Abstract
Keratocystic odontogenic tumors (KCOTs) are locally aggressive odontogenic neoplasms with recurrence rates of up to 60%. Approximately 5% of KCOTs are associated with nevoid basal cell carcinoma (Gorlin) syndrome and 90% of these show genomic inactivation of the PTCH1 gene encoding Patched 1. Sporadic KCOTs reportedly have PTCH1 mutations in 30% of cases, but previous genomic analyses have been limited by low tumor DNA yield. The aim of this study was to identify recurrent genomic aberrations in sporadic KCOTs using a next-generation sequencing panel with complete exonic coverage of sonic hedgehog (SHH) pathway members PTCH1, SMO, SUFU, GLI1, and GLI2. Included were 44 sporadic KCOTs from 23 female and 21 male patients with a median age of 50 years (range, 10 to 82 y) and located in the mandible (N = 33) or maxilla (N = 11). Sequencing identified PTCH1 inactivating mutations in 41/44 (93%) cases, with biallelic inactivation in 35 (80%) cases; 9q copy neutral loss of heterozygosity targeting the PTCH1 locus was identified in 15 (34%) cases. No genomic aberrations were identified in other sequenced SHH pathway members. In summary, we demonstrate PTCH1 inactivating mutations in 93% of sporadic KCOTs, indicating that SHH pathway alterations are a near-universal event in these benign but locally aggressive neoplasms. The high frequency of complete PTCH1 loss of function may provide a rational target for SHH pathway inhibitors to be explored in future studies.
Keywords: odontogenic keratocyst, odontogenic cyst, odontogenic tumor, sonic hedgehog, Gorlin
Keratocystic odontogenic tumors (KCOTs) are benign, locally aggressive odontogenic neoplasms and are among the most common lesions of odontogenic origin in the jaws.1,2 Most KCOTs (~90%) are incidental and solitary; an estimated 10% of patients may develop ≥ 2 KCOTs, and up to 5% of affected individuals have nevoid basal cell carcinoma (Gorlin) syndrome (NBCCS).3,4
KCOTs have a predilection for the posterior mandible and asymptomatic posteroanterior growth with cortical expansion occurring later in the disease course.5 The optimal surgical management of these patients is unclear due to the large size of some tumors at the time of diagnosis, the cystic and friable nature of the tissue, and the propensity for satellite cyst formation.6 On the basis of retrospective studies, recurrence rates are as high as 60% with curettage or enucleation, ~20% with adjunctive treatment such as peripheral ostectomy or cryotherapy, and approach 0% with en bloc or segmental resection.7 Carnoy solution, commonly used previously for chemical cauterization following enucleation and curettage, has been abandoned since the Food and Drug Administration banned the use of chloroform for compounding, and use of modified Carnoy solution (without chloroform) has yielded inadequate results.8 The management of syndromic patients or those with recurrent, synchronous or metachronous disease can be particularly challenging.
NBCCS, first described in 1960 by Gorlin and Goltz,9 results from germ-line pathogenic variants in the PTCH1 gene encoding patched 1, and, less commonly, in SUFU and PTCH2, all of which encode proteins crucial for sonic hedgehog (SHH) signaling.10–16 Accordingly, PTCH1 inactivation has been identified in 90% of syndromic KCOTs.17,18 In contrast, only 30% of sporadic KCOTs have been shown to have PTCH1 genomic inactivation.17–26 Most studies investigating sporadic KCOT report small sample sizes with a low yield of tumor DNA; however, a more recent study identified PTCH1 inactivation in 16/19 (84%) of KCOTs.27 Nevertheless, the paucity of published data and the heterogeneity of reported mutational findings resulted in the renaming of KCOT as “odontogenic keratocyst (OKC)” in the 2017 World Health Organization (WHO) classification.1,28
The aim of this study was to perform comprehensive mutational profiling of sporadic KCOT using a large next-generation sequencing (NGS) panel targeting cancer-associated genes including those of the SHH signaling pathway with the goal to improve histopathologic classification and nomenclature and to foster the discovery of novel therapeutic approaches.
MATERIALS AND METHODS
Case Selection
Cases of KCOT/OKC diagnosed between 2012 and 2018 were retrospectively identified in surgical pathology archives at University Hospitals Cleveland Medical Center/Case Western Reserve University School of Medicine and StrataDX, a surgical pathology laboratory in Lexington, MA affiliated with Harvard School of Dental Medicine. Hematoxylin and eosin-stained slides were reviewed by 2 specialists in oral and maxillofacial pathology (I.J.S. and R.S.M.) for diagnostic confirmation based on previously described diagnostic criteria.29 Cases selected were associated with no or minimal inflammation and had sufficient tumor content (ie, neoplastic cyst lining) of at least 30% tumor content following macro-dissection. This study was performed with approval by the Institutional Review Board at University Hospitals Cleveland Medical Center (Cleveland, OH).
Targeted NGS
NGS was performed using the targeted sequencing platform of Brigham and Women’s Hospital, OncoPanel, which interrogates the exonic sequences of 447 cancer-associated genes for mutations and copy number variations, and 191 introns across 60 genes for gene rearrangements.30,31 Single nucleotide polymorphisms known to be heterozygous in the population were targeted at 4 Mbp intervals. DNA extraction from formalin-fixed paraffin-embedded tissue sections of the tumor (QIAamp DNA mini kit; Qiagen, Valencia, CA), construction of hybrid-capture libraries, sequencing using the Illumina HiSeq. 2500 (Illumina, San Diego, CA), and sequence data analysis were performed as previously described.31 Sequencing was performed on tumor DNA only, without a paired non-neoplastic tissue section. All detected alterations (including single nucleotide variants, copy number alterations, and translocation calls) were reviewed manually and annotated as previously reported.31 Copy neutral loss of heterozygosity (CN-LOH) was determined based on deviation of single nucleotide polymorphism allele fractions from the 50% variant allele fraction expected in a diploid sample.
A total of 56 cases were included for sequencing analyses. Ten cases failed quality metrics due to low-sequencing quality and were excluded from the study.
RESULTS
A total of 46 cases were successfully analyzed by NGS, with a mean estimated tumor percentage of 34% (range, 10% to 50%), and mean target coverage of 256 (range, 24 to 402). Two cases with PTCH1 mutations at an allele frequency of ~0.5 and a clinical history suspicious for NBCCS were subsequently omitted from further analysis.
Clinical Findings
Table 1 summarizes the clinical findings of 44 sporadic KCOTs in 23 female and 21 male patients with a median age of 50 years (range, 10 to 82 y). Tumors were located in the mandible (N = 33) or maxilla (N = 11). Follow-up information was not available.
TABLE 1.
Clinicopatholoqic Findings in 44 Sporadic KCOTs
| Case # | Age (y) | Sex | Site | Size(cm) |
|---|---|---|---|---|
| 1 | 56 | M | R mandible | 3.0 |
| 2 | 59 | M | L mandible | 2.5 |
| 3 | 61 | F | R mandible | 2.3 |
| 4 | 66 | M | L mandible | 2.0 |
| 5 | 76 | F | R mandible | 0.5 |
| 6 | 64 | M | Ant. mandible | 3.0 |
| 7 | 34 | M | R maxilla | 2.5 |
| 8 | 38 | F | L mandible | 2.5 |
| 9 | 59 | F | L mandible | 0.6 |
| 10 | 66 | F | L mandible | 3.0 |
| 11 | 72 | M | R maxilla | 4.5 |
| 12 | 82 | F | L mandible | 3.0 |
| 13 | 45 | F | R maxilla | 1.6 |
| 14 | 17 | M | R maxilla | 1.5 |
| 15 | 20 | M | L mandible | 4.0 |
| 16 | 11 | M | L mandible | 3.5 |
| 17 | 20 | F | R mandible | 2.0 |
| 18 | 64 | M | Ant. mandible | 1.5 |
| 19 | 10 | F | L maxilla | Unknown |
| 20 | 35 | M | L mandible | 5.0 |
| 21 | 31 | M | L mandible | 3.0 |
| 22 | 69 | F | L maxilla | 2.0 |
| 23 | 72 | F | R mandible | Unknown |
| 24 | 18 | M | R mandible | 6.0 |
| 25 | 13 | M | Ant. mandible | Unknown |
| 26 | 21 | M | L mandible | Unknown |
| 27 | 25 | M | L mandible | 5.0 |
| 28 | 13 | F | R mandible | 1.0 |
| 29 | 19 | F | L mandible | Unknown |
| 30 | 60 | M | L mandible | Unknown |
| 31 | 54 | F | R mandible | 5.0 |
| 32 | 56 | F | R mandible | 5.0 |
| 33 | 28 | F | L maxilla | 6.0 |
| 34 | 21 | F | R mandible | 2.0 |
| 35 | 24 | M | Ant. Mandible | Unknown |
| 36 | 67 | F | R maxilla | Unknown |
| 37 | 57 | F | R mandible | Unknown |
| 38 | 54 | F | Ant. Mandible | Unknown |
| 39 | 70 | M | R mandible | 1.5 |
| 40 | 41 | F | L maxilla | Unknown |
| 41 | 71 | M | L maxilla | 2.0 |
| 42 | 50 | F | L mandible | Unknown |
| 43 | 56 | M | R maxilla | Unknown |
| 44 | 33 | F | L mandible | 2.0 |
Ant. indicates anterior; F, female; L, left; M, male; R, right.
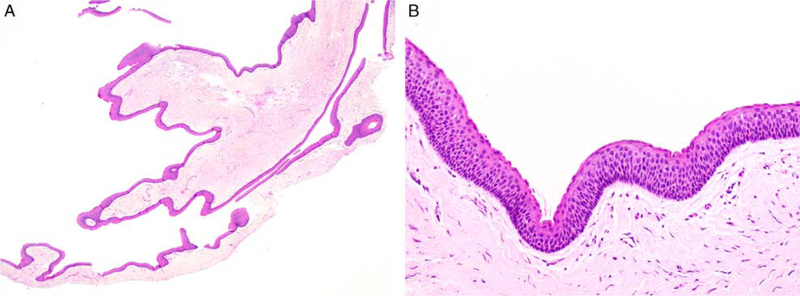
Histologically, all KCOTs consisted of benign, thinly parakeratinized stratified squamous epithelium with a palisaded and variably hyperchromatic basal cell layer supported by underlying fibrous connective tissue and devoid of significant inflammation (Fig. 1). Keratinaceous debris was occasionally present within the cyst lumen.
FIGURE 1.
All KCOTs were characterized by uniformly thin epithelial lining supported by fibrous connective tissue with minimal inflammation (A). The cystic lining is thinly parakeratinized and the basal cell layer exhibits nuclear hyperchromasia and focal palisading (B).
Targeted NGS Results
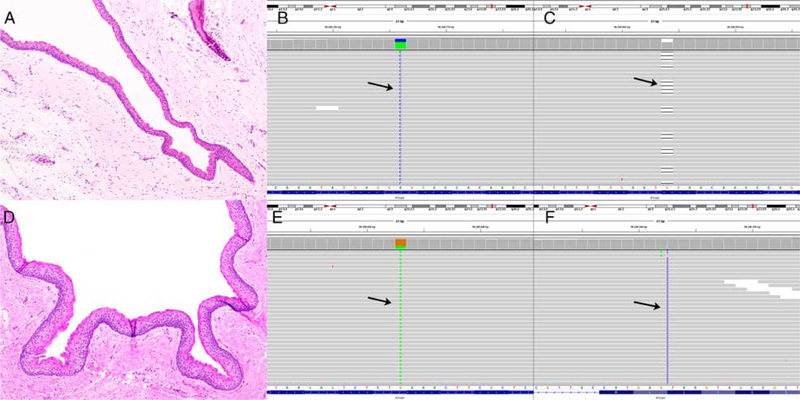
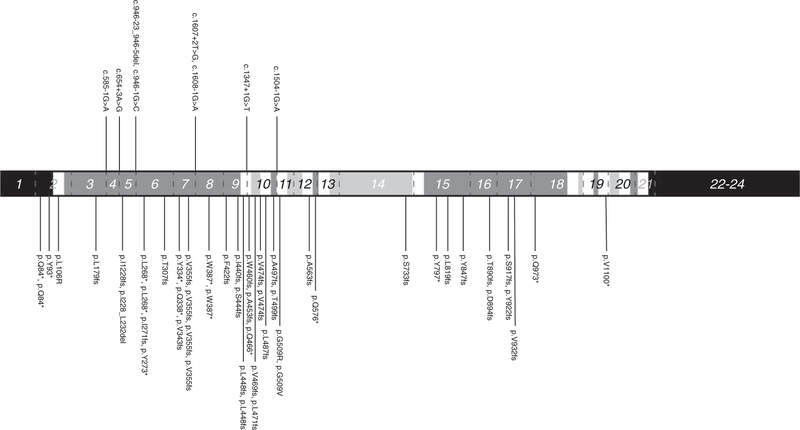
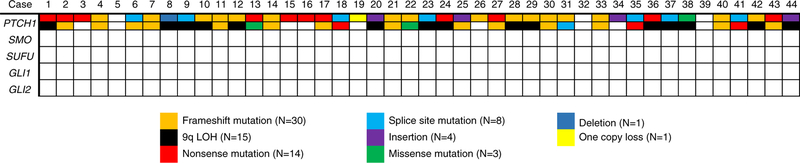
NGS results are summarized in Table 2. PTCH1 alterations were detected in 41/44 (93%) cases; no other somatic SHH pathway variants were detected (Figs. 2–4). All 3 PTCH1 wild-type cases were located in the mandible. The distribution of single nucleotide variants within the PTCH1 (NM_000264) coding region is shown in Figure 3. PTCH1 alterations resulted from frameshift (N = 30), nonsense (N = 14), splice site (N = 8), insertion (N = 4), missense (N = 3) mutations or deletions (N = 1). One case (#19) demonstrated 1 copy loss of the PTCH1 gene only. Twenty cases harbored 2 PTCH1 mutations and 15 cases harbored 9q CN-LOH resulting in biallelic PTCH1 inactivation (Fig. 5) resulting in a total of 35/44 (79.5%) cases with biallelic PTCH1 inactivation. Very rare additional somatic mutations and copy number alterations of uncertain significance were detected across the cohort.
TABLE 2.
Results of Targeted NGS in 44 Sporadic KCOTs
| Case # | Tumor (%) |
PTCH1 Single Nucleotide Variants/Insertion-Deletions |
PTCH1 Copy Number Alterations/LOH |
Biallelic PTCH1 Inactivation |
|---|---|---|---|---|
| 1 | 50 | c.819_822del;p.Y273* (VAF 0.58) | 9q CN-LOH | Yes |
| 2 | 40 | c.1012C>T;p.Q338* (VAF 0.26) c.2763delC;p.Y922fs (VAF 0.23) | — | Yes |
| 3 | 40 | c.1160G>A;p.W387* (VAF 0.21) | — | No |
| 4 | 35 | c.1064_1067del;p.V355fs (VAF 0.37) c.1410delG;p.L471fs (VAF 0.32) | — | Yes |
| 5 | 30 | — | — | No |
| 6 | 45 | c.946-1G>C (VAF 0.29) c.2454dupA;p.L819fs (VAF 0.24) | — | Yes |
| 7 | 40 | c.920delC;p.T307fs (VAF 0.27) c.1408_1420dup;p.V474fs (VAF 0.05) | — | Yes |
| 8 | 50 | c.682_696del;pI228_L232del (VAF 0.40) | 9q CN-LOH | Yes |
| 9 | 30 | c.654+3A>G (VAF 0.41) | 9q CN-LOH | Yes |
| 10 | 35 | c.1318delA;p.I440fs (VAF 0.46) | 9q CN-LOH | Yes |
| 11 | 35 | c.1357dupG;p.A453fs (VAF 0.19) c.1488dupC;p.A497fs (VAF 0.24) | — | Yes |
| 12 | 40 | c.681_684del;p.I228fs (VAF 0.72) | 9q CN-LOH | Yes |
| 13 | 40 | c.279delC;p.Y93* (VAF 0.34) c.317T> G;p.L106R (VAF 0.29) | — | Yes |
| 14 | 35 | c.812_815del;p.I271fs (VAF 0.22) c.1329_1336del;p.S444fs (VAF 0.14) | — | Yes |
| 15 | 32 | c.250C>T;pQ84* (VAF 0.16) | — | No |
| 16 | 40 | c.2391C>G;p.Y797* (VAF 0.24) | — | No |
| 17 | 30 | c.250C>T;p.Q84* (VAF 0.27) c.1341dupA;p.L448fs (VAF 0.23) | — | Yes |
| 18 | 30 | c.1067+2T>G (VAF 0.18) c.2917C>T;p.Q973* (VAF 0.14) | — | Yes |
| 19 | 40 | — | 9q22.32, 1 copy loss | No |
| 20 | 30 | c.3275_3297dup;p.V1100* (VAF 0.27) | 9q CN-LOH | Yes |
| 21 | 35 | c.1405delG;p.V469fs (VAF 0.21) c.2677_2680dup;p.D894fs (VAF 0.24) | — | Yes |
| 22 | 40 | c.1457dupG;p.L487fs (VAF 0.16) c.1525G>C;p.G509R (VAF 0.21) | — | Yes |
| 23 | 35 | c.1347+1G>T (VAF 0.38) | 9q CN-LOH | Yes |
| 24 | 35 | c.1160G>A;p.W387* (VAF 0.36) | 9q CN-LOH | Yes |
| 25 | 35 | c.1353_1377dup; p.W460fs (VAF 0.15) c.2793dupC;p.V932fs (VAF 0.24) | — | Yes |
| 26 | 40 | c.536delT;p.L179fs (VAF 0.16) | — | No |
| 27 | 40 | c.1002T>A;p.Y334* (VAF 0.32) c.1266_1269del;p.F422fs (VAF 0.25) | — | Yes |
| 28 | 35 | c.1412_1419dup; p.V474fs (VAF 0.14) | 9q CN-LOH | Yes |
| 29 | 40 | c.1062_1063delCG; p.V355fs (VAF 0.41) | 9q CN-LOH | Yes |
| 30 | 30 | c.1062_1063insA; p.V355fs (VAF 0.30) c.1342_1345del;p.L448fs (VAF 0.33) | — | Yes |
| 31 | 35 | c.1062delC;p.V355fs (VAF 0.16) c.1068-1G>A (VAF 0.15) | — | Yes |
| 32 | 30 | — | — | No |
| 33 | 30 | c.2197dupT;p.S733fs (VAF 0.08) c.2748delC;p.S917fs (VAF 0.10) | — | Yes |
| 34 | 35 | c.2522_2538dup;p.Y847fs (VAF 0.12) | — | No |
| 35 | 35 | c.585-1G>A (VAF 0.19) c.803T> G;p.L268* (VAF 0.21) | — | Yes |
| 36 | 30 | c.803T> A;p.L268* (VAF 0.42) | 9q CN-LOH | Yes |
| 37 | 30 | c.1504-1G>A (VAF 0.46) | 9q CN-LOH | Yes |
| 38 | 30 | c.1526G> T;p.G509V (VAF 0.47) | 9q CN-LOH | Yes |
| 39 | 30 | — | — | No |
| 40 | 30 | c.1026dupT;p.V343fs (VAF 0.32) c.1686delC;p.A563fs (VAF 0.30) | — | Yes |
| 41 | 20 | c.946-23_946-5del (VAF 0.07) c.1396C>T;p.Q466* (VAF 0.16) | — | Yes |
| 42 | 30 | c.1493dupC;p.T499fs (VAF 0.44) | 9q CN-LOH | Yes |
| 43 | 10 | c.1726C>T;p.Q576* (VAF 0.06) c.2668dupA;p.T890fs (VAF 0.05) | — | Yes |
| 44 | 30 | Insertion PTCH1 exon 10: PTCH1 exon 10 | 9q CN-LOH | Yes |
LOH indicates loss of heterozygosity; VAF, variant allele fraction.
FIGURE 2.
Case #13: KCOT (A) characterized by a PTCH1 missense mutation (c.317T > G;p.L106R) (B, arrow) and a PTCH1 nonsense mutation (c.279delC;p.Y93*) (C, arrow) resulting in biallelic PTCH1 inactivation. Case #17: KCOT (D) characterized by a PTCH1 nonsense mutation (c.250C >T;p.Q84*) (E, arrow) and a PTCH1 frameshift mutation (c.1341dupA;p.L448Tfs49*) (F, arrow) resulting in biallelic PTCH1 inactivation. Images show the forward strand of 9q, corresponding to the PTCH1 template strand.
FIGURE 4.
Distribution of non-synonymous PTCH1 mutations by protein domain in sporadic KCOT. cDNA position based on transcript NM_000264. Dotted lines indicate exon boundaries. Black, white, light gray, and dark gray shades represent C/N terminals, transmembrane domains, intracellular domains, and extracellular domains, respectively. Exons are numbered and exons 1, 22, 23, and 24 are displayed in abbreviated lengths.
FIGURE 3.
Overview of genomic events in 44 cases of sporadic KCOT showing near-universal PTCH1 inactivation and no identification of variants in other SHH-associated genes. LOH indicates loss of heterozygosity.
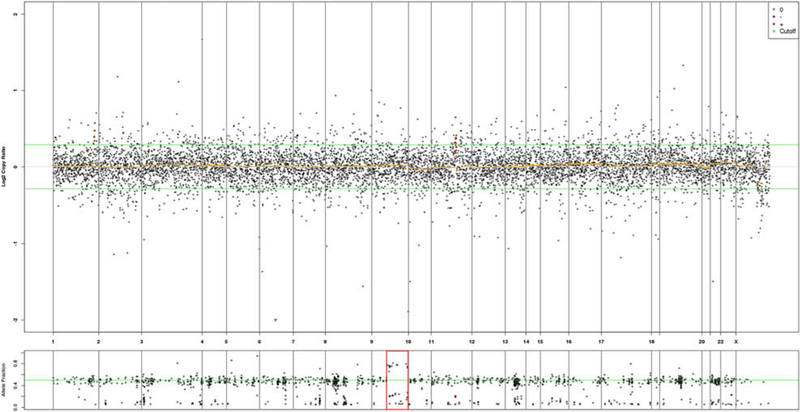
FIGURE 5.
9q CN-LOH represented a major mechanism of PTCH1 biallelic inactivation in sporadic KCOT. Chromosomal copy number variation plot (case #38) shows no copy number alterations but corresponding 9q allele frequency shows 2 heterozygous alleles with deviation from 50% allele frequency, consistent with CN-LOH.
DISCUSSION
KCOT was originally described as a distinct odontogenic cyst in 1926 by the name “cholesteatoma.”32 In subsequent decades it was also referred to as “primordial cyst” due to presumed origin from dental lamina (dental primordium), before being formally described as “odontogenic keratocyst” (OKC) in 1956 by Philipsen.33,34 Philipsen’s original series of 7 cases demonstrated the characteristic histopathologic features of thinly parakeratinized and uniformly thin stratified squamous epithelium with a conspicuously palisaded and hyperchromatic basal cell layer but also presented at least 1 case with surface orthokeratin.33 Thereafter, OKC was divided into parakeratinized and orthokeratinized variants, before the orthokeratinized odontogenic cyst was recognized as a distinct entity on the basis of distinguishing histopathologic features, uniformly indolent biological behavior, and lack of syndromic association.35,36
In appreciation of its aggressive behavior and emerging association with PTCH1 inactivation, the WHO reclassified OKC as “keratocystic odontogenic tumor” in 2005.36 Original reports predominantly included syndromic cases and subsequent reports cumulatively identified PTCH1 inactivating mutations in only 30% of sporadic cases, prompting reclassification as a “developmental” odontogenic cyst (OKC) by the WHO in 2017.18,28,37 Identification of inactivating PTCH1 mutations in 93% of sporadic KCOT in this study, with biallelic inactivation in 80% of cases, suggests an important role of SHH pathway dysregulation in tumor development and confirms that KCOT represents a true neoplasm rather than a developmental/reactive condition. These findings support those of Qu et al27 and stand in contrast to older studies, which were limited by low-sensitivity sequencing methods and insufficient neoplastic DNA yield.17–22 This cohort consisted of cases containing at least 30% neoplastic DNA, but the frequency of PTCH1 alterations in sporadic KCOT may nevertheless remain underestimated as our sequencing panel has incomplete intronic coverage of PTCH1 and may miss structural variants or intronic variants introducing cryptic splice sites. In addition, epigenetic silencing or posttranslational silencing of PTCH1 would require alternative testing strategies.
As in NBCCS cohorts, the observed loss-of-function mutations in our cohort were localized across the entire coding region of PTCH1, consistent with its tumor suppressor function. The most common mechanisms of inactivation were frameshift (N = 30), nonsense (N = 14), and splice site (N = 8) mutations.38 Three PTCH1 missense mutations were identified, 2 (p.L106R and p.G509R) previously reported in NBCCS patients and the other (p.G509V) exhibiting dominant-negative activity.39–41 Nearly all mutations occurred in the coding regions of the 2 large extracellular loops required for SHH ligand binding and in the sterol sensing domain that may play a role in SMO inhibition, corroborating previous reports mapping PTCH1 mutations in sporadic KCOT.18,27,42,43 The large intracellular loop was the site of only 1 mutation (case #33, c.2197dupT;p. S733fs*), the only reported mutation within this domain in sporadic KCOTs to date. 9q CN-LOH occurred in 15/ 44 (34%) cases and represented an important mechanism contributing to PTCH1 biallelic inactivation. 9q CN-LOH has not been previously reported in sporadic KCOT, likely because earlier studies did not detect this type of alteration. SUFU mutations were not identified in this cohort, although they account for up to 5% of NBCCS. However, to date, KCOTs have not been reported in NBCCS patients with germ-line SUFU mutations.44
The pattern of SHH pathway mutations in KCOT resembles that of basal cell carcinoma (BCC), in which up to 90% harbor inactivation of at least 1 PTCH1 allele and 10% to 20% harbor activating SMO mutations.45–49 These genomic similarities may be explained by the overlapping roles of SHH signaling in folliculogenesis and odontogenesis.50 Although PTCH1 and other SHH gene mutations in BCC are often caused by UV light, the KCOTs studied here did not exhibit a UV-related hypermutational signature. Approximately one third of medulloblastomas is characterized by SHH dysregulation, resulting from biallelic PTCH1 or SUFU inactivation, activating SMO mutations, or GLI amplification.51,52 Like-wise, embryonal and fusion-gene negative alveolar rhabdomyosarcoma demonstrate SHH pathway perturbation.53–55 The role of monoallelic PTCH1 inactivation has been supported by in vivo studies.56–58 BCC, medulloblastoma, and rhabdomyosarcoma, like KCOT, may all occur sporadically or in the context of NBCCS.
Occasionally, KCOTs pursue a more rapidly aggressive clinical course, characterized by early cortical perforation and pain/paresthesia, and further studies are necessary to better define this subset genomically.5
The identification of PTCH1 inactivation in sporadic KCOTs has significant potential implications for patient management. SHH inhibition in BCC has been well studied and vismodegib (GDC-0449) reduces BCC tumor burden in NBCCS patients (NCT00957229) and is associated with tumor response in locally advanced or metastatic BCC (NCT00833417), albeit with significant side effects.59–62 Among NBCCS patients treated with vismodegib, incidental KCOT shrinkage has been reported.63,64 Itraconazole and posaconazole are potent SHH inhibitors that inhibit BCC carcinoma growth in clinical trials and animal studies, respectively.65,66 Further studies are needed to determine their role in KCOT management.
In conclusion, we demonstrate PTCH1 inactivating mutations in 93% of sporadic KCOTs, indicating that SHH pathway alterations are a near-universal event and supporting their classification as a neoplasm with cystic growth. Recurrent PTCH1 inactivation in KCOT provides a rational target for SHH pathway inhibitors to be explored in future studies. Additional genomic studies are necessary to better define the small subset of rapidly aggressive KCOT.
ACKNOWLEDGMENTS
The authors thank the Center for Advanced Molecular Diagnostics in the Department of Pathology at Brigham and Women’s Hospital/Harvard Medical School and the Department of Pathology at University Hospitals Cleveland Medical Center/ Case Western Reserve University School of Medicine for supporting this project.
Source of Funding: Supported by grants from the Chalmers J. Lyons Academy of Oral and Maxillofacial Surgery and the HEARTS Foundation. I.-M.S. is supported by a Ruth L. Kirchstein NRSA Institutional Research Training Grant (T32).
Footnotes
Conflicts of Interest: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumors. Lyon, France: IARC Press; 2017. [Google Scholar]
- 2.Johnson NR, Gannon OM, Savage NW, et al. Frequency of odontogenic cysts and tumors: a systematic review. J Investig Clin Dent. 2014;5:9–14. [DOI] [PubMed] [Google Scholar]
- 3.Shear M, Speight PM. Cysts of the Oral and Maxillofacial Regions. Oxford, UK: Blackwell Munksgaard; 2007. [Google Scholar]
- 4.Woolgar JA, Rippin JW, Browne RM. The odontogenic keratocyst and its occurrence in the nevoid basal cell carcinoma syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:727–730. [DOI] [PubMed] [Google Scholar]
- 5.Boffano P, Ruga E, Gallesio C. Keratocystic odontogenic tumor (odontogenic keratocyst): preliminary retrospective review of epidemiologic, clinical, and radiologic features of 261 lesions from University of Turin. J Oral Maxillofac Surg. 2010;68:2994–2999. [DOI] [PubMed] [Google Scholar]
- 6.Sharif FN, Oliver R, Sweet C, et al. Interventions for the treatment of keratocystic odontogenic tumours. Cochrane Database Syst Rev. 2015;11:CD008464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrcanovic BR, Gomez RS. Recurrence probability for keratocystic odontogenic tumors: an analysis of 6427 cases. J Craniomaxillofac Surg. 2017;45:244–251. [DOI] [PubMed] [Google Scholar]
- 8.Dashow JE, McHugh JB, Braun TM, et al. Significantly decreased recurrence rates in keratocystic odontogenic tumor with simple enucleation and curettage using Carnoy’s versus modified Carnoy’s solution. J Oral Maxillofac Surg. 2015;73:2132–2135. [DOI] [PubMed] [Google Scholar]
- 9.Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908–912. [DOI] [PubMed] [Google Scholar]
- 10.Farndon PA, Del Mastro RG, Evans DG, et al. Location of gene for Gorlin syndrome. Lancet. 1992;339:581–582. [DOI] [PubMed] [Google Scholar]
- 11.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. [DOI] [PubMed] [Google Scholar]
- 13.Fan Z, Li J, Du J, et al. A missense mutation in PTCH2 underlies dominantly inherited NBCCS in a Chinese family. J Med Genet. 2008;45:303–308. [DOI] [PubMed] [Google Scholar]
- 14.Pastorino L, Ghiorzo P, Nasti S, et al. Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A. 2009;149A:1539–1543. [DOI] [PubMed] [Google Scholar]
- 15.Fujii K, Ohashi H, Suzuki M, et al. Frameshift mutation in the PTCH2 gene can cause nevoid basal cell carcinoma syndrome. Fam Cancer. 2013;12:611–614. [DOI] [PubMed] [Google Scholar]
- 16.Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32:4155–4161. [DOI] [PubMed] [Google Scholar]
- 17.Pan S, Dong Q, Sun LS, et al. Mechanisms of inactivation of PTCH1 gene in nevoid basal cell carcinoma syndrome: modification of the two-hit hypothesis. Clin Cancer Res. 2010;16:442–450. [DOI] [PubMed] [Google Scholar]
- 18.Guo YY, Zhang JY, Li XF, et al. PTCH1 gene mutations in keratocystic odontogenic tumors: a study of 43 Chinese patients and a systematic review. PLoS One. 2013;8:e77305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreto DC, Gomez RS, Bale AE, et al. PTCH gene mutations in odontogenic keratocysts. J Dent Res. 2000;79:1418–1422. [DOI] [PubMed] [Google Scholar]
- 20.Ohki K, Kumamoto H, Ichinohasama R, et al. PTC gene mutations and expression of SHH, PTC, SMO, and GLI-1 in odontogenic keratocysts. Int J Oral Maxillofac Surg. 2004;33:584–592. [DOI] [PubMed] [Google Scholar]
- 21.Gu XM, Zhao HS, Sun LS, et al. PTCH mutations in sporadic and Gorlin-syndrome-related odontogenic keratocysts. J Dent Res. 2006; 85:859–863. [DOI] [PubMed] [Google Scholar]
- 22.Sun LS, Li XF, Li TJ. PTCH1 and SMO gene alterations in keratocystic odontogenic tumors. J Dent Res. 2008;87:575–579. [DOI] [PubMed] [Google Scholar]
- 23.Levanat S, Gorlin RJ, Fallet S, et al. A two-hit model for developmental defects in Gorlin syndrome. Nat Genet. 1996;12:85–87. [DOI] [PubMed] [Google Scholar]
- 24.Lench NJ, High AS, Markham AF, et al. Investigation of chromosome 9q22.3-q31 DNA marker loss in odontogenic keratocysts. Eur J Cancer B Oral Oncol. 1996;32B:202–206. [DOI] [PubMed] [Google Scholar]
- 25.Zedan W, Robinson PA, Markham AF, et al. Expression of the sonic hedgehog receptor “PATCHED” in basal cell carcinomas and odontogenic keratocysts. J Pathol. 2001;194:473–477. [DOI] [PubMed] [Google Scholar]
- 26.Pan S, Xu LL, Sun LS, et al. Identification of known and novel PTCH mutations in both syndromic and non-syndromic keratocystic odontogenic tumors. Int J Oral Sci. 2009;1:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu J, Yu F, Hong Y, et al. Underestimated PTCH1 mutation rate in sporadic keratocystic odontogenic tumors. Oral Oncol. 2015;51:40–45. [DOI] [PubMed] [Google Scholar]
- 28.Wright JM, Odell EW, Speight PM, et al. Odontogenic tumors, WHO 2005: where do we go from here? Head Neck Pathol. 2014;8:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part II. Histologic features. Oral Surg Oral Med Oral Pathol. 1977;43:233–255. [DOI] [PubMed] [Google Scholar]
- 30.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. [DOI] [PubMed] [Google Scholar]
- 32.Hauer A Ein Cholesteatom im linken Unterkiefer unter einem retinierten Weisheitszahn [A cholesteatoma in the left lower jaw under a retracted wisdom tooth]. Z Stomat. 1926;24:40–59. [Google Scholar]
- 33.Philipsen H Om keratocyster (kolesteatom) i kaeberne [On keratocysts (cholesteatoma) in the jaws]. Tandlaegebladet. 1956;60:963–981. [Google Scholar]
- 34.Soskolne WA, Shear M. Observations on the pathogenesis of primordial cysts. Br Dent J. 1967;123:321–326. [PubMed] [Google Scholar]
- 35.Wright JM. The odontogenic keratocyst: orthokeratinized variant. Oral Surg Oral Med Oral Pathol. 1981;51:609–618. [DOI] [PubMed] [Google Scholar]
- 36.Barnes LEJ, Reichart P, Sidransky D, eds. WHO Classification of Head and Neck Tumors. Lyon, France: IARC Press; 2005. [Google Scholar]
- 37.Wright JM, Vered M. Update from the 4th edition of the World Health Organization Classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianferante DM, Rotunno M, Dean M, et al. Whole-exome sequencing of nevoid basal cell carcinoma syndrome families and review of Human Gene Mutation Database PTCH1 mutation data. Mol Genet Genomic Med. 2018;6:1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hime GR, Lada H, Fietz MJ, et al. Functional analysis in Drosophila indicates that the NBCCS/PTCH1 mutation G509V results in activation of smoothened through a dominant-negative mechanism. Dev Dyn. 2004;229:780–790. [DOI] [PubMed] [Google Scholar]
- 40.Li TJ, Yuan JW, Gu XM, et al. PTCH germline mutations in Chinese nevoid basal cell carcinoma syndrome patients. Oral Dis. 2008;14:174–179. [DOI] [PubMed] [Google Scholar]
- 41.Chidambaram A, Goldstein AM, Gailani MR, et al. Mutations in the human homologue of the Drosophila patched gene in Caucasian and African-American nevoid basal cell carcinoma syndrome patients. Cancer Res. 1996;56:4599–4601. [PubMed] [Google Scholar]
- 42.Marigo V, Davey RA, Zuo Y, et al. Biochemical evidence that patched is the hedgehog receptor. Nature. 1996;384:176–179. [DOI] [PubMed] [Google Scholar]
- 43.Gong X, Qian H, Cao P, et al. Structural basis for the recognition of sonic hedgehog by human Patched1. Science. 2018;361:eaas8935. [DOI] [PubMed] [Google Scholar]
- 44.Evans DG, Oudit D, Smith MJ, et al. First evidence of genotype-phenotype correlations in Gorlin syndrome. J Med Genet. 2017;54: 530–536. [DOI] [PubMed] [Google Scholar]
- 45.Gailani MR, Stahle-Backdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. [DOI] [PubMed] [Google Scholar]
- 46.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. [DOI] [PubMed] [Google Scholar]
- 47.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. [DOI] [PubMed] [Google Scholar]
- 48.Reifenberger J, Wolter M, Weber RG, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 49.Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. [DOI] [PubMed] [Google Scholar]
- 50.Seppala M, Fraser GJ, Birjandi AA, et al. Sonic hedgehog signaling and development of the dentition. J Dev Biol. 2017;5:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017; 31:737.e6–754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwalbe EC, Lindsey JC, Nakjang S, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 2017;18:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tostar U, Malm CJ, Meis-Kindblom JM, et al. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. [DOI] [PubMed] [Google Scholar]
- 54.Zibat A, Missiaglia E, Rosenberger A, et al. Activation of the hedgehog pathway confers a poor prognosis in embryonal and fusion gene-negative alveolar rhabdomyosarcoma. Oncogene. 2010;29: 6323–6330. [DOI] [PubMed] [Google Scholar]
- 55.Pressey JG, Anderson JR, Crossman DK, et al. Hedgehog pathway activity in pediatric embryonal rhabdomyosarcoma and undifferentiated sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zibat A, Uhmann A, Nitzki F, et al. Time-point and dosage of gene inactivation determine the tumor spectrum in conditional Ptch knockouts. Carcinogenesis. 2009;30:918–926. [DOI] [PubMed] [Google Scholar]
- 57.Zhai J, Zhang H, Zhang J, et al. Effect of the sonic hedgehog inhibitor GDC-0449 on an in vitro isogenic cellular model simulating odontogenic keratocysts. Int J Oral Sci. 2019;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davari P, Hebert JL, Albertson DG, et al. Loss of Blm enhances basal cell carcinoma and rhabdomyosarcoma tumorigenesis in Ptch1 +/− mice. Carcinogenesis. 2010;31:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012; 366:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang JY, Ally MS, Chanana AM, et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1720–1731. [DOI] [PubMed] [Google Scholar]
- 62.Xie P, Lefrancois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089.e17–1100.e17. [DOI] [PubMed] [Google Scholar]
- 63.Goldberg LH, Landau JM, Moody MN, et al. Resolution of odontogenic keratocysts of the jaw in basal cell nevus syndrome with GDC-0449. Arch Dermatol. 2011;147:839–841. [DOI] [PubMed] [Google Scholar]
- 64.Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. [DOI] [PubMed] [Google Scholar]
- 66.Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016; 15:866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]