Abstract
Opioid addition/dependence is associated with impulsive and risky behavior. Moreover, opioids can increase impulsive choice in pre-clinical studies with non-humans. The objective of this study was to investigate a potential behavioral mechanism of opioids: a change in the impact of reinforcement magnitude on choice. Rats (n=7) chose between smaller and larger reinforcers under a continuous-choice (concurrent-chains) procedure. The levers associated with the smaller and larger reinforcers alternated every five sessions. During baseline under this procedure, rats showed a reliable preference for the larger reinforcer. Effects of several doses (0.1-1.7 mg/kg, s.c.) of the prescription opioid, oxycodone, were examined on preference based upon reinforcement magnitude. Oxycodone dose-dependently decreased preference for the larger reinforcer (i.e., decreased sensitivity to reinforcement magnitude). The decrease in sensitivity to reinforcement magnitude was selective in that the intermediate doses did not affect, or had minimal impact on, other measures of performance (e.g., on general motivation to respond). These data suggest that a decrease in the sensitivity to reinforcement magnitude is a reliable outcome of μ-opioid administration, an effect that has important implications for the impact of these drugs on both impulsive and risky behavior.
Keywords: Impulsive Choice, Risky Choice, Reinforcement Magnitude, Behavioral Mechanisms, Oxycodone, Opioids, Rats
Introduction
Misuse and abuse of opioids, including prescription opioid medications, has become a significant health concern; the U.S. Department of Health and Human Services declared opioid abuse a public health emergency in 2017. Drug abuse, including opioid-use disorder (American Psychiatric Association, 2013), is associated with impulsive and risky behavior (Madden et al., 1997; Petry, 2001; Perry & Carroll, 2008; Carroll et al. 2010; Yi et al., 2010; Weafer et al., 2014). These behaviors place individuals at risk of contracting and transmitting diseases such as HIV/AIDS and at risk of continued drug abuse, which can increase the chances of dependence and overdose. Although evidence clearly indicates that impulsive and risky characteristics are pre-existing factors related to drug abuse (Perry & Carroll, 2008; Weafer et al., 2014), evidence also suggests that an increased likelihood of impulsive and risky behaviors can occur as a direct result of drug use (Simon et al., 2007; de Wit, 2009; Weafer et al., 2014). Therefore, it is important to characterize how opioids affect the fundamental reinforcement processes involved in making impulsive and risky decisions.
Nonhuman laboratory models have been useful in characterizing effects of drugs of abuse on impulsive and risky behavior (de Wit & Mitchell, 2010; Weafer et al., 2014). Impulsive-choice procedures provide a subject with two response options: 1) a smaller reinforcer delivered immediately or after a relatively short delay (the smaller, sooner reinforcer, or SSR); and 2) a larger reinforcer delivered after a longer delay (the larger, later reinforcer, or LLR). Choosing the SSR is impulsive because it results in reduction in overall reinforcement; whereas, choosing the LLR is said to show self-control because it increases long-term gain. As the delay to the LLR increases, the likelihood of choosing it decreases. The process by which delay decreases the value of a reinforcer is called delay discounting (see Madden & Johnson, 2010). A steeper delay-discounting function indicates that the delay has a bigger impact on choice, and thus, is associated with impulsive choice. In nonhuman animal studies, acute administration of opioids (e.g., morphine) typically increases impulsive choice (Kieres et al., 2004; Pitts and McKinney, 2005; Pattij et al., 2009; Eppolito et al., 2013; Maguire et al., 2016), although in some cases that effect can depend upon factors such as dose or pretreatment time (e.g., Eppolito et al., 2013; Maguire et al., 2016).
In studies of risky choice, individuals are given a choice between a smaller, more certain reinforcer (SCR, the safer option) and a larger, but uncertain reinforcer (LUR, the riskier option). As the probability of receiving the LUR decreases (i.e., the odds against receiving it increases), the likelihood of choosing it decreases. The process by which probability decreases the value of a reinforcer is called probability discounting (Rachlin et al., 1991; Green et al., 1999; Green & Myerson, 2004, 2010, 2013). Individuals whose choices are relatively sensitive to effects of reinforcement probability (i.e., who show relatively steep probability discounting) are said to be risk averse; whereas individuals who are relatively insensitive to effects of reinforcement probability are said to be risky. Although opioid-use disorder is associated with increased risk taking (e.g., Odum et al., 2000; Bechara, 2003; Bickel et al., 2004, Yan et al., 2014), little is known about the direct effects of opioids in nonhuman laboratory models of risky behavior. One study (Mitchell et al., 2011) reported a trend toward increased risky choice in rats, but the reliability of this effect is uncertain.
Understandably, most interpretations of drug effects on impulsive and risky choice have focused on the impact of drugs on delay and probability discounting, respectively (e.g., see de Wit & Mitchell, 2010). Relatively little research, however, has focused on drug effects on the impact of reinforcement magnitude, even though both types of choices involve differences in this variable. Thus, a complete understanding of drug effects on impulsive and risky behavior necessitates examining how a drug interacts with reinforcement magnitude to control choice. Indeed, a given effect on sensitivity to magnitude would be expected to have opposite effects on impulsive and risky choices. For example, under an impulsive-choice procedure, a reduction in sensitivity to reinforcement magnitude would be expected to shift preference toward the SSR, thus increasing impulsive choice. In contrast, a reduction in sensitivity would be expected to shift preference toward the SCR, thus decreasing risky choice.
Several studies have provided evidence that drugs can affect the impact of reinforcement magnitude on impulsive choice. For example, in the common Evenden and Ryan (1996) procedure used to study impulsive choice in nonhumans, the SSR always is presented immediately; whereas, the delay to the LLR increases (e.g., from 0- to 60-s) across blocks of trials within each session. During the first block, both the SSR and the LLR are presented immediately, and subjects typically show an exclusive, or near exclusive, preference for the LLR. As the delay to the LLR increases the likelihood of choosing it decreases and, thus, a delay-discount function is obtained within each session. Interestingly, some studies have reported that some drugs, including opioids, can decrease preference for the larger reinforcer during the first block when the SSR and LLR both are presented immediately (e.g., Pattij et al., 2009; Eppolito et al., 2013; Slezak et al., 2014), suggesting that drugs can affect control of choice by (i.e., decreased sensitivity to) reinforcement magnitude.
Unfortunately, a disadvantage of using single-response, discrete-trial choice procedures to investigate effects of drugs on control of choice by dimensions of reinforcement is that these types of procedures often produce exclusive, or near-exclusive, preference for one alternative over another. This can make it difficult to scale the degree of preference based upon the dimension of reinforcement under study (e.g., Mazur, 2010) and, thus, to determine precisely the effect of drugs. For example, when both reinforcers are presented immediately, a choice between 1 food pellet and 2 food pellets typically yield the same preference (100%) as a choice between 1 food pellet and 5 food pellets. Therefore, under a discrete-trial procedure, when a drug changes not only delay discounting, but also choice when both the larger and smaller reinforcers are presented immediately, it is difficult to determine the degree to which the impact of reinforcement magnitude has been altered by the drug.
Continuous-choice procedures (e.g., concurrent variable-interval, or VI, schedules) are better suited than discrete-trials procedures for quantifying choice controlled by dimensions of reinforcement (e.g., see Baum, 1974; Mazur, 1987, 2010; Davison & McCarthy, 1988; Dallery & Soto, 2013; Grace & Hucks, 2013). In continuous-choice procedures, a subject is presented with two response options and can respond on either option for a period of time. Reinforcers for each option are presented intermittently, and preference is scaled by measuring the proportion of responses on each option or the ratio of responses on one option versus the other (see Dallery & Soto, 2013). These procedures have been used to assess effects of drugs and other neurobiological manipulations on impulsive and risky choices (e.g., Pitts & Febbo, 2004; Locey & Dallery, 2011; Aparicio et al., 2013; Yates et al., 2019). A few studies have focused on effects of stimulants on choice controlled by reinforcement magnitude (Maguire et al., 2009; Pitts et al., 2016); even fewer have focused on opioids and reinforcement magnitude (Fetzer, 2015).
Oxycodone is a semi-synthetic opioid, typically prescribed to treat moderate-to-severe pain (Riley et al. 2008). Although there has been some debate regarding its pharmacological profile (e.g., Kalso, 2007; Nielsen et al., 2007), oxycodone has been shown to be a potent agonist at the μ-opioid receptor, with a behavioral profile similar to other μ-opioid agonists (Beardsley et al. 2004). Despite the fact that it is one of the most abused prescription opioids (Johnston et al., 2018) and that it has played a key role in the much publicized opioid crisis (Van Zee, 2009), there is relatively little research investigating its effects on impulsive and risky choice. In one study (Zacny & de Wit, 2009) with humans, none of the oxycodone doses (5, 10, or 20 mg capsules) affected any of the measures of impulsive or risky choice taken (including measures of delay and probability discounting). These results are somewhat surprising given oxycodone’s behavioral profile as a μ-opioid receptor agonist, the association between opioid-use disorder and impulsivity, and that morphine has shown to affect delay discounting in non-humans.
Because reinforcement magnitude plays such an important role in both impulsive and risky choice, the purpose of the present study was to examine effects of the prescription opioid oxycodone on sensitivity to reinforcement magnitude. Rats responded under a concurrent-chains arrangement (a continuous-choice procedure), and effects of oxycodone on sensitivity to reinforcement magnitude were quantified with the generalized matching law (Baum, 1974).
Methods
Subjects
Seven experimentally naïve male Sprague-Dawley rats served as subjects. The rats were approximately 90 days old at the start of the study. Rats were individually housed in cages in a colony room which functioned under a 12-/12-hr reverse light/dark cycle (lights off at 7:00 a.m.). The rats were provided with water and approximately 15 g of food (LabDiet® Rodent 5001) after each session (or at the corresponding time on days when sessions were not run). The water was removed after 3 hr such that the rats had been without water for approximately 20 hr at the start of each session.
All procedures used in this study were approved by the University of North Carolina Wilmington Institutional Animal Care and Use Committee (UNCW IACUC). The IACUC evaluates all nonhuman animal research at UNCW and insures that all procedures are conducted in accordance with the Eighth Edition of The Guide for the Care and Use of Laboratory Animals (2011) of the National Research Council of the National Academies and in accordance with UNCW’s Assurance Agreement with the Office of Laboratory Animal Welfare of the National Institutes of Health.
Apparatus
Seven operant-conditioning chambers were used for this study. Four were manufactured by Med Associates (model ENV-008) and three were manufactured by Coulbourn Instruments (model H10–11R-TC). The dimensions of the internal working area of the Med Associates chambers were 29.0 cm high, 29.5 cm wide, and 25.0 cm deep, while the internal dimensions of the Coulbourn chambers were 30.5 cm high, 30.5 cm wide, and 25.5 cm deep. Otherwise, all chambers had the same general configuration. The front wall, back wall, and ceiling were composed of aluminum, the right side entry door and left side wall were composed of Plexiglas, and the floor was made of stainless steel rods. The front wall contained a house light, two retractable levers (Med Associates model ENV-112CM), two lever lights, and an opening for access to a liquid dipper (Med Associates model ENV-202M-S). The house light was centered 3.0 cm from the ceiling; this light was on continuously throughout each session except when indicated. Each lever (one left and one right) was positioned 4.0 cm above the floor and 1.0 cm from its adjacent side; when extended, each required 30 N of downward force to operate. A stimulus light was located 2.0 cm directly above each lever. Centered between the two levers and 2.5 cm from the floor grid was a 3.0 cm high by a 4.0 cm wide opening that allowed access to the dipper cup. The dipper cup was raised by a motorized arm which resulted in delivery of a 0.02 cc drop of a 20% (w/v) sucrose solution. When the dipper cup was raised, a light illuminated the dipper cup and all other lights in the chamber were off. A third retractable lever was centered on the opposite, rear wall, 11.0 cm above the floor; there was no lever light above this lever.
Each chamber was enclosed in a sound-attenuating cubicle equipped with a ventilation fan, and white noise was broadcast continuously in the experimental room to mask extraneous sounds. The experimental room was adjacent to a general laboratory area that contained Windows-operated computers and interfacing equipment. The chambers were programmed and session data were recorded by Med Associates software (MED PC-IV).
Behavioral Procedure
Preliminary training.
This phase consisted of adaptation, dipper training, and lever-press training. During the five adaptation sessions, rats were placed in their chambers for 15 min with only the house light on (all levers were retracted, and lever lights were off). Following each of these sessions, the rats were given the sucrose solution manually through a syringe. During dipper training, the house light was on and the dipper was raised periodically (on average every 15 s). Dipper training continued until all rats were consuming the sucrose solution immediately upon presentation. For the remainder of the study, each individual dipper presentation lasted 2.5 s.
Next, the rats were trained to press all three levers (rear, front-left, and front-right) individually, that is, only one lever was extended into the chamber, and the house light and corresponding lever light were on. Throughout lever-press training, reinforcement consisted of a single dipper presentation. Immediately following each lever press, the lever retracted, the lights turned off, and the dipper was presented. These sessions ended following the 40th reinforcer presentation. Once the rats were consistently and quickly responding on each lever, a multiple chained schedule was implemented in which a press on the rear lever retracted that lever and extended either the left- or right-front lever (determined randomly) and turned the corresponding lever light on. A press on that lever retracted it, turned off the lever and house lights, and presented the dipper. Sessions ended after the 40th dipper presentation (20 left and 20 right). Once lever pressing was reliably maintained under the multiple schedule, the rats moved to the experimental procedure.
Experimental procedure.
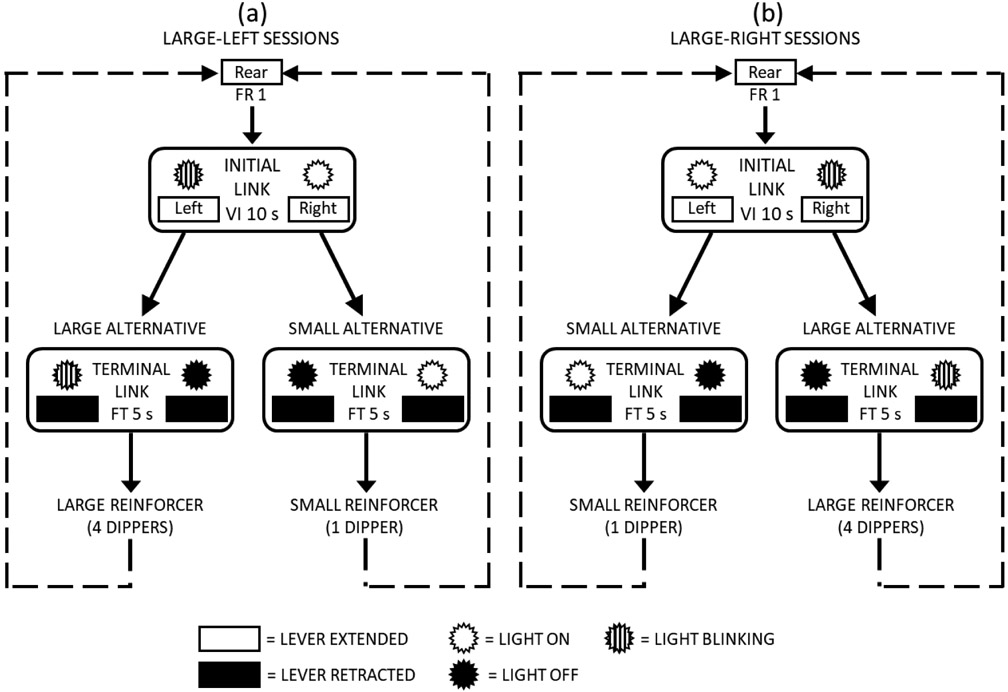
In this phase, each session consisted of a series of concurrent-chains cycles; each cycle comprised an initial (choice) link followed by a terminal (outcome) link. Each cycle began by extending the rear lever and turning on the house light; the rear lever was used to reset the rat’s position in the chamber so that it was directly across from and centered between the two front levers at the beginning of each cycle. A press on the rear lever, fixed ratio (FR) 1, retracted that lever and initiated the initial (choice) link. During the initial link, both the left and right levers were extended, the lights above them were turned on, and a VI schedule was in effect. After the interval had elapsed, a response on the preassigned lever produced entry into the terminal (outcome) link associated with that lever. The preassigned lever was pseudo-randomly determined such that for every six cycles the left lever was assigned three times and the right lever was assigned three times. A 1-s changeover delay (COD) was in effect during the initial link such that a press on the preassigned lever could not produce access to the terminal link until 1 s had passed since the last changeover to that lever. During the terminal link, both levers were retracted, the light above the unassigned lever was turned off, and a fixed-time (FT) 5-s schedule was in effect. After 5 s had elapsed, the reinforcer magnitude associated with that terminal link was provided (i.e., initial-link responses for both options had a 5-s signaled delay to reinforcement). One terminal link ended in a larger reinforcer (4 consecutive dipper presentations), and the other terminal link ended in a smaller reinforcer (1 dipper presentation). Thus, choice responses for one initial link were reinforced with the larger reinforcer and choice responses for the other initial link were reinforced with the smaller reinforcer. During the initial link, the light above the lever associated with the larger reinforcer blinked (0.5-s on, 0.5-s off); it also blinked whenever the terminal link for that option was in effect. After reinforcement presentation, the house light was turned on and the rear lever was extended, signaling the start of the next cycle. At first, the initial-link VI schedule was 5 s; this was gradually increased to a VI 10 s over 5 sessions, where it remained for the rest of the experiment. A diagram of this concurrent-chains procedure is presented in Fig. 1.
Fig.1.
Diagram of the Behavioral Procedure (see “Behavioral Procedure” section for details). Sessions consisted of 60 choice cycles; each choice cycle was comprised of an initial (choice) link, during which choice responses were made on either lever, and a terminal (outcome) link, which provided the consequences for each choice. Each choice cycle began with a press of the rear lever. (a) Shows large-left sessions, in which the larger reinforcer was associated with the left lever and the smaller reinforcer was associated with the right lever; (b) shows large-right sessions, in which the larger reinforcer was associated with the right lever and the smaller reinforcer was associated with the left lever. Large-left and Large-right sessions alternated every 5 sessions; rats learned which sides were associated with the larger and smaller reinforcers based upon the outcomes of their choices. Rectangles indicate levers and circular starbursts indicate lever lights. Unfilled and filled rectangles indicate when a lever was extended (available) and retracted (unavailable), respectively; unfilled and filled starbursts indicate when a lever light was on and off, respectively. The lever light associated with the larger reinforcer blinked during the initial link and during its associated terminal link (indicated by striped starbursts).
Each session began with four forced cycles, during which a rear-lever press resulted in extension of only one of the front levers (i.e., 2 left and 2 right, presented in random order), to ensure the rats sampled each of the initial-/terminal-links prior to experiencing a choice. Following the 4 forced trials, the remainder of each session consisted of 60 concurrent-chains choice cycles, 30 for which the left lever was preassigned and 30 for which the right lever was preassigned. That is, dependent scheduling was used in the initial links to insure equal exposure to each of the terminal links (e.g., Stubbs & Pliskoff, 1969; Yates et al., 2019).
In order to estimate sensitivity to reinforcement magnitude, two types of sessions, large-left and large-right, were arranged. During large-left sessions (Fig. 1a), the left terminal link resulted in the larger reinforcer and the right terminal link resulted in the smaller reinforcer (i.e., responses on the left lever during the initial link were reinforced with the larger reinforcer); during large-right sessions (Fig. 1b), the contingencies were reversed (i.e., responses on the right lever during the initial link were reinforced with the larger reinforcer). Initially, these two session types alternated irregularly and unpredictably in the same manner as in Exp. 2 of Pitts et al. (2016). The aim was for the rats to learn to prefer the lever associated with the larger reinforcer within each session. Under these conditions, however, several of the rats did not show consistent session-by-session preference for the larger reinforcer. Thus, the procedure was modified so that the two session types alternated every five sessions (sessions for M-F of one week were large-left, and sessions for M-F of the next week were large-right, and so on). This procedure, in which the sides associated with the larger and smaller reinforcers alternated every five sessions continued for the remainder of the study (including during the Pharmacological Procedure).
Once performance under the five-session alternation procedure was considered both suitable and stable, the Pharmacological Procedure was initiated. An individual rat’s baseline was considered suitable if there was a greater allocation of responses in the initial link on the lever associated with the larger reinforcer by the fifth session of each five-session block, such that the sensitivity estimate was greater than 0.2 (see Data Analysis for a description of the method used to estimate sensitivity). A baseline was determined to be stable when the sensitivity estimate was greater than 0.2 across four consecutive 5-session blocks (i.e., two blocks of each session type).
Pharmacological Procedure
Oxycodone (Sigma-Aldrich®) was dissolved in 0.9% sodium chloride (saline). Injections of saline or oxycodone were administered subcutaneously 15 min prior to the selected experimental sessions. Initially, doses of 0.3, 1.0, and 1.7 mg/kg were administered, based on the findings of Beardsley et al. (2004) and preliminary studies conducted in this laboratory. Injections were scheduled on Fridays (i.e., the fifth session of each block), provided that the previous session’s data were consistent with baseline data and the rat completed at least 75% of the choice cycles (note that on two occasions, a rat was inadvertently injected when it had completed just slightly less than the 75% criterion the day before). If a scheduled injection was cancelled, the session was still conducted. At least four injections of saline were administered prior to oxycodone administration, two for large-left sessions and two for large-right sessions. Oxycodone doses were first administered in an ascending order, followed by at least two saline administrations, and then administered again in a descending order. During each administration cycle (ascending then descending), each dose of oxycodone was given twice (once prior to a large-left session and once prior to a large-right session) before progressing to a higher or lower dose. Thus, altogether, each dose of oxycodone was administered at least four times to each subject (with the exception of Subject W20) such that the effects of each dose were examined twice on large-left sessions and twice on large-right sessions. After two administrations, injections of 1.7 mg/kg were discontinued for Subject W20 due to dermatophagia. Scheduled no-injection sessions from the fifth session of each five-session block served as control sessions. These sessions occurred before each administration cycle and occasionally within a cycle.
Following completion of the ascending/descending series described above, data analysis revealed an effect on sensitivity at the lowest dose (0.3 mg/kg) for several of the rats. Therefore, 0.1 mg/kg of oxycodone was then added to the drug administration procedure (so that the smallest dose tested had little or no effect on sensitivity). Four administrations of 0.1 mg/kg (two prior to large-left sessions and two prior to large-right sessions) were conducted for each rat.
Data Analysis
For each rat, the total number of responses during the initial link for both the front left and front right levers were recorded for each session. With these data, the log ratio of left-lever to right-lever responses was determined for both types of sessions, large-left and large-right. The log response ratios for both large-left and large-right sessions were determined for all sessions within each condition (no-injection control, saline, and each drug dose). These log response ratios from each condition were analyzed using the generalized matching equation (Baum, 1974),
| (1) |
where the variables R and M denote responses and reinforcement magnitude, respectively, on the left and right levers (subscripts L and R, respectively); the derived parameters SM and log b quantify the sensitivity to reinforcement magnitude and bias, respectively. Overall initial-link response rate was calculated by dividing the total number of responses on both levers during the initial link by the total time, in minutes, spent in the initial link. Latency (s) to press the rear lever to initiate the choice cycle also was obtained. Dose-effect functions were then constructed for sensitivity, bias, overall initial-link response rate, and rear-lever latency. One-way, repeated-measures analyses of variance (ANOVAs) were conducted for each measure using the data at saline and at each dose. Post-hoc analyses were conducted using Tukey’s honestly significant difference test. A p value of less than .05 was used for all significance testing. A selective drug effect of a given dose on sensitivity to reinforcement magnitude was indicated by a significant difference in sensitivity between that dose and saline, without a significant effect on any of the other measures.
Results
Baseline performance.
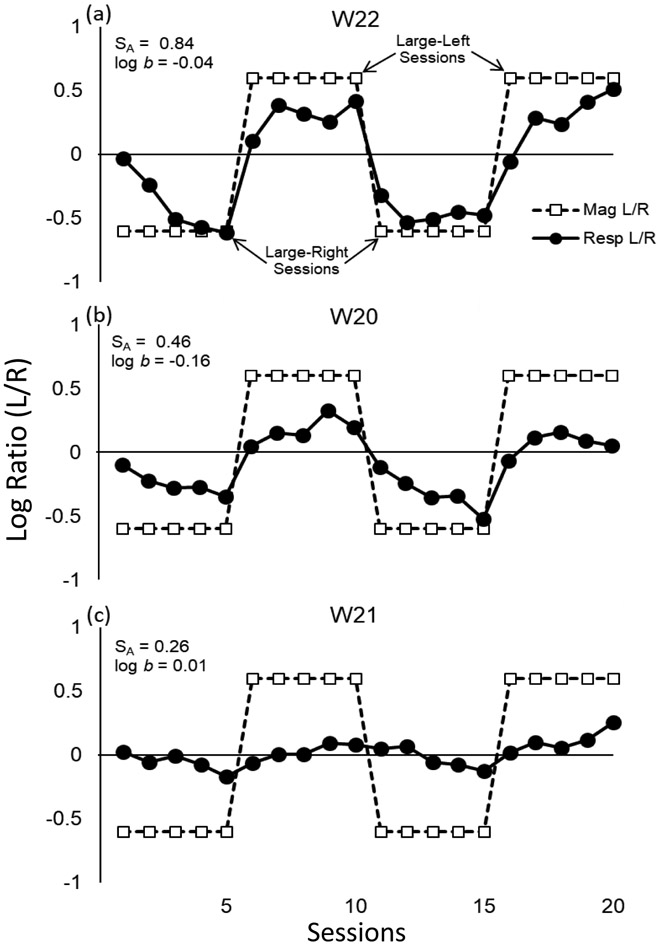
Suitable and stable baselines were obtained for Subjects W15, W19, W20, W21, and W22 in 98 to 109 sessions, and for Subjects W17 and W18 in 139 and 238 sessions, respectively. Fig. 2 shows the programmed amount ratios (unfilled squares) and the corresponding initial-link response ratios (filled circles) for three representative rats across the last 20 sessions (i.e., the last four 5-session blocks) before the first administration of oxycodone. Saline was administered every 5th session (i.e., on the final session of each block of 5 sessions). Log reinforcement ratios of 0.6 (denoted by the unfilled squares above the dashed line at 0) indicate large-left sessions, and log reinforcement ratios of −0.6 (denoted by unfilled squares below the dashed line at 0) indicate large-right sessions. The degree to which the response ratio changed along with the arranged reinforcer magnitude ratio is referred to as tracking. These three rats were chosen because their tracking performances represent the range of those obtained across all of the rats. Rat W22 (Fig. 2a) tracked reinforcer magnitude most closely; the response ratio (filled circles) changed relatively quickly (usually within one or two sessions), and preference for the larger reinforcer reached a substantial level which was maintained over the final two or three sessions of each five-session block. Sensitivity to reinforcement magnitude (obtained by using the four final sessions of each block shown in Fig. 2a) for this rat was 0.84. Rat W20 (Fig. 2b) was selected because it showed an intermediate degree of control by reinforcement magnitude during baseline. For this rat, preference took slightly longer to develop within each five-session block and did not quite reach the level obtained by W22. Sensitivity to magnitude for W20 was 0.46. This rat also showed a slight bias for the right lever (log b for this rat was −0.16); that is, preference for the larger reinforcer was stronger during large-right sessions than during large-left sessions. Rat W21 (Fig. 2c) showed the weakest control by reinforcement magnitude of all the rats; preference was slowest to change and reached the lowest level within each block. Nevertheless, albeit lower than the other rats, Rat W21 did show reliable control by reinforcement magnitude; sensitivity for this rat was 0.26.
Fig. 2.
Log reinforcement magnitude ratios (L/R), indicated by unfilled squares, and initial-link response ratios, indicated by filled circles, for three representative rats over the 20 baseline sessions prior to the initiation of the regimen of oxycodone administration. Saline was administered on the 5th, 10th, 15th, and 20th of these sessions (i.e., the last session of each 5-session block). Sensitivity to reinforcement magnitude (SA) and bias (log b) for each rat, determined using Equation 1, is indicated. Rat W22 (a) showed the highest sensitivity, Rat W20 (b) showed an intermediate sensitivity, and Rat W21 (c) showed the lowest sensitivity, compared to all rats in the study.
In summary, all rats acquired a reliable preference based upon reinforcement magnitude within each five-session block, which provided an effective and sensitive baseline against which to assess effects of oxycodone on sensitivity to reinforcement magnitude. Mean sensitivity (based upon the fifth session of each block) across rats was 0.46 (95% CI = 0.16). Overall, bias was negligible (M = −0.07; 95% CI = 0.09).
Effects of oxycodone.
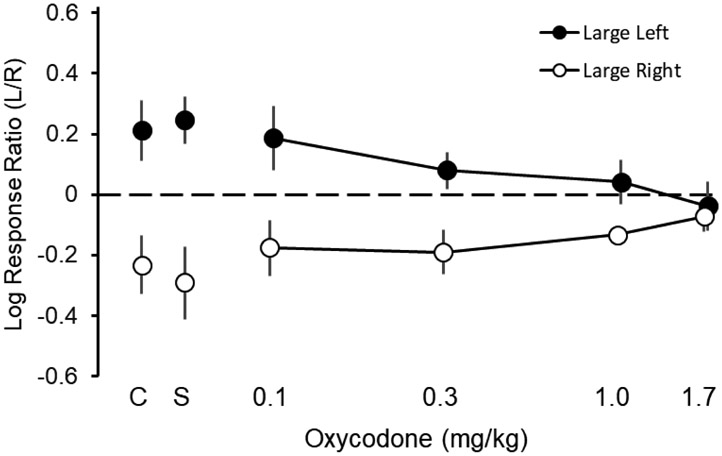
Fig. 3 shows the group dose-effect functions for oxycodone on initial-link response ratios across both session types. Filled circles show log response ratios from large-left sessions and unfilled circles show log response ratios from large-right sessions. Data points above C and S show data from control and saline sessions, respectively. Under control and saline conditions, there was a preference for the larger reinforcer, indicated by the positive values during large-left sessions (filled circles) and negative values during large-right sessions (unfilled circles). Oxycodone produced a dose-related decrease in preference for the larger reinforcer, indicated by the convergence of the functions toward zero as a function of dose. The 1.0 and 1.7 mg/kg doses reliably decreased preference for the larger reinforcer, as indicated by the fact that the means at these doses were outside of the 95% CI for saline; indeed, the highest dose (1.7 mg/kg) completely eliminated preference.
Fig. 3.
Oxycodone dose-effect functions on choice. Log response ratios (L/R) during the initial links for large-left sessions (filled circles) and for large-right sessions (unfilled circles) are plotted as a function of oxycodone dose. Points above C are data from no-injection control sessions, and points above S are data from sessions preceded by saline injections. Data points are means and error bars show 95% CIs; the absence of an error bar indicates that the 95% CI falls within the size of the data point. The dashed line at zero indicates the point at which the number of responses on the left and right lever during the initial links are equal (i.e., indifference).
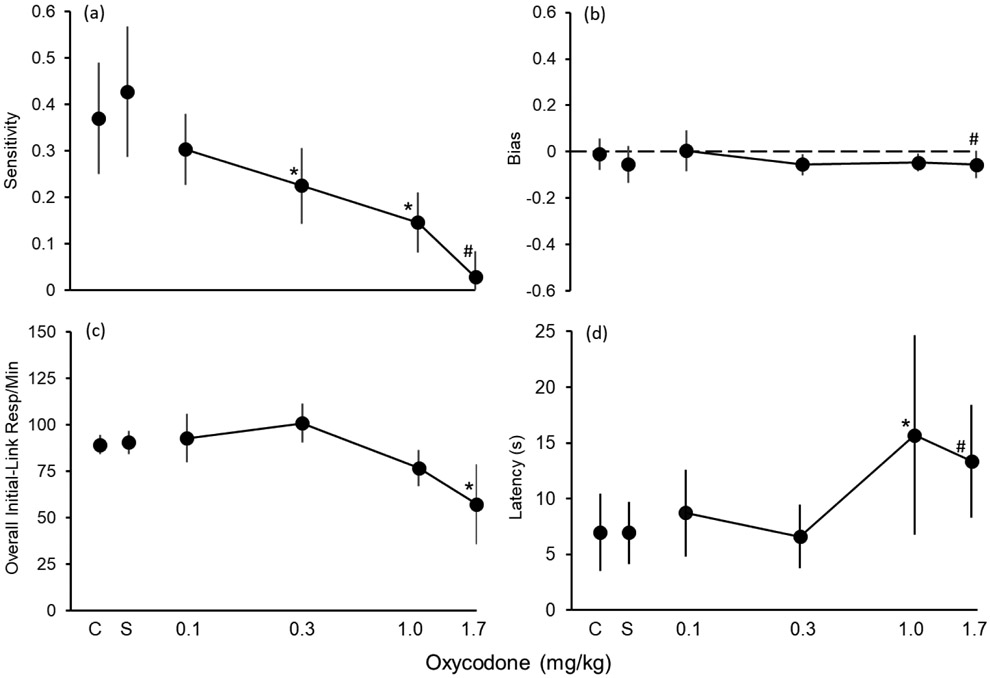
Fig. 4 shows dose-effect functions for oxycodone on sensitivity (a), bias (b), overall initial-link response rates (c), and rear-lever latency (d). Consistent with the data shown in Fig. 3, Fig. 4a shows that oxycodone produced a dose-related decrease in sensitivity to reinforcement magnitude. The 0.3 and 1.0 mg/kg doses decreased sensitivity by nearly, and by over, 50%, respectively. Sensitivity was near 0 at 1.7 mg/kg (i.e., control by reinforcement amount was almost completely eliminated). A one-way, repeated-measures ANOVA revealed a significant effect on sensitivity, F (3,18) = 9.54, p < .001; post-hoc analyses indicated that data for both 0.3 and 1.0 mg/kg were significantly different than those for saline (indicated by asterisks in Fig. 4a). On average, oxycodone produced very little effect on bias (Fig. 4 b; F (3,18) = 1.29, p = .31). It is important to note that, although it produced substantial effects on sensitivity, the 1.7 mg/kg dose was not included in the ANOVAs for sensitivity and bias (indicated by a # in Fig. 4 a & b). This was because a) this dose substantially affected overall initial-link response rates (see Fig. 4c) and rear-lever latency (see Fig. 4d), thereby reducing the number of cycles completed, and b) Rat W20 did not receive a sufficient number of administrations of this dose to obtain a sensitivity value.
Fig. 4.
Oxycodone dose-effect functions for sensitivity to reinforcement magnitude (a), bias (b), overall initial-link response rates (c), and rear-lever press latency (d). Data points are means, and error bars are 95% CIs. Asterisks (*) indicate data at that dose were significantly different than the data at saline (p < 0.05). Pound signs (#) indicate that those data from 1.7 mg/kg were not included in the statistical analysis. See text for an description of the methods used to derive sensitivity and bias and the characteristics of the statistical analyses.
On average, oxycodone produced a dose-related decrease in overall initial-link response rates (F (4,24) = 6.22, p < .01); post-hoc analyses indicated, however, that only the data for 1.7 mg/kg were significantly different than the data for saline. Oxycodone also increased rear-lever response latency (F (3,18) = 4.77, p = .01); data at 1.0 mg/kg were significantly different than those at saline (data from 1.7 mg/kg were not included in the ANOVA because Subject W20 did not always respond at this dose).
Table 1 displays the average number of cycles per session (out of a possible 60) completed for control, saline, and each dose of oxycodone. Under control and saline, subjects usually completed almost all of the cycles (M = 98.1%) within the session time limit. At 0.1 and 0.3 mg/kg oxycodone, all subjects continued to complete almost all cycles per session (M = 98.8%). For some of the subjects (e.g., W15, W20, and W21), 1.0 mg/kg decreased the number of cycles completed by 20–25%, which is consistent with the effects of this dose on initial-link response rates and rear-lever latency for these subjects. The 1.7 mg/kg dose decreased number of cycles completed for all subjects, often considerably.
Table 1.
Average cycles completed per session for each condition (control, saline, and oxycodone doses).
| Subject | Control | Saline | 0.1 | 0.3 | 1.0 | 1.7 |
|---|---|---|---|---|---|---|
| W15 | 57.13 | 55.56 | 59.5 | 57.25 | 41.75 | 38.75 |
| W17 | 59.67 | 60 | 59 | 60 | 58.25 | 28.75 |
| W18 | 57.5 | 58.17 | 60 | 60 | 54.75 | 25.5 |
| W19 | 60 | 57.45 | 60 | 60 | 60 | 48 |
| W20 | 60 | 58.54 | 60 | 60 | 42.75 | 3.5 |
| W21 | 60 | 60 | 54.5 | 59.75 | 44.5 | 22 |
| W22 | 60 | 60 | 60 | 60 | 59 | 56.5 |
| Mean | 59.19 | 58.25 | 59 | 59.57 | 51.57 | 31.86 |
| 95% CI | 0.95 | 1.35 | 0.76 | 0.26 | 6.09 | 13.10 |
Overall, the data in Fig. 4, combined with those in Table 1 show that, at the level of group, 0.3 mg/kg oxycodone decreased sensitivity to reinforcement magnitude without affecting bias, overall initial-link response rates, or the latency to initiate a choice cycle. The 1.0 mg/kg oxycodone dose also decreased sensitivity to reinforcement magnitude without affecting bias or overall initial-link response rates. But it did increase the overall latency to initiate a choice cycle; for three of the seven rats, this increase in latency did reduce the total number of choice cycles within the session.
Discussion
The baseline sensitivities to reinforcement magnitude obtained in this study are consistent with the values reported in previous studies using similar procedures with pigeons (Maguire et al., 2007; Fetzner, 2015) and rats (Aparicio et al., 2016). The present findings provide additional support for the utility of these types of procedures for determining sensitivity to reinforcement magnitude and for their use as a baseline to study effects of drugs on choice.
Oxycodone produced dose-related decreases in sensitivity to reinforcement magnitude; indeed, in some instances, a dose nearly eliminated preference of the larger reinforcer. The reduction in sensitivity was selective at 0.3 mg/kg; that is, 0.3 mg/kg oxycodone significantly decreased sensitivity to reinforcement magnitude without significantly affecting overall initial-link response rate or latency to initiate a choice cycle. In addition, in four of the seven rats, 1.0 mg/kg affected sensitivity to magnitude without substantially affecting these other measures (e.g., see Table 1). Thus, the oxycodone-produced decrease in sensitivity to reinforcement magnitude was not a by-product of, for example, impaired motor functioning or a decrease in effectiveness of the sucrose solution as a reinforcer.
The oxycodone-produced hyposensitivity to reinforcement magnitude obtained in the present study replicates previous findings from discrete-trial, delay-discounting studies showing that morphine decreases the percentage of choices of a larger reinforcer when both the larger and smaller reinforcers were presented immediately (Pattij et al., 2009; Eppolito et al., 2013; Slezak et al., 2014). The present data extends those findings by quantifying this effect under a continuous-choice arrangement and by providing evidence that this effect is selective (i.e., not a by-product of other general effects on behavior). The present data also replicate and extend those reported by Fetzner (2015) showing that morphine decreased sensitivity to reinforcement magnitude in a concurrent-chains procedure with pigeons. Taken together, these findings suggest that a hyposensitivity to reinforcement magnitude is a reliable and general behavioral mechanism of the actions of opioids, including oxycodone.
A reduction in sensitivity to reinforcement magnitude has important implications for predicting and interpreting oxycodone’s effects, and those of other opioids, on both impulsive and risky choice. In the case of impulsive choice, the present data predict that, all else being equal, administration of oxycodone would shift preference toward the SSR, thereby increasing impulsive choice. This prediction is consistent with those studies showing that acute morphine administration increases impulsive choice (Kieres et al., 2004; Pitts and McKinney, 2005; Pattij et al., 2009; Maguire et al., 2016). In the case of risky choice, however, a decrease in sensitivity to reinforcement magnitude would shift preference toward the SCR, thereby decreasing risky choice. All else being equal, such an effect would be expected to decrease risky choice. Although opioid-use disorder clearly is associated with increased risk taking (Odum et al., 2000; Bechara, 2003; Bickel et al., 2004; Yan et al., 2014), the impact of opioid administration on risky choice, and a determination of the behavioral mechanisms involved, awaits further investigation.
In is important to emphasize the all-else-being-equal proviso in the above predictions/interpretations. Both impulsive and risky choice involve a tradeoff between reinforcement magnitude and another dimension of reinforcement: delay (impulsive choice) or probability (risky choice). A complete account of the behavioral mechanisms involved in effects of opioids, indeed of any drug, on impulsive or risky choice necessarily will involve a characterization of the effects on the nature of control by the relevant dimensions of reinforcement (see Pitts, 2014). With respect to impulsive choice, for example, data showing that morphine administration shifts delay-discount functions to the left (e.g., Pitts and McKinney, 2005; Pattij et al., 2009; Maguire et al. 2016) combined with the data from the present study, indicate that opioids increase impulsive choice by both enhancing the discounting effects of delay) and by reducing effects of reinforcement magnitude. When combined, these two mechanisms would be expected to produce a profound increase in impulsive choice. It should be noted, however, that in the studies showing leftward shifts in delay discounting, the two different reinforcement magnitudes typically remain constant as the delay is manipulated, and thus, it is difficult to disentangle, and therefore quantify, the relative contributions of magnitude and delay. Continuous-choice procedures, along with quantitative analyses of the sort presented in the present study, are better suited to determine the contribution of each dimension to choice and the degree to which each contribution is changed by drug administration.
The predictions, based upon the present data, that oxycodone would increase impulsive choice and decrease risky choice are not consistent with data reported by Zacny and de Wit (2009) that oxycodone administration did not affect either delay or probability discounting in humans. At this point, the specific reason(s) for this discrepancy are not clear. One obvious possibility is the different species (rats versus verbal humans). Preliminary evidence of this possibility is provided by data from a Master’s thesis conducted in our laboratory showing that 0.3 mg/kg oxycodone shifted delay-discounting functions in rats to the left (Aikman, 2012) – an effect similar to that typically found with morphine. Another set of possibilities relates to the substantial procedural differences between the present study and the study by Zacny and de Wit, including the type of consequence used (sucrose solution vs. money), the delivery of the consequence (immediately after each choice vs. the outcome of a randomly selected trial delivered at the end of the study), and the different routes of oxycodone administration (s.c. injection vs oral ingestion). Furthermore, given that impulsive/risky choices involve tradeoffs between reinforcer magnitude and reinforcer delay/probability, it also is possible that the effects of oxycodone on any study of impulsive or risky choice will always reflect the interaction among those different mechanisms. For example, oxycodone may decrease sensitivity to both reinforcer magnitude and delay, which would impact impulsive choice in opposite directions; the former shifting choice toward the SSR and the latter shifting choice toward the LLR. These effects could cancel each other, resulting in a net outcome of no effect. Indeed, a similar kind of interaction may have played a role in some of the exceptions to morphine’s typical increasing effects on impulsive choice (e.g., Eppolito et al., 2013; Maguire et al. 2016).
Although the finding that oxycodone decreased sensitivity to reinforcement magnitude certainly seems promising as a potential behavioral mechanism of opioid effects on impulsive and risky choice, the present data do not rule out other potential behavioral mechanisms. For example, it is possible that the reduction in sensitivity obtained here reflected an oxycodone-induced failure of stimulus control by reinforcement magnitude or by the blinking stimulus light that was associated with the larger reinforcer. The present data also do not address the potential pharmacological mechanisms involved in opioid effects on impulsive/risky choice. Several of oxycodone’s behavioral effects, including those related to its abuse liability, appear to be mediated by activity at μ-opioid receptors (Beardsley et al., 2004). The specific receptor mechanisms associated with oxycodone’s effects in the present study, however, remain to be clarified. Indeed, there is much work to be done to characterize both the acute and chronic effects of oxycodone administration on impulsive and risky choice, isolate the conditions under which those effects occur, identify the specific behavioral and pharmacological mechanisms involved, and determine how those mechanisms interact to determine choice. It will be important, for example, to determine if effects of oxycodone and other opioids on impulsive and risky behavior, and the mechanisms associated with those effects, differ for males and females.
In summary, in the present study, oxycodone decreased sensitivity to reinforcement magnitude. Because reinforcement magnitude plays an important role in both impulsive and risky choice, the present data suggest that a reduction in sensitivity to reinforcement magnitude may be a potentially important behavioral mechanism involved in the relation between opioids and impulsive and risky choice. Characterizing and quantifying the relative roles of these reinforcement mechanisms will help inform the search for neurobiological bases of impulsive and risky choice, and of the effects of opioid drugs. For example, da Costa Araújo et al. (2010) reported that effects of reinforcement delay on choice were associated with enhanced activity in both the orbital prefrontal cortex and the nucleus accumbens core; whereas, effects of reinforcement magnitude were associated with enhanced activity only in the nucleus accumbens core. Both acute and chronic effects of opioids, and effects of opioid withdrawal, on impulsive and risky choice may be related to their differential impacts on those two neurobiological mechanisms. Furthermore, identification of the specific reinforcement mechanisms involved in effects of opioids and other drugs on impulsive choice may help us identify potential therapeutic targets. There is a current emphasis on developing treatments to reduce delay discounting and related processes (see Bickel et al., 2015). Identifying the specific reinforcement mechanisms involved in effects of drugs on impulsive and risky choice will facilitate efforts to develop behavioral and pharmacological interventions that target specific deficits (e.g., hyposensitivity to reinforcer magnitude) that might determine and/or result from opioid abuse.
Acknowledgements
All authors of this study made significant contributions to the preparation of this manuscript. The final manuscript has been read and approved by all authors.
This work was supported by the National Institutes of Health, National Institute on Drug Abuse (Grant R15-DA045960). The data presented here were collected by the first author as part of a Master’s thesis at the University of North Carolina Wilmington under the mentorship of the second and third authors. The authors would like to thank the other members of the thesis committee, Drs. Thomas Cariveau and Wendy Donlin Washington, for their valuable input. Portions of these data were previously presented in 2018 at the 35th Annual Meeting of the Southeastern Association for Behavior Analysis in Chattanooga, TN and in 2019 at the 45th Annual Convention of the Association for Behavior Analysis International in Chicago, IL. The authors wish to thank all graduate and undergraduate students in the UNCW Experimental Analysis of Behavior Lab who assisted with data collection for this study. The authors also extend a special thank you to Tiffany Kronenwetter for providing excellent animal care for the subjects of this study.
Source of funding: Grant DA045960 from the National Institute on Drug Abuse (National Institutes of Health)
Footnotes
Conflicts of interest: None declared
References
- American Psychiatric Association. (2013). Substance-related and addictive disorders In Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596.dsm16 [DOI] [Google Scholar]
- Aikman AN (2012). Effects of acute and chronic oxycodone administration and withdrawal on impulsive behavior. Unpublished master’s thesis, University of North Carolina Wilmington, Wilmington, NC. [Google Scholar]
- Aparicio CF, Hughes CE, Pitts RC (2013). Impulsive choice in Lewis and Fischer 344 rats: effects of extended training. Conductual 1: 22–46. [Google Scholar]
- Aparicio CF, Baum WM, Hughes CE, Pitts RC (2016). Limits to preference and the sensitivity of choice to rate and amount of food. J Exp Anal Behav 105: 322–37. 10.1002/jeab.198 [DOI] [PubMed] [Google Scholar]
- Baum WM (1974). On two types of deviation from the matching law: bias and undermatching. J Exp Anal Behav 22: 231–42. 10.1901/jeab.1974.22-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS (2004). Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12: 163–72. 10.1037/1064-1297.12.3.163 [DOI] [PubMed] [Google Scholar]
- Bechara A (2003). Risky business: emotion, decision-making, and addiction. J Gambl Stud 19: 23–51. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Giordano LA, Badger GJ (2004). Risk-sensitive foraging theory elucidates risky choices made by heroin addicts. Addiction 99: 855–61. 10.1111/j.1360-0443.2004.00733.x [DOI] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Moody L. Wilson AG (2015). Therapeutic opportunities for self-control repair in addiction and related disorders: change and the limits of change in trans-disease processes. Clin Psychol Sci 3:140–153. 10.1177/2167702614541260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL (2010). Delay discounting as a predictor of drug abuse In: Impulsivity: the behavioral and neurological science of discounting. Madden G, Bickel W (editors). Washington, DC: APA; pp. 243–72. 10.1037/12069-009 [DOI] [Google Scholar]
- da Costa Araujo S, Bod S, Valencia Torres L, Olarte Sanchez CM, Bak VK, Deakin JFW, et al. (2010). Choice between reinforcer delays versus choice between reinforcer magnitudes: differential Fos expression in the orbital prefrontal cortex and nucleus accumbens core. Behav Brain Res 213: 269–277. 10.1016/j.bbr.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Soto PL (2013). Quantitative description of environment-behavior relations In: APA handbook of behavior analysis, vol. 1. Methods and principles. Madden G, Dube W, Hackenberg T, Hanley G, Lattal K (editors). Washington, DC: APA; pp. 219–49. 10.1037/13937-010 [DOI] [Google Scholar]
- Davison M, McCarthy D (1988). The matching law: a research review. Hillsdale: Lawrence Erlbaum Associates, Inc; 10.4324/9781315638911 [DOI] [Google Scholar]
- de Wit H (2009). Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14: 22–31. 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Mitchell SH (2010). Drug effects on delay discounting In: Impulsivity: the behavioral and neurological science of discounting. Madden G, Bickel W (editors). Washington, DC: APA; pp. 213–41. 10.1037/12069-008 [DOI] [Google Scholar]
- Eppolito AK, France CP, Gerak LR (2013). Effects of acute and chronic morphine on delay discounting in pigeons. J Exp Anal Behav 99: 277–89. 10.1002/jeab.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN (1996). The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 28: 161–70. 10.1007/s002130050121 [DOI] [PubMed] [Google Scholar]
- Fetzner WD (2015). Rapid acquisition of preference in concurrent chains: effects of morphine on sensitivity to reinforcement amount. Explorations 10: 92–11 [Google Scholar]
- Grace RC, Hucks AD (2013). The allocation of operant behavior In: APA handbook of behavior analysis, vol. 1. Methods and principles. Madden G, Dube W, Hackenberg T, Hanley G, Lattal K (editors). Washington, DC: APA; pp. 307–37. 10.1037/13937-014 [DOI] [Google Scholar]
- Green L, Myerson J (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull 130: 769–92. 10.1037/0033-2909.130.5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J (2010). Experimental and correlational analyses of delay and probability discounting In: Impulsivity: the behavioral and neurological science of discounting. Madden G, Bickel W (editors). Washington, DC: APA; pp. 67–92. [Google Scholar]
- Green L, Myerson J (2013). How many impulsivities? A discounting perspective. J Exp Anal Behav 99: 4–13. 10.1002/jeab.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P (1999). Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes. J Exp Psychol Learn Mem Cogn 25: 418–27. 10.1037//0278-7393.25.2.418 [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME (2018). Monitoring the future national survey results on drug use, 1975–2017: overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 10.3998/2027.42/142406 [DOI] [Google Scholar]
- Kalso E (2007). How different is oxycodone from morphine? Pain 132: 227–28. 10.1016/j.pain.2007.09.027 [DOI] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB (2004). Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology 173: 167–74. 10.1007/s00213-003-1697-2 [DOI] [PubMed] [Google Scholar]
- Locey ML, Dallery J (2011). Nicotine and behavioral mechanisms of intertemporal choice. Behav Processes 87: 18–24. 10.1016/j.beproc.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS (2010). A delay-discounting primer In: Impulsivity: the behavioral and neurological science of discounting. Madden G, Bickel W (editors). Washington, DC: APA; pp. 11–37. 10.1037/12069-001 [DOI] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK (1997). Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: drug and monetary rewards. Exp Clin Psychopharmacol 5: 256–62. 10.1037/1064-1297.5.3.256 [DOI] [PubMed] [Google Scholar]
- Maguire DR, Hughes CE, Pitts RC (2007). Rapid acquisition of preference in concurrent schedules: effects of reinforcement amount. Behav Processes 75: 213–19. 10.1016/j.beproc.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Maguire DR, Rodewald AM, Hughes CE, Pitts RC (2009). Rapid acquisition of preference in concurrent schedules: effects of d-amphetamine on sensitivity to reinforcement amount. Behav Processes 81: 238–43. 10.1016/j.beproc.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP (2016). Effect of daily morphine administration and its discontinuation on delay discounting of food in rhesus monkeys. Behav Pharmacol 27: 155–64. 10.1097/fbp.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987). An adjusting procedure for studying delayed reinforcement In: Quantitative analyses of behavior, vol. 5. The effect of delay and of intervening events on reinforcement value. Commons M, Mazur J, Nevin J, Rachlin H (editors). Hillsdale: Lawrence Erlbaum Associates, Inc; pp. 55–73. [Google Scholar]
- Mazur JE (2010). Distributed versus exclusive preference in discrete-trial choice. J Exp Psychol Anim Behav Process 36: 321–333. 10.1037/a0017588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B (2011). Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology 218: 703–12. 10.1007/s00213-011-2363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT (2007). Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain 132: 289–300. 10.1016/j.pain.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK (2000). Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend 60: 259–66. 10.1016/s0376-8716(00)00111-3 [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MCW, Wiskerke J, Schoffelmeer ANM (2009). Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 205: 489–502. 10.1007/s00213-009-1558-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008). The role of impulsive behavior in drug abuse. Psychopharmacology 200: 1–26. 10.1007/s00213-008-1173-0 [DOI] [PubMed] [Google Scholar]
- Petry NM (2001). Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 154: 243–250. 10.1007/s002130000638 [DOI] [PubMed] [Google Scholar]
- Pitts RC (2014). Reconsidering the concept of behavioral mechanisms of drug action. J Exp Anal Behav 101: 422–41. 10.1002/jeab.80 [DOI] [PubMed] [Google Scholar]
- Pitts RC, Febbo SM (2004). Quantitative analyses of methamphetamine’s effects on self-control choices: implications for elucidating behavioral mechanisms of drug action. Behav Processes 66: 213–33. 10.1016/j.beproc.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP (2005). Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav 83: 297–314. 10.1901/jeab.2005.47-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R, Cummings CW, Cummings C, Woodcock RL, Hughes CE (2016). Effects of methylphenidate on sensitivity to reinforcement delay and to reinforcement amount in pigeons: implications for impulsive choice. Exp Clin Psychopharmacol 24: 464–76. 10.1037/pha0000092 [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D (1991). Subjective probability and delay. J Exp Anal Behav 55: 233–44. 10.1901/jeab.1991.55-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J, Eisenberg E, Muller-Schwefe G, Drewes AM, Arendt-Nielsen L (2008). Oxycodone: A review of its use in the management of pain. Curr Med Res Opin 24: 175–92. Oxycodone: A review of its use in the management of pain [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B (2007). Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci 121: 543–9. 10.1037/0735-7044.121.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak JM, Ricaurte GA, Tallarida RL, Katz JL (2014). Methylphenidate and impulsivity: a comparison of effects of methylphenidate enantiomers on delay discounting in rats. Psychopharmacol 231: 191–8. 10.1007/s00213-013-3220-8 [DOI] [PubMed] [Google Scholar]
- Stubbs DA, Pliskoff SS (1969). Concurrent responding with fixed relative rate of reinforcement. J Exp Anal Behav 12: 887–95. 10.1901/jeab.1969.12-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zee A (2009). The promotion and marketing of Oxycontin: commercial triumph, public health tragedy. Am J of Public Health 99: 221–7. 10.2105/ajph.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Mitchell SH, de Wit H (2014). Recent translational findings on impulsivity in relation to drug abuse. Curr Addict Rep 1: 289–300. 10.1007/s40429-014-0035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W-S, Li Y-H, Xiao L, Zhu N, Bechara A, Sui N (2014). Working memory and affective decision making in addition: a neurocognitive comparison between heroin addicts, pathological gamblers, and healthy controls. Drug Alcohol Depend 134: 194–200. 10.1016/j.drugalcdep.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Yates JR, Prior NA, Chitwood MR, Day HA, Heidel JR, Hopkins SE, et al. (2019).Using a dependent schedule to measure risky choice in male rats: effects of d-amphetamine, methylphenidate, and methamphetamine. Exp Clin Psychopharmacol 10.1037/pha0000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Mitchell SH, Bickel WK (2010). Delay discounting and substance abuse-dependence In: Impulsivity: the behavioral and neurological science of discounting. Madden G, Bickel W (editors). Washington, DC: APA; pp. 67–92. 10.1037/12069-007 [DOI] [Google Scholar]
- Zacny JP, de Wit H (2009). The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav 94: 108–13. 10.1016/j.pbb.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]