Abstract
Objective
The aim of this study was to investigate the value of the combined expression of the gastric mucosal differentiation protein pepsinogen C (PGC) and gastric cancer (GC)-associated antigen MG7 for the diagnosis of GC and prediction of the development from precancerous conditions to GC.
Methods
The gastric mucosal biopsies of 285 subjects enrolled from a region with a high incidence of GC were obtained and histopathologically examined. Subjects testing negative for GC (n=208) were followed up from 1998 to 2015. The levels of PGC and MG7 in the biopsies were determined by immunohistochemistry.
Results
PGC was positive in 91.4% of the non-atrophic gastritis, 26.5% of the atrophic gastritis, and 0% of the GC. MG7 was positive in 15.0% of the non-atrophic gastritis, 82.4% of the atrophic gastritis, and 94.8% of the GC. The non-atrophic gastritis group was predominantly “PGC+MG7−”. The atrophic gastritis and GC groups were predominantly “PGC−MG7+”. The rate of GC in subjects with “PGC−MG7+” staining was 113.4-fold higher [95% confidence interval (95% CI): 15.3−869.4, P<0.001] than that in subjects with other staining patterns. The sensitivity and specificity of the “PGC−MG7+” pattern were 92.2% and 78.8% for the detection of GC and 77.2% and 97.9% for GC and precancerous disease, respectively. In the follow-up cohort of non-GC subjects, the risk of developing GC was higher in those with the “PGC−MG7+” staining pattern.
Conclusions
Our data suggest that the “PGC−MG7+” pattern can be employed as a useful follow-up panel for detecting individuals with a high risk of GC, and the dynamic assessment of the follow-up panel needs multi-centre large-scale validation in the future.
Keywords: Pepsinogen C, gastric cancer-associated antigen MG7, gastric cancer, diagnosis, carcinogenesis
Introduction
Gastric cancer (GC) is highly prevalent in Asia and is the second leading cause of cancer mortality in China (1,2). An effective screening strategy can reduce GC mortality through early detection, diagnosis, and treatment. The search for biological biomarkers that could predict GC risk has been a hot research topic in the related research fields. It is well known that pepsinogen C (PGC) and the GC-associated antigen MG7, measured either in tissue or serum, have potential to be biomarkers associated with GC (3-5). PGC belongs to the aspartic proteinase family and is the precursor of pepsin C, which is the terminal product of mature differentiated gastric mucosa cells and is mainly expressed in the stomach and proximal duodenum (6,7). PGC expression in situ was previously found to be significantly decreased in precancerous gastric diseases and almost absent in GC, suggesting that the lack of PGC expression in gastric mucosa is an ideal “negative marker” of GC (8-12). MG7 belongs to a group of non-specific membrane surface antigens (13). Previous studies have found that MG7 was absent in normal gastric mucosa, but the expression of MG7 gradually increased from benign gastric diseases to precancerous diseases, suggesting that the presence of MG7 expression in gastric mucosa is a “positive marker” of GC (14,15).
Gastric carcinogenesis is a multi-step procedure that involves various factors. Although a single biomarker would provide a simpler diagnostic test, the combined detection of multiple biomarkers could maximize the advantages of the component biomarkers to increase diagnostic accuracy (16-18). The latest findings of our studies showed that low PGC combined with high MG7-Ag may be key molecular events during the malignant transformation of gastric mucosa (19). However, it is still unclear whether the promising combination of PGC and MG7-Ag could be used as a follow-up panel for monitoring the dynamic progression of precancerous gastric diseases and what its detection efficiency for high-risk individuals is. The above outstanding matters require further evaluation and verification. The goal of this study was to analyze the expression of PGC and MG7 during the initiation and progression of GC to assess their combined diagnostic value. In addition, long-term follow-up information was collected with a gastroscopy examination and a pathological diagnosis to further evaluate the potential application for GC prediction.
Materials and methods
Study subjects
This study was a retrospective follow-up analysis of 285 individuals from a region with a high incidence of GC (Zhuanghe, Liaoning Province, China). A total of 172 male patients and 113 female patients were included, with age ranging from 21 to 84 (mean age, 51.8±13.4) years old. All subjects underwent gastroscopic examination. The histopathological diagnosis of the gastric mucosa biopsies was performed by at least two experienced pathologists based on the new Sydney System for the classification of gastritis and the World Health Organization gastric cancer classification. The study was approved by the Institute Research Medical Ethics Committee of the First Affiliated Hospital of China Medical University. Written informed consent was obtained from each study participant.
Cohort follow-up
Altogether, 208 subjects without GC were included in the follow-up cohort analysis, and these subjects underwent follow-up with gastroscopic examination and histopathological diagnosis of gastric mucosa biopsy one to seven times (1998, 1999, 2002, 2004, 2010, 2011, and 2015) after the first examination (1997). In addition, the occurrence of GC and death due to GC were obtained from the yearly “Cancer incidence and death registration report of Zhuang He City” for the monitored subjects during the follow-up. The follow-up period ended on December 31, 2015. There were no patients lost to follow-up in this research.
Immunohistochemistry assay
Immunohistochemical staining
PGC antibody (anti-pepsinogen C antibody, product name 2D5) was kindly provided by the Institute of Clinical Examination of Japan. MG7 monoclonal antibody was kindly provided by the Digestive Disease Research Laboratory of the Fourth Military Medical University (Xijing Hospital of Digestive Diseases). Immunohistochemical staining was performed using the two-step streptavidin-peroxidase method (Maixin, Kit-9801D2, Fuzhou, China).
Four-μm-thick tissue sections from paraffin-embedded tissues were mounted onto poly-L-lysine-coated glass slides and then baked at 70 °C overnight. The tissue sections were deparaffinized in xylene, rehydrated in ethanol, and then immersed in citrate buffer for antigen retrieval. Endogenous peroxidase was quenched using 3% hydrogen peroxide for 10 min. To decrease nonspecific staining, 2% normal non-immune animal serum was subsequently used to block tissue collagen for 10 min. The tissue sections were then incubated at 4 °C overnight with either the PGC or MG7 antibodies (5 μg/mL) or phosphate buffer solution (PBS) alone. Next, the sections were incubated with biotinylated anti-mouse secondary antibody for 30 min and then washed with PBS. The slides were stained with diaminobenzidine chromogenic reagent (DAB-0031, Maixin Inc.) for 3−10 min, and the reaction was stopped with water.
Criterion of immunohistochemical staining
Immunohistochemical staining was evaluated using a previously published comprehensive scoring method based on the staining intensity and positive cell number (19). The staining intensity of the mucosa cells was assessed according to the pattern of staining characteristic of the majority of cells. The staining intensity was classified as 0 (no staining), 1 (light brown staining), 2 (brown staining), and 3 (heavy brown staining). The percentage of stained cells was classified as 0 (no staining), 1 (1%−33%), 2 (34%−66%), and 3 (>66%). The two scores were combined and then classified into 4 grades: 0 was negative (−); 2−3, (+); 4, (++); 5−6, (+++). A score greater than two was considered positive expression.
Evaluation of diagnostic accuracy of PGC-MG7 by relative operator characteristic (ROC)
A ROC curve was used to evaluate the diagnostic utility of PGC-MG7 detection for GC and its precancerous state.
Statistical analysis
Statistical analysis was performed using SPSS software (Version 18.0, SPSS Inc., Chicago, IL, USA). The Chi-squared test was applied to assess the differences in individual or combined expression of PGC and MG7 in different gastric diseases. The Youden index (YD) = (sensitivity + specificity) −1 is used in evaluation of diagnostic efficacy of PGC and MG7 biomarkers. Logistic regression analysis was conducted to calculate odds ratio (OR) and 95% confidence interval (95% CI). P<0.05 was regarded as statistically significant.
Results
Subject baseline information
A total of 140 non-atrophic gastritis (NAG) cases, 68 atrophic gastritis (AG) cases, and 77 GC cases were included. The bassline information of these subjects was shown in Table 1 . There were statistical differences in the age and gender among three groups, so they were corrected in the subsequent statistical analysis.
1. Characteristics of subjects (N=285).
| Characteristics | NAG (n=140) | AG (n=68) | GC (n=77) | P* |
| *, compared among three groups; NAG, non-atrophic gastritis; AG, atrophic gastritis; GC, gastric cancer. | ||||
Age (
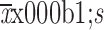 ) (year)
) (year)
|
45.6±10.7 | 53.5±12.3 | 61.5±12.6 | <0.05 |
| Sex [n (%)] | <0.05 | |||
| Male | 76 (54.3) | 41 (60.3) | 55 (71.4) | |
| Female | 64 (45.7) | 27 (39.7) | 22 (28.6) | |
PGC and MG7 protein expression in different gastric diseases
Expression of PGC and MG7 protein alone
The positive rate of PGC gradually decreased from NAG (91.4%) to AG (26.5%) to GC (0%). The differences between each group were all statistically significant (P<0.05). In contrast, the MG7 positive rate gradually increased from NAG (15.0%) to AG (82.4%) to GC (94.8%). The differences between each group were all statistically significant (P<0.05) (Figure 1 , Table 2 ).
1. Immunohistochemical staining for PGC/MG7 in different diseases. (A) Photomicrograph of NAG showing +6 score, PGC expression; (B) Photomicrograph of AG showing +0 score, PGC expression; (C) Photomicrograph of GC showing +0 score, PGC expression; (D) Photomicrograph of NAG showing +0 score, MG7 expression; (E) Photomicrograph of AG showing +6 score, MG7 expression; (F) Photomicrograph of GC showing +6 score, MG7 expression. (×100). PGC, pepsinogen C; NAG, non-atrophic gastritis; AG, atrophic gastritis; GC, gastric cancer.
2. Expression of PGC and MG7 protein in different gastric diseases.
| Gastric diseases | Number | PGC | MG7 | |||
| Positive rate [n (%)] | P | Positive rate [n (%)] | P | |||
| *, vs. NAG; #, vs. AG; PGC, pepsinogen C; NAG, non-atrophic gastritis; AG, atrophic gastritis; GC, gastric cancer. | ||||||
| NAG | 140 | 128 (91.4) | − | 21 (15.0) | − | |
| AG | 68 | 18 (26.5) | <0.001* | 56 (82.4) | <0.001* | |
| GC | 77 | 0 (0) | <0.001*, <0.001# | 73 (94.8) | <0.001*, 0.016# | |
Combined expression of PGC and MG7
Four combinations of PGC and MG7 expression levels were defined: “A type” (PGC+MG7−), “B type” (PGC−MG7−), “C type” (PGC+MG7+), and “D type” (PGC−MG7+). The NAG group was predominantly the “A type.” The AG and GC groups were predominantly the “D type” (60.3% and 92.2%, respectively). The “A type” staining was significantly higher in the NAG group than in the other groups (P<0.001), while no significant difference was observed between the AG and GC groups (P=0.500). The rate of the “D type” staining was significantly different among the three groups (P<0.001). After further logistic regression analysis adjusted by age and sex, the “A type” samples had a 0.009-fold risk of GC (95% CI: 0.001−0.064, P<0.001), and 0.110-fold risk (95% CI: 0.031−0.445, P=0.002) for the “B type” samples, 0.033-fold risk (95% CI: 0.007−0.151, P<0.001) for the “C type” samples, compared with the “D type” samples (Table 3 ).
3. Combined expression of PGC-MG7 in different gastric diseases.
| PGC-MG7 combination | Gastric diseases [n (%)] | GC risk | ||||
| NAG | AG | GC | OR (95% CI) | P** | ||
| *, vs. other combinations; **, D type as control; adjusted by age and sex; PGC, pepsinogen C; NAG, non-atrophic gastritis; AG, atrophic gastritis; GC, gastric cancer; OR, odds ratio; 95% CI, 95% confidence interval. | ||||||
| A type | 111 (79.3)* | 2 (2.9) | 1 (1.3) | 0.009 (0.001−0.064) | <0.001 | |
| B type | 8 (5.7) | 10 (14.7) | 3 (3.9) | 0.110 (0.031−0.445) | 0.002 | |
| C type | 18 (12.9) | 15 (22.1) | 2 (2.6) | 0.033 (0.007−0.151) | <0.001 | |
| D type | 3 (2.1) | 41 (60.3)* | 71 (92.2)* | 1.0 | − | |
| Total | 140 (100) | 68 (100) | 77 (100) | − | − | |
Diagnostic accuracy of PGC and MG7 expression levels
A ROC curve was calculated to assess the accuracy of the PGC and MG7 biomarkers at different immunohistochemistry score cut-off values. The optimal cut-off values were 2 for PGC and 3 for MG7. The sensitivity, specificity, and YD were 96.1%, 70.2%, and 0.663 for PGC and 87.0%, 78.4%, and 0.654 for MG7, respectively. For the “D type” combination, the sensitivity, specificity, and YD were 92.2%, 78.8%, and 0.711, respectively (Table 4 ).
4. Evaluation of diagnostic efficacy of PGC and MG7 proteins in GC.
| Gastric diseases | Biomarker | Sensitivity (%) | Specificity (%) | YD | AUC | P* |
| *, for AUC; PGC, pepsinogen C; GC, gastric cancer; AG, atrophic gastritis; YD, Youden index; AUC, area under the curve. | ||||||
| GC | PGC≤2 | 96.1 | 70.2 | 0.663 | 0.837 | <0.001 |
| MG7≥3 | 87.0 | 78.4 | 0.654 | 0.892 | <0.001 | |
| D type | 92.2 | 78.8 | 0.711 | − | <0.001 | |
| AG+GC | PGC≤2 | 97.2 | 82.9 | 0.801 | 0.943 | <0.001 |
| MG7≥2 | 89.0 | 85.0 | 0.740 | 0.911 | <0.001 | |
| D type | 77.2 | 97.9 | 0.751 | − | <0.001 | |
In addition, a ROC curve for the diagnosis of GC and precancerous disease (AG+GC) was drawn using PGC and MG7 as biomarkers. The optimal cut-off value was 2 for both PGC and MG7. The sensitivity, specificity, and YD were 97.2%, 82.9%, and 0.801 for PGC and 89.0%, 85.0%, and 0.740 for MG7, respectively. For the “D type” combination, the sensitivity, specificity, and YD were 77.2%, 97.9%, and 0.751, respectively (Table 4 ).
Association of combined expression of PGC and MG7 with GC risk in follow-up cohort
A total of 208 non-GC individuals were followed up from January 1, 1998, to December 31, 2015. Seven GC cases developed during that time: one in the NAG group (0.71%) and six in the AG group (8.8%). After comparing the risk of gastric carcinogenesis in different PGC-MG7 combinations, the rate of carcinogenesis was higher in the “D type” (6.818%) than in the “A type” (0.885%), “B type” (5.556%), and “C type” (6.061%) (Table 5 ).
5. Gastric carcinogenesis in different PGC-MG7 combination cohorts.
| Gastric diseases | Gastric carcinogenesis in follow-up [n (%)] | |||
| A type (N=113) | B type (N=18) | C type (N=33) | D type (N=44) | |
| PGC, pepsinogen C; NAG, non-atrophic gastritis; AG, atrophic gastritis. | ||||
| NAG | 1 (0.885) | − | − | − |
| AG | − | 1 (5.556) | 2 (6.061) | 3 (6.818) |
| Total | 1 (0.885) | 1 (5.556) | 2 (6.061) | 3 (6.818) |
Discussion
Few biomarkers are available for large-scale GC screening or early diagnosis. Conventional tumor markers such as carcinoembryonic antigen (CEA) and cancer antigens carbohydrate antigen (CA) 19-9, CA 50, and CA 72-4 are not tissue specific. They are expressed in many gastrointestinal cancers; moreover, their sensitivities and specificities are not ideal (12,20).
Both the absence of normal differentiation antigens and the presence of tumor-associated antigens are significant characteristics of cancer cells. These characteristics can be used as biomarkers for the prediction and early diagnosis of cancer (21,22). As a mature differentiation marker of gastric mucosa, PGC is absent in GC tissue (3). On the contrary, as a GC-related antigen, MG7 is frequently present in GC tissue (5). In this study, we, for the first time, observed the joint expression of PGC and MG7 in long-term follow-up population with GC risk and further evaluated its potential prediction for GC carcinogenesis. This research could provide some valuable data to support the clinical application of the combined detection of PGC-MG7 for the early diagnosis of GC and its precancerous condition.
In this study, four combinations of PGC and MG7 levels were defined. The “PGC+MG7−” or “A type” combination was the dominant type in the NAG group, while the “PGC−MG7+” or “D type” combination was the dominant type in the AG and GC groups. The risk of GC in the “PGC+MG7−” group was 0.009-fold lower than that in the “PGC−MG7+” group. In addition, ROC curve analysis suggested that the diagnostic accuracy of their combined detection was higher than the accuracy of either single biomarker. Based on these findings, we conducted a 15-year follow-up study of a high GC incidence population. The rate of gastric carcinogenesis was higher in the “PGC−MG7+” group than in the other groups, suggesting increased MG7 expression and decreased PGC expression could reflect abnormal hyperplasia, poor differentiation, and the degree of malignancy of gastric mucosa, and these markers could be used to monitor the initiation and progression of GC. In the future, expanded sample validation studies should be carried out.
The occurrence of gastric cancer is a process from normal to pre-cancerous stage and to cancerous stage according to the Correa’s pattern (23). If precancerous lesions can be identified early, the patient can be treated before its progression to GC. Chronic atrophic gastritis is defined as a precancerous lesion of GC by the World Health Organization (24). The combination of serum PGC and other biomarkers was found to be a useful tool for GC screening and AG detection (3). In addition, combinations of serum pepsinogen, gastrin-17, and H. pylori antibodies can also be used to detect AG more effectively (25-27). Our study assessed the diagnostic utility of PGC and MG7 protein expression in GC and precancerous lesions (AG and GC) by ROC curve analysis. The optimal cut-off value of the immunohistochemistry scores was 2 for both MG7 and PGC. The sensitivity and specificity of the individual markers were high. Combining the markers improved the specificity to 97.9% and therefore boosted the negative predictive value of the assay. Thus, the combined detection of PGC and MG7 could increase the diagnostic utility for GC and its precancerous disease state.
Conclusions
The combined detection of PGC and MG7 more accurately detected GC and its precancerous disease state than a single biomarker. These results suggested that the PGC-MG7 combination had a potentially significant role for identifying people at high risk of developing GC. The implementation of PGC and MG7 detection could help clinicians detect, diagnose, and treat GC early, thereby reducing the number of deaths caused by this disease.
Acknowledgements
This study was supported by grants from the National Science and Technology Support Program (No. 2015BAI13B07), and the Science Technology Project in Liaoning Province (No. 2012225016).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Park CH, Kim EH, Jung DH, et al The new modified ABCD method for gastric neoplasm screening. Gastric Cancer. 2016;19:128–35. doi: 10.1007/s10120-015-0473-4. [DOI] [PubMed] [Google Scholar]
- 4.Tu H, Sun L, Dong X, et al Temporal changes in serum biomarkers and risk for progression of gastric precancerous lesions: a longitudinal study. Int J Cancer. 2015;136:425–34. doi: 10.1002/ijc.29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Z, Fu S, Hu P, et al The diagnostic value of monoclonal gastric cancer 7 antigen: a systematic review with meta-analysis. Clin Exp Med. 2014;14:337–43. doi: 10.1007/s10238-013-0246-5. [DOI] [PubMed] [Google Scholar]
- 6.Shen S, Jiang J, Yuan Y Pepsinogen C expression, regulation and its relationship with cancer. Cancer Cell Int. 2017;17:57. doi: 10.1186/s12935-017-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mescoli C, Gallo Lopez A, Taxa Rojas L, et al Gastritis staging as a clinical priority. Eur J Gastroenterol Hepatol. 2018;30:125–9. doi: 10.1097/meg.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 8.Sipponen P, Graham DY Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2–10. doi: 10.1080/00365520600863720. [DOI] [PubMed] [Google Scholar]
- 9.Sipponen P Biomarkers in clinical practice: a tool to find subjects at high risk for stomach cancer. A personal view. Adv Med Sci. 2006;51:51–3. [PubMed] [Google Scholar]
- 10.Miki K Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 11.Yu G, Wang GX, Wang HG, et al The value of detecting pepsinogen and gastrin-17 levels in serum for pre-cancerous lesion screening in gastric cancer. Neoplasma. 2019;66:637–40. doi: 10.4149/neo_2018_180825N647. [DOI] [PubMed] [Google Scholar]
- 12.He CY, Sun LP, Gong YH, et al Serum pepsinogen II: a neglected but useful biomarker to differentiate between diseased and normal stomachs. J Gastroenterol Hepatol. 2011;26:1039–46. doi: 10.1111/j.1440-1746.2011.06692.x. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Chen Z, Juan SJ, et al Detection of circulating gastric carcinoma-associated antigen MG7-Ag in human sera using an established single determinant immuno-polymerase chain reaction technique. Cancer. 2000;88:280–5. doi: 10.1002/(SICI)1097-0142(20000115)88:2<280::AID-CNCR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Ren J, Pan K, et al Detection of gastric carcinoma-associated MG7-Ag by serum immuno-PCR assay in a high-risk Chinese population, with implication for screening. Int J Cancer. 2010;126:469–73. doi: 10.1002/ijc.24739. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Tie J, Xie Y, et al Detection of gastric carcinoma-associated antigen MG7-Ag in human sera using surface plasmon resonance sensor. Cancer Epidemiol. 2010;34:648–51. doi: 10.1016/j.canep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Sagaert X, Topal B, et al Gastric cancer. Lancet. 2016;388:2654–64. doi: 10.1016/s0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu Y, Zhang J, et al Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters. Chin J Cancer Res. 2018;30:623–32. doi: 10.21147/j.issn.1000-9604.2018.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M, Song J, Zhou Z, et al Comparison of serum microRNA21 and tumor markers in diagnosis of early non-small cell lung cancer. Dis Markers. 2016;2016:3823121. doi: 10.1155/2016/3823121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Shen S, Dong N, et al Correlation between negative expression of pepsinogen C and a series of phenotypic markers of gastric cancer in different gastric diseases. Cancer Med. 2018;7:4068–76. doi: 10.1002/cam4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui L, Rixv L, Xiuying Z A system for tumor heterogeneity evaluation and diagnosis based on tumor markers measured routinely in the laboratory. Clin Biochem. 2015;48:1241–5. doi: 10.1016/j.clinbiochem.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Zheng TH, Zhao JL, Guleng B Advances in molecular biomarkers for gastric cancer. Crit Rev Eukaryot Gene Expr. 2015;25:299–305. doi: 10.1615/critreveukaryotgeneexpr.2015014360. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Dou M, Yao X, et al Potential biomarkers in diagnosis of human gastric cancer. Cancer Invest. 2016;34:115–22. doi: 10.3109/07357907.2015.1114122. [DOI] [PubMed] [Google Scholar]
- 23.Correa P Human gastric carcinogenesis: a multistep and multifactorial process — First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 24.Hamilton S, Aaltonen L. World Health Organization classification of tumours. Pathology and Genetics of Turnouts of the Digestive System. IARC Press: Lyon, 2000;37-40.
- 25.Syrjänen KJ, Sipponen P, Härkönen M, et al Accuracy of the GastroPanel test in the detection of atrophic gastritis. Eur J Gastroenterol Hepatol. 2015;27:102–4. doi: 10.1097/meg.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNicholl AG, Forne M, Barrio J, et al Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol. 2014;26:941–8. doi: 10.1097/meg.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31:707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]



