Abstract
Background:
During myocardial ischemia, hypoxia-inducible factors (HIFs) are stabilized and provide protection from ischemia and reperfusion injury. Recent studies show that myocyte-specific HIF2A promotes myocardial ischemia tolerance through induction of epidermal growth factor, amphiregulin. Here, we hypothesized that HIF2A may enhance epidermal growth factor receptor 1 (ERBB1) expression in the myocardium that could interface between growth factors and its effect on providing tolerance to ischemia and reperfusion injury.
Methods:
Human myocardial tissues were obtained from ischemic heart disease patients and normal control patients to compare ERBB1 expression. Myocyte-specific Hif2a or ErbB1 knockout mice were generated to observe the effect of Hif2a knockdown in regulating ERBB1 expression and to examine the role of ERBB1 during myocardial ischemia and reperfusion injury.
Results:
Initial studies of myocardial tissues from patients with ischemic heart disease showed increased ERBB1 protein (1.12 ± 0.24 vs 13.01 ± 2.20, p<0.001). In contrast, ERBB1 transcript was unchanged. Studies utilizing shRNA repression of HIF2A or Hif2aloxP/loxP Myosin Cre+ mice directly implicated HIF2A in ERBB1 protein induction during hypoxia or following myocardial ischemia, respectively. Repression of RNA-binding protein 4 (RBM4) abolished HIF2A-dependent induction of ERBB1 protein. Moreover, ErbB1loxP/loxP Myosin Cre+ mice experienced larger infarct sizes (22.46 ± 4.06 vs 46.14 ± 1.81, p<0.001) and could not be rescued via amphiregulin treatment.
Conclusions:
These findings suggest that HIF2A promotes transcription-independent induction of ERBB1 protein and implicate epidermal growth factor signaling in protection from myocardial ischemia and reperfusion injury.
Introduction
Myocardial ischemia and reperfusion injury is among the leading causes of death in the Western countries,1 and is commonly attributed to coronary artery blockage, which leads to profound hypoxia in the myocardium.2 Local myocardial tissue hypoxia during myocardial ischemia is associated with the stabilization of hypoxia-inducible transcription factors (HIFs).3 HIFs are heterodimeric transcription factor with two major isoforms of the alpha-subunit – HIF1A and HIF2A. During normoxic conditions, both isoforms are immediately degraded through proteasomal degradation. When oxygen levels fall, both isoforms are stabilized and subsequently form a heterodimer with HIF1B that is transcriptionally active and induces HIF-target genes.2,4
HIFs are implicated in mediating adaptive responses during conditions of acute hypoxia. They are widely expressed in many tissues and mediate anti-inflammatory effects in a wide range of diseases, including acute kidney injury, lung injury or myocardial ischemia and reperfusion injury.4-7 The protective effects of HIFs during myocardial ischemia are attributed to the induction of HIF target genes. For example, HIF1A has been shown to induce enzymes of the glycolytic pathway, thereby enhancing the anaerobic capacity of myocytes to generate ATP and providing increased myocardial resistance to ischemia.8,9 The HIF-pathway can be targeted pharmacologically utilizing compounds that cause “normoxic” stabilization of HIFs. For example, previous studies demonstrated that pharmacologic HIF stabilization, using pharmacological agent dimethyloxaloylglycine (DMOG) is associated with protection from ischemia and reperfusion injury (referred to here as “cardio-protection”).3,10 Together, these studies provide evidence that HIF can be targeted to render the myocardium more resistant to the detrimental effects of ischemia and reperfusion injury. This is of importance for the field of anesthesiology and perioperative medicine, as it is foreseeable that HIF activators could be used in surgical patients. For example, pharmacologic HIF activators could be given to elective patients undergoing cardiac surgery for a time course of 2 weeks before cardiac surgery and improve outcomes in cardiac surgery patients.11-13
Only recently, studies identified a functional role of HIF2A in providing cardio-protection during ischemia and reperfusion injury.10 These studies demonstrate that mice with myocyte-specific deletion of Hif2a experience significantly larger infarct sizes in comparison to control mice or mice with myocyte-specific Hif1a deletion, thereby implicating HIF2A directly in attenuating myocardial ischemia and reperfusion injury. Subsequent studies indicate that myocyte-specific Hif2a provides cardio-protection through induction of the HIF2A target gene amphiregulin (AREG).10,14,15 Gene-targeted mice for Areg experienced larger myocardial infarct sizes, and treatment with recombinant AREG was associated with attenuated myocardial ischemia and reperfusion injury.10 AREG is a known ligand for the epidermal growth factor receptor (EGFR) ERBB116, which induces downstream signaling events including the phosphatidylinositol 3-kinase and Akt/Protein Kinase B (PI3K/Akt) pathway as a direct target.17 In the current study, we hypothesized that HIF2A-mediated cardio-protection through AREG could include a functional role of HIF2A in controlling ERBB1 expression, and thereby enhancing cardio-protection. Surprisingly, our studies provided evidence for a post-transcriptional function for HIF2A in mediating the induction of ERBB1 during myocardial ischemia and provide genetic evidence for a functional role of myocyte-specific ERBB1 signaling in attenuating myocardial infarction.
Materials and Methods
Cell culture.
Human cardiac myocytes (HCM, Cat#6200) from ScienCell (Carlsbad, CA) were cultured according to the manufacturer instructions with cardiac myocyte medium (CMM). For hypoxia experiments, HCM were incubated in a hypoxia incubator (1% O2 and 5% CO2) from Coy laboratory products (Grass Lake, MI) for specific individual time points.
Recombinant human/mouse amphiregulin treatment.
Carrier-free, recombinant human amphiregulin protein (AREG, Cat#262-AR-100/CF) and mouse amphiregulin protein (AREG, Cat#989-AR-100/CF) was purchased from R&D Systems (Minneapolis, MN). After exposure to normoxic or hypoxic conditions, HCM were washed with PBS, and cardiac myocyte medium with 20 nM AREG was applied to HCM for 10 minutes under normoxic conditions. For in vivo treatment, murine AREG was prepared and given to mice as described previously.10
Knockdown of HCM by lentivirus.
HCM were transfected with lentiviral particles containing target shRNAs (MISSION shRNA lentiviral particles, Sigma, MO) as specified: shControl; non-targeting control shRNA (SHC001), shHIF1A; Sigma’s MISSION pLKO.1 lentiviral vectors targeting HIF1A (TRCN0000003809), shHIF2A; Sigma’s MISSION pLKO.1 lentiviral vectors targeting HIF2A (TRCN0000003807), shRBM4; Sigma’s MISSION pLKO.1 lentiviral vectors targeting RBM4 (TRCN0000164640), shERBB1; Sigma’s MISSON pLKO.1 lentiviral vectors targeting ERBB1 (TRCN0000039633, TRCN0000039634, TRCN0000010329). Before shRNA transfection into HCM, a puromycin kill curve was determined for HCM, which showed 1.25 μg/mL puromycin concentration as the working concentration range. HCM were transduced with shRNAs on passage 2, and experiments were performed on passage 3. Specifically, shERBB1 cardiac myocytes were knocked down with three different targeting shRNA to enhance the efficacy of knockdown.
Mice.
Mice 8 to 16 weeks-old and both sex were used for these studies. The weights of animals used were 25 ± 5 g. Myosin Cre (B6.FVB(129)-Tg(Myh6-cre/Esr1), Hif2aloxP/loxP (Epas1tm1Mcs/J) and C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). ErbB1loxP/loxP mice were kindly provided by Maria Sibilia, PhD, Medical University of Vienna.18 Hif2aloxP/loxP Myosin Cre+, ErbB1loxP/loxP Myosin Cre+ mice, and Myosin Cre+ mice were injected with tamoxifen for 5 consecutive days (1 mg/day) for Cre-recombinase activation and allowed to recover for 7 days before the start of the experiments. Genotyping was performed by GeneTyper (New York, NY). Animal experiments and procedures were approved by the University of Colorado Denver and the University of Texas Health Science Center at Houston (UTHealth) Institutional Animal Care and Use Committee and performed in an AAALAC-accredited facility.
Murine model for myocardial ischemia.
For myocardial ischemia and reperfusion injury, we followed previously described protocols from our laboratory.10,19 Per our primary hypothesis, pilot experiments were performed to find the optimal ischemia time. The 45 minute ischemia and 2 hour reperfusion model was selected to analyze the difference in infarct size and cardiac troponin I. Experiments were performed in the mouse surgery suite during day time hours, ranging from 9 am to 5 pm. Mice were anesthetized by pentobarbital on a dosage of 70 mg/kg. To maintain anesthesia, pentobarbital was given at 10 mg/kg/h, as described previously.20-22 Mice were then intubated and connected to a ventilator (Servo 900C, Siemens from DRE veterinary, Louisville, KY). Ventilation was set on pressure control, with frequency of 110 breaths/min, positive end-expiratory pressure (PEEP) of 5 mbar, peak inspiratory pressure above PEEP of 10 mbar, and FiO2 of 0.4. The body temperature of mice was maintained at 37 ˚C throughout the experiments utilizing a temperature controlled surgical table with a feedback loop to a rectal thermometer. A PE10 (Tip O.D. (mm/”), Tapered>.024 mm/.011”) catheter was placed into the carotid artery for fluid replacement, hemodynamic monitoring or treatment with medications (e.g. reconstitution experiments). A left lateral thoracotomy was performed, and an 8-0 nylon suture was placed right around the left coronary artery (LCA). The suture was threaded through a small piece of tube and from both sides weights were applied by Eppendorf tubes for total LCA occlusion, as described earlier.22 Successful occlusion of the LCA occlusion was confirmed by immediate change of color of the myocardium that is supplied by the LCA. This change can be visualized by observation of the myocardium turning from bright red to pale white.10,23,24 After 45 minutes of ischemia, the weights were removed and reperfusion was initiated. During ischemia, normal saline with an infusion rate of 0.1 mL/hour was applied via the carotid artery catheter to apply proper fluid replacement. During reperfusion, the infusion rate was changed to 0.8 mL/hour to maintain a MAP above 60 mmHg and to ensure sufficient reperfusion for successful infarct staining. After the end of experiment, blood was obtained from the portal vein and centrifuged at 13,000 (x g), 10 minutes for serum collection. Serum was stored at −80 ˚C before usage.
Infarct staining.
To assess the percentage of the infarcted area relative to the area at risk, infarcted tissues and area at risk were measured using staining techniques.10 For this purpose, the following protocol was used, as described previously.3,22,25 After applying myocardial ischemia and reperfusion, the heart was flushed with 5 ml of normal saline via the carotid artery catheter and LCA was re-occluded. 800 ul of Evan’s blue dye (1%) was injected through the carotid artery catheter. The Area-at-Risk (AAR; area of myocardium that is perfused by the coronary artery) was determined by the area that was not stained by Evan’s blue. Following the injection of Evan’s blue, the heart was excised and kept in −20 ˚C for 15 minutes. Hearts were then sliced into 1 mm sections using a heart matrix (1 mm thickness) and incubated with 1% triphenyltetrazolium chloride (TTC) for 10 minutes at 37 ˚C. Stained heart slices were placed in 10% neutral-buffered formalin overnight for fixation. Pictures of the heart slices were captured after they were placed between two glass slides with both slide sides being clamped with Nikon D5300 camera at 16x magnification. AAR and infarct size was determined using ImageJ software. If a bubble was unintentionally existent in the catheter during Evan’s blue injection thus resulting in unsuitable AAR determination, sample was excluded. Even so, blood was obtained and used for cardiac TnI analysis.
cTnI ELISA.
In addition to staining for infarct size, myocardial injury was also determined by measuring cardiac troponin via ELISA, as we have done previously.22,23 Cardiac troponin I were measured by ELISA using cTnI ELISA Kit (Cat#CTNI-1-HS) from Life Diagnostics (West Chester, PA) following the manufacturer’s instructions.
Human cardiac tissue.
To study expression of ERBB1 in human tissues, we examined ERBB1 transcript and protein concentrations in human cardiac tissues. As described previously,9,10 human cardiac tissues were provided by the University of Colorado at Denver Division of Cardiology biobank, with all specific patient information being de-identified. For studying conditions of myocardial ischemia, ischemic heart disease (IHD) tissues were obtained from the left ventricle of explanted hearts of patients with ischemic cardiomyopathy, undergoing orthotropic heart transplantation. For normal control tissues, control (C) tissues were obtained from the left ventricle of cardiac allografts that were planned for transplantation but could not be utilized due to logistic reasons. The utilization of these tissues was approved by the University of Colorado Denver Colorado Multiple Institutional Review Board (COMIRB).
Transcriptional analysis.
For studying transcript expression, mRNA was extracted from HCM or tissue using RNeasy mini kit (Cat#74106, Qiagen), as described previously.10 cDNA was synthesized using iScript cDNA synthesis kit (Cat#170-8891, Biorad). Primers were purchased from Qiagen. The sequences of the primers are as follows: murine EGFR (Cat#QT00101584), human EGFR (Cat#QT00085701), human RBM4 (Cat#QT00235186), murine β-actin (Cat#QT00095242), and human β-actin (Cat#QT01680476).
Immunoblotting experiments.
To study the expression of proteins, immunoblotting experiments were performed as described previously.10 For tissues, murine hearts were first flushed with 5 ml of ice-cold saline and LCA was re-occluded. Evan’s blue dye was injected via carotid artery catheter. The area that was not stained by Evan’s blue was cut out and snap frozen in liquid nitrogen. HCM were lysed by Lysis buffer (150 mM NaCl, 25 mM Tris, pH 8.0, 5 mM EDTA, 2 % Triton X-100). Heart tissues were lysed using T-PER (Cat#78510, ThermoFisher). Lysed samples were centrifuged at 13,000 (x g) for 20 minutes and the supernatant collected and stored at −80 ˚C. Protein concentration was measured by BCA assay (Cat#23225, ThermoFisher). 8% or 10% polyacrylamide gels were used for SDS-PAGE. Protein samples for SDS-PAGE were prepared by mixing 4x Laemmli sample buffer (Cat#1610747, Biorad) and 2-mercaptoethanol with equal concentrations of protein per sample followed by 5 minute incubation in 95 ˚C. Proteins were transferred to nitrocellulose membranes by Trans-Blot Turbo system (Cat#1704150, Biorad) and were detected using specific primary antibodies as specified: Human ERBB1 (Cell signaling, Cat#4267; Figure 1B, 2B, 3B, 4B, and 6B), murine ERBB1 (Abcam, Cat#ab52894; Figure 2F, 3E, and 5A), RBM4 (Santa Cruz, Cat#sc373852), phosphorylated AKT (Cell signaling, Cat#9271), total AKT (Cell signaling, Cat#9272), β-actin (EMD Millipore, Cat#cp01). The specificity of ERBB1 antibodies to human and mouse samples were validated through comparing the expression of ERBB1 in shControl HCM to shERBB1 treated HCM and tamoxifen-induced Myosin Cre+ to ErbB1loxP/loxP Myosin Cre+ mouse, respectively. Proteins were detected using ChemiDoc Imaging system (Cat#12003153, Biorad) using chemiluminescent detection. Densitometry was performed using ImageJ software. ERBB1 protein expression were quantified by capturing the whole band, whether it was in a single or double band form.
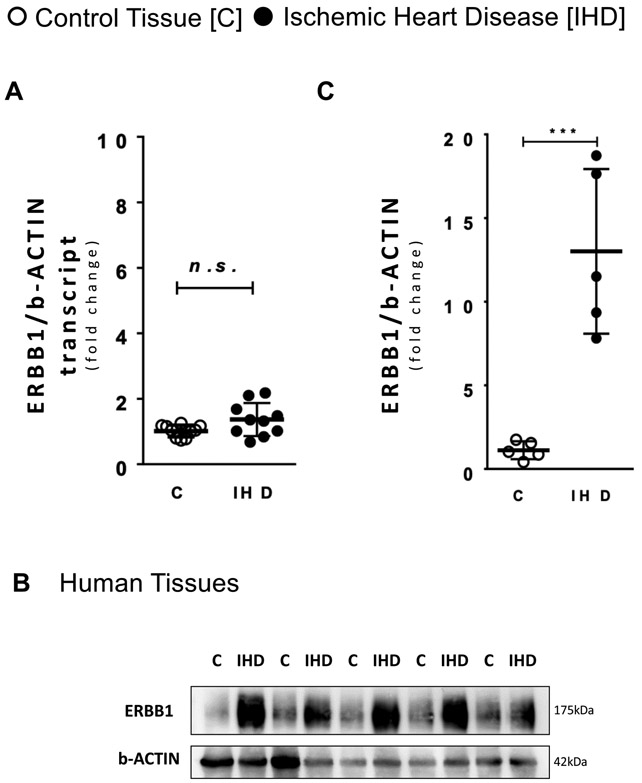
Figure 1. Ischemic heart disease patients have higher ERBB1 protein levels.
A ERBB1 transcript levels comparing healthy control (C) tissues and tissues from ischemic heart disease (IHD) patients (n=10 in both groups). B ERBB1 protein levels comparing C tissues and IHD patients’ tissue; β-ACTIN was used as a loading control. One representative blot from two independent experiments is shown. C Densitometry of ERBB1 western blots for C tissues and IHD patients’ tissue (n=5 in both groups). ***p<0.001, n.s., nonsignificant. Data are presented as the mean ± SD. (A, C) Two-tailed Satterthwaite t-test. A t=−2.07, df=11.32 c t=−5.37, df=4.09
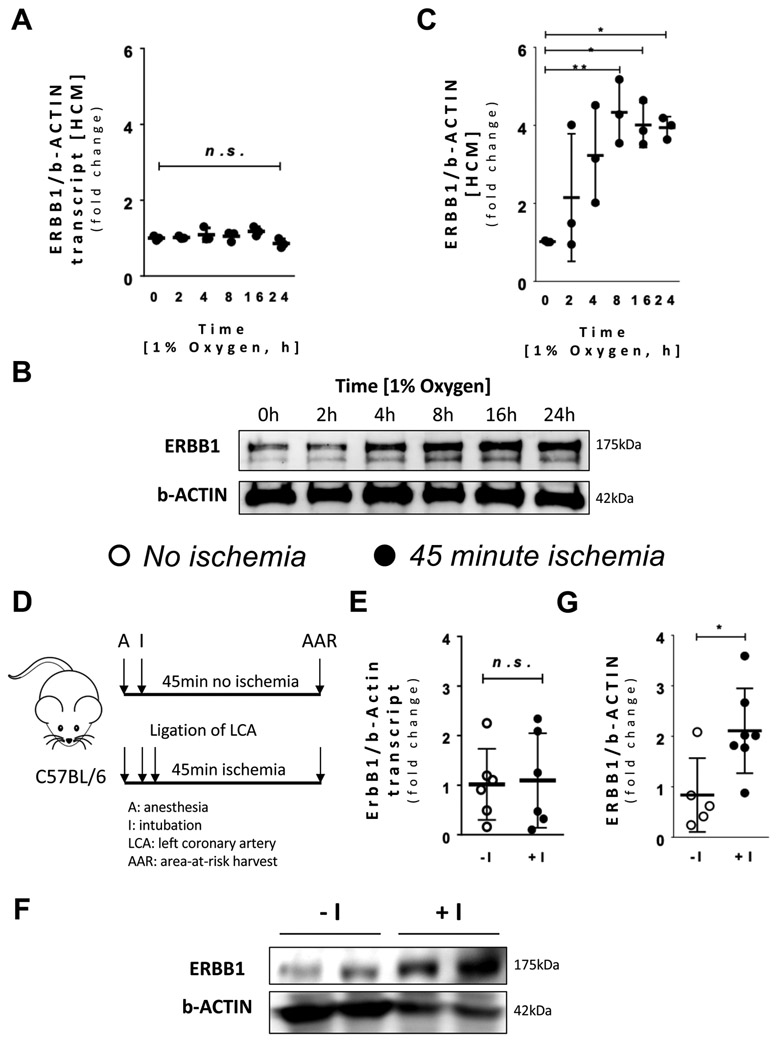
Figure 2. Hypoxia increases ERBB1 protein expression.
Human cardiac myocytes (HCM) were exposed to hypoxia (1% oxygen) and different time points were assessed. A ERBB1 transcript levels at different time points during hypoxia (n=3 per time point). B ERBB1 protein levels at different time points during hypoxia; β-ACTIN was used as a loading control. One representative blot from three independent experiments is shown. C Densitometry of ERBB1 western blots. A significant fold change was seen beginning at 8 hours of hypoxia (n=3 per time point). D Schematic illustrating the experimental design of ischemia in mice. Mice receiving no ischemia (−I) were intubated and put on ventilation. Carotid artery catheter was placed, and normal saline was supplied. After thoracotomy, a suture was placed underneath the left coronary artery (LCA). However, no hanging weight was applied. Mice receiving ischemia (+I) was treated the same way, but hanging weight was applied for total occlusion of the LCA. E ErbB1 transcript levels comparing no ischemia (−I) and 45 minutes’ ischemia (+I) in the Area-at-Risk (AAR) (n=7 per group). One observation from each group was removed as outliers. F ERBB1 protein levels comparing no ischemia (−I) and ischemia (+I) in the AAR; β-ACTIN was used as a loading control. One representative blot from three independent experiments is shown. G Densitometry of ERBB1 western blots (n≥5 per group). *p<0.05, **p<0.01, n.s., nonsignificant. Data are presented as the mean ± SD. (A, C) One-way ANOVA and Bonferroni adjustment for comparisons with values at time zero (E, G) unpaired, two-tailed t-test. A total of 24 mice were used in the studies for Figure 2, and two results were excluded from the analysis. A F5,12=2.37, p=0.102 C F5,12=5.62, p=0.007 E t=−0.16, df=10 G t=−2.72, df=10
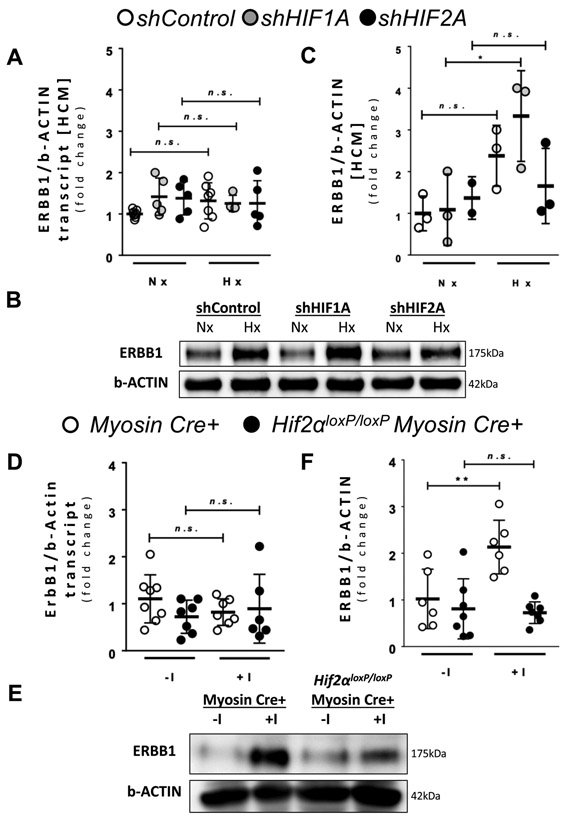
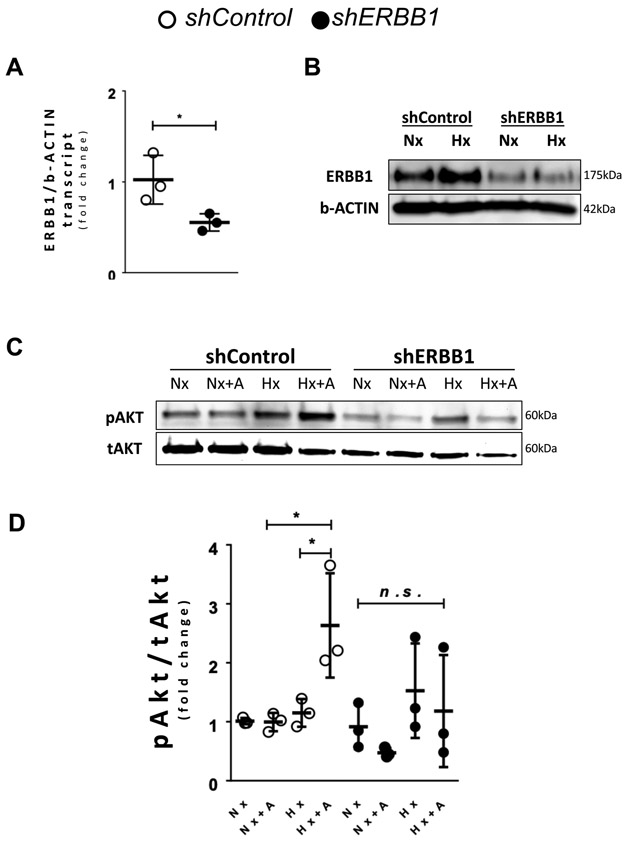
Figure 3. HIF2A upregulates ERBB1 protein levels during hypoxia.
(A-C) HIF1A and HIF2A were knocked down in HCM using short hairpin RNAs (shRNA) by lentiviral transfection. shControl HCM were transfected with lentivirus containing an empty vector. A ERBB1 transcript levels in normoxic (Nx) and hypoxic (1% oxygen; Hx) conditions (n≥4 per group). HCM were exposed to normoxia or hypoxia for 16 hours. B ERBB1 protein levels in normoxic (Nx) and hypoxic (Hx) conditions; β-ACTIN was used as a loading control. One representative blot from two independent experiments is shown. C Densitometry of ERBB1 western blots (n≥2 per group). No significant difference was found comparing Nx and Hx conditions in shHIF2A HCM. D ErbB1 transcript levels comparing no ischemia (−I) and ischemia (+I) in the AAR in Myosin Cre+ and Hif2aloxP/loxP Myosin Cre+ mice. Mice underwent no ischemia or 45 minutes of ischemia by total occlusion of the LCA. Heart samples were collected from n≥6 mice per group. One observation was removed as an outlier from the +I group of Hif2aloxP/loxP Myosin Cre+ mice. E ERBB1 protein levels in the AAR comparing no ischemia (−I) and ischemia (+I) in the AAR in Myosin Cre+ and Hif2aloxP/loxP Myosin Cre+ mice; β-ACTIN was used as a loading control. One representative blot from three independent experiments is shown. F Densitometry of ERBB1 western blots (n≥6 per group). *p<0.05, **p<0.01, n.s., nonsignificant. One observation was removed as an outlier from the −I group of Myosin Cre+ mice. Two animals did not survive and were excluded from the −I group of Hif2aloxP/loxP Myosin Cre+ mice. Data are presented as the mean ± SD. (A, C, D, and F) Two-way ANOVA and Bonferroni adjustment for comparisons between Nx and Hx, −I and +I. A total of 42 mice were used in the studies for Figure 3, and four results were excluded from the analysis. A F5,27=0.93, p=0.474 C F5,11=3.68, p=0.033 D F3,24=0.83, p=0.490 F F3,22=8.99, p<0.001
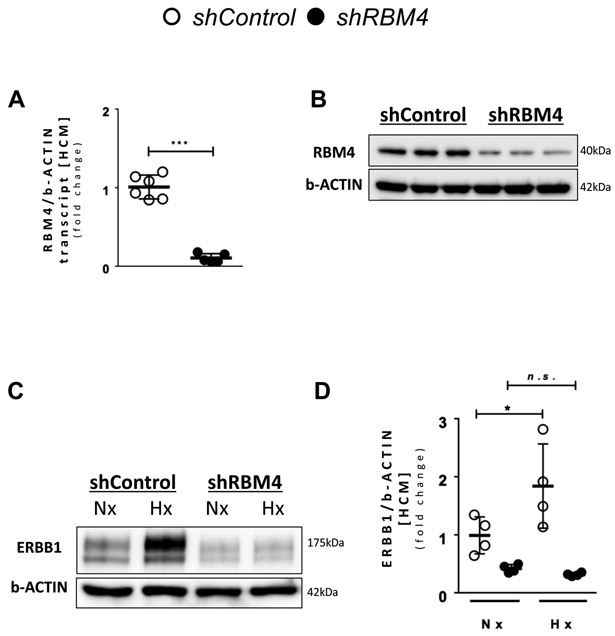
Figure 4. RBM4 is required for ERBB1 protein upregulation during hypoxia.
A RBM4 transcript levels after knockdown of RBM4 (n≥5 per group). B RBM4 protein levels after knockdown of RBM4. One representative blot from three independent experiments is shown. C ERBB1 protein levels in Nx and Hx; β-ACTIN was used as a loading control. One representative blot from three independent experiments is shown. D Densitometry of ERBB1 western blots (n=4 per group). *p<0.05, ***p<0.001, n.s., nonsignificant. Data are presented as the mean ± SD. A unpaired, two-tailed t-test. D Two-way ANOVA and Bonferroni adjustment for comparisons between Nx and Hx. A t=12.56, df=9 D F3,12=12.32, p<0.001
Figure 6. Upregulation of phosphorylated Akt levels occur after hypoxia and concomitant AREG treatment.
(A-D) ERBB1 was knocked down using shRNA in HCM via lentiviral transfection. The shControl was transfected with lentivirus containing an empty vector. A ERBB1 transcript levels after knockdown of ERBB1 (n=3 per group). B ERBB1 protein levels in normoxia (Nx) and hypoxia (Hx); β-ACTIN was used as a loading control. HCM cells were exposed to normoxia (Nx) or hypoxia (1% oxygen) for 16 hours. One representative blot from two independent experiments is shown. C pAkt (Ser473) levels were assessed in shControl and shERBB1 HCM after 16 hours of normoxia (Nx) or hypoxia (Hx), followed with or without subsequent 20 nM amphiregulin (AREG) treatment for 10 minutes (+A). One representative blot from three independent experiments is shown. D Densitometry of phosphorylated Akt western blots, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n=3 per group). *p<0.05, n.s., nonsignificant. Data are presented as the mean ± SD. A unpaired, two-tailed t-test. D Three-way ANOVA and Bonferroni adjustment for comparisons between Nx and Hx, as well as between with and without +A. A t=2.87, df=4 D F7,16=3.8, p=0.013
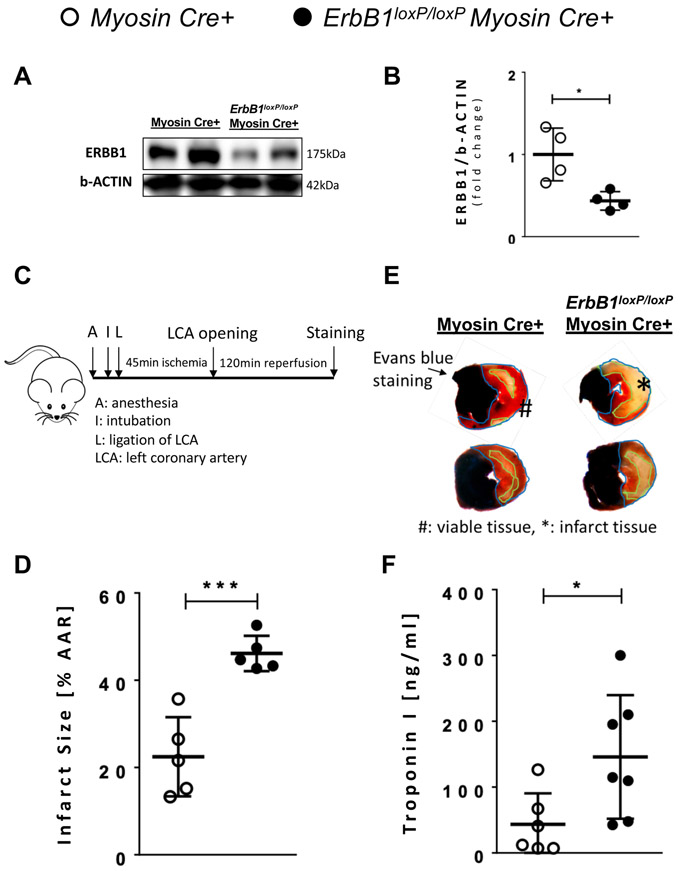
Figure 5. Tamoxifen induced knockout of ERBB1 in cardiac myocyte results in larger infarct size.
Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice were given tamoxifen daily at a dose of 1 mg via intraperitoneal injection for 5 consecutive days. Animals were then given a recovery period of 7 days. A ERBB1 protein levels after tamoxifen-induced in vivo knockout of ErbB1 in cardiac myocyte; β-ACTIN was used as a loading control. Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice underwent 45 minutes of ischemia by total occlusion of LCA. One representative blot from three independent experiments is shown. B Densitometry of ERBB1 western blots (n=4 per group). C Schematic of how surgery was performed. Infarct size was measured after double staining with 1% Evan’s blue and 1% triphenyltetrazolium chloride (TTC). Infarct sizes were normalized by percentage of infarct size to the AAR. D Infarct size after 45 minutes of ischemia and 2 hours of reperfusion in Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice (n=5 per group). E Representative infarct staining results from Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice. F Cardiac troponin I (TnI) levels determined by ELISA. Blood was collected from the portal vein after 45 minutes of ischemia and 2 hours of reperfusion in Myosin Cre+ (n=6) and ErbB1loxP/loxP Myosin Cre+ mice (n=7). The difference between infarct size measurement and cardiac TnI sample number are addressed in the methods section. *p<0.05, ***p<0.001, n.s., nonsignificant. Data are presented as the mean ± SD. (B, D, and F) unpaired, two-tailed t-test. A total of 10 mice were used in the studies for Figure 5, and none of the results were excluded from the analysis. B t=3.32, df=6 D t=−5.33, df=8 F t=−2.67, df=12
Statistical Analysis.
The primary hypothesis is evaluated in the experiment of measuring infarct sizes on Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice. It was estimated that these two groups would be respectively associated with a mean infarct size difference of 20% with common standard deviation 8%. Mean comparison should be evaluated by the two-sided t-test. At the 5% significance level, the size of 5 mice per group provides the power 97% for detecting significant difference between Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice. Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice were randomly assigned to amphiregulin-treated or PBS-treated groups. The investigator was not blinded to amphiregulin treatment but blinded to the genotype of mice to which the treatment was given at the time of surgery. Outliers were detected by Grubb’s test and removed from data. In addition, a post hoc analysis was performed without outliers removed, which can be found in Supplemental Figure 2. All data approximately followed normal distribution and were summarized as mean and standard deviation (SD). Equal variance was evaluated by F test and Levene’s test for two experimental group setting and ANOVA setting, respectively. Mean comparison between two experimental groups was evaluated by the two-sample equal-variance t-test, or the Satterthwaite t-test when the equal variance assumption was violated. ERBB1 fold changes collected from six experimental groups at six time points were analyzed by one-way ANOVA, followed by comparing mean at each study time point to mean at time zero. Experiments involving two or three factors utilized the complete factorial design. All factors were between-subject factors. Data from these experiments were analyzed by ANOVA including main factors and all possible interactions. Pairwise comparisons of study interest were subsequently conducted. Within each ANOVA framework, Bonferroni method was applied to adjust for multiple pairwise comparisons of study interest. Two-sided p values were reported and p value less than 0.05 was considered statistically significant. Data reporting and analysis was reviewed by a faculty-level statistician. Statistical analyses were performed by using the SAS software (version 9.4, the SAS Institute) and Prism (version 7, GraphPad Software Inc.)
Results
Cardiac ERBB1 protein expression is elevated in patients with ischemic heart disease
Previous studies have shown that during myocardial ischemia and reperfusion injury, HIFs are stabilized and provide cardioprotection.3,9,11 Recent studies implicate HIF2A in myocardial protection. These studies show that mice with myocyte-specific Hif2a deletion are more vulnerable to ischemia and reperfusion injury. As a mechanism for HIF2A-elicited cardio-protection, they identified that HIF2A provides cardio-protection through the transcriptional induction of epidermal growth factor, amphiregulin (AREG).10 Here, we pursued the hypothesis that HIF2A could also function to provide cardio-protection by enhancing AREG signaling through induction of AREG receptors. In the heart, AREG binds and signals solely through the ERBB1 receptor.16,26 ERBB1 is highly expressed in cardiac myocytes, while its expression is undetectable in other cellular compartments of the heart, such as vascular endothelia.27 Therefore, we pursued studies to assess ERBB1 transcript and protein content during myocardial ischemia. As first step, we examined the expression of human ERBB1 in left ventricular tissue samples from patients with ischemic heart disease. For this purpose, we measured ERBB1 mRNA and protein via qRT-PCR and Western blot, respectively. Cardiac tissues were obtained through the University of Colorado at Denver Biobank9,10 from patients who underwent orthotropic heart transplantation for ischemic heart disease. Control tissues were obtained from the left ventricle of healthy donor hearts that were destined to be cardiac allograft but could not be used due to logistic reasons. We found that ERBB1 mRNA levels were not different between the two groups (Figure 1A). In contrast, ERBB1 protein levels were significantly elevated in ischemic heart disease patients as compared to healthy controls (Figure 1B and 1C, 1.12 ± 0.24 vs 13.01 ± 2.20, p<0.001). Taken together, these findings demonstrate a transcription-independent induction of ERBB1 protein in human cardiac tissues of patients with ischemic heart disease.
ERBB1 protein expression is induced by ambient hypoxia or myocardial ischemia.
After having shown that ERBB1 protein expression is elevated in patients experiencing myocardial ischemia, we next performed studies to confirm these findings in in vitro and in vivo models. We first examined ERBB1 transcript and protein levels in human cardiac myocytes (HCM) exposed to ambient hypoxia (1% oxygen) over a time course of 0 to 24 hours. In line with the above studies in human cardiac tissues, we found that ERBB1 mRNA levels were not altered between baseline and different time points in hypoxia (Figure 2A). In contrast, ERBB1 protein levels significantly increased compared to baseline (0 hours) after 8 hours of hypoxia exposure and remained elevated throughout the time course study (Figure 2B and 2C). In subsequent studies, we exposed C57BL/6 mice to 45 minutes of myocardial ischemia by occluding the left coronary artery (Figure 2D) and assessed ERBB1 expression in tissues from the area-at-risk (AAR). Consistent with our findings in human cardiac tissues, ErbB1 mRNA levels were unchanged following myocardial ischemia (Figure 2E), whereas ERBB1 protein levels were significantly elevated within the AAR (Figure 2F and 2G). Of note, there was one experimental result in both groups of Figure 2E which were excluded by Grubb’s test. The results of analysis remained similar with and without exclusion of outliers. Taken together, these findings further suggest that cardiac ERBB1 protein expression is elevated during conditions of limited oxygen availability (hypoxia or myocardial ischemia) without concomitant induction of ErbB1 transcript levels, indicating a transcription-independent mechanism of induction.
HIF2A mediates ERBB1 protein induction during ambient hypoxia or myocardial ischemia
Based on previous findings implicating HIF2A in enhancing cardiac AREG signaling,10 we examined a potential role of HIF2A in also mediating ERBB1 protein induction. For this purpose, we used previously described cardiac myocyte cell lines (HCM) with shRNA-mediated repression of HIF1A or HIF2A.10 HCM were exposed to ambient hypoxia (1% oxygen) for 16 hours and ERBB1 transcript and protein were quantified. We observed no changes of ERBB1 mRNA in HIF1A or HIF2A deficient HCM or respective controls upon exposure to ambient hypoxia (Figure 3A). Consistent with our previous findings,10 HIF1A deficient HCM demonstrated increased ERBB1 protein after 16 hours of ambient hypoxia exposure. In contrast, HCM with shRNA-mediated HIF2A repression did not show significant elevation in ERBB1 protein (Figure 3B and 3C). These findings implicate HIF2A in the induction of ERBB1 protein during conditions of ambient hypoxia.
As next step, we examined the role of HIF2A as an inducer of ERBB1 protein in a murine model of myocardial ischemia. We exposed Myosin Cre+ or Hif2aloxP/loxP Myosin Cre+ mice to myocardial ischemia. We previously generated and characterized these transgenic mice that allow for deletion of Hif2a in myocytes following tamoxifen treatment.10 To achieve Hif2a deletion in the myocardium, Hif2aloxP/loxP Myosin Cre+ mice (or Cre controls) were treated daily with tamoxifen injections for 5 consecutive days followed by a 7 day recovery period. Mice were then subjected to 45 minutes of ischemia and ErbB1 expression was assessed in the AAR. Similar to our in vitro findings above in HCMs with HIF2A deletion, we observed that ErbB1 mRNA expression did not change with myocardial ischemia in both Myosin Cre+ and Hif2aloxP/loxP Myosin Cre+ mice (Figure 3D). Of note, there was one experimental result in the Hif2aloxP/loxP Myosin Cre+ ischemia (+I) group of Figure 3D which was excluded by Grubb’s test. The results of analysis remained similar with and without exclusion of outliers. In contrast, ERBB1 protein expression was elevated in Myosin Cre+ mice after ischemia, whereas ERBB1 protein did not increase in in Hif2aloxP/loxP Myosin Cre+ mice (Figure 3E and 3F). Of note, there was one experimental result in the Myosin Cre+ no ischemia (−I) group of Figure 3F which was excluded by Grubb’s test. The results were different when outlier data was excluded versus when outlier data was included in analysis. Taken together, these results implicate HIF2A in the transcription-independent induction of ERBB1 protein during conditions of oxygen deficiency – such as occurs during ambient hypoxia or myocardial ischemia.
RNA Binding Motif Protein 4 (RBM4) in conjunction with HIF2A mediates increase of ERBB1 protein during hypoxia.
In most instances, HIF2A increases target gene expression through its binding to hypoxia-response elements (HREs) located within the gene’s promoter. Recent studies have identified a transcription-independent mechanism of HIF2A-elicted protein induction. Here, these studies demonstrated RNA binding motif protein 4 (RBM4) to recruit HIF2A in hypoxia to the ERBB1 mRNA reverse HRE (rHRE) site, whereby the HIF2A-RBM4-eIF4E2 (Eukaryotic Translation Initiation Factor 4E Family Member 2) complex actively translates ERBB1 mRNA.28 Based on these findings, we hypothesized a functional role of RBM4 in mediating HIF2A-dependent ERBB1 protein induction in the heart. To address this hypothesis, we generated HCM with shRNA-mediated repression of RBM4. Knockdown of RBM4 was confirmed by RT-PCR (Figure 4A) and Western blot (Figure 4B). Subsequently, RBM4 knockdown HCMs and respected control (non-targeting shRNA) HCM were exposed to ambient hypoxia (1% oxygen) for 16 hours. We noted that increases of ERBB1 protein following hypoxia exposure of control cells were completely abolished in RBM4 knockdown HCMs (Figure 4C and 4D). These data indicate the likelihood that elevated ERBB1 protein in human myocytes following hypoxia exposure involve an interaction between HIF2A and RBM4.
Conditional deletion of ErbB1 in cardiac myocytes is associated with increased myocardial injury following ischemia and reperfusion.
After having shown that HIF2A coordinates the post-transcriptional induction of ERBB1 protein expression during hypoxia or myocardial ischemia, we next performed studies to address the functional role of ERBB1 signaling during myocardial injury. For this purpose, we generated mice with inducible deletion of ErbB1 in cardiac myocytes by crossing previously described ErbB1loxP/loxP mice18 with Myosin Cre+ mice. Indeed, ERBB1 protein was repressed in ErbB1loxP/loxP Myosin Cre+ mice compared to Myosin Cre+ mice after giving 45 minutes of ischemia (Figure 5A and 5B) to 44%. The fact that we don’t see a complete reduction of ERBB1 protein in ErbB1loxP/loxP Myosin Cre+ mice compared to Myosin Cre+ mice may be related to the fact that ERBB1 could also be expressed in other tissues than cardiac myocytes such as fibroblasts. As next step, we proceeded with studies of myocardial injury induced by ischemia and reperfusion. As shown in Figure 5C, Myosin Cre+ or ErbB1loxP/loxP Myosin Cre+ mice underwent 45 minutes of ischemia and 2 hours of reperfusion, followed by measurements of infarct sizes and circulating cardiac injury markers. Infarct size was significantly larger in ErbB1loxP/loxP Myosin Cre+ as compared to Myosin Cre+ mice (Figure 5D and 5E, 22.46 ± 4.06 vs 46.14 ± 1.81, p<0.001). Similarly, serum concentrations of the cardiac injury marker troponin I were significantly elevated in ErbB1loxP/loxP Myosin Cre+ mice compared to controls following myocardial ischemia and reperfusion injury (Figure 5F). Taken together, these data indicate a cardio-protective role of myocyte-specific ERBB1 signaling during myocardial ischemia and reperfusion injury.
ERBB1-mediated cardio-protection involves Akt pathway activity.
Previous studies in myocardial ischemia and reperfusion injury have implicated an important role of phosphatidylinositol 3-kinase and Akt/Protein Kinase B (PI3K/Akt) signaling 29 in providing cardio-protection and the PI3K/Akt pathway has been previously shown to function as a downstream signaling target of ERBB1.30 Based on these findings, we hypothesized that ERBB1 induces cardio-protective effects through activation of PI3K/Akt. To address this hypothesis, we first generated ERBB1 knockdown HCM. HCM were transfected with ERBB1-targeting shRNA containing lentivirus. For control, non-targeting shRNA containing lentivirus was transfected into HCM. RT-PCR confirmed knockdown of ERBB1 transcript levels are shown in Figure 6A. Subsequently, we exposed HCM to normoxia or hypoxia (1% oxygen). Control HCM showed an increase in ERBB1 protein after 16 hours of hypoxia, whereas ERBB1 knockdown HCM showed repression of ERBB1 protein at baseline and following hypoxia exposure (Figure 6B). Next, we assessed phosphorylated Akt in control and ERBB1 knockdown HCM prior to and after 16 hours of hypoxia treatment, in the absence or presence of AREG stimulation. Dose finding studies showed that 10 minutes of 20 nM recombinant AREG treatment after 16 hours of hypoxia in HCM induces the highest phosphorylated Akt levels (Supplementary figure 1A and 1B). Hypoxia itself did not significantly increase phosphorylation of Akt (Figure 6C and 6D) in HCM. However, when 20 nM recombinant AREG was applied to HCM for 10 minutes following hypoxia exposure, levels of phosphorylated Akt were significantly increased in control HCM. This effect was abolished in ERBB1 knockdown HCM. These data suggest that increased ERBB1 protein during hypoxia enhance AREG-dependent Akt phosphorylation through ERBB1 signaling. To test whether other growth factors could elicit similar results, epidermal growth factor (EGF), epiregulin, heparin binding EGF like growth factor (HBEGF), and transforming growth factor alpha (TGF-α) were treated to HCM at same conditions. While EGF, epiregulin, HBEGF, and TGF-α significantly increased phosphorylated Akt, treatment with the monoclonal ERBB1 antibody cetuximab resulted in a moderate decrease in pAkt in EGF and HBEGF treated HCM. On the other hand, cetuximab treatment in epiregulin and TGF-α treated HCM reduced pAkt levels to baseline, similar to AREG (Supplementary figure 1C and 1D). Taken together, these data suggest that AREG, epiregulin and TGF-α are the growth factors that significantly increase phosphorylated Akt after hypoxia exclusively via the ERBB1 receptor.
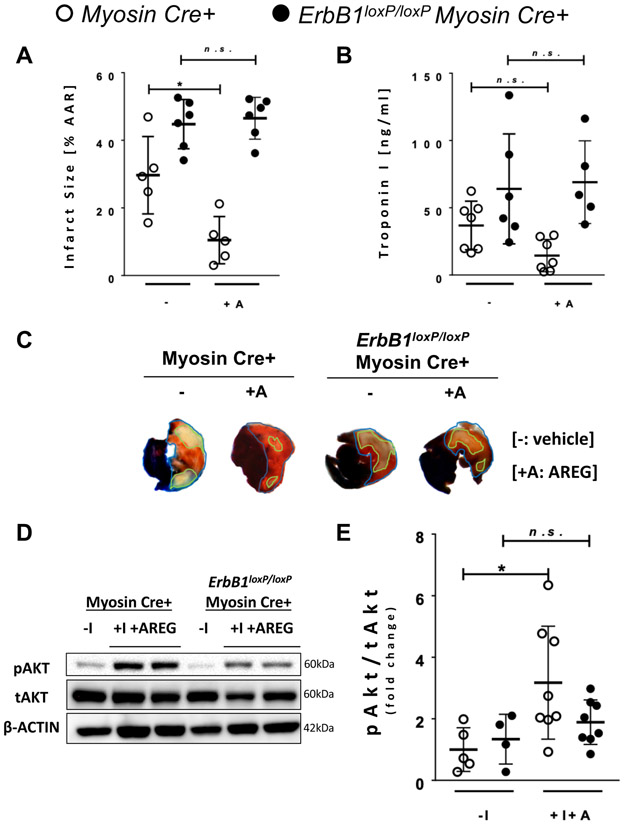
Cardio-protective effects of AREG treatment are abolished in ErbB1loxP/loxP Myosin Cre+ mice.
Based on the above in vitro findings implicating ERBB1 signaling in mediating AREG elicited Akt phosphorylation during hypoxia, we proceeded with in vivo studies to address role of ERBB1 signaling in AREG-dependent cardio-protection and concomitant Akt phosphorylation. For this purpose, Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice underwent 45 minutes of ischemia followed by 2 hours of reperfusion after treatment with 10 ug recombinant AREG via a carotid artery catheter or vehicle control. Consistent with previous findings,10 AREG treatment decreased the infarct size from 29.1% to 10.46% in Myosin Cre+ mice. In contrast, the cardio-protective effect of AREG treatment was abolished in ErbB1loxP/loxP Myosin Cre+ mice, which showed similar infarct size with or without Areg treatment (47.9% to 46.53%, Figure 7A). Similarly, attenuation of plasma troponin I by AREG treatment were abolished in ErbB1loxP/loxP Myosin Cre+ mice (Figure 7B). We next examined phosphorylated Akt in the AAR following myocardial ischemia and reperfusion. Consistent with our in vitro data above, Myosin Cre+ mice showed increased phosphorylation of Akt after 45 minutes of ischemia and 2 hours of reperfusion when compared to baseline levels. In contrast, phosphorylated Akt in ErbB1loxP/loxP Myosin Cre+ mice after 45 minutes of ischemia and 2 hours of reperfusion were not significantly increased from baseline (Figure 7D and 7E). The slight but not statistically significant increase in phosphorylated Akt may be explained by the incomplete knockdown of ERBB1 in myocytes, or by the activation of ERBB1 expressed on different cellular components of the murine heart. Of note, there was one experimental result in the Myosin Cre+ normoxic (−I) group of Figure 7E which was excluded by Grubb’s test. The results of analysis remained similar with and without exclusion of outliers. Taken together, these findings indicate that AREG-ERBB1-mediated signaling induces cardio-protection by increasing Akt phosphorylation.
Figure 7. Cardioprotective effects of AREG via upregulation of phosphorylated Akt requires the ERBB1 receptor.
A Infarct size after 45 minutes of ischemia and 2 hours of reperfusion in Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice treated with normal saline (N/S) or AREG (n≥5 per group). N/S (−) or 10 ug AREG (+A) dissolved in N/S were given to the mouse via the carotid catheter before the initiation of ischemia. One animal did not survive and was excluded from the - group of Myosin Cre+ mice. B Cardiac troponin I (TnI) levels after myocardial ischemia and reperfusion injury in Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice with N/S (−) or AREG treatment (+A) (n≥5 per group). The difference between infarct size measurement and cardiac TnI sample number are addressed in the methods section. C Representative infarct staining results from Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice with or without AREG treatment. D pAkt (Ser473) protein levels in normoxic (−I) tissue or AAR from mice heart that underwent 45 minutes of hypoxia plus 2 hours of reperfusion with AREG treatment (+I+A) in Myosin Cre+ and ErbB1loxP/loxP Myosin Cre+ mice; β-ACTIN was used as a loading control. 10 ug AREG dissolved in N/S were given to the mouse via the carotid catheter before the initiation of ischemia. One representative blot from three independent experiments is shown. E Densitometry of phosphorylated Akt western blot, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n≥4 per group). One observation was removed as an outlier from the −I group of Myosin Cre+ mice. *p<0.05, n.s., nonsignificant. Data are presented as the mean ± SD. (A, B and E) Two-way ANOVA and Bonferroni adjustment for comparisons between with and without +A, −I and +I+A. A total of 49 mice were used in the studies for Figure 7, and two results were excluded from the analysis. A F3,18=23.42, p<0.001 B F3,21=5.55, p<0.001 E F3,21=4.00, p=0.021
Discussion
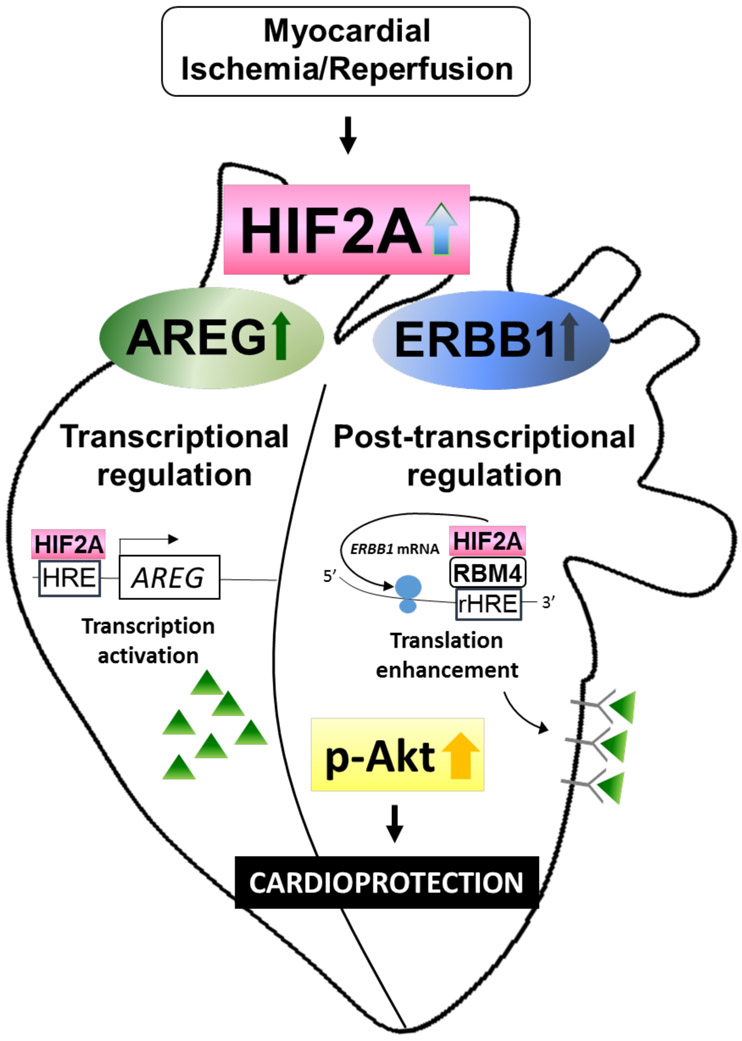
In the present study, we demonstrate a functional role for HIF2A in mediating cardio-protection through the induction of AREG receptor ERBB1 in human cardiac myocytes and murine heart. Based on our initial studies demonstrating elevated ERBB1 receptor protein expression in patients with ischemic heart disease, we performed in vitro and in vivo studies to examine the role of HIF2A and ERBB1 signaling during myocardial ischemia and reperfusion injury. We found that ERBB1 protein was also elevated following ambient hypoxia exposure of human myocytes or in the ischemic myocardial tissue from mice. Together, these studies demonstrated that ERBB1 protein was elevated during conditions of limited availability of oxygen without concomitant changes in ERBB1 transcript expression. Subsequent studies with in vitro knockdown of HIF2A in human myocytes or in mice with myocyte-specific deletion of Hif2a indicate a functional role of HIF2A in mediating ERBB1 protein induction. Based on previous reports showing that HIF2A can mediate post-transcriptional induction of ERBB1 during hypoxia,28 we identified a functional role of RBM4 in mediating cardiac induction of ERBB1 protein. Subsequent studies to address the functional role of ERBB1 signaling during myocardial ischemia demonstrated that induced myocyte-specific deletion of ErbB1 is associated with increased myocardial injury during ischemia and reperfusion. Moreover, the cardio-protective effects of the ERBB1 ligand AREG were abrogated in gene-targeted mice for ErbB1. Together with previous studies implicating HIF2A in the induction of AREG during conditions of myocardial ischemia,10 the present studies highlight a functional role for HIF2A in coordinating enhanced AREG signaling through the transcriptional induction of AREG and post-transcriptional induction of the AREG receptor, ERBB1 (Figure 8).
Figure 8. Suggested mechanism of ERBB1 and cardioprotection during myocardial ischemia and reperfusion injury.
During myocardial ischemia and reperfusion injury, the heart experiences significant hypoxia. Hypoxia stabilizes transcription factor HIF2A, which is then recruited by RBM4 to the RNA hypoxia response element (rHRE). This complex initiates the translation of ErbB1 mRNA to produce ERBB1 protein. Also upregulated during myocardial injury is AREG. HIF2A binds to the AREG promotor and increases the transcription of AREG gene. Finally, increased AREG protein binds to the increased ERBB1 receptors and activate important ERBB1 downstream signaling cascades, such as Akt, to provide cardio-protection.
Several previous studies have highlighted functional roles of HIFs in providing cardio-protection from ischemia and reperfusion injury. For example, pharmacologic studies with the HIF activator dimethyloxalylglycine (DMOG) are associated with cardiac stabilization of HIF1A and attenuated myocardial infarct sizes following ischemia and reperfusion.3 Subsequent studies have highlighted several potential mechanisms on how HIF1A can provide cardio-protection. HIF1A is known to enhance the production of the extracellular signaling molecule adenosine and the adenosine A2B receptor (Adora2b).31,32 Adenosine signaling through its receptors are reported to have anti-inflammative and protective roles during organ injury.33 In line with these observations, studies of myocardial ischemia and reperfusion injury indicate that cardio-protective effects of HIF1A depend on purinergic signaling events, including enhanced production of extracellular adenosine and Adora2b signaling.20,21,34 Other studies suggest that HIF1A could provide cardio-protection from ischemia and reperfusion through interaction with the circadian rhythm protein period 2 and thereby enhance myocardial resistance to ischemia by optimizing cardiac carbohydrate metabolism.9,11
Only recently has a functional role of HIF2A in cardio-protection from ischemia and reperfusion injury emerged.10 Mice with siRNA-mediated repression of HIF1A or with myocyte specific deletion of HIF2A show abolished cardio-protection when treated with DMOG prior to myocardial ischemia, suggesting that both HIF-isoforms contribute to cardio-protection.3,10 Based on our current study, the signaling mechanisms for HIF-dependent cardio-protection are likely controlling different signaling pathways. While HIF1A appears to provide cardio-protection through enhancing purinergic signaling pathways,21 HIF2A-dependent cardio-protection involves epidermal growth factor signaling, including the transcriptional induction of epidermal growth factor, AREG10 and the post-transcriptional induction of its receptor, ERBB1.
Previous studies have identified functional roles of ERBB1 in the heart. The ERBB1 receptor, one of the four subtypes of ErbB receptor tyrosine kinases (ErbB 1-4), is expressed in cardiac tissues and deletion of ErbB1 in mouse embryonic stem cells result in failure of mice to survive.18,35 ERBB1 is mainly expressed in cardiac myocytes, but they are also found in fibroblasts.36 While fibroblasts have been reported to contribute to the healing process by expanding and modulating the extracellular matrix, their role during the post-ischemic inflammatory phase are limited.37 Previous studies showed that increased human ErbB1 (hEGFR) expression in the mouse myocardium leads to heart hypertrophy and semilunar valve defects.38 Cardiac knockout of ErbB1 affects the phosphorylation and activation of both receptors ErbB1 and ErbB2, thus resulting in ventricular dilation, hypertrophy, and abnormal cardiac function in the adult mouse heart.39 Deletion of ErbB1 in vascular smooth muscle cells (VSMC) led to dilation of the aorta and coronary artery as well as disruption of the basal VSMC homeostasis.40 Previous studies also showed functional roles of ERBB1 and its ligands in the heart during ischemia and reperfusion injury. Such studies provide evidence that during reperfusion, bradykinin activates ERBB1 and thus protects the heart by increased phosphorylation of Akt and preservation of mitochondrial membrane potential.41 Other studies observed increases in epidermal growth factor (EGF) in the heart during stress-induced injury in mice and showed that it protects the heart possibly through ERBB1 activation.42 Additional studies have searched for a relationship between ERBB1 and the adenosine A1 receptor during ischemic preconditioning and suggest that the cardio-protective effect of ischemic preconditioning may include ERBB1 signaling, since the effects were abolished after treatment with AG-1478, a pharmacologic antagonist of ERBB1 signaling.43 The reason we investigated in AREG and its effect on ERBB1 was because both were simultaneously upregulated through transcription factor HIF2A during hypoxia. AREG was the highest differentially regulated transcript in the microarray study comparing post-ischemic heart tissues from Myosin Cre+ and Hif2aloxP/loxP Myosin-Cre+ mice, and we sought to investigate the effect of AREG-ERBB1 interaction during myocardial ischemia and reperfusion injury.10
Previous studies have examined transcriptional responses of ERBB1 during conditions of limited oxygen availability. For this purpose, the authors compared mRNA expression of ERBB1 in the left ventricle (hypoxic) to the left atrium (normoxic) in biopsies from each patient’s hearts undergoing coronary artery bypass operation, which showed downregulation of HER2 and upregulation of ERBB1.44 In contrast, the present studies suggest that ERBB1 mRNA levels did not change during hypoxia, while ERBB1 protein levels increased, which is suggestive of a post-transcriptional mechanism. The discrepancy in results may come from the regional difference of where the normoxic control was harvested, as well as methodological differences of how the tissue was processed. Consistent with our findings, previous studies identified HIF2A as mediator of this response, including HIF2A dependent increase of ERBB1 in various human cell lines after hypoxia exposure. Here, metabolic labeling techniques showed that increased translation of ERBB1 mRNA was the driving force behind increased ERBB1 expression.45 The mechanism was further studied in glioblastoma cells, which showed that HIF2A along with RBM4 and cap-binding eIF4E2 creates a complex and that this complex binds to the RNA hypoxic response element (rHRE) on the ERBB1 mRNA, thereby initiating selective cap-dependent protein synthesis and escaping the repression of protein synthesis during hypoxia.28
Our current study provides evidence for PI3K/Akt signaling as an endpoint of ERBB1-mediated cardio-protection. This is consistent with previous studies that demonstrate Akt promotes cellular survival during ischemia-reperfusion injury in the mouse heart.46 Akt specifically falls into a category called “reperfusion injury salvage kinase (RISK)” pathway.47 These studies demonstrate that increased phosphorylation of PI3K/Akt during reperfusion apparently functions to activate several cardio-protective mechanisms, including phosphorylation of the pro-apoptotic protein Bcl-2-associated death promoter (BAD)48, inhibition of mitochondrial cytochrome c release49, or inhibition of the opening of mitochondrial permeability transition pore (MPTP) and maintenance of the mitochondrial membrane potential50.
Our study has several limitations. First, ERBB1 expression in the human heart may vary within different regions, and the spatiotemporal kinetics of ERBB1 upregulation during myocardial ischemia and reperfusion injury needs further investigation. Second, the ERBB1 receptor can potentially bind to ligands other than AREG, such as epiregulin and TGF-α. Although we demonstrate the specific contribution of AREG-ERBB1 interaction in activating the PI3K/Akt signaling pathway in this study, other ligands may play a part in this process and contribute to cardio-protection. Future studies may be necessary to investigate the effect of other ligands, where there might be synergistic effects from the combination of multiple growth factors.
In summary, we have identified HIF2A-dependent induction of ERBB1 protein and concomitant cardio-protection through activation of the PI3K/Akt signaling pathway as a novel mechanism for HIF2A in providing cardio-protection from ischemia and reperfusion injury. Interestingly, it appears that HIF2A coordinates enhanced signaling effects of AREG through transcriptional and post-transcriptional mechanism. Indeed, previous studies from our laboratory implicate HIF2A as a transcriptional regulator of AREG induction during myocardial ischemia10 and our current study implicates HIF2A as an enhancer of ERBB1 protein expression. Taken together, these findings indicate that AREG signaling through ERBB1 is controlled by HIF2A during myocardial ischemia and can be targeted for attenuating myocardial injury following ischemia and reperfusion. Clinical studies will have to determine if these findings can be translated into patients experiencing ischemic myocardial tissue injury, such as during myocardial infarction or during cardiac surgery. Such translational studies could include pharmacologic activators of HIFs or could directly target the amphiregulin-ERBB1 signaling pathway.
Supplementary Material
Supplementary Figure 1. Dose finding studies of AREG treatment and the effect of other ligands. A Phosphorylated Akt (Ser473) levels at different time points. shControl HCM were exposed to 16 hours of hypoxia and subsequently given 20 nM AREG in normoxia for 0, 5, 10, 20, 30 and 60 minutes (1h). Total Akt is shown. B pAkt (Ser473) levels after 10, 20, and 40 nM concentrations of AREG treatment. Total Akt is shown. C pAkt (Ser473) levels in 1 hour vehicle or Cetuximab (C) treated HCM after 15 hours of normoxia (Nx) or hypoxia (Hx), followed with or without subsequent 20 mM amphiregulin (AREG), epidermal growth factor (EGF), epiregulin, heparin binding EGF like growth factor (HBEGF) or transforming growth factor alpha (TGF-α) treatment for 10 minutes. One representative blot from three independent experiments is shown. D Densitometry of phosphorylated Akt western blots, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n=3 per group). *p<0.05, **p<0.01, ***p<0.001, n.s., nonsignificant. Data are presented as the mean ± SD.
Supplementary Figure 2. Post hoc analysis of Figures 2E, 3D, 3F and 7E 2E ErbB1 transcript levels comparing no ischemia (−I) and 45 minutes’ ischemia (+I) in the Area-at-Risk (AAR) (n=8 per group). t=−0.09, df=12 3D ErbB1 transcript levels comparing no ischemia (−I) and ischemia (+I) in the AAR in Myosin Cre+ and Hif2aloxP/loxP Myosin Cre+ mice. Mice underwent no ischemia or 45 minutes of ischemia by total occlusion of the LCA. Heart samples were collected from n≥6 mice per group. F3,25=0.47, p=0.707 3F Densitometry of ERBB1 western blots (n≥6 per group). F3,23=3.05, p=0.049 7E Densitometry of phosphorylated Akt western blot, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n≥4 per group). F3,22=1.98, p=0.147
Acknowledgements
The authors would like to thank Kelley Brodsky, MS (Department of Cardiology, University of Colorado School of Medicine, Aurora, CO, USA) and Jessica Wang, PhD (Department of Anesthesiology, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA) for reviewing the original data.
Funding Statement: The present studies are supported by National Institute of Health Grants R01 DK097075, POI-HL114457, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE; Deutsche Forschungsgemeinschaft (DFG) research fellowship to M.K.; International Anesthesia Research Society Mentored Research Award and National Institutes of Health Grants P50-CA098258 and DK056338 to J.L.B; National Heart, Lung, and Blood Institute (NIH-NHLBI) Grant 1K08HL102267 and 1R01HL122472-01 to T.E.; Austrian Science Fund (FWF) grant P18421 and DKW1212, Austrian Federal Government’s GEN-AU program “Austromouse” (GZ 200.147/1-VI/1a/2006 and 820966) to M.S.
Footnotes
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Eltzschig HK, Eckle T: Ischemia and reperfusion--from mechanism to translation. Nat Med 2011; 17: 1391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Carmeliet P: Hypoxia and inflammation. N Engl J Med 2011; 364: 656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK: Hypoxia-Inducible Factor-1 Is Central to Cardioprotection: A New Paradigm for Ischemic Preconditioning. Circulation 2008; 118: 166–175 [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Bratton DL, Colgan SP: Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 2014; 13: 852–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckle T, Brodsky K, Bonney M, Packard T, Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M, Eltzschig HK: HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol 2013; 11: e1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig HK: Adenosine: an old drug newly discovered. Anesthesiology 2009; 111: 904–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK: Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 2011; 22: 14–20 [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL, Roth PH, Fang HM, Wang GL: Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 1994; 269: 23757–63 [PubMed] [Google Scholar]
- 9.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK: Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 2012; 18: 774–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeppen M, Lee JW, Seo SW, Brodsky KS, Kreth S, Yang IV, Buttrick PM, Eckle T, Eltzschig HK: Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat Commun 2018; 9: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Bonney SK, Eckle T: Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med 2013; 19: 345–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK: Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth Analg 2018; 126: 308–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helwani MA, Amin A, Lavigne P, Rao S, Oesterreich S, Samaha E, Brown JC, Nagele P: Etiology of acute coronary syndrome after noncardiac surgery. Anesthesiology 2018; 128: 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiehl DP, Bordoli MR, Abreu-Rodriguez I, Wollenick K, Schraml P, Gradin K, Poellinger L, Kristiansen G, Wenger RH: Non-canonical HIF-2alpha function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene 2012; 31: 2283–97 [DOI] [PubMed] [Google Scholar]
- 15.Bordoli MR, Stiehl DP, Borsig L, Kristiansen G, Hausladen S, Schraml P, Wenger RH, Camenisch G: Prolyl-4-hydroxylase PHD2- and hypoxia-inducible factor 2-dependent regulation of amphiregulin contributes to breast tumorigenesis. Oncogene 2011; 30: 548–60 [DOI] [PubMed] [Google Scholar]
- 16.Johnson GR, Kannan B, Shoyab M, Stromberg K: Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem 1993; 268: 2924–31 [PubMed] [Google Scholar]
- 17.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW: Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003; 284: 31–53 [DOI] [PubMed] [Google Scholar]
- 18.Natarajan A, Wagner B, Sibilia M: The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A 2007; 104: 17081–17086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckle T, Koeppen M, Eltzschig H: Use of a hanging weight system for coronary artery occlusion in mice. Journal of visualized experiments: JoVE 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK: CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 2007; 116: 1784–94 [DOI] [PubMed] [Google Scholar]
- 21.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK: Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation 2007; 115: 1581–90 [DOI] [PubMed] [Google Scholar]
- 22.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK: Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 2006; 291: H2533–40 [DOI] [PubMed] [Google Scholar]
- 23.Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L, Eckle T: Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol 2015; 195: 1732–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T: Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One 2013; 8: e71493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP: Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 2009; 113: 224–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willmarth NE, Ethier SP: Amphiregulin as a novel target for breast cancer therapy. J Mammary Gland Biol Neoplasia 2008; 13: 171–9 [DOI] [PubMed] [Google Scholar]
- 27.Russell KS, Stern DF, Polverini PJ, Bender JR: Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol 1999; 277: H2205–11 [DOI] [PubMed] [Google Scholar]
- 28.Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, Payette J, Holcik M, Pause A, Lee S: An oxygen-regulated switch in the protein synthesis machinery. Nature 2012; 486: 126–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster K, Kuno A, Solenkova N, Felix SB, Krieg T: The delta-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. Am J Physiol Heart Circ Physiol 2007; 293: H1604–8 [DOI] [PubMed] [Google Scholar]
- 30.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H: Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem 2000; 275: 30934–42 [DOI] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP: Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 2003; 198: 783–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP: HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 2006; 20: 2242–50 [DOI] [PubMed] [Google Scholar]
- 33.Ohta A, Sitkovsky M: Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414: 916. [DOI] [PubMed] [Google Scholar]
- 34.Eltzschig HK, Sitkovsky MV, Robson SC: Purinergic signaling during inflammation. N Engl J Med 2012; 367: 2322–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC: Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 1995: 230–230 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Jin X, Hu C-F, Zhang Y-P, Li R, Shen C-X: Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating EGFR-dependent pathway. Basic Res Cardiol 2018; 113: 12. [DOI] [PubMed] [Google Scholar]
- 37.Shinde AV, Frangogiannis NG: Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 2014; 70: 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF: Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development 2003; 130: 4515–4525 [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan V, Zucker IH, Jones JA, Carlson M, Ma YJ: Cardiac ErbB-1/ErbB-2 mutant expression in young adult mice leads to cardiac dysfunction. Am J Physiol Heart Circ Physiol 2008; 295: H543–54 [DOI] [PubMed] [Google Scholar]
- 40.Schreier B, Döhler M, Rabe S, Schneider B, Schwerdt G, Ruhs S, Sibilia M, Gotthardt M, Gekle M, Grossmann C: Consequences of epidermal growth factor receptor (ErbB1) loss for vascular smooth muscle cells from mice with targeted deletion of ErbB1. Arterioscler Thromb Vasc Biol 2011; 31: 1643–1652 [DOI] [PubMed] [Google Scholar]
- 41.Methner C, Donat U, Felix SB, Krieg T: Cardioprotection of bradykinin at reperfusion involves transactivation of the epidermal growth factor receptor via matrix metalloproteinase-8. Acta Physiol 2009; 197: 265–71 [DOI] [PubMed] [Google Scholar]
- 42.Pareja M, Sanchez O, Lorita J, Soley M, Ramirez I: Activated epidermal growth factor receptor (ErbB1) protects the heart against stress-induced injury in mice. Am J Physiol Regul Integr Comp Physiol 2003; 285: R455–62 [DOI] [PubMed] [Google Scholar]
- 43.Williams-Pritchard G, Knight M, Hoe LS, Headrick JP, Peart JN: Essential role of EGFR in cardioprotection and signaling responses to A1 adenosine receptors and ischemic preconditioning. Am J Physiol Heart Circ Physiol 2011; 300: H2161–8 [DOI] [PubMed] [Google Scholar]
- 44.Munk M, Memon AA, Goetze JP, Nielsen LB, Nexo E, Sorensen BS: Hypoxia changes the expression of the epidermal growth factor (EGF) system in human hearts and cultured cardiomyocytes. PLoS One 2012; 7: e40243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S: Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A 2007; 104: 13092–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K: Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 2000; 101: 660–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hausenloy DJ, Yellon DM: New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 2004; 61: 448–60 [DOI] [PubMed] [Google Scholar]
- 48.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91: 231–41 [DOI] [PubMed] [Google Scholar]
- 49.Kennedy SG, Kandel ES, Cross TK, Hay N: Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 1999; 19: 5800–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davidson SM, Hausenloy D, Duchen MR, Yellon DM: Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 2006; 38: 414–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dose finding studies of AREG treatment and the effect of other ligands. A Phosphorylated Akt (Ser473) levels at different time points. shControl HCM were exposed to 16 hours of hypoxia and subsequently given 20 nM AREG in normoxia for 0, 5, 10, 20, 30 and 60 minutes (1h). Total Akt is shown. B pAkt (Ser473) levels after 10, 20, and 40 nM concentrations of AREG treatment. Total Akt is shown. C pAkt (Ser473) levels in 1 hour vehicle or Cetuximab (C) treated HCM after 15 hours of normoxia (Nx) or hypoxia (Hx), followed with or without subsequent 20 mM amphiregulin (AREG), epidermal growth factor (EGF), epiregulin, heparin binding EGF like growth factor (HBEGF) or transforming growth factor alpha (TGF-α) treatment for 10 minutes. One representative blot from three independent experiments is shown. D Densitometry of phosphorylated Akt western blots, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n=3 per group). *p<0.05, **p<0.01, ***p<0.001, n.s., nonsignificant. Data are presented as the mean ± SD.
Supplementary Figure 2. Post hoc analysis of Figures 2E, 3D, 3F and 7E 2E ErbB1 transcript levels comparing no ischemia (−I) and 45 minutes’ ischemia (+I) in the Area-at-Risk (AAR) (n=8 per group). t=−0.09, df=12 3D ErbB1 transcript levels comparing no ischemia (−I) and ischemia (+I) in the AAR in Myosin Cre+ and Hif2aloxP/loxP Myosin Cre+ mice. Mice underwent no ischemia or 45 minutes of ischemia by total occlusion of the LCA. Heart samples were collected from n≥6 mice per group. F3,25=0.47, p=0.707 3F Densitometry of ERBB1 western blots (n≥6 per group). F3,23=3.05, p=0.049 7E Densitometry of phosphorylated Akt western blot, which is expressed as a ratio of phosphorylated Akt to total Akt (pAkt/tAkt) (n≥4 per group). F3,22=1.98, p=0.147