Abstract
Inter-alpha Inhibitor proteins (IAIPs) are naturally occurring immunomodulatory molecules found in most tissues. We have reported ontogenic changes in the expression of IAIPs in brain during development in sheep and abundant expression of IAIPs in fetal and neonatal rodent brain in a variety cellular types and brain regions. Although a few studies identified bikunin, light chain of IAIPs, in adult human brain, the presence of the complete endogenous IAIP protein complex has not been reported in human brain. In this study, we examined the immunohistochemical expression of endogenous IAIPs in human cerebral cortex from early in development through the neonatal period and in adults using well-preserved postmortem brains. We examined total, nuclear and cytoplasmic staining of endogenous IAIPs and their expression in neurofilament light polypeptide (NFL) positive neurons and glial fibrillary acidic protein (GFAP) positive astrocytes. IAIPs were ubiquitously detected for the first time in cerebral cortical cells at 24–26, 27–28, 29–36 and 37–40 weeks of gestation and in adults. Quantitative analyses revealed that IAIPs were predominately localized in the nucleus in all age groups, but cytoplasmic IAIP expression was more abundant in adults than in the younger ages. Immunoreactivity of IAIPs was expressed in neurons and astrocytes in all age groups. In addition, IAIP co-localization with GFAP-positive astrocytes was more abundant in adults than in the developing brain. We conclude that IAIPs exhibit ubiquitous expression, and co-localize with neurons and astrocytes in the developing and adult human brain suggesting a potential role for IAIPs in development and endogenous neuroprotection.
Keywords: brain development, human autopsy, Inter-alpha Inhibitors, immunohistochemistry, neurons, astrocytes
1. INTRODUCTION
Inter-alpha Inhibitor Proteins (IAIPs) are structurally related proteins that are key elements of innate immunity (Fries & Blom, 2000; Fries & Kaczmarczyk, 2003; Garantziotis et al., 2007; Kobayashi, 2006; Salier, Rouet, Raguenez, & Daveau, 1996). IAIPs are found in the brain and in most peripheral tissues including liver, kidney, intestine, lung, and connective tissues (Bourguignon et al., 1999; Daveau et al., 1998; Fries & Blom, 2000; Ide et al., 1999; Itoh et al., 1996; Kobayashi, Sun, & Terao, 1999; Sjoberg & Fries, 1992; Takano et al., 1999; Yoshida et al., 1989; Zhuo & Kimata, 2008). The two major forms of IAIPs in human plasma are Inter-alpha Inhibitor (IαI), which consists of two heavy chains (H1 and H2) and a single light chain (bikunin), and Pre-alpha Inhibitor (PαI), which consists of one heavy chain (H3) and one light chain (Enghild, Thogersen, Pizzo, & Salvesen, 1989; Lim, Josic, Callanan, Brown, & Hixson, 2005; Odum, Halkier, Hojrup, & Schousboe, 1989). High levels of IAIPs are normally present in human plasma of adults, newborns, and even in premature infants, suggesting that these circulating IAIP complexes are important (Baek et al., 2003; Fries & Blom, 2000; Lim et al., 2003). Moreover, IAIPs have been shown to have numerous essential immunomodulatory roles (Fries & Blom, 2000; Fries & Kaczmarczyk, 2003; Lim, 2013).
Although many of the mechanisms underlying the functional importance of IAIPs are not known, increases in IAIP expression are correlated with a diverse spectrum of pathological conditions including tissue injury and repair (Huth et al., 2015; Plaas et al., 2011; Tan, McGrouther, Day, Milner, & Bayat, 2011), and inflammation (Daveau et al., 1998; Garantziotis et al., 2007; Itoh et al., 1994; Kobayashi, 2006; Mizon, Mairie, Balduyck, Hachulla, & Mizon, 2001; Okroj et al., 2012; Seifert, Kurzinger, Hopt, & Wittel, 2013; Wakahara et al., 2005). Recent studies have also reported changes in the level of IAIPs after stroke (Kashyap et al., 2009) and Alzheimer’s disease (Shi et al., 2019), suggesting that endogenous IAIPs may be integrally related to the pathogenesis of inflammatory-related brain disorders. In addition, systemic levels of IAIPs have been reported to be decreased in sepsis in neonates and adults (Chaaban et al., 2009; Degraeuwe & Nieman, 2010; Opal et al., 2007) and in necrotizing enterocolitis in neonates (Chaaban et al., 2010; Shah et al., 2017). Sepsis and necrotizing enterocolitis are both associated with impaired neurodevelopmental outcomes in premature infants (O’Shea, 2002; Stoll et al., 2004). Inflammatory responses in the fetal systemic and central nervous systems appear to contribute to the development of many types of brain injury in the newborn (Ferriero, 2004). Furthermore, we previously reported time dependent changes in the endogenous expression of IAIPs in the brain after exposure to hypoxia-ischemia and hypoxia alone in neonatal rats (Disdier et al., 2018) and in the cerebral cortex and cerebellum of fetal sheep after exposure to brain ischemia (Spasova et al., 2017). Studies have also suggested that IAIPs could be important in neuroplasticity (Duman, Aghajanian, Sanacora, & Krystal, 2016; Gaudet, Lim, Stonestreet, & Threlkeld, 2016; Goldschmied & Gehrman, 2019; Normann, Schmitz, Furmaier, Doing, & Bach, 2007; Popoli, Gennarelli, & Racagni, 2002). Other studies have reported that genetically determined variants in the heavy chains of IAIPs are related to psychiatric disorders including schizophrenia and bipolar disorders (Brandl et al., 2016; Cross-Disorder Group of the Psychiatric Genomics, 2013; Hamshere et al., 2013; Sasayama et al., 2014; Schizophrenia Psychiatric Genome-Wide Association Study, 2011) and that mice deficient in bikunin (Ambp/bikunin knock out mice) exhibit specific behavioral phenotypes such as increased anxiety-like behavior (Goulding et al., 2019). Taken together, these findings suggest that endogenous IAIPs could have important roles in a variety of brain related abnormalities.
In contrast to studies examining the potential roles of IAIPs under pathologic conditions, little attention has been given to the properties of endogenous IAIPs under physiological conditions, particularly in the brain. Although a few studies have reported the presence of the light chain of IAIPs (bikunin) in human adult brain, the complete endogenous IAIP protein complex has not been previously reported in the brain of humans. IAIP related proteins and mRNA have been detected in various cells including neurons, astrocytes, and meningeal cells in the central nervous system (CNS) (Cai, Yu, Monga, Mishra, & Mishra, 1998; Chan, Risler, Raguenez, & Salier, 1995; Daveau et al., 1998; Hyman, Tanzi, Marzloff, Barbour, & Schenk, 1992; Kashyap et al., 2009; Kato et al., 2001; Mizushima, Nii, Kato, & Uemura, 1998; Sanchez, Martinez, Lindqvist, Akerstrom, & Falkenberg, 2002; Takano et al., 1999; Werbowetski-Ogilvie et al., 2006; Yoshida, Yoshimura, Ito, & Mihara, 1991). Taken together, these results suggest that IAIPs could be pivotal moieties in the brain and have critical functional roles in brain physiology. However, there is a paucity of systematic information available regarding the existence of endogenous IAIPs in human brain.
In an earlier study, we have demonstrated relatively high levels of IAIPs in the cerebral cortex, and choroid plexus from early in fetal and through the neonatal period up to maturity in adult sheep (Spasova, Sadowska, Threlkeld, Lim, & Stonestreet, 2014). Each IAIP moiety exhibited specific ontogenic patterns of expression unique to each molecular species during development (Spasova et al., 2014). We have also recently identified IAIPs in cultured cortical mouse neurons and rat neurons, microglia, and astrocytes in vitro (Chen et al., 2016). The genes of both the light chain and heavy chain mRNA transcripts and IAIPs proteins likewise were expressed in the cultured mouse neurons (Chen et al., 2016). IAIP immunoreactivity was also identified in neurons, microglia, astrocytes and oligodendroglia in most brain regions (Chen et al., 2016). Overall, these findings support the concept that IAIPs are endogenous components of the normal brain, and also could play important roles in brain development and maturation. However, studies investigating the presence of endogenous IAIPs in the human brain over a wide range of ages are currently not available.
Therefore, we examined the immunohistochemical expression and localization of IAIPs specific to brain cells for the first time in preterm, newborn, and adult human brain on archival autopsy material, as an initial step to study IAIPs in the human brain. Furthermore, we characterized and quantified the immunohistochemical staining profiles of IAIPs in nucleus and cytoplasm, and in neurons and astrocytes during human brain development.
2. MATERIALS AND METHODS
2.1. Human Subjects
The Institutional Review Boards of Women & Infants Hospital of Rhode Island and Rhode Island Hospital approved the use of the residual archival tissue for this study from premature, full-term and adult subjects. The brain tissue samples were obtained from archival postmortem cases by the pathology departments of Women & Infants Hospital of Rhode Island and Rhode Island Hospital. Brain tissue samples were paraffin-embedded, sectioned by the Division of Pathology at Women & Infants Hospital of Rhode Island or Division of Neuropathology at the Rhode Island Hospital using their standard clinical protocols. The criteria for exclusion of cases included infants with severe asphyxia, severe chorioamnionitis, necrotizing enterocolitis, grade III or IV intraventricular hemorrhage or congenital anomalies of the brain. In addition, the brain samples were included only if the prospective autopsy report (reviewed by board certified neuropathologists - E.G.S. or S.D.) indicated the presence of limited neuropathological abnormalities.
2.2. Brain Tissue Collection and Preparation
The archival brain samples were included from 26 subjects between 24 and 40 weeks of gestation and five adult subjects (Table 1). We categorized the infants into maturational stages of 24–26, 27–28, 29–36 and 37–40 weeks of gestation to represent different gestational age groups from extremely preterm infants up to full term gestation. The different gestational age groups were selected based upon clinically relevant periods of gestation as a result of their impact on survival and neurodevelopmental outcomes.(El-Metwally, Vohr, & Tucker, 2000; Hintz et al., 2011; Stephens, Tucker, & Vohr, 2010; Vohr, 2014; Vohr, Wright, Poole, & McDonald, 2005) The preterm infants were also compared to the adults to determine the effects of immaturity on our immunohistochemical determinations of IAIPs. The brains of the adult patients did not exhibit neuropathological evidence of any neurodegenerative disorders and were considered relatively normal by the neuropathologists (E.G.S. or S.D.). The characteristics of the study subjects are summarized in Table 1. Cerebral cortical tissues from the somatosensory cortex were fixed in 10% neutral buffered formalin, embedded in paraffin and then sectioned at a thickness of 4 μm.
TABLE 1.
Baseline characteristics of the subjects.
| Sex | Age (wks or years) | Age at death (day or years) | LB/SB | PMI (h) | Cause of death |
|---|---|---|---|---|---|
| M | 24 | NA | SB | NA | Hydrops fetalis with pulmonary hypoplasia |
| M | 24 | 0 | SB | NA | Undetermined |
| F | 24 | 0 | SB | NA | Fetal vascular malperfusion-cord obstruction |
| M | 26 | 8 | LB | 0 | Bronchopulmonary dysplasia |
| M | 27 | 7 | LB | 2 | Intrauterine growth restriction |
| M | 27 | 0 | LB | 0 | Arthrogryposis with cardiomegaly |
| F | 28 | 0 | LB | 0 | Hydrops fetalis |
| F | 28 | 43 | LB | 1 | Conjoined twins, thoracopagus type |
| F | 28 | 0 | SB | NA | Fragile X syndrome |
| F | 28 | 0 | SB | NA | Feral and maternal vascular malperfusion |
| M | 29 | 4 | LB | 1 | Gastric perforation; pneumonia |
| M | 30 | 0 | SB | 0 | Multiple congenital anomalies |
| M | 30 | 31 | LB | 1 | Bronchopneumonia |
| F | 34 | 0 | SB | NA | Undetermined |
| F | 35 | 0 | SB | NA | Fetal vascular malperfusion-cord obstruction |
| M | 37 | 0 | SB | 0 | Hydrops fetalis |
| M | 37 | 0 | SB | 2 | Sepsis |
| M | 38 | 0 | LB | 0 | Congenital diaphragmatic hernia |
| M | 38 | 0 | SB | NA | Oligohydramnios |
| F | 39 | 0 | SB | NA | Sepsis |
| F | 40 | 0 | SB | NA | Fetal vascular malperfusion-cord obstruction |
| M | Adult | 59 | - | 6 | Acute myocardial infarction, lung cancer, normal brain |
| F | Adult | 79 | - | 20 | Congestive heart failure |
| M | Adult | 35 | - | 8 | Adenocarcinoma., normal brain |
| M | Adult | 70 | - | 59 | Lung abscesses |
| M | Adult | 85 | - | 60 | Lung abscesses, normal brain |
LB/SB: liveborn/stillborn; PMI: post-mortem interval; NA: not available
2.3. Generation of Polyclonal Rabbit Anti-Rat IAIPs Antibody
Anti-serum against IAIPs was generated by immunizing rabbits with purified rat-IAIPs through a custom polyclonal antibody production service provided by 21st Century Biochemicals, Inc. (Marlboro, MA, USA). Rat IAIPs were extracted and purified from rat serum (Millipore Sigma, St. Louis, MO) using an anion exchange chromatographic column (Toyopearl GigaCap Q-650M, Tosoh Bioscience, King of Prussia, PA, USA). Briefly, rat serum was diluted in 20 mM Tris-HCl + 200 mM NaCl, pH 6.8 and passed through the column. The column was subsequently washed with buffer containing 50 mM Acetate + 250 mM NaCl, pH 4.2. Bound IAIPs were eluted from the column using 20 mM Tris-HCl + 750 mM NaCl, pH 6.8. Eluted proteins were buffer exchanged to phosphate buffered saline (20 mM Phosphate + 150 mM NaCl, pH 7.4) using the Amicon Ultra Centrifugal Filter Units with regenerated cellulose membranes with molecular weight cut offs of 30 kDa (Millipore Sigma, Burlington, MA, USA). Rabbits were immunized five times with purified IAIPs (200 ug) mixed with Freund’s adjuvant. Rabbits were bled following each immunization boost for the titer analysis and the final bleed from the rabbit was used in this study (R-22-c pAb). The reactivity of R-22-c pAb was assessed by Western immunoblot analysis using purified IAIPs from other species and determined that the antibody is cross-reactive with purified IAIPs from human, mouse and sheep (data not shown).
2.4. Validation of Purified R-22c pAb by Western Immunoblotting
Western immunoblot was used to validate the specificity of the rabbit polyclonal antibody, R-22c pAb against the rat IAIPs. Residual archival frozen adult human cerebral cortex was obtained for Western immunoblot analysis of IAIPs. Fifteen μg of total human brain protein per well were fractionated on SDS-polyacrylamide gel. The proteins on the gel were then transferred onto one polyvinylidene difluoride membrane (PVDF, 0.2 μm, Bio-Rad Laboratories, Hercules, CA, USA) using a semi-dry transfer technique. Thereafter, one half of the membrane was incubated with pre-immune serum (PIS, 1:5000, ProThera Biologics Inc., Providence, RI, USA) as a negative control for the R22c pAb, whereas the other half of the membrane with same loading proteins was incubated with R-22c pAb (1:5000, ProThera Biologics Inc.) overnight at 4 °C. After washing three times with TBS-T/0.1% Tween-20), the membrane was incubated with peroxidase-labeled goat anti-rabbit secondary antibody (1:20,000, Alpha Diagnostic, San Antonio, TX, USA) for one hour at room temperature. After incubating the membrane with enhanced chemiluminescence (ECL plus, Western Blotting Detection Reagents, Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA), they were exposed to autoradiography film (Daigger, Vernon Hills, IL, USA). Purified human IAIPs (0.05 μg), human serum containing IAIPs (1:2000), IAIP-depleted serum (1:2000), and human cerebral cortical samples (50 and 60 μm) were loaded to serve as positive and negative controls. The amounts of each sample loaded on both PIS and R22c pAb incubated membranes were the same and exposed for the same amount of time.
The human IAIP-depleted serum was obtained by passing diluted human serum in the running buffer (20 mM Tris-HCl + 200 mM NaCl, pH 6.8) onto an anion exchange chromatographic column (Toyopearl GigaCap Q-650M, Tosoh Bioscience, King of Prussia, PA, USA) and capturing IAIPs on the column. Although IAIPs are bound tightly to the resin, almost all other serum proteins are found in the flow through fraction. Salt was subsequently removed from the flow through fraction (IAIP-depleted serum) by using a tangential flow filtration (Labscale TFF system, MilliporeSigma, Burlington, MA, USA) and Pellicon XL ultrafiltration cassette (Ultracel 10 kDa, MilliporeSigma).
2.5. Validation of Purified R-22c pAb by Immunofluorescence Staining of the Light Chain of IAIPs (Bikunin)
We performed immunohistochemical analysis to verify that the immunoreactivity pattern of the light chain of IAIPs, termed bikunin, exhibited a similar pattern to that of IAIPs to confirm further the specificity of highly purified R-22c pAb. Immunostaining was performed using polyclonal rabbit-anti-bikunin N-terminal antibody (1:100, Abcam) according to the same protocol used in the immunostaining of IAIPs.
2.6. Immunohistochemical Analyses
Brain sections for immunohistochemical analysis were deparaffinized in xylene and rehydrated in descending series of ethanol (100%, 95%, and 70%). Subsequently, a heat-induced antigen retrieval procedure was performed using an antigen unmasking solution (citrate-based, pH=6.0, Vector Laboratory, Burlingame, CA, USA). The solution was preheated for 10 minutes and then heated slides were submerged in the solution for 20 minutes in a microwave according to the manufacturer’s protocol. Thereafter, the slides were immersed in Superblock T20 (TBS) blocking buffer (Thermo Fisher Scientific, Waltham, MA, USA) for 1 hour at room temperature to reduce non-specific binding. Each step was followed by washing in Tris-buffered saline (TBS, pH=7.6).
We performed double immunofluorescence staining to elucidate the expression pattern of IAIP immunoreactivity and its localization in neurons and astrocytes in cerebral cortical tissues from the various age groups. Neurofilament light peptide (NFL) and glial fibrillary acidic protein (GFAP) were used as neuronal and astrocytic markers, respectively. NFL is a well-established neuronal marker (Perrot, Berges, Bocquet, & Eyer, 2008; Yuan et al., 2012) used in fetal post-mortem immunohistochemical brain analysis because of their early appearance in human brain development and their overall resistance to prolonged post-mortem intervals (Sarnat, 2013). Moreover, findings have also suggested that the immunoreactivity of NFL is specific for neuronal cells in human brain ranging between 18 and 40 weeks of gestation, as well as, in the adult brain (Nakamura et al., 2003). In addition, GFAP has been used extensively in developmental studies (Corvetti, Capsoni, Cattaneo, & Domenici, 2003; Guo et al., 2015; Robertson, 2014; Rochefort, Quenech’du, Ezan, Giaume, & Milleret, 2005; Rozovsky, Finch, & Morgan, 1998). The immunoreactivity of GFAP in brain has been also reported over a wide range of gestational ages from early the fetal period up to maturity in the adult (Nakamura et al., 2003). Therefore, NFL and GFAP are ideal markers to examine immunohistochemical staining profiles of neurons and astrocytes throughout a wide range of ages obtained from archival human autopsy materials in our study.
Although autofluorescence can make interpretation of stains in archival formalin-fixed tissue more difficult, we included negative controls with each of our immunofluorescence studies, the results of which suggested that autofluorescence was not a major problem. Even though a bright field approach using a chromogen such as DAB (3,3′-Diaminobenzidine) could also be an option to eliminate autofluorescence in postmortem tissue, immunofluorescent staining is the optimal method to examine double labelling signals.
Briefly, each section was incubated with purified IgG of the polyclonal rabbit anti-rat IAIPs (1:100, R-22c pAb, ProThera Biologics Inc., Providence, RI, USA) diluted in TBS with 0.05%Tween (TBST) containing 1% bovine serum albumin (Thermo Fisher Scientific) overnight at 4 °C. The stained slides were then incubated overnight at 4 °C with either diluted chicken anti-neurofilament light peptide (NFL) polyclonal antibody (1:1000, Abcam, Cambridge, MA, USA) to detect neurons or mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (1:1000, Abcam) to detect astrocytes. The specifics for primary antibodies used in immunohistochemical analyses are described in Table 2. The immunoreactivity was then visualized by incubating the stained slides with a mixture of diluted secondary antibodies, Alexa-488 tagged anti-rabbit IgG (1:500, Invitrogen, Grand Island, NY, USA) in combination with either Alexa-555 tagged anti-chicken IgG (1:500, Invitrogen) or Alexa-594 tagged anti-mouse IgG (1:500, Invitrogen) for 1 hour at room temperature. Mounting media with DAPI was then applied (Vector Laboratories). Negative control slides were included along with each set of experimental slides to ensure that the immunohistochemical analysis detected the specific signals. The negative controls were performed with pre-immune serum (PIS, 1:100, ProThera Biologics, Inc.) and with omission of either the anti-NFL or anti-GFAP antibodies followed by incubation with the secondary antibodies. The PIS was obtained from a rabbit before immunization with rat IAIPs and used as a negative control for the specificity of the polyclonal rabbit anti-rat IAIPs antibody. All immunofluorescence staining was completed in one run and included appropriate negative controls, thereby avoiding any variability that could have arisen from different runs.
TABLE 2.
List of primary antibodies used in immunohistochemical analysis
| Antibody | Product# | Immunogen | Manufacturer | Dilution |
|---|---|---|---|---|
| Rabbit anti-IAIP | R-22c PAb | Purified rat plasma IAIP | ProThera Biologics | 1: 100 |
| Rabbit anti-Bikunin N-terminal | ab189431 | Synthetic peptide within Human Bikunin aa 76–104 (N terminal): EAEISMTSTRWRKGVCEETSGAYEKTDTD |
Abcam | 1:100 |
| Chicken anti-neurofilament lightpeptide (NFL) | ab72997 | Tissue, cells or virus corresponding to Cow 68kDa Neurofilament/NF-L | Abcam | 1:1000 |
| Mouse anti-glial fibrillary acidicprotein (GFAP) | ab8975 | Glial fibrillary acidic protein (full length) from human brain | Abcam | 1:1000 |
2.7. Image Acquisition
The stained slides were independently examined by two investigators (Boram Kim and Virginia Hovanesian) without knowledge of the ages of the study subjects. We used both conventional fluorescence and confocal laser scanning microscopes to acquire images by each of the investigators. The conventional fluorescence microscope was used to acquire the images that were used for cellular quantification (Boram Kim). The confocal laser-scanning microscope (Virginia Hovanesian) was used to verify the images from the conventional fluorescent microscope, to substantiate the cellular localization of the IAIPs and to acquire the images for Figures 2, 3, 5, and 7.
Figure 2.
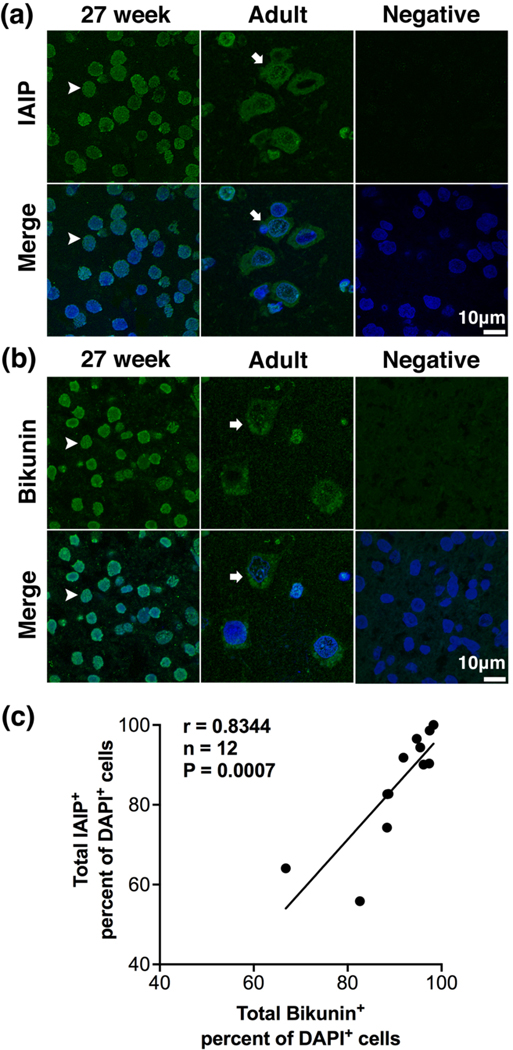
Comparison of immunofluorescence staining patterns of IAIP and the light chain of IAIP termed bikunin in the cerebral cortex of human brain acquired by confocal microscopy.
(a) Representative high-power (180 x), confocal Z-stack projection images of immunofluorescence staining of IAIP and pre-immune serum (PIS) as a negative control for IAIP in in the cerebral cortex of human brain. Cortical sections incubated with purified polyclonal rabbit anti-rat IAIPs exhibited IAIP immunoreactivity (green) in the preterm at 27 weeks of gestation and the adult subjects. IAIP and DAPI counterstains were shown (green blue, Merge). In contrast, no IAIP immunoreactivity was detected in the PIS incubated section (Negative). Nuclear IAIP staining was identified in the cerebral cortical cells in the preterm infant at 27 weeks of gestation (arrowheads). Cytoplasmic staining was observed in the cerebral cortex in the adult (arrows). Scale bar = 10 μm. (b) Representative high-power (180 x), confocal Z-stack projection images of immunofluorescence staining of bikunin and negative control in in the cerebral cortex of human brain. Bikunin immunoreactivity (green) was observed in the preterm infant at 27 weeks of gestation and in the adult. IAIP and DAPI counterstains were shown (green blue, Merge). In contrast, no immunoreactivity was detected in the cerebral cortical section incubated with the secondary antibody without the specific rabbit anti-bikunin N-terminal polyclonal antibody (Negative). The cells exhibiting nuclear bikunin immunoreactivity was identified in the cerebral cortex in the preterm at 27 weeks of gestation (arrowheads). The cell with cytoplasmic bikunin immunoreactivity was observed in the cerebral cortex in the adult (arrows). Scale bar = 10 μm. (c) Correlation between the percent of total bikunin positive cells and total IAIP positive cells in the cerebral cortex of human brain (Pearson’s correlation coefficient, r = 0.8344, n = 12, P = 0.0007).
Figure 3.
Immunohistochemical distribution and localization of IAIP in the cerebral cortex of human brain.
(a) Representative relative low power (60 x), confocal Z-stack projections of IAIP in the cerebral cortex of human brain in the different age groups. IAIP staining (green) was widely distributed in the cerebral cortical cells in the preterm and newborns at 24, 28, 34, and 39 weeks of gestation and in the adult. IAIP and DAPI counterstains were shown (green blue, Merge). The arrows indicate cells with cytoplasmic staining of IAIP. The arrowheads show cells with nuclear staining of IAIPs. Scale bar = 20 μm. (b) Representative high-power, confocal Z-stack projection images of IAIP staining in the preterm at 24 weeks of gestation and in the adult and negative control in the cerebral cortex of human brain to better illustrate cytoplasmic and nuclear staining. IAIP staining (green) and IAIP and DAPI counterstains (green blue, Merge) were clearly observed in both the preterm infant and the adult. The cell with nuclear staining of IAIP (arrowheads) was observed in the preterm infants at 24 weeks of gestation when viewed through the high-power confocal microscope (400 x). The cells with cytoplasmic staining of IAIP (arrows) were identified in the adult cerebral cortex under the high-power viewed microscope (200 x). The XZ panels show a slice from the mid-point of each of the selected cells of a confocal acquisition in the XZ plane. In the image from the 24 weeks of gestation, premature infant (arrowhead) the staining is within the nuclear membrane, whereas in the adult (arrow) the IAIP staining is present both in and outside the nuclear membrane. No immunoreactivity was detected in the brain section incubated with pre-immune serum as a negative control for IAIP (Negative). Scale bar = 5 μm (24 weeks of gestation), 10 μm (Adult and Negative).
Figure 5.

Immunohistochemical distribution and localization of neurons and IAIP expressing neurons.
(a) Representative relative low-power (60 x) confocal images of NFL and double immunofluorescence staining of NFL with IAIP in the cerebral cortex of human brain in the different age groups. The arrows indicate NFL staining (red) and IAIP staining (green) and the arrowheads designate double staining of NFL with IAIP along with DAPI counterstains in the preterm and newborns at 26, 28, 34, and 37 weeks of gestation and in the adult (red green blue, Merge). Scale bar = 20 μm. (b) Representative high-power (180 x), confocal images of immunofluorescence staining of NFL and double immunofluorescence staining of NFL with IAIP from the cerebral cortex of the human brain in an infant at 37 weeks of gestation and in an adult. NFL positive neurons (red, arrows), IAIP staining (green, arrows), and IAIP expressing NFL stained neurons along with counterstained DAPI (red green blue, Merge, arrowheads) were observed in both the preterm infant at 24 weeks of gestation and the adult. Scale bar = 10 μm.
Figure 7.
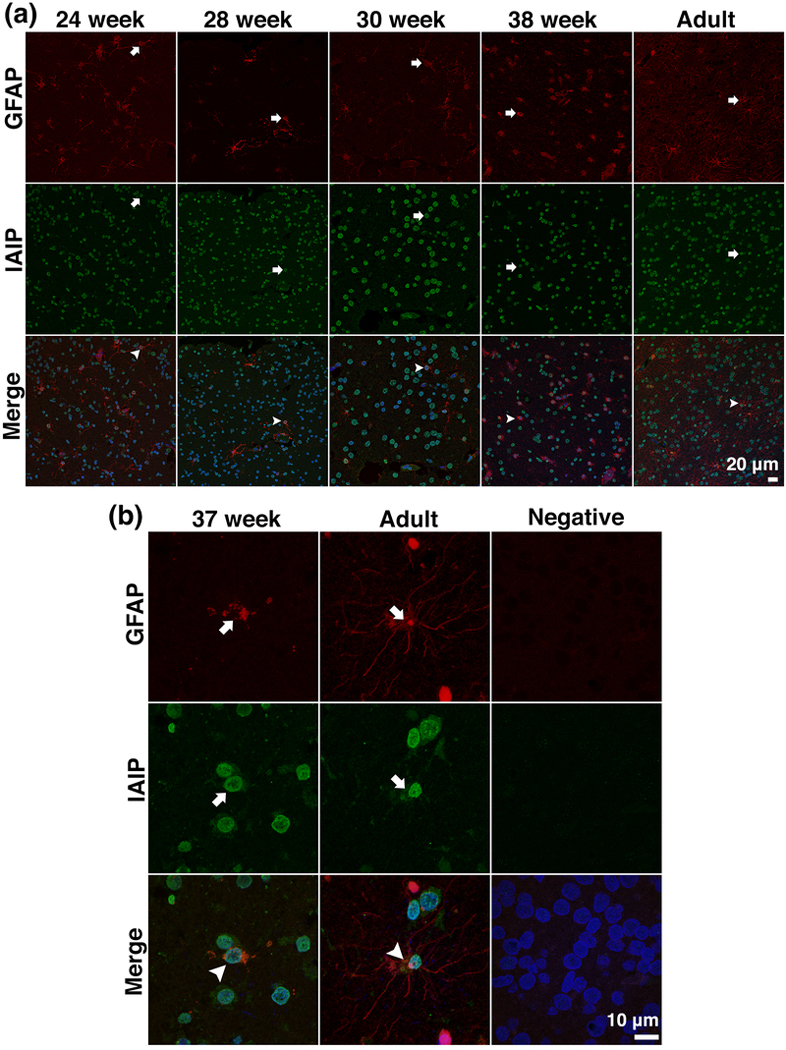
Immunohistochemical distribution and localization of astrocytes and IAIP expressing astrocytes
(a) Representative relative low-power (40 x) confocal images of GFAP and double immunofluorescence staining of GFAP with IAIP in the cerebral cortex of human brain in the different age groups. GFAP staining (red, arrows), IAIP staining (green, arrows), and double staining of GFAP with IAIP along with DAPI counterstains (red green blue, Merge, arrowheads) were observed in the cerebral cortical cells in preterm and newborns at 24, 28, 30, and 38 weeks of gestation and in the adult. Scale bar = 20 μm. (b) Representative high power (180 x), confocal images of immunofluorescence staining of GFAP and double immunofluorescence staining of GFAP with IAIP along with counterstained DAPI nucleus from the cerebral cortex of the human brain in an infant at 37 weeks of gestation and in an adult. GFAP stained astrocytes (red, arrows), IAIP staining (green, arrows), and IAIP expressing GFAP stained astrocytes (red green blue, Merge, arrowheads) were identified in both the preterm infant at 24 weeks of gestation and the adult. Scale bar = 10 μm.
A Zeiss Axio Imager M2 imaging system microscope (Carl Zeiss, Inc., Jena, Germany) connected with computer software Stereo Investigator 10.0 (MicroBrightField, Inc., Williston, VT, USA) was used for image acquisition for the purpose of quantification. Ten fields within each cerebral cortical sample from each case were first randomly selected based upon the DAPI channel. The randomly selected RGB images of cerebral cortex were acquired with cy3 (Red), FITC (Green), and DAPI (Blue) filters and a 40x objective from the Zeiss Axio Imager M2 imaging system microscope. Each wavelength was acquired separately using the same camera settings for each channel and then pseudo-merged into a RGB image for analysis in either ImageJ or Adobe Photoshop. The area of each field was 35242.1875 μm2, so that the total area of ten fields were 352421.875 μm2. The optical conditions were rigorously maintained the same across all analyses sessions.
Randomly selected Red/Green/Blue (RGB) images of cerebral cortex in each case were collected with a Nikon C1si confocal microscope (Nikon Inc., Mellville, NY, USA) using diode lasers. 561 (Red), 488 (Green), and 402 (Blue). Each wavelength was acquired separately by invoking the frame lamda, which reduces the potential bleed through when the emission spectra overlap. Each frame in an acquisition was viewed through Elements version 3.20 (Nikon Inc.).
2.8. Image Processing and Cellular Quantification
All images were acquired as described above with the Zeiss Axio Imager M2 imaging system microscope (Carl Zeiss, Inc.). Each channel was acquired separately and then pseudo-merged into a RGB image for analysis in either ImageJ or Adobe Photoshop using methods similar to those previously described (Jiang et al., 2019; Otxoa-de-Amezaga et al., 2019; Plowey & Ziskin, 2016; Wassink et al., 2017). First, the total number of cells defined as DAPI stained nuclei were automatically counted in NIH ImageJ. The split channel command was used to separate out each channel. The blue channel (DAPI) was selected and positive staining was defined through intensity thresholding. The resulting binary images were quantified with ImageJ’s, “Analyze Particles” for total cell counts.
The number of immunoreactive cells were manually counted with a count tool in Photoshop CC. In brief, after arranging RGB images in each color channel, total IAIP positive cells were defined, when viewed through both of green and blue channels, and counted if the nuclei in the blue channel were co-localized with IAIP staining or surrounded by IAIP staining in the green channel. Nuclear IAIP positive cells were defined as the cells with IAIP positive staining only within the nuclear compartment, based upon the subcellular localization of IAIP immunoreactivity. Cytoplasmic IAIP positive cells were defined as cells with IAIP positive staining within cytoplasmic compartment along with presence or absence of IAIP staining within nuclear compartment. Total bikunin, nuclear bikunin, and cytoplasmic bikunin positive cells were counted using the same methodology as for the IAIPs positive cells. The quantity of positively stained cells was calculated in ten selected counting fields from each slide.
NFL positive cells were defined, when viewed through both red and blue channels, and counted if the nuclei in the blue channel were co-localized with NFL staining or surrounded by a NFL positive cell body in the red channel. Similarly, GFAP positive cells were defined, when viewed through double red with blue channels, and counted if the nuclei in the blue channel were co-localized with GFAP staining or surrounded by a GFAP positive cell body in the red channel. Double positive cells were defined, when viewed through the three channels, red, green, and blue channels, and counted if the nuclei in the blue channel contained staining signals in both red and green channels.
2.9. Statistical Analysis
We calculated the cytoplasmic IAIP, nuclear IAIP, total IAIP (cytoplasmic plus nuclear IAIP), NFL, and GFAP positively stained cells as a percent of the DAPI counterstained nuclei. IAIP+ NFL+ cells determined as a percent of NFL positively stained cells. IAIP+ and GFAP+ cells were determined as a percent of GFAP positively stained cells in the cerebral cortex of human brain in each age group.
Study groups were categorized based upon maturation, as follows: 24–26, 27–28, 29–26, and 37–40 weeks of gestation, and adult. Statistical analysis was performed with StatSoft STATISTICA (StatSoft, Tulsa, OK, USA) and all data were represented as mean ± standard deviation. Statistical significance was considered as P < 0.05, as detected using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Relationships between the immunoreactivity of IAIP and bikunin were also examined by Pearson correlation coefficient analysis. A P value of P < 0.05 was considered as a statistical significance.
3. RESULTS
3.1. Validation of R-22c pAb Specificity by Western Immunoblot
Figure 1 contains the Western immunoblot validation for the specificity of the polyclonal rabbit anti-rat IAIPs antibody (R-22c pAb) that we used to detect IAIPs by immunohistochemical analysis in the brain. The R-22c pAb detected positive bands for the 250 (IαI) and 125 kDa (PαI) IAIP in the purified hIAIP (lane A). The R-22c pAb also detected two specific IαI and PαI bands in the human serum (lanes B and C). In contrast, the R-22c pAb did not detect the 250 and 125 kDa IAIP bands in the depleted serum (lanes D and E). The R-22c pAb also detected the 250 and 125 kDa bands in tissue extracts from the two different adult human brains (lanes F and G).
Figure 1.
Western immunoblot to verify R-22 pAb specificity.
0.05 μg of pure human IAIP protein was loaded onto lane A. The same quantity of human serum (1:2000) was loaded onto lanes B and C. The same amount of IAIP-depleted human serum (1:2000) was loaded onto lanes D and E. Fifty and 65 μg of two different human cerebral cortical samples were loaded onto lanes F and G. The pre-immune serum (PIS) used as a negative control for the R-22C pAb did not recognize IAIP bands. The R-22C pAb detected two bands, at approximately 250 and 125 kDa in the human IAIP protein (lanes A, R22c). Two bands, one was approximately 250 kDa and the other is relatively lower than 125 kDa compared with the pure human IAIP protein, were detected in the human serum (Lane B and C, R22c). Bands were not detected in the IAIP-depleted human serum (lanes D and E, R22c). The similar bands were identified in the human brain samples, when compared with the pure human IAIP protein (lanes E and F, R22c).
3.2. Comparison of the immunoreactivity between anti-IAIP and anti-bikunin antibodies
In order to further validate the use of the purified polyclonal rabbit anti-rat IAIPs (R-22c pAb), we also determined the immunoreactivity of the light chain of IAIPs termed bikunin with a commercially available rabbit polyclonal bikunin-N terminal antibody (Abcam, Cambridge, MA) and compared the immunohistochemical staining patterns of IAIPs and bikunin in cells in the cerebral cortex of human brain.
Figure 2a contains high-power images of the cerebral cortical sections incubated with IAIPs and pre-immune serum (PIS) as a negative control. The section incubated with IAIP widely exhibited immunoreactivity for IAIP positively stained cerebral cortical cells both at 27 weeks of gestation and in the adult subject (Figure 2a, IAIP, Merge). In contrast, the PIS incubated brain section did not show immunoreactivity to IAIP (Figure 2a, Negative). IAIP positive staining was mainly localized in the nucleus at 27 weeks of gestation (Figure 2a, arrowheads) and in the cytoplasm in the adult subject (Figure 2a, arrows). Figure 2b contains high-power images of bikunin stained cerebral cortical sections at 27 weeks of gestation and in an adult subject and a negative control. Similarly, the specific rabbit anti-bikunin N-terminal polyclonal antibody identified abundant bikunin positive stained cells in the cerebral cortex in both the neonate at 27 weeks of gestation and in the adult subject (Figure 2b, Bikunin, Merge). In contrast, immunoreactivity to bikunin was not detected in the cerebral cortical section that was incubated with the secondary antibody without the specific rabbit anti-bikunin N-terminal polyclonal antibody as a negative control (Figure 2b, Negative). In addition, bikunin immunoreactivity was predominantly localized in the nucleus at 27 weeks of gestation (Figure 2b, arrowheads) and localized to the cytoplasm in the adult cerebral cortex (Figure 2b, arrows). Consequently, IAIPs and bikunin immunoreactivity appeared to exhibit similar staining patterns in the cerebral cortical cells both in the preterm and adult brains.
Total IAIP+ and bikunin+ cells plotted as a percent of the DAPI positive cells exhibited a positive linear relationship (Figure 2c, Pearson correlation coefficient, n = 12, r = 0.8344, P = 0.0007). Therefore, the highly purified polyclonal rabbit anti-rat IAIPs (R-22c pAb) and commercially available rabbit polyclonal anti-bikunin-N terminal antibody (Abcam, Cambridge, MA) exhibited similar patterns of staining for cells positive for IAIPs and the light chain bikunin in the human cerebral cortex. These findings further confirm the specificity of the purified polyclonal rabbit anti-rat IAIPs (R-22c pAb) to detect IAIPs in fixed human cerebral cortical tissue.
3.3. Study Subjects
Table 1 summarizes the baseline characteristics of the study subjects including the sex, age, age at death, live born (LB)/still born (SB), and post-mortem interval (PMI) and cause of death.
3.4. Immunohistochemical Expression of IAIPs during Development
Immunofluorescence staining with a specific anti-IAIP antibody (R-22c pAb) identified cells positively stained for IAIP in randomly selected areas from the representative cerebral cortex samples in the human brain of subjects 24–26, 27–28, 29–36 and 37–40 and in the adult brain (Figure 3a). The positive immunofluorescent staining for IAIPs appeared to be predominately localized in the nucleus of the cerebral cortical cells in the preterm and newborn infants at 24, 28, 34 and 39 weeks of gestation and in the adult cerebral cortex (Figure 3a, IAIP, arrowheads) because the IAIP immunoreactivity was co-localized with the DAPI stained nuclei (Figure 3a, Merge, arrowheads). Positive IAIP staining was also observed to a limited degree in the cytoplasm of cerebral cortical cells in the preterm and newborns at 24, 28, 34 and 39 weeks of gestation and in the adult (Figure 3a, arrows). Figure 3b contains randomly selected high-power images of IAIP immunostaining in the cerebral cortex of human brain in a preterm infant at 24 weeks of gestation and in the adult cerebral cortex to elucidate IAIP immunoreactivity specific to the subcellular localizations and a PIS incubated negative control. The arrowheads designate cells with IAIP positive immunoreactivity in the nucleus of a preterm infant at 24 weeks of gestation and the arrows designate cells with cytoplasmic immunoreactivity in an adult subject. In contrast, no immunoreactivity was observed in the PIS incubated negative control brain section.
The IAIP positive cells in cytosolic and nuclear and compartments were calculated as a percent of the total number of cells, which was determined by counting the DAPI positive nuclei within the different age groups (Fig 4 a, b, c). The relative number of DAPI positive stained nuclei was similar among the different randomized areas examined within each subject. The fluorescent expression of IAIPs in the cytoplasm differed among the age groups (Figure 4a, ANOVA, F (4, 21) = 7.7660, n = 26, P = 0.0005). Specifically, the percent of cytoplasmic IAIP positive cells was higher in the cerebral cortex of the adult subjects (n = 5) than in the preterm and newborn infants at 24–26, 27–28, 29–36, and 37–40 weeks of gestation (n = 4, P = 0.0011; n = 6, P = 0.0057; n = 5, P = 0.0011; n = 6, P = 0.0175; respectively). However, differences were not observed in the immunofluorescence expression of IAIPs in the nucleus among the age groups (Figure 4b, ANOVA, F (4, 21) = 1.8708, n =26, P = 0.1532). Likewise, the total number of IAIP positive cells also did not differ across the age groups (Figure 4c, ANOVA, F (4, 21) = 0.8207, n = 26, P = 0.5265). Further analysis did not detect significant differences in the cytoplasmic IAIP (data not shown, ANOVA, F (3, 17) = 1.3755, n = 21, P = 0.2841), nuclear IAIP (data not shown, ANOVA, F (3, 17) = 1.0348, n = 21, P = 0.4022), or total IAIP expression (data not shown, ANOVA, F = (3, 17) = 0.6218, n = 21, P = 0.6105) among the infants at 24–26, 27–28, 29–36, and 37–40 weeks of gestation.
Figure 4.

Quantitative analysis of IAIP expression in the cerebral cortical cells of human brain (n = 4, 6, 5, and 6 from 24–26, 27–28, 29–36, and 37–40 weeks of gestation, respectively and 5 in the adult group).
(a) The percent of cytoplasmic IAIP+ cells were greater in the adults than in infants at 24–26, 27–28, 29–36, and 37–40 weeks of gestation. (b) The percent of nuclear IAIP+ and (c) total IAIP+ cells to DAPI+ cells (sum of a plus b) did not differ among the different age groups. Dot plot, mean ± SD. One-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P<0.01
3.5. Expression of Neurons and IAIP Positive Neurons during Development
Neuronal staining was examined by immunofluorescence detection with chicken polyclonal anti-NFL antibody in the cerebral cortices of the preterm and newborn infants at 26, 28, 30, and 38 weeks of gestation and in the adult subjects (Figure 5a, NFL, arrows). IAIP staining was shown by green fluorescence (Figure 5a, IAIP, arrows). Cells double stained with NFL and IAIP were detected in the cerebral cortex of preterm and newborn infants at 26, 28, 34, and 37 weeks of gestation and in the adult subjects (Figure 5a, Merge, arrowheads). Figure 5b contains high-power images of NFL, IAIP, and NFL IAIP and DAPI positive stained cells in representative cerebral cortices of a neonate at 37 weeks of gestation and in an adult and a negative control. NFL (Figure 5b, NFL, arrows) and IAIP immunoreactivities (Figure 5b, IAIP, arrows) were observed in the cerebral cortex. Cerebral cortical cells double stained with NFL and IAIP were detected in both the neonates and adult subjects (Figure 5b, Merge, arrowheads). Consequently, IAIP stained neurons appeared to be distributed in a wide range of ages in human brain.
Figure 6 contains the analysis of NFL+ as the percent of DAPI+ cells and IAIP+ NFL+ as a percent of NFL+ cells in the preterm and newborn infants at 24–26 (n = 4), 27–28 (n = 6), 29–36 (n = 5) and 37–40 (n = 6) weeks of gestation and in the adult (n = 5) subjects. Significant differences were detected in the NFL+ as a percent of DAPI+ cells (Figure 6a, ANOVA, F (4, 21) = 2.8482, n = 26, P = 0.0496). However, differences between the age groups were not detected by Tukey’s post-hoc analysis. Furthermore, IAIP+ NFL+ cells as a percent of NFL+ cells were greater in the neonates at 29–36 weeks of gestation than 37–40 weeks of gestation (Figure 6b, P = 0.0459). After inspection of Figs. 6a and 6b additional analysis was performed to investigate the developmental profiles of IAIP-expressing neurons in preterm and full-term infants at 24–26 (n = 4), 27–28 (n = 6), 29–36 (n = 5), and 37–39 (n = 6) weeks of gestation. NFL+ neuronal expression differed across the age groups (data not shown, ANOVA, F (3,17) = 5.399, n = 21, P = 0.0085). The percent of NFL+ neurons was higher at 29–36 weeks of gestation compared to those at 24–26 and 37–40 weeks of gestation (data not shown, P = 0.0089 and P = 0.0307, respectively). In addition, IAIP+NFL+ neuronal expression differed among the age groups (data not shown, ANOVA, F (3, 17) = 3.386, n = 21, P =0.0424). However, specific differences between age groups were not detected by Tukey’s post-hoc test. These findings suggest that the number of neurons, as identified by NFL staining, reached a peak at 29–36 weeks of gestation and IAIPs are potentially present in NFL+ neurons over a wide span of neonatal gestational ages and in adults.
Figure 6.

Quantitative analysis of neurons and IAIP-expressing neurons in the cerebral cortex of human brain (n = 4, 6, 5, and 6 from 24–26, 27–28, 29–36, and 37–40 weeks of gestation, respectively and 5 from the adult group). (a) The percent of NFL+ cells to DAPI positive cells changed with age (ANOVA, P < 0.05). However, specific differences were not detected among the different age groups. (b) The percent of IAIP+ NFL+ cells to NFL+ cells were greater in the neonates at 29–36 weeks of gestation than at 37–40 weeks of gestation. Dot plot, mean ± SD. One-way ANOVA with Tukey’s multiple comparisons. **P<0.01.
3.6. Expression of Astrocytes and IAIP Positive Astrocytes during Development
Astrocytes were visualized with mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibody and double labeled with the specific anti-IAIP antibody (R-22c pAb) for IAIP immunodetection. Figure 7a contains representative immunofluorescent images of double labeled GFAP and IAIP in cerebral cortices of the preterm and newborn infants at 24–26, 27–28, 29–36 and 37–40 weeks of gestation and in the adult subjects. GFAP-expressing astrocytes were detected as expected in all age groups (Figure 7a, GFAP, arrows). IAIP positive staining was shown by green fluorescence (Figure 7a, IAIP, arrows). Cells positively stained with GFAP and IAIP were observed in the cerebral cortices of the preterm and newborn infants at 24, 28, 30, and 38 weeks of gestation and in the adult (Figure 7a, Merge, arrowheads). Figure 7b contains confocal images of the positive staining for GFAP (Figure 7b, GFAP, arrowheads), IAIP (Figure 7b, IAIP, arrowheads), and co-localization staining for IAIP and GFAP along with DAPI stained cellular nuclei (Figure 7b, arrowheads) in subjects at 37 weeks of gestation and in the adult subjects. In contrast, the negative control section did not show immunoreactivity for either GFAP or IAIP.
The expression and developmental profiles of astrocytes and IAIP-expressing astrocytes were evaluated by the quantities of cells stained with GFAP and double stained with IAIP and GFAP in the cerebral cortex of human brain at different ages. Figure 8 contains the GFAP+ as a percent of DAPI+ cells and IAIP+ GFAP+ as a percent of GFAP+ cells in cerebral cortical cells plotted for preterm and newborn infants at 24–26 (n = 4), 27–28 (n = 6), 29–36 (n =5) and 37–40 (n = 6) weeks of gestation and in the adult (n = 5) subjects. Significant differences were observed in the GFAP+ cells as a percent of DAPI+ cells (Figure 8a, ANOVA, F (4, 21) = 13.457, n = 26, P < 0.0001). Specifically, the percent of GFAP+ cells was higher in the adult subjects than at 24–26 (P = 0.0001), 27–28 (P = 0.0002), 29–36 (P = 0.0015) and 37–40 (P = 0.001) weeks of gestation. In addition, the percent of IAIP+ GFAP+ cells as a percent of the GFAP+ cells was also significantly different across the age groups (Figure 8b, ANOVA, F (4, 21) =3.267, n = 26, P = 0.0313). Specifically, the percent of IAIP+ GFAP+ cells was higher in adults than in the preterm infants at 27–28, weeks of gestation (P = 0.0230). Consequently, the number of astrocytes detected and IAIP co-localization with astrocytes increased with development in human brain.
Figure 8.

Quantitative analysis of astrocytes and IAIP-expressing astrocytes in the cerebral cortex of human brain (n = 4, 6, 5, and 6 from 24–26, 27–28, 29–36, and 37–40 weeks of gestation, respectively and 5 in the adult group). (a) The percent of GFAP+ cells to DAPI positive cells were greater in the adults compared with the neonates at 24–26, 27–28, 29–36, and 37–40 weeks of gestation. (b) The percent of IAIP+ GFAP+ cells to GFAP+ cells were greater in the adults than in the neonates at 27–28 weeks of gestation. Dot plot, mean ± SD. One-way ANOVA with Tukey’s multiple comparisons. *P<0.05, **P<0.01, ***P<0.001.
4. DISCUSSION
The primary objective of the current study was to identify the presence of endogenous IAIPs in human brain tissue during development. In this study, we have quantitatively examined the immunohistochemical expression of IAIPs in the cerebral cortex of post-mortem human brain tissue obtained from various developmental ages, including preterm and full-term neonates, and adult subjects. Here, we provide the first report of the immunohistochemical expression of IAIPs in human brain tissue from very early in gestation up to maturity in adult subjects. The major findings of this study were as follows. First, IAIP positive cells are abundantly distributed in the cerebral cortex in preterm and full term neonates ranging from 24 to 40 weeks of gestation and in adult subjects. Second, IAIP positive staining was expressed in both the cell nucleus and cytoplasm of neonatal and adult cerebral cortex. Third, sixty to eighty percent of cerebral cortical cells expressed IAIPs in the nucleus. Fourth, the quantity of IAIP expression in the cytoplasm increased as a function of age from 24 weeks of gestation up to maturity in the adult. Fifth, positive IAIP staining was observed in neurons and astrocytes in cerebral cortex of all subjects and sixth, IAIP expression in neurons and astrocytes differed among the age groups.
Although IAIP related proteins and genes have been previously detected in adult human brain (Hyman et al., 1992; Werbowetski-Ogilvie et al., 2006; Yoshida et al., 1991), the presence of complete IAIPs within the brain of infants or adults has not been previously reported. In this study, we report for the first time the immunoreactivity of endogenous IAIPs in the cerebral cortex across a wide age range including infants between 24–40 weeks of gestation and in adults. Moreover, our quantitative assessment showed that the IAIPs are distributed in relatively large numbers of cells in the cerebral cortex in all age groups. These findings are consistent with our previous studies in sheep, in which we showed by Western immunoblotting that IAIP moieties were highly expressed in the cerebral cortex of sheep during development (Spasova et al., 2014). The finding that IAIP expression was detected as early as 24 weeks of gestation in humans is also consistent with our previous report showing that IAIP genes and proteins were detected in the cultured brain cells and brain parenchyma of both embryonic mice and neonatal rats (Chen et al., 2016). Taken together, our findings of abundant amounts of IAIPs in the brain of fetal sheep, embryonic mouse, neonatal rat, and human infants during development suggest that early expression of these proteins could implicate a previously unidentified functional role for IAIPs during brain development (Chen et al., 2016; Spasova et al., 2014).
Even though many of the physiological properties of IAIPs remain to be elucidated, particularly with reference to its endogenous presence in the brain, prior work suggests that these molecules are part of the innate immune system and important during inflammation (Fries & Blom, 2000). Specifically, IAIPs have been shown to inhibit destructive serine proteases and complement activation, down-regulate pro-inflammatory cytokines, and augment anti-inflammatory cytokine production during inflammation (Fries & Blom, 2000; Singh et al., 2010). Studies have demonstrated that the light chain of IAIPs, bikunin, exhibits protective effects against ischemic injury in various somatic organs (Cao et al., 2000; Kaga, Katsuki, Futamura, Obata, & Shibutani, 1996; Kakinuma et al., 1997; Koga et al., 2010; Yano, Anraku, Nakayama, & Ushijima, 2003). n the brain, we have shown that endogenous IAIPs were reduced 4 hours after ischemia in the fetal sheep, suggesting that changes in endogenous IAIPs could participate in the response to brain ischemia (Spasova et al., 2017), and findings showing decreased plasma levels of inter-alpha trypsin inhibitor heavy chain 4 in adult patients with ischemic stroke agree with our previous findings (Kashyap et al., 2009). In addition, recent work examined behavioral phenotypes in mice deficient in Ambp/bikunin, the light chain of IAIPs (Goulding et al., 2019). The loss of Ambp/bikunin resulted in augmented anxiety-like behavior, decreased exploratory activity, increased sex-dependent acoustic startle responses, and alterations in social approach in mice suggesting a role for the heavy chains, ITIH1/ITIH3, in the development of mood disorders (Goulding et al., 2019). Polymorphisms of inter-alpha inhibitor heavy chain 3 are also associated with increased incidences of psychiatric disorders and suicide attempts in patients (Hamshere et al., 2013; Sasayama et al., 2014). Therefore, IAIPs have important immunomodulatory properties and reductions in endogenous IAIPs could have a role in ischemic brain injury and the loss of endogenous Ambp/bikunin predisposes to behavioral and mood disorders (Goulding et al., 2019). Thus, based on our current findings showing the existence of endogenous IAIPs in a large numbers of cells in the cerebral cortex of human brain over a wide range of ages, we speculate that endogenous IAIPs could have physiological roles in both developing and mature brain. Nonetheless, the potential role of IAIPs during development in the human brain cannot be determined by the current study. Future in vivo and in vitro studies are required to delineate the roles of endogenous IAIPs and the use of mice deficient in Ambp/bikunin could also help to elucidate the potential roles of bikunin in brain (Goulding et al., 2019).
Our analysis of IAIPs throughout human development unexpectedly detected these proteins both in the cytoplasm, as well as in the nucleus, early in development. Moreover, the relative quantities of IAIPs increased with age specifically in the cytoplasmic compartment. In agreement with our findings, earlier studies have reported the expression IAIP-related proteins in subcellular compartments by immunohistochemistry (Kobayashi et al., 1999; Moriyama, Glenton, & Khan, 2001). Specifically, the heavy chains of IAIPs and the light chain, bikunin, have previously been localized in the cytoplasm of cells in lung, kidney, and liver in mice (Kobayashi et al., 1999; Moriyama et al., 2001). In our previous work, we identified cells with both cytoplasmic and perinuclear IAIP staining in the fetal and neonatal rodent brain (Chen et al., 2016). It is noteworthy that the subcellular localization of IAIPs in the cerebral cortex was predominately confined to the nucleus, rather than the cytoplasm, in the human brain from 24 weeks of gestation up to maturity in the adult. There are no previous reports regarding quantitative comparisons of IAIP expression specific for subcellular localization in the brain of any species. However, considering that much of the IAIP immunohistochemical expression appears to be mainly located in the nucleus, it remains possible that IAIPs could perform their function within the nucleus. Therefore, although we cannot be certain of the implications of our findings, we speculate that the relatively large number of cells containing nuclear IAIPs in cerebral cortex throughout a wide range of developmental ages suggests that endogenous nuclear IAIPs could potentially have a role in nuclear regulation of gene expression and/or other essential nuclear processes similar to those of histones and/or the high-mobility group box-1 protein (Chen, Nakada, et al., 2019; Deng, Scott, Fan, & Billiar, 2019; Tolsma & Hansen, 2019). In addition, the potential exists that IAIPs could be important binding partners for other nuclear proteins such as histones and the high-mobility group box-1 protein (Chaaban et al., 2015; Chen, Zhang, et al., 2019). However, we also cannot rule out the possibility that some of the nuclear appearing staining could not impart represent staining in the endoplasmic reticulum. Limitations on the amount of residual brain tissue available from the clinical autopsy material restricted the number of slides available for our study.
Another novel finding of our study is the increase in endogenous cytoplasmic, but not nuclear IAIPs, in the human cerebral cortex with advancing age. Our previous findings demonstrated that both the 250 kDa IαI and 125 kDa PαI IAIP complexes, were expressed in brain in an age dependent manner during ovine development (Spasova et al., 2014). For example, the IαI moiety in the cerebral cortex was more highly expressed in adult sheep than in the fetus and newborn lambs (Spasova et al., 2014). In contrast, the expression of PαI was higher in the preterm fetus and adult sheep than in newborn lambs (Spasova et al., 2014). Although we have not yet clarified the profiles of each IAIP moiety in human brain from our initial residual autopsy samples, we can speculate that the molecular moieties of IAIP could potentially change in the course of human brain development. Moreover, intracytoplasmic staining of the IAIP light chain, bikunin, has previously been observed in interstitial cells, reactive astrocytes and macrophages in human brain tumors (Yoshida et al., 1989). However, the significance of the increases in cytoplasmic IAIPs with increasing age remains to be determined.
Our quantitative analysis demonstrated that NFL positive neuronal cells changed with development in the cerebral cortex with the largest number of NFL positive neurons present amongst the early gestation subjects at 29 to 36 weeks of gestation. Our findings are consistent with a previous report showing that neuronal cells in the human cortex increased to reach a maximum at 28–32 weeks of gestation and then gradually decreased until around full-term gestation (Rabinowicz, de Courten-Myers, Petetot, Xi, & de los Reyes, 1996). Furthermore, the relatively high amount of IAIP-expressing neuronal cells in infants supports the contention that endogenous IAIPs are present in neurons during brain development.
Quantitative analysis also demonstrated that the percent of GFAP positive cells in the cerebral cortex gradually increased from less than 1% in the preterm period at 24–26 weeks of gestation up to approximately 24% in the adult subjects. The percent of GFAP positive cells in the cerebral cortex were significantly higher in the adult than at 24–26, 27–28, 29–36, and 37–40 weeks of gestation. These findings are consistent with previous reports showing that astrocytic populations in the cerebral cortex are higher in the mature than in the developing brain (Ge & Jia, 2016). Likewise, the expression of astrocytic IAIPs demonstrated a similar developmental pattern signifying that endogenous IAIPs could have important maturational roles in several different cell types during brain development.
In the current study, we used both confocal and epifluorescent microscopy and were able detect IAIPs in the cerebral cortex using both methods. We also verified the use of the purified IgG of the polyclonal rabbit anti-rat IAIPs antibodies by several methods. We demonstrated very good correlations between the positive IAIP staining with our purified IgG polyclonal rabbit anti-IAIP antibody and positive bikunin staining with the polyclonal rabbit-anti bikunin N-terminal antibodies. Moreover, we were able to show that the purified IgG of the polyclonal rabbit anti-IAIP antibodies appropriately identified the pure human IAIPs and IAIPs in residual frozen adult brain tissue but did not show any staining when the pre-immune serum was applied.
There are several limitations to our current study. First, fresh frozen tissues are not obtained for routine autopsies at Women & Infants Hospital of Rhode Island. Therefore, we did not have tissue available from the different age groups to permit analysis by Western immunoblotting and were restricted to analyzing IAIP protein expression by Western immunoblot in just a few adult brain samples obtained in Department of Pathology at Rhode Island Hospital. Therefore, we were not able to determine the relative amounts of IAIP expression in the cerebral cortex similar to our previous report (Spasova et al., 2014). The limited fresh tissue available also prevented further analysis of IAIPs’ subcellular localization by cell fractionation methods. Second, we did not determine the nuclear versus cytoplasm localization of IAIPs in the NFL positive neurons and GFAP positive astrocytes. Therefore, we cannot comment upon the potential differential localizations of IAIPs in these two cell types. Third, we did not have sufficient numbers of brain sections available to examine whether endogenous IAIPs are expressed in other types of brain cells such as microglia and oligodendrocytes. Fourth, we were not able to demonstrate the distribution and localization of the individual IAIP moieties or heavy chains of IAIPs because the R-22c pAb used in the present study detected both the IαI and PαI complexes. However, we did demonstrate that bikunin expression correlated with IAIPs expression by using a specific antibody against bikunin, suggesting that other IAIP related molecules are also present in human brain. Fifth, we recognize that there are limitations to the use of human autopsy material. We cannot be certain that the localization of IAIPs in the brain were not affected by disease states prior to the subjects’ demise, the timing of demise and/or the basic nature of autopsy material. Lastly, we have not examined the differences in IAIP expression in the brain as a function of sex because of the limited numbers of samples available within each age group category. Nonetheless, the current study is important because we have identified the patterns of IAIP expression in human brain for the first time over a wide range of gestational ages and in adults.
We conclude that endogenous IAIPs are expressed at cellular and subcellular levels in human brain during development from 24 weeks of gestation up to maturity in the adult. Although the specific functions of endogenous IAIPs in the brain during development are not known, the presence of IAIPs in relatively large numbers of cerebral cortical cells including neurons and astrocytes suggest that these proteins most likely have important roles in human brain physiology including their potential role in development, maturation and endogenous neuroprotection.
Significance:
This paper provides the first immunohistochemical staining profiles of IAIPs in human brain over a wide range of ages. The results show abundant expression of IAIPs in the cell nucleus and cytoplasm in preterm and full term infants ranging from 24 to 40 weeks of gestation and in adults. IAIPs were expressed in neurons and astrocytes at all ages. The ubiquitous expression of IAIPs in major cell types of the cerebral cortex supports a potential role for IAIPs in human brain physiology including potential roles in development, maturation and endogenous neuroprotection.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under the following award numbers: Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P30GM114750, National Institutes of Health 1R21NS095130, 1R21NS096525 and R44 NS084575-02. The authors assume all responsibility for the study, and assert that the contents herein do not represent the National Institutes of Health’s official views. We would like to dedicate this work to Edward G Stopa, M.D., who was a superb neuropathologist, instrumental in this work, a true friend and key consultant to our laboratory. We also gratefully acknowledge the helpful editorial assistance of Kimberly Alonge, PhD.
Funding Information: National Institute of General Medical Sciences of the National Institutes of Health: Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P30GM114750, National Institutes of Health 1R21NS095130, 1R21NS096525 and R44 NS084575.
Footnotes
CONFLICTS OF INTEREST
Y-P Lim is employed by ProThera Biologics, Inc. and has a significant financial stake in the company. All other authors declare no conflicts of interest.
DATA ACCESSIBLITY
Further information regarding the resources, reagents and data availability should be directed to the corresponding author and will be considered upon request.
Associate Editor: Jerome Badaut, PhD, University of Bordeaux, France
REFERENCES
- Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, & Lim YP (2003). Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr, 143(1), 11–15. doi: 10.1016/S0022-3476(03)00190-2 [DOI] [PubMed] [Google Scholar]
- Bourguignon J, Borghi H, Sesboue R, Diarra-Mehrpour M, Bernaudin JF, Metayer J, … Thiberville L. (1999). Immunohistochemical distribution of inter-alpha-trypsin inhibitor chains in normal and malignant human lung tissue. J Histochem Cytochem, 47(12), 1625–1632. doi: 10.1177/002215549904701214 [DOI] [PubMed] [Google Scholar]
- Brandl EJ, Lett TA, Chowdhury NI, Tiwari AK, Bakanidze G, Meltzer HY, … Muller DJ (2016). The role of the ITIH3 rs2535629 variant in antipsychotic response. Schizophr Res, 176(2–3), 131–135. doi: 10.1016/j.schres.2016.06.032 [DOI] [PubMed] [Google Scholar]
- Cai T, Yu P, Monga SP, Mishra B, & Mishra L. (1998). Identification of mouse itih-4 encoding a glycoprotein with two EF-hand motifs from early embryonic liver. Biochim Biophys Acta, 1398(1), 32–37. [DOI] [PubMed] [Google Scholar]
- Cao ZL, Okazaki Y, Naito K, Ueno T, Natsuaki M, & Itoh T. (2000). Ulinastatin attenuates reperfusion injury in the isolated blood-perfused rabbit heart. Ann Thorac Surg, 69(4), 1121–1126. [DOI] [PubMed] [Google Scholar]
- Chaaban H, Keshari RS, Silasi-Mansat R, Popescu NI, Mehta-D’Souza P, Lim YP, & Lupu F. (2015). Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood, 125(14), 2286–2296. doi: 10.1182/blood-2014-06-582759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, & Padbury JF (2010). Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr, 157(5), 757–761. doi: 10.1016/j.jpeds.2010.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, & Padbury JF (2009). The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr, 154(4), 620–622 e621. doi: 10.1016/j.jpeds.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Chan P, Risler JL, Raguenez G, & Salier JP (1995). The three heavy-chain precursors for the inter-alpha-inhibitor family in mouse: new members of the multicopper oxidase protein group with differential transcription in liver and brain. Biochem J, 306 ( Pt 2), 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nakada S, Donahue JE, Chen RH, Tucker R, Qiu J, … Stonestreet BS (2019). Neuroprotective effects of inter-alpha inhibitor proteins after hypoxic-ischemic brain injury in neonatal rats. Exp Neurol, 317, 244–259. doi: 10.1016/j.expneurol.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rivard L, Naqvi S, Nakada S, Padbury JF, Sanchez-Esteban J, … Stonestreet BS (2016). Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience, 324, 69–81. doi: 10.1016/j.neuroscience.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Kim B, Jaitpal S, Meng SS, Adjepong K, … Stonestreet BS (2019). High-mobility group box-1 translocation and release after hypoxic ischemic brain injury in neonatal rats. Exp Neurol, 311, 1–14. doi: 10.1016/j.expneurol.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvetti L, Capsoni S, Cattaneo A, & Domenici L. (2003). Postnatal development of GFAP in mouse visual cortex is not affected by light deprivation. Glia, 41(4), 404–414. doi: 10.1002/glia.10194 [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics, C. (2013). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet, 381(9875), 1371–1379. doi: 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daveau M, Jean L, Soury E, Olivier E, Masson S, Lyoumi S, … Salier JP (1998). Hepatic and extra-hepatic transcription of inter-alpha-inhibitor family genes under normal or acute inflammatory conditions in rat. Arch Biochem Biophys, 350(2), 315–323. [DOI] [PubMed] [Google Scholar]
- Degraeuwe PL, & Nieman FH (2010). The diagnostic value of inter-alpha inhibitor proteins for neonatal sepsis. J Pediatr, 156(2), 341; author reply 341. doi: 10.1016/j.jpeds.2009.08.040 [DOI] [PubMed] [Google Scholar]
- Deng M, Scott MJ, Fan J, & Billiar TR (2019). Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol, 106(1), 161–169. doi: 10.1002/JLB.3MIR1218-497R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disdier C, Zhang J, Fukunaga Y, Lim YP, Qiu J, Santoso A, & Stonestreet BS (2018). Alterations in inter-alpha inhibitor protein expression after hypoxic-ischemic brain injury in neonatal rats. Int J Dev Neurosci, 65, 54–60. doi: 10.1016/j.ijdevneu.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, & Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med, 22(3), 238–249. doi: 10.1038/nm.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Metwally D, Vohr B, & Tucker R. (2000). Survival and neonatal morbidity at the limits of viability in the mid 1990s: 22 to 25 weeks. J Pediatr, 137(5), 616–622. doi: 10.1067/mpd.2000.109143 [DOI] [PubMed] [Google Scholar]
- Enghild JJ, Thogersen IB, Pizzo SV, & Salvesen G. (1989). Analysis of inter-alpha-trypsin inhibitor and a novel trypsin inhibitor, pre-alpha-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J Biol Chem, 264(27), 15975–15981. [PubMed] [Google Scholar]
- Ferriero DM (2004). Neonatal brain injury. N Engl J Med, 351(19), 1985–1995. doi: 10.1056/NEJMra041996 [DOI] [PubMed] [Google Scholar]
- Fries E, & Blom AM (2000). Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol, 32(2), 125–137. [DOI] [PubMed] [Google Scholar]
- Fries E, & Kaczmarczyk A. (2003). Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim Pol, 50(3), 735–742. doi:035003735 [PubMed] [Google Scholar]
- Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, & Schwartz DA (2007). Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol, 179(6), 4187–4192. [DOI] [PubMed] [Google Scholar]
- Gaudet CM, Lim YP, Stonestreet BS, & Threlkeld SW (2016). Effects of age, experience and inter-alpha inhibitor proteins on working memory and neuronal plasticity after neonatal hypoxia-ischemia. Behav Brain Res, 302, 88–99. doi: 10.1016/j.bbr.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, & Jia JM (2016). Local production of astrocytes in the cerebral cortex. Neuroscience, 323, 3–9. doi: 10.1016/j.neuroscience.2015.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmied JR, & Gehrman P. (2019). An Integrated Model of Slow-Wave Activity and Neuroplasticity Impairments in Major Depressive Disorder. Curr Psychiatry Rep, 21(5), 30. doi: 10.1007/s11920-019-1013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding DR, Nikolova VD, Mishra L, Zhuo L, Kimata K, McBride SJ, … Garantziotis S.(2019). Inter-alpha-inhibitor deficiency in the mouse is associated with alterations in anxiety-like behavior, exploration and social approach. Genes Brain Behav, 18(1), e12505. doi: 10.1111/gbb.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JH, Ma W, Yang JW, Gao Y, Liang Z, Liu J, … Li LY (2015). Expression pattern of NeuN and GFAP during human fetal spinal cord development. Childs Nerv Syst, 31(6), 863–872. doi: 10.1007/s00381-015-2713-7 [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, … O’Donovan MC (2013). Genome-wide significant associations in schizophrenia to ITIH¾, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry, 18(6), 708–712. doi: 10.1038/mp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz SR, Kendrick DE, Wilson-Costello DE, Das A, Bell EF, Vohr BR, … Network NNR (2011). Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics, 127(1), 62–70. doi: 10.1542/peds.2010-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth S, Heise R, Vetter-Kauczok CS, Skazik C, Marquardt Y, Czaja K, … Baron JM (2015). Inter-alpha-trypsin inhibitor heavy chain 5 (ITIH5) is overexpressed in inflammatory skin diseases and affects epidermal morphology in constitutive knockout mice and murine 3D skin models. Exp Dermatol, 24(9), 663–668. doi: 10.1111/exd.12704 [DOI] [PubMed] [Google Scholar]
- Hyman BT, Tanzi RE, Marzloff K, Barbour R, & Schenk D. (1992). Kunitz protease inhibitor-containing amyloid beta protein precursor immunoreactivity in Alzheimer’s disease. J Neuropathol Exp Neurol, 51(1), 76–83. [DOI] [PubMed] [Google Scholar]
- Ide H, Itoh H, Yoshida E, Kobayashi T, Tomita M, Maruyama H, … Nawa Y. (1999). Immunohistochemical demonstration of inter-alpha-trypsin inhibitor light chain (bikunin) in human mast cells. Cell Tissue Res, 297(1), 149–154. [DOI] [PubMed] [Google Scholar]
- Itoh H, Ide H, Kataoka H, Tomita M, Yoshihara H, & Nawa Y. (1994). cDNA sequencing of mouse alpha 1-microglobulin/inter-alpha-trypsin inhibitor light chain and its expression in acute inflammation. J Biochem, 116(4), 767–772. [DOI] [PubMed] [Google Scholar]
- Itoh H, Tomita M, Kobayashi T, Uchino H, Maruyama H, & Nawa Y. (1996). Expression of inter-alpha-trypsin inhibitor light chain (bikunin) in human pancreas. J Biochem, 120(2), 271–275. [DOI] [PubMed] [Google Scholar]
- Jiang L, Ash PEA, Maziuk BF, Ballance HI, Boudeau S, Abdullatif AA, … Wolozin B. (2019). TIA1 regulates the generation and response to toxic tau oligomers. Acta Neuropathol, 137(2), 259–277. doi: 10.1007/s00401-018-1937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga N, Katsuki Y, Futamura Y, Obata M, & Shibutani Y. (1996). Role of urinary trypsin inhibitor in the maintenance of pregnancy in mice. Obstet Gynecol, 88(5), 872–882. doi: 10.1016/0029-7844(96)00268-2 [DOI] [PubMed] [Google Scholar]
- Kakinuma C, Kuwayama C, Kaga N, Futamura Y, Katsuki Y, & Shibutani Y. (1997). Trophoblastic apoptosis in mice with preterm delivery and its suppression by urinary trypsin inhibitor. Obstet Gynecol, 90(1), 117–124. doi: 10.1016/S0029-7844(97)00176-2 [DOI] [PubMed] [Google Scholar]
- Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, & Daginawala HF (2009). Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta, 402(1–2), 160–163. [DOI] [PubMed] [Google Scholar]
- Kato M, Seki N, Sugano S, Hashimoto K, Masuho Y, Muramatsu M, … Nakafuku M. (2001). Identification of sonic hedgehog-responsive genes using cDNA microarray. Biochem Biophys Res Commun, 289(2), 472–478. doi: 10.1006/bbrc.2001.5976 [DOI] [PubMed] [Google Scholar]
- Kobayashi H. (2006). Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem, 387(12), 1545–1549. doi: 10.1515/BC.2006.192 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sun GW, & Terao T. (1999). Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice. Cell Tissue Res, 296(3), 587–597. [DOI] [PubMed] [Google Scholar]
- Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, … Maekawa T. (2010). Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res, 32(9), 925–932. doi: 10.1179/016164110X12645013515133 [DOI] [PubMed] [Google Scholar]
- Lim YP (2013). ProThera Biologics, Inc.: a novel immunomodulator and biomarker for life-threatening diseases. R I Med J (2013), 96(2), 16–18. [PubMed] [Google Scholar]
- Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, & Palardy JE (2003). Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis, 188(6), 919–926. doi: 10.1086/377642 [DOI] [PubMed] [Google Scholar]
- Lim YP, Josic D, Callanan H, Brown J, & Hixson DC (2005). Affinity purification and enzymatic cleavage of inter-alpha inhibitor proteins using antibody and elastase immobilized on CIM monolithic disks. J Chromatogr A, 1065(1), 39–43. [DOI] [PubMed] [Google Scholar]
- Mizon C, Mairie C, Balduyck M, Hachulla E, & Mizon J. (2001). The chondroitin sulfate chain of bikunin-containing proteins in the inter-alpha-inhibitor family increases in size in inflammatory diseases. Eur J Biochem, 268(9), 2717–2724. [DOI] [PubMed] [Google Scholar]
- Mizushima S, Nii A, Kato K, & Uemura A. (1998). Gene expression of the two heavy chains and one light chain forming the inter-alpha-trypsin-inhibitor in human tissues. Biol Pharm Bull, 21(2), 167–169. [DOI] [PubMed] [Google Scholar]
- Moriyama MT, Glenton PA, & Khan SR (2001). Expression of inter-alpha inhibitor related proteins in kidneys and urine of hyperoxaluric rats. J Urol, 165(5), 1687–1692. [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto M, Oda E, Yamamoto A, Kanemura Y, Hara M, … Okano H. (2003). Expression of tubulin beta II in neural stem/progenitor cells and radial fibers during human fetal brain development. Lab Invest, 83(4), 479–489. [DOI] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Furmaier A, Doing C, & Bach M. (2007). Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry, 62(5), 373–380. doi: 10.1016/j.biopsych.2006.10.006 [DOI] [PubMed] [Google Scholar]
- O’Shea TM (2002). Cerebral palsy in very preterm infants: new epidemiological insights. Ment Retard Dev Disabil Res Rev, 8(3), 135–145. doi: 10.1002/mrdd.10032 [DOI] [PubMed] [Google Scholar]
- Odum L, Halkier T, Hojrup P, & Schousboe I. (1989). Characterization of urinary proteinase inhibitors with segments of amino acids sequences identical to sequences of pancreatic secretory trypsin inhibitor. Int J Biochem, 21(12), 1319–1327. [DOI] [PubMed] [Google Scholar]
- Okroj M, Holmquist E, Sjolander J, Corrales L, Saxne T, Wisniewski HG, & Blom AM (2012). Heavy chains of inter alpha inhibitor (IalphaI) inhibit the human complement system at early stages of the cascade. J Biol Chem, 287(24), 20100–20110. doi: 10.1074/jbc.M111.324913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Lim YP, Siryaporn E, Moldawer LL, Pribble JP, Palardy JE, & Souza S. (2007). Longitudinal studies of inter-alpha inhibitor proteins in severely septic patients: a potential clinical marker and mediator of severe sepsis. Crit Care Med, 35(2), 387–392. doi: 10.1097/01.CCM.0000253810.08230.83 [DOI] [PubMed] [Google Scholar]
- Otxoa-de-Amezaga A, Miro-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, … Planas AM (2019). Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol, 137(2), 321–341. doi: 10.1007/s00401-018-1954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot R, Berges R, Bocquet A, & Eyer J. (2008). Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol, 38(1), 27–65. doi: 10.1007/s12035-008-8033-0 [DOI] [PubMed] [Google Scholar]
- Plaas A, Sandy JD, Liu H, Diaz MA, Schenkman D, Magnus RP, … Galante JO (2011). Biochemical identification and immunolocalizaton of aggrecan, ADAMTS5 and inter-alpha-trypsin-inhibitor in equine degenerative suspensory ligament desmitis. J Orthop Res, 29(6), 900–906. doi: 10.1002/jor.21332 [DOI] [PubMed] [Google Scholar]
- Plowey ED, & Ziskin JL (2016). Hippocampal phospho-tau/MAPT neuropathology in the fornix in Alzheimer disease: an immunohistochemical autopsy study. Acta Neuropathol Commun, 4(1), 114. doi: 10.1186/s40478-016-0388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, & Racagni G. (2002). Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord, 4(3), 166–182. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, de Courten-Myers GM, Petetot JM, Xi G, & de los Reyes E. (1996). Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol, 55(3), 320–328. [PubMed] [Google Scholar]
- Robertson JM (2014). Astrocytes and the evolution of the human brain. Med Hypotheses, 82(2), 236–239. doi: 10.1016/j.mehy.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Rochefort N, Quenech’du N, Ezan P, Giaume C, & Milleret C. (2005). Postnatal development of GFAP, connexin43 and connexin30 in cat visual cortex. Brain Res Dev Brain Res, 160(2), 252–264. doi: 10.1016/j.devbrainres.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, & Morgan TE (1998). Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging, 19(1), 97–103. [DOI] [PubMed] [Google Scholar]
- Salier JP, Rouet P, Raguenez G, & Daveau M. (1996). The inter-alpha-inhibitor family: from structure to regulation. Biochem J, 315 ( Pt 1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez D, Martinez S, Lindqvist A, Akerstrom B, & Falkenberg C. (2002). Expression of the AMBP gene transcript and its two protein products, alpha(1)-microglobulin and bikunin, in mouse embryogenesis. Mech Dev, 117(1–2), 293–298. [DOI] [PubMed] [Google Scholar]
- Sarnat HB (2013). Clinical neuropathology practice guide 5–2013: markers of neuronal maturation. Clin Neuropathol, 32(5), 340–369. doi: 10.5414/NP300638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hori H, Yamamoto N, Nakamura S, Teraishi T, Tatsumi M, … Kunugi H. (2014). ITIH3 polymorphism may confer susceptibility to psychiatric disorders by altering the expression levels of GLT8D1. J Psychiatr Res, 50, 79–83. doi: 10.1016/j.jpsychires.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study, C. (2011). Genome-wide association study identifies five new schizophrenia loci. Nat Genet, 43(10), 969–976. doi: 10.1038/ng.940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Kurzinger RP, Hopt UT, & Wittel UA (2013). Systemic differential gene regulation of the inter-alpha-trypsin inhibitor family in acute necrotizing pancreatitis in mice. J Surg Res, 180(2), e83–90. doi: 10.1016/j.jss.2012.03.061 [DOI] [PubMed] [Google Scholar]
- Shah BA, Migliori A, Kurihara I, Sharma S, Lim YP, & Padbury J. (2017). Blood Level of Inter-Alpha Inhibitor Proteins Distinguishes Necrotizing Enterocolitis From Spontaneous Intestinal Perforation. J Pediatr, 180, 135–140 e131. doi: 10.1016/j.jpeds.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Ohta Y, Liu X, Shang J, Morihara R, Nakano Y, … Abe K. (2019). Acute Anti-Inflammatory Markers ITIH4 and AHSG in Mice Brain of a Novel Alzheimer’s Disease Model. J Alzheimers Dis. doi: 10.3233/JAD-181218 [DOI] [PubMed] [Google Scholar]
- Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, … Lim YP (2010). Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res, 68(3), 242–247. doi:10.1203/00006450-201011001-0047410.1203/00006450-201011001-0047410.1203/PDR.0b013e3181e9fdf010.1203/PDR.0b013e3181e9fdf0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg EM, & Fries E. (1992). Biosynthesis of bikunin (urinary trypsin inhibitor) in rat hepatocytes. Arch Biochem Biophys, 295(2), 217–222. [DOI] [PubMed] [Google Scholar]
- Spasova MS, Chen X, Sadowska GB, Horton ER, Lim YP, & Stonestreet BS (2017). Ischemia reduces inter-alpha inhibitor proteins in the brain of the ovine fetus. Dev Neurobiol, 77(6), 726–737. doi: 10.1002/dneu.22451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasova MS, Sadowska GB, Threlkeld SW, Lim YP, & Stonestreet BS (2014). Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med (Maywood), 239(6), 724–736. doi: 10.1177/1535370213519195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens BE, Tucker R, & Vohr BR (2010). Special health care needs of infants born at the limits of viability. Pediatrics, 125(6), 1152–1158. doi: 10.1542/peds.2009-1922 [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, … Human Development Neonatal Research, N. (2004). Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA, 292(19), 2357–2365. doi: 10.1001/jama.292.19.2357 [DOI] [PubMed] [Google Scholar]
- Takano M, Mori Y, Shiraki H, Horie M, Okamoto H, Narahara M, … Shikimi T. (1999). Detection of bikunin mRNA in limited portions of rat brain. Life Sci, 65(8), 757–762. [DOI] [PubMed] [Google Scholar]
- Tan KT, McGrouther DA, Day AJ, Milner CM, & Bayat A. (2011). Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin. J Eur Acad Dermatol Venereol, 25(3), 317–327. doi: 10.1111/j.1468-3083.2010.03792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolsma TO, & Hansen JC (2019). Post-translational modifications and chromatin dynamics. Essays Biochem, 63(1), 89–96. doi: 10.1042/EBC20180067 [DOI] [PubMed] [Google Scholar]
- Vohr BR (2014). Neurodevelopmental outcomes of extremely preterm infants. Clin Perinatol, 41(1), 241–255. doi: 10.1016/j.clp.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Poole WK, & McDonald SA (2005). Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics, 116(3), 635–643. doi: 10.1542/peds.2004-2247 [DOI] [PubMed] [Google Scholar]
- Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, … Terao, T. (2005). Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J Infect Dis, 191(6), 930–938. doi: 10.1086/428134 [DOI] [PubMed] [Google Scholar]
- Wassink G, Davidson JO, Dhillon SK, Fraser M, Galinsky R, Bennet L, & Gunn AJ (2017). Partial white and grey matter protection with prolonged infusion of recombinant human erythropoietin after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab, 37(3), 1080–1094. doi: 10.1177/0271678X16650455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Agar NY, Waldkircher de Oliveira RM, Faury D, Antel JP, Jabado N, & Del Maestro RF (2006). Isolation of a natural inhibitor of human malignant glial cell invasion: inter alpha-trypsin inhibitor heavy chain 2. Cancer Res, 66(3), 1464–1472. doi: 10.1158/0008-5472.CAN-05-1913 [DOI] [PubMed] [Google Scholar]
- Yano T, Anraku S, Nakayama R, & Ushijima K. (2003). Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology, 98(2), 465–473. [DOI] [PubMed] [Google Scholar]
- Yoshida E, Sumi H, Maruyama M, Tsushima H, Matsuoka Y, Sugiki M, & Mihara H. (1989). Distribution of acid stable trypsin inhibitor immunoreactivity in normal and malignant human tissues. Cancer, 64(4), 860–869. [DOI] [PubMed] [Google Scholar]
- Yoshida E, Yoshimura M, Ito Y, & Mihara H. (1991). Demonstration of an active component of inter-alpha-trypsin inhibitor in the brains of Alzheimer type dementia. Biochem Biophys Res Commun, 174(2), 1015–1021. [DOI] [PubMed] [Google Scholar]
- Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, … Nixon RA (2012). Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J Neurosci, 32(25), 8501–8508. doi: 10.1523/JNEUROSCI.1081-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, & Kimata K. (2008). Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res, 49(5), 311–320. doi: 10.1080/03008200802325458 [DOI] [PubMed] [Google Scholar]




