Abstract
The purpose of this investigation was to determine the role of extracellular vesicles (EVs), released from articular chondrocytes in a physiological or pathological state, in cell-cell communication with other articular chondrocytes or chondrocytic precursor cells. Conditioned medium from interleukin-1beta (IL-1β-treated human articular chondrocytes stimulated catabolic events and inhibited type II collagen expression in articular chondrocytes to a much greater degree than medium from IL-1β-treated chondrocytes after complete removal of EVs. Vehicle-treated and IL-1β-treated human articular chondrocytes released EVs of similar size; however the number of EVs released by IL-1β-treated chondrocytes was markedly higher than the number of EVs released from vehicle-treated cells. Furthermore, our findings demonstrate that similar to medium from IL-1β-treated chondrocytes containing EVs, EVs isolated from medium of IL-1β-treated chondrocytes stimulated catabolic events in articular chondrocytes, whereas EVs isolated from the medium of vehicle-treated chondrocytes inhibited catabolic events and increased mRNA levels of aggrecan and type II collagen in IL-1β-treated chondrocytes. Furthermore, medium containing EVs from vehicle-treated articular chondrocytes or EVs isolated from this medium stimulated chondrogenesis of C3H10T1/2 cells, whereas medium containing EVs from IL-1β-treated chondrocytes or EVs isolated from this medium inhibited chondrogenesis. Our findings suggest that EVs released by articular chondrocytes play a key role in the communication between joint cells and ultimately in joint homeostasis, maintenance, pathology, and repair.
Keywords: Extracellular vesicles, articular chondrocytes, chondrogenesis, catabolic events
Introduction
Extracellular vesicles (EVs) are cell-derived phospholipid membrane-based nanoparticles that present with functional surface/membrane proteins and contain protein and RNA species (mRNA, miRNA, and small noncoding RNAs), and that dynamically reflect the states of the parent cell and tissue.1–3 EVs are being produced and released by most cells of the body, including articular chondrocytes, and serve to transmit biological signals, transfer proteins/nucleic acids, and induce biological effects on target cells via cell surface interactions, membrane fusion or endocytosis of the EVs by target cells.4 EVs can be categorized into two main classes based on their cellular origin. Exosomes (Exo) are released by cells when intracellular multi-vesicular bodies fuse with the cell membrane, while micro vesicles (MV) are released via direct outward budding of the cell membrane.1–3
It has become evident that EVs play crucial role in the regulation of physiological and pathological processes in many tissues. For example, it has been shown that EVs released from cancer cells promote cancer progression and metastasis.5 In contrast, EVs isolated from mesenchymal stem cells (MSCs) may have therapeutic and regenerative potentials, and may be more advantageous for therapeutic use than stem cells because of the avoidance of potential safety, regulatory and practicality issues of cellular incorporation, such as the use of stem cells with damaged/mutated DNA, and potential erroneous differentiation of injected stem cells.6 Very little, however, is known about the role of EVs released from articular chondrocytes in articular cartilage homeostasis, maintenance and pathology.
Through communication between joint cells, EVs may modulate gene expression and change downstream functions of recipient cells, as well as physiological or pathological processes. For example, synovial fibroblast-derived EVs from osteoarthritic (OA) patients stimulate macrophages to produce a spectrum of pro-inflammatory cytokines and chemokines, which leads to a further acceleration of cartilage degradation.7 In contrast, EVs released from MSCs have been suggested to have therapeutic properties and inhibit cartilage degradation and inflammation in OA.8–14 These findings suggest that the cargo of EVs, which is responsible for EVs’ actions, not only depends on the parent cell type, but also on the state of the cell from which the EVs are released. Since EVs play a key role in affecting tissue homeostasis and pathology, we determined how EVs released from articular chondrocytes in a physiological or pathological state affect recipient chondrocyte and chondrocyte precursor functions. Our findings suggest that EVs released from articular chondrocytes in a normal or pathological state may play important roles in the regulation of cartilage homeostasis and pathology.
Methods
Cell Culture –
The multipotential murine C3H/10T1/2 mesenchymal stem cell line (ATTCC) was induced to chondrogenic differentiation in micromass cultures in the presence of BMP-2 (100ng/ml) and insulin, human transferrin and selenous acid (ITS)+ (100ng/ml) in medium containing 5% EV-free FBS or a 1:1 mixture of medium containing 10% EV-free FBS and serum-free conditioned medium from articular chondrocytes.
Human articular chondrocytes were isolated from articular cartilage samples obtained from patients (donor age range 48 – 67) undergoing total knee replacement surgery at NYU Langone Orthopedic Hospital. Knee cartilage was harvested from regions with no macroscopically evident degeneration. The collection of tissue from patients undergoing knee replacement surgery was approved by the Institutional Regulatory Board at NYU School of Medicine. Human chondrocytes were isolated from these cartilage samples as described by us.15 Cells were plated at density of 1 × 106 cells/well into 6-well tissue culture plate and grown in monolayer in complete medium. For collection of conditioned media or isolation of EVs from conditioned media, human articular chondrocytes after they reached confluency were cultured in serum-free medium for 48h.
For IL-1β treatment, articular chondrocytes, after they reached confluency were serum starved for 24 h followed by treatment with vehicle PBS / 0.1% bovine serum albumin (BSA) or different concentrations of human recombinant IL-1β (2ng/ml, 5ng/ml, 10ng/ml) in PBS/0.1% BSA and cultured in serum-free medium. After 24 h treatment with various concentrations of IL-1β the medium was removed and fresh serum-free medium without IL-1β was added. After 24 h of culture the medium was collected and half of the medium was centrifuged at 100,000 × g for 18h for complete removal of EVs.
Isolation of EVs from conditioned media –
To isolate or remove EVs from conditioned medium collected from chondrocytes, medium was first centrifuged at 3,000 × g for 10 min to remove debris. For the isolation of EVs the supernatant was then centrifuged at 100,000 × g for 2h at 4°C. For complete removal of EVs the medium was centrifuged at 100,000 × g for 18h at 4°C. The isolated EVs were washed with PBS followed by centrifugation at 100,000 × g for 2h at 4°C. EV pellets were resuspended in PBS for direct use in subsequent experiments.
Nanoparticle tracking analysis of EVs –
EVs were measured by nanoparticle tracking analysis (NTA) using Nanosight LM10 (Malvern) with a laser wavelength of 533 nm and analyzed by NanoSight NTA software v3.2. All measurements were performed at 25°C under constant slow flow setting of 100. Three videos of 1 min were analyzed per sample. The software calculated the number and size of particles from the three videos and expressed as mean and standard deviation (SD).
Transmission electron microscopy –
Ten microliters of purified EVs, corresponding to approximately 500 × 106 particles, were absorbed on Formvar carbon-coated grids for 10 min. The drops were then blotted with filter paper and negatively stained with 2% uranyl acetate in aqueous suspension for 10 min. Excess of uranyl was removed by touching the grid to a filter paper. The grid was dried at room temperature. Grids were examined with a transmission electron microscope (TALOS L120C Thermo Fisher Scientific, Waltham, MA, USA) at 120 kV.
RT-PCR and real-time PCR analysis –
Total RNA was isolated from cell cultures using the RNeasy minikit (Qiagen, Valencia, CA). mRNA levels of aggrecan, cyclooxygenase (Cox)-2, IL-6, inducible nitric oxide synthase (iNOS), matrix metalloproteinase (MMP)-13, type I collagen and type II collagen were quantified by real-time PCR as described previously.16 Briefly, 1 μg of total RNA was reverse transcribed in a total volume of 30 μl by using an Omniscript RT kit (Qiagen). A 1:100 dilution of the cDNA resulting from the reverse transcription of 1 μg of total RNA was used as the template to quantify the relative content of mRNA by real-time PCR (StepOnePlus™ System; Applied Biosystems, Foster City, CA) with the appropriate primers and SYBR Green. PCRs were performed with a SYBR Green PCR Master Mix kit (Applied Biosystems), with 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min, and 1 cycle at 95°C for 15 s and 60°C for 1 min. The 18S RNA was amplified at the same time and used as an internal control. The cycle threshold values for 18S RNA and the samples were measured and calculated by computer software. Transcript levels were calculated according to the equation x = 2–ΔCt, where ΔCt = Ctexp – Ct18S. Relative transcript levels were calculated as x = 2–ΔΔCt, in which ΔΔCt = ΔE – ΔC, ΔE = Ctexp – Ct18S, and ΔC = Ctctl – Ct18S.
SDS PAGE and immunoblotting –
Twenty μg of total EV protein were analyzed by SDS PAGE and immunoblotting with antibodies specific for AnxA6 (Abcam, ab7671), CD63 (Abcam, ab59479), flotillin-1 (Abcam, ab41927) as described previously.17
Cell viability assay –
Cells were treated with various concentrations of IL-1β (0, 2, 5, 10 ng/ml) for 24 h. The percentage of viable cells was determined using the Cell Counting Kit-8 (CCK-8) assay (APExBIO, Houston, TX)) following the manufacturer’s instructions. The absorbance of WST-8 formazan obtained for vehicle-treated cells was set as 100%.
Solid phase ELISA assay –
10 μl of medium collected from chondrocytes cultured in conditioned medium containing EVs or medium after complete removal of EVs from vehicle-treated or IL-1β-treated chondrocytes was used to determine the concentration of the proenzyme form of MMP-13 (pro-MMP-13) released into the medium using a solid phase ELISA assay (R & D Systems, Minneapolis, MN) following the manufacturer’s instructions. The concentration of pro-MMP-13 was normalized to the total RNA concentration of the cells.
Statistical analysis –
Data was analyzed using PRISM statistical analysis software. Descriptive statistics (mean, standard deviations, medians, and interquartile ranges) were generated for each outcome variable. The descriptive statistics was generated for each group separately. Analysis of the variance (ANOVA) was used to assess differences in means among 3 or more groups, while comparisons of two groups was made using t tests. If there were significant differences in ANOVA, pairwise tests were conducted to assess specific differences using Tukey’s multiple comparison procedure. A p-value < 0.05 was used as a threshold for statistical significance.
Results
To mimic inflammatory conditions in OA joints and stimulate catabolic events in articular chondrocytes in vitro in cell culture, IL-1β at various concentrations has been used. A previous study, which used IL-1β in a concentration range from 0.001 ng/ml to 10 ng/ml to treat articular chondrocytes showed that 10 ng/ml IL-1β was the most effective concentration to stimulate phospholipase A2 (PLA2) and Cox-2 mRNA levels and prostaglandin E2 (PGE2) release.18 Furthermore, another study showed that IL-1β at a concentration of 10 ng/ml was the most effective in stimulating catabolic events in cartilage explants.19 Similarly, our study shows that IL-1β at various concentrations increased the mRNA levels of catabolic markers, including Cox-2, IL-6 and MMP-13 in articular chondrocyte cultures with 10 ng/ml IL-β being the most effective in stimulating these mRNA levels (Suppl Fig. 1). IL-1β at a concentration of 2, 5 or 10 ng/ml only slightly but not significantly reduced the viability of articular chondrocytes compared to vehicle-treated cells (Suppl. Fig. 1). Finally, analysis of MMP-13 released into the medium using ELISA revealed that treatment of articular chondrocytes with 10ngml IL-1β resulted in the highest concentration of the pro-form of MMP-13 released into the medium (Suppl. Fig. 1).
The effect of conditioned medium containing EVs or after complete removal of EVs from IL-1β-treated chondrocytes on catabolic events of articular chondrocytes.
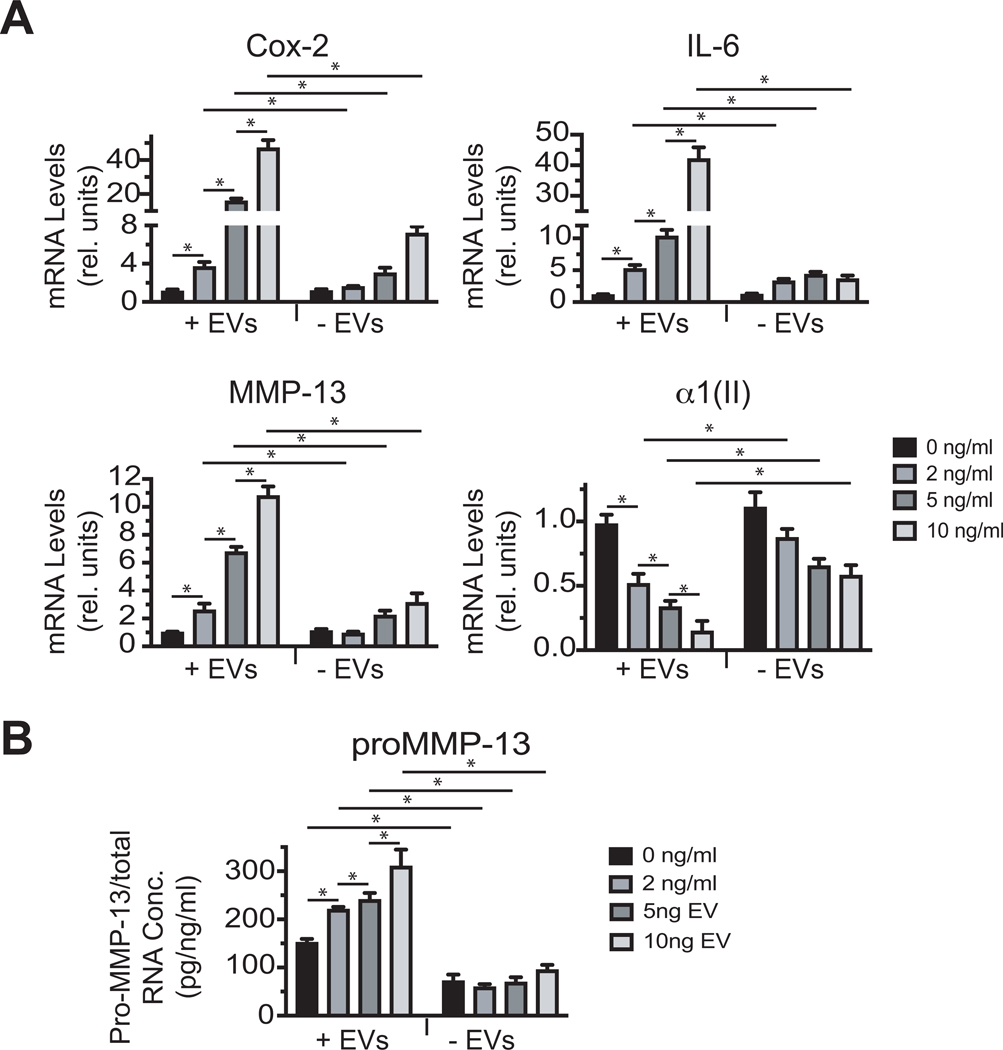
Most if not all cells, including articular chondrocytes release EVs into the culture medium. First, we tested how conditioned medium containing EVs and conditioned medium after complete removal of EVs from IL-1β-treated human articular chondrocytes affect human articular chondrocytes. For these experiments conditioned medium was collected from human articular chondrocytes cultures that were treated in serum-free medium with different concentrations of IL-1β. To avoid the presence of residual IL-1β from the treatment in the collected medium, medium was changed after 24 h treatment with IL-1β to fresh serum-free medium without IL-1β, which was collected after 24h. Complete removal of EVs was achieved by ultracentrifugation of the medium for 18 h at 100,000 × g. Conditioned medium containing EVs from IL-1β-treated chondrocytes increased the mRNA levels of the catabolic markers Cox-2, IL-6 and MMP-13 and decreased the mRNA levels of type II collagen with medium from 10 ng/ml IL-1β-treated chondrocytes being the most effective compared to medium from vehicle-treated chondrocytes (Fig. 1A). Complete removal of EVs from the medium resulted in a reduced increase of the mRNA levels of Cox-2, IL-6 and MMP-13 and a reduced decrease in the mRNA levels of type II collagen compared to EV-containing medium from chondrocytes treated with various concentrations of IL-1β (Fig. 1A). Furthermore, the concentration of the released proenzyme form of MMP-13 (pro-MMP-13) increased from chondrocytes that were treated with medium containing EVs from IL-1β-treated chondrocytes, compared to cells, which were treated with medium containing EVs from vehicle-treated cells. Medium containing EVs from chondrocytes treated with 10 ng/ml IL-1β led to the highest concentration of pro-MMP-13 released into the medium (Fig. 1B). Removal of EVs from the medium of IL-1β-treated chondrocytes resulted in a markedly reduced concentration of pro-MMP-13 released from chondrocytes (Fig. 1B). These findings establish the important role of EVs released by chondrocytes in the communication with other chondrocytes. Since 10 ng/ ml IL-1β treatment was the most effective concentration to stimulate catabolic events in articular chondrocytes without resulting in significant cell death the following experiments were all performed with 10 ng/ml IL-1β.
Figure 1: The effect of conditioned medium from IL-1β-treated chondrocytes containing EVs or after complete removal of EVs on the expression of catabolic markers and type II collagen.
A: mRNA levels of Cox-2, IL-6, MMP-13, and type II collagen (α1(II)) of human articular chondrocytes that were cultured in conditioned EV-containing medium (+ EVs) or conditioned medium after complete removal of EVs (-EVs) from articular chondrocytes treated for 24h with various concentrations of IL-1β (0, 2, 5, and 10 ng/ml) in serum-free medium. mRNA levels were determined by real time PCR analysis using SYBR Green and are expressed as relative units with the mRNA levels of chondrocytes cultured in conditioned medium from vehicle-treated chondrocytes set as 1. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01. B: The concentration of pro-MMP-13 released into the medium from articular chondrocytes cultured in EV-containing (+ EVs) conditioned medium or conditioned medium after complete removal of EVs from articular chondrocytes cultured in the presence of various concentrations of IL-1β (0, 2, 5, 10 ng/ml). The concentration of pro-MMP-13 released into the medium was determined by solid phase ELISA. The concentration of pro-MMP-13 was normalized to the total RNA concentration and is expressed as pg / ng total RNA / ml. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01.
Characterization of EVs released from vehicle-treated and IL-1β-treated human articular chondrocytes.
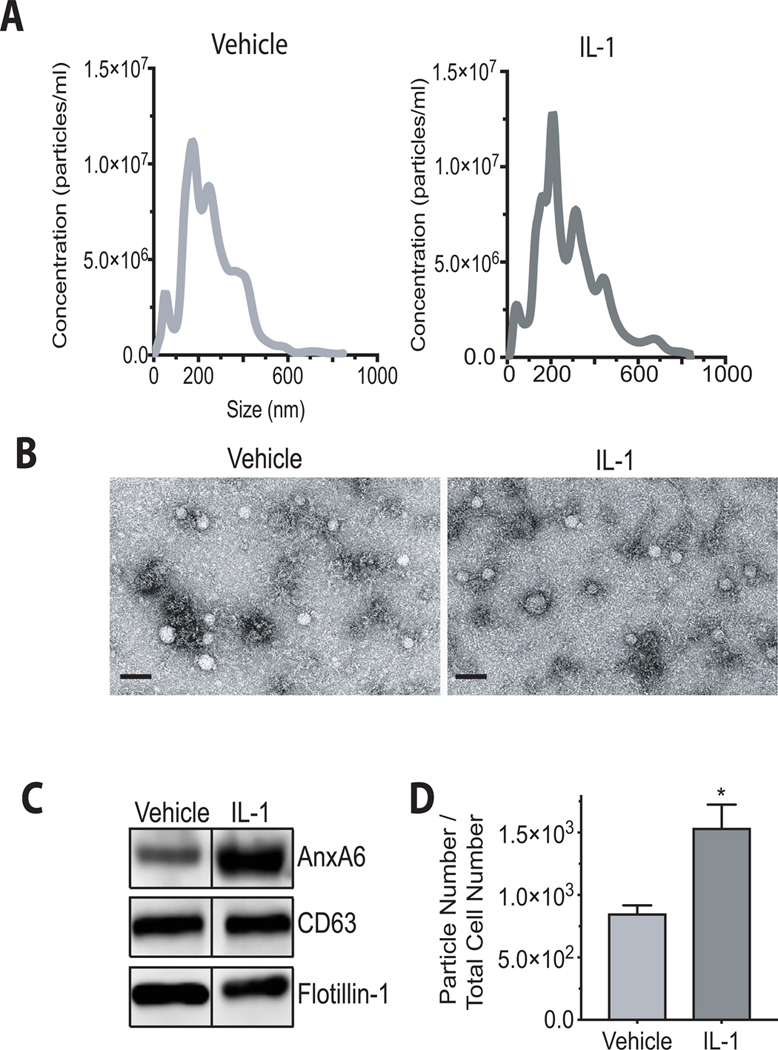
EVs were isolated from vehicle-treated or IL-1β-treated human articular chondrocytes. These cells were treated under serum-free conditions with IL-1β for 24 h. The size distribution of these particles and the number of particles released by chondrocytes were analyzed by NTA. EVs isolated from vehicle-treated or IL-1β-treated chondrocytes showed a similar size distribution with an average size of 204 ± 7 nm for EVs released from vehicle-treated chondrocytes and 191 ± 6 nm for EVs released from IL-1β-treated chondrocytes (Fig. 2A), as confirmed by transmission electron microscopy (Fig. 2B). We also evaluated the presence of EV-specific markers in our EV preparations by immunoblotting with antibodies specific for annexin A6 (AnxA6), CD63 and flotillin-1. These three markers were detected in lysates from EVs isolated from the medium of vehicle-treated and IL-1β-treated human articular chondrocytes (Fig. 2C). The number of EVs released by IL-1β-treated chondrocytes was markedly increased compared to the number of EVs released from vehicle-treated chondrocytes. The EV numbers were normalized to the total cell numbers (Fig. 2D).
Figure 2: Characterization of EVs released from vehicle-treated and IL-1β-treated human articular chondrocytes.
EVs released into the medium from human articular chondrocytes in the absence or presence of IL-1β. Human articular chondrocytes were serum starved for 24h followed by treatment with IL-1β (10ng/ml) in PBS/0.1%BSA (IL-1) or PBS/0.1%PBS (Vehicle) for 24h. A: Size distribution of EVs in the medium of vehicle-treated (Vehicle) and IL-1β-treated (IL-1) human articular chondrocytes was measured using nanoparticle tracking analysis. B: Representative transmission electron microscopy images of EVs isolated from the medium of vehicle-treated (Vehicle) and Il-1b-treated human articular chondrocytes. Bar, 200 nm. C: Immunoblot analysis of EV markers, AnxA6, CD63, and flotillin-1 in isolated EVs from the medium of vehicle-treated (Vehicle) and IL-1β-treated (IL-1) human articular chondrocytes. D: The numbers of EVs released into the medium of IL-1β-treated or vehicle-treated cells were analyzed by nanoparticle tracking analysis and normalized to the total cell number. Data were obtained from four different experiments and expressed as mean ± SD. *p < 0.01 vs. vehicle-treated cells.
The effect of EVs isolated from the medium of IL-1β-treated chondrocytes on articular chondrocytes.
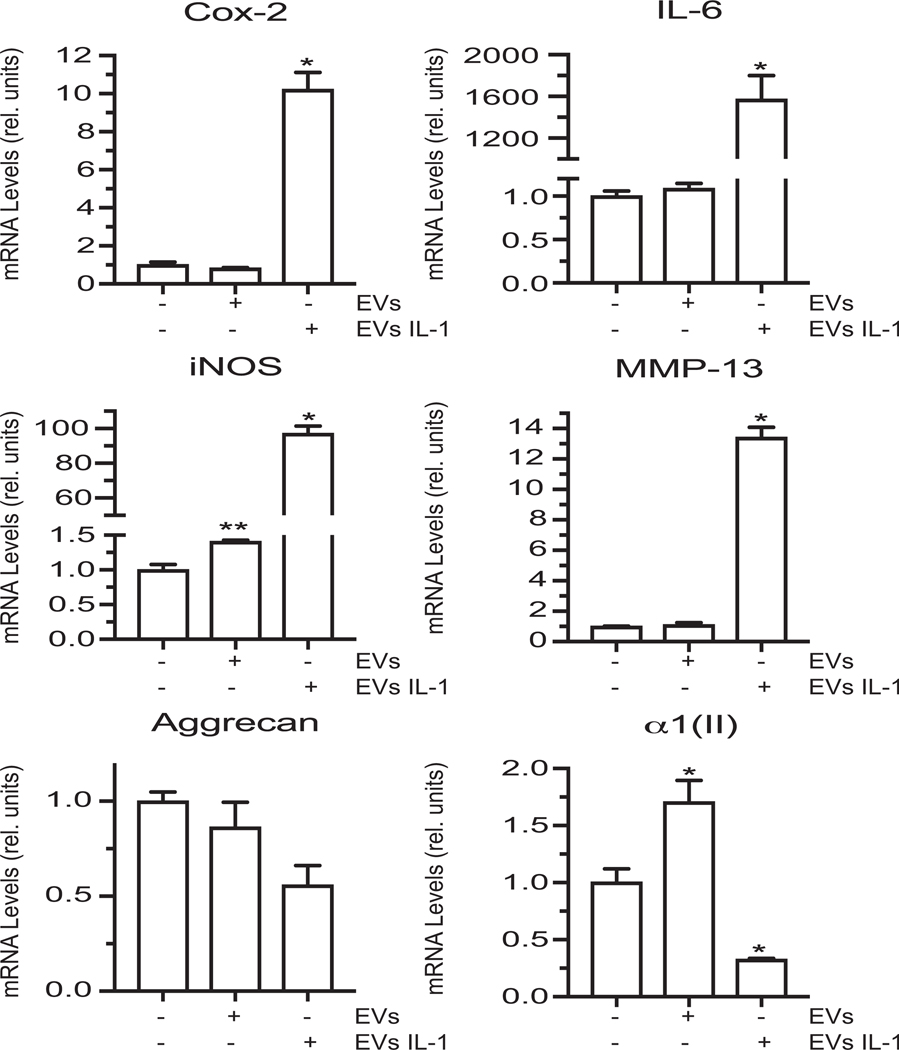
EVs were isolated by ultracentrifugation from the medium of vehicle-treated and IL-1β-treated human articular chondrocytes. Human articular chondrocytes were cultured in the presence of EVs (10μg of total protein) isolated from vehicle-treated or IL-1β-treated chondrocytes. EVs released from IL-1β-treated articular chondrocytes stimulated catabolic events in articular chondrocytes as indicated by the increased mRNA levels of catabolic markers, Cox-2, IL-6, iNOS and MMP-13, and the decreased mRNA levels of articular cartilage markers aggrecan and type II collagen, whereas EVs released from vehicle-treated ACs did not alter or only slightly increased the mRNA levels of Cox-2, IL-6, iNOS, MMMP-13, and aggrecan compared to articular chondrocytes not treated with EVs (Fig. 3). EVs isolated from the medium of vehicle-treated chondrocytes increased the mRNA levels of type II collagen compared to the levels of cells not treated with EVs (Fig. 3).
Figure 3: The effect of EVs isolated from the medium of vehicle-treated or IL-1β-treated articular chondrocytes on catabolic and anabolic marker mRNA levels of articular chondrocytes.
mRNA levels of catabolic markers Cox-2, IL-6, iNOS, MMP-13 and articular cartilage markers aggrecan and type II collagen (α1(II)) in human articular chondrocytes cultured in the absence or presence of EVs isolated from vehicle-treated (EVs) or IL-1β-treated human chondrocytes (EVs IL-1). mRNA levels were determined by real time PCR analysis using SYBR Green and normalized to the levels of 18S RNA. Values are expressed as relative units with the mRNA levels of human articular chondrocytes cultured in the absence of EVs set as 1. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01.
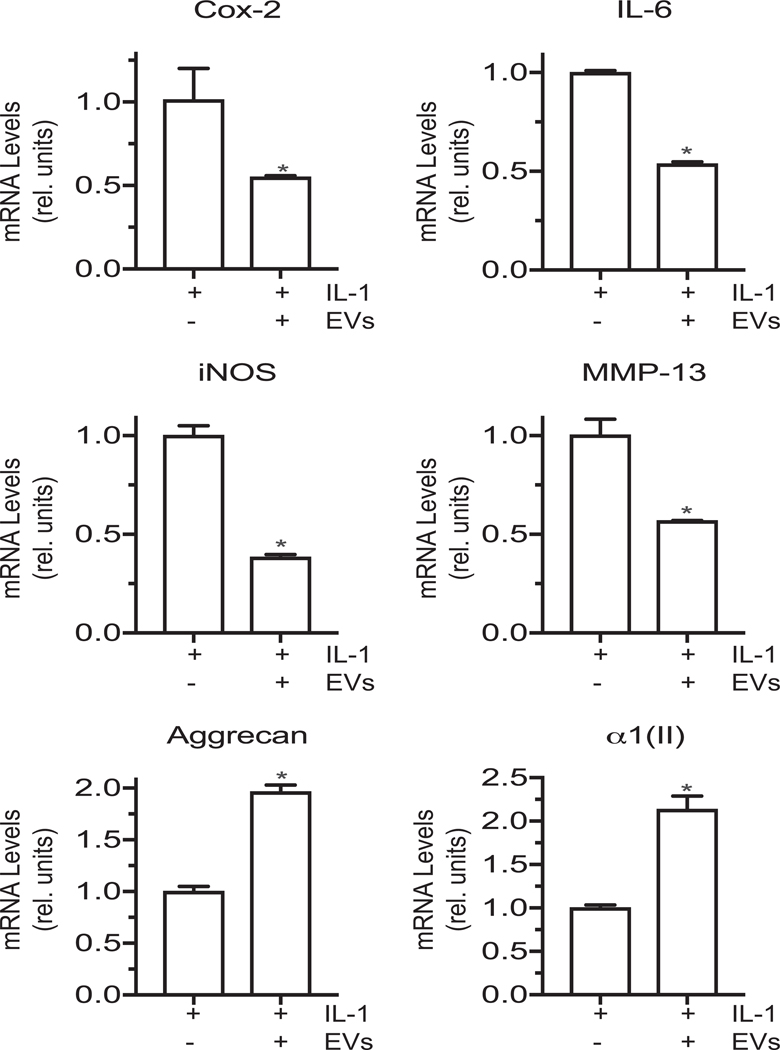
The effect of EVs isolated from the medium of vehicle-treated chondrocytes on IL-1β-treated articular chondrocytes.
EVs isolated from the medium of vehicle-treated human articular chondrocytes reduced the mRNA levels of Cox-2, IL-6, iNOS and MMP-13, and increased the mRNA levels of articular cartilage markers aggrecan, type II collagen in IL-1β-treated articular chondrocytes (Fig. 4).
Figure 4: The effect of EVs isolated from the medium of vehicle-treated articular chondrocytes on catabolic and anabolic marker mRNA levels of articular chondrocytes.
mRNA levels of catabolic markers Cox-2, IL-6, iNOS, MMP-13 and articular cartilage markers aggrecan and type II collagen (α1(II)) in IL-1β-treated human articular chondrocytes cultured in the absence or presence of EVs isolated from vehicle-treated (EVs) human articular chondrocytes. mRNA levels were determined by real time PCR analysis using SYBR Green and normalized to the levels of 18S RNA. Values are expressed as relative units with the mRNA levels of IL-1β-treated human articular chondrocytes set as 1. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01 vs. IL-1β treated cells.
The effect of conditioned medium containing EVs or after complete removal of EVs from vehicle-treated or IL-1β-treated chondrocytes on chondrogenic differentiation of C3H10T1/2 cells.
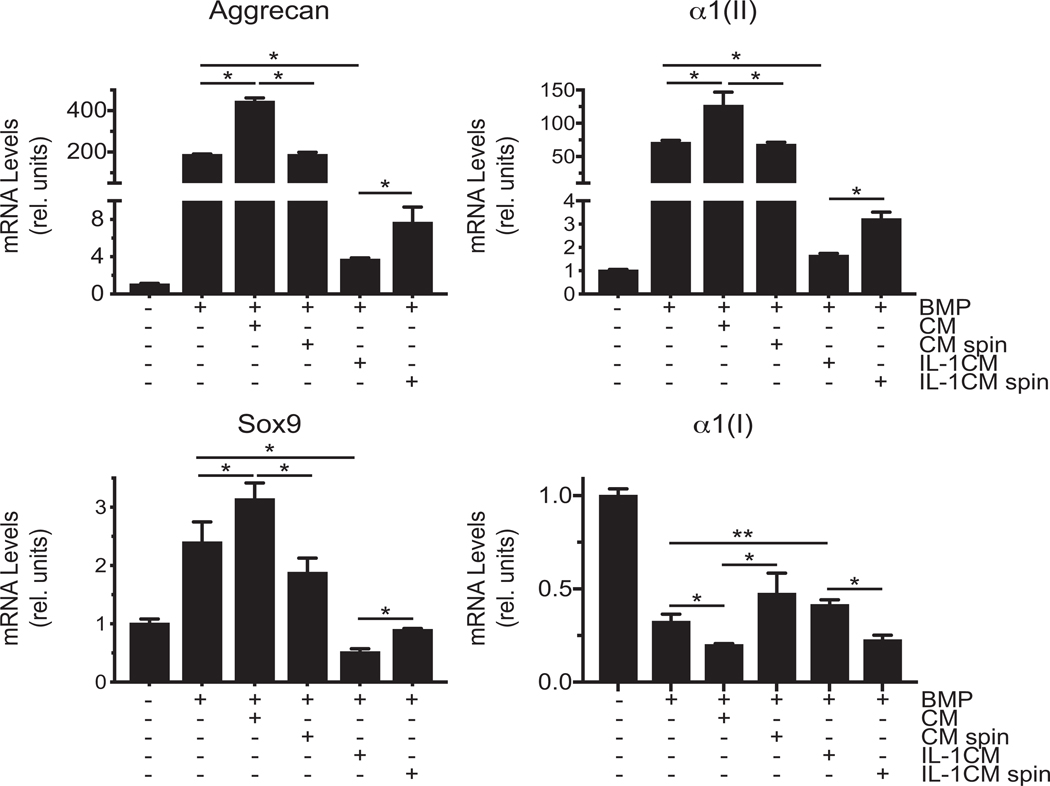
Next, we determined how conditioned medium containing EVs and conditioned medium after complete removal of EVs from vehicle-treated or IL-1β-treated articular chondrocytes affect chondrogenic differentiation of C3H10T1/2 cells. To induce chondrogenic differentiation, C3H10T1/2 cells were cultured in chondrogenic differentiation medium in the presence of BMP-2 in micromass cultures. The degree of chondrogenic differentiation was determined by increased mRNA levels of aggrecan, Sox-9 and type II collagen and decreased type I collagen mRNA levels in micromass cultures of C3H10T1/2 cells in the presence of BMP-2 compared to micromasses cultured in the absence of BMP-2 (Fig. 5). Conditioned medium from vehicle-treated articular chondrocytes further increased mRNA levels of aggrecan, type II collagen and Sox9 and further decreased mRNA levels of type I collagen in BMP-treated micromasses, whereas conditioned medium from IL-1β-treated chondrocytes decreased mRNA levels of these chondrogenic markers and increased mRNA levels of type I collagen (Fig. 5). Conditioned medium from vehicle-treated chondrocytes from which EVs were completely removed did not stimulate chondrogenic differentiation as indicated by similar mRNA levels of aggrecan, type II collagen and Sox9 as in BMP-2-treated micromasses. Complete removal of EVs from conditioned medium from IL-1β-treated chondrocytes resulted in increased mRNA levels of aggrecan, type II collagen and Sox9 and decreased mRNA levels of type I collagen compared to EV containing conditioned medium from IL-1β-treated chondrocytes (Fig. 5).
Figure 5: The effect of conditioned medium from the medium of vehicle-treated or IL-1β-treated articular chondrocytes containing EVs or after complete removal of EVs on chondrogenic differentiation of C3H/10T1/2 cells.
mRNA levels of aggrecan, type II collagen (α1(II)), Sox9, and type I collagen (α1(I)) during chondrogenic differentiation of C3H/10T1/2 cells cultured in micromasses in the absence or presence of BMP-2 (BMP) and conditioned medium containing EVs from vehicle-treated (CM) or IL-1β-treated (IL-1CM) mouse articular chondrocytes or conditioned medium from vehicle-treated (CM spin) or IL-1β-treated (IL-1CM spin) mouse articular chondrocytes, from which EVs were completely removed by ultracentrifugation. mRNA levels were determined by real time PCR analysis using SYBR Green and are expressed as relative units with the mRNA levels of untreated micromasses set as 1. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01; **p < 0.05.
The effect of EVs isolated from the medium of vehicle-treated or IL-1β-treated chondrocytes on chondrogenic differentiation of C3H10T1/2 cells.
C3H10T1/2 micromasses cultured in the presence of BMP-2 and EVs (10μg of total protein) isolated from vehicle-treated articular chondrocytes showed increased alcian blue staining compared to micromasses cultured in the presence of BMP-2 only (Figs. 6B, D). When BMP-2-treated micromasses were cultured in the presence of EVs isolated from IL-1β-treated articular chondrocytes alcian blue staining was markedly reduced to level similar to untreated micromass cultures (Figs. 6A, C). Similarly, immunostaining with antibodies specific for type II collagen was increased in sections of micromasses cultured in the presence of BMP-2 and EVs isolated from vehicle-treated chondrocytes compared to micromasses cultured in BMP-2 alone (Figs. 6F, H). When micromasses were treated with BMP-2 and EVs isolated from IL-1β-treated chondrocytes little immunostaining for type II collagen was detected similar to micromasses cultured in the absence of BMP-2 (Figs. 6E, G). mRNA levels of aggrecan and type II collagen were also markedly reduced in BMP-2-treated C3H10T1/2 micromassses in the presence of EVs isolated from the medium of IL-1β-treated articular chondrocytes, whereas EVs isolated from the medium of vehicle-treated chondrocytes increased mRNA levels of aggrecan and type II collagen in BMP-2-treated C3H10T1/2 micromass cultures (Fig. 6I). These findings reveal that EVs released from articular chondrocytes stimulate chondrogenic differentiation of precursor cells, whereas EVs released from articular chondrocytes in an inflammatory environment inhibit chondrogenic differentiation.
Figure 6: The effect of EVs isolated from the medium of vehicle-treated or IL-1β-treated articular chondrocytes on chondrogenic differentiation of C3H/10T1/2 cells.
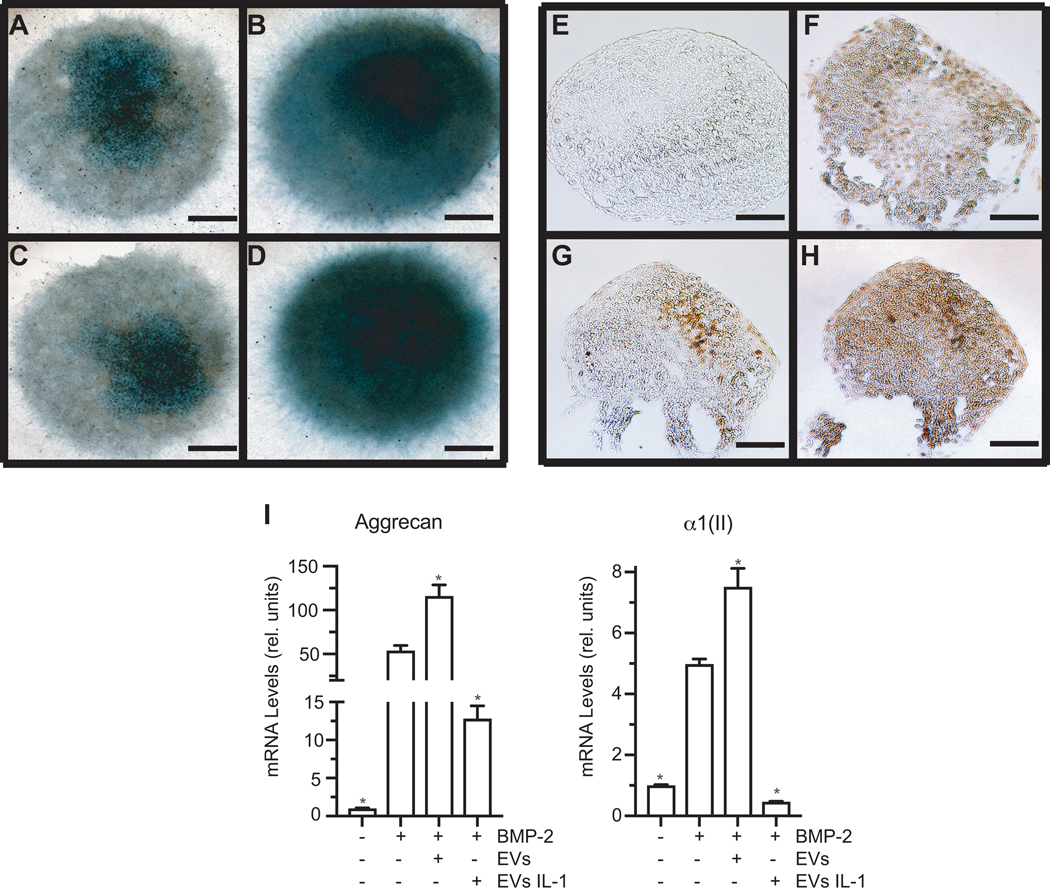
A – H: Alcian blue (Alcian Blue) staining and immunostaining for type II collagen (Type II Collagen) of C3H10T1/2 micromasses cultured in chondrogenic differentiation medium in the absence (A, E) or presence of BMP-2 (B, F), BMP-2 plus EVs isolated from the medium of IL-1β-treated human articular chondrocytes (C, G), or BMP-2 plus EVs isolated from the medium of vehicle-treated articular chondrocytes (D, H). I: mRNA levels of aggrecan and type II collagen (α1(II)) of C3H10T1/2 micromasses cultured in chondrogenic differentiation medium in the absence or presence of BMP-2, BMP-2 and EVs isolated from the medium of IL-1β-treated human articular chondrocytes, or BMP-2 and EVs isolated from the medium of vehicle-treated articular chondrocytes. mRNA levels were determined by real time PCR analysis using SYBR Green and are expressed as relative units with the mRNA levels of untreated micromasses set as 1. Data were obtained from four different experiments and are expressed as mean ± SD. *p < 0.01 vs. BMP-2-treated micromass cultures.
Discussion
The findings of this study indicate that EVs released by articular chondrocytes play a major role in communication among chondrocytes, and between chondrocytes and stem cells. In addition, our findings show that the effects of EVs released by articular chondrocytes on other chondrocytes or stem cells depend on the state of the chondrocyte. EVs released from articular chondrocytes cultured in a normal environment were chondro-protective and decreased catabolic events in IL-1β-treated chondrocytes, whereas EVs released from chondrocytes cultured in an inflammatory environment stimulated catabolic events in articular chondrocytes. Furthermore, EVs released from IL-1β-treated articular chondrocytes inhibited chondrogenesis of precursor cells, whereas EVs released from vehicle-treated articular chondrocytes stimulated chondrogenesis. These findings confirm previous studies showing that the nature of EV cargo is cell-type specific and is influenced by the physiological and pathological state of the donor cell, the stimuli that modulates their production and release, and the molecular mechanism that leads to their biogenesis.2, 3, 20–23 More importantly, our findings suggest that in an inflammatory environment such as after joint injury or during osteoarthritis, articular chondrocytes and, as shown in other studies, synovial fibroblasts release EVs that will accelerate cartilage degradation and will inhibit endogenous precursor cells present in the joint from repairing cartilage lesions.4, 24
Our findings demonstrate that EVs released into the medium from vehicle-treated chondrocytes were responsible for stimulating chondrogenic differentiation of C3H10T1/2 cells as only medium containing EVs stimulated mRNA levels of aggrecan, Sox-9 and type II collagen in BMP-2-treated micromass cultures. Medium from which EVs were completely removed did not stimulate chondrogenic differentiation. In contrast, medium from IL-1β-treated articular chondrocytes containing EVs and also medium from which EVs were completely removed inhibited chondrogenic differentiation of BMP-2-treated micromass cultures. However, medium containing EVs was more effective in inhibiting chondrogenesis than medium which did not contain EVs. These findings suggest that articular chondrocytes in a physiological state release EVs that promote chondrogenic differentiation of precursor cells, whereas articular chondrocytes in an inflammatory state release soluble factors and EVs that both inhibit chondrogenic differentiation of precursor cells.
Our data showing that conditioned medium from IL-1β-treated chondrocytes after complete removal of EVs and wash out of IL-1β was still able to reduce chondrogenic differentiation of C3H10T1/2 cells even though to a lesser degree than EV-containing medium suggest that articular chondrocytes in an inflammatory state release a variety of factors and cytokines, including but not limited to IL-6, IL-8, tumor necrosis factor-alpha (TNF-α) and MMPs and EVs that inhibit chondrogenic differentiation. Our findings are consistent with previous findings showing reduced chondrogenic differentiation of MSCs in the presence of inflammatory cytokines.25, 26
Since, as discussed above, EVs enclose a protected microenvironment, these particles carry proteins, growth factors and cytokines and also mRNAs, non-coding RNAs and microRNAs.1, 2 More importantly, EVs are more stable than soluble factors released by a cell in an in vivo environment and therefore can act much more efficiently on other cells. Furthermore, EVs have various ways to interact with recipient cells and affect their functions and phenotype. First, EVs similar to secreted factors can bind to cell surface receptors on the recipient cells via EV surface proteins or glycosaminoglycans, and induce signaling events in the recipient cell similar to soluble factors. Second, EVs can be taken up by recipient cells and release their cargo inside the recipient cell. The microRNAs from the EV cargo can then affect gene expression in the recipient cell. Third, full length mRNA released from the EVs can be translated into protein by the recipient cell. Therefore, it is very likely that EVs have a greatly enhanced ability to affect the recipient cell, especially in vivo, relative to soluble factors released by a cell.
As with any study, limitations were noted. Similar to most in vitro studies using IL-1β, the concentrations of IL-1β used in our study do not necessarily reflect pathophysiological conditions in vivo. Furthermore, for the determination of the changes in the expression levels of catabolic and anabolic markers in human articular chondrocytes, we mostly analyzed the mRNA levels and not the protein levels except for the proenzyme form of MMP-13, which was analyzed by a solid phase ELISA.
Conclusion
Our findings show that EVs released by articular chondrocytes affect recipient articular chondrocytes and MSCs. The effect of EVs on recipient cells depends on the state of the articular chondrocyte from which these particles are released. EVs released from an articular chondrocyte cultured in a non-inflammatory state protects articular chondrocytes against an inflammatory state and promotes chondrogenesis of precursor cells, whereas EVs released from articular chondrocytes in an inflammatory state stimulate catabolic and inflammatory events in recipient chondrocytes and inhibit chondrogenesis of precursor cells. Our findings suggest that the understanding of the biogenesis of EVs released from joint cells may provide a novel therapeutic strategy for the treatment of joint pathologies and injuries.
Supplementary Material
Acknowledgements:
This study was partially funded by a grant from the National Institutes of Arthritis, Musculoskeletal and Skin Diseases (1R01AR064186 to T.K.). Xiaoming Liu was supported by China Scholarship Council (CSC NO. 201606260106).
References
- 1.Sedgwick AE, D’Souza-Schorey C. The biology of extracellular microvesicles. Traffic 2018; 19: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol 2016; 36: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desrochers LM, Antonyak MA, Cerione RA. Extracellular Vesicles: Satellites of Information Transfer in Cancer and Stem Cell Biology. Dev Cell 2016; 37: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 2016; 18: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabalee J, Towle R, Garnis C. The Role of Extracellular Vesicles in Cancer: Cargo, Function, and Therapeutic Implications. Cells 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant 2018; 27: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenis R, Zanutel R, Caponnetto F, Toffoletto B, Cifu A, Pistis C, et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediators Inflamm 2017; 2017: 4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J 2018. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther 2018; 9: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tofino-Vian M, Guillen MI, Perez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell Physiol Biochem 2018; 47: 11–25. [DOI] [PubMed] [Google Scholar]
- 12.Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep 2017; 7: 16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140–5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017; 7: 180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 2017; 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arth. Rheum. 1992; 35: 806–811. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol 2002. June 10; 157: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell KA, Minashima T, Zhang Y, Hadley S, Lee YJ, Giovinazzo J, et al. Annexin A6 Interacts With p65 and Stimulates NF-kappaB Activity and Catabolic Events in Articular Chondrocytes. Arthritis Rheum 2013; 65: 3120–3129. [DOI] [PubMed] [Google Scholar]
- 18.Lyons-Giordano B, Pratta MA, Galbraith W, Davis GL, Arner EC. Interleukin-1 differentially modulates chondrocyte expression of cyclooxygenase-2 and phospholipase A2. Exp Cell Res 1993; 206: 58–62. [DOI] [PubMed] [Google Scholar]
- 19.McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res 2013; 31: 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018; 75: 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol 2018; 28: R435–R444. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm 2014; 71: 537–543. [PubMed] [Google Scholar]
- 23.Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 2014; 12: e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther 2014; 16: R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagani S, Borsari V, Veronesi F, Ferrari A, Cepollaro S, Torricelli P, et al. Increased Chondrogenic Potential of Mesenchymal Cells From Adipose Tissue Versus Bone Marrow-Derived Cells in Osteoarthritic In Vitro Models. J Cell Physiol 2017; 232: 1478–1488. [DOI] [PubMed] [Google Scholar]
- 26.Borem R, Madeline A, Bowman M, Gill S, Tokish J, Mercuri J. Differential Effector Response of Amnion- and Adipose-Derived Mesenchymal Stem Cells to Inflammation; Implications for Intradiscal Therapy. J Orthop Res 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.