devcel.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Prevedel R, Ruocco G, Antonacci G, Biology C, Unit B, Molecular E, Unit DB, Molecular E, Unit N, Molecular E, Brillouin microscopy – an emerging tool for mechanobiology, Nat. Methods 16 (2019) 969–977. doi: 10.1038/s41592-019-0543-3. [DOI] [PubMed] [Google Scholar]
- [156].Zhang J, Raghunathan R, Rippy J, Wu C, Finnell RH, Larin KV, Scarcelli G, Tissue biomechanics during cranial neural tube closure measured by Brillouin microscopy and optical coherence tomography, Birth Defects Res. (2018) 1–8. doi: 10.1002/bdr2.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Nikolopoulou E, Galea GL, Rolo A, Greene NDE, Copp AJ, Neural tube closure: cellular, molecular and biomechanical mechanisms, Development. 144 (2017) 552–566. doi: 10.1242/dev.145904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Galea GL, Nychyk O, Mole MA, Moulding D, Savery D, Nikolopoulou E, Henderson DJ, Greene NDE, Copp AJ, Vangl2 disruption alters the biomechanics of late spinal neurulation leading to spina bifida in mouse embryos, Dis. Model. Mech 11 (2018) dmm.032219. doi: 10.1242/dmm.032219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Anderson MJ, Schimmang T, Lewandoski M, An FGF3-BMP Signaling Axis Regulates Caudal Neural Tube Closure, Neural Crest Specification and Anterior-Posterior Axis Extension, PLoS Genet. 12 (2016) e1006018. doi: 10.1371/journal.pgen.1006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Mansour SL, Targeted disruption of int 2 (fgf-3) causes developmental defects in the tail and inner ear, Mol. Reprod. Dev 39 (1994) 62–68. doi: 10.1002/mrd.1080390111. [DOI] [PubMed] [Google Scholar]
- [161].Morsli H, Choo D, Ryan A, Johnson R, Wu DK, Development of the Mouse Inner Ear and Origin of Its Sensory Organs, J. Neurosci 18 (1998) 3327–3335. doi: 10.1523/jneurosci.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kelly M, Chen P, Shaping the mammalian auditory sensory organ by the planar cell polarity pathway, Int. J. Dev. Biol 51 (2007) 535–547. doi: 10.1387/ijdb.072344mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ruben R, No Title, Acta Otolaryngol. Suppl 220 (1967) 1–44. [PubMed] [Google Scholar]
- [164].Chen P, Segil N, p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti., Development. 126 (1999) 1581–90. [DOI] [PubMed] [Google Scholar]
- [165].Chacon-Heszele MF, Ren D, Reynolds AB, Chi F, Chen P, Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin, Development. 139 (2012) 968–978. doi: 10.1242/dev.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chen P, Johnson JE, Zoghbi HY, Segil N, The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination, Development. 129 (2002) 2495–2505. [DOI] [PubMed] [Google Scholar]
- [167].McKenzie E, Krupin A, Kelley MW, Cellular Growth and Rearrangement during the Development of the Mammalian Organ of Corti, Dev. Dyn 229 (2004) 802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- [168].Driver EC, Northrop A, Kelley MW, Cell migration, intercalation and growth regulate mammalian cochlear extension, (2017). doi: 10.1242/dev.151761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yamamoto N, Okano T, Ma X, Adelstein RS, Kelley MW, Myosin II regulates extension, growth and patterning in the mammalian cochlear duct, Development. 136 (2009) 1977–1986. doi: 10.1242/dev.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ossipova O, Chu CW, Fillatre J, Brott BK, Itoh K, Sokol SY, The involvement of PCP proteins in radial cell intercalations during Xenopus embryonic development, Dev. Biol (2015). doi: 10.1016/j.ydbio.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wilson PA, Oster G, Keller R, Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants., Development. 105 (1989) 155–66. [DOI] [PubMed] [Google Scholar]
- [172].Andrew DJ, Ewald AJ, Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration, Dev. Biol 341 (2010) 34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R, Yang L, How do you get six meters of review epididymis inside a human scrotum?, J. Androl 32 (2011) 558–564. doi: 10.2164/jandrol.111.013029. [DOI] [PubMed] [Google Scholar]
- [174].Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R, Yang L, Review How Do You Get Six Meters of Epididymis Inside a Human Scrotum?, J Androl. 32 (2011) 558–564. doi: 10.2164/jandrol.111.013029. [DOI] [PubMed] [Google Scholar]
- [175].Hirashima T, Pattern formation of an epithelial tubule by mechanical instability during epididymal development, Cell Rep. 9 (2014) 866–873. doi: 10.1016/j.celrep.2014.09.041. [DOI] [PubMed] [Google Scholar]
- [176].Joseph A, Yao H, Hinton BT, Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns, Dev. Biol 325 (2009) 6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Xu B, Washington AM, Domeniconi RF, Ferreira Souza AC, Lu X, Sutherland A, Hinton BT, Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct, Dev. Biol 412 (2016) 219–233. doi: 10.1016/j.ydbio.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Dyche WJ, A comparative study of the differentiation and involution of the mullerian duct and wolffian duct in the male and female fetal mouse, J. Morphol 162 (1979) 175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- [179].Nelson CM, Epithelial Packing: Even the Best of Friends Must Part, Curr. Biol 28 (2018) R1197–R1200. doi: 10.1016/j.cub.2018.08.055. [DOI] [PubMed] [Google Scholar]
- [180].Gómez-Gálvez P, Vicente-Munuera P, Tagua A, Forja C, Castro AM, Letrán M, Valencia-Expósito A, Grima C, Bermúdez-Gallardo M, Serrano-Pérez-Higueras Ó, Cavodeassi F, Sotillos S, Martín-Bermudo MD, Márquez A, Buceta J, Escudero LM, Scutoids are a geometrical solution to three-dimensional packing of epithelia, Nat. Commun 9 (2018) 2960. doi: 10.1038/s41467-018-05376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Tomaszewski J, Joseph A, Archambeault D, Yao HH-C, Essential roles of inhibin beta A in mouse epididymal coiling, Proc. Natl. Acad. Sci 104 (2007) 11322–11327. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]