Abstract
Purpose.
To examine the in vivo microanatomy of retinal folds and geographic lesions in dogs with acquired or inherited retinal dysplasia.
Material and methods.
Thirteen dogs had retinal microanatomy evaluation under general anesthesia using cSLO/sdOCT; two eyes had non-inherited multifocal retinal folds, five had inherited multifocal retinal folds (drd1 or drd2), and 10 geographic retinal dysplasia. Retinas from two drd2 carrier dogs were examined by histology and immunohistochemistry (IHC) after in vivo imaging.
Results.
Retinal folds are the common feature of acquired focal/multifocal or geographic retinal dysplasia, are indistinguishable structurally from those associated with syndromic oculo-skeletal dysplasia, and represent outer nuclear layer invaginations and rosettes visible by sdOCT. In dogs heterozygous for oculo-skeletal dysplasia, the folds form clusters in a perivascular distribution along superior central vessels. IHC confirmed photoreceptor identity in the retinal folds. The geographic dysplasia plaques are not focally detached, but have inner retinal disorganization and intense autofluorescence in cSLO autofluorescence mode that is mainly limited to the geographic lesion, but is not uniform and in some extends beyond the plaques.
Conclusion.
We propose that the autofluorescent characteristic of the geographic lesions is associated with an inner retinal disruption associated with perivascular or infiltrating macrophages and phagocytosis of cellular debris. As well, we suggest restructuring the examination forms to distinguish the folds that are sporadically distributed from those that have a perivascular distribution as the latter likely represent carriers for drd. In this latter group, DNA testing would be a helpful tool to provide specific breeding advice.
Keywords: dog, geographic plaques, oculo-skeletal dysplasia, retinal dysplasia, retinal folds
Introduction
Retinal dysplasia (RD) is a defect of retinal differentiation which groups a number of unrelated abnormalities with different phenotypic characteristics and genetic, environmental or unknown etiologies.[1–5] In the dog, the generalized form of RD is characterized by detachment from the retinal pigment epithelium (RPE) of the abnormally developed retina at birth, or during the first 6 weeks of life, the time period when the retina undergoes the major period of postnatal maturation.[6, 7] Autosomal recessive inheritance of generalized RD is well supported for some though not in all reported breeds, e.g. Labrador retriever[8], Sealyham[9], Yorkshire[10] and Bedlington[11] terriers. The focal/multifocal form is characterized by a wide spectrum of ophthalmoscopically visible abnormalities ranging from retinal folds that appear as streaks, dots and/or vermiform lesions present singly or in multiples, and located in the tapetal or non-tapetal regions. Non-syndromic focal/multifocal retinal dysplasia is recognized as a familial trait in the American cocker spaniel[12], and as an autosomal recessive disorder in the English springer spaniel[13, 14]; in the latter breed, the folds are only part of a more complex pattern of retinal defects.[15, 16] In multiple other breeds, e.g. Beagle[17], Rottweiler[18] and Golden Retriever[19] possible inheritance of the trait is suspected but there is lack of supporting formal or convincing genetic studies.
Lastly, a version of the focal/multifocal form of RD, termed geographic RD, is recognized as a distinct clinical entity. The characteristic lesion is a thickened circular plaque of retinal tissue located in the posterior pole and/or equatorial regions of the fundus.[20] In the tapetal region, they appear darker and alter the normal tapetal reflectivity; in the non-tapetal region the lesions appear as circular gray to white plaques. Regardless of their location, retinal folds partially or completely surround the lesions. Unlike the generalized or focal/multifocal forms of RD, some dogs with geographic RD lesions do not have clinically evident fundus lesions early, before 10 weeks of age, and, more often, the lesions develop later when they are young adults.[20]
Retinal dysplasia is also a component of more severe inherited syndromic diseases, the so called oculo-skeletal dysplasias.[21–23] The disease loci are termed drd1 and drd2 (dwarfism with retinal dysplasia 1 and 2) in the Labrador retriever and Samoyed breeds, and represents non-allelic oculoskeletal disorders caused, respectively, by mutations in the COL9A3 and COL9A2 genes.[24] Homozygous affected dogs exhibit short-limbed dwarfism affecting the forelimbs, and a constellation of ocular changes which, in the most severe form, are characterized by complete retinal detachment and cataracts. Some heterozygous dogs have lesions limited to the retina, and consist of vitreal strands and focal/multifocal RD.[22, 23, 25] Although described as retinal folds, the essential clinical features of RD in dogs heterozygous for either mutation differ from the “garden variety” retinal folds found sporadically in dogs. In these dogs the retinal folds generally are located in the posterior pole and with a perivascular distribution along the superior venules and arterioles.[21] In contrast to the specific phenotypes present in homozygous and heterozygous drd1 and drd2 dogs, a recent study described a possibly syndromic RD found in a research colony derived from a single affected female Pit Bull terrier. The disease is inherited as autosomal dominant, and affected dogs are shorter in stature and have a spectrum of retinal lesions ranging from multifocal and geographic lesions to retinal detachment. To date, the disease-causing gene/mutation has not been identified.[26]
Because most of the milder phenotypes associated with RD, focal/multifocal folds or geographic lesions, cause little or no visual impairment, there is a paucity of histologic studies that can complement the clinically observed fundus changes. A detailed histologic study of retinal folds in the Beagle has been carried out[27], but, to our knowledge, the structural lesions of geographic RD have yet to be reported. Given that very high resolution in vivo imaging modalities are now available, e.g. confocal scanning laser ophthalmoscopy and spectral domain optical coherence tomography (cSLO and sdOCT), we have carried out detailed non-invasive imaging to define the in vivo retinal microanatomy of sporadic and inherited RD in the dog. For the purpose of this study we have used the term acquired to distinguish the sporadic disorders from those that truly are inherited. Our results show that, in spite of being etiologically distinct, retinal folds are structurally similar, and that the lesions of geographic RD point to an inner rather than outer retinal abnormality.
Material and methods
Dogs
Thirteen dogs were included in this study; summary details and clinical diagnoses for each are in Table 1. The 10 service dog candidates from The Seeing Eye, Inc. included German shepherds, Labrador retrievers and Labrador retriever/Golden retriever crosses. These dogs were bred as service dogs for the blind, and had their first ophthalmic examination (biomicroscopy and indirect ophthalmoscopy after pharmacologic mydriasis) at 5–6 weeks of age before leaving the breeding station for a “puppy raiser” home. With the exception of 1 dog, F379 (Table 1), in which retinal folds were noted at 5–6 weeks of age in the non-tapetal junction of the right eye, the remaining 9 dogs were normal at this first examination. Subsequently, F379 developed geographic retinal dysplasia in the tapetal region of the left eye, and the retinal folds disappeared from the right eye. At 14–15 months of age the dogs returned to the training center, and during routine pre-training ophthalmic examination using the same methods, the lesions noted in Table 1 were identified. The late development of geographic retinal dysplasia lesions is consistent with what we have previously reported.[20] None of the parents of dogs from The Seeing Eye, Inc. were affected with retinal dysplasia (focal, multifocal, geographic or generalized), or any other inherited ocular disorder as determined by yearly eye examinations, pre-breeding ERGs, and DNA testing for prcd in Labrador retrievers if parental status is unknown. In all but one case, the non-invasive retinal imaging was done at a single time point. The exception (Table 1- B191) was examined 3 times in the 15–29 month age interval.
Table 1.
Retinal dysplasia-affected dogs included in the study.
| Dog ID | Source | Gender | Breed | Age (months) at Diagnosis/study | Clinical Diagnosis | Genotype at drd1 and drd2 |
|---|---|---|---|---|---|---|
| K234 | Service dog candidate | Female | German shepherd | 17m | Multifocal RD OD | WT |
| B191-OD | Service dog candidate | Female | German shepherd | 15, 18, 29 m | Multifocal RD OD | WT |
| M111 | Penn Working Dog Center | Male | Labrador retriever | 14m | Multifocal RD OU | drd1 carrier |
| D355 | RDSF | Male | Mixed breed (Samoyed derived) | 4m | Multifocal RD OU | drd2 carrier |
| D358 | RDSF | Female | Mixed breed (Samoyed derived) | 4m | Multifocal RD OD | drd2 carrier |
| B191-OS | Service dog candidate | Female | German shepherd | 15, 18, 29 m | Geographic RD OS | WT |
| F045 | Service dog candidate | Female | Labrador retriever | 19m | Geographic RD OD | WT |
| X335 | Service dog candidate | Female | Labrador/Golden retriever cross | 14m | Geographic RD OS | WT |
| Y346 | Service dog candidate | Female | German shepherd | 15m | Geographic RD OU | WT |
| F379 | Service dog candidate | Female | Labrador retriever | 19m | Geographic RD OS | WT |
| Z186 | Service dog candidate | Male | Labrador/Golden retriever cross | 16m | Geographic RD OS | WT |
| Z187 | Service dog candidate | Male | Labrador/Golden retriever cross | 16m | Geographic RD OS | WT |
| M305 | Service dog candidate | Male | Labrador retriever | 16m | Geographic RD OS | |
| F051 | Service dog candidate | Male | German shepherd | 16m | Geographic RD OS | WT |
OD=right eye; OS=left eye; OU=both eyes
RD=retinal dysplasia
RDSF=Retinal Disease Studies Facility
WT=wildtype for drd1 and drd2 loci by DNA testing
One dog (Table 1, M111) being trained at the Penn Working Dog Center was identified during the annual ACVO/StokesRx National Service Animal Eye Exam program. Lastly, two dogs were part of the breeding colony at the Retinal Disease Studies Facility (RDSF) of the University of Pennsylvania, School of Veterinary Medicine. They were mixed breed with Samoyed background, and had originated from the colony used to map the drd2 locus that identified the causal COL9A2 gene/mutation responsible for oculo-skeletal dysplasia in the Samoyed breed (Table 1).[24] In none of the dogs was antibody or PCR testing for canine herpesvirus 1 performed.
Examination procedure
Ophthalmic examination was performed in all study dogs using binocular indirect ophthalmoscopy and biomicroscopy after mydriasis. Fundus photographs were taken with a hand-held Genesis-D Camera (Genesis; Kowa Company Ltd; Tokyo; Japan) directly or indirectly through the Volk 20 diopter lens[20], or with the RetCam wide field retinal imaging system using a 130° lens (Clarity Medical Systems, Inc.; Pleasanton; CA; USA). All procedures were in full compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approval by the University of Pennsylvania Institutional Animal Care and Use Committee (804956).
Genetic test for oculo-skeletal dysplasia syndrome
A 3ml blood sample was collected in EDTA tubes and sent to OptiGen, LLC (www.Optigen.com) from all dogs, and tested for COL9A2 and COL9A3 gene mutations. The results of the testing are summarized in Table 1. Except for 3 dogs that were carriers for drd1 or drd2, the remaining dogs were found to be homozygous wildtype (WT) at both loci.
Non-invasive in vivo retinal imaging
In vivo retinal imaging was performed with dogs under general anesthesia. Briefly, the dogs were pre-medicated with SQ acepromazine (Phoenix, Clipper Distributing Company, St-Joseph, MO) and atropine (Med-Pharmex Incorporated, Pomona, CA), and induced with IV propofol (4–6mg/kg; Zoetis, Kalamazoo, MI) and maintained in a semi-closed circuit of 2–3% Isoflurane (Akorn Inc., Lake Forest, IL). Imaging was performed using a combined confocal scanning laser ophthalmoscopy (cSLO) and spectral domain optical coherence tomography (sdOCT) Spectralis™ HRA/OCT instrument (Heidelberg Engineering, Germany). Near-infrared (IR) en face and autofluorescence (AF) images of the fundus were acquired using a 55° lens and 820 nm and 488 nm wavelengths, respectively. Vertical and horizontal sdOCT raster scans from the lesions and from normal retinal areas were obtained using a 30° lens. Settings were fixed in 49 sequential B-scans separated by a 120 μm distance and ART (automatic real time) mean of 9 for each scan; however, size of area was adjusted according to the size of lesion. Single B-scans were also performed from some lesions with settings fixed as 30° line and ART mean of 20. In all but one case (B191) raster and single scans were acquired using the high-resolution mode. In B191’s case raster and single scans were acquired using the high-speed mode due to strong eye movement, but following the same settings previously described. Detailed anesthesia and the cSLO and sdOCT procedures have been described in several of our recent publications.[28–30] To further characterize the geographic retina dysplasia lesions, we carried out manual segmentation of the OCT scans. Three retinal thickness measurements, each 250 μm apart, were taken in the approximate center of the lesion and in adjacent normal retina on the temporal and nasal sides in areas lacking prominent retinal vessels. The measurements included the distance from the internal to external limiting membrane (ILM-ELM) and ILM to vitreal border of outer nuclear layer (ILM-vONL).
Tissue processing, histopathology and immunohistochemistry (IHC)
After imaging, the two drd2 carrier dogs from the research colony were euthanatized with an overdose of pentobarbital sodium injected intravenously and the eyes collected. The right globes were fixed in 4% paraformaldehyde (PFA) in 0.1M phosphate-buffered saline at 4°C after a slit was made with a razor blade anterior to the ora serrata. After 3 hours in 4% PFA, the posterior segment was isolated, the vitreous gently removed, and the eyecup fixed for additional 24 hours in 2% PFA at 4°C. Tissues were then trimmed and cryoprotected for 24 hours each in 15% and 30% sucrose in 0.1M sodium phosphate and 0.15M sodium chloride at 4°C, and embedded in optimal cutting temperature medium, frozen, and stored at −80°C. Ten μm-thick retinal cryosections extending horizontally in the tapetal area through the retinal lesions were cut and used for immunohistochemistry (IHC), as previously reported.[31] Double labeling IHC using primary antibodies directed against rod opsin (Rho), human cone arrestin (hCA) and RPE65 was performed as previously described[31] to identify the presence of photoreceptors and RPE cells in the dysplastic lesions.
The left globes were fixed in Alcoholic Bouin’s 80% solution and transferred to 70% ethanol after 72 hours. Globes were then sent to Excalibur Pathology Inc. (Norman, OK) for further processing. Six μm-thick retinal sections extending horizontally in the tapetal area through the retinal folds were cut, stained with H&E and used for standard histological evaluation.
Results
Non-syndromic multifocal retinal dysplasia
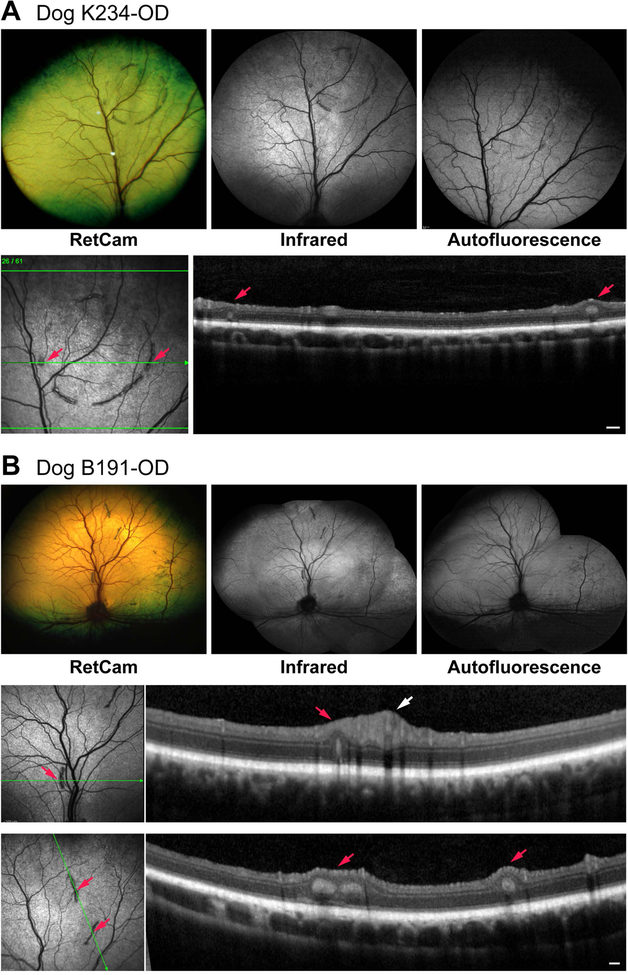
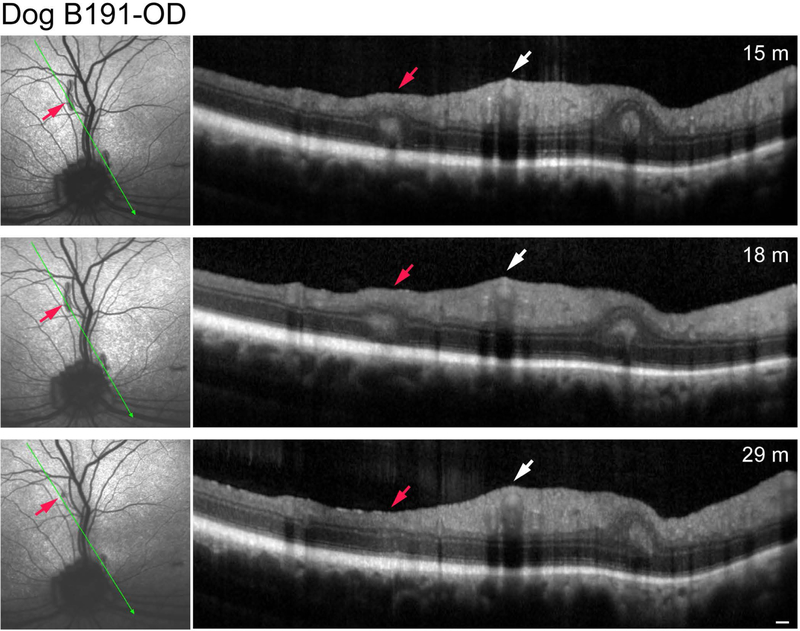
Two dogs (2 eyes) were diagnosed with acquired multifocal RD (Table 1). The lesions were typical of the retinal folds diagnosed clinically in dogs. In one (K234), the folds formed an incomplete ring-like pattern in the superior tapetal region just nasal to the superior vessels. This finding was suggestive of geographic RD except that the retina inside the ring was completely normal (Fig. 1A). The right eye of the second dog, B191, had the retinal folds scattered throughout the tapetal region (Fig. 1B). Non-invasive imaging of the dogs at 17 and 15 months, respectively, showed absence of AF in the folds or elsewhere in the retina (Table 2); sdOCT clearly identified the folds and rosettes in the lesion areas, while the remaining retina was unaffected. Follow up examinations of B191-OD at 18 and 29 months indicated faint AF of some folds at 29 months (Table 2; images not shown), and complete or partial disappearance of some folds and re-establishment at those sites of normal retinal structure by OCT at that age (Fig. 2).
Figure 1.
Clinical and imaging findings in dogs with focal/multifocal retinal dysplasia. In both dogs (A, B), there is lack of autofluorescence in the folds or in the adjoining normal retina. For each group of images, the lower set represent the sdOCT scans illustrated for the plane of the green arrow. Small red arrows identify the specific folds identified in the en face image and OCT scans. Inside and outside of the folds, the retina is structurally normal. White arrow (B) points to the normal thickness of inner retina at the site of the superior venule and arterioles. Scale bar = 200 μm for OCT scan in A, and both scans in B.
Table 2.
Summary of clinical, cSLO and sdOCT findings in the study dogs.
| Dog ID | Clinical findings | confocal Scanning Laser Ophthalmoscopy (cSLO) | Optical Coherence Tomography (sdOCT) | |
|---|---|---|---|---|
| AF mode | near IR | |||
| Multifocal Retinal Dysplasia | ||||
| K234 | folds in a circular pattern-tapetal region-OD | no AF | folds appear as dark gray streaks | retinal folds and rosettes; adjacent retina normal |
| B191-OD | linear folds-tapetal region-OD | faint AF within some folds at 29 months | folds appear as dark gray streaks; some improved at 29 months | retinal folds and rosettes; adjacent retina normal. Some folds became smaller and partially disappeared at 29 months |
| Syndromic Multifocal Retinal Dysplasia | ||||
| M111 | linear folds perivascular-OU | no AF | folds appear as dark gray streaks in perivascular pattern | retinal folds and rosettes; increased retinal thickness surrounding folds in supero-central retinal vessel region |
| D355 | linear folds perivascular-OU | no AF | folds appear as dark gray streaks in perivascular pattern | retinal folds and rosettes; increased retinal thickness surrounding folds in supero-central retinal vessel region |
| D358 | linear folds perivascular-OD | no AF | folds appear as dark gray streaks in perivascular pattern | retinal folds and rosettes; increased retinal thickness surrounding folds in supero-central retinal vessel region |
| Geographic Retinal Dysplasia | ||||
| B191-OS | linear folds-tapetal region and dysplastic plaque with surrounding folds-OS | no AF in plaque at 15 month; faint, punctate AF in folds around plaque and elsewhere in fundus | folds appear as dark gray streaks in perivascular pattern | retinal folds and rosettes; adjacent retina normal in non-plaque region; folds and rosettes surround plaque and inner retina thickened and disorganized |
| F045 | dysplastic plaque with surrounding folds-OD | AF in plaque and inferior extensions | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| X335 | dysplastic plaque with surrounding folds-OS | AF in lower 2/3 of plaque | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| Y346 | dysplastic plaque with surrounding folds-OU | Mild AF in inferior 2/3 of both plaques | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| F379 | dysplastic plaque with surrounding folds-OS | AF in 4/5 of plaque and temporal extension | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized; outer/inner retina in area of intense AF outside the plaque |
| Z186 | dysplastic plaque with surrounding folds-OS | AF in plaque and short temporal extension | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| Z187 | dysplastic plaque with surrounding folds-OS | AF in plaque with short finger-like extensions superiorly | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| M305 | dysplastic plaque with surrounding folds-OS | AF outside of plaque in surrounding retinal folds | folds are dark gray and plaque is distinct | folds and rosettes surround plaque; inner retina thickened and disorganized |
| F051 | dysplastic plaque with surrounding folds-OS | no AF | folds are dark gray and surround distinct plaque | folds and rosettes surround plaque; inner retina thickened and disorganized |
AF mode=autofluorescent mode; 488 nm wavelength
near IR=infrared mode; 820 nm wavelength
OD=right eye
OS=left eye
Figure 2.
Serial sdOCT scans of the same retinal fold of Dog B191-OD; plane of scan noted by thin green arrow. The red arrow points to the retinal fold that partially disappeared by 29 months of age. White arrow points to the normal thickness of inner retina at the site of the superior venule and arterioles. Note that the small fold present adjacent to and nasal to the optic disc remains unchanged. Scale bar = 200 μm for OCT scans.
Syndromic multifocal retinal dysplasia
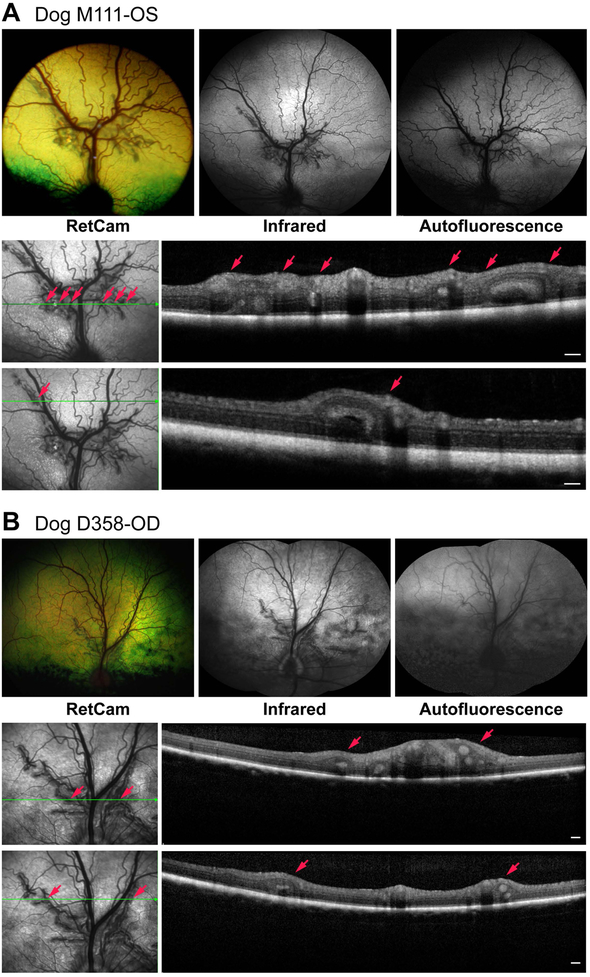
Both eyes of drd1 carrier dog (M111), and 3 of 4 eyes of two drd2 carrier dogs (D355, D358) had multifocal retinal folds that clustered in superior retina beginning ~1–2 disc diameters from superior disc edge. These clusters were located perivascularly around the branches of the principal superior venule and arterioles (Fig. 3) in a pattern that was the same as illustrated in the initial report of Labrador retrievers that were carriers for the disease eventually termed drd1.[21, 24] In all cases, the lesions presented as simple retinal folds or rosettes in the tapetal fundus, but no changes were observed in the retinal thickness or morphology on sdOCT outside of these lesions. Note, however, that as the retinal folds clustered around the superior central vessels, this region appeared thicker. Near-infrared images revealed the lesions as grey dark streaks, but no autofluorescence was observed in AF mode in any of them (Table 2, Fig. 3).
Figure 3.
Clinical and imaging findings in dogs with multifocal retinal dysplasia and having a heterozygous mutation in drd1 (A, Dog M111) or drd2 (B, Dog D358). In both cases the folds cluster perivascularly mainly along the superior venules and arterioles. For each group of images, the lower set represent the sdOCT scans illustrated for the plane of the horizontal green arrow. Small red arrows identify the specific folds identified in the en face image and OCT scans. Scale bar = 200 μm for OCT scans.
Retinal folds, rosettes and photoreceptors morphology in syndromic multifocal retinal dysplasia: drd2 carriers
Histopathology sections of drd2 carrier eyes were obtained approximately from same areas as the sdOCT scans taken during the in vivo imaging studies. The conventional H&E stained sections showed the retinal folds and rosettes characteristic of RD. These were located above the superior edge of the optic disc; elsewhere, retinal structure was well preserved and normal (Fig. 4A–C). Double fluorescent IHC confirmed the presence of rod and cone photoreceptors in the folds and rosette lumen using photoreceptor specific antibodies (Rho, hCA). Labeling against RPE65, an RPE-specific protein, showed that this cell layer was intact and did not extend into the lumen of the retinal folds (Fig. 4D–F).
Figure 4.
Histopathological and immunohistochemical findings in eyes of dogs heterozygous for drd2. A. Dog D355-OS. Vertical section of the globe through the pupil/optic disc plane. The large red rectangle illustrates the areas illustrated at higher magnification. A deep retinal fold is present close to the optic disc (lower small red rectangle) while the retina is normal more peripherally (upper small red rectangle). B and C. Illustration of single (B) and multiple (C) retinal folds present in Dog # D358-OD and D355-OD, respectively. Alcoholic Bouin’s fixation, paraffin embedding, H&E stained. D-F. Immunohistochemistry results of Dog D355-OD (D and F) and Dog D358-OD (E). The area sampled and antibodies used are shown in the images. The rhodopsin (Rho) and human cone arrestin (hCA) label specifically the rod outer segment (Rho) and cones (hCA), and shows that these cells are present in the folds and in the rosette lumen. RPE65 labeling is present exclusively in the RPE, and these cells are excluded from the folds and rosettes. Scale bars=50 μm.
Geographic retinal dysplasia
Geographic retinal dysplasia was clinically diagnosed in 9 dogs (Tables 1 and 2). Although the disease occurs in both the tapetal and non-tapetal regions, in our experience it is more frequently observed superiorly.[20] In the present study, all dogs imaged had geographic RD in the tapetal region. The lesion was unilateral in seven dogs, bilateral in one, and in one dog the fellow eye had multifocal RD.
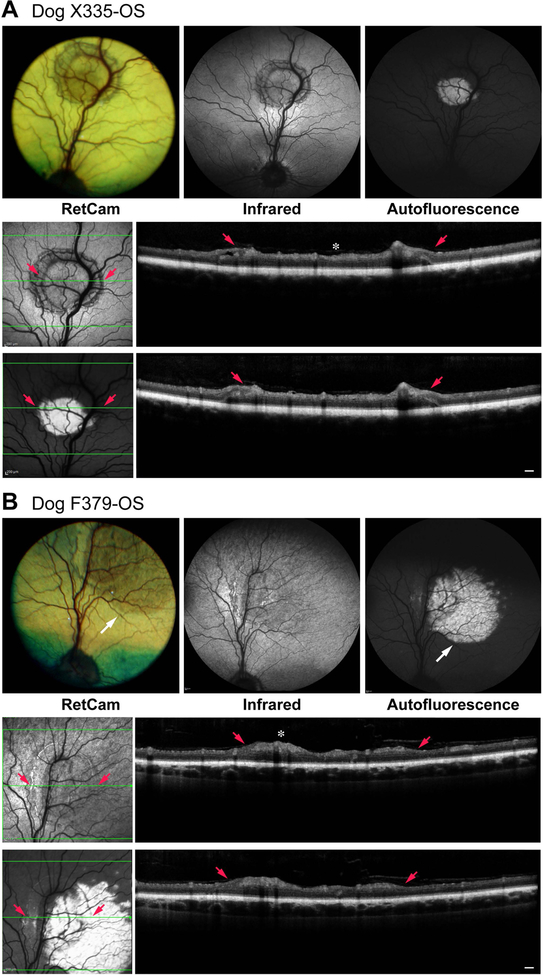
The characteristic fundus lesions of geographic RD are illustrated in Figs. 5 and 6; summary details of clinical and imaging results are presented in Table 2. The plaque-like lesions were present in the tapetal region and appeared as darker, circular lesions surrounded by a partial or complete ring of retinal folds. They appeared thicker, but showed no evidence of separation of the neural retina from the RPE. Some eyes showed extensions from the geographic plaque to presumably non-involved areas which showed brown discoloration (Fig. 5, F379), or finger like extensions from the plaque (Fig. 6, Z187). In most cases, the geographic RD plaques were associated with the major superior retinal venule, or a smaller tributary (Figs. 5 and 6).
Figure 5.
Clinical and imaging findings in dogs with geographic retinal dysplasia. The lesion plaque shows intense autofluorescence which is present in the lower 2/3 of the plaque (Dog X335) or extends beyond the plaque borders (Dog F379 white arrow). In Dog F379, the extension of the autofluorescence is paralleled by a change in the tapetal reflection visible in the fundus photograph. For each group of images, the lower set represent the sdOCT scans illustrated for the plane of the horizontal green arrow. Small red arrows point to specific folds at the borders of the geographic lesion, and identified in the en face image and OCT scans. Between the red arrows, the plaque lesion shows that IPL, GCL and NFL are thickened and irregular (white *). Outside the folds/plaque, retinal structure is normal even in areas that show intense autofluorescence (Dog F379, temporal to plaque). Retinal thickness measurements (ILM-ELM/ILM-vONL; see methods) in the center of the geographic lesion in the plane of the horizontal green arrow are: 162/107 μm for X335, and 143/101 μm for F379. The non-affected retina on either side of the lesion is ~123/75 in both eyes confirming that the increased retinal thickness involves the inner retina. Scale bar = 200 μm for OCT scans.
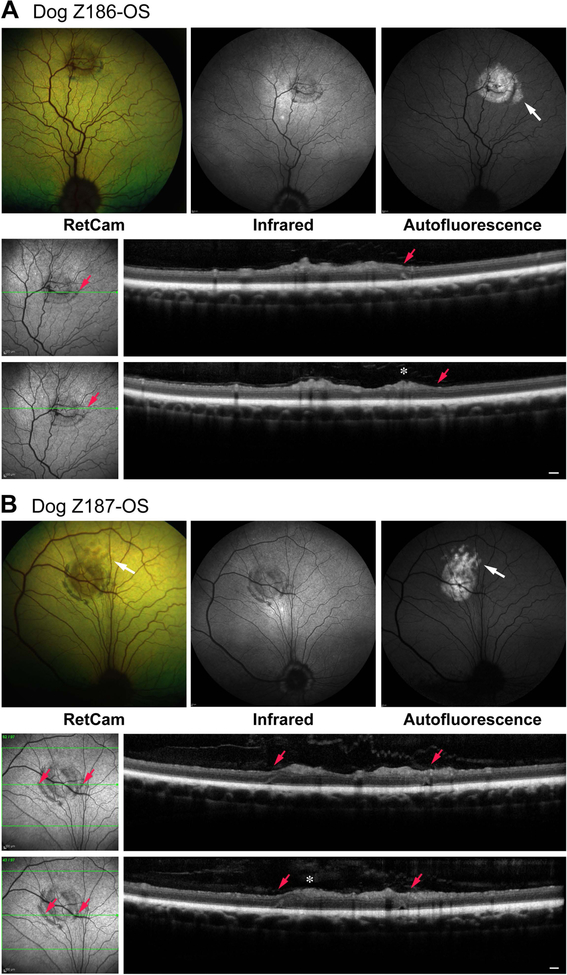
Figure 6.
Clinical and imaging findings in dogs with geographic retinal dysplasia. The lesion plaque shows intense autofluorescence which extends beyond the lower temporal border of the plaque (Dog Z186-OS) or as finger like projections (Dog Z187-OS) (white arrows). These finger like projections are readily visible in the fundus photograph of Dog Z187-OS. For each group of images, the lower set represent the sdOCT scans illustrated for the plane of the horizontal green arrow. Small red arrows point to specific folds at the borders of the geographic lesion, and identified in the en face image and OCT scans. Between or next to the red arrows, the plaque lesion shows that IPL, GCL and NFL are thickened and irregular (white *). Outside the folds/plaque, retinal structure is normal even in areas that show intense autofluorescence (Z186-OS, temporal to plaque). Retinal thickness measurements (ILM-ELM/ILM-vONL; see methods) of the geographic lesion taken in the plane of the horizontal green arrow for Dog Z186 (upper of 2 scans) and Dog Z187 (lower of 2 scans) are: 198/120 μm for Z186 and 186/108 μm for Z187. The non-affected retina on either side of the lesion is ~133/72 μm in both eyes confirming that the increased retinal thickness involves the inner retina. Scale bar = 200 μm for OCT scans.
Autofluorescent imaging with the cSLO showed that most of the lesions were intensely AF. When AF was limited to the plaque, in most cases it did not extend uniformly throughout the lesion, and instead was present in ~2/3–3/4 of the plaque (Fig. 5, X335). In other cases, e.g. F379 and Z187 (respectively Figs. 5 and 6; Table 2), AF extended beyond the clinically visible retinal plaque as a smooth circular patch, or finger like projections, and these corresponded to the tapetal color changes visible on the clinical examinations and documented in the color fundus images. The AF extensions appeared ‘deeper’ in the retina and were more intense and regular than the AF plaques. Two dogs (B191-OS and F051) did not have any evidence of AF in the geographic RD plaques when imaged at 15–16 months of age, and in a third dog (M305), faint AF was observed outside the plaque and limited to the surrounding retinal folds (Table 2). Re-examination of B191-OS at 18 and 29 months of age demonstrated that punctate AF developed in the folds around the plaque by 29 months of age.
Spectral domain OCT scans of the plaques and surrounding normal retinal regions showed changes limited to the inner retina-inner plexiform (IPL), ganglion cell (GCL) and nerve fiber (NFL) layers; these layers were thicker and apparently disorganized in all eyes. This area of abnormal inner retina was bordered by retinal folds and rosettes that structurally were indistinguishable from the focal/multifocal RD folds (Figs. 5 and 6; Table 2). In those cases where AF extended beyond the plaque border, sdOCT analysis of this region failed to identify any structural abnormalities in the retina or RPE (Fig. 5, F379). Lastly, in all cases, there was no evidence of separation of the neural retina from the RPE.
Discussion
Non-syndromic focal/multifocal, geographic and generalized RD are considered by many ophthalmologists as inherited retinal defects grouped under the RD rubric in the eye examination registries run by the ACVO (CAER-OFA) and ECVO (Eye Scheme). Even though the diseases are phenotypically and causally distinct, there has been the common misconception that the diseases may represent a continuum, hence the grouping under RD, with retinal folds representing the mildest and most insignificant form, and generalized RD representing the most severe because it causes complete blindness at an early age. Although named dysplasia to indicate abnormal development, so far the early developmental defect was only characterized in the English springer spaniel[14], and the generalized form as clinical and histologic abnormalities are recognized in the first 6 weeks of development, the critical period of postnatal retinal maturation in the dog.[6, 7]
Focal/multifocal RD often is seen at the time of the first clinical examination, i.e. ~5–6 weeks of age for some breeds; however, many times it is not as, for example, highlighted by the normal findings at 5–6 weeks in the eyes of 2 dogs (Table 1, K234-OD and B191-OD) in this study. As well, geographic retinal dysplasia fails to meet the criteria of dysplasia given that 22 of 23 dogs in a prior study[20], and all the dogs in the present study had a normal retinal examination at ~5–6 weeks of age in eyes that eventually developed geographic lesions. Obviously, the absence of disease using indirect ophthalmoscopy does not mean absence of microanatomic changes given that indirect ophthalmoscopy, at best, is not an absolute method of assessing retinal integrity. Regardless, while the term dysplasia is ingrained in the clinical ophthalmology lexicon, we need to be cautious and not assume that all of the disorders grouped under the dysplasia umbrella term represent developmental abnormalities that are congenital defects. That is certainly not the case for geographic RD.
Autosomal recessive inheritance has been clearly shown in generalized RD, e.g. in Bedlington terriers[11] and in another now uncommon disease found in Labrador retrievers.[8, 32] For the focal/multifocal and geographic forms of RD, breeding recommendations depend on the clinical diagnosis, being “breeder option” for focal/multifocal and “no” for the geographic form, yet these recommendations are made without any formal genetic studies. Furthermore, as focal/multifocal and geographic RD lesions generally are asymptomatic in dogs, there is very limited supporting histopathologic material. To begin to address questions about this very common but poorly understood group of diseases, we have taken advantage of high resolution in vivo imaging modalities to better characterize the features of the disease.
So what do the acquired and non-syndromic forms of focal/multifocal and geographic RD have in common? The one common finding in all is retinal folds. These appear as single or multiple in the focal/multifocal form, and encircling, either fully or partially, the characteristic retinal plaque of geographic RD. These folds also are indistinguishable from those associated with the drd1 or drd2 heterozygous state of syndromic RD (see below). Confocal SLO imaging in near IR mode shows the folds as dark gray streaks that have variable length, and their sides can be regular and smooth or wavy. Spectral domain OCT shows that the folds represent outer nuclear layer invaginations and rosettes which are the in vivo counterpart of the histologic abnormalities elegantly described by Weisse and associates [27] and illustrated in Fig. 4 of the present study.
In spite of finding that the structural features of retinal folds in acquired and non-syndromic forms of focal/multifocal and geographic RD are clinically and structurally indistinguishable, there are striking differences between the folds, and these are in their distribution pattern. Sporadic focal/multifocal folds can occur anywhere in the fundus, and the adjacent retina is normal by clinical examination or high resolution cSLO/sdOCT. In contrast, the folds present in carriers of drd1 or drd2 are located specifically in the posterior pole, above and near the optic disc, and appear as clusters of folds in a perivascular distribution along the superior venule and arterioles. The significance of this characteristic distribution is missed in routine screening examinations carried out by ACVO and ECVO diplomates as the default finding for retinal dysplasia is ‘folds’ and does not address the specific distribution of the lesions. Lastly, in regards to geographic RD, the folds are always adjacent to the plaque lesion, and OCT scans that extend through the major lesion show disorganized inner retina on one side of the fold, and structurally normal retina on the other (Figs. 5 and 6).
The results of the in vivo imaging studies of geographic RD were unexpected. The plaque-like lesion is thicker than surrounding normal retina, and the structural changes are limited to the inner retinal layers where the IPL, GCL and NFL are thickened and disorganized. In most cases, the geographic RD plaques are associated with the major superior retinal venule, or smaller tributaries. This vascular-associated distribution also was noted in our previous study for lesions occurring in the tapetal and non-tapetal regions.[20]
As well, the majority of the plaque lesions showed intense autofluorescence in cSLO AF mode. The AF generally was limited to the geographic lesions although not all lesions showed uniform AF throughout; indeed in some the AF extended beyond the plaque into regions with no abnormalities detected on in vivo cross-sectional imaging (Table 2 and Fig. 5). In the absence of supporting histopathology, it is tempting to speculate on the origin of the AF material. The inner retinal disruption associated with the plaque may be associated with perivascular or infiltrating macrophages and phagocytosis of cellular debris.[33] For AF outside the plaque, it is possible that this is located at the RPE level and represents photoreceptor cellular debris that has extended into the normal retina from the geographic lesion. However, these are only suggestions which must await rigorous histopathology and IHC assessment to transition this observation from speculation to fact.
Lastly, we did have eyes from heterozygous drd2 dogs for histopathologic examination. The eyes with retinal folds had characteristic folds and rosettes located superior to and near the optic disc in the regions where the superior central retinal venules and arterioles are located. As expected, the folds had rod and cone photoreceptors but no RPE, thus confirming that the folds form from invagination of the 9 inner layers of the neuroretina without involvement of the RPE.
To our knowledge this is the first cSLO/sd-OCT in vivo imaging study that demonstrates the microanatomic differences of the geographic and the multifocal forms of retinal dysplasia in dogs. As well, we suggest that it is important to more specifically define the distribution pattern of folds in examination forms to distinguish those that are sporadically distributed from those that have a perivascular distribution superiorly as the latter is likely to represent carriers for drd. In this latter group DNA testing is important to provide specific breeding advice.
Acknowledgement
Authors are grateful to the staff of The Seeing Eye, Inc. for assisting with animal transportation and medical record information, Theresa Jordan and all RDSF staff for assistance in the imaging studies, Svetlana Savina for wonderful technical assistance, Evelyn Santana for assistance with the images, Lydia Melnyk for research coordination and Dr. Charles-Antoine Assenmacher for helpful discussions on sources of autofluorescence in tissues. Authors appreciate the discounted research rate provided by OptiGen LLC for drd1 and drd2 molecular testing. This study was supported in part by Foundation Fighting Blindness and NEI/NIH grant EY-06855, and the Van Sloun Fund for Canine Genetic Research. The content of the project supported by the NEI/NIH is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
References
- 1.Lahav M, Albert DM, Wyand S. Clinical and histopathologic classification of retinal dysplasia. American Journal of Ophthalmology. 1973; 75: 648–667. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein AM, Osburn BI, Prendergast RA. The pathogenesis of retinal dysplasia. American Journal of Ophthalmology. 1971; 72: 13–21. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein AM, Parshall CJ Jr., Osburn BI, Prendergast RA. An experimental, virus-induced retinal dysplasia in the fetal lamb. American Journal of Ophthalmology. 1971; 72: 22–34. [DOI] [PubMed] [Google Scholar]
- 4.Percy DH, Carmichael LE, Albert DM, King JM, Jonas AM. Lesions in puppies surviving infection with canine herpesvirus. Veterinary Pathology. 1971; 8: 37–53. [DOI] [PubMed] [Google Scholar]
- 5.Shively J, Phemiste rR, Epling G, Jensen R. Pathogenesis of radiation-induced retinal dysplasia. Investigative Ophthalmology. 1970; 9: 888–900. [PubMed] [Google Scholar]
- 6.Aguirre GD, Rubin LF, Bistner SI. Development of the canine eye. American Journal of Veterinary Research. 1972; 33: 2399–2414. [PubMed] [Google Scholar]
- 7.Acland GM, Aguirre GD. Retinal degenerations in the dog: IV. Early retinal degeneration (erd) in Norwegian elkhounds. Experimental Eye Research. 1987; 44: 491–521. [DOI] [PubMed] [Google Scholar]
- 8.Kock E. Retinal dysplasia: a comparative study in human beings and dogs. Karolinska Institutet, Stockholm, 1974. [Google Scholar]
- 9.Ashton N, Barnett KC, Sachs DD. Retinal dysplasia in the Sealyham terrier. Journal of Pathology and Bacteriology. 1968; 96: 269–272. [DOI] [PubMed] [Google Scholar]
- 10.Stades FC. Hereditary retinal dysplasia (RD) in a family of Yorkshire terriers. Tijdschrift voor Diergeneeskunde. 1978; 103: 1087–1090. [PubMed] [Google Scholar]
- 11.Rubin L. Heredity of retinal dysplasia in bedlington terriers. Journal of the American Veterinary Medical Association. 1968; 152: 260–262. [Google Scholar]
- 12.MacMillan AD, Lipton DE. Heritability of multifocal retinal dysplasia in American Cocker Spaniels. Journal of the American Veterinary Medical Association. 1978; 172: 568–572. [PubMed] [Google Scholar]
- 13.Schmidt G, Ellersieck M, Wheeler C, Blanchard G, Keller W. Inheritance of retinal dysplasia in the English springer spaniel. Journal of the American Veterinary Medical Association. 1979; 174: 1089–1090. [PubMed] [Google Scholar]
- 14.Whitely HE. Dysplastic canine retinal morphogenesis. Investigative Ophthalmology and Visual Science. 1991; 32: 1492–1498. [PubMed] [Google Scholar]
- 15.Lavach J, Murphy J, Severin G. Retinal dysplasia in the English springer spaniel. Journal of the American Animal Hospital Association. 1978; 14: 192–199. [Google Scholar]
- 16.O’Toole D, Young S, Severin GA, Neumann S. Retinal dysplasia of English springer spaniel dogs: light microscopy of the postnatal lesions. Veterinary Pathology. 1983; 20: 298–311. [DOI] [PubMed] [Google Scholar]
- 17.Heywood R, Wells GA. A retinal dysplasia in the Beagle dog. Veterinary Record. 1970; 87: 178–180. [DOI] [PubMed] [Google Scholar]
- 18.Bedford PG. Multifocal retinal dysplasia in the rottweiler. Veterinary Record. 1982; 111: 304–305. [DOI] [PubMed] [Google Scholar]
- 19.Long SE, Crispin SM. Inheritance of multifocal retinal dysplasia in the golden retriever in the UK. Veterinary Record. 1999; 145: 702–704. [PubMed] [Google Scholar]
- 20.Holle DM, Stankovics ME, Sarna CS, Aguirre GD. The geographic form of retinal dysplasia in dogs is not always a congenital abnormality. Veterinary Ophthalmology. 1999; 2: 61–66. [DOI] [PubMed] [Google Scholar]
- 21.Nelson D, MacMillan A. Multifocal retinal dysplasia in field trial Labrador retrievers. Journal of the American Animal Hospital Association. 1983; 19: 388. [Google Scholar]
- 22.Meyers VN, Jezyk PF, Aguirre GD, Patterson DF. Short-limbed dwarfism and ocular defects in the Samoyed dog. Journal of the American Veterinary Medical Association. 1983; 183: 975–979. [PubMed] [Google Scholar]
- 23.Carrig CB, MacMillan A, Brundage S, Pool RR, Morgan JP. Retinal dysplasia associated with skeletal abnormalities in Labrador Retrievers. Journal of the American Veterinary Medical Association. 1977; 170: 49–57. [PubMed] [Google Scholar]
- 24.Goldstein O, Guyon R, Kukekova A, Kuznetsova TN, Pearce-Kelling SE, Johnson J, Aguirre GD, Acland GM. COL9A2 and COL9A3 mutations in canine autosomal recessive oculoskeletal dysplasia. Mammalian Genome. 2010; 21: 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrig CB, Sponenberg DP, Schmidt GM, Tvedten HW. Inheritance of associated ocular and skeletal dysplasia in Labrador retrievers. Journal of the American Veterinary Medical Association. 1988; 193: 1269–1272. [PubMed] [Google Scholar]
- 26.Rodarte-Almeida AC, Petersen-Jones S, Langohr IM, Occelli L, Dornbusch PT, Shiokawa N, Montiani-Ferreira F. Retinal dysplasia in American pit bull terriers--phenotypic characterization and breeding study. Veterinary Ophthalmology. 2016; 19: 11–21. [DOI] [PubMed] [Google Scholar]
- 27.Weisse I, Stotzer H, Seitz R. Die neuroepithelile invagination, eine form der netzhaut-dysplasie beim Beagle-hund. Zentralblatt für Veterinärmedizin. 1973; 20: 89–99. [PubMed] [Google Scholar]
- 28.Beltran WA, Cideciyan AV, Guziewicz KE, Iwabe S, Swider M, Scott EM, Savina SV, Ruthel G, Stefano F, Zhang L, Zorger R, Sumaroka A, Jacobson SG, Aguirre GD. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PloS One. 2014; 9: e90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltran WA, Cideciyan AV, Iwabe S, Swider M, Kosyk MS, McDaid K, Martynyuk I, Ying G-S, Shaffer J, Deng W-T, Boye SL, Lewin AS, Hauswirth WW, Jacobson SG, Aguirre GD. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. Proceedings of the National Academy of Sciences of the United States of America. 2015; 112: E5844–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cideciyan AV, Sudharsan R, Dufour VL, Massengill MT, Iwabe S, Swider M, Lisi B, Sumaroka A, Marinho LF, Appelbaum T, Rossmiller B, Hauswirth WW, Jacobson SG, Lewin AS, Aguirre GD, Beltran WA. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proceedings of the National Academy of Sciences of the United States of America. 2018; 115: E8547–E8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beltran WA, Hammond P, Acland GM, Aguirre GD. A frameshift mutation in RPGR exon ORF15 causes photoreceptor degeneration and inner retina remodeling in a model of X-linked retinitis pigmentosa. Investigative Ophthalmology and Visual Science. 2006; 47: 1669–1681. [DOI] [PubMed] [Google Scholar]
- 32.Barnett K, Bjorck G, Kock E. Hereditary retinal dysplasia in the Labrador retriever in England and in Sweden. Journal of Small Animal Practice. 1970; 10: 755–759. [Google Scholar]
- 33.Mendes-Jorge L, Ramos D, Luppo M, Llombart C, Alexandre-Pires G, Nacher V, Melgarejo V, Correia M, Navarro M, Carretero A, Tafuro S, Rodriguez-Baeza A, Esperanca-Pina JA, Bosch F, Ruberte J. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Investigative Ophthalmology and Visual Science. 2009; 50: 5997–6005. [DOI] [PubMed] [Google Scholar]