Abstract
Background.
Pediatric cerebral sinovenous thrombosis (CSVT) is a treatable cause of brain injury, acute symptomatic seizures and remote epilepsy. Our objective was to prospectively study epilepsy and neurologic outcomes in neonates and children one year after CSVT diagnosis.
Methods.
Patients with CSVT were enrolled prospectively from 21 international sites through the Seizures in Pediatric Stroke (SIPS) Study. Clinical data including acute symptomatic seizures and CSVT risk factors were collected at diagnosis. A blinded neuroradiologist reviewed acute imaging. At one year, outcomes including seizure recurrence, epilepsy diagnosis, anticonvulsant use, and modified Engel score were collected. Neurological outcomes were assessed using the modified Rankin score (mRS) and the King’s Outcome Scale of Childhood Head Injury (KOSCHI).
Results.
Twenty-four participants with CSVT were enrolled (67% male, 21% neonates). Headache was the most common presenting symptom in non-neonates (47%, 9/19). Nine (37.5%) presented with acute symptomatic seizures. Six (25%; 95% CI = 10%– 47%) developed epilepsy by 1-year follow-up. No clinical predictors associated with epilepsy were identified. KOSCHI and mRS scores at 1 year were favorable in 71%. Half of CSVT patients who developed epilepsy (3/6) did not have infarcts, hemorrhage, or seizures identified during the acute hospitalization
Conclusion.
Our study provides a prospective estimate that epilepsy occurs in approximately one quarter of patients by one year after diagnosis of CSVT. Later epilepsy can develop in the absence of acute seizures or parenchymal injury associated with the CSVT.
Keywords: pediatric stroke, cerebral sino-venous thrombosis, outcomes, seizures
Introduction
Cerebral sinovenous thrombosis (CSVT) is an uncommon but serious cause of acute brain injury, acute symptomatic seizures and remote epilepsy in children. In the large adult International Study on Cerebral Vein and Dural Sinus Thrombosis, nearly 40% experienced seizures at or before diagnosis[1]. Seizures are also reported as a common presenting sign in pediatric CSVT case series and registry data[2–5], but prospective studies of seizures after neonatal and childhood CSVT have been limited and few studies report epilepsy and neurologic outcomes beyond discharge.[6] In a retrospective Northern California pediatric stroke cohort that included children with arterial ischemic stroke and CSVT, approximately one quarter of children with an infarct due to CSVT developed epilepsy by 2 years after stroke, and epilepsy incidence rates were similar in patients with arterial or venous infarcts. [7]. In prior series, 8–38% of pediatric patients with CSVT have been reported to develop epilepsy after variable lengths of follow-up, [2,8,9] suggesting that epilepsy is a frequent sequelae. However, prospective, systematically collected data regarding epilepsy frequency and severity after pediatric CSVT are lacking.
Our objective was to prospectively study outcomes in neonates and children one year after CSVT diagnosis, including epilepsy and standardized scoring of neurologic disability. In this descriptive paper we report on epilepsy and developmental outcomes in neonates and children who present with acute symptomatic seizures at the time of their CSVT diagnosis.
Methods
The Seizures in Pediatric Stroke (SIPS) Study was a prospective cohort study that enrolled term neonates (birth at ≥ 37 weeks gestational age to 28 days of life) and children (29 days to 18 years of life) with arterial ischemic stroke or CSVT from 21 international sites between June 2011 and December 2012 as previously described[10,11]. All sites obtained informed consent from guardians and study approval from local institutional review boards. Only participants with CSVT were included in this analysis.
Inclusion criteria for patients with CSVT were (1) term birth through 18 years of age; (2) clinical evidence of any transient neurological dysfunction such as headache, seizure, decreased level of consciousness, or focal neurological signs consistent with CSVT; and (3) evidence of thrombosis of the cerebral veins or venous sinuses seen on MRI, MRV, or CTV.
Local site investigators abstracted demographics, patient characteristics, clinical presentation, and CSVT risk factors from the medical record onto standardized case report forms with secure online data entry. CSVT risk factors included the presence of a chronic underlying medical disorder, acute systemic illness, dehydration, hypercoagulability and family history of thrombosis. Acute symptomatic seizure was defined as a seizure occurring at or within 7 days of CSVT diagnosis. Epilepsy was defined as at least one unprovoked seizure occurring > 1 month after CSVT in accordance with definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy.[12] Local investigators determined acute seizures diagnosed either clinically or electrographically on EEG performed for clinical indications, and documented (1) the number of acute seizures (single, 2 – 10, or >10 seizures), (2) the duration of the longest acute seizure in minutes, (3) whether a patient was seizing on arrival to medical attention, and (4) whether rescue medications were required. Imaging data was recorded in pre-established data forms and collected from clinical reports. When available, clinically obtained brain imaging studies (CT or MRI) performed within 14 days of diagnosis were collected and reviewed by a single neuroradiologist (MW) to confirm CSVT, and determine the presence of ischemic infarcts and intracranial hemorrhage.
At hospital discharge, guardians were given seizure diaries with instructions to document all seizures. Longitudinal follow-up data were obtained by review of health records and the pediatric stroke recurrence and recovery questionnaires (RRQ),[13] a standardized parental questionnaires administered at 3-months and 12-months. Data including recurrence or worsening of CSVT after discharge, anticonvulsant use, and number and characteristics of remote seizures after hospital discharge were documented. Neurologic examinations were performed and epilepsy outcomes assessed at the one-year follow-up. Functional outcome was scored using the modified Rankin Score (mRS), a 6-grade (0 – 6) scale that measures degree of disability after stroke where 0 = no symptoms, 1 = no disability, 2 = slight disability, 3 = moderate disability, 4 = moderately severe disability, 5 = severe disability, and 6 = death,[14] and the Kings Outcome Scale after Childhood Head Injury (KOSCHI), a 5-grade (1 – 5) scale that measures outcome following pediatric traumatic brain injury, where 5 = good recovery, 4 = moderate disability, 3 = severe disability, 2 = vegetative state, and 1 = death[15]. Abnormal outcomes were defined as KOSCHI < 5 or mRS > 2.
Epilepsy outcome was classified using the modified Engel score previously used in non-surgical, pediatric stroke populations.[16,17] Patients were scored as Class 0 if they are seizure-free and off antiepileptic medication for at least 6 months, Class 1 if they were seizure-free for at least 6 months while on medication or seizure-free for less than 6 months off medication, Class 2 if they had less than 1 seizure per month, Class 3 if they had 1 – 4 seizures per month, Class 4 if they had 5 – 30 seizures per month, and Class 5 if they had more than 30 seizures per month.
Summary statistics were used to describe demographics and clinical characteristics. The presence of ischemic infarcts and intracranial hemorrhage were analyzed as dichotomous variables and were only included when identified as “definitely present” after review of acute brain imaging studies by the study neuroradiologist (MW). Infarcts were further classified as single or multiple, and as subcortical only if no infarcts included the cortex. Intracranial hemorrhages were further described by location (intraparenchymal and/or subarachnoid haemorrhage). Proportions were compared using a two-tailed Fisher exact test. Significance was set at p < 0.05
Results
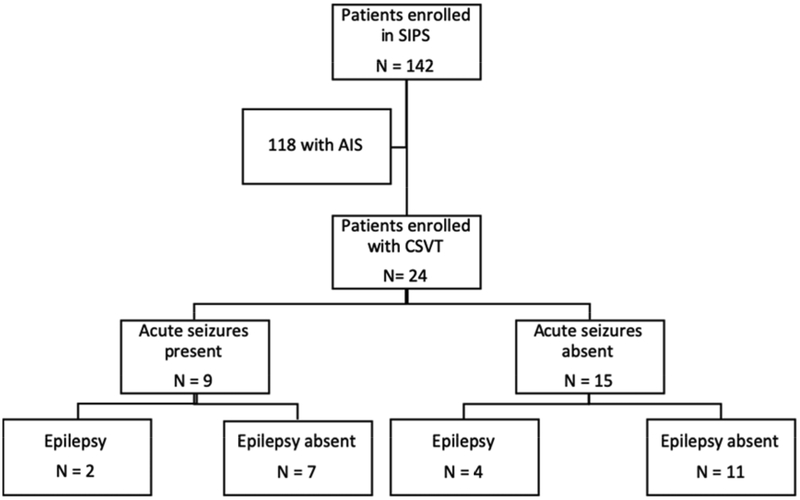
We identified 24 patients with CSVT from a total of 142 with ischemic stroke enrolled in the SIPS study[10] (Figure 1). Sixteen (67%) were male and five (21%) were neonates. Headache was the most common clinical presentation in non-neonates with CSVT (47%, 9/19 children). The most common risk factor for childhood CSVT was a chronic disorder, identified in 42% (10/24). These included suspected or confirmed genetic syndromes and chronic hematologic conditions or malignancies. Baseline demographics and clinical characteristics were similar in those patients with compared to those without acute seizures (Table 1).
Figure 1:
Flow diagram of pediatric stroke patients from which 24 CSVT patients were identified. Acute seizures and epilepsy outcomes are reported. SIPS, seizures in pediatric stroke study; AIS, arterial ischemic stroke; CSVT, cerebral sinovenous thrombosis.
Table 1:
Demographics, clinical and radiological features of CSVT cohort. IQR, interquartile range; CSVT, cerebral sinovenous thrombosis; LOC, level of consciousness.
| Acute seizure | No acute seizure | Epilepsy | No epilepsy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=9 | N=15 | N=6 | N=18 | |||||||
| Demographic/Characteristic | n | (%) | n | % | P value | n | % | n | % | P-value |
| Male | 5 | (56) | 11 | (73) | 0.371 | 3 | (50) | 13 | (72) | 0.317 |
| Neonate | 2 | (22) | 3 | (20) | 0.897 | 1 | (17) | 4 | (22) | 0.772 |
| Age at diagnosis (median y, IQR) | 0.9 | (0.1 – 3.2) | 1.7 | (0.2 – 6.1) | 0.999 | 1.0 | (0.4 – 12.8) | 1.5 | (0.1 – 4.6) | 0.640 |
| CSVT risk factors | ||||||||||
| Chronic medical disorder | 4 | (44) | 6 | (40) | 0.999 | 3 | (50) | 7 | (39) | 0.633 |
| Acute systemic illness | 6 | (67) | 7 | (47) | 0.340 | 3 | (50) | 10 | (56) | 0.813 |
| Dehydration | 4 | (44) | 3 | (33) | 0.202 | 2 | (33) | 5 | (28) | 0.795 |
| Local infection* | 2 | (22) | 5 | (56) | 0.562 | 2 | (33) | 5 | (28) | 0.795 |
| Acute clinical presentation | ||||||||||
| Hemiparesis | 4 | (44) | 2 | (13) | 0.15 | 2 | (33) | 4 | (22) | 0.586 |
| Visual field defect | 0 | 1 | (7) | 0.999 | 0 | 0 | 0.999 | |||
| Decreased LOC | 2 | (22) | 4 | (27) | 0.999 | 1 | (17) | 5 | (28) | 0.586 |
| Headache | 2 | (22) | 7 | (47) | 0.389 | 2 | (33) | 7 | (39) | 0.808 |
| Papilledema | 1 | (11) | 2 | (13) | 0.999 | 0 | 3 | (17) | 0.546 | |
| Imaging Findings (N = 22) | N=9 | N=13 | N=6 | N=16 | ||||||
| Ischemic infarct | 2 | (25) | 3 | (23) | 0.999 | 1 | (17) | 8 | (50) | 0.157 |
| Multiple infarcts | 0 | 3 | (23) | 0.257 | 1 | (17) | 2 | (13) | 0.800 | |
| Subcortical infarcts only | 0 | 2 | (15) | 0.505 | 0 | 2 | (13) | 0.999 | ||
| Hemorrhage | 3 | (38) | 1 | (8) | 0.253 | 2 | (17) | 2 | (13) | 0.259 |
Meningitis, sinusitis, otitis media or mastoiditis
Nine patients (38%, including 2 of five neonates and 7 of 12 older children) presented with an acute seizure. Most of the acute symptomatic seizures were clinical (56%) or electro-clinical (33%). One was electrographic only. All clinical seizures were focal motor in semiology. One patient had both focal motor and bilaterally convulsive seizures. Seizures were recurrent in the acute period in 7 cases (78%) (Table 2). Three patients were seizing on arrival to medical care, and six of nine were treated with a rescue medication to stop acute seizures. A prolonged acute symptomatic seizure was recorded in four patients, who had a seizure duration ranging from 4 – 20 minutes. All nine patients with acute symptomatic seizures were discharged on at least one anticonvulsant. Six (67%) were discharged on more than one anticonvulsant.
Table 2:
Characteristics of acute seizures presentation.
| Acute Seizure N=9(%) | |
|---|---|
| Clinical only | 5 (56) |
| Electrographic only | 1 (11) |
| Electroclinical | 3 (33) |
| Focal motor | 8 (89) |
| Bilaterally convulsive | 1 (11) |
| Single seizure | 1 (11) |
| 2–10 seizures | 5 (56) |
| >10 seizures | 2 (22) |
| Unknown frequency | 1 (11) |
Twenty-two of the 24 patients with CSVT (92%) had imaging available for central review. Of those, five (21%) had CT imaging, 16 (67%) had MR imaging, and one had both CT and MR imaging. Acute infarct was definitely present in 5/22 (21%). In the five patients with definite infarcts, three (60%) had multifocal infarcts. Two had subcortical infarcts only. Four of 22 (18%) patients had hemorrhage; two patients with parenchymal, one with subarachnoid, and one patient with both parenchymal and subarachnoid hemorrhage. Twenty-one (88%) of the 24 patients were anticoagulated. One patient had extension of thrombus documented two days after CSVT diagnosis and prior to initiation of anticoagulation.
One-year outcomes are shown in (Table 3). No patients had recurrence or new CSVT after hospital discharge. Six patients (25%; 95% CI = 10% - 47%) were diagnosed with epilepsy by one year. Patients with and without acute seizures had a similar frequency of epilepsy at one year (22% and 27%, respectively, p=0.999). Of the nine patients with acute seizures, seven (78%) remained on a maintenance anticonvulsant one year after CSVT diagnosis. Of the 15 patients without acute seizure, two (13%) were receiving a maintenance anticonvulsant at the one-year follow-up. Patients who presented with acute seizures were more likely to be on an anticonvulsant at one-year follow-up (p = 0.003) than those who did not present with acute symptomatic seizures.
Table 3:
Acute and one year Epilepsy and mRS and KOSCHI score comparisons for patients with CSVT.
| Acute seizure | No acute seizure | Epilepsy | No epilepsy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=9 | N=15 | N=6 | N=18 | |||||||
| n | (%) | n | (%) | p-value | n | % | n | % | p-value | |
| Acute seizure | - | - | - | - | - | 2 | (33) | 7 | (39) | |
| Epilepsy | 2 | (22) | 4 | (27) | 0.999 | - | - | - | - | na |
| Anticonvulsant at 1 year | ||||||||||
| none | 2 | (22) | 13 | (87) | 0.003 | 2 | na | |||
| 1 | 4 | (44) | 2 | (13) | 0.15 | 3 | na | |||
| >1 | 3 | (33) | 0 | 0.042 | 1 | na | ||||
| mRS | N=8 | N=13 | N=6 | N=15 | ||||||
| 0 | 3 | (33) | 8 | (53) | 0.387 | 2 | (33) | 9 | (60) | 0.269 |
| 1–2 | 3 | (33) | 4 | (27) | 0.999 | 1 | (17) | 6 | (40) | 0.306 |
| 3–5 | 2 | (22) | 1 | (7) | 0.531 | 3 | (50) | 0 | 0.015 | |
| 6 | 0 | 0 | 0.999 | 0 | 0 | 0.999 | ||||
| KOSCHI Score | N=8 | N=13 | N=6 | N=15 | ||||||
| 5 | 6 | (67) | 9 | (60) | 0.999 | 3 | (50) | 12 | (80) | 0.169 |
| 4 | 0 | 2 | (13) | 0.505 | 0 | (0) | 2 | (13) | 0.999 | |
| 3 | 2 | (22) | 2 | (13) | 0.618 | 3 | (50) | 1 | (7) | 0.053 |
| 1–2 | 0 | 0 | 0.999 | 0 | 0 | 0.999 | ||||
mRS= modified Rankin Score, KOSCHI=Kings Outcome Scale after Childhood Head Injury, na = not applicable
Modified Rankin and KOSCHI scores were available for 21 patients (88%). Moderate disability was found in 6 of 21 children (29%). The mRS and KOSCHI scores were similar in patients with and without acute seizures. No differences were found in mRS or KOSCHI scores in those with compared to those without versus without epilepsy (Table 3). No deaths were recorded acutely or at follow up.
The six patients with epilepsy at one-year follow up are described in Table 4. Three had abnormal neurological outcomes; all three children had Engel score > 0. One patient had had seizures in the last 6 months (Engel 1), one had 1 – 4 seizures per month (Engel 3), and one had 5 – 30 seizures per month (Engel 4). All three were taking anticonvulsants. Three patients had epilepsy but no history of acute symptomatic seizures or infarct or hemorrhage identified in the acute period. There was no family history of epilepsy in any of the patients with CSVT.
Table 4:
Clinical characteristics of patients diagnosed with epilepsy.
| Patient | Age (years) | Stroke | Acute seizure | Seizure at 3 months | Seizure at one year | Anticonvulsant one year | mRS | KOSCHI | mEngel |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | No | No | Yes | Yes | PB | 4 | 3a | 1 |
| 2 | 0.2 | Hemorrhagic†* | Yes | No | Yes | TPX | 4 | 3b | 4 |
| 3 | 0.9 | Hemorrhagic† | Yes | No | Yes | LEV, clonazepam | 5 | 3a | 3 |
| 4 | 16.7 | lschemic^ | No | No | Yes | LEV | 1 | 5a | 0 |
| 5 | 17.9 | No | No | Yes | No | None | 0 | 5b | 0 |
| 6 | 1.1 | No | No | No | Yes | None | 0 | 5b | 0 |
AED, antiepileptic drug; TPX, topiramate; LEV, levetiracetam; PB, phenobarbital, mRS, modified rankin score; KOSCHI, Kings Outcome Scale after Childhood Head Injury; mEngel, modified Engel.
Intraparenchymal haemorrhage;
subarachnoid haemorrhage;
mul1ple ischemic strokes
Discussion
In this prospective study of 24 pediatric patients diagnosed with CSVT, a quarter of patients were diagnosed with epilepsy by 12-month follow-up. This is consistent with previous reported prevalence of epilepsy after CSVT of 8 – 38%.[2,7–9] Previous studies in patients with arterial ischemic stroke, demonstrated that those with acute seizures at onset of stroke are at higher risk of developing epilepsy[7,18]. Prolonged and recurrent seizures in the acute period also predict increased epilepsy risk in pediatric patients with arterial ischemic stroke [10]. Studies have also shown that adult patients with CSVT and acute seizures have an increase the risk of remote seizures[19]. In a case series of Swiss children, 5 of 13 neonates who were followed for at least 18 months after CSVT developed epilepsy; none of the 24 older children in the study developed epilepsy.[8] Among 37 childhood CSVT survivors identified in European stroke registries, three (8%) were identified with epilepsy after a median follow-up of 1 year.[2] Despite a 24% overall risk of developing epilepsy, those patients in our study with acute seizures did not demonstrate a higher risk of epilepsy. Our sample number limited our ability to detect a difference if a small effect size exists. As well, many of the patients who did not meet our criteria for epilepsy were maintained on an anticonvulsant for the first year after acute seizure, possibly contributing to an underestimate of the true rate of epilepsy after pediatric CSVT. At 12-month follow up, over three-quarters of patients with an acute seizure were still taking an anticonvulsant, whereas only 13% of patients with no acute seizures were receiving an anticonvulsant.
Previous case series and registries have examined developmental outcomes in children with CSVT and epilepsy. In a single center case series in Canada, epilepsy was reported in 18% of neonatal CSVT survivors after a median of 2.5 years, and all patients who developed epilepsy had neurologic deficits documented on follow-up.[9] Our results from this prospective study are consistent with these previous findings suggesting neurological deficits in 29% (mRS >2 and KOSCHI <5). Neurological deficits at follow up after CSVT have been reported in 38% in other studies [2,20]. High rates of poor neurological outcomes in neonates specifically, have been suggested[8,9,21], with older children more likely having intellectual abilities within the normal range 18 months after CSVT[8]. Although, long-term outcomes, in our study, based on KOSCHI and mRS at 1 year, are overall, favorable, patients with epilepsy and those who were on one or more anticonvulsant have documented neurological sequelae. This is consistent with previous pediatric CSVT studies suggesting seizures as a predictor of poor outcome[20,22]. This study was under-powered to determine the effect of specific anticonvulsant management on epilepsy and developmental outcomes and further research focusing on outcome are suggested. No deaths occurred in our study, in keeping with previously reported low mortality rates of 3 – 12%[2–4,8,20].
At time of presentation with CSVT, 38% of our cohort presented with acute seizures. Previous pediatric stroke registries have reported that acute symptomatic seizures occur in 26 – 58% of patients with CSVT.[2,4,8,22] Acute seizures were the most common presentation of CSVT in neonates. The proportion of neonates with acute seizures in our study was lower than described in several prior studies that reported acute seizures in 57 – 69% of neonates with CSVT [9,23–25]. This may reflect the small number of neonates with CSVT enrolled in SIPS. In addition, variation in EEG monitoring could have led to an underestimate of acute seizures. Headache was the most common presentation of CSVT in children in our study. Identification of headache is likely higher in older children who can report headache as a symptom; workup for headache may also lead to the initial diagnosis of CSVT and anticoagulation prior to potentially developing a seizure.
We found that the patients that presented with acute seizures had a high likelihood to be seizing on arrival to medical attention (33%; 3/9), to require rescue medications (67%; 6/9) and had multiple seizures in the acute period (89%; 8/9). The high frequency of repeated acute seizures during the first seven days after diagnosis raises the question of whether short-term prophylactic anticonvulsant treatment should be considered in children with CSVT. Brain Trauma Foundation guidelines[26] and the guidelines for the management of pediatric severe traumatic brain injury [18] recommend prophylactic anticonvulsant treatment after severe traumatic brain injury to decrease the incidence of early posttraumatic seizures; the rationale for the recommendation is partly based on an estimated incidence of clinical seizures in 12% and electrographic seizures in 20–25%. At least one study of adults with CSVT suggests possible benefit of preventing recurrent acute symptomatic seizures by treating with an anticonvulsant[1] but few data are available to guide seizure prevention in the pediatric population. Risk and benefit of long-term anticonvulsant management as well as screening for epileptic encephalopathy and the potential effects on long-term outcome require further study.
In this cohort, a chronic underlying disorder was identified in 42%, consistent with previous literature.[2,8,20] Many potential risk factors for CSVT such as chronic illness, recent infection, dehydration, anemia, and prothrombotic states [2,4,5,20,27] may influence the likelihood of acute seizures, but our small numbers limited the power to identify these as predictors of seizure in our study. We did not identify any acute clinical factors associated with epilepsy. Half of the patients with epilepsy at one-year follow up had no parenchymal injury (infarct or hemorrhage) identified in the acute period. We did not review follow-up imaging, and it is possible that infarct or haemorrhage may have occurred later in these patients. However, parents were asked about recurrence of new stroke or thrombosis during follow-up and no recurrences or progression was identified through their reports. Even when macroscopic injury is not identified on early imaging, poor venous drainage from pediatric CSVT may trigger a functional alteration of excitatory and inhibitory neuronal networks that chronically increases seizure susceptibility. Other comorbid or genetic factors likely also play a role in whether patients develop early epilepsy.
There are several limitations to our study. The majority of SIPS participants were enrolled after arterial ischemic stroke. Small numbers of participants with CSVT included in these analyses resulted in a lack of power to identify potential differences in developmental outcomes in those with versus without seizures or epilepsy. We included clinical seizures reported by investigators even if they occurred when not monitored on EEG. Our study provides prospective data, where previously published papers are retrospective in nature. The focus on seizures a priori, results in better ascertainment of these outcomes. We had limited data regarding specifics of the underlying disorders, but some chronic disorders may have contributed to a higher epilepsy incidence.
Our prospective study highlights the high proportion of pediatric patients with CSVT present with acute seizures. Clinical predictors of acute seizure and later diagnosis of epilepsy are yet to be identified. Future studies focusing on neonates and children with CSVT are needed to guide clinical decision making for acute treatment and prevention of seizures, as well as optimal duration of treatment with anticonvulsants.
Supplementary Material
Sources of support:
Pediatric Epilepsy Research Foundation (grant 112010-007)
Auxilium Foundation
National Institutes of Health (2K12NS001692-11 and KL2TR000143)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: The authors declare no potential conflict of interest with respect to research, authorship, and/or publication of this article.
See Appendix I for Seizures in Pediatric Stroke (SIPS) sites and investigators.
References
- [1].Ferro José M., Canhão Patrícia, Bousser Marie-Germaine, Stam Jan, Barinagarrementeria Fernando. Early Seizures in Cerebral Vein and Dural Sinus Thrombosis. Stroke 2008;39:1152–8. doi: 10.1161/STROKEAHA.107.487363. [DOI] [PubMed] [Google Scholar]
- [2].Sebire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain 2005;128:477–89. [DOI] [PubMed] [Google Scholar]
- [3].Kenet G, Waldman D, Lubetsky A, Kornbrut N, Khalil A, Koren A, et al. Paediatric cerebral sinus vein thrombosis. A multi-center, case-controlled study. Thromb Haemost 2004;92:713–8. [DOI] [PubMed] [Google Scholar]
- [4].Ichord RN, Benedict SL, Chan AK, Kirkham FJ, Nowak-Göttl U, International Paediatric Stroke Study Group. Paediatric cerebral sinovenous thrombosis: findings of the International Paediatric Stroke Study. Arch Dis Child 2015;100:174–9. doi: 10.1136/archdischild-2014-306382. [DOI] [PubMed] [Google Scholar]
- [5].Lolli V, Molinari F, Pruvo J-P, Soto Ares G. Radiological and clinical features of cerebral sinovenous thrombosis in newborns and older children. J Neuroradiol 2016;43:280–9. doi: 10.1016/j.neurad.2015.12.001. [DOI] [PubMed] [Google Scholar]
- [6].Ichord R Cerebral Sinovenous Thrombosis. Front Pediatr 2017;5:163. doi: 10.3389/fped.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fox CK, Glass HC, Sidney S, Lowenstein DH, Fullerton HJ. Acute seizures predict epilepsy after childhood stroke. Ann Neurol 2013;74:249–56. doi: 10.1002/ana.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grunt S, Wingeier K, Wehrli E, Boltshauser E, Capone A, Fluss J, et al. Cerebral sinus venous thrombosis in Swiss children. DevMedChild Neurol 2010;52:1145–50. [DOI] [PubMed] [Google Scholar]
- [9].Moharir MD, Shroff M, Pontigon AM, Askalan R, Yau I, MacGregor D, et al. A prospective outcome study of neonatal cerebral sinovenous thrombosis. JChild Neurol 2011;26:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fox CK, Mackay MT, Dowling MM, Pergami P, Titomanlio L, Deveber G, et al. Prolonged or recurrent acute seizures after pediatric arterial ischemic stroke are associated with increasing epilepsy risk. Dev Med Child Neurol 2017;59:38–44. doi: 10.1111/dmcn.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fox CK, Jordan LC, Beslow LA, Armstrong J, Mackay MT, deVeber G. Children with post-stroke epilepsy have poorer outcomes one year after stroke. International Journal of Stroke 2018;13:820–3. doi: 10.1177/1747493018784434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- [13].Lo WD, Ichord RN, Dowling MM, Rafay M, Templeton J, Halperin A, et al. The Pediatric Stroke Recurrence and Recovery Questionnaire: validation in a prospective cohort. Neurology 2012;79:864–70. doi: 10.1212/WNL.0b013e318266fc9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. n.d:4. [DOI] [PubMed] [Google Scholar]
- [15].Paget SP, Beath AWJ, Barnes EH, Waugh M-C. Use of the King’s Outcome Scale for Childhood Head Injury in the evaluation of outcome in childhood traumatic brain injury. Developmental Neurorehabilitation 2012;15:171–7. doi: 10.3109/17518423.2012.671381. [DOI] [PubMed] [Google Scholar]
- [16].Golomb MR, Garg BP, Carvalho KS, Johnson CS, Williams LS. Perinatal stroke and the risk of developing childhood epilepsy. JPediatr 2007;151:409–13, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Billinghurst LL, Beslow LA, Abend NS, Uohara M, Jastrzab L, Licht DJ, et al. Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology 2017;88:630–7. doi: 10.1212/WNL.0000000000003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fox CK, Glass HC, Sidney S, Smith SE, Fullerton HJ. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology 2016;86:2179–86. doi: 10.1212/WNL.0000000000002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Davoudi V, Keyhanian K, Saadatnia M. Risk factors for remote seizure development in patients with cerebral vein and dural sinus thrombosis. Seizure 2014;23:135–9. doi: 10.1016/j.seizure.2013.10.011. [DOI] [PubMed] [Google Scholar]
- [20].deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001;345:417–23. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- [21].Kersbergen KJ, Groenendaal F, Benders MJ, de Vries LS. Neonatal cerebral sinovenous thrombosis: neuroimaging and long-term follow-up. JChild Neurol 2011;26:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wasay M, Dai AI, Ansari M, Shaikh Z, Roach ES. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol 2008;23:26–31. [DOI] [PubMed] [Google Scholar]
- [23].Nwosu ME, Williams LS, Edwards-Brown M, Eckert GJ, Golomb MR. Neonatal sinovenous thrombosis: presentation and association with imaging. Pediatr Neurol 2008;39:155–61. doi: 10.1016/j.pediatrneurol.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [24].Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. ArchNeurol 2006;63:405–9. [DOI] [PubMed] [Google Scholar]
- [25].Jordan LC, Rafay MF, Smith SE, Askalan R, Zamel KM, deVeber G, et al. Antithrombotic Treatment in Neonatal Cerebral Sinovenous Thrombosis: Results of the International Pediatric Stroke Study. J Pediatr 2010;156:704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- [27].Heller C, Heinecke A, Junker R, Knofler R, Kosch A, Kurnik K, et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation 2003;108:1362–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.