Abstract
BACKGROUND & PURPOSE
Perinatal hemorrhagic stroke in late preterm and term neonates is understudied. We describe 2-month and 2-year neurological outcomes in a prospective cohort.
METHODS
Neonates ≥36 weeks’ gestation with spontaneous hemorrhagic stroke (parenchymal and intraventricular) presenting at ≤28 days of age were enrolled between 03/2007 and 05/2015 at three tertiary pediatric centers. Hemorrhagic transformation of arterial ischemic stroke or cerebral sinovenous thrombosis was excluded. The Pediatric Stroke Outcome Measure (PSOM) assessed outcomes. Wilcoxon signed-rank tests evaluated change over time.
FINDINGS
Twenty-six neonates were included (median age: 1 day, IQR 0–16; median gestational age: 38.3 weeks, IQR 37.0–39.0). Hemorrhage was isolated intraventricular in 7 (27%), isolated intraparenchymal in 6 (23%), and a combination in 10 (39%). Three neonates (12%) died during hospitalization; one died later due to cardiac disease. Among 22 survivors, outcomes were assessed at a median of 2.1 months (IQR 1.7–3.3) in 96% and 1.9 years (IQR 1.3–2.0) in 73%. Median PSOM scores were 0.0 (IQR 0.0–1.0) and 0.25 (IQR 0.0–1.3), respectively. At 2 years, 45% of patients had no or non-impairing deficits (PSOM <1.0), 30% had mild (PSOM 1.0–2.0), and 5% had moderate deficits (PSOM 2.5–4.5). Over time, 31% worsened and 6% improved. Although total PSOM scores did not change significantly (p=0.08), language sub-scores worsened (p=0.009). No child developed epilepsy.
CONCLUSIONS
Perinatal hemorrhagic stroke survivors had favorable outcomes in early childhood; at 2 years moderate-severe deficits occurred in 5%. Language deficits may emerge over time, warranting close follow-up.
Keywords: perinatal hemorrhagic stroke, term newborn, outcomes, seizure
Introduction
Perinatal stroke is a common cause of lifelong neurologic morbidity in late preterm and term neonates, with an estimated overall incidence of 1 in 2200–2800 live births1,2. Recent data suggest that perinatal hemorrhagic stroke may be more common than previously thought, with reported incidences between 1 in 6000–9000 live births2,3. Although of comparable incidence to perinatal arterial ischemic stroke (1 in 2300–5000 live births), perinatal hemorrhagic stroke is understudied and comparatively little is understood about its risk factors, etiologies, and outcomes4. While IVH in premature neonates originates from a fragile germinal matrix, the mechanisms responsible for late preterm and term hemorrhagic strokes remain unclear. Over two thirds of cases are consistently described as idiopathic in cohort studies despite extensive evaluations1,2.
To date, studies have largely focused on elucidating clinical presentation and risk factors, which include fetal distress, post-maturity, congenital heart disease, and bleeding diatheses1,5. Recently, one population-based nested case-control study further identified lower maternal age, primiparity, prior spontaneous abortion, difficult transition, and small for gestational age as risk factors for idiopathic perinatal hemorrhagic stroke, suggesting a multifactorial etiology2. Although a few larger population-based cohort studies have been published1,2, outcomes after perinatal hemorrhagic stroke have mostly been described in small retrospective cohorts6–8. Additionally, the wide follow-up ranges within many studies (several months up to a couple decades for different individuals within the same cohort) makes interpretation of outcome data across development challenging. While some retrospective studies have reported poor outcomes in over 40%, one prospective study of perinatal hemorrhagic stroke noted that, despite the significant mortality rate (20%), only a minority of the cohort experienced moderate or severe deficits at a median of 1 year9.
Overall our understanding of perinatal hemorrhagic stroke outcomes and how neurological function changes over time remain limited, posing challenges to the study of novel interventions in this population as well as to effective counseling of parents and caregivers. The aims of this prospective multicenter cohort study were to describe early outcome trajectories of spontaneous perinatal hemorrhagic stroke and to identify the distribution of deficits across neurologic domains over time.
Materials & Methods
Case Identification
Neonates ≥36 weeks’ gestation at birth with spontaneous hemorrhagic stroke (intraparenchymal or intraventricular hemorrhage), presenting at ≤28 days of life between March 2007 and May 2015 were enrolled prospectively at three pediatric tertiary care centers (Monroe Carell Jr. Hospital at Vanderbilt, Children’s Hospital of Philadelphia, Johns Hopkins Children’s Center). All cases were confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). Exclusion criteria were known trauma, isolated subdural, epidural, or subarachnoid hemorrhage, and hemorrhagic transformation of arterial ischemic stroke, cerebral venous sinus thrombosis, or hypoxic ischemic encephalopathy. Identification of cases was aided by prior implementation of a stroke protocol at each institution and strong relationships between neurology, neonatology, and neurosurgery departments at all participating institutions. Families were approached for consent and enrollment during the initial hospitalization or at the infant’s first follow-up. Institutional Review Board Approval was obtained at each site.
Clinical Data
Data collected from the initial hospitalization included demographics, maternal and birth history, clinical presentation, laboratory studies, neuroimaging results, and interventions. Study pediatric neurologists reviewed original neuroimaging as well as reports to determine hemorrhage characteristics and etiology using all available clinical, neuroimaging, and surgical data, if applicable. Hemorrhage volume was estimated and reported as a percentage of total brain volume (TBV) as described previously10. Serial imaging and review at pediatric neurovascular conferences at each institution was performed at the discretion of the treating pediatric neurologist and used to refine final etiology classification. Given the observational nature of the study, diagnostic and treatment choices were made by the treatment team; however, a common approach for evaluation at initial presentation as well as during follow-up is described in the Supplement. De-identified data were compiled into a single data collection tool shared across institutions in Research Electronic Data Capture (REDCap)11.
Outcome Assessment
Outcomes were assessed at pediatric stroke clinic visits at 2 months and 2 years using the Pediatric Stroke Outcome Measure (PSOM), a standardized composite score of neurologic function developed for pediatric ischemic stroke previously used in both prospective and retrospective studies of perinatal and pediatric hemorrhagic stroke12,13. Five domains (right and left sensorimotor, expressive and receptive language, and cognitive/behavioral) are each assigned a score between 0 and 2 representing increasingly severe functional impairment. Domain scores are then added for a total score (range 0 [no deficit] to 10 [maximum deficit)])14. Outcome status was dichotomized as “good” if total PSOM score was <1.0, corresponding to either complete recovery or at most a mild deficit in a single domain not impacting function, or “poor” if total PSOM score was ≥1.0 or if the patient died. This dichotomization has been used by other groups studying perinatal hemorrhagic stroke15. Outcomes were also categorized at a more granular level to describe the level of neurologic function: no or non-impairing deficits (PSOM<1.0), mild deficits (PSOM 1.0–2.0), moderate deficits (PSOM 2.5–4.5), and severe deficits (PSOM 5.0–10.0, including death). Sub-scores were tracked to examine domain-specific deficits and their relative trajectories over time. For the purpose of analysis, right and left sensorimotor sub-scores and expressive and receptive language sub-scores were combined, ultimately leading to three sub-score measures: sensorimotor combined, language combined, and cognitive/behavioral. Outcomes were also assessed qualitatively via assessment of rehabilitative service use as well as a short parent survey regarding the child’s functional recovery15. Subgroup analyses were performed in children who died during the initial hospitalization and in a subset of children with longer follow-up (3.5–5.5 years post-hemorrhage).
Statistical Analyses
Descriptive statistics including frequencies with percentages and median with interquartile ranges (IQR) were used for categorical and continuous variables, respectively. Wilcoxon signed-rank tests were used to compare mean PSOM scores over time. Associations between possible predictors of poor outcome or of death were evaluated with exact logistic regression or Fisher exact tests. Unpaired student t-tests were used to compare means between neonates who died during the initial hospitalization and those who did not. All statistical analyses were conducted in STATA 15.0 (StataCorp College Station, TX, USA).
Results
Cohort Characteristics & Clinical Presentation
Twenty-six neonates met inclusion criteria, with a median age at presentation of 1 day (IQR 0.0–3.6) and estimated gestational age of 39.1 (IQR 38.3–40.0) weeks. Clinical presentation and outcomes of 14 of these 26 were previously reported at a median of 1 year9. Table 1 summarizes demographic, obstetric, birth history, known precipitating factors, and clinical presentation data. Seventeen neonates (65%) were male, and 23 (88%) were Caucasian. Obstetric history was available for only 18 cases due to hospital transfers or lack of prenatal care, with a notable proportion of pregnancies complicated by maternal hypertension (23%), tobacco use (27%), or recreational drug use (23%). Most deliveries were uncomplicated vaginal deliveries (18/26, 70%), with induction in half of these. Only a single case was an assisted delivery (1/26, 3.9%). Median 1- and 5-minute Apgar scores were known for 20 neonates and were 8 (IQR 7–8) and 9 (IQR 9–9), respectively. Perinatal distress was observed in 10 of the 22 cases in which this information was available (45%). Seizure was the most common presenting sign (58%), followed by apnea (38%), abnormal level of consciousness (27%), and abnormal tone (27%).
Table 1.
Summary of cohort demographics, birth and medical history, clinical presentation, and univariable associations with poor 2-year outcome (N=26)
| N (%) | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, days (median & IQR) | 1.0 (0.0 – 3.6) | 1.0 | 0.9 – 1.1 | 0.9 |
| EGA, weeks (median & IQR) | 39.1 (38.3 – 40.0) | 0.4 | 0.1 – 1.0 | 0.04* |
| Birth weight, kg (median & IQR) | 3.24 (3.03 – 3.56) | 0.06 | 0.002 – 0.9 | 0.04* |
| Male sex | 17 (65) | 1.3 | 0.1 – 14 | 1.0 |
| Race & Ethnicity | ||||
| Caucasian (reference) | 23 (88) | |||
| African-American | 3 (12) | 1.7 | 0.08 – 118 | 1.0 |
| Hispanic | 2 (7.7) | |||
| Obstetric and Birth History | ||||
| Maternal age (median & IQR) (n=18) | 29 (25 – 34) | 1.0 | 0.8 – 1.2 | 1.0 |
| Antenatal concerns (n=18) | ||||
| GDM | 1 (5.6) | |||
| Maternal HTN | 6 (33) | 0.8 | 0.4 – 16 | 1.0 |
| Maternal tobacco use | 7 (39) | 1.0 | 0 – 3.2 | 0.9 |
| Maternal drug use | 6 (33) | 1.0 | 0 – 3.2 | 0.9 |
| Maternal infection | 5 (28) | |||
| Abnormal bleeding | 1 (5.6) | 1.9 | 0.8 – 137 | 1.0 |
| Delivery history (n=22) | ||||
| PROM >24h | 2 (7.7) | 0.3 | 0 – 4.5 | 0.4 |
| Prolonged second stage | 2 (7.7) | |||
| Cord abnormalities | 9 (35) | 0.3 | 0.05 – 2.1 | 0.4 |
| Placental abnormalities | 1 (3.9) | |||
| Delivery method (n=26) | ||||
| Spontaneous/induced vaginal | 18 (69) | 0.9 | 0.09 – 7.8 | 1.0 |
| Assisted vaginal (vacuum) | 1 (3.9) | |||
| Unscheduled/urgent C/S | 7 (27) | |||
| Apgar (median & IQR) (n=20) | ||||
| 1 min | 8 (7 – 8) | |||
| 5 min | 9 (9 – 9) | |||
| Fetal distress (n=22) | 10 (45) | 1.6 | 0.2 – 17 | 1.0 |
| Medical History & Precipitating Factors | ||||
| CHD | 4 (15) | 2.8 | 0.2 – 177 | 0.7 |
| Bleeding disorder | 1 (3.9) | |||
| Renal failure | 1 (3.9) | |||
| Sepsis | 3 (12) | 0.5 | ||
| Coagulopathy | 9 (35) | 1.7 | 0.8 – 118 | 1.0 |
| Thrombocytopenia | 4 (15) | |||
| ECMO (heparin) | 1 (3.9) | |||
| Thrombolytic therapy | 1 (3.9) | |||
| Vitamin K refusal | 3 (12) | |||
| Surgery | 2 (7.7) | |||
| Clinical Presentation | ||||
| Apnea | 10 (38) | 0.8 | 0.01 – 24 | 1.0 |
| Difficulty feeding | 6 (23) | 2.1 | 1.0 – 157 | 1.0 |
| Abnormal tone/reflexes | 7 (27) | 8.0 | 0.4 – 588 | 0.2 |
| Altered mental status | 7 (27) | 6.1 | 0.5 – 354 | 0.2 |
| Seizure | 15 (58) | 1.2 | 0.1 – 11 | 1.0 |
p<0.05.
EGA – Estimated gestational age; SGA – Small for gestational age; LGA – Large for gestational age; AMA – Advanced maternal age; GDM – Gestational diabetes mellitus; HTN – Hypertension; PROM – Premature rupture of membranes; US – ultrasound; NSVD – normal spontaneous vaginal delivery C/S – Cesarean section; CHD – Congenital heart disease; ECMO – extracorporeal membrane oxygenation
Neuroimaging Data & Etiology
Table 2 summarizes neuroimaging data, treatment received, and hospital course. Hemorrhage was isolated intraventricular in 8 neonates (31%), isolated parenchymal in 6 (23%), a combination of parenchymal and intraventricular in 10 (39%), and a combination of parenchymal and subarachnoid in 2 (8%). Hemorrhage was supratentorial in nearly all cases (96%). Intraparenchymal hemorrhages were located in the thalamus (2), basal ganglia (5), cerebellum (1), and cerebral hemispheres (13; frontal lobe in 3, temporal lobe in 2, parietal lobe in 4, occipital in 4). Hemorrhage volume could be confidently calculated in 16 cases (89% of cases with intraparenchymal hemorrhage). Of these, hemorrhage volume was <2% of TBV in 11, 2–4% of TBV in 1, and ≥4% of TBV in 4. Six cases of IVH originated from the choroid plexus or germinal matrix. Vascular imaging was performed during the acute hospitalization in 21 cases (81%). Nine underwent both magnetic resonance angiography (MRA) and venography (MRV) (35%), 7 had MRA only (27%), 3 MRV only (12%), 1 had CT angiogram (4%), and 1 had conventional angiography (4%). No vascular lesions were identified via initial imaging; autopsy of one neonate revealed abnormal vessels in the territory of the parenchymal hemorrhage, possibly a diffuse telangiectasia or cavernoma, but definitive lesion characterization remained challenging. Follow-up vascular imaging was obtained in 8 survivors (36%) and was unrevealing in all except one who underwent conventional angiography due to persisting concern for a vascular lesion on MRI. Review in a multispecialty pediatric vascular conference confirmed no arterial vascular abnormalities and ultimately cavernoma was felt to be the cause of the hemorrhage.
Table 2.
Summary of hemorrhage characteristics, treatment, hospital course, outcomes, and univariable association with poor 2-year outcome (N=26)
| Hemorrhage Characteristics | N (%) | OR | 95% CI | p-value |
|---|---|---|---|---|
| Hemorrhage pattern | ||||
| Isolated IVH (1 IVH+SAH) | 8 (31) | |||
| Isolated ICH | 6 (23) | |||
| ICH + another compartment (IVH or SAH) | 12 (46) | |||
| Supratentorial | 25 (96) | |||
| Hemorrhage volume (n=14) | ||||
| <2% TBV | 11 (69) | |||
| 2–4% TBV | 1 (6.3) | |||
| ≥4% TBV | 4 (25) | 1.1 | 0.8 – 1.6 | 0.7 |
| Complicating findings | ||||
| Edema | 12 (46) | 0.5 | 0.05 – 3.8 | 0.7 |
| Hydrocephalus | 9 (35) | 3.9 | 0.4 – 56 | 0.3 |
| Herniation syndrome | 3 (11) | |||
| Etiology | ||||
| Cavernoma | 1 (3.9) | |||
| Coagulopathy | 9 (35) | 2.0 | 0.3 – 15 | 0.5 |
| Choroid plexus/germinal matrix bleed | 6 (23) | 0.3 | 0.04 – 2.1 | 0.2 |
| Idiopathic | 10 (42) | 1.1 | 0.2 – 7.3 | 0.9 |
| Treatment & Hospital Course | ||||
| Interventions | 9.6 | 0.9 – 105 | 0.06 | |
| Mannitol/hyperosmolar saline | 1 (3.9) | NA | ||
| AED | 16 (62) | 1.0 | ||
| Transfusion | 9 (35) | 8.5 | 0.7 – 495 | 0.1 |
| Hematoma evacuation | 2 (7.7) | |||
| Ventriculostomy | 1 (3.9) | |||
| Seizures during hospitalization | 14 (54) | 1.2 | 0.1 – 15 | 1.0 |
| Hospital LOS | 10.5 (7 – 18) | 1.0 | 1.0 – 1.1 | 0.3 |
| ICU LOS | 7 (6 – 13) | 1.0 | 0.9 – 1.2 | 0.5 |
| Outcomes | ||||
| PSOM (median and IQR) | ||||
| 2 month (n=22) | 0 (0 – 1) | |||
| 2 year (n=16) | 0.25 (0 – 1.25) | |||
| 4 year (n=7) | 1 (0 – 1.5) | |||
| Overall mortality | 4 (20) | |||
p<0.05.
IVH – intraventricular hemorrhage; SAH – subarachnoid hemorrhage; ICH – intracerebral hemorrhage; TBV – total brain volume; AED – Antiepileptic drug; LOS – length of stay; ICU – intensive care unit; PSOM – Pediatric Stroke Outcome Measure
Bleeding diatheses caused hemorrhage in 9 patients (35%). Three had hemorrhagic disease of the newborn due to vitamin K refusal, 1 had neonatal alloimmune thrombocytopenia, 2 had mild thrombocytopenia in the context of sepsis, one of which was complicated by a renal artery thrombus requiring thrombolytic therapy, 1 had isolated mild thrombocytopenia, 1 was anticoagulated in the setting of post-surgical extracorporeal membrane oxygenation, and 1 had von Willebrand Factor deficiency. Four neonates had congenital heart disease (15%), 2 of whom had recently undergone repair. Supplemental Table 1 summarizes the cardiac and anticoagulation history of these neonates. A clear etiology could not be determined in the remaining 10 cases (42%) despite careful imaging review. In 2 of the 5 newborns in whom vascular imaging was not obtained, imaging suggested a clear choroid plexus or germinal matrix hemorrhage. Hematologic abnormalities were present in 2 others (severe thrombocytopenia with platelets of 9,000 due to sepsis in one, INR of 1.7 and PTT 51 in the setting of vitamin K refusal in the other). Finally, etiology remained idiopathic in a newborn with unrepaired congenital heart disease in whom support was withdrawn following the development of cardiac failure superimposed on renal failure.
Treatment & Hospital Outcome
Acute life-threatening increased intracranial pressure requiring medical or surgical treatment occurred in 3 cases (12%). Sixteen patients were treated with antiseizure medications (62%), monotherapy in 14, 2 critically ill neonates required 5 medications. Monotherapy was with phenobarbital in 13 and levetiracetam in 1. Median hospital length of stay was 11 days (IQR 7–18); median length of intensive care unit was 7 days (IQR 6–13). Perinatal hemorrhagic stroke was identified as the primary cause of death in 1 neonate and contributed to the decision to withdraw care in 2 neonates with medical comorbidities. A fourth child with a history of congenital heart disease died prior to the 1-year follow-up visit due to underlying cardiac disease.
Neurologic Outcomes
Twenty-two of the 23 survivors (96%) were evaluated at pediatric stroke clinics at a median of 2.1 months (IQR 1.7–3.3). Sixteen of the 22 patients alive at 2 years (73%) were evaluated at a median of 1.9 years (IQR 1.3–2.0).
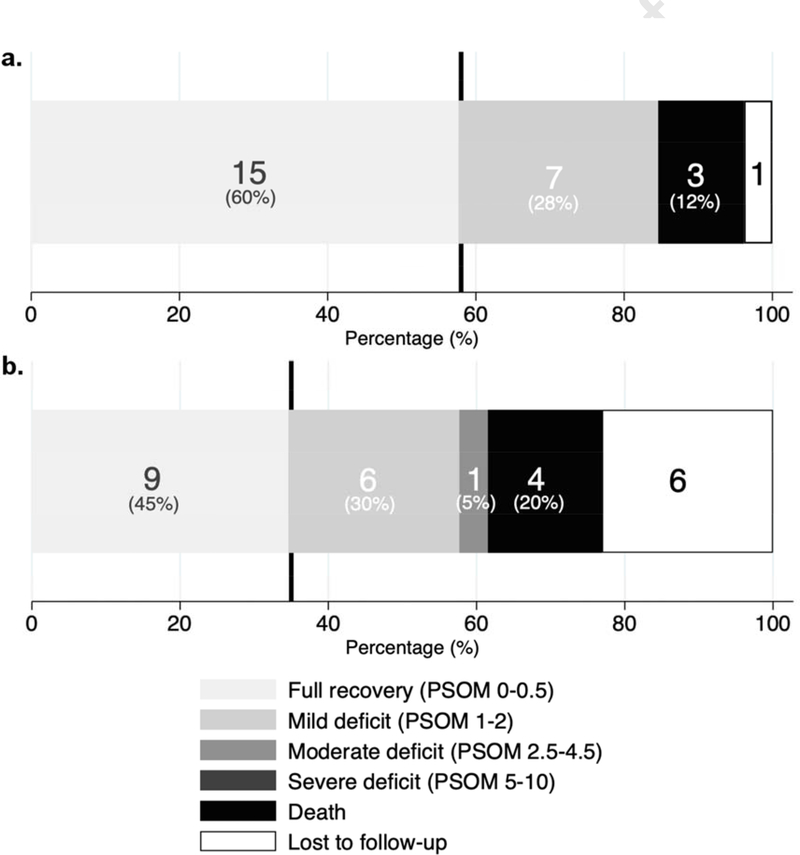
At 2-month follow-up, the median total PSOM was 0 (IQR 0.0–1.0) (Table 2). Neurologic outcome was poor (total PSOM ≥1.0) in 7 infants in addition to the 3 neonates who died during the initial hospitalization (10/25, 40%). Overall, 15 (60%) had no or non-impairing deficits (PSOM <1.0), 7 (28%) had mild deficits (PSOM 1.0–2.0), and no patient had moderate or severe deficits (PSOM 2.5–10). At 2-year follow-up, the median total PSOM among survivors was 0.25 (IQR 0.0–1.3). Neurologic outcome was poor (total PSOM ≥1.0) in 7 in addition to the 4 who died during hospitalization or in the follow-up period (11/20, 55%). Overall, 9 (45%) had no or non-impairing deficits (PSOM <1.0), 6 (30%) had mild deficits (PSOM 1.0–2.0), and 1 (5%) had moderate deficits (PSOM 2.5–4.5). Figure 1 illustrates the distribution of PSOM scores at 2 months and 2 years. In univariable analyses, poor outcome was inversely associated with estimated gestational age (OR 0.4, 95% CI 0.1–1.0, p=0.04) as well as with birth weight (OR 0.06, 95% CI 0.002–0.9, p=0.04). No associations with demographic, maternal, obstetric, precipitating, clinical presentation, or treatment factors were present.
Figure 1.
Distribution of total PSOM scores at a) 2 months, b) 2 years (N=26 at both timepoints; bar labels include number of cases per category as well as percentage of known outcomes; vertical line represents poor outcome cut-off).
PSOM scores were compared between the two time points in 16 patients. While median total PSOM score did not significantly increase over time (p=0.08), language combined sub-scores worsened in 44% (p=0.009). Sensorimotor combined and cognitive/behavioral sub-scores remained stable (69% and 88%, respectively). Over time, 31% of patients had more deficits compared to improvement in 6%. Direction of change for both the overall PSOM score as well as sub-scores is illustrated in Supplemental Figure 1. Supplemental Figure 2 further illustrates shift in PSOM scores over time.
Qualitative Outcomes
There were no recurrent hemorrhages at 2 months or 2 years. None of the 22 infants experienced seizures by the 2-month follow-up, but 50% remained on an antiseizure medication (phenobarbital monotherapy in 9/11, levetiracetam monotherapy in 2/11). Fifteen of the 22 infants (68%) were receiving rehabilitative services. At 2-year follow-up, one child had experienced a seizure with fever; all children had stopped daily antiseizure medications. A second child experienced two seizures, each with fever, after 2-year follow-up but remained off antiseizure medication. From a functional standpoint, 44% of parents reported impairment compared to peers. Eight of the 16 children seen at 2 years (50%) continued to receive rehabilitative services, and 2 used assistive devices (13%).
Sub-analyses: Patients Who Died and Patients with Later Follow-up
All neonates who died during initial hospitalization were small for gestational age, however, this association was not statistically significant (p=0.5). Risk of death was not associated with congenital heart disease or bleeding diathesis, but there was a trend towards association with sepsis (OR 30, 95% CI 1.0–2701, p=0.05). Hemorrhage was intraparenchymal plus intraventricular in two cases, with volumes of <2% TBV and ≥4% TBV. In the remaining case, hemorrhage did not have an intraparenchymal component.
Seven of the 22 (32%) survivors were also evaluated at a median of 4.5 years (IQR 3.5–4.8). At this later time, median total PSOM score was 1 (IQR 0.0–1.5). Neurologic outcome was poor (total PSOM ≥1.0) in 5/7 (71%). Overall, 2/7 had no or non-impairing deficits (29%) (PSOM <1.0), 5 had mild deficits (PSOM 1.0–2.0), and none had moderate or severe deficits. When compared to 2-year outcomes in these same 7 children, median total PSOM scores did not change significantly over time with the majority of total PSOM scores remaining stable (67%), and an equal proportion of scores improving or worsening (17%). While sample size limited analysis of sub-scores, a similar pattern of relative worsening of the language relative to the sensorimotor and cognitive sub-scores was observed. Language sub-scores worsened in 3/7 (43%) of cases compared to worsening in 2/7 (29%) and 1/7 (14%) in the sensorimotor and cognitive sub-scores respectively. Conversely, improvement was seen only in the sensorimotor sub-score (3/7, 43% of cases). No patients had recurrent hemorrhage, and none had restarted an antiseizure medication. From a functional standpoint, 71% of parents reported impairment compared to peers, with all noting increased need for help with day-to-day activities and one report of emotional and behavioral dysregulation (14%). Four children (57%) continued to receive rehabilitative services, with use of assistive devices in 1 (14%). For the 4 school-aged children, all were in age-appropriate classes with 2 receiving in-class services.
Discussion
In this multicenter prospective cohort study we describe the clinical features and outcome trajectories of perinatal primary hemorrhagic stroke. In our cohort of 26 neonates, 12% died in the newborn period. Survivors, however, had a relatively favorable outcome at 2 years with moderate-severe deficits in only 5% and no or non-impairing deficits in over 40% of cases. Differences in inclusion criteria among studies of perinatal hemorrhagic stroke make comparisons challenging (estimated gestational age, inclusion/exclusion of hemorrhagic transformation of arterial ischemic strokes and cerebral sinovenous thrombosis, inclusion/exclusion of isolated IVH). The mortality rate in our cohort is comparable to the 25% rate reported by Brouwer et al8 but significantly higher than the 4% reported by Cole et al2. Of note, these studies variably included hemorrhagic conversion of ischemic strokes as well as presumed perinatal hemorrhagic stroke in the latter case.
Comparison of outcomes in hemorrhagic stroke in children and infants has also been historically challenging due to inconsistent outcome measures16. More recently, Cole et al2 and Bruno et9 al have used the PSOM in perinatal hemorrhagic stroke. Although cut-offs used vary slightly, our findings of functional deficits (PSOM ≥1) in 55% of cases and moderate-severe deficits in 5% of survivors are consistent with the poor outcome in 56% reported by Cole et al (PSOM ≥1)2.
Our cohort was largely seizure-free during follow-up, with no diagnoses of epilepsy. Although lower than the 6% incidence of epilepsy reported by Cole et al2, this finding is overall consistent with reports in the literature17. Epilepsy incidence after primary perinatal hemorrhagic stroke is clearly lower than the rate described in perinatal arterial ischemic stroke (38–46%)18. Outcomes were also favorable when compared with those described in a parallel cohort of children with hemorrhagic stroke (ages 29 days–18 years) at these same institutions, where over a third of patients had significant neurologic deficits (PSOM >2) at 3-year follow-up, and over 10% developed symptomatic epilepsy by 2 years19,20. Overall, these data suggest favorable outcomes for survivors of perinatal hemorrhagic stroke, particularly when contrasted with those of perinatal arterial ischemic stroke and pediatric hemorrhagic stroke. Though seizures are a common presenting or early symptom, the consistent observation that incidence of epilepsy is low suggests that early weaning of antiseizure medications may be appropriate in most cases.
Although outcomes in survivors are encouraging, two caveats should be emphasized. First, the high percentage of both functional difficulties impacting daily activities and use of rehabilitative services underscores the importance of consistent follow-up and access to services. Second, language sub-scores significantly worsened in our cohort between 2-month and 2-year follow-up. Given challenges assessing early language skills, this finding may simply reflect limited assessment. Language sub-scores did not significantly change across time in the subset of patients with 4–5-year follow-up; however, our sample size at later time points was underpowered to demonstrate significant worsening in the language domain. Notably, in this group language sub-scores did worsen more frequently (43%) when compared to sensorimotor (29%) and cognitive sub-scores (14%), and in no cases did they improve. Language deficits may emerge over time or may be appreciated more easily as the cognitive demands children face at school-age increase. Data regarding long-term educational placement and outcomes in perinatal hemorrhagic stroke are scarce, although outcomes in pediatric hemorrhagic stroke suggest a similar pattern of either worsening or more appreciable deficits over time19,21.
A hemorrhage etiology could not be identified in over 40% of cases, which is consistent with reports in the literature1,2. Follow-up imaging was generally unrevealing but did uncover one cavernoma in a highly suspect case. Among the cases with a clear etiology, bleeding diatheses accounted for the majority, followed by choroid plexus and germinal matrix hemorrhages. Vascular malformations were rare. Despite fetal distress being common (45% of cases), the vast majority of deliveries were vaginal with only one assisted delivery. This is consistent with recent reports1,9 and is in contrast with other studies that suggest an association with assisted deliveries22. Maternal hypertension, prenatal infections, and substance exposures, all factors associated with fetal distress, were also common.
Our data underscore the limited understanding of the pathophysiology of primary perinatal hemorrhagic stroke. Some have suggested underlying unique vascular development and anatomy, which in combination with increased pressures occurring during the fetal transition and perinatal stressors lead to rare events that are difficult to predict2. The contribution of bleeding diatheses, however, is clearly significant. Although this study was not designed to quantify hemorrhage risk or provide specific screening and treatment recommendations, the fact that 10% of cases occurred in the context of vitamin K refusal is noteworthy. While intracranial bleeding is known to be the predominant manifestation of “late” vitamin K deficiency bleeding (VKDB) occurring between 2 and 12 weeks of life, manifestations in the first 24 hours and week of life (“early” and “classical” VKDB, respectively) are thought to be mainly gastrointestinal and umbilical23. All cases of hemorrhagic stroke following refusal in our cohort occurred within the first 24 hours of life. While it is possible that additional unknown factors were present, this observation suggests a possible role of early VKDB and highlights the importance of parental counseling.
Limitations of our study include small sample size, which impacted our ability to identify predictors of poor outcome and track longer-term outcome trajectories at school-age. The subgroup for whom data was available 4–5 years post hemorrhage was particularly small (7/22 survivors). Data for these individuals could be biased towards worse outcomes, as children with more significant functional difficulties may be more likely to follow-up. Prolonged and consistent follow-up, however, may also reflect greater access to care. Although the PSOM has been validated for infants, capturing language and cognitive deficits at early ages is difficult14. This limitation, which affects other studies in the field, further underscores that deficits may not be apparent during early follow-up. Finally, our ability to quantify rehabilitative services was limited. Despite these limitations, the prospective nature of this study is a significant strength, particularly given evidence that case identification for pediatric stroke via ICD-code based searches is poor24. The ability to track outcomes at consistent time-points and evaluate trajectories over time is also notable
Conclusions
In this prospective cohort study of primary perinatal hemorrhagic stroke, over 40% of cases were considered idiopathic even after careful imaging review. Bleeding diatheses represented the most commonly identified etiology. Despite a significant mortality rate (20%), survivors had relatively favorable outcomes with moderate-severe deficits in only 5% at 2 years. At a median follow-up of 2 years, no child developed epilepsy, and all assessed children had successfully weaned off antiseizure medication. Total PSOM scores did not change significantly over time; however, language sub-scores worsened while sensorimotor and cognitive/behavioral sub-scores remained stable. Overall, these data suggest that deficits, particularly in the language domain, may emerge over time. Larger prospective cohort studies of neurocognitive outcomes in school-aged survivors of primary perinatal hemorrhagic stroke are needed to better delineate the vulnerability of this population over time and optimal approach to screening and intervention.
Supplementary Material
Acknowledgments
Funding: This work was funded by NINDS K23-NS062110 (LCJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and Predictors of Perinatal Hemorrhagic Stroke: Results From the Kaiser Pediatric Stroke Study. Pediatrics. 2009. doi: 10.1542/peds.2008-0874 [DOI] [PubMed] [Google Scholar]
- 2.Cole L, Dewey D, Letourneau N, et al. Clinical Characteristics, Risk Factors, and Outcomes Associated With Neonatal Hemorrhagic Stroke. JAMA Pediatr. 2017. doi: 10.1001/jamapediatrics.2016.4151 [DOI] [PubMed] [Google Scholar]
- 3.Takenouchi T, Kasdorf E, Engel M, Grunebaum A, Perlman JM. Changing pattern of perinatal brain injury in term infants in recent years. Pediatr Neurol. 2012. doi: 10.1016/j.pediatrneurol.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 4.Raju TNK, Nelson KB, Ferriero D, Lynch JK. Ischemic Perinatal Stroke: Summary of a Workshop Sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007. doi: 10.1542/peds.2007-0336 [DOI] [PubMed] [Google Scholar]
- 5.Gupta SN, Kechli AM, Kanamalla US. Intracranial Hemorrhage in Term Newborns: Management and Outcomes. Pediatr Neurol. 2009. doi: 10.1016/j.pediatrneurol.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 6.Winkelhorst D, Kamphuis MM, Steggerda SJ, et al. Perinatal Outcome and Long-Term Neurodevelopment after Intracranial Haemorrhage due to Fetal and Neonatal Alloimmune Thrombocytopenia. Fetal Diagn Ther. 2019. doi: 10.1159/000488280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhawar BS, Ranger A, Steven DA, Del Maestro RF. A follow-up study of infants with intracranial hemorrhage at full-term. Can J Neurol Sci. 2005. doi: 10.1017/S0317167100004224 [DOI] [PubMed] [Google Scholar]
- 8.Brouwer AJ, Groenendaal F, Koopman C, Nievelstein RJA, Han SK, De Vries LS. Intracranial hemorrhage in full-term newborns: A hospital-based cohort study. Neuroradiology. 2010. doi: 10.1007/s00234-010-0698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno CJ, Beslow LA, Witmer CM, et al. Haemorrhagic stroke in term and late preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2014. doi: 10.1136/archdischild-2013-304068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beslow LA, Ichord RN, Kasner SE, et al. ABC/XYZ estimates intracerebral hemorrhage volume as a percent of total brain volume in children. Stroke. 2010;41(4):691–694. doi: 10.1161/STROKEAHA.109.566430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40(5):1666–1671. doi: 10.1161/STROKEAHA.108.541383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: A prospective consecutive cohort study. Stroke. 2010;41(2):313–318. doi: 10.1161/STROKEAHA.109.568071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure: A Validation and Reliability Study. Stroke. 2012;43(6):1602–1608. doi: 10.1161/STROKEAHA.111.639583 [DOI] [PubMed] [Google Scholar]
- 15.Lo WD, Ichord RN, Dowling MM, et al. The pediatric stroke Recurrence and Recovery Questionnaire: Validation in a prospective cohort. Neurology. 2012;79(9):864–870. doi: 10.1212/WNL.0b013e318266fc9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmann KA, Jordan LC. Outcome measures used in pediatric stroke studies: A systematic review. Arch Neurol. 2012. doi: 10.1001/archneurol.2011.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesan C, Millichap JJ, Krueger JM, et al. Epilepsy Following Neonatal Seizures Secondary to Hemorrhagic Stroke in Term Neonates. In: Journal of Child Neurology. 2016. doi: 10.1177/0883073815600864 [DOI] [PubMed] [Google Scholar]
- 18.Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: Presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014. doi: 10.1016/j.pediatrneurol.2014.07.031 [DOI] [PubMed] [Google Scholar]
- 19.Porcari GS, Beslow LA, Ichord RN, Licht DJ, Kleinman JT, Jordan LC. Neurologic Outcome Predictors in Pediatric Intracerebral Hemorrhage: A Prospective Study. Stroke. 2018. doi: 10.1161/STROKEAHA.118.021845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beslow LA, Abend NS, Gindville MC, et al. Pediatric intracerebral hemorrhage: Acute symptomatic seizures and epilepsy. JAMA Neurol. 2013. doi: 10.1001/jamaneurol.2013.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawks C, Jordan LC, Gindville M, Ichord RN, Licht DJ, Beslow LA. Educational Placement After Pediatric Intracerebral Hemorrhage. Pediatr Neurol. 2016. doi: 10.1016/j.pediatrneurol.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman S, Pavlakis S. Pediatric and newborn stroke. Curr Treat Options Neurol. 2008. doi: 10.1007/s11940-008-0045-6 [DOI] [PubMed] [Google Scholar]
- 23.Sankar MJ, Chandrasekaran A, Kumar P, Thukral A, Agarwal R, Paul VK. Vitamin K prophylaxis for prevention of Vitamin K deficiency bleeding: A systematic review. J Perinatol. 2016. doi: 10.1038/jp.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology. 2006;67(11):2053–2055. doi: 10.1212/01.wnl.0000247281.98094.e2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.