Abstract
BACKGROUND:
It is unclear whether maintaining pulmonary perfusion and ventilation during cardiopulmonary bypass (CPB) reduces pulmonary inflammatory tissue injury compared with standard CPB where the lungs are not ventilated and are minimally perfused. In this study, we tested the hypothesis that maintenance of lung perfusion and ventilation during CPB decreases regional lung inflammation, which may result in less pulmonary structural damage.
METHODS:
Twenty-seven pigs were randomly allocated into a control group only submitted to sternotomy (n = 8), a standard CPB group (n = 9), or a lung perfusion group (n = 10), in which lung perfusion and ventilation were maintained during CPB. Hemodynamics, gas exchanges, respiratory mechanics, and systemic interleukins (ILs) were determined at baseline (T0), at the end of 90 minutes of CPB (T90), and 180 minutes after CPB (T180). Bronchoalveolar lavage (BAL) ILs were obtained at T0 and T180. Dorsal and ventral left lung tissue samples were examined for optical and electron microscopy.
RESULTS:
At T90, there was a transient reduction in PaO2/FIO2 in CPB (126 ± 64 mm Hg) compared with the control and lung perfusion groups (296 ± 46 and 244 ± 57 mm Hg; P < 0.001), returning to baseline at T180. Serum ILs were not different among the groups throughout the study, whereas there were significant increases in BAL IL-6 (P < 0.001), IL-8 (P < 0.001), and IL-10 (P < 0.001) in both CPB and lung perfusion groups compared with the control group. Polymorphonuclear counts within the lung tissue were smaller in the lung perfusion group than in the CPB group (P = 0.006). Electron microscopy demonstrated extrusion of surfactant vesicles into the alveolar spaces and thickening of the alveolar septa in the CPB group, whereas alveolar and capillary histoarchitecture was better preserved in the lung perfusion group.
CONCLUSIONS:
Maintenance of lung perfusion and ventilation during CPB attenuated early histologic signs of pulmonary inflammation and injury compared with standard CPB. Although increased compared with control animals, there were no differences in serum or BAL IL in animals receiving lung ventilation and perfusion during CPB compared with standard CPB.
Respiratory dysfunction after cardiac surgery using cardiopulmonary bypass (CPB) is a frequent postoperative complication associated with an increased length of mechanical ventilation, hospital stay, and mortality.1–5 During standard aortobicaval CPB, intense pulmonary inflammation is initiated by multiple events including the contact of blood with foreign surfaces of the CPB circuit and ischemia-reperfusion injury.6,7 Pulmonary inflammation is further worsened by pulmonary ischemia because of diversion of blood flow from the pulmonary circulation.8 These events lead to an increase in lung permeability,6 interstitial pulmonary edema,9 and respiratory cell dysfunction.10 Taken together with interruption of mechanical ventilation, the impact of CPB-associated pulmonary inflammation is clinically expressed as a highly prevalent postoperative worsening in gas exchanges, formation of atelectasis, and augmentation of extravascular pulmonary lung water,2,9,11,12 which can be particularly significant in high-risk patients.5
Extracorporeal circulation using biventricular bypass while maintaining lung ventilation and perfusion eliminates the requirement for a membrane oxygenator and avoids lung ischemia-reperfusion by maintenance of pulmonary blood flow through the pulmonary artery. This technique has been associated with a reduction in systemic inflammation and better preservation of gas exchange and pulmonary mechanics both experimentally13 and clinically.14 However, it is uncertain whether continued lung perfusion and ventilation during extracorporeal circulation might reduce the magnitude and distribution of the inflammatory response occurring within the lung tissue.
In this study, we tested the hypothesis that maintenance of lung perfusion and ventilation during CPB decreases regional lung inflammation and that this reduction in inflammation would result in less pulmonary structural damage. To test this hypothesis, we evaluated optical and electronic histologic markers of regional lung inflammation, blood and bronchoalveolar lavage (BAL) cytokines, gas exchange, and lung mechanics in pigs undergoing sternotomy, conventional aortobicaval CPB, or biventricular bypass.
METHODS
After approval of the study by the Ethics Committee (Comissão de Ética no Uso de Animais da Faculdade de Medicina da Universidade de São Paulo; protocol number 396/03), 28 male Large White and Landrace crossbred pigs were studied. All animals used in this study were 12 weeks old. All procedures were performed according to the recommendations of Brazilian Society of Laboratory Animal Science guidelines for ethical animal research.15 The animals were fasted for 12 hours with free access to water. On the morning of the experiment, a veterinarian examined all animals before transportation to the laboratory.
The animals were randomly allocated into 1 of 3 study groups: (1) control group (n = 8), in which mechanical ventilation and sternotomy were performed but no cardiac or pulmonary bypass used; (2) a conventional aortobicaval CPB group (CPB group, n = 9), in which CPB was maintained for 90 minutes without mechanical ventilation, followed by a 90-minute period of open chest and mechanical ventilation after separation from CPB; and (3) lung perfusion group (lung perfusion group, n = 10), in which biventricular bypass was performed for 90 minutes followed by open chest mechanical ventilation for 90 minutes after weaning from bypass. Mechanical ventilation was maintained throughout the study in the lung perfusion group. The random sequence of group allocation in blocks of different sizes was obtained using Stata version 10 statistical software (StataCorp, College Station, TX), and allocation of each animal was enclosed in a brown numbered envelope and revealed at the start of each experiment.
Animal Preparation
Animals were premedicated with 5 mg·kg−1 ketamine and 0.25 mg·kg−1 midazolam IM and positioned supine. General anesthesia was induced with 3 to 5 mg·kg−1 propofol through a marginal ear vein and maintained with 1.2% isoflurane, 3 μg·kg−1·h−1 fentanyl, and 5 μg·kg−1 h pancuronium. Lactated Ringer’s solution was infused at 8 mL·kg−1·h−1. The animal’s trachea was intubated with a 6.5-mm cuffed tube, and tidal volume was set at 10 mL·kg−1, respiratory rate at 15 breaths per minute, positive end-expiratory pressure (PEEP) of 5 cm H2O, and inspired oxygen fraction of 0.6. Inspiratory time was 1 second with an inspiratory pause of 0.3 seconds (Primus, Dräger, Lubeck, Germany).
An 18-gauge catheter was inserted in the femoral artery via cut down for arterial blood pressure measurements and blood sampling, and a thermodilution pulmonary artery catheter (catheter model 131 HF7; Baxter Healthcare Corporation, Westlake Village, CA) was introduced through the right internal jugular vein. Heart rate, continuous electrocardiogram, vascular pressures, esophageal temperature, and pulse oximetry were monitored with a multiparametric monitor (IntelliVue MP40, Philips Medical Systems, Netherlands). End-tidal carbon dioxide, inspiratory oxygen, and isoflurane concentrations were monitored using a gas analyzer (Criticare Systems Inc., Waukesha, WI). Peak, plateau, and end-expiratory pressure were obtained from the calibrated pneumotachograph from the ventilator. A heat and moisture exchanger (Humid-Vent Compact-S®, Gibeck, Sweden) was placed between the Y piece connecting the respirator circuit and the tracheal tube. After anesthesia induction, a lung expansion maneuver was performed with 30 cm H2O airway pressure for 15 seconds, which was repeated for 3 times and repeated on reinitiation of mechanical ventilation in the CPB group.16 The supine position was used for the whole length of the study. Body surface area in square meters was calculated using a conversion factor applicable to pigs (k·× body weight2/3, where k = 0.09).17
Surgical Preparation
After general anesthesia and placement of monitoring cannulas, animals’ lungs were ventilated for 30 minutes for stabilization. A median sternotomy was then performed, and the pericardium and both pleurae were opened.
In the control group, the sternotomy was covered with sterile sheets and kept open throughout the experimental procedure. In the extracorporeal circulation groups, heparin (500 IU·kg−1) was administered IV, and cannulation was performed. In the CPB group, extracorporeal circulation was established using cannulas inserted into the superior (24F) and inferior (26F) vena cava and in the ascending aorta (20F). The extracorporeal circuit was connected to a centrifugal pump and membrane oxygenator (ECOBEC, Braile Biomédica, São José do Rio Preto, Brazil) previously primed with 1500 mL lactated Ringer’s solution, 20% mannitol solution (1 g·kg−1), and heparin (10,000 IU). After initiation of bypass, ventilation was stopped, and the pulmonary artery was clamped. Pump flow rate was maintained at 2.2 to 2.4 L·min−1·m−2.
In the lung perfusion group, left heart bypass was established by placing a cannula in the left atrium (30F) and in the ascending aorta (20F) with the left heart bypass circuit connected to a centrifugal pump (Biomedicus 520D, Medtronic, Eden Prairie, MN) primed with 800 mL lactated Ringer’s solution. Right heart bypass was established by connecting the superior and inferior cava cannulas to the pulmonary artery (20F) using a roller pump (ECOBEC, BraileBiomédica, SJRP, SP, Brazil) primed in the same way as in the CPB group. During cardiovascular manipulation, atrial arrhythmias were reverted by intrathoracic cardio-version with 2 J. After 90 minutes of extracorporeal circulation, animals were separated from bypass. No vasoactive drugs were used during separation from extracorporeal circulation.
Measurements
Arterial and venous blood samples for assessment of blood gases and circulating interleukin (IL) concentrations, standard hemodynamic measurements, and respiratory mechanics were obtained at 3 time points: Baseline (T0), after anesthesia induction and surgical instrumentation; 90 minutes after baseline (T90), which corresponded to immediately after the complete weaning from circulatory bypass in the CPB and lung perfusion groups; and 180 minutes after baseline (T180). BAL fluid samples were obtained at T0 and T180 for evaluation of IL. At the end of the experiment, the animals were euthanized with 2.5 mEq·kg−1 potassium chloride according to the guidelines from the Report of the American Veterinary Association Panel on Euthanasia.18 The lungs were then inflated to 20 cm H2O, and the main left bronchium was clamped. Lung tissue samples were collected from the ventral and dorsal regions of the left lower lobe.
Blood Gas Analysis and Respiratory Mechanicals
The ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2/Fio2), pulmonary shunt (shunt), and alveolar-arterial gradient of oxygen (AaDo2) were calculated using standard formulae.19 Airway pressure, expiratory tidal volume, PEEP, and plateau pressure were recorded from the mechanical ventilator. The static compliance was calculated as expiratory tidal volume/(plateau pressure − PEEP).
Serum and BAL Interleukins
Samples of blood and from BAL for analysis of IL6, IL8, and IL10 were centrifuged, and the supernatant was frozen at −70°C and analyzed using a commercial ELISA swine kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions. The BAL IL concentrations were normalized with plasmatic urea. Although BAL is a powerful technique for sampling the epithelial lining fluid of the lower respiratory tract, it results in a significant dilution of lower airway fluid. To quantify the apparent volume of epithelial lining fluid obtained by BAL, urea was used as an endogenous marker of dilution. Because urea diffuses readily through the body, plasma and in situ epithelial lining fluid urea concentrations are identical; thus, epithelial lining fluid volume can be calculated using simple dilution principles.20 Considering the degree of dilution promoted by the instillation of BAL fluid, we can compute the actual epithelial lining fluid cytokine concentrations.
BAL fluid samples were collected using a flexible bronchoscope; the tip was wedged into a subsegment of the right cranial lobe, which was then lavaged after instilling and gently aspirating 20 mL saline solution. This procedure was repeated for 3 subsegments; the final lavage fluid was then a mix of the subsegment samples.
Microscopic Tissue Examination
After sampling and fixation in 10% formaldehyde, lung tissue was embedded in paraffin and cut into 5-μm thin sections. The sections were stained with hematoxylin and eosin and examined under light microscopy. Neutrophil infiltration was reported as the number of multilobular nuclear cells divided by the area of lung parenchyma (alveolar septa) evaluated. Morphometric analysis was performed using an integrated eyepiece with a coherent system consisting of a 100-point and 50-line grid (known length) coupled to a conventional light microscope (Axioplan, Zeiss, Oberkochen, Germany). The number of polymorphonuclear neutrophils (PMNs) and amount of pulmonary tissue edema were determined using the point-counting technique.21 Briefly, counting was performed in 10 random field digital images obtained using a light microscope with a magnification of 400 times the lung tissue slice. With the aid of Image Pro-Plus software (Media Cybernetics, Rockville, MD), each lung parenchyma image was superposed with a grid of dots with an area per dot 7.2 × 104 μm2. The number of PMN in each image was divided by area of lung parenchyma (overall dot area). The edema fraction area was the calculated area of edema over the lung parenchyma area in each field.
Transmission electron microscopy was performed in 3 slices of lung tissue (2 × 2 × 2 mm) incised from the dorsal and ventral regions of the left lung to obtain a stratified random sample. The specimens were then fixed in 2.5% glutaraldehyde and phosphate buffer. Ultrathin sections were analyzed using a transmission electron microscope (JEOL 1010 Transmission Electron Microscope, JEOL Ltd., Tokyo, Japan).
Statistical Analysis
Five pilot experiments were performed to estimate the required sample size. The main outcome was a 40% PMN infiltrate increase in the CPB group. Eight animals were required per group with a type 1 error (α) of 0.05 and a power of 0.80 for a 1-tailed test.
On the basis of possible losses because of life-threatening arrhythmias during cardiovascular manipulations, we decided to use 10 animals in groups subjected to extracorporeal circulation. Temperature, hemodynamics, respiratory variables, urinary output, fluid balance, arterial and venous blood gas analysis, BAL, and serum IL were studied among the groups using the generalized estimating equation model with the within-subject variable being the study time point or lung region when PMN count and edema were analyzed. The covariance matrix was set as a model-based estimator, the working matrix correlation structure was autoregressive lag 1, and the link function used was identity. If the main effects or interactions were significant, a pairwise post hoc analysis using the Sidak test was performed.
PMN counts and edema assessment in the ventral and dorsal lung fragments were compared among the groups with a Kruskal-Wallis test and between dorsal and ventral regions with a Wilcoxon test. Differences were considered significant at P < 0.05. The results were expressed as mean ± SD, median (interquartile range, 25%–75%), or as defined otherwise. All analyses were conducted using IBM-SPSS software (version 20, IBM Corp., Armonk, NY).
RESULTS
The groups were similar in weight (control: 41 ± 3 kg, CPB: 41.2 ± 3 kg, and lung perfusion: 41.3 ± 4.3 kg; P = 0.97) and body surface area (control: 1.07 ± 0.05 m2, CPB: 1.07 ± 0.05 m2, and lung perfusion: 1.07 ± 0.07 m2; P = 0.98). Urine output was larger (P = 0.003) in the CPB (880 ± 515 mL) and lung perfusion (582 ± 931 mL) groups than in the control group (170 ± 44 mL), whereas median values of fluid balance were smaller in the CPB (478 mL; 276–718 mL) and lung perfusion (725 mL; 548–1019 mL) groups than in the control group (958 mL; 901–1042 mL; P < 0.001). Temperature decrease was more pronounced in the lung perfusion than in the CPB or the control groups (P < 0.001) as shown in Table 1. The hematocrit of both groups undergoing bypass was lower by 2% to 4% at the end of CPB compared with the control group (P < 0.01), as shown in (Table 1). One animal was excluded from the CPB group because of ventricular fibrillation.
Table 1.
Blood Temperature and Hematocrit in the Control, CPB, and Lung Perfusion groups
| Parameter | Baseline | End of bypass | 90 min after bypass | Interaction P value |
|---|---|---|---|---|
| Blood temperature (°C) | ||||
| Control group | 37.8 ± 0.8 | 38.2 ± 0.5 | 38.6 ± 1.2 | <0.001 |
| CPB group | 37.8 ± 0.6 | 37.8 ± 0.4 | 37.9 ± 0.8 | |
| LP group | 38.3 ± 1.1 | 36 ± 1.4*† | 36.6 ± 1*† | |
| Hematocrit (%) | ||||
| Control group | 29 ± 3 | 29 ± 3 | 30 ± 3 | 0.006 |
| CPB group | 28 ± 4 | 26 ± 3* | 27 ± 4 | |
| LP group | 29 ± 2 | 25 ± 3* | 26 ± 2* | |
CPB = cardiopulmonary bypass; LP = lung perfusion group.
P < 0.01 compared with baseline.
P < 0.001 compared with control group.
Hemodynamic Variables
Hemodynamic variables were similar among all groups at baseline, except for heart rate that was nonsignificantly slower in the lung perfusion group (Table 2). At the end of extracorporeal circulation, heart rate was increased in the CPB group compared with the control and lung perfusion groups (P < 0.001). Left ventricular systolic work index (LVSWI) was reduced in the CPB group compared with the control group (P = 0.01), whereas right ventricular systolic work index increased in the lung perfusion group compared with the CPB group (P < 0.001). Ninety minutes after extracorporeal circulation, the CPB group had lower LVSWI compared with the control group (P < 0.001). Although LVSWI was reduced in the lung perfusion group when compared with the control group, cardiac index was maintained within the normal range because of a reduction in systemic vascular resistance index. No other alterations were observed in other hemodynamic variables (Table 2).
Table 2.
Hemodynamic Variables in the Control, CPB, and Lung Perfusion Groups During the Study
| Parameter | Baseline | End of bypass | 90 min after bypass | Interaction P value |
|---|---|---|---|---|
| HR (bpm) | ||||
| Control group | 109 ± 18 | 111 ± 16 | 118 ± 19 | <0.001 |
| CPB group | 101 ± 17 | 128 ± 17* | 124 ± 22 | |
| LP group | 93 ± 10 | 90 ± 14†‡ | 101 ± 17‡ | |
| MAP (mm Hg) | ||||
| Control group | 69 ± 6 | 79 ± 9 | 74 ± 7 | 0.11 |
| CPB group | 66 ± 9 | 67 ± 14 | 61 ± 5 | |
| LP group | 68 ± 6 | 66 ± 17 | 56 ± 6 | |
| PAP mean (mm Hg) | ||||
| Control group | 18 ± 1 | 19 ± 2 | 19 ± 4 | 0.08 |
| CPB group | 17 ± 2 | 17 ± 2 | 17 ± 2 | |
| LP group | 19 ± 2 | 22 ± 3 | 21 ± 3 | |
| PCWP (mm Hg) | ||||
| Control group | 11 ± 2 | 11 ± 1 | 12 ± 2 | 0.37 |
| CPB group | 10 ± 3 | 9 ± 4 | 9 ± 3 | |
| LP group | 12 ± 2 | 12 ± 2 | 12 ± 1 | |
| CI (L·min−1·m−2) | ||||
| Control group | 3.9 ± 0.8 | 4.2 ± 0.8 | 4.2 ± 1 | 0.03 |
| CPB group | 4 ± 0.6 | 3.6 ± 0.8 | 3.2 ± 0.5† | |
| LP group | 3.8 ± 0.9 | 4.1 ± 0.7 | 3.6 ± 0.4 | |
| SVRI (dynes·s·cm−5·m−2) | ||||
| Control group | 1314 ± 332 | 1415 ± 291 | 1315 ± 248 | 0.02 |
| CPB group | 1204 ± 206 | 1348 ± 385 | 1350 ± 208 | |
| LP group | 1290 ± 292 | 1070 ± 294 | 1038 ±162‡ | |
| PVRI (dynes·s·cm−5·m−2) | ||||
| Control group | 144 ± 52 | 154 ± 66 | 150 ± 72 | 0.36 |
| CPB group | 152 ± 83 | 187 ± 61 | 210 ± 72 | |
| LP group | 155 ± 44 | 191 ± 38 | 209 ± 61 | |
| RVSWI (g·m−2·beat−1) | ||||
| Control group | 5 ± 1 | 6 ± 1 | 6 ± 2 | <0.001 |
| CPB group | 6 ± 1 | 4 ± 2 | 4 ± 1 | |
| LP group | 5 ± 2 | 8 ± 3*‡ | 6 ± 1 | |
| LVSWI (g·m−2·beat−1) | ||||
| Control group | 28 ± 6 | 34 ± 7 | 30 ± 5 | <0.001 |
| CPB group | 31 ± 9 | 22 ± 4† | 19 ± 4*† | |
| LP group | 31 ± 8 | 35 ± 13 | 22 ± 4*† | |
bpm = beats per minute; CI = cardiac index; CPB = cardiopulmonary bypass; HR = heart rate; LP = lung perfusion; LVSWI = left ventricular systolic work index; MAP = mean arterial pressure; PAP = pulmonary arterial pressure; PCWP = pulmonary artery occlusion pressure; PVRI = pulmonary vascular resistance; RVSWI = right ventricular systolic work index; SVRI = systemic vascular resistance index.
P < 0.05 compared with baseline.
P < 0.05 compared with control group at the same moment.
P < 0.05 compared with CPB group at the same moment.
Gas Exchange and Respiratory Mechanics
Gas exchange and respiratory mechanics were similar in all groups at baseline. At the end of the extracorporeal circulation period, the CPB group had a pronounced reduction in Pao2/Fio2 ratio (P < 0.001) and arterial pH (P = 0.01) and an increase in Paco2 (P = 0.04), pulmonary shunt (P < 0.001), and alveolar-arterial oxygen gradient (P = 0.001) when compared with the control and lung perfusion groups (Table 3). These changes reverted at 90 minutes after the end of extracorporeal circulation. The CPB group showed an increase in plateau pressure (P < 0.001) and reduction in static compliance of the respiratory system (P < 0.001) after the end of extracorporeal circulation, which returned to normal values 90 minutes later (Table 3).
Table 3.
Respiratory and Biological Variables in the Control, CPB, and Lung Perfusion Groups During the Study
| Parameter | Baseline | End of bypass | 90 min after bypass | Interaction P value |
|---|---|---|---|---|
| Pao2/Fio2 (mm Hg) | ||||
| Control group | 390 ± 96 | 464 ± 52 | 444 ± 38 | <0.001 |
| CPB group | 430 ± 37 | 201 ± 103*† | 354 ± 80 | |
| LP group | 351 ± 72 | 372 ± 77‡ | 379 ± 58 | |
| Paco2 (mm Hg) | ||||
| Control group | 39 ± 5 | 38 ± 2 | 39 ± 5 | 0.02 |
| CPB group | 41 ± 3 | 45 ± 5† | 41 ± 4 | |
| LP group | 38 ± 4 | 37 ± 4‡ | 37 ± 4 | |
| Arterial pH | ||||
| Control group | 7.46 ± 0.04 | 7.48 ± 0.03 | 7.49 ± 0.05 | 0.001 |
| CPB group | 7.46 ± 0.03 | 7.39 ± 0.04*† | 7.45 ± 0.04 | |
| LP group | 7.46 ± 0.04 | 7.45 ± 0.04‡ | 7.47 ± 0.04 | |
| Svo2 (%) | ||||
| Control group | 75 ± 5 | 76 ± 5 | 76 ± 4 | 0.001 |
| CPB group | 75 ± 6 | 63 ± 6*† | 60 ± 6*† | |
| LP group | 74 ± 6 | 79 ± 8‡ | 70 ± 6‡ | |
| Pulmonary shunt (%) | ||||
| Control group | 10 ± 5 | 7 ± 4 | 8 ± 3 | <0.001 |
| CPB group | 9 ± 2 | 22 ± 10*† | 9 ± 3 | |
| LP group | 12 ± 6 | 13 ± 5‡ | 10 ± 3 | |
| AaDo2 (mm Hg) | ||||
| Control group | 125 ± 60 | 74 ± 30 | 89 ± 25 | <0.001 |
| CPB group | 94 ± 26 | 234 ± 70*† | 140 ± 51 | |
| LP group | 155 ± 61 | 142 ± 55‡ | 134 ± 36 | |
| Airway pressure (cm H2O) | ||||
| Control group | 19 ± 3 | 19 ± 2 | 19 ± 2 | <0.001 |
| CPB group | 17 ± 3 | 21 ± 4* | 20 ± 2* | |
| LP group | 18 ± 3 | 20 ± 3* | 20 ± 3* | |
| Plateau pressure (cm H2O) | ||||
| Control group | 17 ± 2 | 17 ± 3 | 17 ± 2 | <0.001 |
| CPB group | 16 ± 3 | 20 ± 4* | 18 ± 2 | |
| LP group | 17 ±2 | 18 ± 4 | 18 ± 4 | |
| Static compliance (mL·cm H2O−1) | ||||
| Control group | 40 ± 7 | 42 ± 8 | 41 ± 6 | 0.01 |
| CPB group | 44 ± 15 | 33 ± 10* | 35 ± 5 | |
| LP group | 39 ± 6 | 36 ± 9 | 36 ± 13 | |
AaDo2 = alveolar-arterial oxygen gradient; CPB = cardiopulmonary bypass; LP = lung perfusion; Svo2 = mixed venous saturation.
P < 0.05 compared with baseline.
P < 0.05 compared with control group at the same moment.
P < 0.05 compared with CPB group at the same moment.
Plasma and Bronchoalveolar Lavage Fluid Cytokines
Serial sequential measurements of serum IL-6, IL-8, and IL-10 did not differ from baseline and had a similar behavior throughout the study for all groups (Fig. 1). In contrast, although BAL fluid IL showed no differences among groups at baseline, a significant increase in IL-6 (P < 0.001), IL-8 (P = 0.001), and IL-10 (P < 0.001) in the CPB and lung perfusion groups was observed 90 minutes after extracorporeal circulation compared with the control group (Fig. 2).
Figure 1.
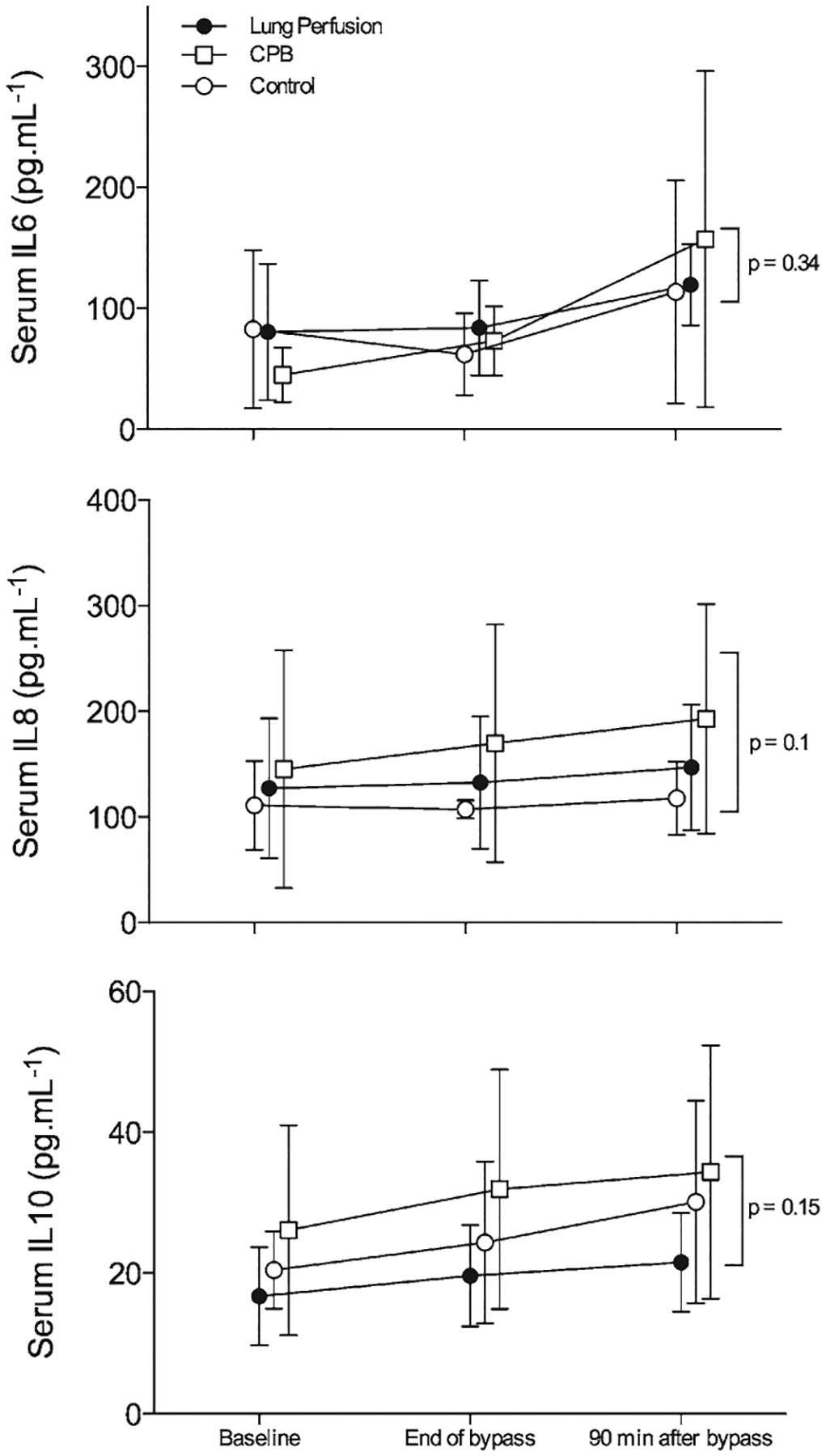
Serum interleukins (ILs; upper), interleukin-8 (middle), and interleukin-10 (lower) levels from the control (open circles), cardiopulmonary bypass (CPB; open squares), and lung perfusion (closed circles) groups throughout the study. Data are presented as mean ± SD.
Figure 2.
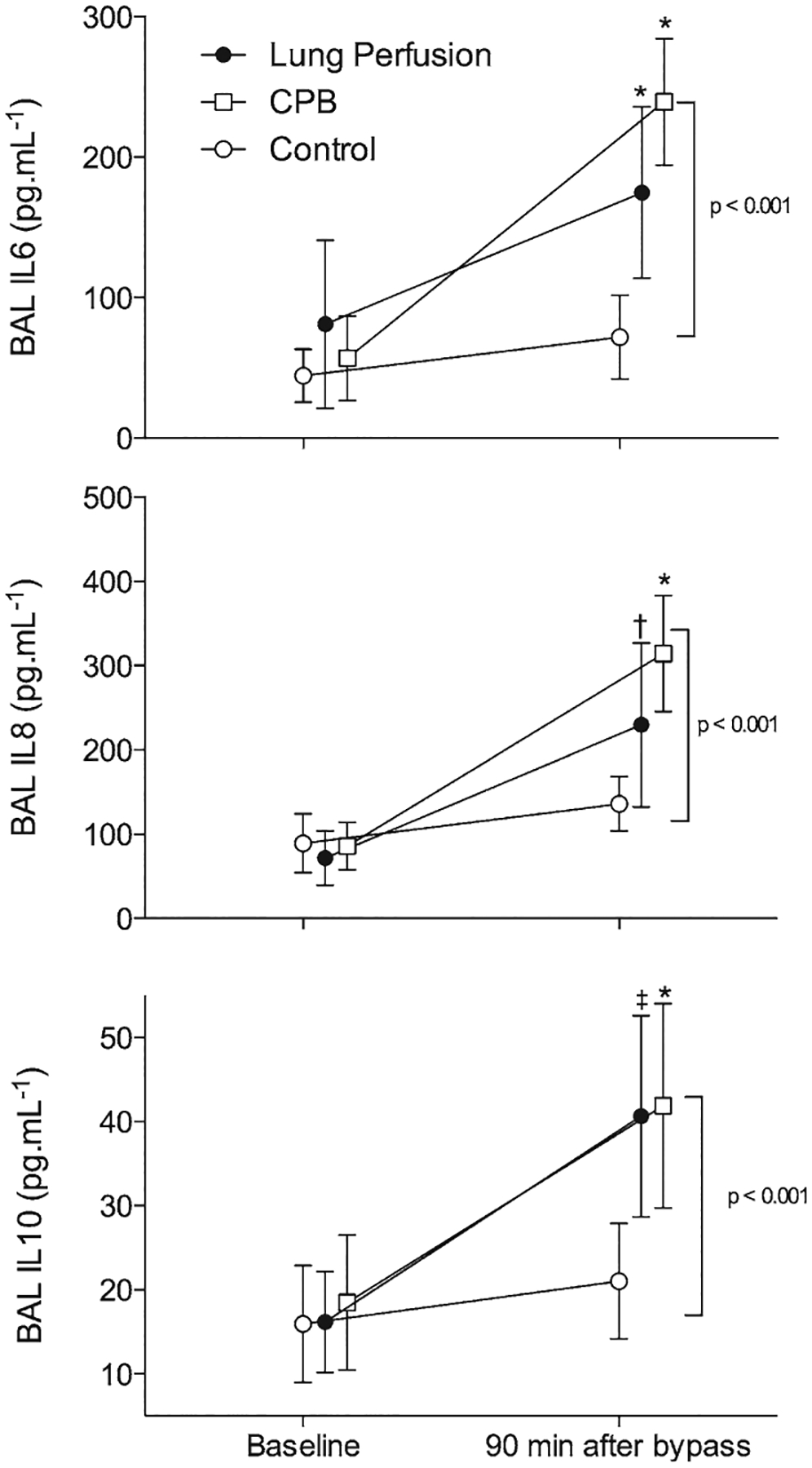
Bronchoalveolar lavage (BAL) interleukins (IL)-6 (upper), interleukin-8 (middle), and interleukin-10 (lower) levels in the control (open circles), cardiopulmonary bypass (CPB; open squares), and lung perfusion (closed circles) groups throughout the study. *Different from control group with P < 0.05, †Different with P = 0.003, and ‡Different with P = 0.001 in the Sidak post hoc test. Data are presented as mean ± SD.
Light and Transmission Electron Microscopy
Quantitative histologic analysis demonstrated that the dorsal regions of the CPB and lung perfusion groups had a larger amount of interstitial edema in the lung parenchyma compared with the control group (P = 0.016; Fig. 3A). In contrast, the same amount of edema was observed in the ventral lung regions of the 3 groups. PMN cell counts in both dorsal and ventral lung tissue samples were higher in the CPB group than in the lung perfusion and control groups (P < 0.001; Fig. 3B). Qualitative analysis of the ventral region of the CPB groups, lung fragments using light microscopy with 400× magnification revealed thickening of the interalveolar septa, edema, and PMN infiltrate while the interalveolar septa were preserved (Fig. 4).
Figure 3.
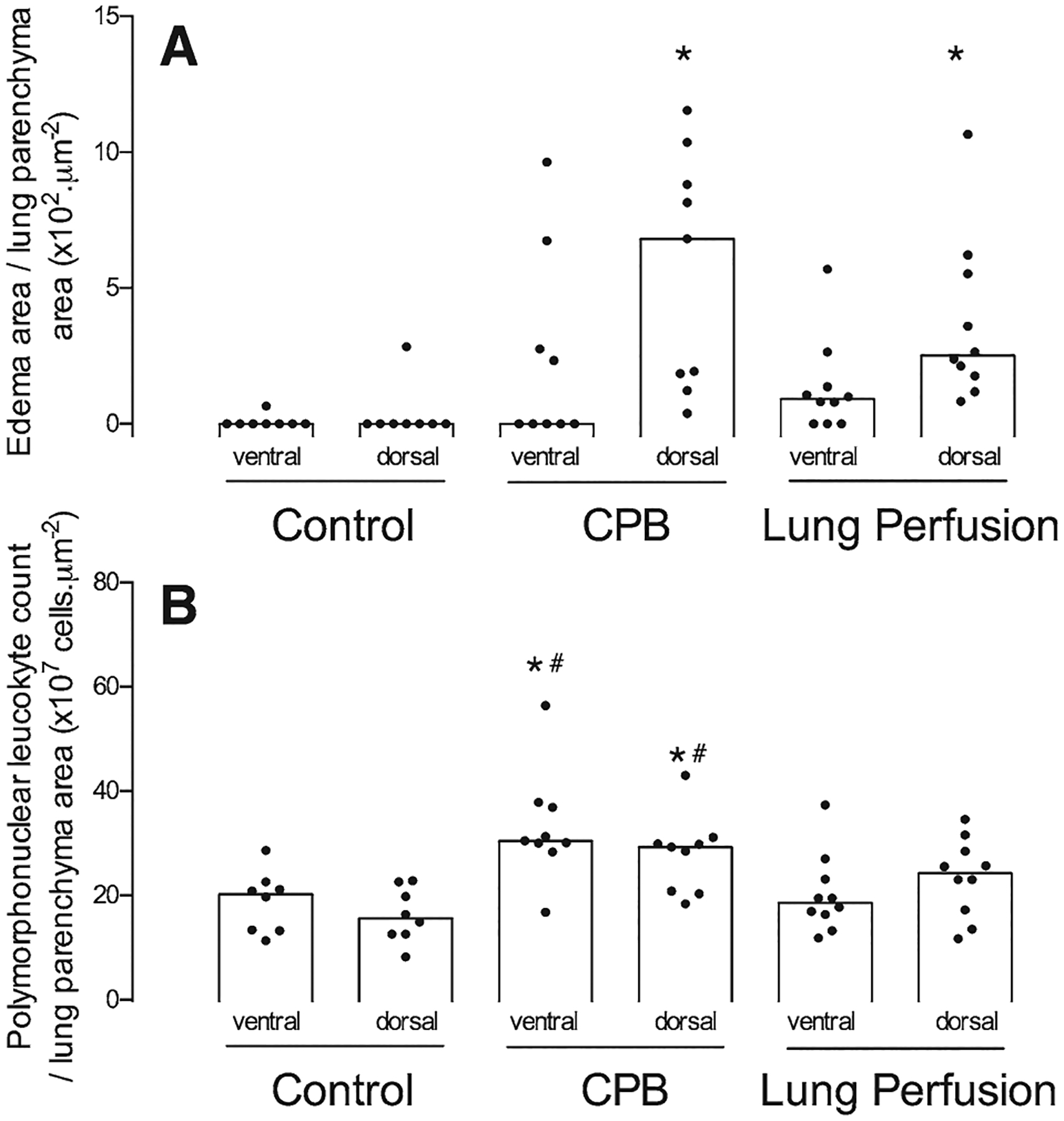
Edema (A) and polymorphonuclear neutrophil (B) counts in the ventral and dorsal regions of the lung from control, cardiopulmonary bypass (CPB), and lung perfusion groups presented as individual values and median (open bars). *Different from the same region in the control group with P < 0.05. #Different from the same region in the lung perfusion group with P < 0.05.
Figure 4.
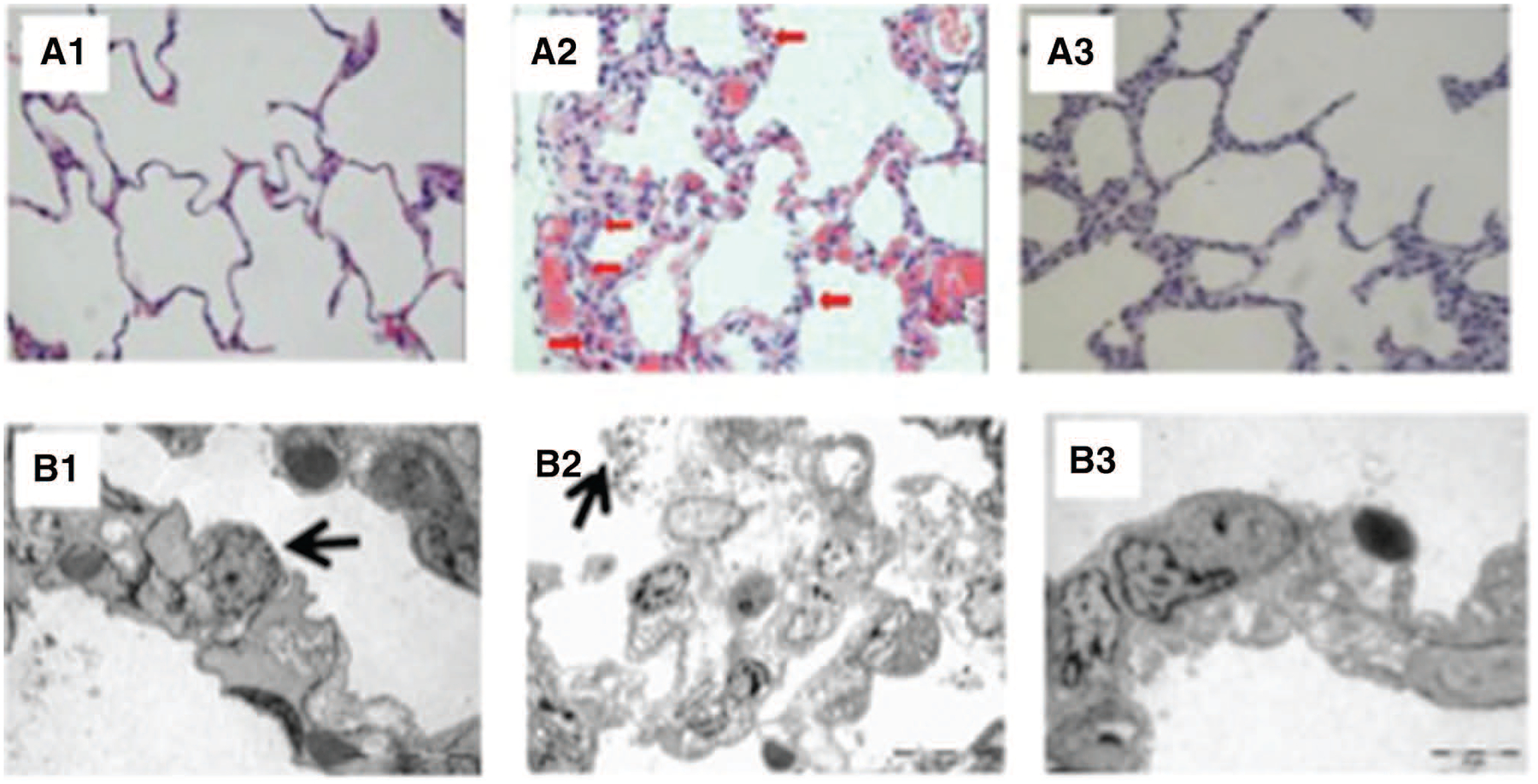
Histologic images obtained from the ventral pulmonary fragments of the control (left), cardiopulmonary bypass (CPB; middle), and lung perfusion (right) groups using light (upper; magnification 400×) and electron (lower) microscopy. As can be seen in the panel A2, the interalveolar septa were thickened by edema and infiltrated by polymorphonuclear (red arrows), thickening of the intraalveolar septa, while the interalveolar septa integrity was preserved in the lung perfusion group (A3). Panel B1 shows an electron micrograph from a lung fragment from the control group where the alveolar septa and capillaries are normal, and the histoarchitecture of the alveolar space is maintained. Note a type II pneumocyte, with lamellar bodies (arrow). Panel B2 shows a lung fragment from the CPB group (970×). Notice the extended edema vacuolization of the alveolar septa resulting in a consequent thickness of interalveolar septa. There are signs of extrusion of surfactant vesicles of surfactant into alveolar spaces (arrow). Panel B3 shows that the histoarchitecture of the interalveolar septa and capillaries was preserved, as well as the absence of interstitial edema (1650×).
Electron microscopy demonstrated preserved histoarchitecture of the interalveolar septa and capillaries, as well as the absence of interstitial edema in the lung perfusion group (Fig. 4). In contrast, signs of extrusion of surfactant vesicles into alveolar spaces and thickening of the alveolar septa were found in the CPB group.
DISCUSSION
In this study, we observed (1) a greater extent of pulmonary histologic and inflammatory signs of tissue damage in animals submitted to traditional CPB than in animals with maintained lung perfusion and ventilation during extracorporeal circulation; and (2) the presence of a regional pulmonary inflammatory response indicated by increased BAL IL with both methods of extracorporeal circulation compared with control, in the absence of a significant change in systemic levels of the same IL.
It is accepted that conventional aortobicaval CPB exacerbates the systemic inflammatory response to surgical trauma that can contribute to deterioration of postoperative pulmonary function.5,6,22 Although the main source of pulmonary artery flow is interrupted during conventional CPB, tissue perfusion is maintained through the bronchial arteries, whose flow decreases from 4.8% of total circulatory flow in physiologic conditions to 1% of total flow during CPB.23 After pulmonary reperfusion, there is an increase in the circulating concentration of proinflamma-tory mediators,6 which triggers endothelial cells to express adhesion molecules that can bind to circulating neutrophils.24,25 Neutrophils are sequestered and activated in the lungs during CPB, producing an increase in endothelial permeability with extravasation of fluid and plasma components.26,27 Neutrophils that penetrate the lung tissue release bioactive lipids, cytokines, oxygen metabolites, and enzymes, such as elastase and matrix metalloproteinases, that injure basement membranes, extracellular matrix, and type I pneumocytes.28,29
In this study, we observed that avoiding pulmonary ischemia-reperfusion injury associated with CPB by the use of biventricular bypass with maintenance of pulmonary perfusion and ventilation reduced pulmonary tissue infiltration by PMN leucocytes. This was accompanied by preserved histoarchitecture of the interalveolar septa and pulmonary capillaries compared with animals having traditional CPB. Indirect evidence of pulmonary inflammation after standard CPB was previously reported by Richter et al.30 who observed a proportionally larger increase in the concentration of IL-6 and IL-8 measured in blood samples drawn from the pulmonary vein compared with that of the right atrium. Based on these results, the authors proposed that pulmonary tissue could also be a site of inflammation during CPB. These findings are corroborated in studies of cardiac surgery patients by an increase in levels of other inflammatory markers in tracheal aspirates compared with plasma.5 In another study, the same group found that IL-6 and IL-8 were significantly increased from 30 minutes up to 4 hours after CPB compared with patients undergoing biventricular bypass. This was associated with an increase in pulmonary shunt and in AaDo2, reduced arterial Pao2, and longer postoperative mechanical ventilation time.14 However, it was unclear in those studies how atelectasis was managed throughout the experiments. This is an important factor because it can explain both worsening in gas exchange4 and pulmonary inflammation during mechanical ventilation.31 In addition, microvascular endothelial disruption was described in atelectatic zones and was associated with increased protein leakage and lung permeability.32 Our results of increased parenchymal edema in dorsal regions in both the lung perfusion and CPB groups suggest the presence of injurious local inflammatory mechanisms associated with atelectasis.4,33 The worsening histologic findings in the CPB group imply that the absence of lung perfusion and ventilation could play an additional role, globally and locally, to exacerbate lung injury.
Another potential source of lung parenchymal injury for patients undergoing cardiac surgery is mechanical ventilation using large tidal volumes.34–36 Because the lung perfusion group’s lungs were ventilated for a longer time with a tidal volume of 10 mL·kg–1, it could be expected that their lungs would present more structural pulmonary damage. Instead, this group showed fewer abnormalities than the CPB group.
To separate the contribution of lung collapse from ischemia-reperfusion injury leading to impaired gas exchange, we performed an alveolar recruitment maneuver with high airway pressures after the reinitiation of mechanical ventilation in the CPB group. This was aimed to restore lung aeration and the consequent gas exchange impairment11 as well as to minimize cyclic derecruitment known to occur in patients during cardiac surgery.4
In contrast to the findings of Massoudy et al.,14 we observed that expansion of atelectatic lung regions in the CPB group entirely restored gas exchange to a condition comparable to that present in the lung perfusion group. Hence, the gas exchange derangements observed in the CPB group in our study might have been related in part to lung collapse occurring when mechanical ventilation was stopped during extracorporeal circulation.
In contrast to previous studies, we did not find a significant difference in systemic (e.g., circulating cytokines) or regional markers of inflammation (e.g., BAL IL, regional pulmonary edema) between the methods of extracorporeal circulation. Several factors could have contributed to this finding. Lack of change in pulmonary cytokine levels would determine a lack of change in systemic cytokine levels if the main source of those cytokines were the lungs. Ninety minutes of bypass may not have been sufficient time to reveal the benefit of one method against the other and allow for distinct intensities in the inflammatory response between the groups. Finally, a larger sample size might have been necessary to detect group differences.
Our study has limitations. First, the period of observation after bypass was short, which limited the evaluation of possible recovery of lung function several hours after extracorporeal circulation. Ninety minutes of extracorporeal circulation may not have been sufficient time to reveal any benefits of maintaining lung perfusion compared with CPB for modulating the intensity of the inflammatory response between the groups. Normothermic CPB is reported to have a protective effect on systemic inflammation37 and could provide benefits for lung mechanics.38,39 In contrast, both mild40 and moderate41 hypothermia help to attenuate lung edema and inflammation. Given that the mean temperature in the lung perfusion group was 36.6°C 90 minutes after bypass, it is unlikely that this would, by itself, explain the observed differences in inflammatory findings. Finally, because this study was designed to specifically evaluate the effects of maintenance of pulmonary perfusion during extracorporeal circulation, we did not include ischemic cardiac arrest that occurs during aortic cross-clamping in human cardiac surgery. Inflammatory mediators, which are produced by the arrested heart42 and that subsequently perfuse the lung,27,43 could modify pulmonary inflammation, and the interactions between bypass and cardiac arrest are still unclear.
In conclusion, we observed that the maintenance of lung perfusion and ventilation during 90 minutes of extracorporeal circulation decreased regional lung injury likely associated with ischemia-reperfusion compared with conventional CPB. This benefit was expressed by a reduction in PMN infiltration in lung parenchyma, preserved histoarchitecture of interalveolar septa and capillaries, and the absence of interstitial edema. Further studies are necessary to investigate possible beneficial long-lasting effects of maintenance of pulmonary perfusion and ventilation during CPB.
Funding:
This study was supported by grants from Brazilian National Council for Scientific and Technological Development (CNPQ; grant number 478485/2004-2).
Footnotes
DISCLOSURES
Name: Claudia Regina da Costa Freitas, MD, PhD.
Contribution: This author helped design the study, conduct the study, collect the data, analyze the data, and prepare the manuscript, and is the author responsible for archiving the study files.
Attestation: Claudia Regina da Costa Freitas approved the final manuscript.
Name: Luiz Marcelo Sa Malbouisson, MD, PhD.
Contribution: This author helped design the study, analyze the data, and prepare the manuscript.
Attestation: Luiz Marcelo Sa Malbouisson approved the final manuscript.
Name: Anderson Benicio, MD, PhD.
Contribution: This author helped design the study and collect the experimental data.
Attestation: Anderson Benicio approved the final manuscript.
Name: Elnara Marcia Negri, MD, PhD.
Contribution: This author analyzed lung fragments.
Attestation: Elnara Marcia Negri approved the final manuscript.
Name: Filipe Minussi Bini, MD.
Contribution: This author helped collect the experimental data.
Attestation: Filipe Minussi Bini approved the final manuscript.
Name: Cristina Oliveira Massoco, DVM, PhD.
Contribution: This author helped conduct the interleukin analysis.
Attestation: Cristina Oliveira Massoco approved the final manuscript.
Name: Denise Aya Otsuki, PhD.
Contribution: This author helped collect the experimental data and conduct the preoperative evaluation of the animals.
Attestation: Denise Aya Otsuki approved the final manuscript.
Name: Marcos Francisco Vidal Melo, MD, PhD.
Contribution: This author helped analyze the data and write the manuscript.
Attestation: Marcos Francisco Vidal Melo approved the final manuscript.
Name: Maria Jose Carvalho Carmona, MD, PhD.
Contribution: This author helped design the study, conduct the study, collect the data, analyze the data, and prepare the manuscript, and is the author responsible for archiving the study files.
Attestation: Maria Jose Carvalho Carmona approved the final manuscript.
This manuscript was handled by: Charles W. Hogue, MD.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
REFERENCES
- 1.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 1999;68:1107–15 [DOI] [PubMed] [Google Scholar]
- 2.Szeles TF, Yoshinaga EM, Alenca W, Brudniewski M, Ferreira FS, Auler JO, Carmona MJ, Malbouisson LM. Hypoxemia after myocardial revascularization: analysis of risk factors. Rev Bras Anestesiol 2008;58:124–36 [DOI] [PubMed] [Google Scholar]
- 3.Yende S, Wunderink R. Causes of prolonged mechanical ventilation after coronary artery bypass surgery. Chest 2002;122:245–52 [DOI] [PubMed] [Google Scholar]
- 4.Carvalho AR, Ichinose F, Schettino IA, Hess D, Rojas J, Giannella-Neto A, Agnihotri A, Walker J, MacGillivray TE, Vidal Melo MF. Tidal lung recruitment and exhaled nitric oxide during coronary artery bypass grafting in patients with and without chronic obstructive pulmonary disease. Lung 2011;189:499–509 [DOI] [PubMed] [Google Scholar]
- 5.de Prost N, El-Karak C, Avila M, Ichinose F, Vidal Melo MF. Changes in cysteinyl leukotrienes during and after cardiac surgery with cardiopulmonary bypass in patients with and without chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 2011;141:1496–502.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest 2001;119:31–6 [DOI] [PubMed] [Google Scholar]
- 7.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003;75:S715–20 [DOI] [PubMed] [Google Scholar]
- 8.Chai PJ, Williamson JA, Lodge AJ, Daggett CW, Scarborough JE, Meliones JN, Cheifetz IM, Jaggers JJ, Ungerleider RM. Effects of ischemia on pulmonary dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1999;67:731–5 [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues RR, Sawada AY, Rouby JJ, Fukuda MJ, Neves FH, Carmona MJ, Pelosi P, Auler JO, Malbouisson LM. Computed tomography assessment of lung structure in patients undergoing cardiac surgery with cardiopulmonary bypass. Braz J Med Biol Res 2011;44:598–605 [DOI] [PubMed] [Google Scholar]
- 10.Massoudy P, Piotrowski JA, van de Wal HC, Giebler R, Marggraf G, Peters J, Jakob HG. Perfusing and ventilating the patient’s lungs during bypass ameliorates the increase in extravascular thermal volume after coronary bypass grafting. Ann Thorac Surg 2003;76:516–21 [DOI] [PubMed] [Google Scholar]
- 11.Magnusson L, Zemgulis V, Wicky S, Tydén H, Thelin S, Hedenstierna G. Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 1997;87:1153–63 [DOI] [PubMed] [Google Scholar]
- 12.Vargas FS, Terra-Filho M, Hueb W, Teixeira LR, Cukier A, Light RW. Pulmonary function after coronary artery bypass surgery. Respir Med 1997;91:629–33 [DOI] [PubMed] [Google Scholar]
- 13.Mendler N, Heimisch W, Schad H. Pulmonary function after biventricular bypass for autologous lung oxygenation. Eur J Cardiothorac Surg 2000;17:325–30 [DOI] [PubMed] [Google Scholar]
- 14.Massoudy P, Zahler S, Tassani P, Becker BF, Richter JA, Pfauder M, Lange R, Meisner H. Reduction of pro-inflamma-tory cytokine levels and cellular adhesion in CABG procedures with separated pulmonary and systemic extracorporeal circulation without an oxygenator. Eur J Cardiothorac Surg 2000;17:729–36 [DOI] [PubMed] [Google Scholar]
- 15.Brazilian Society of Laboratory Animal Science. Guidelines for Ethical Animal Research 2013. [Google Scholar]
- 16.Rothen HU, Neumann P, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth 1999;82:551–6 [DOI] [PubMed] [Google Scholar]
- 17.Holt JP, Rhode EA, Kines H. Ventricular volumes and body weight in mammals. Am J Physiol 1968;215:704–15 [DOI] [PubMed] [Google Scholar]
- 18.American Veterinary Medical Association. 2000 report of the AVMA panel on euthanasia. J Am Vet Med Assoc 2001;218:669–96 [DOI] [PubMed] [Google Scholar]
- 19.Bach JF. Hypoxemia: a quick reference. Vet Clin North Am Small Anim Pract 2008;38:423–6, vii [DOI] [PubMed] [Google Scholar]
- 20.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60:532–8 [DOI] [PubMed] [Google Scholar]
- 21.Gil J Models of Lung Disease—Microscopy and Structural Methods. CRC Press, 1990 [Google Scholar]
- 22.Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47–55 [DOI] [PubMed] [Google Scholar]
- 23.Schlensak C, Doenst T, Preusser S, Wunderlich M, Kleinschmidt M, Beyersdorf F. Bronchial artery perfusion during cardiopulmonary bypass does not prevent ischemia of the lung in piglets: assessment of bronchial artery blood flow with fluorescent microspheres. Eur J Cardiothorac Surg 2001;19:326–31 [DOI] [PubMed] [Google Scholar]
- 24.Huber AR, Kunkel SL, Todd RF III, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 1991;254:99–102 [DOI] [PubMed] [Google Scholar]
- 25.Springer TA. Adhesion receptors of the immune system. Nature 1990;346:425–34 [DOI] [PubMed] [Google Scholar]
- 26.Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest 1989;83:1122–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Richter JA, Meisner H. Significant leukocyte and platelet retention during pulmonary passage after declamping of the aorta in CABG patients. Eur J Med Res 1999;4:178–82 [PubMed] [Google Scholar]
- 28.Butler J, Parker D, Pillai R, Westaby S, Shale DJ, Rocker GM. Effect of cardiopulmonary bypass on systemic release of neutrophil elastase and tumor necrosis factor. J Thorac Cardiovasc Surg 1993;105:25–30 [PubMed] [Google Scholar]
- 29.Carney DE, Lutz CJ, Picone AL, Gatto LA, Ramamurthy NS, Golub LM, Simon SR, Searles B, Paskanik A, Snyder K, Finck C, Schiller HJ, Nieman GF. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation 1999;100:400–6 [DOI] [PubMed] [Google Scholar]
- 30.Richter JA, Meisner H, Tassani P, Barankay A, Dietrich W, Braun SL. Drew-Anderson technique attenuates systemic inflammatory response syndrome and improves respiratory function after coronary artery bypass grafting. Ann Thorac Surg 2000;69:77–83 [DOI] [PubMed] [Google Scholar]
- 31.Zaher TE, Miller EJ, Morrow DM, Javdan M, Mantell LL. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radic Biol Med 2007;42:897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duggan M, McNamara PJ, Engelberts D, Pace-Asciak C, Babyn P, Post M, Kavanagh BP. Oxygen attenuates atelectasis-induced injury in the in vivo rat lung. Anesthesiology 2005;103:522–31 [DOI] [PubMed] [Google Scholar]
- 33.de Prost N, Costa EL, Wellman T, Musch G, Winkler T, Tucci MR, Harris RS, Venegas JG, Vidal Melo MF. Effects of surfactant depletion on regional pulmonary metabolic activity during mechanical ventilation. J Appl Physiol 2011;111:1249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis 1993;148:1194–203 [DOI] [PubMed] [Google Scholar]
- 35.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S; IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428–37 [DOI] [PubMed] [Google Scholar]
- 36.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frering B, Philip I, Dehoux M, Rolland C, Langlois JM, Desmonts JM. Circulating cytokines in patients undergoing normothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg 1994;108:636–41 [PubMed] [Google Scholar]
- 38.Ranucci M, Soro G, Frigiola A, Menicanti L, Ditta A, Candido G, Tambalo S. Normothermic perfusion and lung function after cardiopulmonary bypass: effects in pulmonary risk patients. Perfusion 1997;12:309–15 [DOI] [PubMed] [Google Scholar]
- 39.Tönz M, Mihaljevic T, Pasic M, von Segesser LK, Turina M. The warm versus cold perfusion controversy: a clinical comparative study. Eur J Cardiothorac Surg 1993;7:623–7 [DOI] [PubMed] [Google Scholar]
- 40.Cruces P, Ronco R, Erranz B, Conget P, Carvajal C, Donoso A, Díaz F. Mild hypothermia attenuates lung edema and plasma interleukin-1β in a rat mechanical ventilation-induced lung injury model. Exp Lung Res 2011;37:549–54 [DOI] [PubMed] [Google Scholar]
- 41.Chin JY, Koh Y, Kim MJ, Kim HS, Kim WS, Kim DS, Kim WD, Lim CM. The effects of hypothermia on endotoxin-primed lung. Anesth Analg 2007;104:1171–8 [DOI] [PubMed] [Google Scholar]
- 42.Ambrosio G, Tritto I. Reperfusion injury: experimental evidence and clinical implications. Am Heart J 1999;138:S69–75 [DOI] [PubMed] [Google Scholar]
- 43.McFarlane T, Kleinloog R, Bennett M. Pulmonary sequestration of cardioplegia administered via the aortic root during aorto-coronary bypass surgery. Perfusion 2007;22:93–101 [DOI] [PubMed] [Google Scholar]


