Abstract
Objective
To investigate the prognostic impact of D2-plus lymphadenectomy including the posterior (No. 8p, No. 12b/p, No. 13, and No. 14v), and para-aortic (No. 16a2, and No. 16b1) lymph nodes (LNs) in subtotal gastrectomy for advanced gastric antral carcinoma.
Methods
A total of 203 patients with advanced gastric cancer (GC) located in the antrum, who underwent R0 gastrectomy with D2 or D2-plus lymphadenectomy between January 2003 and December 2011 were enrolled. Propensity score matching was used to reduce the strength of the confounding factors to accurately evaluate prognoses. The therapeutic value index (TVI) was calculate to evaluate the survival benefit of dissecting each LN station.
Results
Of 102 patients with D2-plus lymphadenectomy, 21 (20.59%) were pathologically identified as having LN metastases beyond the extent of D2 lymphadenectomy. After matching, the overall survival (OS) was significantly better in the D2-plus than the D2 group (P=0.030). In the multivariate survival analysis, D2-plus lymphadenectomy (hazard ratio, 0.516; P=0.006) was confirmed to significantly improve the survival rate. In the logistic regression analysis, pN stage [odds ratio (OR), 2.533; 95% confidence interval (95% CI), 1.368−4.691; P=0.003] and extent of LNs metastasis (OR, 5.965; 95% CI, 1.335−26.650; P=0.019) were identified as independent risk factors for LN metastases beyond the extent of D2 lymphadenectomy. The TVI of patient with metastasis to LNs station was 7.1 (No. 8p), 5.7 (No. 12p), 5.1 (No. 13), and 7.1 (both No. 16a2 and No. 16b1), respectively.
Conclusions
D2-plus lymphadenectomy may improve the prognoses of some patients with advanced GC located in the antrum, especially for No. 8p, No. 12b, No. 13, and No. 16.
Keywords: Stomach, neoplasm, lymphadenectomy, prognosis, metastasis
Introduction
Gastric cancer (GC) is one of the most commonly observed digestive malignant tumors, worldwide, and is associated with a high mortality (1). In China, more than 410,000 new GC cases and 290,000 GC-associated deaths are observed each year (2). The most effective treatment for curable GC is surgical resection. However, GC shows a strong tendency for lymph node (LN) involvement and local spread. Therefore, lymphadenectomy has crucially important clinical significance in such settings, and the extent of lymphadenectomy also directly influences survival outcomes. Subtotal gastrectomy with D2 lymphadenectomy is considered the standard treatment procedure for curative advanced GC located in the antrum (3-5). However, it is controversial whether D2-plus lymphadenectomy including dissection of the posterior (No. 8p, No. 12b/p, No. 13, and No. 14v), and para-aortic (No. 16a2, and No. 16b1) LNs can contribute to improving patient outcomes.
The Japan Clinical Oncology Group (JCOG) 9501 trial (6) showed no survival benefit in GC patients after D2-plus para-aortic LN dissection compared with after D2 lymphadenectomy. Meanwhile, clinical trials conducted in Poland and eastern Asia (7,8) also showed that D2-plus lymphadenectomy did not significantly improve survival in GC patients. The No. 14v LN station, the inclusion in D2 lymphadenectomy of which was controversial in Japan, is now not included in D2 lymphadenectomy for gastric antral carcinoma, according to the latest treatment guidelines (9). However, gastric antral carcinoma has a strong tendency to invade the duodenal region LN stations, including No. 5, No. 6, No. 12, No. 13, and No. 14v (10). A recent investigation showed that the dissection of the No. 14v LN could improve the 5-year survival rates in patients with advanced gastric antral carcinoma (11). In addition, several observational studies have reported that the dissection of the No. 8p, No. 12p, No. 13, No. 14v and No. 16 LN stations may be significantly correlated with better prognoses in advanced GC after curative surgery (12-14). Therefore, the authors of the above-mentioned reports suggest it should be reconsidered whether D2 lymphadenectomy is the optimal extent for advanced gastric antral carcinoma. Accordingly, we aimed to investigate the prognostic impact of D2-plus lymphadenectomy including dissection of the posterior (No. 8p, No. 12b/p, No. 13, No. 14v), and para-aortic (No. 16a2, and No. 16b1) LNs to elucidate the optimal extent of lymphadenectomy for patients with advanced gastric antral carcinoma and to analyze the possible risk factors for LN metastases beyond the extent of D2 lymphadenectomy.
Materials and methods
Patients
We retrospectively reviewed 1,744 patients with advanced GC who had undergone R0 distal gastrectomy with D2 or D2-plus lymphadenectomy at Tianjin Medical University Cancer Institute & Hospital, between January 2003 and December 2011. Patient data were retrieved retrospectively from the patients’ hospital records. Eligibility criteria included: 1) proven primary carcinoma located in the antrum, histologically; 2) T2 or more advanced stage; 3) curative gastrectomy with pathologically negative resection margins (R0 resection); 4) D2 or D2-plus lymphadenectomy; and 5) remaining alive at the initial hospital stay and the first postoperative month. The exclusion criteria were: 1) history of gastrectomy or other malignancies; 2) history of neoadjuvant chemotherapy; 3) distant metastases or peritoneal dissemination; 4) loss of follow-up; or 5) death due to other diseases or accidents. Ultimately, 203 patients in total were enrolled in this study. Among these patients, 101 patients underwent D2 lymphadenectomy (D2 group) and 102 underwent D2-plus lymphadenectomy (D2-plus group).
The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute & Hospital. All patients provided written informed consent before any enrolling procedures were initiated.
Surgical management
Curative gastrectomy with lymphadenectomy was performed in all patients. Curative resection was defined as the complete absence of grossly visible tumor tissue and pathologically negative resection margins. Primary tumors were resected en bloc with D2 lymphadenectomy, according to Japanese Gastric Cancer Association guidelines (15). We carried out more radical surgery in some cases including dissection of the posterior LN stations along the hepatic artery (No. 8p), the hepato-duodenal ligament (No. 12b/p), the pancreatic head (No. 13) and the superior mesenteric vein (No. 14v), because of the stronger tendency to invade the duodenal region LN stations. The dissection of these LNs additionally depended on intraoperative examination (invasion beyond the muscolaris propria or suspicious LN metastases), based on surgeon decision. The additional para-aortic LNs (No. 16a2, and No. 16b1) were dissected in some patients, with positive para-aortic nodes on preoperative CT scan or intraoperative examination (16). D2-plus lymphadenectomy was defined as the D2 lymphadenectomy with any LN station dissection beyond D2 region. The chosen surgical procedures were based mainly on Japanese GC treatment guideline (15).
Follow-up
After curative surgery for GC, all patients were followed up every 3 or 6 months for 2 years, and annually, thereafter, until death. The median follow-up time for the entire cohort was 44 (range, 4−138) months. The follow-up of all the patients in this study was completed in December 2015. At every visit, patients underwent ultrasonography, computed tomography, chest radiography, and endoscopy. Overall survival (OS) served as the primary end-point, and was defined as the time interval between the date of surgery and the date of either death as a result of GC or the last follow-up. During the follow-up period, 150 patients (74.9%) died.
Propensity score matching (PSM)
To overcome possible selection bias between the D2 and D2-plus groups, we performed one-to-one matching using PSM (17,18). The propensity score, defined as the conditional probability of patients being treated given the covariates, can be used to balance the covariates in two groups and therefore reduce such bias (19,20). It has also been reported that potential confounding variables that are unrelated to the exposure but related to the outcome should be included in the propensity score model, and that this will decrease the variance of an estimated exposure effect without increasing the bias (21). The propensity scores were estimated by using a nonparsimonious multiple logistic regression model. Accordingly, in our study, which aimed to obtain more reliable results, the following covariates were selected for the calculation of the propensity score: sex, age, tumor size, Lauren type, pT stage, pN stage, pTNM stage. Eventually, 38 pairs of exact matching and 14 pairs of nearest neighbor matching patients were included after matching.
Therapeutic value of LN dissection
The therapeutic value of each LN dissection was determined by a therapeutic value index (TVI), which was calculated by multiplication of the frequency of metastasis to the station and the 5-year survival rate of patients with metastasis to that station (22). The frequency of metastasis to each station was calculated by dividing the number of patients with metastasis at that station by the number in whom the station was dissected. The cumulative 5-year survival rate of patients with LN metastasis was calculated for each nodal station by the life-table method, irrespective of metastasis to other LN stations (22).
Statistical analysis
The χ2 or Fisher’s exact test used for categorical variables, and a t test was used for continuous variables. Factors that showed significant difference in the univariate analysis (P<0.05) were included in the multivariate analysis. Multivariate analysis was performed using a logistic regression model for the evaluation of the predictive risk factors. OS was determined using the Kaplan-Meier method, and a log-rank test was used to evaluate significance. Multivariate analyses of OS were performed to calculate the hazard ratios (HRs) and 95% confidence interval (95% CI) through the Cox regression model. In all the other statistical analyses, significance was defined as P<0.05 (two-sided). All statistical analyses were performed using the statistical analysis program package IBM SPSS Statistics (Version 24.0; IBM Corp., New York, USA).
Results
Clinical characteristics before and after PSM
The clinical characteristics of GC patients in the D2 and D2-plus groups are listed in Table 1 . A total of 101 (49.8%) and 102 (50.2%) patients were assigned to the D2 group and D2-plus group, respectively. Before matching, we observed some difference between the groups in terms of sex (P=0.033), Lauren type (P=0.061), and pN stage (P=0.084). After PSM (Table 1 ), 52 pairs of patients were enrolled. The strength of the selective bias between the two groups was reduced after matching, including sex (P=0.836), Lauren type (P=0.807), and pN stage (P=0.877). This implicated that the confounding factors were balanced. In addition, the number of total LNs dissected (before P=0.266, after P=0.604), the proportion of LNs metastasis (before P=0.311, after P=0.406) and the number of LNs metastasis in the D2 lymphadenectomy region (before P=0.635, after P=0.511) showed no significant difference between D2 and D2-plus group both before and after PSM, which showed good comparability of two groups (Table 1 ).
1. Clinical characteristics of patients in D2 and D2-plus groups before and after PSM.
| Characteristics | Entire cohort | P | PSM | P | ||
| D2 group (n=101) | D2-plus group (n=102) | D2 group (n=52) | D2-plus group (n=52) | |||
| PSM, propensity score matching; LN, lymph node; *, Fisher’s exact test. | ||||||
| Sex | 0.033 | 0.836 | ||||
| Male | 71 | 57 | 35 | 34 | ||
| Female | 30 | 45 | 17 | 18 | ||
| Age (year) | 0.413 | 0.502 | ||||
| ≤65 | 69 | 75 | 40 | 37 | ||
| >65 | 32 | 27 | 12 | 15 | ||
| Tumor size (cm) | 0.623 | 0.844 | ||||
| ≤4.0 | 49 | 53 | 26 | 25 | ||
| >4.0 | 52 | 49 | 26 | 27 | ||
| Lauren type | 0.061* | 0.807 | ||||
| Intestinal | 28 | 20 | 10 | 11 | ||
| Diffuse | 72 | 77 | 42 | 41 | ||
| Mixed | 1 | 5 | 50 | 50 | ||
| pT stage | 0.790 | 1.000 | ||||
| T2 | 20 | 19 | 59 | 59 | ||
| T3 | 5 | 7 | 51 | 51 | ||
| T4a | 74 | 72 | 41 | 41 | ||
| T4b | 2 | 4 | 51 | 51 | ||
| pN stage | 0.084 | 0.877* | ||||
| N0 | 50 | 37 | 24 | 25 | ||
| N1 | 13 | 26 | 10 | 12 | ||
| N2 | 21 | 16 | 7 | 4 | ||
| N3a | 10 | 17 | 8 | 9 | ||
| N3b | 7 | 7 | 3 | 2 | ||
| pTNM stage | 0.600 | 0.847* | ||||
| IA | 0 | 0 | 0 | 0 | ||
| IB | 16 | 12 | 7 | 8 | ||
| IIA | 6 | 6 | 2 | 0 | ||
| IIB | 29 | 25 | 16 | 19 | ||
| IIIA | 33 | 33 | 16 | 14 | ||
| IIIB | 10 | 19 | 8 | 9 | ||
| IIIC | 7 | 7 | 3 | 2 | ||
| Number of total LNs dissected | 0.266 | 0.604 | ||||
| <16 | 22 | 16 | 10 | 8 | ||
| ≥16 | 79 | 86 | 42 | 44 | ||
| Proportion of LNs metastasis | 0.311 | 0.406 | ||||
| 0 | 49 | 37 | 24 | 25 | ||
| <10% | 14 | 17 | 9 | 9 | ||
| 10%−40% | 26 | 36 | 11 | 15 | ||
| >40% | 12 | 12 | 8 | 3 | ||
| Extent of LNs metastasis | 0.140 | 0.512 | ||||
| Peri-gastric | 75 | 66 | 39 | 36 | ||
| Extra-gastric | 26 | 36 | 13 | 16 | ||
Number of LNs metastasis in D2 lymphadenectomy region (
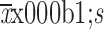 )
)
|
3.06±4.67 | 3.42±6.10 | 0.635 | 3.92±5.89 | 3.20±5.18 | 0.511 |
Dissection range and distribution of LN metastases beyond the extent of D2 lymphadenectomy
Supplementary Figure S1 shows the regions of D2-plus lymphadenectomy, and the distribution of LN metastasis beyond the extent of D2 lymphadenectomy in patients with advanced gastric antral carcinoma. In 102 patients who underwent D2-plus lymphadenectomy, the No. 12b (54/102), No. 13 (39/102), No. 12p (35/102) and No. 14v (25/102) LN stations were the most frequently dissected sites. Finally, 21 (20.59%) of the 102 patients were pathologically identified as having LN metastases beyond the extent of D2 lymphadenectomy. The highest LN metastasis proportions beyond the extent of D2 lymphadenectomy contained the No. 8p (28.6%), No. 14v (20.0%) and No. 12p (17.1%) stations.
S1. Distribution and metastasis rate of each LN station. No. 8p, the hepatic artery; No. 12b/p, the hepato-duodenal ligament; No. 13, the pancreatic head; No. 14v, the superior mesenteric vein; No. 16a2 and No. 16b1, para-aortic LNs. LN, lymph node.
Correlation analysis of risk factors for LN metastases beyond the extent of D2 lymphadenectomy
The univariate analysis showed that LN metastases beyond the extent of D2 lymphadenectomy were significantly correlated with age (P=0.048), pN stage (P<0.001), proportion of LNs metastasis (P<0.001), and extent of LNs metastasis (P<0.001) (Table 2 ). These factors which have the significant difference (P<0.05) in the univariate analysis were included in the multivariate analysis. We adopted logistics regression method with forward step procedures in the multivariate analysis. pN stage [odds ratio (OR), 2.533; 95% CI, 1.368−4.691; P=0.003] and extent of LNs metastasis (OR, 5.965; 95% CI, 1.335−26.650; P=0.019) were identified as independent risk factors for LN metastases beyond the extent of D2 lymphadenectomy in patients with advanced gastric antral carcinoma (Table 2 ).
2. Correlation analysis of risk factors for LN metastasis beyond the extent of D2 lymphadenectomy (N=102).
| Characteristics | Univariate analysis | Multivariate analysis | ||||||
| No. | LN metastasis beyond the extent of D2 lymphadenectomy (n) | P | OR | 95% CI | P | |||
| Positive
(n=21) |
Negative
(n=81) |
|||||||
| LN, lymph node; OR, odds ratio; 95% CI, 95% confidence interval; *, Fisher’s exact test; **, continuity correction. | ||||||||
| Sex | 0.896 | |||||||
| Male | 57 | 12 | 45 | |||||
| Female | 45 | 9 | 36 | |||||
| Age (year) | 0.048 | |||||||
| ≤65 | 75 | 19 | 56 | |||||
| >65 | 27 | 2 | 25 | |||||
| Tumor size (cm) | 0.966 | |||||||
| ≤4.0 | 53 | 11 | 42 | |||||
| >4.0 | 49 | 10 | 39 | |||||
| Lauren type | 1.000* | |||||||
| Intestinal | 20 | 4 | 16 | |||||
| Diffuse | 77 | 16 | 61 | |||||
| Mixed | 5 | 1 | 4 | |||||
| pT stage | 0.194* | |||||||
| T2 | 19 | 1 | 18 | |||||
| T3 | 7 | 2 | 5 | |||||
| T4a | 72 | 17 | 55 | |||||
| T4b | 4 | 1 | 3 | |||||
| pN stage | <0.001* | 2.533 | 1.368−4.691 | 0.003 | ||||
| N0 | 37 | 0 | 37 | |||||
| N1 | 25 | 3 | 22 | |||||
| N2 | 16 | 5 | 11 | |||||
| N3a | 17 | 7 | 10 | |||||
| N3b | 7 | 6 | 1 | |||||
| Number of total LNs dissected | 0.593** | |||||||
| <16 | 16 | 2 | 14 | |||||
| ≥16 | 86 | 19 | 67 | |||||
| Proportion of LNs metastasis | <0.001* | |||||||
| 0 | 37 | 0 | 37 | |||||
| <10% | 17 | 4 | 13 | |||||
| 10%−40% | 36 | 11 | 25 | |||||
| >40% | 12 | 6 | 6 | |||||
| Extent of LNs metastasis | <0.001 | 5.965 | 1.335−26.650 | 0.019 | ||||
| Peri-gastric | 66 | 3 | 63 | |||||
| Extra-gastric | 36 | 18 | 18 | |||||
Survival analysis before and after PSM
The prognostic value of D2-plus lymphadenectomy in gastric antral carcinoma was determined. During the follow-up, 150 patients died and 53 patients remained alive. Kaplan-Meier analyses showed no significant difference in terms of prognosis between the D2 and D2-plus groups (P=0.417) before PSM. After PSM, 104 patients were enrolled in this study. The median survival times of patients with D2 lymphadenectomy and those with D2-plus lymphadenectomy were 34±3 months and 53±6 months, respectively. Kaplan-Meier analysis showed that OS of patients in the D2-plus group was significantly superior to that of those in the D2 group (P=0.030) (Figure 1 ). To confirm the survival factors, we performed univariate analysis and Cox regression analysis (Table 3 ). In the univariate survival analysis, tumor size (P=0.004), pT stage (P=0.002), pN stage (P<0.001), and D2-plusvs. D2 LN dissection (P=0.039) were confirmed as prognostic factors for OS, whereas other clinicopathological characteristics, such as sex, age, and Lauren type had no prognostic significance for OS. The results of the Cox regression analysis were showed in Table 3 , Figure 2 . These results showed that more advanced T stage (pT4a vs. pT2: HR, 2.791; 95% CI, 1.248−6.244; P=0.012; and pT4bvs. pT2: HR, 12.714; 95% CI, 2.462−65.644; P=0.002) and more advanced pN stage (pN3avs. pN0: HR, 2.473; 95% CI, 1.316−4.646; P=0.005; and pN3bvs. pN0: HR, 9.379; 95% CI, 3.332−26.395; P<0.001) were significantly associated with poor prognoses, and that D2-plus lymphadenectomy (HR, 0.516; 95% CI, 0.323−0.825; P=0.006) should provide superior survival.
1. Kaplan-Meier curves for overall survival before (A) (P=0.417) and after (B) (P=0.030) propensity score matching.
3. Survival analysis of prognostic factors in patients with distal GC after matching.
| Predictors | Univariate analysis | Cox regression analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| GC, gastric cancer; LN, lymph node; HR, hazard ratio; 95% CI, 95% confidence interval. | |||||
| Time of surgery (year) | 0.469 | ||||
| 2006−2008vs. 2003−2005 | 1.246 (0.738−2.104) | 0.410 | |||
| 2009−2011vs. 2003−2005 | 0.893 (0.504−1.583) | 0.699 | |||
| Sex (female vs. male) | 1.113 (0.701−1.767) | 0.649 | |||
| Age (>65vs. ≤65) (year) | 1.245 (0.765−2.026) | 0.377 | |||
| Tumor size (>4vs. ≤4) (cm) | 1.940 (1.236−3.044) | 0.004 | |||
| Lauren type (diffuse vs. intestinal) | 1.226 (0.699−2.153) | 0.477 | |||
| pT stage | 0.002 | 0.011 | |||
| pT3 vs. pT2 | 3.464 (0.718−16.717) | 0.122 | 4.026 (0.786−20.613) | 0.095 | |
| pT4a vs. pT2 | 3.158 (1.447−6.889) | 0.004 | 2.791 (1.248−6.244) | 0.012 | |
| pT4b vs. pT2 | 7.830 (1.586−38.662) | 0.012 | 12.714 (2.462−65.664) | 0.002 | |
| pN stage | <0.001 | <0.001 | |||
| pN1 vs. pN0 | 1.575 (0.883−2.812) | 0.124 | 1.471 (0.808−2.679) | 0.206 | |
| pN2 vs. pN0 | 1.811 (0.833−3.939) | 0.134 | 2.164 (0.979−4.784) | 0.057 | |
| pN3a vs. pN0 | 2.509 (1.370−4.595) | 0.003 | 2.473 (1.316−4.646) | 0.005 | |
| pN3b vs. pN0 | 8.080 (3.003−21.739) | <0.001 | 9.379 (3.332−26.395) | <0.001 | |
| D2-plus vs. D2 LN dissection | 0.625 (0.400−0.977) | 0.039 | 0.516 (0.323−0.825) | 0.006 | |
2. Forest plot of pT stage, pN stage and extent of lymph node (LN) metastases in advanced gastric antral carcinoma after propensity score matching. In pT stage, other stages vs. pT2 stage; in pN stage, other stages vs. pN0 stage; in extent of LN metastases, D2-plus lymphadenectomy vs. D2 lymphadenectomy. OR, odds ratio; 95% CI, 95% confidence interval.
TVI
We next used TVI to evaluate the survival benefit of each LN in the D2-plus lymphadenectomy region. The TVI values were 7.1 for No. 8p, 5.7 for No. 12p, 5.1 for No. 13, 7.1 for No. 16a2 and 7.1 for No. 16b1, respectively, which were better than some LNs station in D2 lymphadenectomy region, such as No. 7 (3.4), No. 8a (5.0) and No. 9 (3.4) (Table 4 ). The 5-year OS information of each LN station was also available in Table 4 .
4. TVI of each LNs station in D2-plus lymphadenectomy region.
| LN station | Proportion of LN metastasis [% (n/N)] | 5-year OS (%) | TVI |
| TVI, therapeutic value index; LN, lymph node; OS, overall survival. TVI was calculated by multiplication of the frequency of metastasis to the station and the 5-year survival rate of patients with metastasis to that station. | |||
| No. 1 | 10.06 (18/179) | 44.4 | 4.5 |
| No. 3 | 30.43 (56/184) | 23.2 | 7.1 |
| No. 4sb | 15.98 (27/169) | 22.2 | 3.5 |
| No. 4d | 2.52 (4/159) | 25.0 | 0.6 |
| No. 5 | 12.50 (23/184) | 26.1 | 3.3 |
| No. 6 | 34.30 (59/172) | 27.1 | 9.3 |
| No. 7 | 17.61 (31/176) | 19.4 | 3.4 |
| No. 8a | 18.01 (29/161) | 27.5 | 5.0 |
| No. 8p | 28.57 (4/14) | 25.0 | 7.1 |
| No. 9 | 13.14 (23/175) | 26.1 | 3.4 |
| No. 11p | 4.47 (8/179) | 0 | 0 |
| No. 12a | 7.09 (10/141) | 10.0 | 0.7 |
| No. 12b | 12.96 (7/54) | 13.2 | 1.7 |
| N0. 12p | 17.14 (6/35) | 33.3 | 5.7 |
| No. 13 | 12.82 (5/39) | 40.0 | 5.1 |
| No. 14v | 20.00 (5/25) | 20.0 | 4.0 |
| No. 16a2 | 14.29 (2/14) | 50.0 | 7.1 |
| No. 16b1 | 14.29 (2/14) | 50.0 | 7.1 |
Discussion
LN metastasis has a key role in determining the OS associated with GC. The extent of lymphadenectomy is also of great clinical significance in GC. Subtotal gastrectomy with D2 lymphadenectomy, including dissection of the No. 1, No. 3, No. 4sb, No. 4d, No. 5, No. 6, No. 7, No. 8a, No. 9, No. 11p and No. 12a LN stations, is considered the standard treatment for curable gastric antral carcinoma cases, according to Japanese GC guidelines (5). Although there has been several researches to investigate the prognostic impacts of D2-plus lymphadenectomy, it has still been controversial whether the D2-plus lymphadenectomy can contribute to improving patients’ outcomes, until now. This may be explained by different patients enrolled and methods adopted. We therefore enrolled the patients with advanced gastric antral carcinoma which has a strong tendency to invade the duodenal region LN stations and adopted PSM to reduce the strength of the confounding factors, in order to accurately evaluate the prognostic impacts of D2-plus lymphadenectomy.
In this study, the extent of lymphadenectomy beyond D2 mainly comprised the No. 8p, No. 12b/p, No. 13, No. 14v, No. 16a2, and No. 16b1 LN stations. Based on our results, the highest frequency of metastatic LNs involved the No. 8p (28.6%), No. 14v (20.0%) and No. 12p (17.1%) stations, similar to the findings of previous investigations in China (23). However, our results are tremendously different from those of an Italian study (24), in which the metastatic proportions associated with the No. 8p. and No. 12b/p stations were 3.1% and 1.6%, respectively; this may be attributed to differences in the environment and race between the two countries. According to a multicenter study in China that enrolled 8,338 GC patients (25), and another multicenter Italian study that enrolled 743 GC patients (24), there are a larger number of GC patients with advanced disease stage (pT4 stage: 54.5% in China vs. 44.8% in Italy; pN3 stage: 28.1% in China vs. 16.6% in Italy) in China. Thus, metastatic LNs beyond the extent of D2 lymphadenectomy should be carefully detected in China, especially in patients with advanced disease stage.
In the present study, the univariate analysis revealed that the presence of LN metastases beyond the extent of D2 lymphadenectomy was significantly correlated with the age (P=0.048), pN stage (P<0.001), proportion of LNs metastasis (P<0.001) and extent of LNs metastasis (P<0.001). In the multivariate logistic analysis, pN stage and extent of LNs metastasis were identified as independent risk factors. That results indicated patients with more advanced pN stage and more extra-gastric metastatic LNs might have more opportunities of LN metastases beyond the extent of D2 lymphadenectomy in advanced gastric antral carcinoma. Our results also showed the prognostic significance of D2-plus lymphadenectomy. Before PSM, our results failed to show any significant difference with regard to the extent of lymphadenectomy (P=0.417) between the groups. This result was in line with those of several previous studies (6-8). To eliminate selection bias, we adopted the PSM method to balance the confounding factors between the D2 and D2-plus groups. After PSM, patients in the D2-plus group had a significantly superior OS than those in the D2 group (P=0.030). Cox regression analysis further confirmed that D2-plus lymphadenectomy was an independent factor associated with prognosis in advanced gastric antral carcinoma. Recently, de Manzoni et al. (12) reported that D2-plus lymphadenectomy, which includes the removal of the No. 12p, No. 13, No. 14v and No. 16 LN stations, was associated with a lower risk of recurrence, and may provide better local control in GC. Kumagai et al. (10) also reported that the dissection of the No. 12b, No. 13, No. 14v, No. 16a2 and No. 16b1 LN stations in potentially curative gastrectomy for patients with gastric antral carcinoma can yield survival benefits. These results are almost similar to those of our study. According to Figure 1 , the prognosis between D2 and D2-plus groups was the same before the matching, but was different after the matching. This result meant that D2-plus lymphadenectomy should be performed for some sort of high-risk patients. Furthermore, we found that sex (P=0.033), Lauren type (P=0.061), and pN stage (P=0.084) were predominantly responsible for the selective bias between the D2 group and D2-plus group. In the entire cohort of GC patients, the D2-plus group tended to enroll a larger number of female patients with mixed-diffuse histology or pN3 stage disease. It has been reported that, in female patients, GC is significantly correlated with diffuse histology and mixed-diffuse histology (26,27) and pN3 stage is associated with poor survival (28-31). This may explain why the above-mentioned studies failed to elucidate the survival benefit of D2-plus lymphadenectomy in patients with gastric antral carcinoma. D2-plus lymphadenectomy may provide survival benefits especially in gastric antral carcinoma patients with diffuse histology or advanced pN stage disease. The results of previous studies (32,33) also support this result. Accordingly, we considered that D2-plus lymphadenectomy could improve survival in some cases with advanced gastric antral carcinoma, especially for patients with diffuse histology or advanced pN stage.
TVI was used to evaluate the survival benefit of dissecting each LN station in the D2 lymphadenectomy region. The results of TVI showed the possible survival benefit of dissecting No. 8p, No. 12p, No. 13, No. 16a2 and No. 16b1 LNs in a D2-plus gastrectomy for advanced gastric antral carcinoma. The high incidence of LN metastasis for No. 8p (28.57%) and high 5-year OS for No. 16a2 (50.00%) and No.16b1 (50.00%) might result in a relatively high TVI for No. 8p station (7.1), No. 16a2 (7.1) and No. 16b1 (7.1). Kumagai’s study (10) also confirmed the possible survival benefit of dissecting No. 13, 16a2 and 16b1 and got the different results for the No. 8p and No. 12p LNs station. Notably, the high TVI in this study may be due to the small number of patients who have that LN station metastasis in this study. Therefore, a study with large sample size is needed further.
With improvements in the surgical techniques used currently, including laparoscopy and da Vinci Robot, the safety of surgery is on the rise. Contrary to some previous studies (34,35), recent studies (36,37) have shown that D2-plus lymphadenectomy is safe, and does not increase the rate of major surgical complications. At present, our results showed that D2-plus lymphadenectomy, when performed by specialized experienced surgeons, may yield better survival. We believe that, with the use of more convenient and efficient devices, D2-plus lymphadenectomy is completely feasible.
This study has several limitations. First, in this study, the endpoint was OS; we did not investigate disease-free survival. Second, our study had a single-center retrospective design. Third, our study did not have a large sample size. Thus, there is a need for a multicenter randomized clinical trial with a larger sample size to confirm our findings.
Conclusions
D2-plus lymphadenectomy should be performed to improve survival in some cases with gastric antral carcinoma, especially for patients with diffuse histology or advanced pN stage. That survival benefits may get from the dissection of No. 8p, No. 12p, No. 13, No. 16a2 and No. 16b1 LN stations. In addition, there is also a need for a multicenter randomized clinical trial with a larger sample size to confirm our findings.
Acknowledgements
This study was supported in part by grants from the Programs of National Natural Science Foundation of China (No. 81572372), National Key Research and Development Program “major chronic non-infectious disease research” (No. 2016YFC1303202), and National Key Research and Development Program “precision medicine research” (No. 2017YFC0908304).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Jingyu Deng, Email: dengery@126.com.
Han Liang, Email: tjlianghan@126.com.
References
- 1.Torre LA, Bray F, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Zheng R, Wang N, et al Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng JY Chinese Expert Consensus: lymph node examination guidance of radical surgery for gastric Cancer (2019) Zhongguo Shi Yong Wai Ke Za Zhi. 2019;39:881–9. doi: 10.19538/j.cjps.issn1005-2208.2019.09.01>. [DOI] [Google Scholar]
- 4.Lee JH, Kim JG, Jung HK, et al Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasako M, Sano T, Yamamoto S, et al D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 7.Kulig J, Popiela T, Kolodziejczyk P, et al Standard D2 versus extended D2 (D2+) lymphadenectomy for gastric cancer: an interim safety analysis of a multicenter, randomized, clinical trial. Am J Surg. 2007;193:10–5. doi: 10.1016/j.amjsurg.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Yonemura Y, Wu CC, Fukushima N, et al Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132–7. doi: 10.1007/s10147-007-0727-1. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Kumagai K, Sano T, Hiki N, et al Survival benefit of “D2-plus” gastrectomy in gastric cancer patients with duodenal invasion. Gastric Cancer. 2018;21:296–302. doi: 10.1007/s10120-017-0733-6. [DOI] [PubMed] [Google Scholar]
- 11.Eom BW, Joo J, Kim YW, et al Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery. 2014;155:408–16. doi: 10.1016/j.surg.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 12.de Manzoni G, Verlato G, Bencivenga M, et al Impact of super-extended lymphadenectomy on relapse in advanced gastric cancer. Eur J Surg Oncol. 2015;41:534–40. doi: 10.1016/j.ejso.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Morita S, Fukagawa T, Fujiwara H, et al The clinical significance of para-aortic nodal dissection for advanced gastric cancer. Eur J Surg Oncol. 2016;42:1448–54. doi: 10.1016/j.ejso.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Roviello F, Pedrazzani C, Marrelli D, et al Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. 2010;36:439–46. doi: 10.1016/j.ejso.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima T Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 16.Marrelli D, Mazzei MA, Pedrazzani C, et al High accuracy of multislices computed tomography (MSCT) for para-aortic lymph node metastases from gastric cancer: a prospective single-center study. Ann Surg Oncol. 2011;18:2265–72. doi: 10.1245/s10434-010-1541-y. [DOI] [PubMed] [Google Scholar]
- 17.Zhao P, Su X, Ge T, et al Propensity score and proximity matching using random forest. Contemp Clin Trials. 2016;47:85–92. doi: 10.1016/j.cct.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–58. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han HS, Shehta A, Ahn S, et al Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J Hepatol. 2015;63:643–50. doi: 10.1016/j.jhep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Brookhart MA, Schneeweiss S, Rothman KJ, et al Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino RB Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Sasako M, McCulloch P, Kinoshita T, et al New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–51. doi: 10.1002/bjs.1800820321. [DOI] [PubMed] [Google Scholar]
- 23.Wu WP, Deng JY, Liang H, et al Regularity of lymph node metastasis in distal gastric cancer and its clinical significance. Zhongguo Zhong Liu Lin Chuang. 2015;42:906–11. doi: 10.3969/j.issn.1000-8179.2015.18.183. [DOI] [Google Scholar]
- 24.Marrelli D, Ferrara F, Giacopuzzi S, et al Incidence and prognostic value of metastases to “posterior” and para-aortic lymph nodes in resectable gastric cancer. Ann Surg Oncol. 2017;24:2273–80. doi: 10.1245/s10434-017-5857-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Sun Z, Deng J, et al Integration and analysis of associated data in surgical treatment of gastric cancer based on multicenter, high volume databases. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:179–85. doi: 10.3760/cma.j.issn.1671-0274.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Kim SM, Min BH, Lee J, et al Protective effects of female reproductive factors on Lauren intestinal-type gastric adenocarcinoma. Yonsei Med J. 2018;59:28–34. doi: 10.3349/ymj.2018.59.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Fang WL, Wang RF, et al Clinicopathological variation of Lauren classification in gastric cancer. Pathol Oncol Res. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 28.Kashihara H, Shimada M, Yoshikawa K, et al Risk factors for recurrence of gastric cancer after curative laparoscopic gastrectomy. J Med Invest. 2017;64:79–84. doi: 10.2152/jmi.64.79. [DOI] [PubMed] [Google Scholar]
- 29.Arai H, Hironaka S, Minashi K, et al Cumulative incidence, risk factors and prognostic impact of venous thromboembolism in Japanese patients with advanced gastric cancer. Jpn J Clin Oncol. 2017;47:942–8. doi: 10.1093/jjco/hyx107. [DOI] [PubMed] [Google Scholar]
- 30.Ueno D, Matsumoto H, Kubota H, et al Prognostic factors for gastrectomy in elderly patients with gastric cancer. World J Surg Oncol. 2017;15:59. doi: 10.1186/s12957-017-1131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verlato G, Marrelli D, Accordini S, et al Short-term and long-term risk factors in gastric cancer. World J Gastroenterol. 2015;21:6434–43. doi: 10.3748/wjg.v21.i21.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang YX, Liang H, Ding XW, et al The prognostic influence of D2 lymphadenectomy with para-aortic lymph nodal dissection for gastric cancer in N3 stage. Zhonghua Wai Ke Za Zhi. 2013;51:1071–6. doi: 10.3760/cma.j.issn.0529-5815.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Cui H, Deng JY Liang H, et al Advantage of D2+ lymph node dissection for distal advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:127–30. doi: 10.3760/cma.j.issn.1671-0274.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Bonenkamp JJ, Hermans J, Sasako M, et al Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 35.Cuschieri A, Weeden S, Fielding J, et al Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng G, Jie ZG, Li ZR, et al Necessity of No.13 lymph node dissection in advanced gastric carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:145–8. doi: 10.3760/cma.j.issn.1671-0274.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Cao B, Wang Y, et al Prospective randomized controlled trial to compare laparoscopic distal gastrectomy (D2 lymphadenectomy plus complete mesogastrium excision, D2 + CME) with conventional D2 lymphadenectomy for locally advanced gastric adenocarcinoma: study protocol for a randomized controlled trial. Trials. 2018;19:432. doi: 10.1186/s13063-018-2790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]





